Abstract
Although the lipoprotein complex of pulmonary surfactant has long been recognized as essential for reducing lung surface tension, its role in lung immune host defense has only relatively recently been elucidated. Surfactant-associated proteins A (SP-A) and D (SP-D) can attenuate bacterial and viral infection and inflammation by acting as opsonins and by regulating innate immune cell functions. Surfactant-associated protein A and D also interact with antigen-presenting cells and T cells, thereby linking the innate and adaptive immune systems. A recent study from our laboratory demonstrated that mice deficient in SP-A have enhanced susceptibility to airway hyper-responsiveness and lung inflammation induced by Mycoplasma pneumonia, an atypical bacterium present in the airways of approximately 50% of asthmatics experiencing their first episode, and further supports an important role for SP-A in the host response to allergic airway disease. Animal and human studies suggest that alterations in the functions or levels of SP-A and SP-D are associated with both infectious and non-infectious chronic lung diseases such as asthma. Future studies are needed to elucidate whether alterations in SP-A and SP-D are a consequence and/or cause of allergic airway disease.
Keywords: surfactant protein, collectin, asthma, inflammation, lung
Introduction
Pulmonary surfactant is composed of phospholipids and neutral lipids, which account for approximately 90% of its mass, and of proteins, which account for approximately 10% of its mass. The protein component consists primarily of serum proteins and four surfactant-associated proteins (SPs). Surfactant-associated protein B and C are hydrophobic and interact with surfactant lipids to reduce surface tension at the air–liquid interface and increase lung compliance. Surfactant-associated protein A and D are relatively hydrophilic and are pattern recognition molecules of the collectin family of C-type lectins that participate in lung innate immunity. Collectins, which contain an N-terminal collagen-like region and a C-terminal lectin domain, opsonize pathogens and enhance immune cell phagocytosis and pathogen neutralization by regulating the production of cell-derived mediators. The most abundant of the surfactant-associated proteins is SP-A, which is an octadecameric (6 trimers) bouquet-like molecule. Surfactant-associated protein A is structurally similar to C1q, a protein component of the complement system, and to mannose binding lectin, a serum collectin that is involved in the innate immune response. Surfactant-associated protein D is a dodecamer (4 trimers) that is assembled in a cruciform shape. Surfactant-associated protein A and SP-D are synthesized and secreted by the alveolar type II cells, the airway Clara cells, submucosal cells and non-pulmonary tissues (reviewed by Wright1). In the absence of SP-A, the structure of surfactant large aggregates is altered, although the surface tension reducing capacity of surfactant appears unaffected. In addition, SP-A null mice survive with no apparent pathology when housed in a pathogen-free environment and respond similarly to wild-type mice in exercised or hyperoxic conditions.2 In comparison, SP-D null mice develop alveolar proteinosis and increased lipid pools at about 3 weeks of age, even when housed in a sterile environment, indicating that SP-D plays a role in surfactant homeostasis. Also, metalloproteinases are elevated in the lungs of SP-D null mice and likely contribute to the development of an emphysema-like phenotype. During an immune challenge, both SP-A and SP-D null mice generally exhibit insufficient pulmonary immune responses. For example, the null mice clear viruses and bacteria at a slower rate and generally have higher lavage levels of pro-inflammatory cytokines than do wild-type mice (reviewed by Wright1).
Surfactant proteins in allergic airway disease
In comparison to our understanding of the role of surfactant proteins in infectious disease models, relatively little is known about their role in non-infectious chronic lung diseases such as asthma. Asthma is a serious, sometimes life-threatening, respiratory disease that affects the quality of life of an estimated 300 million people world-wide.3 Asthma is characterized by variable airflow obstruction, airway inflammation and hyper-responsiveness, and reversibility either spontaneously or as a result of treatment.4 A myriad of inciting factors have been proposed such as genetic polymorphisms, viral exposure during infancy, and occupational exposures as an adult (reviewed by Lemanske and Busse4 and Dougherty and Fahy5). Symptoms can be exacerbated by such factors as allergen exposure, respiratory tract infections, exercise, environmental irritants, ingestion of non-steroidal anti-inflammatory agents, psychosocial stress as well as others.5 An individual may be susceptible to all or one of these exacerbating factors and susceptibility may vary over time. Airway inflammation in asthma is heterogeneous, with different phenotypes. Atopic asthma is the most common form of asthma and is associated with eosinophilia driven by T helper 2 (Th2) cells and dendritic cells (DCs), and mast-cell sensitization by IgE that is accompanied with the release of multiple bronchoconstrictors.6 In people with non-atopic asthma, who often have a more severe form of the disease, as well as in those asthmatics that smoke, the pattern of inflammation tends to be more neutrophilic in nature.7 Inflammation is primarily localized to the larger conducting airways, although the smaller airways can be affected in more severe disease. There are currently two basic approaches for the treatment of asthma: (i) long-acting medications to prevent exacerbations such as inhaled corticosteroids, long-acting bronchodilators, or a combination of the two, leukotriene inhibitors, IgE binding inhibitors, mast cell stabilizers, phosphodiesterase inhibitors and adenosine receptor antagonists; and (ii) rescue medications for use during exacerbations such as short-acting bronchodilators and intravenous corticosteroids. However, despite advances in medical therapies, suboptimal control of asthma exists for many individuals. Anadan et al.8 examined international trends in asthma prevalence in children and adults by conducting a systematic review of epidemiological studies published from 1990–2008. The authors concluded that reports of reductions in emergency health-care utilization in some economically developed countries do not signal a declining trend in asthma prevalence but rather reflect improvements in quality of care. In fact, asthma prevalence is either increasing or remaining stable in most parts of the world and the burden on health services for the provision of asthma care is thus expected to remain high. Taken together, these facts highlight the imperative need to elucidate further the pathogenesis of the disease and to develop more efficacious interventions.
