Abstract
Importance
Randomized clinical trials have shown that implantable cardioverter-defibrillator (ICD) therapy saves lives. Whether the survival of patients who received an ICD in primary prevention clinical trials differs from that of trial-eligible patients receiving a primary prevention ICD in clinical practice is unknown.
Objective
To determine whether trial-eligible patients who received a primary prevention ICD as documented in a large national registry have a survival rate that differs from the survival rate of similar patients who received an ICD in the 2 largest primary prevention clinical trials, MADIT-II (n=742) and SCD-HeFT (n=829).
Design, Setting, and Patients
Retrospective analysis of data for patients enrolled in the National Cardiovascular Data Registry ICD Registry between January 1, 2006, and December 31, 2007, meeting the MADIT-II criteria (2464 propensity score–matched patients) or the SCD-HeFT criteria (3352 propensity score–matched patients). Mortality data for the registry patients were collected through December 31, 2009.
Main Outcome Measures
Cox proportional hazards models were used to compare mortality from any cause.
Results
The median follow-up time in MADIT-II, SCD-HeFT, and the ICD Registry was 19.5, 46.1, and 35.2 months, respectively. Compared with patients enrolled in the clinical trials, patients in the ICD Registry were significantly older and had a higher burden of comorbidities. In the matched cohorts, there was no significant difference in survival between MADIT-II–like patients in the registry and MADIT-II patients randomized to receive an ICD (2-year mortality rates: 13.9% and 15.6%, respectively; adjusted ICD Registry vs trial hazard ratio, 1.06; 95% CI, 0.85–1.31; P=.62). Likewise, the survival among SCD-HeFT–like patients in the registry was not significantly different from survival among patients randomized to receive ICD therapy in SCD-HeFT (3-year mortality rates: 17.3% and 17.4%, respectively; adjusted registry vs trial hazard ratio, 1.16; 95% CI, 0.97–1.38; P=.11).
Conclusions and Relevance
There was no significant difference in survival between clinical trial patients randomized to receive an ICD and a similar group of clinical registry patients who received a primary prevention ICD. Our findings support the continued use of primary prevention ICDs in similar patients seen in clinical practice.
Trial Registration
clinicaltrials.gov Identifier: NCT00000609
The implantable cardioverter-defibrillator (ICD) is a highly effective therapy for pre-venting sudden cardiac death in patients with heart failure.1–4 However, the outcomes of this therapy in routine clinical practice are largely uncertain. Because randomized clinical trials generally enroll patients with fewer comorbidities and are usually conducted in highly controlled and monitored settings, the results of the primary prevention ICD trials may not be generalizable to routine clinical practice. Some studies have demonstrated the lack of generalizability of randomized clinical trials’ findings to clinical practice in acute coronary syndromes, heart failure, hypertension, and depression.5–11 Whether the findings of randomized clinical trials of primary prevention ICD therapy are generalizable to clinical practice needs to be investigated, especially given the cost and potential complications associated with this device, such as infection and lead and device failure.
We conducted this study to determine whether a difference in survival exists between trial-eligible patients who receive a primary prevention ICD as documented in a large national registry and similar patients randomized to receive ICD therapy in primary prevention clinical trials.
METHODS
Data Sources
MADIT-II and SCD-HeFT
The Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) was a randomized clinical trial of patients with a history of myocardial infarction and a left ventricular ejection fraction (LVEF) of 30% or less. After a mean follow-up of 20 months, ICD therapy reduced mortality compared with medical therapy (14.2% vs 19.8%; hazard ratio [HR], 0.69; 95% CI, 0.51–0.93; P=.02).3
The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) was a multicenter, randomized clinical trial of patients with heart failure due to ischemic or nonischemic cardiomyopathy and LVEF 35% or less despite optimal medical therapy.4 After a median follow-up of 45.5 months, ICD therapy reduced mortality compared with placebo and amiodarone (22% vs 29%; HR, 0.77; 95% CI, 0.62–0.96; P=.007).4
The original patient-level data from MADIT-II and SCD-HeFT were used for this analysis.
National Cardiovascular Data Registry ICD Registry
In 2005, the Centers for Medicare & Medicaid Services announced its decision to expand coverage for ICD implantation for the primary prevention of sudden cardiac death and mandated entering data on all primary prevention ICD implants in Medicare beneficiaries into a national ICD registry. In response to this mandate, the National Cardiovascular Data Registry (NCDR) ICD Registry was launched in June 2005. Of the 1448 participating hospitals, 78% submit data on all ICD implants. Because these hospitals are generally the larger participating hospitals, they account for 90% of all ICD implants entered into the registry.12–14
Details of the ICD Registry have been published previously.14 After formal training on data collection and entry by the NCDR, participating hospitals submit data directly to the NCDR via a secure website. To ensure quality, submitted data undergo rigorous electronic quality checks. Annually, up to 10% of participating sites are randomly selected for an on-site audit.14
Death Master File Data
The NCDR contracted with Yale University to independently conduct deterministic matching between the ICD Registry and the Death Master File from the Social Security Administration. Yale created a limited data set by determining the vital status of patients in the ICD Registry through December 31, 2009. To accomplish the deterministic matching, patient Social Security numbers, names, and dates of birth were used. The search was limited to patients with a valid Social Security number, and then vital status was determined in a stepwise fashion. First, records with an exact match of Social Security number were identified. Second, additional matches were identified in which there was an imperfect match by Social Security number (at least 7 matching digits) and an exact match by name and date of birth. The created data set was deidentified and submitted to the NCDR, which then sent the database to the investigators to conduct this research.
