Abstract
Objective
α-Klotho (α-KL), a protein with antiaging properties, regulates phosphate, calcium, and bone metabolism, induces resistance to oxidative stress, and may participate in insulin signaling. The role of α-KL in neonates, known to be prone to metabolic disturbances and oxidative stress, is not known. The aim of this study was to evaluate circulating soluble α-KL concentrations in preterm and full-term neonates and unravel possible correlations with growth, metabolism, and indices of oxidative stress.
Design
Prospective study.
Methods
Plasma-soluble α-KL levels were determined by specific ELISA in 50 healthy neonates (25 preterm, mean (S.D.) gestational age (GA) 33.7 (1.1) weeks, and 25 full-term infants) at days 14 and 28 of life. Associations of α-KL with anthropometric, metabolic parameters, and indices of oxidative stress were examined.
Results
α-KL levels were significantly higher in full-term than in preterm infants at both days 14 (1099 (480) pg/ml vs 884 (239) pg/ml respectively; P<0.05) and 28 (1277 (444) pg/ml vs 983 (264) pg/ml respectively; P<0.01). In both preterm and full-term infants, α-KL levels increased significantly from day 14 to 28 of life (P<0.001). Circulating α-KL concentrations correlated with GA (β=0.32, P=0.001), body weight (β=0.34, P=0.001), body length (β=0.33, P=0.001), 1,25-dihydroxy-vitamin D level (β=0.24, P<0.05), and malondialdehyde level (β=0.20, P<0.05) but not with glucose, insulin, or homeostasis model assessment index of insulin resistance values.
Conclusions
Soluble α-KL levels rise as GA and postnatal age advance in neonates and may have an impact on vitamin D metabolism and oxidative stress. Whether α-KL may have a role in the regulation of infants’ growth should be further studied.
Introduction
In Greek mythology, Klotho – the daughter of Zeus and Themis – was combing and spinning the thread of life. In science, the name Klotho was given to a gene (α-klotho (α-kl) gene), first identified in 1997, whose disruption resulted in premature aging-related phenotypes in a mouse model (1, 2). The α-kl mutant mice exhibited early progressive arteriosclerosis, neural degeneration, skin and gonadal atrophy, infertility, calcification of soft tissues, pulmonary emphysema, and a short life span.
The α-kl gene encodes a 130 kDa (1014 amino acids) type I membrane protein (α-KL protein) expressed mainly in the kidneys, parathyroid glands, and choroid plexus of the brain but also at lower levels in other organs, including the liver, skeletal muscles, adipose tissue, and the placenta (1, 3, 4). The extracellular domain of α-KL protein is shed and secreted into the blood (soluble α-KL protein), exerting hormonal actions (2, 5, 6). This fragment is also detectable in the cerebrospinal fluid and urine (6, 7). α-KL protein participates in the regulation of parathyroid hormone (PTH) secretion and vitamin D biosynthesis, in the transepithelial transport of calcium ions (Ca2+) in the choroid plexus and kidney, and also in phosphate reabsorption by the kidney (8, 9, 10).
Although its molecular mechanisms of action have not been fully elucidated, α-KL protein acts as a cofactor of fibroblast growth factor 23 (FGF23), a hormone produced by osteoblasts, that enhances renal phosphate excretion and suppresses circulating 1,25-dihydroxy-vitamin D (1,25(OH)2D) levels (9, 11, 12). In addition, α-KL protein plays a critical role in transepithelial Ca2+ transport by regulating the abundance of transient receptor potential vanilloid 5 (TRPV5) channels and by recruiting Na+/K+-ATPase to the cell surface membrane (7, 10, 12, 13). A decrease in α-KL protein in mice and humans results in severe hyperphosphatemia and increased 1,25(OH)2D concentrations followed by increased PTH levels, hypercalcemia, and elevated FGF23 serum concentrations in compensation for the impaired FGF23 signaling (10). On the other hand, an increase in circulating α-KL protein concentrations led to elevation of FGF23 signaling, phosphaturia, severe hypophosphatemia, and decreased 1,25(OH)2D circulating levels (hypophosphatemic rickets) associated with increased PTH circulating levels and marked parathyroid hyperplasia (10, 14).
Although the majority of studies have focused on the role of α-KL protein in calcium and phosphorus homeostasis, there is also evidence that α-KL induces resistance against oxidative stress (15) while it possibly suppresses insulin signaling and participates in the pathogenesis of insulin resistance (IR) (2, 16). Moreover, it has been reported that α-KL promotes adipocyte differentiation (17) while, interestingly, leptin, the ob gene product secreted by adipocytes, is involved in the control of calcium, phosphate, and 1,25(OH)2D homeostasis via stimulation of FGF23 synthesis (18).
