Abstract
Background
We prospectively applied the Surgical Treatment of Ischemic Cardiomyopathy (STICH) trial entry criteria to an observational database to determine whether coronary artery bypass grafting (CABG) decreases mortality compared to medical therapy (MED) for patients suffering coronary artery disease (CAD) and depressed left ventricular ejection fraction (LVEF).
Methods
This was a retrospective, observational, cohort study of prospectively collected data from the Duke Databank for Cardiovascular Disease. Long-term mortality was the main outcome measure. Between January 1, 1995 and July 31, 2009, 86,874 patients underwent cardiac catheterization for suspected ischemic heart disease and were evaluated for inclusion in the analysis.
Results
A total of 2,624 patients were found to have LVEF <35%, CAD amenable to CABG and no left main stenosis ≥50%. After exclusions including ongoing Class III angina and acute myocardial infarction, 763 patients were included for propensity score analysis including 624 who received MED and 139 CABG. Adjusted mortality curves were constructed for those patients in the three quintiles most likely to receive CABG. The curves diverged early, with risk-adjusted mortality rates at 5 years of 46% for MED versus 29% for CABG, and the survival benefit of CABG over MED continued through 10 years follow-up (hazard ratio 0.63, 95% CI 0.45 – 0.88).
Conclusions
Among a propensity-matched, risk-adjusted observational cohort of patients with CAD, LVEF < 35%, and no left main disease ≥ 50%, CABG is associated with a survival advantage over MED through 10 years follow-up.
Introduction
Heart failure is a growing and well-recognized public health problem [1, 2]. Coronary artery disease (CAD) is the primary etiology in two-thirds of heart failure cases [1, 3], with uncontrolled ischemic disease a frequent underlying cause of heart failure exacerbation [4, 5]. Current American College of Cardiology/American Heart Association guidelines highlight recent advances in pharmacologic, surgical, and device therapies that have improved the quality of life and survival of patients with CAD and heart failure [1, 6]. Multiple methodologically sound randomized trials have established the benefits of coronary artery bypass grafting (CABG) for CAD [7, 8]; however, prior to the report of the STICH result in April 2011, no randomized data were available which specifically evaluate the important subgroup of patients with left ventricular (LV) systolic dysfunction [9, 10].
The Surgical Treatment for Ischemic Heart Failure (STICH) trial was a National Heart, Lung, and Blood Institute-funded multicenter international randomized trial addressing two pressing clinical and policy questions regarding the management of patients with surgically revascularizable CAD and LV systolic dysfunction [11, 12]. STICH hypothesis 1 addressed the question of whether contemporary CABG is superior to contemporary medical/secondary prevention therapy in prolonging survival in 1212 patients with CAD/LV systolic dysfunction [12]. The present study was designed to evaluate the survival benefit of CABG for patients with ischemic cardiomyopathy in an observational cohort where the choice of CABG resulted from clinical decision and not randomization by applying the STICH trial’s enrollment criteria and propensity models developed in the Duke Cardiovascular Disease Databank [13].
Material and Methods
Study Design
This was a retrospective, observational, cohort study of prospectively collected data from patients treated at Duke University Medical Center. We sought to identify patients with left ventricular ejection fraction (EF) ≤ 35% and CAD amenable to CABG from all patients undergoing cardiac catheterization and assessment of left ventricular function at Duke University Medical Center between January 1, 1995 and July 31, 2009. Patients with left main coronary artery stenosis ≥ 50% were excluded based upon clinical standards of early randomized trials [7]. Patient outcomes were compared between those receiving medical (MED) versus CABG therapy. This study was approved by the Duke University Health System Institutional Review Board (IRB), and the requirement for individual consent was waived (IRB Registry Number 7399-05-7R0ER). The study end point (mortality) and statistical methodology were specified prior to data retrieval and analysis. All analyses were completed prior to the availability of the STICH hypothesis 1 outcome.
The CABG treatment arm was comprised of any patient receiving CABG within 30 days following initial cardiac catheterization without percutaneous coronary intervention. Time 0 for the CABG group was the date of cardiac catheterization. MED patients were defined as those receiving neither PCI nor CABG within 30 days of the initial catheterization. Patients who died within 5 days (median time to CABG) were excluded to address waiting time bias. Time 0 for the MED group was day 6 following catheterization - thus, the patient must have survived to have been eligible to receive a procedure. Any patients progressing to cardiac transplantation or destination left ventricular assist device placement were planned to be excluded.
Data Source
Pertinent baseline variables (Table 1) from the history, physical examination, laboratory tests, imaging exams, and 12-lead electrocardiogram (ECG) were collected prospectively on standardized forms as part of the patient care process and stored in the Duke Databank for Cardiovascular Disease (DDCD). The results of cardiac catheterization, the procedural details of each CABG, and detailed follow-up were also collected prospectively.