Surfactant-associated protein A and D are secreted in both the larger and smaller airways and have been shown to interact with the multitude of cell types implicated in asthmatic inflammatory processes. Thus, SP-A and -D have the potential to play significant roles in modulating asthma pathogenesis (reviewed elsewhere1,9–13). One of the mechanisms by which SP-A and -D could lessen inflammatory consequences in allergic airway disease is to alter allergen uptake and thereby modify the cellular response to allergen. Surfactant-associated protein A has been shown to bind pollen grains and inhibit mast cell degranulation in vitro,14 and SP-D enhances allergen uptake by alveolar macrophages.15 Surfactant-associated protein A and D bind dust mite allergens and inhibit allergen-specific IgE binding to the dust mite allergens.16 Surfactant-associated protein A and D can inhibit IgE binding to Aspergillus fumigatus, an opportunistic airborne fungal pathogen, and block histamine release from human basophils.17
Another mechanism by which SP-A and -D could thwart inflammatory consequences associated with allergic airway disease is to modulate the functions of DCs and CD4+ Th2 cells (Fig. 1). These adaptive immune cells are purported to play critical roles in asthma pathogenesis. For example, rodents injected intratracheally with ovalbumin-pulsed DCs, and subsequently challenged with ovalbumin (OVA) aerosols developed CD4+ Th2 cell-dependent airway eosinophilia, goblet cell hyperplasia, and bronchial hyper-reactivity.18 When DCs were depleted from the airways of thymidine kinase transgenic OVA-sensitized mice, the secondary response to inhaled OVA, consisting of airway eosinophilia and goblet cell hyperplasia, was abolished, suggesting that airway DCs were necessary for the generation of effector functions in previously sensitized animals.19 In addition, CD4+ T cells accumulate in the lung to generate an inflammatory response that has a strong Th2 component, which contributes to airway hyper-responsiveness. We have shown that SP-A and -D can modify the functions of both DCs and T cells. For example, SP-A inhibited bone-marrow derived DC (BMDC) maturation and phagocytic and chemotactic function while SP-D enhanced antigen uptake and presentation by BMDCs in vitro (reviewed by Wright1). Additionally, Hortobágyi et al.20 demonstrated that, when added exogenously, recombinant SP-D inhibited maturation and tumor necrosis factor (TNF)-α production of BMDCs in vitro. Surfactant-associated protein A and D inhibited T-cell proliferation via an interleukin (IL)-2-dependent mechanism observed with accessory cell-dependent T cell mitogens and specific antigen in an IL-2-independent manner (reviewed by Wright1). In contrast to the results obtained using BMDCs, we also demonstrated that SP-D decreased antigen presentation by DCs isolated from the mouse lung during both basal and inflammatory conditions.21 Wang et al.22 established that SP-A and -D reduce allergen-induced lymphocyte proliferation and histamine release in peripheral blood mono-nuclear cells harvested from children with asthma. Despite these studies, how the surfactant proteins regulate the in vivo function of DCs and T cells during allergen-mediated inflammation require further delineation.
Fig. 1.