Neonates, especially preterm ones, are prone to metabolic disturbances of calcium, phosphate, glucose, and vitamin D and are also susceptible to oxidative stress due to immature antioxidant defense mechanisms (19, 20). Moreover, preterm infants are at risk for the later development of IR (21). Indeed, prepubertal children aged between 4 and 10 years old, who had been born prematurely, had a reduction in insulin sensitivity compared with children born at term (22). Interestingly, a previous study showed that IR may be present even at birth in preterm infants (23). To our knowledge, α-KL protein has been little studied in neonates; its circulating levels were determined only in a study of full-term babies at birth and/or at day 4 of life (4).
The aim of this study was to evaluate the circulating concentrations of α-KL protein during the first month of age in preterm and full-term infants and to unravel possible associations with anthropometric (body weight and length) and metabolic parameters (serum calcium, phosphate, FGF23, 1,25(OH)2D, PTH, glucose, insulin, homeostasis model assessment index of IR (HOMA-IR)), and indices of oxidative stress (malondialdehyde (MDA) concentration and superoxide dismutase (SOD) activity).
Materials and methods
Subjects and study protocol
The study population consisted of 50 healthy neonates admitted to our unit after birth: 25 preterm babies of mean (S.D.) gestational age (GA) 33.7 (1.1) weeks, birth weight 1726 (268) g, and male:female ratio 12:13 and 25 full-term infants (GA 39.1 (1.3) weeks and birth weight 3033 (460) g) who had similar gender distribution to that of preterm infants. Ten out of 50 neonates (five preterm and five full-term babies) were small for GA (SGA; birth weight below the 10th percentile after adjusting for GA and gender). GA was estimated from the last menstrual period and confirmed by fetal ultrasound measurements and clinical examination of the neonate according to the new Ballard score (24).
Criteria for eligibility in the study included: i) absence of congenital malformations or major neonatal morbidities (respiratory distress requiring assisted ventilation or oxygen supplementation after day 3 of life in preterm and full-term infants respectively, hypotension with need for inotropes, intraventricular hemorrhage> grade I (criteria Volpe), sepsis, and necrotizing enter-ocolitis); and ii) tolerance of full enteric feeding (≥ 150 ml/kg per day) up to day 3 of life for full-term infants and day 10 of life for preterm infants. All the infants were fed with breast milk and formula during the experiment. In order to avoid differences in nutrient intake that might have had an impact on α-KL levels or other blood measurements, the same commercial formula (S26Gold; Wyeth Nutritionals, Askeaton, Ireland) was used. In all the infants, anthropometric parameters were obtained periodically by the same investigator. Body weight was obtained daily in preterm infants and weekly in full-term infants using a standard electronic scale. Recumbent length was measured using a standardized length board.
Blood samples were drawn from a forearm vein in all the infants before feeding on the morning of days 14 and 28 of life for routine blood tests as well as for determining plasma levels of soluble α-KL protein; serum concentrations of calcium, phosphate, FGF23, 1,25(OH)2D, PTH, glucose, and insulin; and oxidative stress indices; MDA level in serum as marker of oxidative stress damage and SOD activity in washed red blood cells as a free radical scavenging system were determined. In addition, HOMA-IR index was calculated as fasting glucose (in mmol/l)× fasting insulin (in mIU/l) divided by 22.5 (25). Plasma and serum samples were stored at −80 °C until assayed. The ‘Aghia Sophia’ Children’s Hospital Ethics Committee approved the study and informed parental consent was also obtained.
Assays
Plasma soluble α-KL protein levels were determined using a sandwich ELISA established by Yamazaki et al. (26); the ELISA kit was provided from Kyowa Hakko-Kirin (Tokyo, Japan). The assay determines the extracellular domain of membrane α-KL protein, which is shed and secreted into the blood (cleaved α-KL). The intra- and interassay coefficients of variation (CV) ranged from 2.7 to 9.8% and the sensitivity limit was 94 pg/ml (26). Serum calcium, phosphate, and glucose levels were measured using the Siemens Advia 1800 Clinical Chemistry System (Siemens Healthcare Diagnostics, Erlangen, Germany). Serum levels of intact FGF23 were determined using a commercial sandwich ELISA kit (Kainos Laboratories, Inc., Tokyo, Japan). The intra- and interassay CV ranged from 2.0 to 2.8% and from 2.4 to 3.8% respectively; the sensitivity limit was 3 pg/ml. Serum 1,25(OH)2D levels were measured using the 1,25(OH)2D RIA kit (Immunodiagnostic Systems Ltd., Boldon, UK). Sensitivity of the method was 5 pmol/l (2.1 pg/ml), whereas CV within and between runs were <12 and <14% respectively. Serum intact PTH levels were determined by a sandwich electrochemiluminescence immunoassay (ECLIA) using the Elecsys PTH assay (Elecsys PTH, Roche Diagnostics). The sensitivity limit was 1.20 pg/ml (0.127 pmol/l). The intra-assay CV was <2%, whereas the interassay CV ranged from 2.9 to 4.4%.