Table 1.
Baseline Characteristics of the Study Cohort (Propensity Quintiles I, II, and III)
| Variable | Medicine N = 327 |
CABG N = 131 |
All N = 458 |
Treatment Comparison P-value |
|---|---|---|---|---|
| Age in years, median (25, 75%ile) | 63.5 (56, 71) |
65.2 (57, 72) |
64.1 (56.3, 71.1) |
0.527 |
| Male | 73.7 | 78.6 | 75.1 | 0.271 |
| Caucasian (%) | 64.2 | 66.2 | 64.7 | 0.187 |
| Ejection fraction, median (25, 75%ile) | 0.27 (0.22, 0.31) |
0.28 (0.24, 0.32) |
0.27 (0.22, 0.31) |
0.068 |
| CHF history (%) | 0.800 | |||
| No CHF | 32 | 34 | 33 | |
| Class I | 6 | 6 | 6 | |
| Class II | 14 | 13 | 14 | |
| Class III | 26 | 22 | 25 | |
| Class IV | 21 | 25 | 22 | |
| Diabetes mellitus (%) | ||||
| Total | 34 | 34 | 34 | 0.942 |
| Type I (% of total) | 5 | 14 | 8 | 0.084 |
| Type II (% of total) | 95 | 86 | 92 | 0.084 |
| Mitral insufficiency (%) | ||||
| 0 | 48 | 44 | 47 | 0. 837 |
| 1+ | 18 | 18 | 18 | |
| 2+ | 19 | 19 | 19 | |
| 3+ | 10 | 13 | 11 | |
| 4+ | 5 | 7 | 6 | |
| Number of diseased vessels | <0.001 | |||
| 0-vessel disease (%) | 0 | 0 | 0 | |
| 1-vessel disease (%) | 30 | 12 | 25 | |
| 2-vessel disease (%) | 30 | 24 | 28 | |
| 3-vessel disease (%) | 40 | 64 | 47 | |
| BMI, median (25, 75) | 26 (24,31) |
27 (24,31) |
26 (24, 31) |
0.948 |
| Renal disease (%) | 3.7 | 3.1 | 3.5 | 0.745 |
| History of MI (%) | 40 | 34 | 38 | 0.256 |
| History of smoking (%) | 61 | 66 | 62 | 0.339 |
| History of CABG (%) | 36 | 4 | 7 | <0.001 |
| History of PCI (%) | 22 | 17 | 18 | 0.206 |
| Duke CAD Severity Index | ||||
| Low | 30 | 11 | 24 | <0.001 |
| Intermediate | 33 | 29 | 32 | |
| High | 38 | 60 | 44 | |
| History of peripheral vascular disease (%) | 11 | 10 | 11 | 0.806 |
| History of cerebrovascular disease (%) | 11 | 10 | 11 | 0.806 |
BMI = body mass index; CABG = coronary artery bypass grafting; CHF = congestive heart failure; PCI = percutaneous coronary intervention.
Patient follow-up was conducted by the Duke Clinical Research Institute Follow-up Services Group, which is responsible for collecting annual follow-up data on death and other end points for the DDCD [14]. Annual surveys collect data on overall health, hospitalizations, myocardial infarction, stroke, cardiac procedures, and medication use. Patients are surveyed 6 months after the index visit and yearly thereafter, by means of a mailed, self-administered questionnaire; non-responders are surveyed by telephone. For mortality data, response rate is 95% and there is an annual search of the National Death Index for patients who are lost to follow-up (2%) or who have requested not to be contacted (3%). Information on death is collected through next-of-kin interviews, reviews of hospital discharge summaries and death certificates, and the National Death Index, which provides the cause of death according to the International Classification of Diseases, 10th Revision, after independent committee review.
Propensity models
To identify those patients most likely to have met the STICH revascularization hypothesis criteria as patients for whom adding CABG to MED was “reasonable but not required” [11], we were able to calculate propensity scores for MED and CABG treatments using patients undergoing catheterization between 1995 and 2005 [15]. The propensity model was then validated using the cohort of patients undergoing catheterization between 2006 and 2009. Table 2a lists the variables used in construction of the multivariable models. Table 2b identifies the most contributory patient variables with respect to MED or CABG treatment selection.
Table 2a.
