Abstract
Puerto Rico has higher rates of a range of endocrine-related diseases and disorders compared to the United States. However, little is known to date about human exposures to known or potential endocrine disrupting chemicals (EDCs) in Puerto Rico. We recruited 105 pregnant women in Northern Puerto Rico who provided urine samples and questionnaire data at three times (20±2, 24±2, and 28±2 weeks) during gestation. We measured the urinary concentrations of five phenols and three parabens: 2,4-dichlorophenol (24-DCP), 2,5-dichlorophenol (25-DCP), benzophenone-3 (BP-3), bisphenol A (BPA), triclosan (TCS), butyl paraben (B-PB), methyl paraben (M-PB), and propyl paraben (P-PB). The frequent detection of these chemicals suggests that exposure is highly prevalent among these Puerto Rican pregnant women. Urinary concentrations of TCS, BP-3 and 25-DCP were higher than among women of reproductive age in the US general population, while concentrations of BPA, 24-DCP and parabens were similar. Intraclass correlation coefficients (ICC) varied widely between biomarkers; BPA had the lowest ICC (0.24) and BP-3 had highest (0.62), followed by 25-DCP (0.49) and TCS (0.47). We found positive associations between biomarker concentrations with self-reported use of liquid soap (TCS), sunscreen (BP-3), lotion (BP-3 and parabens), and cosmetics (parabens). Our results can inform future epidemiology studies and strategies to reduce exposure to these chemicals or their precursors.
Keywords: biomarker, endocrine disruptor, environment, epidemiology, exposure
INTRODUCTION
There is growing evidence that exposure to endocrine disrupting chemicals (EDCs) may contribute to various human diseases and disorders, such as adverse pregnancy outcomes, altered reproductive development or function, hindered brain development, obesity, and increased risk of metabolic syndrome and diabetes.[1, 2] In the past two decades, Puerto Rico has experienced a steep increase in the rate of preterm birth, where rates have gone from being similar to the US average in the 1990s (12%) to now being the highest (18%) among all US states and territories.[3, 4] As a nation, Puerto Rico would have the third highest preterm birth rate worldwide behind only Malawi and Congo.[5] Compared to the United States, Puerto Rico also has higher rates of childhood obesity and asthma,[6-8] as well as of obesity, metabolic syndrome, and diabetes in adults.[9, 10] There is some evidence for widespread endocrine disruption on the island, manifest in the form of elevated rates of developmental anomalies such as premature thelarche.[11, 12] However, little is known to date about human exposures to EDCs in Puerto Rico.
Exposure to certain phenols (or their precursors) and parabens is widespread in the United States based on the detection of urinary biomarkers of exposure to these chemicals in virtually everyone tested in the National Health and Nutrition Examination Survey (NHANES), a large-scale study representative of the US population.[13] Use of consumer and personal care products are thought to contribute to exposure, but this remains unclear due to the lack of research to date. Among the environmental phenols, BPA represents the most studied. It is used in the manufacture of polycarbonate plastics and epoxy resins, and may be found in a range of consumer products as well as in canned and other foods. The primary pathway of exposure for most people is likely through the diet, though other sources and pathways are possible.[14] BPA is weakly estrogenic but may impact multiple endocrine-related pathways, and has been associated with a range health effects in animal and human studies.[15, 16] Triclosan (TCS) is used as a preservative and antiseptic agent added to a range of products including soaps, toothpaste, mouthwash, and other personal care products.[17] This widespread use has resulted in contamination of the aquatic environment through residential wastewaters; TCS can also be further transformed into other toxic chemicals.[18, 19] TCS has demonstrated effects on thyroid function and possibly reproduction in animal studies,[19] but there have been very few human studies. Benzophenone-3 (BP-3) is a UV filter and stabilizer used in sunscreens, lotions, conditioners, cosmetics and plastics, which, like TCS, has led to its detection in surface and drinking waters.[13] It has been shown to be weakly estrogenic, anti-androgenic, and/or impact thyroid function in experimental research, but human studies of BP-3 are also lacking.[20]
Dichlorophenols are also prevalent in human urine samples.[13] 2,4-dichlorophenol (24-DCP) is a minor metabolite of the common herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) and other environmental chemicals (including TCS[21]), while 25-DCP is a metabolite of 1,4-dichlorobenzene (paradichlorobenzene) that has been used in moth balls, as a room/toilet deodorizer and previously as an insecticide.[13] Urinary 25-DCP (but not 24-DCP) was associated with obesity in children[22] and with age at menarche in adolescent girls[23] in recent reports utilizing NHANES data. Inverse associations between 25-DCP and infant birth weight, particularly among male infants, have also been reported.[24, 25] Finally, parabens are a class of chemicals widely used as preservatives in cosmetics and other personal care products, and are also used as antimicrobials in various foods and pharmaceuticals.[26] They are suspected EDCs with demonstrated adverse impacts on endocrine and reproductive function in animal studies, but research on human health impacts have been extremely limited.[27, 28]
With the exception of two recent studies of BPA [29, 30] and a related study on parabens,[27] data on temporal variability and/or predictors of exposure to these chemicals related to product use in pregnant women are lacking. Given their ubiquity and potential to contribute to adverse human health, exposure characterization studies are needed to inform epidemiology studies, especially among susceptible populations such as pregnant women and children. Information is also needed on sources of exposure to inform potential interventions aimed at reducing exposures and associated health risks. The objective of this study was to determine distributions, variability, and predictors of urinary biomarkers of environmental phenols and parabens measured at multiple times during pregnancy among women living in Northern Puerto Rico.