Patient Population and Outcomes
In this analysis, we included all patients enrolled in MADIT-II (n=1232) and patients randomized to receive placebo or ICD therapy in SCD-HeFT (n=1676). The ICD Registry was queried to identify adult patients who were enrolled in the registry from January 1, 2006, through December 31, 2007, and who had a history of a myocardial infarction and an LVEF 30% or less. As in MADIT-II, we excluded patients with New York Heart Association (NYHA) class IV symptoms, patients who received an ICD within 40 days after a myocardial infarction, and patients who received an ICD within 3 months after coronary revascularization.
The ICD Registry was also queried to identify adult patients who were enrolled in the registry from January 1, 2006, through December 31, 2007, and who had NYHA class II or III heart failure symptoms and an LVEF 35% or less. As in SCD-HeFT, patients were excluded if they had class I or IV heart failure symptoms or if they received an ICD within 40 days after a myocardial infarction or within 30 days after coronary revascularization.
At the time of trial or registry enrollment, data collected for each patient included demographics, medical history, presenting physical examination and laboratory test findings, and medications. Individual sites established the race and ethnicity of patients and submitted these data to the ICD Registry and to the MADIT-II and SCD-HeFT data coordinating centers. Because it was a National Institutes of Health–funded study, collecting data on race in SCD-HeFT was mandatory. Instructions in the ICD Registry clarified that data on race should be collected as determined by the patient or family. No additional specifications were given.
Because one of the main purposes of the ICD Registry was to define how the outcomes of Medicare beneficiaries receiving a primary prevention ICD compare with those of patients enrolled in the primary prevention ICD randomized clinical trials, we prespecified patients 65 years and older as a subgroup of interest.
The end point for this study was mortality from any cause.
Statistical Methods
Baseline characteristics were compared between patients in each trial vs trial-eligible patients in the registry using the Wilcoxon rank sum test for continuous variables and the likelihood ratio χ2 test for categorical variables and by examining the standardized difference (defined as the absolute value of the difference in group means or proportions, divided by the average standard deviation, and expressed as a percentage) between groups for each variable. Initially, for each trial, comparisons were made between trial patients and all ICD Registry patients who met the trial entry criteria. These initial comparisons showed appreciable imbalances for most baseline variables for both MADIT-II and SCD-HeFT.
Therefore, to ensure a valid comparison of similar patients, we proceeded with propensity score matching. For each trial, we selected a subset of ICD Registry patients, in a 2:1 registry-trial ratio, who most closely matched the trial patients in terms of baseline variables. Baseline characteristics were compared again to confirm the effectiveness of the matching (eFigure, available at http://www.jama.com). These matched subsets were used for all outcome analyses.
Matching was carried out using the propensity score–based method of Rosenbaum and Rubin.15 For each trial, the process was as follows. First, the ICD Registry data were trimmed of extreme values (ie, any ICD Registry patients whose age or LVEF was below the minimum or above the maximum for the trial patients were excluded from the process).
Second, a propensity model was developed using logistic multiple regression in which the dependent (outcome) variable was an indicator of whether each patient was a registry or a trial patient, and the independent (predictor) variables were baseline characteristics available in both the registry and the clinical trials. These variables included age, sex, race (white vs other), QRS duration, LVEF, NYHA class, ischemic heart disease (SCD-HeFT model only), prior coronary artery bypass graft procedure, prior percutaneous coronary intervention, prior atrial arrhythmias, prior ventricular arrhythmias (nonsustained), systolic blood pressure, blood urea nitrogen, creatinine, diabetes, hypertension, and medications including β-blockers. All trial patients and ICD Registry patients who met the trial entry criteria (except those excluded in the first step) were included. From the logistic regression model, an estimated propensity score (the probability p of being a trial patient) and a corresponding logit for the propensity score (loge[p/(1 − p)]) were calculated for each patient.
Third, for the first match (1:1), a caliper width of 0.25 (standard deviation of the logit) was used. For a given trial patient, all ICD Registry patients were considered whose logit differed from the trial patient’s logit by less than the caliper width; among these patients, the ICD Registry patient with the shortest (Mahalanobis) distance from the trial patient was selected as the match. Fourth, trial patients for whom no ICD Registry patients were within the caliper width were matched with the ICD Registry patient who had the closest logit. Fifth, and finally, for the second match (2:1), the caliper width was set to 0.40 (standard deviation of the logit); the process was otherwise the same. Each trial patient was matched with 2 ICD Registry patients, and each ICD Registry patient could be matched only once for each trial.
The primary analyses compared trial patients randomized to receive an ICD to all of the matched ICD Registry patients for that trial. The secondary analyses compared trial patients randomized to medical therapy to all of the matched ICD Registry patients for that trial. Cox proportional hazards models were used to examine mortality in ICD Registry patients compared with trial patients, after controlling for baseline clinical variables. Although the matching process was expected to produce similar groups, covariate adjustment was used to control for any remaining imbalances. All of the baseline variables listed above for the propensity analysis were included as covariates in each model. Risk relationships are shown as HRs and 95% CIs from the Cox models. We tested the proportionality assumption for the variable identifying whether each patient was a trial participant or a registry patient for the 4 main models, and it was met in all cases. As a prespecified subgroup analysis, these comparisons were repeated in the subset of patients 65 years and older.