Serum insulin concentrations were measured by an ECLIA using the automated analyser Cobas e 411 and the Elecsys Insulin Kit (Roche Diagnostics). The intra-and interassay CV did not exceed 2.0 and 2.8% respectively; the sensitivity limit was 0.2 mIU/l.
Serum MDA was measured by a HPLC system using a Chromsystems reagent kit (Chromsystems Instruments and Chemical GmbH, Munich, Germany). The intra-assay and interassay CV were 1.8 and 6.2% respectively. The sensitivity limit of the assay was 0.01 μmol/l. SOD activity was determined in washed erythrocytes using the Ransod kit (RANDOX Laboratories Ltd., Crumlin, UK). The assay employs xanthine and xanthine oxidase to generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride to form a red formazan dye. The SOD activity is measured by the degree of inhibition of this reaction. The sensitivity limit was 0.06 U/ml and intra- and interassay CV were 3.5–4.6 and 5.9–7.0% respectively. Results obtained in SOD units per ml of diluted blood were converted to SOD units per gram of hemoglobin by the type: SOD (in units/ml) divided by hemoglobin (in g/ml).
Statistical analyses
The calculation of sample size of the study was based on a previous report of soluble α-KL concentrations in healthy children (26). Assuming an alpha risk of 0.05, a power of 0.80, and a bilateral test, it was estimated that ~ 15 neonates was the minimum number of infants needed in each group to detect a significant difference of one S.D. in mean α-KL levels between full-term and preterm infants.
Data are presented as mean (S.D.), apart from insulin and HOMA-IR values expressed as median (25th–75th percentiles) due to non-normal distribution. Groups were compared for quantitative variables by the Student’s t or Mann–Whitney U test as appropriate. Univariate and multiple regression analyses were used to examine relations among the variables of interest. Values of α-KL protein were normally distributed (Kolmogorov–Smirnov test) both overall and for pre-term and full-term infants separately, so that a logarithmic transformation was not required. To assess the effects of both the prematurity and the postnatal age on α-KL protein levels, a one-way repeated measures ANOVA was used to compare the values of α-KL protein on days 14 and 28 of life in preterm and full-term infants. Levels of statistical significance were set at P<0.05. All statistical analyses were performed using the SPSS statistical package (SPSS, version 19.0, Chicago, IL, USA).
Results
Body weight and length, metabolic measurements, indices of oxidative stress, and serum FGF23 levels in preterm and full-term infants are shown in Table 1. A significant effect of preterm birth (F=6.0, P=0.01) and postnatal age – day 14 vs 28 of life – (F=27.7, P<0.001) on soluble α-KL protein levels was shown by one-way repeated measures ANOVA. Mean (S.D.) soluble α-KL protein levels were significantly higher in full-term than preterm infants at both days 14 (1099 (480) pg/ml vs 884 (239) pg/ml respectively; P<0.05) and 28 of life (1277 (444) pg/ml vs 983 (264) pg/ml respectively; P<0.01; Fig. 1). In both preterm and full-term infants, α-KL levels increased significantly from day 14 to 28 of life (P<0.001; Fig. 1). No significant interaction between preterm birth and postnatal age on soluble α-KL protein levels was observed. Plasma α-KL levels did not differ significantly between SGA and appropriate for GA neonates. Mean (S.D.) plasma concentrations of soluble α-KL protein on day 14 of life were 794 (112) pg/ml in SGA preterm infants and 963 (286) pg/ml in SGA full-term infants whereas, on day 28 of life, soluble α-KL protein levels were 921 (182) pg/ml and 1196 (103) pg/ml in SGA preterm and full-term infants respectively. As shown in Table 1, there was a rise in 1,25(OH)2D levels, in parallel with the rise in α-KL concentrations from the 14th to the 28th day of life, while a parallel decline in FGF23 and phosphate levels was observed in both preterm and full-term infants.