METHODS
Study Participants
This study was conducted among pregnant women participating in the “Puerto Rico Testsite for Exploring Contamination Threats (PROTECT)” project, an ongoing prospective birth cohort in the Northern Karst Region of Puerto Rico, which is designed to evaluate the relationship between environmental toxicants and risk of preterm delivery. Study participants were recruited at approximately 14±2 weeks of gestation at seven prenatal clinics and hospitals throughout Northern Puerto Rico during 2010-2012. Women were eligible if they were between the ages of 18 to 40 years, resided in a municipality within the Northern karst region, didn’t use oral contraceptives three months prior to pregnancy or in vitro fertilization as a method of assisted reproductive technology, and were free of known medical/obstetrics complications. Women provided spot urine samples at three separate study visits (20±2 weeks, 24±2 weeks, and 28±2 weeks of gestation). Questionnaires to collect demographic information and data on self-reported product use in the 48 hours preceding urine sample collection were also administered at each visit.
The present analysis reflects the first105 women recruited into the study who had urinary biomarker data as of June 2012. The research protocol was approved by the Ethics and Research Committees of the University of Puerto Rico and participating clinics, the University of Michigan School of Public Health, and Northeastern University. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research. The study was described in detail to all participants, and informed consent was obtained prior to study enrollment.
Measurement of Phenols and Parabens in Urine
Urine was collected in polypropylene containers, divided into aliquots, and frozen at -80 °C until shipped overnight to the CDC. All urine samples were analyzed at the National Center for Environmental Health of the CDC for five phenols (BPA, TCS, BP-3, 24-DCP, and 25-DCP) and three parabens (butyl paraben (B-PB), methyl paraben (M-PB), propyl paraben (P-PB)) by online solid phase extraction-high-performance liquid chromatography-isotope dilution tandem mass spectrometry.[31, 32] The analytical method details are provided in the Supporting Information. To monitor for accuracy and precision, each analytical run included calibration standards, reagent blanks, and quality control materials of high and low concentrations. The limits of detection (LODs) varied slightly between analytes, but were generally in the low ng/mL range. Concentrations below the LOD were assigned a value of LOD divided by the square root of 2. Specific gravity (SG) was measured at the University of Puerto Rico Medical Sciences Campus using a handheld digital refractometer (Atago Co., Ltd., Tokyo, Japan). For data analyses utilizing SG-corrected metabolite concentrations, the following formula was used: Pc=P[(1.019-1)/(SG-1)] where Pc is the SG-adjusted urinary concentration (ng/mL), P is the measured urinary concentration, and SG is the specific gravity of the urine sample. An SG of 1.019 was the median SG value for this group of urine samples.
Statistical Analysis
Geometric means and selected percentiles were calculated to describe the distributions of urinary biomarkers of phenols and parabens among study participants and for comparison with other published reports. We compared concentrations measured in the present study with those measured in NHANES. We utilized publicly accessible urinary phenols and parabens concentration data from NHANES 2007-2008 and 2009-2010 among females between the ages of 18 and 40 years, along with appropriate sampling weights, to tabulate geometric means and selected percentiles.
Pearson and Spearman rank correlations were calculated to assess relationships between study visits and between the various biomarkers. Differences in geometric mean biomarker concentrations between study visits (i.e. time points in gestation) were tested using one-way ANOVA. To assess temporal variability in urinary biomarker concentrations intraclass correlation coefficients (ICCs) and their 95% confidence intervals were calculated.[33] ICC is a measure of the reliability of repeated measures over time, defined as the ratio of between-subject variance to total (between-subject plus within-subject) variance. ICC ranges from zero to one, with values near zero indicating poor temporal reliability and values near one indicating high temporal reliability.[34]
Geometric means were compared between categories for maternal age, maternal education, marital status, household income, parity, pre-pregnancy body mass index (BMI), and time of day at urine collection. We examined the association between urinary concentrations of the biomarkers and demographic, sampling time, and 48-hour recall of product use variables using linear mixed effects models with the compound symmetry covariance structure. Demographic factors were included as fixed time-invariant effects in our mixed models. Time of day of sample collection and product use variables were modeled as fixed time-dependent factors. Natural log-transformed unadjusted or SG-adjusted urinary concentrations of phenols or parabens were the dependent variable in mixed models, with separate models for each independent variable. Data were analyzed using SAS 9.2 (SAS Institute Inc. Cary, NC).
RESULTS
A total of 279 urine samples from 105 women were analyzed. Data on SG was missing for 2 samples. Statistical analysis was conducted for both unadjusted and SG-adjusted urinary concentrations, and results were highly consistent between the two approaches throughout. Demographic characteristics of our study sample are shown in Table 1. The mean age was 27.2 years; 82% of the women had an education above the high school level, and 73% were either married or in a domestic partnership. The majority of women reported a household income below $40,000 per year and nearly all women did not smoke during pregnancy.
Table 1.