Studies from several laboratories are consistent with a model in which lung collectins minimize the inflammatory consequences associated with allergic airway disease by binding to allergens and antigens and regulating the functions of immune cells implicated in the pathogenesis of asthma (i.e. dendritic cells [DCs], T cells, and eosinophils [EOS]). Surfactant-associated protein D interacts with DCs to enhance the uptake and presentation of antigen, whereas SP-A affects the production of regulatory molecules (accessory molecules and cytokines) by DCs during allergen or antigen contact that can affect T-cell function. However, if the DCs remain in a less mature state in the lungs, migration may be inhibited to the lymph nodes, the site where DCs primarily present antigen to activate T cells. Concomitantly, in the lungs, SP-A and -D interact directly with T cells, preferentially T effector memory cells (TEMs), to inhibit proliferation and effector function. In addition, SP-D inhibits eosinophil chemotaxis and degranulation. We postulate that, during allergen challenge, SP-A and -D minimize the inflammatory responses that could damage the delicate alveolar epithelium. Thus, deficiencies or inactivation of surfactant proteins could contribute to the development of chronic inflammatory lung diseases. Modified from Wright.1
One of the key effector cells in allergic inflammation is the eosinophil and both SP-A and -D have been shown to regulate eosinophil functions. For example, SP-A represses the production and secretion of IL-8 by isolated eosinophils stimulated with ionomycin.23 Additionally, SP-A expression is up-regulated in patients with allergic rhinitis as compared to controls and positively correlated with eosinophil numbers in the basement membrane of the epithelium, suggesting a key role in mediating inflammation associated with this disease. Several immunomodulatory roles have been described for SP-D interactions with eosinophils: inhibition of eosinophil chemotaxis to eotaxin, regulation of eosinophil degranulation to Ca2+ ionophore and immobilized IgG and serum from allergic patients, and interaction via direct binding to eosinophils.24
Surfactant protein levels in bronchoalveolar lavage fluid (BALF) from humans with asthma have been reported to be both increased and decreased (reviewed by Erpenbeck et al.12). However, a recently published, well-controlled study by Erpenbeck et al.25 concluded that segmental allergen challenge in subjects with asthma resulted in massive eosinophil influx with specific increases in SP-B, SP-C, and SP-D and a decrease in SP-A BALF levels. These levels were compared with baseline and saline control challenge in the same subjects. Importantly, these changes occurred in the absence of a concomitant change in total phospholipid levels in the cell-free BALF, suggesting that SP levels are regulated independently from surfactant phospholipids synthesis and secretion. A specific decrease in SP-A levels during allergic inflammation was also observed in mouse studies using either A. fumigatus or dust mite allergen.26,27 It remains unclear as to whether the surfactant protein alterations are a consequence or a cause of disease or are associated with disease severity.
Studies using SP-A and -D null mice in various allergen models suggest that SP-A and -D deficiencies do contribute to allergic airway disease. Both SP-A and -D null mice are more susceptible to allergic bronchopulmonary aspergillosis (ABPA), an allergic disorder induced by A. fumigatus, and exogenous administration of SP-A and of SP-D reduced A. fumigatus-associated, T cell-mediated, inflammatory indices.28 Aspergillus fumigatus has both allergenic components and fungal pathogenic components and, unlike other allergens, contains intrinsic protease activity that can act as an adjuvant to prolong Th2 responses.29
Surfactant-associated protein A and D null mice also have enhanced susceptibility to allergic airway disease induced by the single antigen model ovalbumin (OVA). We showed that SP-A null mice treated with OVA have greater eosinophilia, T helper 2 (Th2)-associated cytokine levels, and IgE levels as well as elevated proportions of CD4+ effector memory T cells in their lungs compared to wild-type mice treated with OVA.30 The function of SP-D, however, has been investigated more thoroughly using the OVA model. Takeda et al.31 described a progressive increase in SP-D lavage levels in mice up to 48 h after the last OVA aerosol challenge. In addition, administration of recombinant SP-D dose-dependently decreased airway hyper-reactivity and eosinophilia. These decreases were mechanistically attributed to increased IL-10 and Th1 cytokine secretion by alveolar macrophages. Kaspar et al.32 also reported that OVA challenge increased SP-D levels and that the increases that were not affected by treatment with the corticosteroid dexamethasone in rats. Interestingly, increased SP-D expression was traced not only to type II pneumocytes but also to Clara cells and hyperplastic goblet cells. Schaub et al.33 demonstrated that SP-D null mice have a heightened response to OVA challenge; early in the response, the lack of SP-D skews the responses in favor of a Th2-like response, while at later times, a rise in interferon (IFN)-γ and a decrease in IL-10 occur. Levels of BALF eosinophils and IL-13 production were increased also at the early time points. Haczku et al.34 recently showed that resolution of the allergic response to OVA and to A. fumigatus is associated with an IL-4/IL-13-mediated increase in SP-D levels. In addition, SP-D null mice displayed heightened CD4 activation with increased IgE production to allergenic sensitization while the addition of SP-D to A. fumigatus-stimulated lymphocyte cultures directly inhibited Th2 cell activation in vitro. Of note, Schaub et al.33 and Haczku et al.34 reported discrepant results. In contrast to Haczku et al.,34 Schaub et al.33 did not see an increase in serum IgE but did observe tissue eosinophilia, illustrating that SP-D has differential effects depending on the allergen or the sensitization protocol used. Similar to many other studies published on the topic of lung immunity, Haczku et al.34 used splenocytes for in vitro cultures to draw conclusions about the interaction of SPs and T cells. It will be important for future studies to perform immunological assays that incorporate cells harvested directly from the airways, lungs, or draining lymph nodes.