Based on the 2-year mortality rate observed in MADIT-II for patients randomized to medical therapy (22.0%) and the number of patients included in our analyses, we would expect to have greater than 80% power to detect differences larger than 5.0 percentage points. Similarly, based on the 2-year mortality rate observed in SCD-HeFT for patients randomized to placebo (22.4%), we would expect to have greater than 80% power to detect differences larger than 4.8 percentage points. These post hoc power calculations assume that the ratio of clinical trial patients to propensity-matched ICD Registry patients is 1:4 and a 2-sided type I error rate of 0.05.
Differences were declared to be statistically significant at P < .05, and all statistical tests were 2-sided. For all analyses, SAS version 9.2 (SAS Institute) was used. This study was approved by the institutional review board of the Duke University Health System, which determined that informed consent was not applicable to data collected by the ICD Registry.
RESULTS
Baseline Characteristics
After excluding patients with an ICD for inducible sustained ventricular tachycardia on electrophysiological testing (n=7061), patients who received an ICD with cardiac resynchronization therapy (n=50 740), and patients who received device replacements (n=4357), 56 985 ICD Registry patients met the criteria for 1 or both clinical trials. In the ICD Registry, there were 28 608 MADIT-II–like patients and 53 351 SCD-HeFT–like patients. MADIT-II and SCD-HeFT included 742 and 829 patients, respectively, who were randomized to receive an ICD. The number of patients randomized to medical therapy in MADIT-II and to placebo in SCD-HeFT was 490 and 847, respectively.
The baseline characteristics of these groups are presented in Table 1 and Table 2, respectively. Patients 65 years and older accounted for 62% of MADIT-II–like patients and 57% of SCD-HeFT–like patients. Compared with patients in the MADIT-II trial, MADIT-II–like patients in the ICD Registry were significantly older and had a higher burden of comorbidities. Likewise, compared with patients in SCD-HeFT, SCD-HeFT–like patients in the ICD Registry were significantly older and had a higher burden of comorbidities.
Table 1.
Baseline Characteristics of Patients in MADIT-II vs MADIT-II–like Patients in the ICD Registry
| Characteristic | MADIT-II (n = 1232) | ICD Registry (n = 28 608)a | P Value for Difference From MADIT-II Patients | Matched ICD Registry Patients, No. (%)(n = 2464)a | P Value for Difference From MADIT-II Patients | ||
|---|---|---|---|---|---|---|---|
| Age, median (IQR), y | 66 (58–72) | 68 (60–76) | <.001 | 65 (58–71) | .36 | ||
|
| |||||||
| Male sex, No. (%) | 1040 (84) | 22 883 (80) | <.001 | 2088 (85) | .80 | ||
|
| |||||||
| White race, No. (%) | 1073 (87) | 24 427 (85) | .11 | 2145 (87) | .98 | ||
|
| |||||||
| QRS duration, median (IQR), ms | 120 (100–140) | 106 (94–122) | <.001 | 112 (98–138) | .20 | ||
|
| |||||||
| Left ventricular ejection fraction, median (IQR), % | 25 (20–28) | 25 (20–30) | <.001 | 25 (20–28) | .16 | ||
|
| |||||||
| Mean (SD) | 23.2 (5.4) | 24.5 (5.2) | 23.4 (5.4) | ||||
|
| |||||||
| NYHA class, No. (%) | |||||||
| I | 442 (36) | 3634 (13) |
|
<.001 | 854 (35) |
|
.07 |
|
|
|
||||||
| II | 425 (35) | 15 500 (54) | 955 (39) | ||||
|
|
|
||||||
| III or IV | 350 (29) | 9474 (33) | 655 (27) | ||||
|
| |||||||
| Prior CABG, No. (%) | 707 (57) | 14 325 (50) | <.001 | 1397 (57) | .69 | ||
|
| |||||||
| Prior PCI, No. (%) | 536 (44) | 14 343 (50) | <.001 | 1092 (44) | .84 | ||
|
| |||||||
| Prior atrial arrhythmias, No. (%) | 324 (27) | 7243 (25) | .23 | 591 (24) | .06 | ||
|
| |||||||
| Prior ventricular arrhythmias, No. (%) | 138 (12) | 5844 (20) | <.001 | 268 (11) | .57 | ||
|
| |||||||
| Systolic blood pressure, median (IQR), mm Hg | 120 (110–133) | 129 (115–144) | <.001 | 121 (110–134) | .10 | ||
|
| |||||||
| Blood urea nitrogen, median (IQR), mg/dL | 20 (16–28) | 20 (15–28) | .85 | 20 (16–26) | .29 | ||
|
| |||||||
| Creatinine, median (IQR), mg/dL | 1.1 (1.0–1.4) | 1.2 (1.0–1.4) | .008 | 1.1 (1.0–1.3) | .02 | ||
|
| |||||||
| Diabetes, No. (%) | 433 (35) | 11 376 (40) | .001 | 902 (37) | .39 | ||
|
| |||||||
| Hypertension, No. (%) | 655 (53) | 22 255 (78) | <.001 | 1377 (56) | .14 | ||
|
| |||||||
| Medications at baseline, No. (%) | |||||||
| ACE inhibitor or ARB | 1092 (89) | 22 517 (79) | <.001 | 2152 (87) | .29 | ||
|
| |||||||
| β-Blocker | 769 (62) | 24 970 (87) | <.001 | 1691 (69) | <.001 | ||
|
| |||||||
| Calcium-channel blocker | 158 (13) | 2140 (7) | <.001 | 299 (12) | .56 | ||
|
| |||||||
| Digoxin | 721 (59) | 6648 (23) | <.001 | 1406 (57) | .40 | ||
|
| |||||||
| Diuretic | 921 (75) | 17 330 (61) | <.001 | 1840 (75) | .97 | ||
|
| |||||||
| Statin | 776 (63) | 21 732 (76) | <.001 | 1627 (66) | .06 | ||
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CABG, coronary-artery bypass graft procedure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; MADIT-II, Multicenter Automatic Defibrillator Implantation Trial II; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
SI conversion factors: To convert blood urea nitrogen to mmol/L, multiply by 0.357; creatinine to μmol/L, multiply by 88.4.