Demographic characteristics of N=105 pregnant women from Puerto Rico (2010-2012)
| Variable | Mean ± SD or n (%) |
|---|---|
| Maternal Age at enrollment (years) | 27.1 ± 4.8 |
| Gravidity (# pregnancies) | 1.9 ± 1.0 |
| Parity (# live births) | 0.6 ± 0.7 |
| Years of Maternal Education | |
| < High school | 12 (11.4) |
| High school/equivalent | 7 (6.7) |
| College | 86 (81.9) |
| Household Income (US$) | |
| Missing | 15 (14.3) |
| < $20,000 | 46 (43.8) |
| ≥$20,000 to < $40,000 | 27 (25.7) |
| ≥ $40,000 | 17 (16.2) |
| Marital Status | |
| Single | 29 (27.6) |
| Married or living together | 76 (73.4) |
| Pre-pregnancy BMI (kg m-2) | |
| ≤ 25 | 60 (57.1) |
| > 25 to ≤ 30 | 32 (30.5) |
| > 30 | 13 (12.4) |
| Smoked During Pregnancy | |
| Missing | 2 (1.9) |
| Yes | 1 (1.0) |
| No | 102 (97.1) |
| Employment | |
| Unemployed | 42 (40.0) |
| Employed | 63 (60.0) |
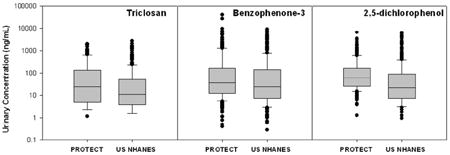
Distributions of urinary biomarker concentrations are presented in Table 2, along with distributions from 18-40 year old women from US NHANES 2007-08 and 2009-10. BPA, BP-3, both dichlorophenols, M-PB and P-PB were detected in between 95% and 100% of samples. TCS was detected in 89% of samples, while B-PB was detected in 58%. When comparing distributions with NHANES women, women in our study had higher geometric mean concentrations of BP-3, TCS, and 25-DCP. Median concentrations of TCS and 25-DCP were two- and six-fold greater, respectively, among women in this study compared to NHANES 2009-10. For BP-3, median concentrations were similar but the populations diverged greatly at the upper end of the distribution, which resulted in a higher geometric mean concentration among Puerto Rican women. Geometric mean and median concentrations of BPA, 24-DCP, and the three parabens were similar between the two populations. When looking across urinary biomarkers there was a strong correlation between 24-DCP and 25-DCP (Spearman r > 0.8) and between the three parabens, particularly between M-PB and P-PB (r = 0.8). There were also weak (r = 0.25 to 0.4) but statistically significant (p < 0.05) correlations between 24-DCP and TCS, and between BP-3 and the parabens.
Table 2.
Urinary phenol and paraben concentrations (ng/mL) in n=105 pregnant women from Puerto Ricoa in 2010-2012 and comparison with U.S. population–based samples of women ages 18-40 from NHANESb.
| %>LOD | GM (95% CI) | Percentiles
|
|||||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 95th | Max | |||
| BPA | |||||||
| PROTECT | 97.9 | 2.6 (2.3, 2.9) | 1.3 | 2.5 | 4.4 | 13.9 | 97.4 |
| NHANES 07-08 | 96.2 | 2.5 (2.2, 2.9) | 1.3 | 2.4 | 4.6 | 14.0 | 64.0 |
| NHANES 09-10 | 92.1 | 2.0 (1.7, 2.2) | 0.8 | 2.0 | 4.1 | 9.2 | 416 |
|
| |||||||
| TCS | |||||||
| PROTECT | 88.9 | 29.9 (23.6, 37.9) | 5.1 | 26.2 | 121 | 944 | 2000 |
| NHANES 07-08 | 84.9 | 18.7 (14.7, 23.8) | 4.4 | 14.0 | 67.7 | 520 | 2780 |
| NHANES 09-10 | 79.0 | 16.9 (12.2, 23.4) | 4.0 | 13.0 | 52.5 | 577 | 2690 |
|
| |||||||
| BP-3 | |||||||
| PROTECT | 100 | 52.2 (41.0, 66.4) | 11.5 | 31.3 | 172 | 2150 | 39700 |
| NHANES 07-08 | 97.8 | 38.6 (28.9, 51.6) | 8.6 | 35.6 | 148 | 1256 | 21500 |
| NHANES 09-10 | 99.3 | 36.3 (22.6, 58.4) | 7.6 | 27.4 | 145 | 3340 | 8970 |
|
| |||||||
| 24-DCP | |||||||
| PROTECT | 97.9 | 1.5 (1.3, 1.8) | 0.6 | 1.3 | 3.3 | 16.3 | 83.3 |
| NHANES 07-08 | 90.1 | 0.9 (0.7, 1.1) | 0.3 | 0.7 | 1.7 | 8.4 | 231 |
| NHANES 09-10 | 85.1 | 0.7 (0.6, 0.9) | 0.2 | 0.6 | 1.5 | 7.1 | 147 |
|
| |||||||
| 25-DCP | |||||||
| PROTECT | 100 | 26.0 (21.4, 31.7) | 7.0 | 19.0 | 82.5 | 650 | 4110 |
| NHANES 07-08 | 99.7 | 8.4 (6.4, 11.0) | 2.6 | 6.1 | 20.1 | 333 | 11300 |
| NHANES 09-10 | 97.6 | 5.1 (3.7, 7.1) | 1.1 | 3.8 | 16.5 | 215 | 3820 |
|
| |||||||
| B-PB | |||||||
| PROTECT | 58.4 | 1.0 (0.8, 1.3) | <0.2 | 0.4 | 5.5 | 36.4 | 148 |
| NHANES 07-08 | 74.8 | 1.1 (0.9, 1.4) | <0.2 | 0.7 | 4.0 | 33.6 | 188 |
| NHANES 09-10 | 59.0 | 0.7 (0.6, 0.8) | <0.2 | 0.4 | 2.2 | 22.1 | 127 |
|
| |||||||
| M-PB | |||||||
| PROTECT | 100 | 140 (117, 167) | 57.6 | 153 | 381 | 1590 | 6040 |
| NHANES 07-08 | 99.7 | 132 (97.1, 179) | 47.8 | 146 | 430 | 1444 | 7550 |
| NHANES 09-10 | 99.5 | 111 (90.3, 138) | 38.6 | 119 | 374 | 1269 | 4840 |
|
| |||||||
| P-PB | |||||||
| PROTECT | 99.3 | 30.0 (24.1, 37.5) | 10.1 | 36.7 | 130 | 493 | 1220 |
| NHANES 07-08 | 98.1 | 28.0 (20.3, 38.6) | 6.4 | 33.6 | 121 | 410 | 1400 |
| NHANES 09-10 | 98.1 | 21.2 (16.0, 28.2) | 4.6 | 24.8 | 110 | 434 | 3490 |
NHANES, National Health and Nutrition Examination Survey; LOD, limit of detection; GM, geometric mean; NA, not applicable
Includes biomarker concentrations for up to 3 repeated samples per woman (n=279 samples).