Although valuable studies have been conducted using SP-D null mice, studies with these mice are confounded by the fact that they begin to show physiological signs as early as 3 weeks of age of what will eventually develop into a complicated emphysemic-like phenotype.35 The features of this phenotype are not typically associated with allergic asthma, making interpretation of the role of SP-D in asthma a challenge. It would be informative to use conditional SP-D null mice in allergic studies to minimize the effects of the activated macrophages of the SP-D null mice.
Whether surfactant modulates airway hyper-reactivity, a classical feature of allergic asthma, is not well understood. Surfactant administration has a bronchodilatory effect in rodent asthma models36 but has achieved limited success in humans with asthma.12,37 The relative contributions of surfactant lipids and proteins in the reported bronchodilatory effects have not been elucidated and whether surfactant can directly affect smooth muscle function is ambiguous.
Surfactant proteins in mediating infection-induced exacerbations in allergic airways
Mounting evidence suggest that pulmonary infections with either the atypical bacterium Mycoplasma pneumonia or Chlamydophila pneumoniae commonly exacerbate symptoms observed in asthmatics, and thus, may contribute to asthma morbidity.38 Additionally, recent studies show that 50% of patients experiencing their first asthmatic episode also have an acute M. pneumoniae airway infection.39 Further evidence from a double-blind, placebo-controlled trial in which pulmonary function improved after antibiotic treatment only in asthmatics with M. pneumoniae infection supports a close link between this atypical bacterial infection and chronic asthma.40 Therefore, a better understanding of the role of infectious agents in asthma and the ability to prevent exacerbations caused by these agents could potentially improve the quality of life for asthma sufferers and help preserve lung function.
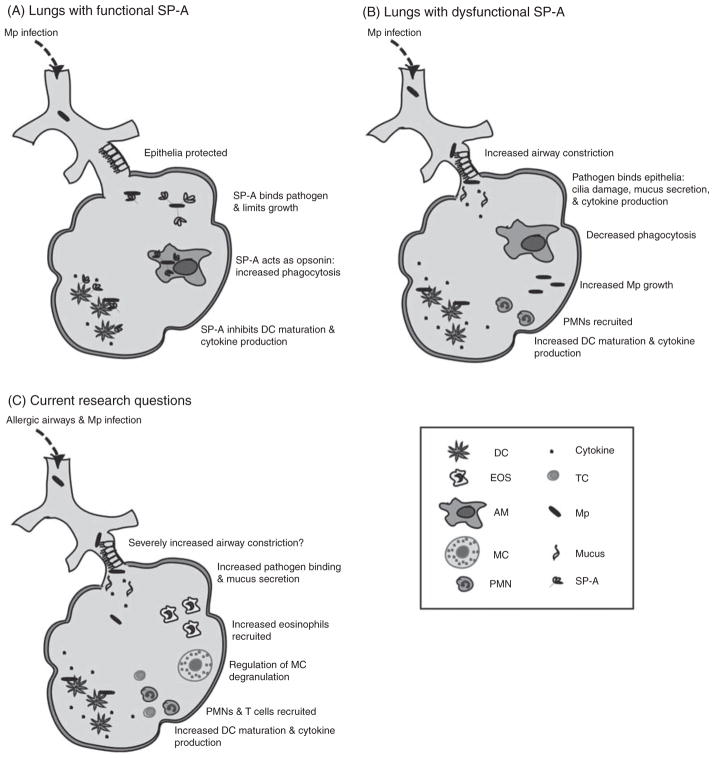
Although previous studies have shown that SP-A null mice have an enhanced susceptibility to infection with Mycoplasma pulmonis,41 studies investigating the role of SP-A in modulating the host response to M. pneumoniae were only recently described. Surfactant-associated protein A has been shown to bind surface disaturated phosphati-dylglycerols42 on M. pneumoniae and to an M. pneumoniae protein known as MPN372.43 Consequences of SP-A/M. pneumoniae binding were inhibition of M. pneumoniae growth in vitro and enhanced M. pneumoniae uptake by macrophages.42 Recent studies from our laboratory showed that SP-A null mice are more susceptible to M. pneumoniae infection than are wild-type mice (Fig. 2). Surfactant-associated protein A null mice had elevated levels of inflammatory cytokines, infiltrating immune cells, mucus production and significantly augmented airway hyper-responsiveness compared to wild-type mice.44 Interestingly, when TNF-α production was inhibited, airway reactivity and mucus production in the SP-A null mice were attenuated to levels approximating those of wild-type mice. We concluded from these studies that SP-A plays a critical role in preserving lung homeostasis and host defense against M. pneumoniae by curtailing inflammatory cell recruitment and limiting an overzealous TNF-α response and that an absence of SP-A or inactivation of SP-A could lead to augmented airway disease in asthmatics. Future studies should be conducted to evaluate the role of SP-A in protecting from M. pneumoniae-induced exacerbations during allergic airway disease.
Fig. 2.