Table 1.
Body weight and length, metabolic measurements, and indices of oxidative stress in preterm and full-term infants. The data represent the mean±S.D., except for insulin and HOMA-IR values expressed as median (25th–75th percentiles).
| Preterm infants (n=25)
|
Full-term infants (n=25)
|
|||
|---|---|---|---|---|
| Day 14 | Day 28 | Day 14 | Day 28 | |
| Body weight (g) | 1934±295§ | 2266±267 | 3052±602*,§ | 3852±631* |
| Body length (cm) | 45.5±2.3§ | 47.8±1.9 | 51.6±2.8*,§ | 54.4±2.9* |
| Glucose (mg/dl) | 83±13 | 89±11 | 82±10 | 89±12 |
| Calcium (mg/dl) | 9.4±0.5 | 9.5±0.5 | 9.6±0.8 | 9.7±0.5 |
| Phosphate (mg/dl) | 7.4±0.6§ | 6.8±0.5 | 7.6±0.7§ | 6.5±0.6‡ |
| FGF23 (pg/ml) | 59.5±32.3¶ | 46.2±18.3 | 77.2±33.8¶ | 51.7±37.9 |
| 1,25(OH)2D (pg/ml) | 48.1±14.7§ | 75.1±12.6 | 60.1±16.1‡,¶ | 71.4±17.7 |
| Parathormone (pg/ml) | 55.7±18.2 | 66.9±28.5 | 38.7±13.8*,|| | 52.1±20.9 |
| Insulin (μU/ml) | 4.9 (2.6–7.3) | 4.7 (2.7–6.2) | 5.0 (2.3–9.2) | 5.9 (2.2–9.7) |
| HOMA-IR | 1.0 (0.6–1.3) | 1.0 (0.6–1.5) | 1.0 (0.5–1.8) | 1.1 (0.5–2.1) |
| MDA (μmol/l) | 0.24±0.10 | 0.22±0.10 | 0.22±0.10 | 0.26±0.16 |
| SOD (U/ml) | 93.1±27.5 | 77.9±25.2 | 93.8±19.4 | 95.4±36.3 |
| SOD (U/g hemoglobin) | 1509±250 | 1696±389 | 1536±214¶ | 1699±304 |
P≤0.001,
P<0.05 compared with preterm infants of same age;
P≤0.001,
P≤0.01,
P<0.05 compared with repeat measurements on day 28.
Figure 1.
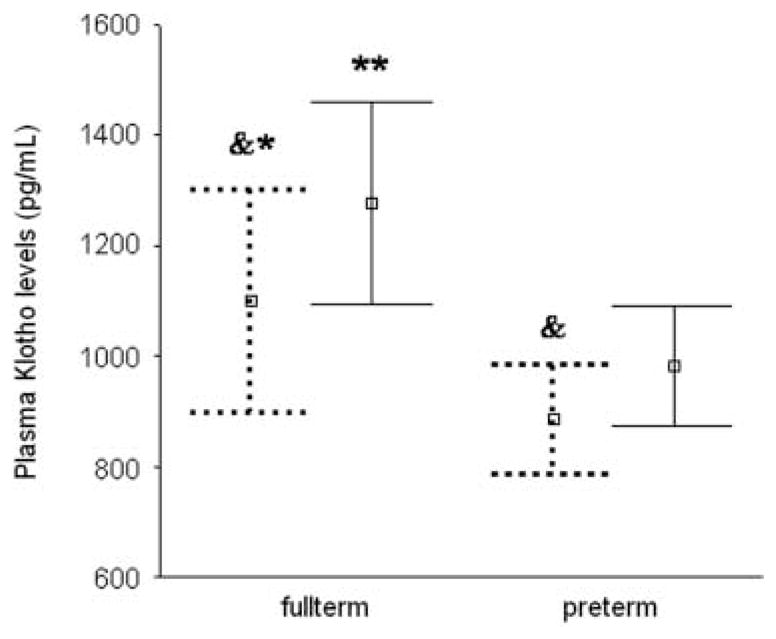
Plasma α-KL levels in full-term and preterm infants. The bars represent mean (95% confidence intervals) of α-KL concentrations at days 14 (dashed lines) and 28 of life (continuous lines). Compared with repeat measurements on day 28 of life; &P<0.001. Compared with preterm infants of same age; **P<0.01, *P<0.05.