Multivariable Model Variables
| Model variables |
|---|
| Propensity of assignment to medication group |
| Age |
| Left ventricular ejection fraction |
| BMI |
| Systolic blood pressure |
| Duke CAD Severity Index |
| Mitral Insufficiency |
| Year |
| CHF Class |
| Any valvular heart disease |
| Male |
| Caucasian |
| Hypertension |
| Charlson Index |
| History of angina |
| Diabetes |
| History of MI |
| History of cerebrovascular disease |
| History of peripheral vascular disease |
| History of smoking |
| History of CABG |
| History of PCI |
| Ventricular gallop |
| Carotid bruit |
BMI = body mass index; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CHF = congestive heart failure; MED = medical therapy; MI = myocardial infarction; PCI = percutaneous coronary intervention; STICH= Surgical Treatment of Ischemic Cardiomyopathy.
Table 2b.
Multivariable Model Variables Predicting Medicine (MED) or CABG Treatment Choice in 763 study Patients at Duke 1995–2005
| For MED Choice | % Information | For CABG Choice | % Information |
|---|---|---|---|
| Prior CABG | 36 | High CAD index | 21 |
| Non-cardiac illness | 9 | Younger age | 16 |
| Higher LVEF | 9 | Recent years | 5 |
| Mitral insufficiency ≥ 2+ | 4 |
CABG = coronary artery bypass grafting; CAD = coronary artery disease; LVEF = left ventricular ejection fraction; MED = medical therapy; MI = myocardial infarction.
Patients were divided into propensity quintiles, with Quintile I representing those least often treated with MED and Quintile V representing those most often treated with MED. Quintiles I–III patients, those with higher probability of CABG treatment, were included for treatment comparison analysis. Patients in Quintiles IV and V, which were almost uniformly treated with MED, were considered unlikely to have been randomized in STICH and were therefore excluded from the final treatment outcome comparison.
Statistical Analysis
In order to account for differences between patients who received MED versus CABG therapy, we fit the data to a logistic-regression model to estimate the probability that patients would receive CABG. All reported P values are two-sided, and in all comparisons, P values of less than 0.05 were considered to indicate statistical significance. Continuous measures were compared with Kruskal-Wallis tests, unordered categorical measures were compared with Pearson Chi-square tests, and ordered categorical measures were compared with Kruskal-Wallis tests. The propensity model was constructed using logistic regression with the outcome of treatment MED or CABG usage and preset list of variables. We calculated unadjusted and risk-adjusted Kaplan– Meier survival estimates for patients receiving MED and for those receiving CABG and compared them using a log-rank test. To estimate the difference in MED and CABG treatments, the time to death analysis using cox regression was constructed from the time of the index cardiac catheterization. The model was adjusted for a preset list of variables and propensity of MED or CABG. For all models the linearity assumptions were assessed graphically and/or with summary statistics and transformations were considered for departures from linearity.
Results
Study Population
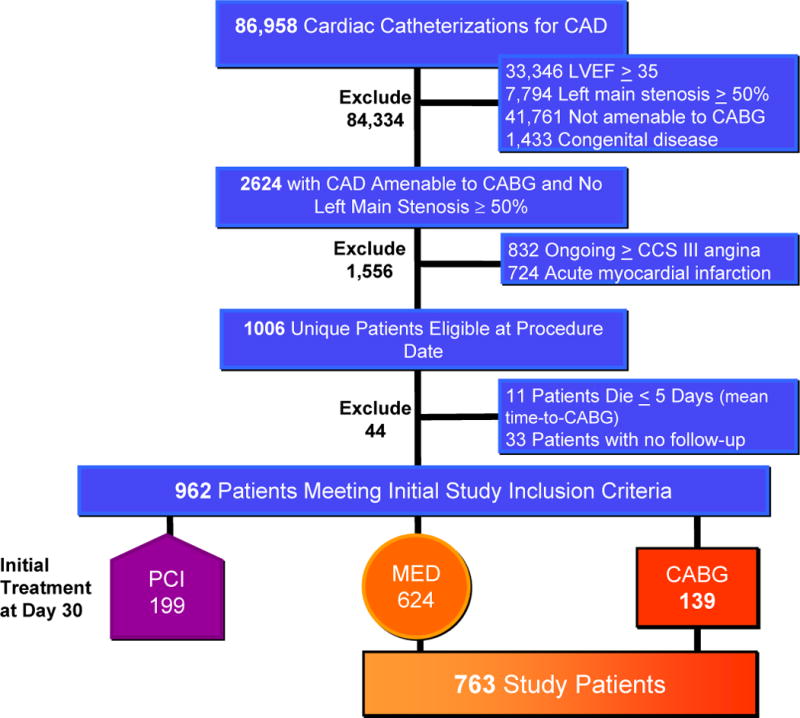
Between January 1, 1995 and July 31, 2009, 86,958 cardiac catheterizations were performed at the Duke Heart Center to evaluate suspected ischemic heart disease. Of these, 2,624 were found to have LVEF<35%, coronary artery disease amenable to CABG and no left main stenosis ≥ 50%. As depicted in Figure 1, 832 were excluded for ongoing ≥ CCS Class III angina and 724 excluded for recent (within 7 days) acute myocardial infarction. The 1068 cardiac catheterizations which met criteria yielded 1006 unique patients. For the 62 patients with more than one cardiac catheterization the first study meeting inclusion criteria was chosen. An additional 11 patients died within the mean time (5 days) between catheterization and CABG and 33 patients without follow-up were excluded. No study patient underwent subsequent cardiac transplantation or destination left ventricular assist device placement. The treatment chosen for the 962 patients screened as “STICH-eligible” included 199 who underwent PCI (18%) and were not included in the analysis. Crossovers from medical therapy to CABG were comprised of 36 patients and median time to CABG among these was 8.5 months. Physicians responsible for the care of included patients chose CABG for 139 patients and medical therapy (MED) for 624 patients. Thus, a total of 763 medical and surgical patients were included for propensity score analysis.
