Abstract
Neural oscillatory anomalies in autism spectrum disorders (ASD) suggest an excitatory/inhibitory imbalance; however, the nature and clinical relevance of these anomalies are unclear. Whole-cortex magnetoencephalography data were collected while 50 children (27 with ASD, 23 controls) underwent an eyes-closed resting-state exam. A Fast Fourier Transform was applied and oscillatory activity examined from 1 to 120 Hz at 15 regional sources. Associations between oscillatory anomalies and symptom severity were probed. Children with ASD exhibited regionally specific elevations in delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and high frequency (20–120 Hz) power, supporting an imbalance of neural excitation/inhibition as a neurobiological feature of ASD. Increased temporal and parietal alpha power was associated with greater symptom severity and thus is of particular interest.
Keywords: Autism, Magnetoencephalography, Resting-state, Oscillations, Alpha, Gamma
Introduction
A disruption in the excitatory/inhibitory balance of neural activity is increasingly thought to characterize autism spectrum disorders (ASD). For instance, mutations in genes involved in the expression of excitatory and inhibitory neurotransmitters (e.g., glutamate and GABA) have been identified in individuals with ASD (Collins et al. 2006; Ramoz et al. 2004), and mutations in genes related to excitatory and inhibitory neurotransmitters result in cognitive and social deficits in animal models of autism (DeLorey et al. 2008). Despite cellular and molecular evidence (Bourgeron 2009; Rubenstein 2010; Rubenstein and Merzenich 2003), very few studies to date have investigated an excitatory/inhibitory imbalance in ASD at a macroscopic cortical circuit scale. This putative imbalance can be assessed noninvasively during a resting-state exam via patterns of neural oscillations, as oscillatory activity reflects the synchronous firing of large populations of neurons mediated by excitatory/inhibitory interactions (Hughes 2008). Oscillatory activity is evident at the scalp at a wide variety of frequencies, from less than 1 Hz to well over 100 Hz and is believed to be a cornerstone of brain function, supporting sensation, perception, and cognition (for reviews, see Uhlhaas et al. 2011; Wang 2010). Resting-state oscillatory activity can be quantified in absolute terms (the amount of power at a given frequency) as well as in relative terms (the proportion of total power accounted for by power at a given frequency).
The few EEG studies employing resting-state exams in ASD all report oscillatory anomalies. Specifically, eyes-open resting-state exams have shown greater relative delta and less relative alpha power in 4- to 12-year-old low-functioning children with ASD (Cantor et al. 1986), and greater 24–44 Hz power in 3- to 8-year-old boys with ASD (Orekhova et al. 2007). Eyes-closed exams have shown greater relative 3–6 Hz and 13–17 Hz power and less 9–10 Hz power in adults with ASD (Murias et al. 2007), and decreased delta (absolute and relative) and beta (absolute) power, as well as increased theta (relative) power, in 6- to 11-year-old children with ASD (Coben et al. 2008).
Although the aforementioned results implicate atypical oscillatory activity in ASD, findings are discrepant, possibly due to between-study differences in age, level of functioning, and medication status of the ASD participants. In addition, key questions remain: (1) Previous findings have been limited to the examination of oscillatory activity in sensor rather than source space. Activity measured at the sensor level may reflect superimposed signals from numerous brain regions and is more susceptible than source activity to muscle and other artifacts. As such, examining neural activity in source space provides better signal separation as well as a greater signal to noise ratio (Scherg et al. 2002). (2) To our knowledge, associations between resting-state oscillatory anomalies in ASD and autistic symptom severity have not been reported, yet are crucial in establishing the clinical significance of oscillatory anomalies in ASD. (3) Almost all ASD resting-state studies have examined activity below 50 Hz, despite growing recognition of the importance of high-frequency oscillations in spontaneous neural network activity (Geisler et al. 2005; Whittington et al. 2000). Excessive resting-state high-frequency oscillatory activity has been reported in epilepsy (Willough by et al. 2003), suggesting that high-frequency activity may also shed light on the pathophysiology of ASD.
In the present study, resting-state 1–120 Hz activity in ASD was investigated using whole-cortex magnetoencephalography (MEG), a technology that provides good temporal and spatial resolution. Based on previous studies, it was hypothesized that children with ASD would exhibit increased low- (<8 Hz) and high-frequency (>20 Hz) activity and decreased alpha (8–12 Hz) activity. Although the loci of oscillatory anomalies have not been consistent across studies, it was hypothesized that anomalies would be regionally specific and most likely to be observed in frontal and parietal regions, as these regions encompass nodes in the default mode network (Raichle et al. 2001), and ASD resting-state anomalies have been observed in these regions using fMRI (Cherkassky et al. 2006; Kennedy et al. 2006). Atypical alpha activity was furthermore predicted to be observed primarily in parietal regions, where alpha waves are most prominent at rest (Salmelin and Hari 1994). Although the lack of precedent in the literature precluded strong a priori predictions, increased resting-state low-frequency (<8 Hz) activity has been reported in a number of neurological disorders (Bosboom et al. 2006; de Jongh et al. 2003; Venables et al. 2009) and thus more likely reflects general brain dysfunction than ASD-specific pathology; therefore, we hypothesized that any observed associations between oscillatory anomalies and ASD symptoms would be evident in frequencies greater than 8 Hz.