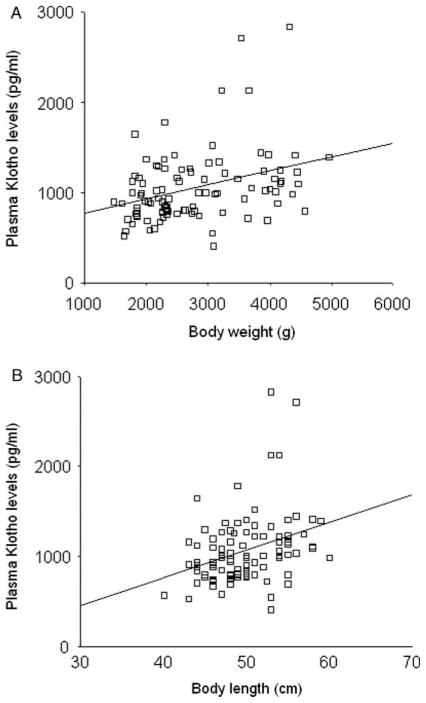
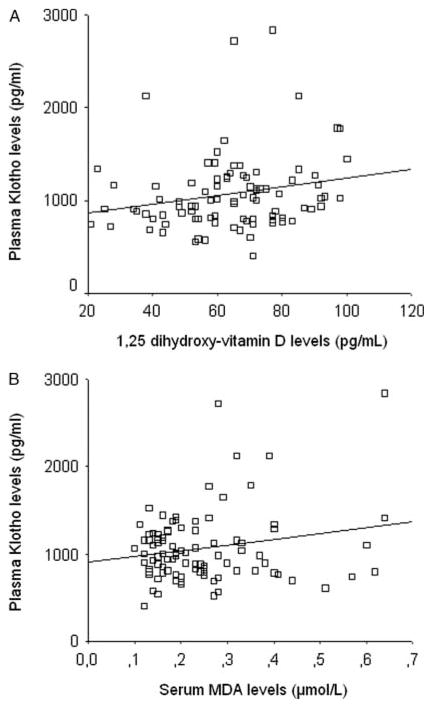
In the total study population, soluble α-KL protein levels correlated positively with GA (β=0.32, P=0.001), body weight (β=0.34, P=0.001; Fig. 2A), and length (β=0.33, P=0.001, Fig. 2B), as well as with 1,25(OH)2D (β=0.24, P<0.05; Fig. 3A) and MDA concentrations (β=0.20, P<0.05; Fig. 3B) but not with calcium, phosphate, FGF23, PTH, SOD, glucose, insulin, or HOMA-IR values. SGA status did not have any significant effect on soluble α-KL protein levels in regression analysis.
Figure 2.
Correlation between plasma α-KL protein concentrations and body weight (A) and length (B) measurements at 14th and 28th day of life. Each open square represents an individual value of each subject. The line represents the regression slope (β=0.34, P=0.001 (A); β=0.33, P=0.001 (B)).
Figure 3.
Correlation between plasma α-KL protein and serum 1,25(OH)2 vitamin D (A) and MDA levels (B). Each open square represents an individual value of each subject. The line represents the regression slope (β=0.24, P<0.05 (A); β=0.20, P<0.05 (B)).
FGF23 concentrations correlated positively with serum calcium concentrations (β=0.35, P=0.001) and negatively with postnatal age (β=−0.29, P=0.003), serum 1,25(OH)2D (β=−0.36, P=0.001), and PTH levels (β=−0.28, P=0.007). Despite the well-known phosphaturic actions of FGF23, no significant correlation was found between FGF23 and serum phosphate levels in univariate regression analysis. However, in multiple regression analysis, FGF23 was an independent predictor of serum phosphate levels (Table 2).
Table 2.
Multiple regression analysis model for serum phosphate levels in the entire study population. Dependent variable: serum phosphate levels; R2 of model=40.7; P<0.0001.
| Independent variables | β | t | P value |
|---|---|---|---|
| α-KL | −0.10 | −1.08 | 0.28 |
| FGF23 | −0.19 | −2.00 | 0.04 |
| 1,25(OH)2D | 0.22 | 2.06 | 0.04 |
| PTH | 0.03 | 0.35 | 0.72 |
| Preterm birth | 0.04 | 0.39 | 0.69 |
| Postnatal age | −0.71 | −6.72 | <0.0001 |
β, standardized regression coefficient.