Figure 1. Identification of Study Cohort from all Patients Undergoing Cardiac Catheterization at Duke Medical Center January 1, 1995–July 31, 2009 and Treated with Medical Therapy (MED) or Coronary Artery Bypass Grafting (CABG).
Medical Therapy Propensity Score
We calculated the propensity score for all patients. Table 2b presents the most contributory variables in the propensity model. The c statistic for the propensity for medical therapy model was 0.88. Figure 2 displays the results of the propensity model for MED versus CABG treatment. Primary survival analysis was completed for those patients in propensity quintiles I, II, and III - those quintiles in which STICH-eligible patients had at least some chance of passing treating clinicians’ gestalt for suitability for CABG. Therefore a total of 458 patients for whom the treatment chosen was MED (n=327) and CABG (n=131) comprise the cohort for whom survival curves are compared.
Figure 2.
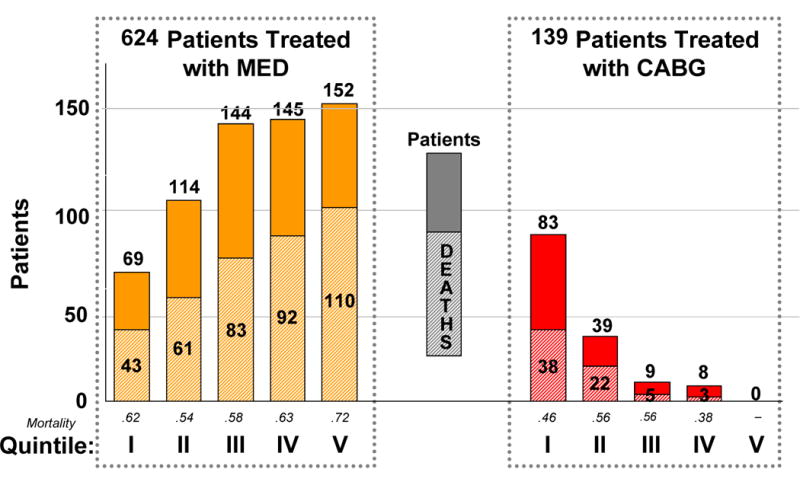
Propensity Quintiles for Selecting Medical Therapy (MED) at Duke 1995–2005. Total Patients and Mortality are Presented for Each Quintile.
Baseline Characteristics for Propensity Quintiles I, II, and III
Table 1 displays the baseline characteristics of the quintiles I, II, and III study patients, comparing the variables between MED versus CABG treatment groups. The overall mean age was 64 years, with 75% male and 65% of Caucasian race. Median LVEF was 27% with 67% of patients having a history of congestive heart failure in the medical record. As expected for an ischemic cardiomyopathy population, risk factors for coronary disease were common, including diabetes mellitus, hypertension, and smoking. Frequent comorbidities included peripheral vascular disease (11%), cerebrovascular disease (11%), and renal disease (4%). These risk factors and comorbidities were similar in prevalence between the MED and CABG groups.
MED patients were significantly less likely to have 3-vessel coronary disease (40% vs. 64%, P<0.0001) than were CABG patients. MED patients also were significantly less likely than CABG patients to have a high Duke CAD Severity Index (38% vs. 60%, P<0.0001). MED patients were more likely to have had prior CABG (36% vs. 4%, P<0.0001) although rates of prior PCI were similar (22% vs. 17%, P=0.206). The number of patients with mitral insufficiency ≥ 2+ did not differ between the groups.