Females 18-40 years of age; n=365 for metabolites measured in 2007-2008; n=415 for 2009-2010.
Box plot comparisons of the concentration distributions for each biomarker between study visits (approximately 20, 24, and 28 weeks gestation) are shown in Figure 1. There were no statistically significant differences between unadjusted or SG-adjusted geometric mean concentrations at the three visits for any of the biomarkers. ICCs, presented in Table 3, varied widely between biomarkers and ranged from weak to moderately strong. BPA had the lowest ICC (0.24) and BP-3 had highest (0.62), followed by 25-DCP (0.49) and TCS (0.47).
Figure 1.
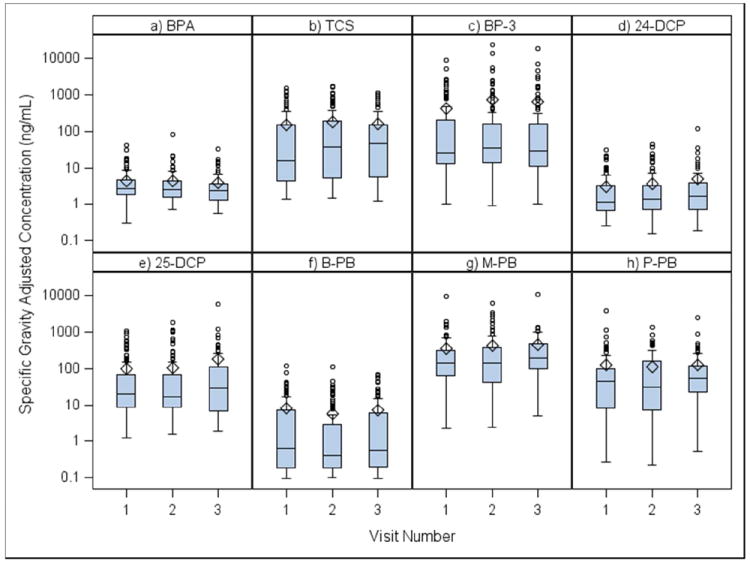
Boxplots comparing SG-adjusted concentrations of urinary biomarkers across study visitsa.
aVisit 1 (20 ±2 weeks gestation), Visit 2 (24 ±2 weeks), Visit 3 (28±2 weeks)
Table 3.
Intraclass correlation coefficients (ICCs) and 95% confidence intervals (95% CIs) for ln-transformed urinary concentrations of phenols and parabens
| Urinary biomarker | Unadjusteda | SG-adjustedb | ||
|---|---|---|---|---|
|
| ||||
| ICC | 95% CI | ICC | 95% CI | |
| BPA | 0.27 | 0.15, 0.42 | 0.24 | 0.13, 0.40 |
| TCS | 0.42 | 0.30, 0.55 | 0.47 | 0.35, 0.59 |
| BP-3 | 0.58 | 0.47, 0.68 | 0.62 | 0.51, 0.71 |
| 24-DCP | 0.37 | 0.25, 0.50 | 0.38 | 0.27, 0.52 |
| 25-DCP | 0.50 | 0.38, 0.62 | 0.49 | 0.38, 0.61 |
| B-PB | 0.45 | 0.33, 0.57 | 0.47 | 0.35, 0.60 |
| M-PB | 0.36 | 0.24, 0.50 | 0.39 | 0.27, 0.53 |
| P-PB | 0.31 | 0.19, 0.46 | 0.32 | 0.20, 0.47 |
n=279 samples from 105 participants
n=277 samples from 105 participants
Urinary biomarker concentrations in relation to sampling and demographic variable categories are presented in Table 4. Only urinary 24-DCP and 25-DCP concentrations were associated with time of day of urine sample collection, where concentrations in samples collected later in the day (between 3:00 and 8:00 PM) were significantly higher (p=0.01 and p=0.006, respectively) compared to the other time categories. There was a trend for increasing BP-3 concentrations with increasing age categories. The oldest age category (>30 years) was also associated with higher TCS and B-PB concentrations. BP-3 and P-PB concentrations were lower among women with <12 years of education. There were increasing trends between BP-3 concentrations and income status, B-PB concentrations and increased parity, and between BPA concentrations and pre-pregnancy BMI. Finally, dichlorophenols concentrations were lower and B-PB concentrations higher among women who were not currently employed.
Table 4.
Geometric means of specific gravity adjusted urinary concentrations of phenols and parabens according to time of urine collection, demographic, and maternal factors.