Discussion
Circulating α-KL protein concentrations were higher in full-term than in preterm neonates and increased during the first month of life. The higher α-KL levels in full-term infants are possibly due to increased production/release of α-KL protein as GA/postconcep-tional age and maturity advance. In rat model studies, the expression of α-KL protein was faintly detected at day 18 of prenatal life and at day 1 after birth but markedly augmented after the 4th day of life (27). Kidney is the predominant organ of α-KL production (1, 3). The majority of nephrons are normally formed during the third trimester of pregnancy and nephrogenesis is completed at the time of birth in full-term infants, whereas it is still ongoing for several weeks after preterm birth (28); in both preterm and full-term infants, renal cellular proliferation and enlargement leading to increase in nephron size and functionality occur during postnatal growth (29). The latter can explain the increase in α-KL protein concentrations during neonatal age in our preterm and full-term infants and also the clearly higher circulating α-KL levels in our full-term infants at days 14 and 28 of life, in comparison with levels reported previously in full-term neonates at day 4 of life (4).
In humans, circulating α-KL protein concentrations were shown to be age dependent declining from childhood to adult life (26). Indeed, circulating α-KL levels in our study population are comparable to those reported in children of mean age 7.1 years, but higher than levels in healthy adults (26). Interestingly, in a recent study, levels of soluble α-KL protein in cord vein blood were markedly higher than the plasma levels of neonates at the 4th day of life, of their mothers, and of healthy adults; this finding was attributed to production of α-KL from the placenta (4). We did not obtain cord blood to determine α-KL levels. However, it is unlikely that α-KL concentrations in our study population at the end of the 2nd and 4th week of life reflect placental origin because we estimated the half-life of soluble α-KL in vivo and found that it is short (20–30 min; Akihiro Imura, 2012; unpublished information).
This study showed that circulating α-KL levels in neonates correlated significantly with 1,25(OH)2D and MDA serum concentrations and also with the infants’ body weight and length. The positive correlation between α-KL and 1,25(OH)2D levels is in accordance with the suggested mutually regulated feedback actions of α-KL and the vitamin D endocrine system. 1,25(OH)2D positively regulates the expression of α-kl, whereas α-KL protein is involved in the signal transduction of FGF23, which suppresses circulating 1,25(OH)2D levels by inhibiting Cyp27b1-mediated production and stimulating Cyp24-mediated catabolism of 1,25(OH)2D (8, 9, 10, 30); the latter explains the negative correlation between FGF23 and 1,25(OH)2D levels in our study population.
Although α-KL protein is a potent regulator of calcium and phosphate metabolism (12, 14), we did not find any significant correlation between α-KL protein and calcium or phosphate levels. However, calcium and phosphate metabolism is regulated by complicated reciprocal actions and feedback mechanisms (10). FGF23 correlated significantly with calcium, phosphate, 1,25(OH)2D, and PTH levels, possibly because FGF23 has a key homeostatic role in maintaining normophosphatemia, some of it through soluble α-KL as a mediator in bone–kidney–parathyroid endocrine axis (11). Interestingly, in the preterm and full-term infants studied, serum phosphate levels decreased at the end of the first month of age simultaneously with the rise in circulating α-KL concentrations and the decline in FGF23 levels. The decrease in phosphate levels is likely related to improvement in renal maturity as postnatal age advances (this hypothesis is supported by the negative influence of postnatal age on serum phosphate levels in multiple regression analysis; Table 2) but can also be attributed, at least in part, to improvement in sensitivity of renal cells to FGF23 signaling and its phosphaturic effects as α-KL levels increase; resistance to FGF23 signaling has been reported in states of decreased α-KL (31).
The positive correlation between α-KL and MDA levels in our infants is possibly suggestive of a role of α-KL as a regulator of oxidative stress in neonates. There is evidence that α-KL protein offers protection against oxidative stress at the cell and organism levels (15, 32). α-KL protein can remove reactive oxygen species and confer resistance against oxidative stress by activating the AMP signaling pathway, increasing SOD expression, and inducing the production of nitric oxide (32); it also causes activation of the transcription factor FOXO leading to upregulation of mitochondrial SOD (15). In our study, no significant correlation between plasma α-KL levels and SOD activity in washed erythrocytes was found. However, the magnitude of stimulation of SOD expression by α-KL protein seems to depend on the type of cells studied. For example, α-KL increased the SOD protein levels in vitro by 1.5, 2.3, and 5.8 times in COS, HeLa, and CHO cells respectively (15). Circulating α-KL levels correlated positively with Sod mRNA in muscle samples in mice (15) but, to our knowledge, the correlation between α-KL levels and SOD activity in erythrocytes has not been reported.