Survival Analysis
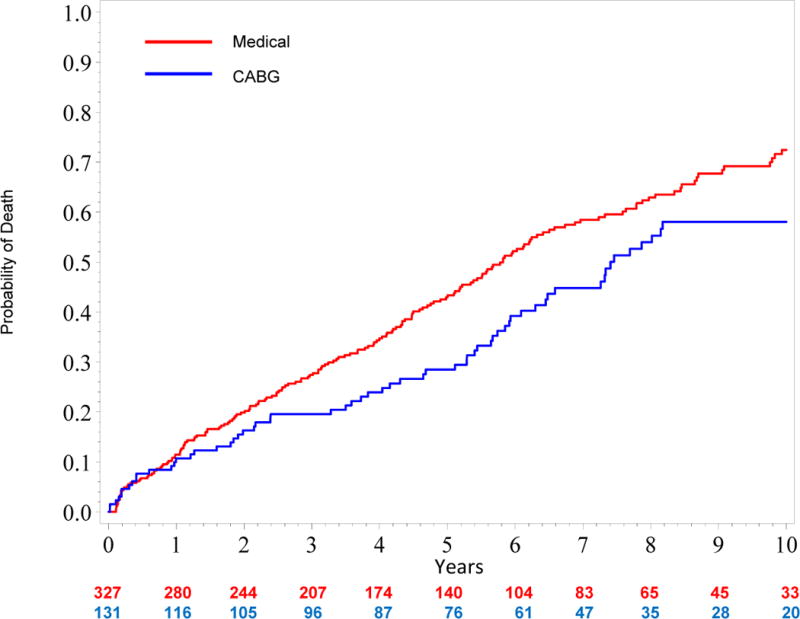
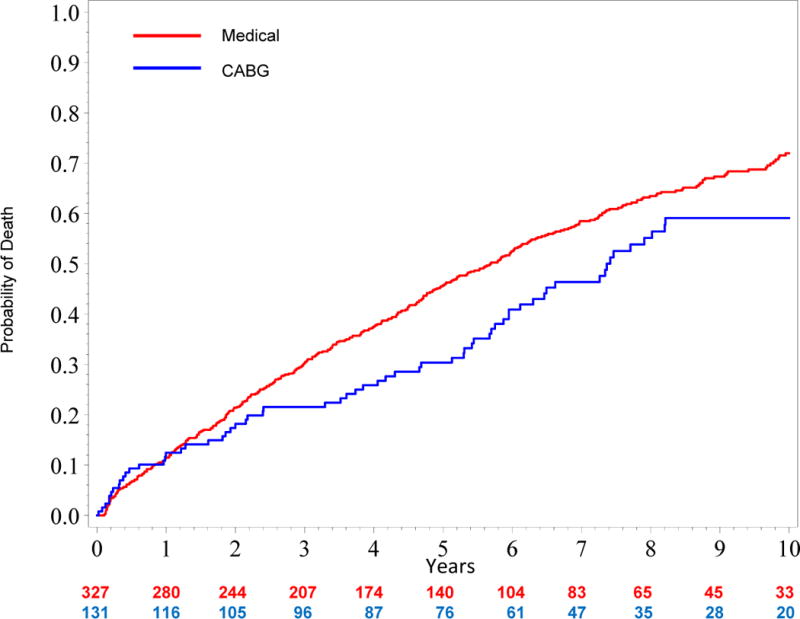
Figure 3 shows an unadjusted all-cause mortality curve for the overall study cohort (quintiles I–V). Figure 4a shows unadjusted mortality curves for the MED and CABG treatment groups (propensity quintiles I–III) through 10 years. Figure 4b shows risk adjusted mortality curves. Both the adjusted and unadjusted curves diverge after one year and the survival benefit of CABG persists through 10 years with a hazard ratio of 0.63 (95% confidence interval = 0.45, 0.88; p=0.006). Corresponding risk-adjusted mortality rates at 5 years were 43% for MED and 29% for CABG, and risk-adjusted mortality rates at 10 years were 72% for MED and 58% for CABG.
Figure 3. Unadjusted Kaplan Meier Estimates of Time to Death in All Study Patients (Propensity Quintiles I–V).