| n (%)a | BPA | TCS | BP-3 | 24-DCP | 25-DCP | B-PB | M-PD | P-PB | |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 277 (100) | 2.8 | 31.8 | 55.8 | 1.7 | 28.0 | 1.1 | 148.4 | 31.8 |
|
| |||||||||
| Time of Day | |||||||||
| 0600–0859 | 37 (13.4) | 2.9 | 37.2 | 68.5 | 1.7 | 31.7 | 0.6 | 188.3 | 29.1 |
| 0900–1159 | 117 (42.2) | 2.5 | 29.1 | 48.3 | 1.7 | 27.4 | 1.1 | 143.8 | 33.8 |
| 1200–1459 | 90 (32.5) | 2.9 | 42.0 | 58.6 | 1.3 | 19.6 | 1.2 | 125.4 | 25.8 |
| 1500–2000 | 33 (11.9) | 3.7 | 17.2 | 64.4 | 2.9 | 69.8 | 1.7 | 201.7 | 50.5 |
| p-valueb | 0.12 | 0.31 | 0.73 | 0.01 | 0.006 | 0.17 | 0.30 | 0.37 | |
|
| |||||||||
| Maternal Age (years) | |||||||||
| <25 | 96 (34.7) | 2.8 | 33.8 | 32.1 | 1.5 | 26.5 | 0.9 | 142.6 | 28.4 |
| 25-30 | 95 (34.3) | 2.8 | 19.9 | 47.3 | 1.6 | 31.6 | 0.8 | 147.0 | 33.1 |
| >30 | 86 (31.0) | 2.8 | 50.1 | 123.8 | 1.9 | 26.0 | 1.8 | 156.9 | 34.7 |
| p-valueb | 0.95 | 0.01 | 0.0003 | 0.35 | 0.99 | 0.04 | 0.90 | 0.76 | |
|
| |||||||||
| Maternal Education (years) | |||||||||
| <12 | 31 (11.2) | 2.6 | 20.1 | 18.6 | 1.4 | 24.1 | 1.2 | 92.9 | 10.9 |
| 12 | 18 (6.5) | 2.5 | 31.2 | 106.8 | 1.6 | 40.6 | 1.9 | 125.2 | 29.8 |
| >12 | 228 (82.3) | 2.8 | 34.0 | 61.5 | 1.7 | 27.7 | 1.0 | 160.4 | 37.0 |
| p-valueb | 0.80 | 0.61 | 0.006 | 0.80 | 0.55 | 0.45 | 0.13 | 0.004 | |
|
| |||||||||
| Marital Status | |||||||||
| Married/Civil Union | 202 (72.9) | 2.7 | 33.4 | 57.1 | 1.7 | 28.4 | 1.1 | 159.3 | 32.8 |
| Unmarried | 75 (27.1) | 3.0 | 27.9 | 52.5 | 1.5 | 26.9 | 1.0 | 122.7 | 29.3 |
| p-valueb | 0.43 | 0.70 | 0.77 | 0.79 | 0.91 | 0.33 | 0.12 | 0.55 | |
|
| |||||||||
| Income Status (US $) | |||||||||
| < $20,000 | 120 (49.8) | 2.7 | 31.0 | 38.3 | 1.4 | 25.0 | 1.2 | 159.2 | 27.0 |
| ≥ $20,000 to < $40,000 | 74 (30.7) | 2.9 | 34.0 | 65.6 | 1.9 | 31.6 | 1.2 | 136.9 | 37.5 |
| ≥ $40,000 | 47 (19.5) | 2.5 | 31.1 | 142.7 | 1.9 | 25.4 | 1.0 | 152.6 | 33.0 |
| p-valueb | 0.66 | 0.94 | 0.003 | 0.39 | 0.89 | 0.88 | 0.68 | 0.67 | |
|
| |||||||||
| Parity | |||||||||
| 0 | 126 (45.5) | 2.8 | 34.3 | 63.3 | 1.7 | 32.4 | 0.8 | 144.4 | 32.4 |
| 1 | 93 (33.6) | 3.0 | 30.5 | 54.9 | 1.6 | 23.3 | 1.0 | 165.1 | 38.1 |
| >1 | 58 (20.9) | 2.5 | 29.1 | 43.4 | 1.6 | 27.4 | 2.4 | 132.8 | 23.0 |
| p-valueb | 0.47 | 0.99 | 0.48 | 0.99 | 0.50 | 0.005 | 0.67 | 0.32 | |
|
| |||||||||
| Prepregnancy BMI (kg m-2) | |||||||||
| ≤ 25 | 158 (57.0) | 2.8 | 31.4 | 50.7 | 1.7 | 29.5 | 1.1 | 173.4 | 36.1 |
| >25 to ≤ 30 | 83 (30.0) | 2.3 | 26.2 | 71.3 | 1.4 | 25.5 | 1.2 | 121.6 | 30.5 |
| > 30 | 36 (13.0) | 4.3 | 52.7 | 48.4 | 1.8 | 27.5 | 0.8 | 118.9 | 20.3 |
| p-valueb | 0.01 | 0.71 | 0.18 | 0.65 | 0.88 | 0.50 | 0.20 | 0.31 | |
|
| |||||||||
| Employment Status | |||||||||
| Unemployed | 109 (39.4) | 2.7 | 35.8 | 48.0 | 1.3 | 19.6 | 1.6 | 148.3 | 26.4 |
| Employed | 168 (60.7) | 2.8 | 29.5 | 61.4 | 1.9 | 35.2 | 0.8 | 148.7 | 36.0 |
| p-valueb | 0.71 | 0.70 | 0.32 | 0.04 | 0.03 | 0.06 | 0.59 | 0.15 | |
n represents number of samples, not participants.
p-values from linear mixed effects models accounting for within-person correlations.
Self-reported use of selected products in the 48 hours preceding urine sample collection that were related to urinary biomarker concentrations are presented in Table 5. Use of hand or body lotion was associated with significantly higher (between 2 and 3-fold) geometric mean concentrations of BP-3, B-PB, M-PB and P-PB. Self reported use of colored cosmetics (make-up) was positively associated with similar changes in all paraben biomarker concentrations. Geometric mean BP-3 concentrations were 10-fold higher among women who reported recent use of sunscreen (503 ng/mL) than among other women (49 ng/mL). Use of mouthwash was associated with significant increases in BP-3 and BPA concentrations. Triclosan concentrations were also higher among women reporting use of liquid soap and hairspray compared to those who did not. The use of bar soap was negatively associated with BP-3 concentrations, and use of bar soap and pesticides were both negatively associated with M-PB concentrations. None of the questionnaire variables, including use of pesticides, were associated with increased dichlorophenol concentrations (not shown). When demographic and product use variables that were associated with concentrations of each biomarker were included simultaneously in multivariate models, results were similar for each product use variable though somewhat attenuated (not shown).