MADIT-II–like patients.
Table 2.
Baseline Characteristics of Patients in SCD-HeFT vs SCD-HeFT–like Patients in the ICD Registry
| Characteristic | Patients in SCD-HeFT (n = 1676) | ICD Registry (n = 53 351)a | P Value for Difference From SCD-HeFT Patients | Matched ICD Registry Patients, No. (%)(n = 3352)a | P Value for Difference From SCD-HeFT Patients |
|---|---|---|---|---|---|
| Age, median (IQR) range, y | 60 (51–68) | 67 (57–75) | <.001 | 59 (51–67) | .41 |
| Male sex, No. (%) | 1294 (77) | 39 599 (74) | .005 | 2611 (78) | .58 |
| White race, No. (%) | 1283 (77) | 41 662 (78) | .11 | 2530 (76) | .43 |
| QRS duration, median (IQR), ms | 112 (96–140) | 106 (94–123) | <.001 | 110 (96–130) | .004 |
| Left ventricular ejection fraction, median (IQR), % | 25 (20–30) | 25 (20–30) | <.001 | 25 (20–30) | .50 |
| Mean (SD) | 23.7 (6.9) | 24.9 (6.4) | 23.9 (6.7) | ||
| NYHA class, No. (%) | <.001 | .26 | |||
| II | 1160 (69) | 31 975 (60) | 2372 (71) | ||
| III | 516 (31) | 21 376 (40) | 980 (29) | ||
| Ischemic heart disease, No. (%) | 884 (53) | 36 856 (69) | <.001 | 1760 (53) | .87 |
| Prior myocardial infarction, No. (%) | 760 (45) | 29 507 (55) | <.001 | 1520 (45) | .99 |
| Prior CABG, No. (%) | 449 (27) | 18 896 (35) | <.001 | 895 (27) | .94 |
| Prior PCI, No. (%) | 344 (21) | 18 099 (34) | <.001 | 685 (20) | .93 |
| Prior atrial arrhythmias, No. (%) | 258 (15) | 14 904 (28) | <.001 | 468 (14) | .18 |
| Prior ventricular arrhythmias, No. (%) | 390 (23) | 12 291 (23) | .82 | 801 (24) | .63 |
| Systolic blood pressure, median (IQR), mm Hg | 118 (106–132) | 129 (114–144) | <.001 | 118 (107–131) | .55 |
| Blood urea nitrogen, median (IQR), mg/dL | 19 (15–26) | 20 (15–28) | <.001 | 19 (15–25) | .54 |
| Creatinine, median (IQR), mg/dL | 1.1 (0.9–1.4) | 1.2 (1.0–1.4) | <.001 | 1.1 (0.9–1.3) | .55 |
| Diabetes, No. (%) | 524 (31) | 20 095 (38) | <.001 | 1023 (31) | .59 |
| Hypertension, No. (%) | 931 (56) | 40 413 (76) | <.001 | 1922 (57) | .23 |
| Medications at baseline, No. (%) ACE inhibitor or ARB |
1610 (96) | 42 189 (79) | <.001 | 3178 (95) | .06 |
| β-Blocker | 1157 (69) | 46 336 (87) | <.001 | 2533 (76) | <.001 |
| Calcium-channel blocker | 189 (11) | 4276 (8) | <.001 | 294 (9) | .005 |
| Digoxin | 1141 (68) | 14 480 (27) | <.001 | 2212 (66) | .14 |
| Diuretic | 1445 (86) | 34 969 (66) | <.001 | 2925 (87) | .29 |
| Statin | 631 (38) | 33 715 (63) | <.001 | 1346 (40) | .08 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB angiotensin receptor blocker; CABG, coronary-artery bypass graft procedure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
SI conversion factors: To convert blood urea nitrogen to mmol/L, multiply by 0.357; creatinine to 3mol/L, multiply by 88.4.
SCD-HeFT–like patients.
As shown in Table 1 and Table 2, propensity score matching provided well-matched samples with only a few remaining differences. Except for β-blocker use, the few differences that were statistically significant did not appear to be clinically meaningful.
Mortality From Any Cause
The median follow-up in MADIT-II and the matched registry patients was 19.5 months (interquartile range [IQR], 10.3–31.9) and 35.8 months (IQR, 30.4–41.2), respectively. The median follow-up in SCD-HeFT and the matched registry patients was 46.1 months (IQR, 35.0–55.1) and 35.0 months (IQR, 29.7–40.6), respectively. During these time periods, across all study cohorts, there were a total of 1614 deaths.