Method
Participants
Participants were 50 children (27 with ASD and 23 age-matched typically developing controls), aged 6–15 years (mean = 9.8, SD = 2.3 in the ASD group; mean = 10.8, SD = 2.5 in the control group). A t test indicated that the groups did not significantly differ in age (p > .17). Participants with ASD were recruited from the Regional Autism Center of The Children's Hospital of Philadelphia (CHOP), the Neuropsychiatry program of the Department of Psychiatry of the University of Pennsylvania School of Medicine, and from local and regional parent support groups such as ASCEND (The Asperger & Autism Alliance for Greater Philadelphia), Autism Society of America—Greater Philadelphia Chapter, and local chapters of Autism Speaks. All children screened for inclusion in the ASD sample had received an ASD diagnosis prior to their involvement in the current research. This prior diagnosis was made by an expert clinician, typically a developmental pediatrician in the Regional Autism Center at CHOP, after an extensive clinical interview, documentation of DSM-IV criteria for ASD, and use of various ASD diagnostic tools, such as the Childhood Autism Rating Scale and, in many cases, the Autism Diagnostic Observation Schedule [ADOS; (Lord et al. 2000)]. Subjects with typical development (TD) were recruited through local newspaper advertisements and from pediatric practices of the CHOP primary care network.
Research participants made two visits to CHOP. During the first visit, clinical and diagnostic testing was performed to confirm the referral ASD diagnosis, to administer neuropsychological tests, and to ensure that the TD children met study inclusion/exclusion criteria. Assessments were performed by a licensed child psychologist with expertise in autism (L.B.). Given the extensive clinical evaluations upon which the original diagnosis was made, an abbreviated diagnostic battery was used to confirm the original diagnosis in the ASD group and to rule out ASD in the TD children. Specifically, ASD was confirmed (or ruled out in the TD children) with the ADOS and parent report on the Social Communication Questionnaire [SCQ; (Rutter et al. 2003)]. Dimensional symptom severity ratings were also obtained by parent report on the Social Responsiveness Scale [SRS; (Constantino and Gruber 2005)]. Asperger's disorder symptomatology was measured with the Krug Asperger's Disorder Index [KADI; (Krug and Arick 2003)]. For final inclusion in the ASD group, children were required to have a confirmatory research diagnosis of ASD by exceeding established cut-offs on both the ADOS and SCQ. Children 1 point below ADOS cut-offs were included if they exceeded cut-offs on at least two parent questionnaires (one ASD subject met ADOS criteria and had a best-estimate diagnosis of ASD by clinician judgment, but exceeded cut-offs on only one parent questionnaire). Mean scores were as follows: ADOS, 13 (SD = 5, range = 7–26); SCQ, 18 (SD = 6, range = 9–26); and SRS (raw), 87 (SD = 20, range = 49–118). To rule out global cognitive delay, all subjects were required to score at or above the 5th percentile (SS > 75) on the Perceptual Reasoning Index (PRI) of the Wechsler Intelligence Scale for Children-IV [WISC-IV; (Wechsler 2003)]. In all subjects, the WISC-IV Verbal Comprehension Index (VCI) was also obtained. Inclusion criteria for the TD control children included scoring below the cut-off for ASD on the ADOS as well as parent questionnaires. Per parent report, they also had never been diagnosed with the following: developmental delay, mental retardation, speech/language disorder/delay, communication disorder, language-based or other learning disability, ADHD, or psychiatric conditions (e.g., bipolar disorder, obsessive compulsive disorder, schizophrenia, conduct disorder, depression, or anxiety disorder).
In addition to the inclusion/exclusion criteria outlined above, per parent report, all subjects and families were native English speakers and had no known genetic syndromes, neurological (e.g., cerebral palsy, epilepsy), or sensory (hearing, visual) impairments. Finally, given known associations between psychotropic medications and brain activity (Blume 2006), all subjects in this study were required to be medication-free. Parental report of medication use was obtained during the initial phone screening and again at the study visit to confirm that participants were medication-free from the time of recruitment and that their medication-free status had not changed between the phone screen and study visit. (Note that although participants were unmedicated for at least 2 months prior to MEG imaging, from our study records it is not possible to determine if subjects were taking medications prior to the phone screen. Nevertheless, 2 months is sufficiently long as to encompass medication washout periods.) The study was approved by the CHOP Institutional Review Board and all participants' families gave written informed consent. As indicated by institutional policy, where competent to do so, children over the age of seven additionally gave verbal assent.
The WISC-IV General Ability Index (GAI) was compared between groups to ensure that any observed group differences in oscillatory activity would not be attributable to group differences in cognitive ability. The mean GAI was 104 (SD = 17, range = 67–138) in the ASD group and 105 (SD = 13, range = 85–132) in the TD group. A t test confirmed that the groups did not differ on GAI (p > .86). The SRS was the measure of ASD symptom severity chosen for investigating the relationship between resting-state oscillatory activity and symptom severity. Whereas the most commonly used ASD diagnostic tools are designed for purposes of categorical classification, the SRS provides dimensional ratings of impairment across a broad range of severity. Mean SRS raw scores were 87 in the ASD group (SD = 20, range = 49–118) and 23 in the TD group (SD = 18, range = 3–62). A t test confirmed that SRS scores were significantly higher in the ASD group than in the TD group (p < .0001).
Procedure
Data were collected using a 275-channel MEG system (VSM MedTech Inc., Coquitlam, BC). Children were scanned in a supine position and were instructed to lie still with their eyes gently closed during a 2-minute resting-state exam. Three head-position indicator coils were attached to the scalp and foam wedges inserted between the side of the participant's head and the inside of the dewar to ensure immobility. The electro-oculogram (EOG; bipolar oblique, upper right and lower left sites) was collected to ensure that participants' eyes remained closed throughout the 2-minute exam. After a band-pass filter (0.03–150 Hz), EOG and MEG signals were digitized at 1,200 Hz with 3rd-order gradiometer environmental noise reduction and downsampled offline to 500 Hz.