(A) Proposed model of M. pneumoniae-infected SP-A functional lungs. Surfactant-associated protein A binds M. pneumoniae and inhibits its growth and pathogenicity. Surfactant-associated protein A acts as an opsonin to increase phagocytosis of M. pneumoniae by alveolar macrophages, which would localize the infection and limit damage to the fragile lung epithelium. Surfactant-associated protein A inhibits M. pneumoniae-induced DC maturation, which would result in less T-cell activation. Airway constriction will be minimal. (B) Proposed model of M. pneumoniae-infected SP-A dysfunctional lungs. Growth and pathogenicity will not be controlled by SP-A. Since M. pneumoniae interacts with TLR2 to increase mucin expression in response to infection and since SP-A also associates with TLR2 on macrophages to limit cytokine release, diminished competition for TLR2 binding in mice lacking SP-A should result in more mucus production and higher levels of TNF-α as compared to wild-type control mice. Decreased levels of SP-A or dysfunctional allelic variants of SP-A would decrease the likelihood of M. pneumoniae being phagocytosed by alveolar macrophages, resulting in M. pneumoniae binding to the airway epithelial cells, maintenance of an extracellular existence and enhanced inflammation and airway reactivity. Dendritic cells will be more mature and consequently T cells will be more activated and airways will present a greater level of inflammation in SP-A deficient mice after pulmonary infection with M. pneumoniae. Airway constriction will be significantly greater with SP-A absent or dysfunctional. (C) Current research questions. In M. pneumoniae-exacerbated allergic lungs, will SP-A limit the number recruited eosinophils infiltrating the airways and protect from M. pneumoniae-stimulated eosinophil cytokine production? Will SP-A inhibit M. pneumoniae-induced DC maturation, T-cell proliferation and activation and chemotaxis of peripheral PMNs? Will SP-A regulate MC functions and protect from acute asthma symptoms? If SP-A modulates any of these inflammatory parameters, it will likely provide protection from M. pneumoniae-induced allergic exacerbations and airway constriction.
Surfactant protein variants associated with lung diseases
Unlike rodents, humans have two SP-A genes, SP-A1 and SP-A2, and SP-A isolated from human lavage consists of both SP-A1 and SP-A2 gene products. More than 30 genetic variants (alleles) have been fully or partially characterized in both SP-A1 and SP-A2 (reviewed by Floros et al.45 and Floros and Wang46). Four of the SP-A1 alleles (6A, 6A2, 6A3, 6A4) and six SP-A2 (1A, 1A0, 1A1, 1A2, 1A3, and 1A5) are observed with a frequency of greater than 1% in the general population. The amino acid changes occur in SP-A1 alleles at positions 19, 50 and 219 and in SP-A2 alleles at amino acids 9, 91 and 223. The amino acid position numbering for all these alleles corresponds to the primary translation product with the initiating Met as position 1. The mature secreted SP-A proteins differ at amino acid positions 19 (Ala or Val), 50 (Leu or Val) or 219 (Arg or Trp) for SP-A1, and positions 9 (Asn or Thr), 91 (Pro or Ala) or 223 (Gln or Lys) for SP-A2.
Several studies by Floros and colleagues have reported associations of SP-A alleles with a variety of lung diseases including tuberculosis, respiratory distress syndrome, COPD, and susceptibility to ozone (reviewed by Pastva et al.13). Recent studies also identified strong associations of SP-A polymorphisms with the severity of respiratory syncytial viral infections.47 A very recent study by Pettigrew and colleagues48 reported an association between wheezing and coughing in newborn infants and concluded that polymorphisms within the SP-A gene may be associated with an increased risk of asthma. Specifically, the 6A/1A haplotype was found to be associated with increased risk of persistent cough or wheeze.48 Interestingly, the structurally homologous mannose binding lectin (MBL), which like SP-A and SP-D is located on the long arm of chromosome 10q25, has been found to have three point mutations in the region coding for the collagen domain of MBL that correlate with low MBL levels in serum.49 In addition, polymorphisms in the promoter region of MBL have been linked to altered levels of serum MBL, and low levels of MBL have been linked to enhanced susceptibility to infection50,51 and severity of lung disease in patients with cystic fibrosis.52 Nagy and co-workers53 reported that the incidence of infection with C. pneumoniae and asthma was increased in children carrying variant MBL alleles.