MADIT-II Analyses
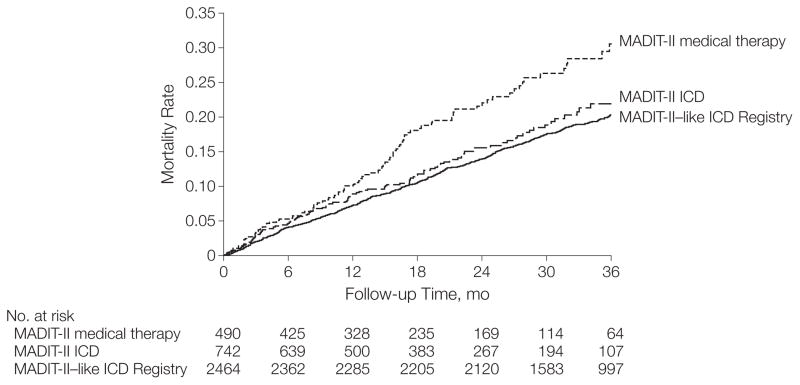
In the adjusted Cox proportional hazards models, there was no significant difference in survival between MADIT-II–like patients in the ICD Registry and MADIT-II patients randomized to receive an ICD (2-year mortality rates: 13.9% vs 15.6%; adjusted registry vs MADIT-II HR, 1.06; 95% CI, 0.85–1.31; P=.62). As shown in Table 3 and Figure 1, the survival of MADIT-II–like patients in the ICD Registry was significantly better than the survival of MADIT-II patients randomized to medical therapy (2-year mortality rates: 13.9% vs 22%; adjusted registry vs MADIT-II HR, 0.73; 95% CI, 0.59–0.92; P=.007).
Table 3.
All-Cause Mortality in the Matched Groups
| Kaplan-Meier Mortality Rate, % (95% CI)
|
ICD Registry vs Trial Patients With ICDs
|
ICD Registry vs Trial Patients Receiving Medical Therapy
|
|||||
|---|---|---|---|---|---|---|---|
| Trial-like Registry Patients | ICD-Randomized Trial Patients | Medical Therapy–Randomized Trial Patients | Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| All Patients | |||||||
| MADIT-II | |||||||
| Year 1 | 7.3 (6.2–8.3) | 8.9 (6.7–11.1) | 10.2 (7.4–13.1) | 1.06 (0.85–1.31) | .62 | 0.73 (0.59–0.92) | .007 |
|
| |||||||
| Year 2 | 13.9 (12.6–15.3) | 15.6 (12.4–18.7) | 22.0 (17.6–26.4) | ||||
|
| |||||||
| Total deaths, No. | 507 | 105 | 97 | ||||
|
| |||||||
| Patients, No. | 2464 | 742 | 490 | ||||
|
| |||||||
| SCD-HeFT | |||||||
| Year 1 | 5.6 (4.8–6.4) | 6.2 (4.5–7.8) | 5.9 (4.3–7.5) | 1.16 (0.97–1.38) | .11 | 0.82 (0.70–0.96) | .01 |
|
| |||||||
| Year 2 | 11.3 (10.3–12.4) | 11.7 (9.5–13.9) | 14.5 (12.1–16.9) | ||||
|
| |||||||
| Year 3 | 17.3 (16.0–18.7) | 17.4 (14.7–20.0) | 22.4 (19.5–25.2) | ||||
|
| |||||||
| Total deaths, No. | 588 | 182 | 244 | ||||
|
| |||||||
| Patients, No. | 3352 | 829 | 847 | ||||
|
| |||||||
| Patients ≥65 y | |||||||
| MADIT-II | |||||||
| Year 1 | 9.2 (7.6–10.8) | 10.8 (7.5–14.1) | 14.1 (9.6–18.7) | 1.02 (0.78–1.33) | .88 | 0.65 (0.49–0.85) | .002 |
|
| |||||||
| Year 2 | 16.5 (14.5–18.5) | 19.8 (14.9–24.7) | 31.5 (24.6–38.5) | ||||
|
| |||||||
| Total deaths, No. | 333 | 70 | 70 | ||||
|
| |||||||
| Patients, No. | 1306 | 397 | 262 | ||||
|
| |||||||
| SCD-HeFT | |||||||
| Year 1 | 7.1 (5.6–8.6) | 8.8 (5.6–12.1) | 7.7 (4.6–10.9) | 1.05 (0.81–1.36) | .73 | 0.76 (0.60–0.96) | .02 |
|
| |||||||
| Year 2 | 14.8 (12.7–16.8) | 16.0 (11.8–20.2) | 18.7 (14.1–23.2) | ||||
|
| |||||||
| Year 3 | 21.8 (19.2–24.4) | 24.8 (19.7–29.9) | 30.2 (24.8–35.7) | ||||
|
| |||||||
| Total deaths, No. | 255 | 94 | 107 | ||||
|
| |||||||
| Patients, No. | 1132 | 294 | 284 | ||||
Abbreviations: HR, hazard ratio; ICD, implantable cardioverter-defibrillator; MADIT-II, Multicenter Automatic Defibrillator Implantation Trial II; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
Figure 1.
Unadjusted Kaplan-Meier Curves for Survival Among MADIT-II Patients and MADIT-II–like Patients in the ICD Registry
Patients compared were MADIT-II–like patients in the ICD Registry, MADIT-II patients randomized to ICD therapy, and MADIT-II patients randomized to medical therapy (for matched patients only). MADIT-II indicates Multicenter Automatic Defibrillator Implantation Trial-II; ICD, implantable cardioverter-defibrillator.