Table 5.
Frequencies of product use reported in the 48-hour recall questionnaire and selecteda SG-adjusted geometric mean concentrations of phenols and parabens (ng/mL) associated with self-reported use (Y) or non-use (N)b
| nb=105 | nc=264 | BPA | TCS | BP-3 | B-PB | M-PB | P-PB | |
|---|---|---|---|---|---|---|---|---|
| Cleaning Products | ||||||||
| Laundry Detergent | 84 | 145 | ||||||
| Fabric Softener | 74 | 132 | ||||||
| General Cleaners | 89 | 163 | ||||||
|
| ||||||||
| Creams and Lotions | ||||||||
| Hand/Body Lotion | 95 | 217 | Y: 62.7; N: 30.1 p=0.05 | Y: 1.1; N: 0.6 p=0.05 | Y: 180; N: 67.6 p=0.0001 | Y: 40.2; N: 11.8 p=0.0001 | ||
| Shaving Cream | 13 | 15 | ||||||
| Sunscreen | 5 | 10 | Y: 503; No: 49.8 p=0.001 | |||||
|
| ||||||||
| Toiletries and Cosmetics | ||||||||
| Perfume/Cologne | 93 | 221 | ||||||
| Colored Cosmetics | 95 | 213 | Y: 1.2; N: 0.6 p=0.01 | Y: 175; N: 82.3 p=0.004 | Y: 40.5; N: 12.6 p=0.0002 | |||
| Bar Soap | 99 | 242 | (negative) p=0.07 | (negative) p=0.05 | ||||
| Liquid Soap | 103 | 223 | Y: 36.7; N: 18.5 p=0.06 | |||||
| Mouthwash | 80 | 146 | Y: 3.2; N: 2.5 p=0.03 | Y: 75.5; N: 37.2 p=0.03 | ||||
|
| ||||||||
| Hair and Nail Products | ||||||||
| Hairspray | 47 | 81 | Y: 50.0; N: 27.5 p=0.05 | |||||
| Conditioner | 97 | 170 | ||||||
| Shampoo | 97 | 169 | ||||||
| Nail Polish | 58 | 86 | ||||||
|
| ||||||||
| Chemical Products | ||||||||
| Pesticides | 21 | 25 | (negative) p=0.09 | |||||
| Pet Grooming Products | 7 | 8 | ||||||
(negative) = negative association; product use associated with lower urinary biomarker concentrations, contrary to hypothesis
Results shown for associations with p-value ≤ 0.1
p-values from linear mixed effects models accounting for within-person correlations.
nb=Total number of participants who answered “yes” at least once
nc=Total number of total responses that were “yes”
DISCUSSION
To our knowledge, this is the first study to report biomarkers of exposure to known or suspected endocrine disrupting environmental phenols and parabens in Puerto Rico, and also the first to report temporal variability and/or predictors of most of these chemicals among pregnant women. We found that exposure to the chemicals measured is highly prevalent among pregnant women in Puerto Rico. We also found evidence that concentrations of TCS, BP-3 and 25-DCP were higher than among women of reproductive age in the US general population. On the other hand, concentrations of BPA, 24-DCP and parabens were similar to those reported among US women.
The urinary biomarker concentrations measured in the present study can also be compared with other studies of pregnant women, though caution must be taken since there were potentially important differences between the studies (e.g., study design, year of sample collection) that may impact these comparisons. Urinary BPA concentrations in this study were somewhat higher than studies in Ohio,[29] New York,[25, 35] Mexico City,[36] Germany[37] and the Netherlands[38] that reported geometric mean and/or median concentrations between 1.0 and 2.0 ng/mL, but similar to studies in Boston,[30] France [24] and Spain[39] that reported median BPA concentrations of 2.6, 2.7 and 2.2 ng/mL, respectively. Studies of the other chemicals we measured have been much more limited in number. Median concentrations of TCS and BP-3 appear to be much higher in this Puerto Rico cohort (26 and 31 ng/mL, respectively) compared to studies of pregnant women in New York (11 and 7.5 ng/mL) [25] and Spain (6.1 and 3.4 ng/mL).[39] Median concentrations of 25-DCP were similar in the Spanish study (17 ng/mL), but higher in the New York study (53 ng/mL), compared to this study (19 ng/mL). A recent pregnancy study in France reported a similar median TCS concentration (24 ng/mL) compared to our study, but BP-3 and 25-DCP concentrations were lower in that study (1.7 and 10.2 ng/mL, respectively).[24] For paraben concentrations in this study, B-PB was somewhat lower, P-PB was somewhat higher, and M-PB was similar compared to the Spanish and French studies.[24, 39]
Characterizing temporal variability in exposure metrics, especially for biomarkers of nonpersistent compounds such as those measured in the present study, is a critical step in designing and interpreting an epidemiology study related to the potential for exposure measurement error. We found that temporal variability in urinary biomarker concentrations was not uniform across the chemicals measured. We found a weak ICC for BPA (ICC=0.24), which is consistent with previous studies among pregnant women in Ohio (ICC=0.10 to 0.28)[29] and Boston (ICC=0.12 to 0.23).[30] This is likely attributable to the rapid metabolism of BPA in addition to intermittent exposure to BPA which occurs mostly through the diet. The ICCs we calculated for M-PB (ICC=0.39) and P-PB (ICC=0.32) were somewhat lower than those recently reported among pregnant women who had sought fertility treatment in Boston (ICC=0.46 and 0.44, respectively).[27] The higher ICCs we reported for BP-3 (0.62), TCS (0.47) and 25-DCP (0.49) suggest more consistent exposure sources over time for these compounds. Only one other study, conducted among children in New York City, has assessed temporal variability of these chemicals.[40] The authors reported lower ICCs than we reported here (0.39, 0.35 and 0.37 for BP-3, TCS and 25-DCP, respectively).