To date, only three polymorphisms in the SP-D gene have been described: Met11Thr, Ala160Thr and Ser270Thr.54 The Thr/Thr11 bi-allelic form of SP-D has reduced viral neutralizing activity, does not properly oligomerize55 and has been associated with an increased susceptibility to M. tuberculosis infections.56 Additionally, studies in a Chinese population found significantly higher rates of the Met11Thr genotype present in patients with allergic rhinitis as compared to control groups, suggesting the Met11Thr polymorphism in SP-D plays a key role in genetic predisposition to allergic rhinitis.57 However, Brandt et al.58 described findings that the Met11Thr variant of SP-D, while affecting SP-D levels and decreasing the ability to bind pathogens, was associated with decreased atopy in black subjects and lower asthma susceptibility in white subjects. While the presence and significance of surfactant protein allelic variants have not been completely elucidated in asthma, existing data suggest that surfactant protein and phospholipid deficiency or dysfunction might play an important role in disease pathogenesis.25,27,59,60
Conclusions and Future Directions
Deficiencies of SP-A and SP-D have been shown to worsen both infectious and non-infectious lung injury in SP-A and SP-D null mice. The roles of SP-A and SP-D in host defense against lung infections is well understood; by virtue of their carbohydrate binding functions, SP-A and SP-D opsonize pathogens and enhance their clearance via increasing phagocytosis and regulating the production of cytokines and free radicals. In contrast, the mechanisms by which SP-A and SP-D regulate immune and epithelial cell responses in non-infectious lung injury are less well understood. Surfactant replacement therapies containing SP-B and SP-C have had a major impact on infant mortality and morbidity in the treatment of infant respiratory distress syndrome. An understanding of the roles of SP-A and SP-D in protecting the lung during acute and chronic lung disease is critical to the development of new and innovative therapies for allergic and inflammatory lung diseases. Moreover, future studies must further clarify the importance of alterations in the functions and levels of SP-A and SP-D, either via inactivation, degradation or polymorphic variation, in causing human lung disease.
Acknowledgments
The work from the authors’ laboratories was supported by the following grants: NIH NIAID 1K08AI068822 (AMP), NIH F32HL091642 (JGL), NIH NHLBI 5P50HL084917 (JRW), NIH NHLBI 5R01HL068072 (JRW), NIH NHLBI 2R01HL030923 (JRW) and NIH 1P01AI81672 (JRW).
References
- 1.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 2.Ikegami M, Jobe AH, Whitsett J, Korfhagen T. Tolerance of SP-A-deficient mice to hyperoxia or exercise. J Appl Physiol. 2000;89:644–648. doi: 10.1152/jappl.2000.89.2.644. [DOI] [PubMed] [Google Scholar]
- 3.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Lemanske RF, Jr, Busse WW. 6. Asthma: factors underlying inception, exacerbation, and disease progression. J Allergy Clin Immunol. 2006;117:S456–S461. doi: 10.1016/j.jaci.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol. 2007;119:1055–1062. doi: 10.1016/j.jaci.2007.01.015. quiz 63–64. [DOI] [PubMed] [Google Scholar]
- 8.Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2009;65:152–167. doi: 10.1111/j.1398-9995.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 9.Haczku A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J Allergy Clin Immunol. 2008;122:861–879. doi: 10.1016/j.jaci.2008.10.014. quiz 80–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen GL, Husby S, Holmskov U. Surfactant protein A and surfactant protein D variation in pulmonary disease. Immunobiology. 2007;212:381–416. doi: 10.1016/j.imbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Kishore U, Greenhough TJ, Waters P, et al. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Erpenbeck VJ, Krug N, Hohlfeld JM. Therapeutic use of surfactant components in allergic asthma. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:217–224. doi: 10.1007/s00210-008-0354-z. [DOI] [PubMed] [Google Scholar]
- 13.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhotra R, Haurum J, Thiel S, Jensenius JC, Sim RB. Pollen grains bind to lung alveolar type II cells (A549) via lung surfactant protein A (SP-A) Biosci Rep. 1993;13:79–90. doi: 10.1007/BF01145960. [DOI] [PubMed] [Google Scholar]
- 15.Erpenbeck VJ, Malherbe DC, Sommer S, et al. Surfactant protein D increases phagocytosis and aggregation of pollen-allergen starch granules. Am J Physiol. 2005;288:L692–L698. doi: 10.1152/ajplung.00362.2004. [DOI] [PubMed] [Google Scholar]