SCD-HeFT Analyses
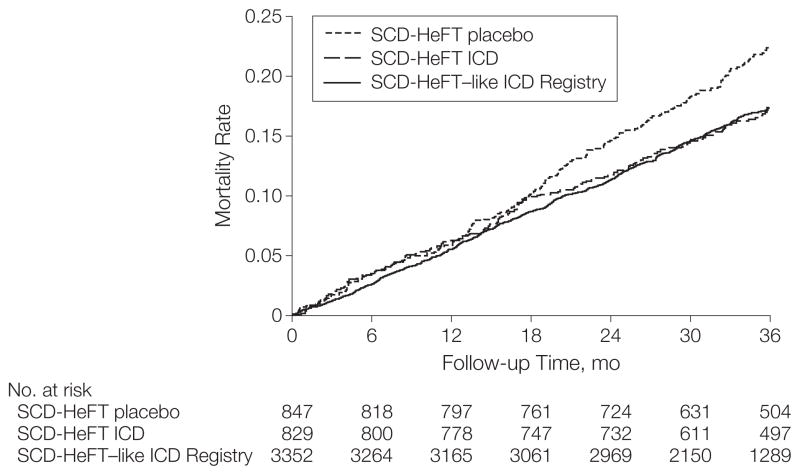
In the adjusted Cox proportional hazards models, there was no significant survival difference between SCD-HeFT–like patients in the ICD Registry and patients randomized to receive ICD therapy in SCD-HeFT (3-year mortality rates: 17.3% vs 17.4%; adjusted registry vs SCD-HeFT HR, 1.16; 95% CI, 0.97–1.38; P=.11) (Table 3 and Figure 2). The survival of SCD-HeFT–like patients in the ICD Registry was significantly better than the survival of SCD-HeFT patients randomized to placebo (3-year mortality rates: 17.3% vs 22.4%; adjusted registry vs SCD-HeFT HR, 0.82; 95% CI, 0.70–0.96; P=.01).
Figure 2.
Unadjusted Kaplan-Meier Curves for Survival Among SCD-HeFT Patients and SCD-HeFT–like Patients in the ICD Registry
Patients compared were SCD-HeFT–like patients in the ICD Registry, SCD-HeFT patients randomized to ICD therapy, and SCD-HeFT patients randomized to medical therapy only (for matched patients only). SCD-HeFT indicates Sudden Cardiac Death in Heart Failure Trial; ICD, implantable cardioverter-defibrillator.
MADIT-II Patients 65 Years and Older
Limiting the analysis to patients within the matched data sets who were 65 years and older, there was no significant difference in survival between MADIT-II–like patients in the ICD Registry and MADIT-II patients randomized to receive an ICD (2-year mortality rates: 16.5% vs 19.8%; adjusted registry vs MADIT-II HR, 1.02; 95% CI, 0.78–1.33; P=.88). Compared with older patients who received medical therapy in MADIT-II, older MADIT-II–like patients in the ICD Registry had significantly better survival (2-year mortality rates: 16.5% vs 31.5%; adjusted registry vs MADIT-II HR, 0.65; 95% CI, 0.49–0.85; P=.002).
SCD-HeFT Patients 65 Years and Older
No significant difference in survival was observed between SCD-HeFT–like patients in the ICD Registry and patients randomized to receive ICD therapy in SCD-HeFT (3-year mortality rates: 21.8% vs 24.8%; adjusted registry vs SCD-HeFT HR, 1.05; 95% CI, 0.81–1.36; P=.73) (Table 3). Older SCD-HeFT–like patients in the registry had significantly better survival than older patients who received placebo in SCD-HeFT (3-year mortality rates: 21.8% vs 30.2%; adjusted registry vs SCD-HeFT HR, 0.76; 95% CI, 0.60–0.96; P=.02) (Table 3).
COMMENT
Comparing the largest registry of ICD implants in the United States with 2 pivotal primary prevention randomized clinical trials, we demonstrated that the adjusted survival of MADIT-II–like and SCD-HeFT–like patients who received a primary prevention ICD in clinical practice was not significantly different from the survival of patients who received an ICD in the clinical trials but was significantly greater than the survival of trial patients randomized to receive medical therapy only. Importantly, the generalizability of these results held even after limiting the analyses to patients 65 years and older.
Prior work in other fields has demonstrated that it is often challenging to generalize the findings from randomized clinical trials to clinical practice.5–11 Our study found that patients receiving ICDs in clinical practice were significantly older and had more co-morbidities than those enrolled in the randomized clinical trials. The rates of use of cardiac medications were also significantly different between the groups. After adjusting for these differences with propensity score matching and Cox proportional hazards models, we found no significant difference in survival between MADIT-II–like patients in the ICD Registry and patients randomized to ICD therapy in MADIT-II and significantly better survival than patients randomized to medical therapy in MADIT-II.
However, there was an appreciable difference in survival between MADIT-II patients who received medical therapy and MADIT-II–like patients in the ICD Registry that appeared early and continued during follow-up (Figure 2). Although the improved survival among the MADIT-II–like patients in the registry is likely due to the ICD, other additional factors may have played a role. Such factors include the (slightly) lower blood urea nitrogen and higher rates of β-blocker and statin use at baseline in the registry patients after matching. Although blood urea nitrogen was not significantly different between the 2 groups, it was the strongest (by χ2 statistic) factor in the mortality model, which is why it likely had an appreciable effect on the results.
Similarly, we observed no significant difference in survival between SCD-HeFT–like patients in the ICD Registry and patients randomized to receive ICD therapy in SCD-HeFT and significantly better survival than patients randomized to placebo in SCD-HeFT. Similar results were found when the analysis was limited to patients 65 years and older. This is important because of the potential for a higher risk of complications from implanting this device in older patients and the presence of other competing risks for mortality. These findings underscore the effectiveness of primary prevention ICD therapy in clinical practice.