In this study, the positive correlation between plasma α-KL levels and infants’ anthropometric parameters (body weight and length) is novel and possibly indicates increased α-KL protein production/release as the size of neonates advances. It has been reported that a direct relationship between birth weight and nephron number exists (33), the kidney being a major source of circulating α-KL (1, 3). Moreover, in neonates, body weight accounts for almost 80% of the variance of fat mass as assessed by dual-energy X-ray absorptiometry (34). As α-KL protein is mainly produced in renal cells and at much lower levels in other organs, including the adipose tissue (1, 3, 4), the positive correlation between α-KL levels and body weight in our study population not only possibly reflects renal α-KL production in association with infants’ body weight variation but may also reflect the production of α-KL by adipose tissue. On the other hand, there is evidence that α-KL protein works as a hormonal factor to promote adipocyte differentiation. In mouse fibroblast cells, mRNA expression and protein levels of adipocyte differentiation markers were increased by α-KL protein stimulation and, inversely, α-kl gene suppression led to decreased mRNA expression of adipocyte differentiation markers (17). Interestingly, in klotho knockout mice, an almost undetectable amount of white adipose tissue was observed (1, 2). Furthermore, α-KL is physiologically important for maintaining normal bone metabolism; α-KL deficiency reduces the osteoblastic population and disturbs bone mineralization (35, 36). The klotho mutant mice have low turnover bone metabolism and decreased cortical bone thickness (35), while they also suffer from growth retardation (1). Soluble α-KL was recently shown to stimulate osteoblastic cell proliferation (37). Thus, the correlations between plasma soluble α-KL levels and body weight and length in our study population may also suggest that α-KL has a role in the regulation of growth in neonates. Determination of other known circulating growth factors, such as insulin-like growth factor 1 (IGF1), might be helpful in elucidating the role of α-KL in neonatal growth.
It was previously reported that insulin stimulates the cleavage and release of α-KL protein (5), while α-KL inhibits insulin and IGF1 signaling and induces IR via suppression of tyrosine phosphorylation of the insulin and IGF1 receptors (2). Conversely, downregulation of α-KL in IR syndromes was shown (38) and, interestingly, gene therapy with α-KL in animals led to, at least in part, correction of metabolic abnormalities typical of the metabolic syndrome, possibly via an improvement in insulin sensitivity (16). However, the results of a recent study argue against a direct role of α-KL in the pathogenesis of IR (3); indeed, neither the expression of α-KL was influenced by the induction of IR in rodent models nor the soluble α-KL protein inhibited IGF1 and/or insulin signaling in insulin-responsive cell lines (3). We did not observe any significant correlation between α-KL and glucose, insulin, or HOMA-IR values.
In conclusion, plasma levels of soluble α-KL protein in neonates rise as GA and postnatal age advance and correlate significantly with 1,25(OH)2D and MDA serum concentrations and also with the infants’ body weight and length but not with glucose and insulin concentrations or HOMA-IR values. Our findings support an impact of α-KL on vitamin D metabolism and oxidative stress in neonates. Whether the correlation between α-KL concentrations and infants’ growth parameters reflects an active role of α-KL in the regulation of neonatal growth or is due to maturation of α-KL-producing tissues remains to be elucidated.
Acknowledgments
Funding
This work was funded by the University of Athens Special Fund. The funding source played no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the report for publication.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenzi O, Veyrat-Durebex C, Wollheim CB, Villemin P, Rohner-Jeanrenaud F, Zanchi A, Vischer UM. Evidence against a direct role of klotho in insulin resistance. Pflügers Archives: European Journal of Physiology. 2010;459:465–473. doi: 10.1007/s00424-009-0735-2. [DOI] [PubMed] [Google Scholar]
- 4.Ohata Y, Arahori H, Namba N, Kitaoka T, Hirai H, Wada K, Nakayama M, Michigami T, Imura A, Nabeshima Y, Yamazaki Y, Ozono K. Circulating levels of soluble α-klotho are markedly elevated in human umbilical cord blood. Journal of Clinical Endocrinology and Metabolism. 2011;96:E943–E947. doi: 10.1210/jc.2010-2357. [DOI] [PubMed] [Google Scholar]