Data Processing and Analysis
A clinical read was first performed to examine the data for gross abnormalities. All eye-closed recordings were read as normal by the clinical team (i.e., epileptiform activity was not observed in any of the recordings). A three step process was employed for removal of muscle and movement artifact. First, participants' raw EOG data were visually examined and epochs contaminated by blinks, saccades, or other significant EOG activity were removed from the MEG data. Second, blind to diagnosis, participants' MEG data were visually inspected for muscle-related activity (focusing especially on data from sensors close to the temporalis muscles), and data containing muscle activity were removed. Third, any additional artifacts were rejected by amplitude and gradient criteria (amplitude 1,200 fT/cm, gradients 4,800 fT/cm/sample). We have found this three-state approach to provide more thorough artifact rejection than automated artifact-rejection routines. All participants had at least 45 s of artifact-free data, with a mean of 86 (SD = 17) seconds in the ASD group and a mean of 85 (SD = 16) seconds in the TD group. A t-test confirmed that the groups did not differ with respect to amount of artifact-free data (p > .84). To ensure that any observed group power differences were not due to group differences in residual eye movement, power in the EOG channel from 1 to 120 Hz was compared between groups. A repeated-measures ANOVA with frequency band (1–4, 4–8, 8–12, 12–20, 20–30, 30–55, 65–90, 90–120 Hz) as a within-subjects factor revealed no main effect or interaction involving group (ps > .71).
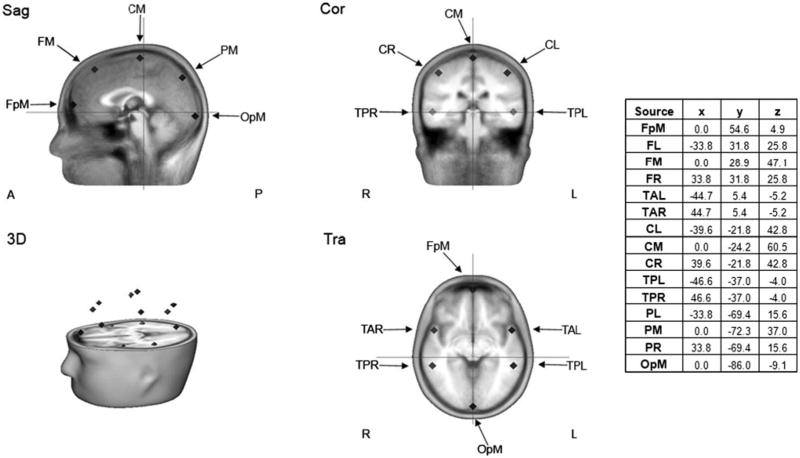
The MEG data were then further processed using BESA 5.2 (MEGIS Software GmbH, Grafelfing, Germany). To decompose the 275 channel data into a smaller number of measures for data analysis, a source model with 15 regional sources (Fig. 1) was applied to project each individual's raw MEG surface data into brain source space where the waveforms are the modeled source activities. These regional sources are not intended to correspond to precise neuroanatomical structures but rather to represent neural activity at coarsely defined regions and to provide measures of brain activity with better signal separation and with a greater signal-to-noise ratio than would be afforded at the sensor level (Scherg et al. 2002). The locations of the regional sources in the model are such that there is an approximately equal distance between sources (> 3 cm), helping to separate signals originating from different brain regions. Goodness of fit for the regional source model (using the two orthogonal orientations of each regional source) over the entire resting dataset ranged from 79 to 91%, with a mean of 85% (SD = 3.0) in the ASD group and 85% (SD = 2.3) in the TD group. A t test confirmed that the groups did not differ with respect to goodness of fit (p > .88).
Fig. 1. Source model showing the locations of the 15 regional sources and their Talairach coordinates. FpM fronto-polar midline, FL frontal left, FM frontal midline, FR frontal right, TAL temporal anterior left, TAR temporal anterior right, CL central left, CM central midline, CR central right, TPL temporal posterior left, TPR temporal posterior right, PL parential left, PM parential midline, PR parential right, OPM occipito-polar midline.
To transform MEG data from the time domain into the frequency domain, a Fast Fourier Transform (FFT) was applied to artifact-free two-second epochs of continuous data for each of the two orthogonally oriented time series at each regional source. Each two-second epoch overlapped 50% with the next epoch, and each epoch was multiplied by a cosine squared window. This combination of overlap and windowing ensured that each time point contributed equally to the mean power spectra. The mean power spectra for the two orthogonally oriented time series at each regional source were summed to yield the power at a given frequency at that source. Total spectral power was computed for each participant by summing the power across all frequencies (1–120 Hz) and all sources. Based on total spectral power, three participants (2 ASD, 1 TD) were identified as outliers (>2 SD from the mean) and their data were excluded from subsequent analyses.
Prior to parceling the remaining participants' data into frequency bands for further analysis, the peak alpha frequency at the parietal midline source (the location of the greatest alpha power for the majority of participants) was identified in each participant to ensure that all peak alpha frequencies were between 8 and 12 Hz. The peak frequency of alpha oscillations is below 8 Hz early in life and increases with development, generally reaching the 8–12 Hz range by approximately 3 years of age (Klimesch 1999). The mean peak frequency was indeed between 8 and 12 Hz for all participants, with a mean of 9.6 Hz (SD = 0.8) in both the ASD and TD groups. A t test confirmed that the groups did not differ in peak alpha frequency (p > .92). Given no group difference, individual spectra were averaged and oscillatory activity at the 15 regional sources was examined in eight standard frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta1 (12–20 Hz), beta2 (20–30 Hz), gamma1 (30–55 Hz), gamma2 (65–90 Hz), and very fast oscillations (VFO; 90–120 Hz).