In this analysis, we focused on the following question: when we restrict the real-world analysis to patients who are similar to trial patients, is their survival different? Although this is a narrower definition of external validity, it is an important one. Patients enrolled in randomized clinical trials of primary prevention ICD therapy were monitored carefully over the course of the trials, and physicians who implanted and followed those devices were highly experienced. This level of care may not occur in real-world practice. A previous report using Medicare Claims data found that only 43% of patients who received an ICD had an initial follow-up visit within the recommended 2- to 12-week window after device implantation, and approximately 20% of surviving patients had no follow-up within a year.16 In another analysis of Medicare Claims data, there was an association between a higher risk of procedural complications and a lower volume of ICD implants.17
Therefore, it is reasonable to question whether the results of the trials can be expected in clinical practice. Through propensity score matching and adjustment for differences between registry patients and patients enrolled in the clinical trials, our matched sample became similar to patients enrolled in the clinical trials. This enabled us to address the concern that the care of patients in the highly controlled and monitored setting of clinical trials compromises the external validity of the results.
One prior retrospective study compared clinical practice patients with an ICD with clinical practice ICD-eligible patients with no ICD, using data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) registry and the Get With The Guidelines–Heart Failure (GWTG-HF) registry, as well as long-term outcome data from Medicare claims files.18 In the 4685 identified patients (mean age, 75 years), mortality was significantly lower among patients who received an ICD compared with those who did not (adjusted hazard ratio, 0.71; 95% CI, 0.56–0.91).18 Our findings support the results of this study and extend them beyond the Medicare population.
One of the main purposes of the ICD Registry, as specified by the Centers for Medicare & Medicaid Services, was to define how the characteristics and outcomes of Medicare beneficiaries receiving a primary prevention ICD compare with those of patients enrolled in the primary prevention ICD randomized clinical trials. Our analysis provides important data on the survival of trial-eligible patients who receive a primary prevention ICD in routine clinical practice, including patients 65 years and older.
Our study has several limitations. Because of appreciable differences in baseline characteristics between registry and randomized clinical trial patients, we were unable to address the question of how the outcomes of patients receiving an ICD in randomized clinical trials compare with the outcomes of all patients seen in clinical practice. Thus, our results may not apply to registry patients who are significantly different from patients in the clinical trials. The most valid approach for examining the effectiveness of ICDs in clinical practice would be through a clinical trial that randomizes patients of similar age and comorbidities to patients seen in clinical practice to an ICD vs no ICD. Propensity score matching was used to create comparable populations. Because of the exclusion of many patients, our cohort may not resemble a true cohort of patients in real-world practice. We could not adjust for clinical factors not captured by the registry nor for unknown confounders. Data were collected by medical record review that was dependent on the accuracy and completeness of documentation and abstraction. Given the limited follow-up in the clinical trials and the merged ICD Registry with the Death Master File, we were unable to examine longer-term survival.
In addition, excluding recipients of cardiac resynchronization therapy may raise concerns about potential fundamental differences between our patient population and patients enrolled in the trials, as some patients with NYHA class III symptoms who would now be eligible for a cardiac resynchronization therapy device were not excluded from MADIT-II and SCD-HeFT; however, patients with NYHA class III symptoms made up only 23% of the MADIT-II population and 30% of the SCD-HeFT population, and a smaller percentage would have qualified for cardiac resynchronization therapy based on a wide QRS.
CONCLUSIONS
Using the largest registry of ICD implants in the United States, we demonstrated that survival among MADIT-II–like and SCD-HeFT–like patients who received a primary prevention ICD in clinical practice was not significantly different from survival among patients who received an ICD in those major primary prevention ICD trials but was significantly greater than trial patients randomized to receive medical therapy. These findings were observed in the overall studied population as well as in patients aged 65 years and older.
Acknowledgments
Funding/Support: This analysis was funded by a grant (1R01-HL093071-01A1) from the National Heart, Lung, and Blood Institute.
Role of the Sponsor: The National Heart, Lung, and Blood Institute had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The manuscript was reviewed by the MADIT-II and SCD-HeFT leadership and the American College of Cardiology NCDR ICD Registry Research and Publications Committee.
Footnotes
Disclaimer: Views expressed in this article are those of the authors and do not necessarily represent the official view of the National Heart, Lung, and Blood Institute. Dr Peterson, a contributing editor for JAMA, was not involved in the editorial review of or the decision to publish this article.
Online-Only Material: The eFigure appears at http://www.jama.com.
Additional Contributions: We thank Yongfei Wang, MS, a statistician at Yale University, for conducting the deterministic matching between the ICD Registry and the Death Master File from the Social Security Administration. Through a subcontract between the NCDR and Yale University, Mr Wang was compensated for his efforts.
Author Contributions: Dr Al-Khatib had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Al-Khatib, Hammill, Mark, Anstrom, L. Curtis, Peterson, Sanders, Lee.
Acquisition of data: Al-Khatib, Bardy, Hall, Mark, Anstrom, Lee, Moss.
Analysis and interpretation of data: Al-Khatib, Hellkamp, Bardy, Hammill, Hall, Mark, Anstrom, J. Curtis, Al-Khalidi, L. Curtis, Heidenreich, Peterson, Sanders, Clapp-Channing, Lee, Moss.
Drafting of the manuscript: Al-Khatib.