We found that only dichlorophenol concentrations were associated with time of day of urine sample collection, where samples collected later in the day had significantly higher concentrations of these biomarkers. The lack of association between urinary BPA and time of day in these women was inconsistent with time of day influences on urinary BPA reported by a previous study of pregnant women in Ohio[29] and an NHANES analysis.[41]. On the other hand, the positive association between BPA and BMI in this study was consistent with previous studies reporting relationships between BPA exposure and obesity.[42, 43] Our observation of increased TCS concentrations among women in their 30s is similar to an NHANES analysis.[17] Finally, our finding that dichlorophenol concentrations were lower among women not currently employed may suggest occupational sources of exposure to the parent chemicals, which may also be reflected in the time of day patterns we observed in these two biomarkers. However, additional detailed studies would be needed to support that conclusion.
Most of the associations we observed between self-reported product use and urinary biomarker concentrations are supported by what is known about the use of these chemicals. Liquid soap use in the 48 hours preceding urine sample collection was positively associated with TCS concentrations in this study, which is consistent with the high concentrations (>1,000 μg/g) of TCS recently measured in samples of “conventional” hand soap and liquid dish soap.[44] TCS concentrations were also higher among women reporting use of hairspray than among women who did not. However, we could not find evidence for the presence of TCS in hairspray.[17, 44, 45] The divergence of the exposure distribution we observed for BP-3 concentrations between women in this study compared to NHANES, but only at the high end of the distribution, may also represent a subpopulation of pregnant women in Puerto Rico with unique and direct exposure to a particular source. Self-reported use of sunscreen and hand/body lotion was positively associated with BP-3 concentrations. The greatest increase for any product use/biomarker combinations in our study was observed for sunscreen use and BP-3. BP-3, a UV filter, has been detected in both “conventional” and “alternative” sunscreens.[44] Many hand and body lotions advertise having a sun protection factor and thus may contain BP-3. We also found higher urinary concentrations of BP-3 and BPA in relation to self-reported mouthwash use. No information relating to the use of these chemicals in mouthwash could be located, and it may be possible that these associations were due to confounding or were chance findings due to multiple comparisons. Concentrations of parabens, which are commonly used in cosmetics and other personal care products,[45] were associated with self-reported use of cosmetics and lotion. Parabens were recently detected in a study that sampled various cosmetic and lotion products.[44]
Strengths of our study include the novel aspects described earlier, in addition to its focus on an understudied and potentially at-risk population, a fairly large sample size for assessing predictors of metabolite concentrations, its important and original contribution to informing future exposure assessment and epidemiology studies, and the collection of repeated data on urinary biomarkers and self-reported product use. The repeated data allowed for an especially powerful analysis for the time varying factors, as each participant can serve as their own reference in mixed effects models. A primary limitation of our study was the inability to ask more detailed questions on the product use form. However, the inclusion of increased detail on the product use form would result in an exponential increase in participant burden, and may introduce additional recall errors. Added detail, such as specific product and brand names, would also result in a diffuse dataset that may lack power to test associations. Another potential limitation may be in our ability to compare our results with other studies or generalize our findings to other populations due to differences in study (e.g., questionnaire) design and differences in product formulation, availability and use by country/region.
In conclusion, we found evidence that urinary concentrations of triclosan, BP-3, and 25-DCP were higher among a group of pregnant women in Puerto Rico compared to women of reproductive age in the US, while concentrations of BPA, 24-DCP and parabens were similar. Although greatly limited, there is some evidence that exposure to these chemicals may be associated with adverse pregnancy outcomes and other health effects. Additional human epidemiology studies of these chemicals are greatly needed. We found positive associations between biomarker concentrations and self-reported use of liquid soap (TCS), sunscreen (BP-3), lotion (BP-3 and parabens), and cosmetics (parabens). This information, coupled with data from studies measuring these chemicals in specific products, may help inform pregnant women and others on how to reduce their exposure. Finally, the degree of temporal reliability observed for the urinary measures varied by analyte. Epidemiology studies utilizing these urinary biomarkers to estimate exposure during pregnancy should include as many repeated measurements at multiple times during gestation as feasible to reduce measurement error and to explore potential windows of susceptibility to adverse health outcomes. However, the collection and analysis of multiple urine samples must be reconciled with budget and logistic constraints of large-scale epidemiology studies given the high costs associated with the additional contact with participants and sensitive analytical chemistry methods required.
Supplementary Material
Acknowledgments
The project described was supported by P42ES017198 and P30ES017885 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of Centers for Disease Control and Prevention, the National Institute of Environmental Health Sciences or the National Institutes of Health. We gratefully acknowledge Xiaoliu Zhou, Tao Jia, and Joshua Kramer for technical assistance in measuring the urinary concentrations of the phenols and parabens.
References
- 1.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meeker JD. Exposure to environmental endocrine disruptors and child development. Archives of pediatrics & adolescent medicine. 2012;166(6):E1–7. doi: 10.1001/archpediatrics.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.March of Dimes. Premature birth report cards. 2012 Dec 4; http://www.marchofdimes.com/mission/prematurity_reportcard.html.