As ASD anomalies have been reported in absolute and relative power, analyses were conducted for both measures between 1 and 120 Hz. At each regional source, relative power was calculated by dividing the power in each frequency band by the total power. This provides a measure of the proportion of power within each frequency band. Absolute power and relative power are thus related but distinct, with group differences in absolute power indicating differences in the raw power spectra, and group differences in relative power indicating group differences in the distributions of power across frequency bands. Thus, it is possible that group differences in absolute and relative power may differ for a given frequency band.
Linear mixed model analyses compared absolute and relative power between groups at each of the 15 regional sources. Fixed effects were frequency band (8 bands), anterior-to-posterior (frontopolar, frontal, anterior temporal, central, posterior temporal, parietal, occipital), and laterality (left hemisphere, midline, right hemisphere), and subject was a random effect. Custom models were created in order to specifically test for group differences; as such, all non-group-related interactions and main effects were excluded. For absolute power, the model included all possible two- and three-way interactions involving group as well as the main effect of group. For relative power, the model included only the following interaction terms: group by frequency band, group by frequency band by anterior-to-posterior, and group by frequency band by laterality. The main effect of group as well the group by laterality and group by anterior-to-posterior interaction terms were excluded from the relative power analysis because at each source the total relative power (summed across frequency bands) must, by definition, equal 1 for every participant. Because of this, F will always equal 0 and p will always equal 1 for these effects. For analyses of both absolute and relative power, highest-order significant interactions are reported. Where group differences in power were observed, linear regressions assessed associations with ASD symptom severity (SRS total raw scores).
Results
Absolute Power
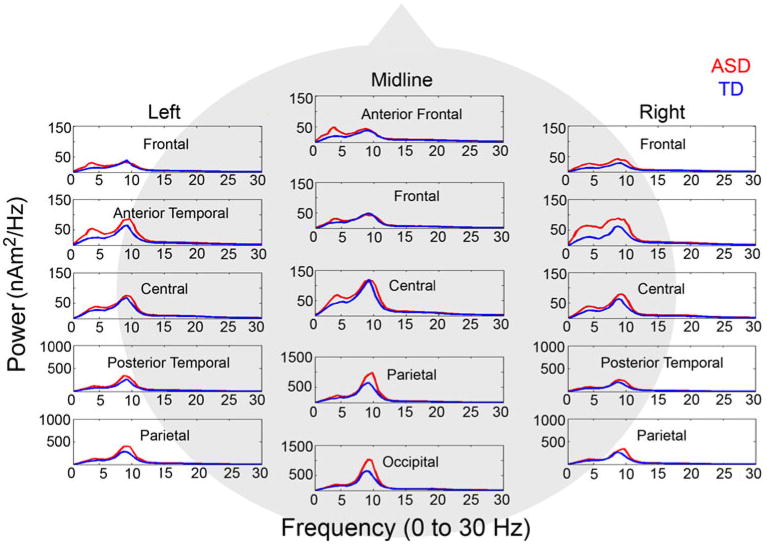
A linear mixed model analysis of absolute power revealed a significant group by frequency band by anterior-to-posterior interaction, F(84, 5439) = 31.34, p < .001, showing greater power in the ASD than in the TD group in the theta band at parietal, F(1,679) = 4.79, p = .029, and occipital, F(1, 2765) = 5.86, p = .016, regions, as well as in the alpha band at posterior temporal, F(1, 1194) = 12.87, p < .001, parietal, F(1, 679) = 81.26, p < .001, and occipital F(1, 2765) = 112.24, p < .001, regions (see Fig. 2). A significant group by frequency band by laterality interaction was also observed, F(28, 5439) = 8.75, p < .001, showing that alpha power was significantly greater in ASD in left hemisphere, midline, and right hemisphere regions, Fs(1,340) = 11.33, 66.10, and 8.44, ps = .001, < .001, and .004, respectively and theta power was significantly greater in ASD in midline regions, F(1,340) = 4.62, p = .032, and marginally greater in the left hemisphere F(1, 340) = 3.02, p = .083.
Fig. 2. Power spectra for the ASD (red) and TD (blue) control groups from 1–30 Hz at all 15 regional sources. Note that due to regional differences in the magnitude of oscillatory activity, the y axis scaling differs across sources (Color figure online).
Given a previous finding of increased resting 24–44 Hz power in children with ASD (Orekhova et al. 2007) and reports of increased power up to and exceeding 100 Hz in epilepsy (Willoughby et al. 2003), the lack of group differences in high-frequency activity was surprising. To further investigate our a priori prediction of greater high-frequency activity in ASD, linear mixed model analyses were conducted individually for beta1, beta2, gamma1, gamma2, and VFO bands, with anterior-to-posterior and laterality fixed factors. A Bonferroni correction was applied, such that a conservative α = 0.01 for each test protected a family-wise α = 0.05. As expected, group differences were observed at all high-frequency bands, demonstrating greater power in ASD than in the TD group. These high-frequency abnormalities in ASD exhibited a regional specificity similar to the low-frequency findings reported above, that is, most robust at temporal, parietal, and occipital regions (see Table 1 for details). Because muscle artifact can contaminate high-frequency MEG and EEG data (Orekhova et al. 2007), high frequency analyses were repeated, excluding the regional sources where muscle activity is most prominent (frontopolar, anterior and posterior temporal, and occipital). For all bands, the group by anterior-to-posterior interactions remained significant (for beta1 and beta2, Fs(4, 360) = 155.32 and 157.15, ps < .001; for low gamma, high gamma, and VFO, Fs(4, 360) = 128.74, 63.40, and 54.81, ps < .001), demonstrating greater high-frequency activity in ASD that became more pronounced from anterior to posterior and was most evident in parietal regions.