- 5.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of klotho by ADAM10 and ADAM17. PNAS. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted klotho protein in sera and CSF: implication for post-translational cleavage in release of klotho protein from cell membrane. FEBS Letters. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 7.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 8.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Molecular Endocrinology. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 9.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 10.Nabeshima Y. Discovery of α-klotho unveiled new insights into calcium and phosphate homeostasis. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 2009;85:125–141. doi: 10.2183/pjab.85.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiological Reviews. 2012;92:131–135. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klotho Kuro-o M. Pflugers Archives: European Journal of Physiology. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 13.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. α-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 14.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased α-klotho level results in hypophosphatemic rickets and hyperparathyroidism. PNAS. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. Journal of Biological Chemistry. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochemical and Biophysical Research Communications. 2000;276:767. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 17.Chihara Y, Rakugi H, Ishikawa K, Ikushima M, Maekawa Y, Ohta J, Kida I, Ogihara T. Klotho protein promotes adipocyte differentiation. Endocrinology. 2006;147:3835–3842. doi: 10.1210/en. 2005-1529. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. Journal of Bone and Mineral Research. 2010;25:1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 19.Georgeson GD, Szony BJ, Streitman K, Varga IS, Kovacs A, Kovacs L, Laszlo A. Antioxidant enzymes activities are decreased in preterm infants and in neonates born via caesarean section. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2002;103:136–139. doi: 10.1016/S0301-2115(02)00050-7. [DOI] [PubMed] [Google Scholar]
- 20.Robles R, Palomino N, Robles A. Oxidative stress in the neonate. Early Human Development. 2001;65(Suppl):S75–S81. doi: 10.1016/S0378-3782(01)00209-2. [DOI] [PubMed] [Google Scholar]
- 21.Hofman PL, Regan F, Cutfield WS. Prematurity – another example of perinatal metabolic programming? Hormone Research. 2006;66:33–39. doi: 10.1159/000093230. [DOI] [PubMed] [Google Scholar]
- 22.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, Cutfield WS. Premature birth and later insulin resistance. New England Journal of Medicine. 2004;351:2179–2186. doi: 10.1056/NEJMoa042275. [DOI] [PubMed] [Google Scholar]
- 23.Martos-Moreno GA, Barrios V, Sáenz de Pipaón M, Pozo J, Dorronsoro I, Martínez-Biarge M, Quero J, Argente J. Influence of prematurity and growth restriction on the adipokine profile, IGF1 and ghrelin levels in cord blood: relationship with glucose metabolism. European Journal of Endocrinology. 2009;161:381–389. doi: 10.1530/EJE-09-0193. [DOI] [PubMed] [Google Scholar]
- 24.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard score, expanded to include extremely premature infants. Journal of Pediatrics. 1991;119:417–423. doi: 10.1016/S0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y. Establishment of sandwich ELISA for soluble α-klotho measurement: age-dependent change of soluble α-klotho levels in healthy subjects. Biochemical and Biophysical Research Communications. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, Nagail R. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochemical and Biophysical Research Communications. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 28.Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: implications for long-term renal health. Reproductive Sciences. 2011;18:322–333. doi: 10.1177/1933719111401659. [DOI] [PubMed] [Google Scholar]
- 29.Davidson AJ. Uncharted waters: nephrogenesis and renal regeneration in fish and mammals. Pediatric Nephrology. 2011;26:1435–1443. doi: 10.1007/s00467-011-1795-z. [DOI] [PubMed] [Google Scholar]
- 30.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. American Journal of Physiology Renal Physiology. 2005;289:F1088–F1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 31.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to FGF-23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111. 053405. [DOI] [PubMed] [Google Scholar]
- 32.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biological Chemistry. 2008;389:233–241. doi: 10.1515/BC.2008. 028. [DOI] [PubMed] [Google Scholar]
- 33.Luycky VA, Brenner BM. The clinical importance of nephron mass. Journal of the American Society of Nephrology. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 34.Koo WW, Walters JC, Hockman EM. Body composition in neonates: relationship between measured and derived anthropometry with dual-energy X-ray absorptiometry measurements. Pediatric Research. 2004;56:694–700. doi: 10.1203/01.PDR. 0000142587.59238.BD. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteo-penia. Journal of Clinical Investigation. 1999;104:229–237. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H, Amizuka N, Oda K, Noda M, Ohshima H, Maeda T. Histological and elemental analyses of impaired bone mineralization in klotho-deficient mice. Journal of Anatomy. 2008;212:275–285. doi: 10.1111/j.1469-7580.2008.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, Richards WG. Fibroblast growth factor 23 (FGF23) and α-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcified Tissue International. 2011;89:140–150. doi: 10.1007/s00223-011-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagishi T, Saito Y, Nakamura T, Takeda S, Kanai H, Sumino H, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. Troglitazone improves endothelial function and augments renal klotho mRNA expression in Otsuka Long-Evans Tokushima Fatty (OLETF) rats with multiple atherogenic risk factors. Hypertension Research. 2001;24:705–709. doi: 10.1291/hypres.24.705. [DOI] [PubMed] [Google Scholar]