- 4.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, Wilson EC. Births: final data for 2009. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 5.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 6.Garza JR, Perez EA, Prelip M, McCarthy WJ, Feldman JM, Canino G, Ortega AN. Occurrence and correlates of overweight and obesity among island Puerto Rican youth. Ethnicity & disease. 2011;21(2):163–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Otero-Gonzalez M, Garcia-Fragoso L. Prevalence of overweight and obesity in a group of children between the ages of 2 to 12 years old in Puerto Rico. Puerto Rico health sciences journal. 2008;27(2):159–61. [PubMed] [Google Scholar]
- 8.Rivera-Soto WT, Rodriguez-Figueroa L, Calderon G. Prevalence of childhood obesity in a representative sample of elementary school children in Puerto Rico by socio-demographic characteristics. Puerto Rico health sciences journal. 2010;29(4):2008. 357–63. [PubMed] [Google Scholar]
- 9.US Centers for Disease Control and Prevention. Increasing prevalence of diagnosed diabetes - United States and puerto rico, 1995-2010. MMWR Morbidity and mortality weekly report. 2012;61:918–21. [PubMed] [Google Scholar]
- 10.Perez CM, Guzman M, Ortiz AP, Estrella M, Valle Y, Perez N, Haddock L, Suarez E. Prevalence of the metabolic syndrome in San Juan, Puerto Rico. Ethnicity & disease. 2008;18(4):434–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Colon I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environmental health perspectives. 2000;108(9):895–900. doi: 10.1289/ehp.108-2556932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larriuz-Serrano MC, Perez-Cardona CM, Ramos-Valencia G, Bourdony CJ. Natural history and incidence of premature thelarche in Puerto Rican girls aged 6 months to 8 years diagnosed between 1990 and 1995. Puerto Rico health sciences journal. 2001;20(1):13–8. [PubMed] [Google Scholar]
- 13.US Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Dec 4; http://www.cdc.gov/exposurereport/
- 14.Geens T, Goeyens L, Covaci A. Are potential sources for human exposure to bisphenol-A overlooked? International journal of hygiene and environmental health. 2011;214(5):339–47. doi: 10.1016/j.ijheh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Braun JM, Hauser R. Bisphenol A and children’s health. Current opinion in pediatrics. 2011;23(2):233–9. doi: 10.1097/MOP.0b013e3283445675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. The Journal of steroid biochemistry and molecular biology. 2011;127(1-2):27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003-2004. Environmental health perspectives. 2008;116(3):303–7. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedoux G, Roig B, Thomas O, Dupont V, Le Bot B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environmental science and pollution research international. 2012;19(4):1044–65. doi: 10.1007/s11356-011-0632-z. [DOI] [PubMed] [Google Scholar]
- 19.Dann AB, Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. Journal of applied toxicology : JAT. 2011;31(4):285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 20.Krause M, Klit A, Blomberg Jensen M, Soeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewiecki KT. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. International journal of andrology. 2012;35(3):424–36. doi: 10.1111/j.1365-2605.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- 21.Tulp MT, Sundstrom G, Martron LB, Hutzinger O. Metabolism of chlorodiphenyl ethers and Irgasan DP 300. Xenobiotica; the fate of foreign compounds in biological systems. 1979;9(2):65–77. doi: 10.3109/00498257909038708. [DOI] [PubMed] [Google Scholar]
- 22.Twum C, Wei Y. The association between urinary concentrations of dichlorophenol pesticides and obesity in children. Reviews on environmental health. 2011;26(3):215–9. doi: 10.1515/reveh.2011.029. [DOI] [PubMed] [Google Scholar]
- 23.Buttke DE, Sircar K, Martin C. Exposures to Endocrine-Disrupting Chemicals and Age of Menarche in Adolescent Girls in NHANES (2003-2008) Environmental health perspectives. 2012;120(11):1613–8. doi: 10.1289/ehp.1104748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Pin I, Charles MA, Cordier S, Slama R. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environmental health perspectives. 2012;120(3):464–70. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environmental health perspectives. 2008;116(8):1092–7. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005-2006. Environmental health perspectives. 2010;118(5):679–85. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, Ye X, Ford J, Keller M, Meeker JD, Hauser R. Predictors and Variability of Urinary Paraben Concentrations in Men and Women, Including before and during Pregnancy. Environmental health perspectives. 2012;120(11):1538–43. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environmental health perspectives. 2011;119(2):252–7. doi: 10.1289/ehp.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environmental health perspectives. 2011;119(1):131–7. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental health perspectives. 2012;120(5):739–45. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844(1):53–9. doi: 10.1016/j.jchromb.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–13. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 33.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer epidemiology biomarkers & prevention. 1995;4(6):649–54. [PubMed] [Google Scholar]
- 34.Rosner B. Fundamentals of Biostatistics. Vol. 5. Duxbury; Pacific Grove, CA: p. 2000. [Google Scholar]
- 35.Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, Wang S. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environmental health perspectives. 2012;120(8):1190–4. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantonwine D, Meeker JD, Hu H, Sanchez BN, Lamadrid-Figueroa H, Mercado-Garcia A, Fortenberry GZ, Calafat AM, Tellez-Rojo MM. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environmental health : a global access science source. 2010;9:62. doi: 10.1186/1476-069X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasper-Sonnenberg M, Wittsiepe J, Koch HM, Fromme H, Wilhelm M. Determination of bisphenol a in urine from mother-child pairs-results from the duisburg birth cohort study, Germany. Journal of toxicology and environmental health Part A. 2012;75(8-10):429–37. doi: 10.1080/15287394.2012.674907. [DOI] [PubMed] [Google Scholar]
- 38.Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VW, Mackenbach JP, Steegers EA, Tiemeier H, Longnecker MP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environmental research. 2008;108(2):260–7. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodriguez LS, Riano I, Tardon A, Vrijheid M, Calafat AM, Sunyer J, Project I. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environment international. 2011;37(5):858–66. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental research. 2008;106(2):257–69. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environmental health perspectives. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shankar A, Teppala S, Sabanayagam C. Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003-2008. ISRN endocrinology. 2012;2012:965243. doi: 10.5402/2012/965243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environmental research. 2011;111(6):825–30. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environmental health perspectives. 2012;120(7):935–43. doi: 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Library of Medicine. Household Products Database. Dec 4; http://hpd.nlm.nih.gov/index.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