Table 1. Group by anterior-to-posterior interaction effects for high frequency bands.
| Frequency band | F | p | Region |
|---|---|---|---|
| Beta1 | 76.97 | <.001 | Parietal* |
| Occipital** | |||
| Beta2 | 59.94 | <.001 | Parietal* |
| Occipital** | |||
| Low gamma | 60.92 | <.001 | Parietal* |
| Occipital** | |||
| High gamma | 38.49 | <.001 | Anterior temporal** |
| Posterior temporal** | |||
| Occipital* | |||
| VFO | 30.97 | <.001 | Anterior temporal** |
| Posterior temporal** | |||
| Occipital* |
p < .10,
p < .05
Relative Power
A linear mixed model analysis of relative power revealed a significant group by frequency band by anterior-to-posterior interaction, F(96, 5496) = 20.15, p < .001, with the ASD group exhibiting greater relative delta at frontal regions, F(1, 5496) = 4.49, p = .034, and greater relative alpha at anterior temporal, F(1, 5496) = 4.80, p = .029 and parietal, F(1, 5496) = 28.75, p < .001, regions. A significant group by frequency band by laterality interaction was also observed, F(32, 5496) = 4.21, p < .001, showing that the ASD group exhibited significantly greater relative delta in the right hemisphere, F(1, 5496) = 4.41, p = .036 and marginally significantly greater relative delta in the left hemisphere, F(1, 5496), p = .093. The ASD group exhibited significantly greater relative alpha in the left hemisphere, midline, and right hemisphere, Fs(1, 5496) = 5.26, 9.79, and 5.12, ps = .022, .002, and .024.
Potential Influences of Age on Spectral Power
Given the substantial brain development that occurs between 6 and 15 years of age, linear mixed model analyses of absolute and relative power were repeated with age entered as a covariate. For absolute power, although age was a significant covariate, F(1, 43) = 4.86, p = .033, indicating developmental changes in oscillatory power between 6 and 15 years of age, the age by group interaction was not significant, F(1, 43) = .75, 00 = .393. In addition, the group by frequency band by anterior-to-posterior interaction remained significant, F(84, 5439) = 31.34, p < .001, as did the group by frequency band by laterality interaction, F(28, 5439) = 8.753, p < .001. Contrasts revealed the same significant alpha band results reported above; however, the result of greater parietal theta power in ASD became marginally significant, F(1, 674) = 3.79, p = .052, as did that of greater midline theta power in ASD, F(1, 336) = 3.47, p = .063. For relative power, age was not a significant covariate and the age by group interaction was not significant, both Fs(1, 5494) = 0.00, ps = 1.00; however, the group by frequency band by anterior-to-posterior interaction remained significant, F(96, 5494) = 20.14, p < .001, as did the group by frequency band by laterality interaction, F(32, 5494) = 4.21, p < .001. Contrasts revealed the same significant group differences reported above.
Associations with Symptom Severity
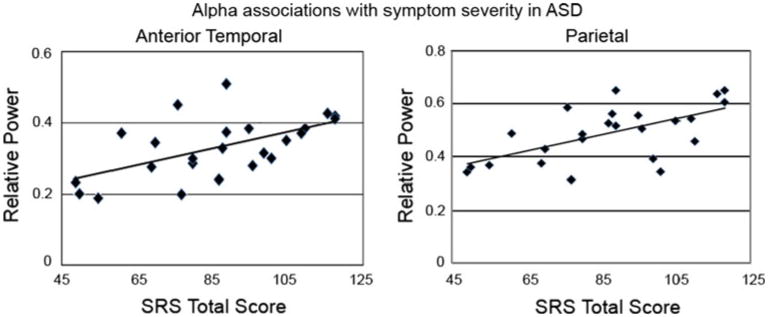
To test the hypothesis that atypical oscillatory activity is associated with ASD symptom severity, associations between the regionally- and spectrally-specific anomalies that emerged in the omnibus tests reported above and SRS scores were probed within the ASD group via linear regression. In addition, as the SRS exhibits a normal distribution across the general population (although with a higher mean score in ASD), associations were also examined in the TD group. For absolute power (5 regressions), a Bonferroni-corrected α = 0.01 protected a family-wise α = 0.05. No significant absolute power associations were observed (all ps > .05). For relative power (3 regressions), a Bonferroni-corrected α = 0.017 protected a family-wise α = 0.05. In ASD, symptom severity (higher SRS scores) was associated with increased anterior temporal relative alpha power, R2 = .34, p = .012, and increased parietal relative alpha power R2 = .39, p = .017 (Fig. 3). No significant associations were observed in the TD group.
Fig. 3. Scatterplots showing associations between elevated alpha power in anterior temporal (left) and parietal (right) regions and symptom severity (indexed by raw SRS scores) in ASD.
Discussion
The current study investigated resting-state neural oscillatory activity in children with ASD, with the aim of characterizing the potential excitation/inhibition imbalance that has been proposed to underlie the behavioral manifestations of ASD (Rubenstein and Merzenich 2003). Our findings indicate that ASD is characterized by atypical neural oscillatory activity. The finding of elevated relative delta power is consistent with Cantor and colleagues' finding in low-functioning children with ASD (Cantor et al. 1986), as well as with Murias and colleagues' finding that relative delta abnormalities are prominent in anterior regions (Murias et al. 2007). Delta abnormalities have been linked to a disconnection between gray and white matter (Huang et al. 2009) as well as thalamic abnormalities (Hughes and Crunelli 2005; O'Connor and Robinson 2005). Given reports of abnormal low-frequency activity in several disorders (Bosboom et al. 2006; de Jongh et al. 2003; Rockstroh et al. 2007; Venables et al. 2009; see also Roberts et al. 2009), and no associations between delta activity and ASD symptom severity in the present study, elevated delta in ASD may be a general indicator of neurological dysfunction rather than an abnormality specific to ASD.