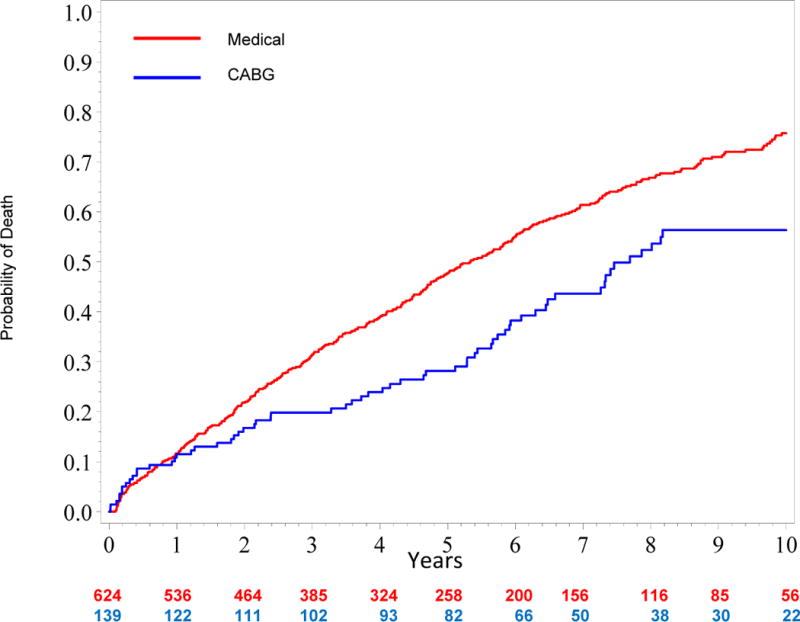
Figure 4. A, Unadjusted and B, Risk-adjusted Kaplan Meier Rate Estimates of Time to Death in 395 Patients with Similar Propensity (Quintiles I–III) for Medical Therapy.
Comment
Among a cohort of patients with ischemic cardiomyopathy meeting enrollment criteria for the Surgical Treatment of Ischemic Cardiomyopathy (STICH) trial, we have found that CABG is associated with a survival advantage over optimal medical therapy (MED) through 10 years follow-up. This finding comes from a propensity-matched, risk-adjusted group of patients with CAD, LVEF ≤ 35%, and no left main disease ≥ 50% captured over a contemporary 15 year period. This represents arguably the most detailed observational evaluation of the clinical outcomes associated with clinically-driven CABG and MED in patients with ischemic cardiomyopathy.
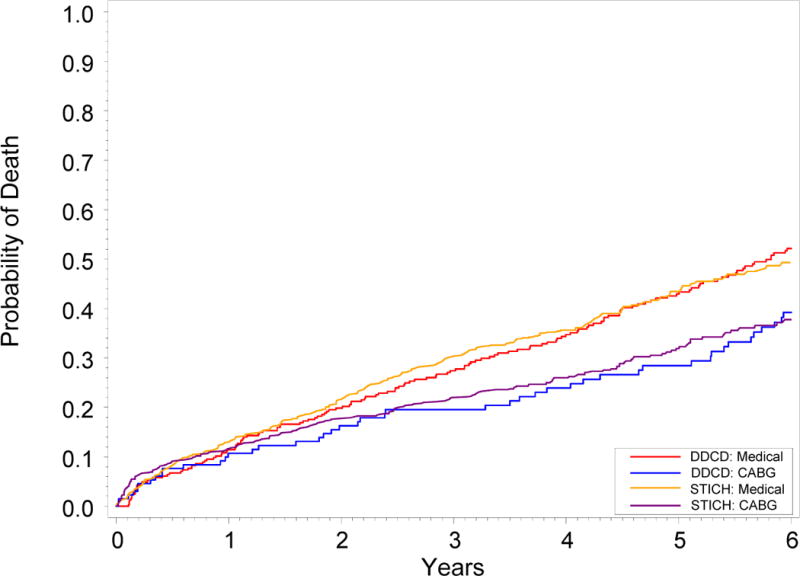
Robust randomized prospective validation that CABG relieves symptoms and prolongs life in patients with advanced coronary artery disease has been repeated in every decade since the first clinical studies enrolled patients in the 1960’s following development of the procedure [7–9, 16, 17]. Indeed, during its 45 year existence, CABG remains the most studied surgical procedure in history. Nonetheless, until the STICH trial, the evidence base remained deficient in identifying whether patients with CAD and LV systolic dysfunction should receive revascularization [10]. Between July 2002 and May 2007, STICH enrolled 1212 patients with an ejection fraction of 35% or less and coronary artery disease amenable to CABG [12]. These patients were randomly assigned to MED alone or medical therapy plus CABG. An “as-treated” analysis compared the 592 patients treated with MED throughout the first year after randomization to the 620 patients who underwent CABG (either because they were randomly assigned to CABG or because they crossed over to CABG during the first year of follow-up) and the hazard ratio for death from any cause with CABG was 0.70 (95% CI 0.58, 0.84; P<0.001) through 6 years of follow-up [12]. We have observed a hazard ratio of 0.63 (95% CI 0.45, 0.88; P=0.006) applying the trial criteria to our detailed prospective observational database. Figure 5 displays unadjusted Kaplan Meier rate estimates of time to death through 6 years by treatment received for both the study patients and from the multinational STICH trial cohort. The mortality curves from our observational cohort treated at a single referral center in North Carolina are remarkably similar to those from a randomized trial conducted at 99 centers in 22 nations across the globe, suggesting generalizability of the STICH result to the U.S. population. While the clinical implications of STICH have been debated because the primary intention to treat analysis failed to demonstrate as hypothesized a statistically significant 25% reduction in the rate of death from any cause in contradistinction to the secondary analyses by treatment received [18], this observational analysis serves as an independent validation of the benefits of CABG over medical therapy.