Critical revision of the manuscript for important intellectual content: Hellkamp, Bardy, Hammill, Hall, Mark, Anstrom, J. Curtis, Al-Khalidi, L. Curtis, Heidenreich, Peterson, Sanders, Clapp-Channing, Lee, Moss.
Statistical analysis: Hellkamp, Anstrom, Al-Khalidi, L. Curtis, Lee.
Obtained funding: Al-Khatib.
Administrative, technical, or material support: Clapp-Channing.
Study supervision: Al-Khatib, Bardy, Hammill, Lee, Moss.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Bardy reported consulting fees, equity, and intellectual property rights in Cameron Health. Dr Mark reported receiving research grants from Eli Lilly, Medtronic, AstraZeneca, and Gilead Sciences. Dr Anstrom reported receiving research support from AstraZeneca, Eli Lilly, and Medtronic; having served as a consultant for Abbott Vascular, AstraZeneca, Bristol Meyer Squibb, and Ikaria; and having served on data monitoring committees for Pfizer and Vertex. Dr J. Curtis reported owning stock in Medtronic and receiving salary support from the American College of Cardiology Foundation, Center for Medicare & Medicaid Services, and National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI). Dr L. Curtis reported receiving research and salary support from Johnson & Johnson, GlaxoSmithKline, Agency for Healthcare Research and Quality, and NHLBI. Dr Curtis has made available online a detailed listing of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp). Dr Heidenreich reported receiving research support from Medtronic. Dr Lee reported receiving modest consulting fees from Medtronic. Dr Moss reported receiving research grants from Boston Scientific and salary support from Boston Scientific, Medtronic, St Jude, and Biotronic. No other disclosures were reported.
References
- 1.Moss AJ, Hall WJ, Cannom DS, et al. Multicenter Automatic Defibrillator Implantation Trial Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335(26):1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341(25):1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, et al. Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Cardinal H, Monfared AA, Dorais M, LeLorier J. A comparison between persistence to therapy in ALLHAT and in everyday clinical practice: a generalizability issue. Can J Cardiol. 2004;20(4):417–421. [PubMed] [Google Scholar]
- 6.Pedone C, Lapane KL. Generalizability of guidelines and physicians’ adherence: case study on the Sixth Joint National Committee’s guidelines on hypertension. BMC Public Health. 2003;3:24. doi: 10.1186/1471-2458-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keitner GI, Posternak MA, Ryan CE. How many subjects with major depressive disorder meet eligibility requirements of an antidepressant efficacy trial? J Clin Psychiatry. 2003;64(9):1091–1093. doi: 10.4088/jcp.v64n0915. [DOI] [PubMed] [Google Scholar]
- 8.Bahit MC, Cannon CP, Antman EM, et al. TIMI 9 Trial and TIMI 9 Registry. Thrombolysis In Myocardial Infarction. Direct comparison of characteristics, treatment, and outcomes of patients enrolled versus patients not enrolled in a clinical trial at centers participating in the TIMI 9 Trial and TIMI 9 Registry. Am Heart J. 2003;145(1):109–117. doi: 10.1067/mhj.2003.43. [DOI] [PubMed] [Google Scholar]
- 9.Walsh CR, Lloyd-Jones DM, Camargo CA, Jr, Giugliano RP, O’Donnell CJ. Clinical trials of unfractionated heparin and low-molecular-weight heparin in addition to aspirin for the treatment of unstable angina pectoris: do the results apply to all patients? Am J Cardiol. 2000;86(9):908–912. doi: 10.1016/s0002-9149(00)01120-6. [DOI] [PubMed] [Google Scholar]
- 10.Hordijk-Trion M, Lenzen M, Wijns W, et al. EHS-CR Investigators. Patients enrolled in coronary intervention trials are not representative of patients in clinical practice: results from the Euro Heart Survey on Coronary Revascularization. Eur Heart J. 2006;27(6):671–678. doi: 10.1093/eurheartj/ehi731. [DOI] [PubMed] [Google Scholar]
- 11.Badano LP, Di Lenarda A, Bellotti P, Albanese MC, Sinagra G, Fioretti PM. Patients with chronic heart failure encountered in daily clinical practice are different from the “typical” patient enrolled in therapeutic trials. Ital Heart J. 2003;4(2):84–91. [PubMed] [Google Scholar]
- 12.The ICD Registry Program. Heart Rhythm Society; [Accessed December 11, 2012.]. http://www.hrsonline.org/Practice-Guidance/Quality-Outcomes-Reporting/ICD-Registry. [Google Scholar]
- 13.ICD Registry. [Accessed August 15, 2010.];National Cardiovascular Data Registry. http://www.ncdr.com/webncdr/ICD/Default.aspx.
- 14.Hammill SC, Kremers MS, Kadish AH, et al. Review of the ICD Registry’s third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6(9):1397–1401. doi: 10.1016/j.hrthm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 16.Al-Khatib SM, Mi X, Qualls L, et al. Follow-up of patients with new cardiovascular implantable electronic devices: are the experts’ recommendations being implemented in routine clinical practice? [abstract] Heart Rhythm. 2012;9(5 suppl):S381. doi: 10.1161/CIRCEP.112.974337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol. 2005;46(8):1536–1540. doi: 10.1016/j.jacc.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez AF, Fonarow GC, Hammill BG, et al. Clinical effectiveness of implantable cardioverter-defibrillators among Medicare beneficiaries with heart failure. Circ Heart Fail. 2010;3(1):7–13. doi: 10.1161/CIRCHEARTFAILURE.109.884395. [DOI] [PubMed] [Google Scholar]