Given reports of decreased alpha in ASD (Cantor et al. 1986; Murias et al. 2007), the present finding of increased posterior alpha was unexpected. Differences in participant characteristics between studies might account for the discrepant findings. For example, whereas participants in the present study were relatively high-functioning children with ASD, Cantor and colleagues studied low-functioning children, and Murias and colleagues studied adults. In addition, whereas all participants in the current study were unmedicated, subjects in Murias' study were receiving medications, and it is unclear whether this was true of participants in Cantor's study. Reports of the effects of numerous medications (including selective serotonin reuptake inhibitors (SSRIs) and methylphenidate, two classes of drugs commonly prescribed to children with ASD) on neural oscillations (Blume 2006; Dumont et al. 2005; Loo et al. 1999), highlight the importance of examining unmedicated participants. Furthermore, alpha activity is modulated by state of arousal, such that it is greatest when individuals are alert but at rest (Fisch 1999) and decreases when individuals are anxious. Several steps were taken to ensure that participants in the current study were alert during the resting-state exam: (1) The exam took place immediately following set-up in the MEG scanner, (2) the exam lasted only 2 minutes; and (3) an experimenter interacted with the child over an intercom just prior to the start of data collection. Experimenters also worked to minimize participant anxiety by remaining calm and friendly throughout the session. The effect of anxiety on alpha activity potentially complicates interpretation of decreased alpha power reported in previous studies (Cantor et al. 1986; Murias et al. 2007), as individuals with ASD are often prone to anxiety (and it was qualitatively observed that ASD participants in the present study tended to be more anxious than control participants during the MEG exam). However, the present finding of increased alpha in ASD is opposite of what would be expected if group differences were due only to anxiety.
In addition to differences in participant characteristics, another possible explanation for the discrepancy in alpha findings is that whereas children in the current study were scanned in an eyes-closed condition, which is optimal for eliciting alpha activity, children in Cantor et al. (1986) study were scanned in an eyes-open condition. Notably, although the present findings of increased alpha were unexpected, alpha power was the only measure associated with ASD symptom severity, suggesting that atypical alpha activity is of potential clinical relevance. The lack of a similar association in the TD group suggests that resting alpha power is not a sensitive index of individual differences in social ability in typically developing children, but rather may be a more specific indicator of ASD symptomatology. Future studies comparing oscillatory activity in ASD and other neurodevelopmental disorders are necessary to determine the specificity and potential diagnostic utility of elevated posterior alpha activity to ASD.
Converging evidence provides insight into potential mechanisms and functional correlates of atypical alpha activity in ASD. First, alpha is inversely related to perception and attention, suggesting that alpha reflects functional inhibition of sensory systems (Jokisch and Jensen 2007). It is possible that increased alpha power at rest renders the ASD brain unprepared for sensory input and thus may be associated with sensory processing abnormalities in ASD, such as delayed auditory M100 evoked responses (Roberts et al. 2010) and deficient rapid temporal auditory processing (Oram Cardy et al. 2005). Future work directly comparing resting alpha activity and sensory processing in children with ASD would address this hypothesis. Second, inhibitory interneurons, which are likely abnormal in ASD (Casanova et al. 2002b; Levitt et al. 2004), play a role in maintaining alpha oscillations (Lorincz et al. 2009), suggesting a potential mechanism for the observed alpha findings. Interestingly, interneuron abnormalities in a mouse model of ASD are specific to frontal and parietal regions (Levitt 2005), demonstrating similar regional specificity to the delta and alpha findings reported in the present study.
The frontal and parietal loci of the atypical delta and alpha activity observed here are also consistent with the loci of ASD resting-state anomalies identified with fMRI (Cherkassky et al. 2006; Kennedy et al. 2006). The spatial correspondence between fMRI and the present findings are interesting, and future studies employing MEG and fMRI (or perhaps simultaneous EEG and fMRI) in the same participants to investigate associations between resting-state findings may provide additional insight into the pathophysiology of ASD. This approach is especially of interest given a previous finding that alpha power, measured with EEG, is negatively correlated with the BOLD fMRI signal in a fronto-parietal network in healthy adults (Laufs et al. 2003). Moreover, fMRI studies have highlighted the role of regions within prefrontal, parietal, and temporal cortex in sensory and social processing (Iacoboni et al. 2004; Peelen et al. 2010), suggesting associations between atypical patterns of neural activity at rest and during basic sensory and higher-level processing in ASD. The relationship observed here between resting alpha power and scores on the SRS is consistent with such an association.
In addition to low-frequency anomalies, in the present study children with ASD exhibited elevated 20–120 Hz activity, predominantly in posterior brain regions. This finding replicates and extends the results of Orekhova et al. (2007), who observed increased EEG resting beta and low gamma activity in ASD. Interestingly, although they focused on the low gamma band, Orekhova also observed increased resting high gamma (up to 70 Hz) in one of their two ASD populations. The current study is the first to report atypical resting-state activity between 70 and 120 Hz in ASD. These findings should be interpreted with caution, however, as they were evident only in post hoc tests and, as such, are not as robust as the lower-frequency findings. In addition, although every effort was made to ensure that group differences in spectral power were not due to artifact, it is not possible to completely rule out potential contributions of residual muscle or motion, as well as anxiety, which can lead to increased high-frequency (particularly beta band) activity (Aftanas and Pavlov 2005). Future studies are needed to replicate the observed elevated high-frequency activity in ASD; this is an important avenue of pursuit given that high-frequency oscillatory activity is believed to underlie signal transmission in cortical microcircuits (Sohal et al. 2009), sensory processing (Tallon-Baudry et al. 1997), and short-range neural communication (Fries 2005). Furthermore, local circuit abnormalities at the microstructural level in ASD, such as atypical size and structure of cortical minicolumns, which play a role in maintaining the excitatory/inhibitory balance (Casanova et al. 2002a, b), are consistent with the pathologically elevated high-frequency activity observed here. In addition, there is a high degree of comorbidity of ASD and epilepsy (Canitano 2007), a disorder characterized by an abnormal excitation/inhibition ratio (Scharfman 2007) and increased high-frequency oscillatory activity (Willoughby et al. 2003). Electrophysiological epileptiform activity is present in a large percentage of children with ASD without epilepsy (Chez et al. 2006; Lewine et al. 1999), which suggests that a similar neural circuit abnormality may be involved in the pathophysiology of ASD.