Figure 5. Unadjusted Kaplan Meier Rate Estimates of Time to Death for STICH and Duke Database (DDCD) Patients.
Furthermore, this study demonstrates that comprehensive rigorous databases such as the Duke Databank for Cardiovascular Disease may approximate the results of randomized controlled trials. While carefully collected prospective observational data has been shown to predict the results of other major randomized trials [19–21], there exist multiple examples of observational data at odds with randomized controlled trials [22, 23]. Without patient randomization, the comparability of treatment groups can be enhanced using propensity methods to correct for imbalances in confounding prognostic factors. This approach was taken here, with final analysis of only the propensity quintiles in which patients had some chance to receive CABG - that is, with some chance to pass the clinician’s unmeasured gestalt for suitability for either therapy. In other words, by analyzing propensity Quintiles I–III (Fig. 2), we included those patients for whom actual clinical equipoise in treatment selection is most likely to exist.
The presence of heart failure among those referred for cardiac surgery is already great, with approximately 40% of CABGs performed in this patient population [24]. High risk patients are generally believed to have the most to gain from CABG, as current practice guidelines indicate [1]. Outcomes for patients with CAD have improved in recent years with advances in both medical therapy [25, 26] as well as advances in CABG therapy [27, 28]. The mortality of CAD patients having depressed LVEF is high as demonstrated in this analysis, with a 72% 10 year mortality in the medically treated population. Among patients with CAD and heart failure, the impact of CABG is likely substantial [12, 29]; however, in the present study, only 14% of patients with CAD amenable to CABG and EF<35% received CABG. Based on our observational findings, had CABG been provided only to the patients from Quintiles I–III which were given medical treatment alone, an additional 36 patients (19%) would have lived to 10 years follow-up.
Limitations
The statistical models used in this analysis estimate the effect of CABG indirectly by the difference in MED and CABG prognoses because the effect of CABG on prognosis cannot be observed directly in an individual patient. While clinical databases collecting data prospectively and according to a protocol with rigorous quality control measures and complete follow-up are most likely to be adequate for guiding patient care decisions, this remains a retrospective, observational study for which causality cannot be inferred as with prospective randomized controlled trials [30]. Further, patients from Duke likely differ from those treated elsewhere, both in described characteristics as well as in characteristics not captured by case report forms. Medical and surgical therapies for ischemic cardiomyopathy have undergone marked changes during the study period [31–33]; however, the present analysis does not attempt to quantify the impact of changes in care over time. Finally, patients in this study were limited to those referred for catheterization and therefore may not include all patients in the community with this condition.
Conclusions
Among a propensity-matched, risk-adjusted cohort of patients meeting STICH trial entry criteria with CAD, LVEF ≤ 35%, and no left main disease ≥ 50%, CABG was associated with a survival advantage over MED through 10 years follow-up. Carefully collected prospective observational data can complement the results of randomized trials.
Acknowledgments
This analysis was funded by the Duke Clinical Research Institute. Dr. Williams is supported in part by training grant T32-HL069749 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt S, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Brozema S. Heart Failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Baker D, Jones R, Hodges J, et al. Management of Heart Failure. III. The role of revascularization in the treatment of patients with moderate or severe left ventricular systolic dysfunction. JAMA. 1994;272:1158–1134. doi: 10.1001/jama.272.19.1528. [DOI] [PubMed] [Google Scholar]
- 4.Chin M, Goldman L. Factors contributing to the hospitalization of patients with congestive heart failure. Am J Public Health. 1997;87:643–648. doi: 10.2105/ajph.87.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghali J, Kadakia S, Cooper R, et al. Precipitating factors leading to decompensation of heart failure. Arch Intern Med. 1988;148:2013–2016. [PubMed] [Google Scholar]
- 6.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114(5):e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 7.Murphy M, Hultgren HN, Detre K, Thomsen J, Takaro T. Treatment of chronic stable angina. A preliminary report of survival data of the randomized Veterans Administration cooperative study. N Engl J Med. 1977;297(12):621–627. doi: 10.1056/NEJM197709222971201. [DOI] [PubMed] [Google Scholar]