- 16.Seitzman GD, Sonstein J, Kim S, Choy W, Curtis JL. Lung lymphocytes proliferate minimally in the murine pulmonary immune response to intratracheal sheep erythrocytes. Am J Respir Cell Mol Biol. 1998;18:800–812. doi: 10.1165/ajrcmb.18.6.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madan T, Kishore U, Shah A, et al. Lung surfactant proteins A and D can inhibit specific IgE binding to the allergens of Aspergillus fumigatus and block allergen-induced histamine release from human basophils. Clin Exp Immunol. 1997;110:241–249. doi: 10.1111/j.1365-2249.1997.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrecht BN, Peleman RA, Bullock GR, Pauwels RA. Sensitization to inhaled antigen by intratracheal instillation of dendritic cells [see comments] Clin Exp Allergy. 2000;30:214–224. doi: 10.1046/j.1365-2222.2000.00818.x. [DOI] [PubMed] [Google Scholar]
- 19.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–4097. [PubMed] [Google Scholar]
- 20.Hortobagyi L, Kierstein S, Krytska K, et al. Surfactant protein D inhibits TNF-alpha production by macrophages and dendritic cells in mice. J Allergy Clin Immunol. 2008;122:521–528. doi: 10.1016/j.jaci.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen S, Lo B, Evans K, Neophytou P, Holmskov U, Wright JR. Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens. Am J Respir Cell Mol Biol. 2007;36:94–102. doi: 10.1165/rcmb.2006-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JY, Shieh CC, You PF, Lei HY, Reid KB. Inhibitory effect of pulmonary surfactant proteins A and D on allergen-induced lymphocyte proliferation and histamine release in children with asthma. Am J Respir Crit Care Med. 1998;158:510–518. doi: 10.1164/ajrccm.158.2.9709111. [DOI] [PubMed] [Google Scholar]
- 23.Cheng G, Ueda T, Nakajima H, et al. Suppressive effects of SP-A on ionomycin-induced IL-8 production and release by eosinophils. Int Arch Allergy Immunol. 1998;117 (Suppl 1):59–62. doi: 10.1159/000053574. [DOI] [PubMed] [Google Scholar]
- 24.von Bredow, Hartl C, Schmid DK, et al. Surfactant protein D regulates chemotaxis and degranulation of human eosinophils. Clin Exp Allergy. 2006;36:1566–1574. doi: 10.1111/j.1365-2222.2006.02598.x. [DOI] [PubMed] [Google Scholar]
- 25.Erpenbeck VJ, Schmidt R, Gunther A, Krug N, Hohlfeld JM. Surfactant protein levels in bronchoalveolar lavage after segmental allergen challenge in patients with asthma. Allergy. 2006;61:598–604. doi: 10.1111/j.1398-9995.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 26.Atochina EN, Beers MF, Tomer Y, et al. Attenuated allergic airway hyperresponsiveness in C57BL/6 mice is associated with enhanced surfactant protein (SP)-D production following allergic sensitization. Respir Res. 2003;4:15. doi: 10.1186/1465-9921-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JY, Shieh CC, Yu CK, Lei HY. Allergen-induced bronchial inflammation is associated with decreased levels of surfactant proteins A and D in a murine model of asthma. Clin Exp Allergy. 2001;31:652–662. doi: 10.1046/j.1365-2222.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- 28.Madan T, Kishore U, Singh M, et al. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. 2001;107:467–475. doi: 10.1172/JCI10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Pastva AM, Mukherjee S, Giamberardino C, Brown B, Lo B, Wright JR. SP-A modulates CD4+ T cell phenotype and function during allergen-mediated inflammation. Am J Respir Crit Care Med. 2009;179:A4243. [Google Scholar]
- 31.Takeda K, Miyahara N, Rha YH, et al. Surfactant protein D regulates airway function and allergic inflammation through modulation of macrophage function. Am J Respir Crit Care Med. 2003;168:783–789. doi: 10.1164/rccm.200304-548OC. [DOI] [PubMed] [Google Scholar]
- 32.Kasper M, Sims G, Koslowski R, et al. Increased surfactant protein D in rat airway goblet and Clara cells during ovalbumin-induced allergic airway inflammation. Clin Exp Allergy. 2002;32:1251–1258. doi: 10.1046/j.1365-2745.2002.01423.x. [DOI] [PubMed] [Google Scholar]
- 33.Schaub B, Westlake RM, He H, et al. Surfactant protein D deficiency influences allergic immune responses. Clin Exp Allergy. 2004;34:1819–1826. doi: 10.1111/j.1365-2222.2004.02068.x. [DOI] [PubMed] [Google Scholar]
- 34.Haczku A, Cao Y, Vass G, et al. IL-4 and IL-13 form a negative feedback circuit with surfactant protein-D in the allergic airway response. J Immunol. 2006;176:3557–3565. doi: 10.4049/jimmunol.176.6.3557. [DOI] [PubMed] [Google Scholar]
- 35.Collins RA, Ikegami M, Korfhagen TR, Whitsett JA, Sly PD. In vivo measurements of changes in respiratory mechanics with age in mice deficient in surfactant protein D. Pediatr Res. 2003;53:463–467. doi: 10.1203/01.PDR.0000049464.46191.BF. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Wang L, Li E, Enhorning G. Pulmonary surfactant given prophylactically alleviates an asthma attack in guinea-pigs. Clin Exp Allergy. 1996;26:270–275. [PubMed] [Google Scholar]
- 37.Erpenbeck VJ, Hagenberg A, Dulkys Y, et al. Natural porcine surfactant augments airway inflammation after allergen challenge in patients with asthma. Am J Respir Crit Care Med. 2004;169:578–586. doi: 10.1164/rccm.200301-104OC. [DOI] [PubMed] [Google Scholar]