A limitation of the current study is that the ASD participants were all high-functioning. This is a limitation common to many neuroimaging studies, because the scanning procedures can be demanding of participants and may not be tolerated by lower-functioning individuals. Thus it is unclear at present whether and how the findings from the current study extend to lower-functioning children with ASD. Fortunately, resting-state studies are, by definition, task-free and are therefore more amenable to the study of low-functioning individuals than task-based experimental designs. Future studies investigating resting-state oscillatory activity in low-functioning children with ASD will be helpful in resolving discrepant findings between studies and in characterizing the neural bases of phenotypic heterogeneity in ASD.
In sum, present findings demonstrate atypical resting-state oscillatory activity in children with ASD, suggest that regionally- and spectrally-specific alpha anomalies are of possible clinical relevance, and support an imbalance of neural excitation/inhibition as a potential ASD biomarker. Although it is not yet known whether the observed pattern of atypical activity represents a core pathology in ASD, it is likely that an imbalance of neural excitation and inhibition early in life has cascading detrimental effects on myriad developmental domains. For instance, this balance has been shown to regulate critical periods in development (Hensch 2005), during which the brain is maximally responsive to specific types of sensory input. In addition, oscillatory activity in local networks is driven by the firing of inhibitory interneurons and is critical for the development of these networks (see Uhlhaas and Singer 2006, for a review). Several neurological disorders are characterized by imbalances of excitation and inhibition (Uhlhaas and Singer 2006), and it is likely that the precise timing during development and/or the mechanisms of the imbalance result in distinct profiles of oscillatory activity that differentiate the disorders. Furthermore, given the protracted developmental trajectory and plasticity of cortical circuits, profiles of oscillatory anomalies associated with neurological disorders may change over the lifespan. Future studies with sample sizes large enough to compare groups cross-sectionally based on age, or longitudinal studies tracking oscillatory activity in the same children over time, would help to specify anomalies in the developmental trajectories of neural oscillations in children with ASD.
Acknowledgments
This study was supported in part by NIH grants R01DC008871 and R01DC008871-02S1 (TR) and T32NS007413 (LC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Neurological Disorders and Stroke or the National Institutes of Health. This research was also supported in part by grants from the Nancy Lurie Marks Family Foundation, the Jeffrey and Christina Lurie Family Foundation, Autism Speaks, and the Pennsylvania Department of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. The authors thank the children and families who participated; Katelyn Cannon, John Dell, Sarah Khan, Peter Lam, Justin Monroe, and Jamie Rundio for their help with data collection; Saba Qasmieh for her help with participant recruitment; and Susan E. Levy and Gregory Miller for clinical insights and helpful discussions. Dr. Roberts gratefully acknowledges the Oberkircher Family for the Oberkircher Family Chair in Pediatric Radiology at Children's Hospital of Philadelphia.
Contributor Information
Lauren Cornew, Lurie Family Foundations MEG Imaging Center, Department of Radiology, Children's Hospital of Philadelphia, 2nd Floor Wood Bldg., Room 2115, Mail Stop W02-1010, Philadelphia, PA 19104-4399, USA.
Timothy P. L. Roberts, Email: robertstim@email.chop.edu, Lurie Family Foundations MEG Imaging Center, Department of Radiology, Children's Hospital of Philadelphia, 2nd Floor Wood Bldg., Room 2115, Mail Stop W02-1010, Philadelphia, PA 19104-4399, USA.
Lisa Blaskey, Lurie Family Foundations MEG Imaging Center, Department of Radiology, Children's Hospital of Philadelphia, 2nd Floor Wood Bldg., Room 2115, Mail Stop W02-1010, Philadelphia, PA 19104-4399, USA; Center for Autism Research, Department of Pediatrics, Children's Hospital of Philadelphia, Philadelphia, PA, USA.
J. Christopher Edgar, Lurie Family Foundations MEG Imaging Center, Department of Radiology, Children's Hospital of Philadelphia, 2nd Floor Wood Bldg., Room 2115, Mail Stop W02-1010, Philadelphia, PA 19104-4399, USA.