- 8.European Coronary Surgery Study Group Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. Lancet. 1982;2:1173–1180. [PubMed] [Google Scholar]
- 9.Alderman E, Fisher LD, Litwin P, Kaiser GC, Myers WO, Maynard C, Levine F, Schloss M. Results of coronary artery surgery in patients with poor left ventricular function (CASS) Circulation. 1983;68(4):785–795. doi: 10.1161/01.cir.68.4.785. [DOI] [PubMed] [Google Scholar]
- 10.Jones R. Is it time for a randomized trial of surgical treatment of ischemic heart failure? J Am Coll Cardiol. 2001;37(5):1210–1213. doi: 10.1016/s0735-1097(01)01123-8. [DOI] [PubMed] [Google Scholar]
- 11.Velazquez E, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. J Thorac Cardiovasc Surg. 2007134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor CM, Velazquez EJ, Gardner LH, Smith PK, Newman MF, Landolfo KP, Lee KL, Califf RM, Jones RH. Comparison of coronary artery bypass grafting versus medical therapy on long-term outcome in patients with ischemic cardiomyopathy (a 25-year experience from the Duke Cardiovascular Disease Databank) Am J Cardiol. 2002;90(2):101–107. doi: 10.1016/s0002-9149(02)02429-3. [DOI] [PubMed] [Google Scholar]
- 14.Harris PJ, Lee KL, Harrell FE, Jr, Behar VS, Rosati RA. Outcome in medically treated coronary artery disease. Ischemic events: nonfatal infarction and death. Circulation. 1980;62(4):718–726. doi: 10.1161/01.cir.62.4.718. [DOI] [PubMed] [Google Scholar]
- 15.D’Agostino RJ. Propensity scores in cardiovascular research. Circulation. 2007115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 16.Rogers W, Coggin CJ, Gersh BJ, Fisher LD, Myers WO, Oberman A, Sheffield LT. Ten-year follow-up of quality of life in patients randomized to receive medical therapy or coronary artery bypass graft surgery. The Coronary Artery Surgery Study (CASS) Circulation. 1990;82(5):1859–1862. doi: 10.1161/01.cir.82.5.1647. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, Davis K, Killip T, Passamani E, Norris R. Effect of coronary artery bypass graft surgery on survival: overview of 10-year resultsfrom randomised trials by the Coronary Artery Bypass Graft Surgery Trialists. Lancet. 1994;344:563–570. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 18.Fang JC. Underestimating medical therapy for coronary disease… again. N Engl J Med. 2011;364(17):1671–1673. doi: 10.1056/NEJMe1103414. [DOI] [PubMed] [Google Scholar]
- 19.Hlatky M, Califf RM, Harrell FE, Jr, Lee KL, Mark DB, Pryor DB. Comparison of predictions based on observational data with the results of randomized controlled clinical trials of coronary artery bypass surgery. J Am Coll Cardiol. 1988;11(2):237–245. doi: 10.1016/0735-1097(88)90086-1. [DOI] [PubMed] [Google Scholar]
- 20.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 21.Echt D, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo The cardiac arrhythmia suppression trial. N Engl J Med. 1991324:781. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 22.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 23.Detre KM, Guo P, Holubkov R, Califf RM, Sopko G, Bach R, Brooks MM, Bourassa MG, Shemin RJ, Rosen AD, et al. Coronary revascularization in diabetic patients: a comparison of the randomized and observational components of the Aypass Angioplasty Revascularization Investigation (BARI) Circulation. 1999;99(5):633–640. doi: 10.1161/01.cir.99.5.633. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 25.Moss A, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 26.Cobb L, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 27.Serruys P, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW. SYNTAX Investigators: Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 28.Shroyer A, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D. Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group: On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361(19):1827–1837. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- 29.Filsoufi F, Rahmanian PB, Castillo JG, Chikwe J, Kini AS, Adams DH. Results and predictors of early and late outcome of coronary artery bypass grafting in patients with severely depressed left ventricular function. Ann Thorac Surg. 2007;84(3):808–816. doi: 10.1016/j.athoracsur.2007.04.117. [DOI] [PubMed] [Google Scholar]
- 30.Doll R. Sir Austin Bradford Hill and the progress of medical science. BMJ. 1992;305(6868):1521–1526. doi: 10.1136/bmj.305.6868.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 32.Williams JB, Delong ER, Peterson ED, Dokholyan RS, Ou FS, Ferguson TB., Jr Secondary prevention after coronary artery bypass graft surgery: findings of a national randomized controlled trial and sustained society-led incorporation into practice. Circulation. 2011;123(1):39–45. doi: 10.1161/CIRCULATIONAHA.110.981068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez AF, Shea AM, Milano CA, Rogers JG, Hammill BG, O’Connor CM, Schulman KA, Peterson ED, Curtis LH. Long-term Outcomes and Costs of Ventricular Assist Devices Among Medicare Beneficiaries. JAMA. 2008;300(20):2398–2406. doi: 10.1001/jama.2008.716. [DOI] [PMC free article] [PubMed] [Google Scholar]