- 38.Pelaia G, Vatrella A, Gallelli L, et al. Respiratory infections and asthma. Respir Med. 2006;100:775–784. doi: 10.1016/j.rmed.2005.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biscardi S, Lorrot M, Marc E, et al. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–1346. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 40.Kraft M, Rex MD, Martin RJ, Chue HW. Elastin density is reduced in the distal lung in patients with nocturnal worsening of asthma. Am J Respir Crit Care Med. 2002;165:416. [Google Scholar]
- 41.Hickman-Davis JM, Gibbs-Erwin J, Lindsey JR, Matalon S. Role of surfactant protein-A in nitric oxide production and mycoplasma killing in congenic C57BL/6 mice. Am J Respir Cell Mol Biol. 2004;30:319–325. doi: 10.1165/rcmb.2003-0246OC. [DOI] [PubMed] [Google Scholar]
- 42.Piboonpocanun S, Chiba H, Mitsuzawa H, et al. Surfactant protein A binds Mycoplasma pneumoniae with high affinity and attenuates its growth by recognition of disaturated phosphatidyl-glycerols. J Biol Chem. 2005;280:9–17. doi: 10.1074/jbc.M411570200. [DOI] [PubMed] [Google Scholar]
- 43.Kannan TR, Provenzano D, Wright JR, Baseman JB. Identification and characterization of human surfactant protein A binding protein of Mycoplasma pneumoniae. Infect Immun. 2005;73:2828–2834. doi: 10.1128/IAI.73.5.2828-2834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ledford JG, Goto H, Potts EN, et al. SP-A preserves airway homeostasis during Mycoplasma pneumoniae infection in mice. J Immunol. 2009;182:7818–7827. doi: 10.4049/jimmunol.0900452. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 45.Floros J, Fan R, Matthews A, et al. Family-based transmission disequilibrium test (TDT) and case-control association studies reveal surfactant protein A (SP-A) susceptibility alleles for respiratory distress syndrome (RDS) and possible race differences. Clin Genet. 2001;60:178–187. doi: 10.1034/j.1399-0004.2001.600303.x. [DOI] [PubMed] [Google Scholar]
- 46.Floros J, Wang G. A point of view: quantitative and qualitative imbalance in disease pathogenesis; pulmonary surfactant protein A genetic variants as a model. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:295–303. doi: 10.1016/s1095-6433(01)00325-7. [DOI] [PubMed] [Google Scholar]
- 47.El Saleeby CM, Quasney MW, DeVincenzo JP. The association of surfactant protein A genotypes and disease severity within an RSV infected population. PAS. 2005;57:710. [Google Scholar]
- 48.Pettigrew MM, Gent JF, Zhu Y, et al. Respiratory symptoms among infants at risk for asthma: association with surfactant protein A haplotypes. BMC Med Genet. 2007;8:15. doi: 10.1186/1471-2350-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner MW, Lipscombe RJ, Levinsky RJ, et al. Mutations in the human mannose binding protein gene: their frequencies in three distinct populations and relationship to serum levels of the protein. Immunodeficiency. 1993;4:285–287. [PubMed] [Google Scholar]
- 50.Summerfield JA, Ryder S, Sumiya M, et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886–889. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 51.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–943. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 52.Garred P, Pressler T, HOM, et al. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104:431–437. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagy A, Kozma GT, Keszei M, Treszl A, Falus A, Szalai C. The development of asthma in children infected with Chlamydia pneumoniae is dependent on the modifying effect of mannose-binding lectin. J Allergy Clin Immunol. 2003;112:729–734. doi: 10.1016/s0091-6749(03)02010-4. [DOI] [PubMed] [Google Scholar]
- 54.DiAngelo S, Lin Z, Wang G, et al. Novel, non-radioactive, simple and multiplex PCR-cRLFP methods for genotyping human SP-A and SP-D marker alleles. Dis Markers. 1999;15:269–281. doi: 10.1155/1999/961430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartshorn KL, White MR, Tecle T, et al. Reduced influenza viral neutralizing activity of natural human trimers of surfactant protein D. Respir Res. 2007;8:9. doi: 10.1186/1465-9921-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Floros J, Lin HM, Garcia A, et al. Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. J Infect Dis. 2000;182:1473–1478. doi: 10.1086/315866. [DOI] [PubMed] [Google Scholar]
- 57.Deng YQ, Tao ZZ, Kong YG, et al. Association between single nucleotide polymorphisms of surfactant protein D and allergic rhinitis in Chinese patients. Tissue Antigens. 2009;73:546–552. doi: 10.1111/j.1399-0039.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 58.Brandt EB, Mingler MK, Stevenson MD, et al. Surfactant protein D alters allergic lung responses in mice and human subjects. J Allergy Clin Immunol. 2008;121:1140–1147. e2. doi: 10.1016/j.jaci.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright SM, Hockey PM, Enhorning G, et al. Altered airway surfactant phospholipid composition and reduced lung function in asthma. J Appl Physiol. 2000;89:1283–1292. doi: 10.1152/jappl.2000.89.4.1283. [DOI] [PubMed] [Google Scholar]
- 60.van de Graaf EA, Jansen HM, Lutter R, et al. Surfactant protein A in bronchoalveolar lavage fluid. J Lab Clin Med. 1992;120:252–263. [PubMed] [Google Scholar]