References
- Aftanas LI, Pavlov SV. Trait anxiety impact on posterior activation asymmetries at rest and during evoked negative emotions: EEG investigation. International Journal of Psychophysiology. 2005;55:85–94. doi: 10.1016/j.ijpsycho.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Blume WT. Drug Effects on EEG. Journal of Clinical Neurophysiology. 2006;23:306–311. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- Bosboom JLW, Stoffers D, Stam CJ, van Dijk BW, Verbunt J, Berendse HW, et al. Resting state oscillatory brain dynamics in Parkinson's disease: An MEG study. Clinical Neurophysiology. 2006;117:2521–2531. doi: 10.1016/j.clinph.2006.06.720. [DOI] [PubMed] [Google Scholar]
- Bourgeron T. A symaptic trek to autism. Current Opinion in Neurobiology. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Canitano R. Epilepsy in autism spectrum disorders. European Child and Adolescent Psychiatry. 2007;16:61–66. doi: 10.1007/s00787-006-0563-2. [DOI] [PubMed] [Google Scholar]
- Cantor DS, Thatcher RW, Hrybyk M, Kaye H. Computerized EEG analyses of autistic children. Journal of Autism and Developmental Disorders. 1986;16:169–187. doi: 10.1007/BF01531728. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. Journal of Child Neurology. 2002a;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002b;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Chez MG, Chang M, Krasne V, Coughlan C, Kominsky M, Schwartz A. Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy & Behavior. 2006;8:267–271. doi: 10.1016/j.yebeh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Coben R, Clarke AR, Hudspeth W, Barry RJ. EEG power and coherence in autism spectrum disorder. Clinical Neurophysiology. 2008;119:1002–1009. doi: 10.1016/j.clinph.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, et al. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale (SRS) Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- de Jongh A, Baayen JC, de Munck JC, Heethaar RM, Vandertop WP, Stam CJ. The influence of brain tumor treatment on pathological delta activity in MEG. NeuroImage. 2003;20:2291–2301. doi: 10.1016/j.neuroimage.2003.07.030. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: A potential model of autism spectrum disorder. Behavioural Brain Research. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont GJH, de Visser SJ, Cohen AF, van Gerven JMA. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. British Journal of Clinical Pharmacology. 2005;59:495–510. doi: 10.1111/j.1365-2125.2005.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch BJ. Fisch and Spehlmann's EEG primer: Basic principles of digital and analog EEG. 3rd. New York: Elsevier; 1999. [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Geisler C, Brunel N, Wang XJ. Contributions of intrinsic membrane dynamics to fast network oscillations with irregular neuronal discharges. Journal of Neurophysiology. 2005;94:4344–4361. doi: 10.1152/jn.00510.2004. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Huang M, Theilmann RJ, Robb A, Angeles A, Nichols S, Drake A, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. Journal of Neurotrauma. 2009;26:1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Gamma, fast, and ultrafast waves of the brain: Their relationships with epilepsy and behavior. Epilepsy & Behavior. 2008;13:25–31. doi: 10.1016/j.yebeh.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, et al. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. NeuroImage. 2004;21:1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. Journal of Neuroscience. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: Resting functional abnormalities in autism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Krug D, Arick JR. Krug Asperger's Disorder Index. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P. Disruption of interneuron development. Epilepsia. 2005;46:22–28. doi: 10.1111/j.1528-1167.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurdevelopmental disorders. Trends in Neurosciences. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Andrews R, Chez M, Patil AA, Devinsky O, Smith M, et al. Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics. 1999;104:405–418. doi: 10.1542/peds.104.3.405. [DOI] [PubMed] [Google Scholar]
- Loo SK, Teale PD, Reite ML. EEG correlates of methylphenidate response among children with ADHD: A preliminary report. Biological Psychiatry. 1999;45:1657–1660. doi: 10.1016/s0006-3223(98)00250-9. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry. 2007;62:270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor SC, Robinson PA. Analysis of the electroencephalographic activity associated with thalamic tumors. Theoretical Biology. 2005;233:271–286. doi: 10.1016/j.jtbi.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Brian J, Roberts TPL. Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. NeuroReport. 2005;16:329–332. doi: 10.1097/00001756-200503150-00005. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, et al. Excess of high frequency electroencephalogram oscillations in boys with autism. Biological Psychiatry. 2007;62:1022–1029. doi: 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Atkinson AP, Vuilleumier P. Supramodal representations of perceived emotions in the human brain. Journal of Neuroscience. 2010;30:10127–10134. doi: 10.1523/JNEUROSCI.2161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, et al. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. American Journal of Psychiatry. 2004;161:662–669. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- Roberts TPL, Edgar JC, Schwartz ES. Magnetoencephalography: Technique, clinical applications and future opportunities. In: Holodny A, editor. Functional neuroimaging: A clinical approach. New York: Informa Healthcare; 2009. [Google Scholar]
- Roberts TPL, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, et al. MEG detection of delayed auditory evoked responses in autism spectrum disorders: Towards an imaging biomarker for autism. Autism Research. 2010;3:8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockstroh B, Wienbruch C, Ray WJ, Elbert TR. Abnormal oscillatory brain dynamics in schizophrenia: A sign of deviant communication in neural network? BMC Psychiatry. 2007;7:44. doi: 10.1186/1471-244X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR. Development of the cerebral cortex: implications for neurodevelopmental disorders. Journal of Child Psychology and Psychiatry. 2010 doi: 10.1111/j.1469-7610.2010.02307.x. Early view, article first published online: 24 aug 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Salmelin R, Hari R. Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalography and Clinical Neurophysiology. 1994;91:237–248. doi: 10.1016/0013-4694(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. The neurobiology of epilepsy. Current Neurology and Neuroscience Reports. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherg M, Ille N, Bornfleth H, Berg P. Advanced tools for digital EEG review: Virtual source montages, whole-head mapping, correlation, and phase analysis. Journal of Clinical Neurophysiology. 2002;19:91–112. doi: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. Journal of Neuroscience. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W. A new look at gamma? High (>60 Hz) γ-band activity in cortical networks: Function, mechanisms, and impairment. Progress in Biophysics and Molecular Biology. 2011;105:14–28. doi: 10.1016/j.pbiomolbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Venables NC, Bernat EM, Sponheim SR. Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophrenia Bulletin. 2009;35:826–839. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Neurophysiological and computational principles of cortical rhythms in cognition. Physiological Reviews. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 3rd. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: Experimental and mathematical observations on network dynamics. International Journal of Psychophysiology. 2000;94:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Willoughby JO, Fitzgibbon SP, Pope KJ, Mackenzie L, Medvedev AV, Clark CR, et al. Persistent abnormality detected in the non-ictal electroencephalogram in primary generalised epilepsy. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:51–55. doi: 10.1136/jnnp.74.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]