SUMMARY
Glioblastomas (GBMs) are highly vascular and lethal brain tumors that display cellular hierarchies containing self-renewing tumorigenic glioma stem cells (GSCs). As GSCs often reside in perivascular niches and may undergo mensenchymal differentiation, we interrogated GSC potential to generate vascular pericytes. Here we show that GSCs give rise to pericytes to support vessel function and tumor growth. In vivo cell lineage tracing with constitutive and lineage specific fluorescent reporters demonstrated that GSCs generate the majority of vascular pericytes. Selective elimination of GSC-derived pericytes disrupts neovasculature and potently inhibits tumor growth. Analysis of human GBM specimens showed that most pericytes are derived from neoplastic cells. GSCs are recruited toward endothelial cells via the SDF-1/CXCR4 axis and induced to become pericytes predominantly by TGF-β. Thus, GSCs contribute to vascular pericytes that may actively remodel perivascular niches. Therapeutic targeting of GSC-derived pericytes may effectively block tumor progression and improve the anti-angiogenic therapy.
INTRODUCTION
Glioblastomas (GBMs) are fatal tumors with florid vascularization that correlates with tumor malignancy and clinical prognosis (Norden et al., 2009). Targeting endothelial cells (ECs) has been a major focus of anti-angiogenic therapeutics, although tumor vessels consist of two distinct but interdependent cellular compartments, ECs and pericytes (Bergers and Song, 2005; Carmeliet and Jain, 2011). However, most current therapies targeting ECs are not curative and may transform tumor growth patterns towards a more invasive phenotype in GBMs (Paez-Ribes et al., 2009), suggesting that targeting ECs alone is not sufficient for effective tumor control. Therefore, further insights into the tumor vascular development and maintenance have direct translational implications.
Vascular pericytes play critical roles in various physiological contexts, including support of vascular structure and function, maintenance of blood-brain barrier, facilitation of vessel maturation, and initiation of vessel sprouting (Armulik et al., 2010; Bell et al., 2010; Bergers and Song, 2005; Winkler et al., 2011). Pericytes and ECs communicate with each other by direct physical contact and reciprocal paracrine signaling to maintain vessel integrity and function (Franco et al., 2012; Carmeliet and Jain, 2011; Song et al., 2005). Altered association between pericytes and ECs has been shown in tumor vessels (Carmeliet and Jain, 2011; Winkler et al., 2011). Tumor vessels with less pericyte coverage appear more vulnerable to radiation and chemotherapy, suggesting that pericytes are critical to protect ECs and may promote therapeutic resistance (Bergers et al., 2003; Franco et al., 2012). When therapies target ECs in tumors, the pericyte network often maintains a functional core of pre-existing blood vessels (Carmeliet and Jain, 2011). The tumor vasculature frequently exhibits structural and functional abnormality with irregular pericytes on endothelial tubules. The pericyte-EC interaction also differs substantially between tumors and normal tissues (Morikawa et al., 2002; Winkler et al., 2011). However, the mechanisms underlying the abnormality and difference are poorly understood. To better understand the vascular development and maintenance in tumors and lay the foundation for improved targeting therapy, it is essential to determine the interplay between cancer cells and vascular compartments.
GBMs display remarkable cellular hierarchies with tumorigenic glioma stem cells (GSCs) at the apex (Bao et al., 2006a; Calabrese et al., 2007; Zhou et al., 2009), although the cancer stem cell (CSC) model remains controversial for some tumor types (Magee et al., 2012). We previously demonstrated that GSCs promote tumor angiogenesis through elevated expression of VEGF (Bao et al., 2006b). This study has been extended by others (Ehtesham et al., 2009; Folkins et al., 2009). GSCs are often located in perivascular niches and interact with ECs in bi-directional manner (Bao et al., 2006b; Calabrese et al., 2007). Within this context, there was an excitement generated by reports suggesting that GSCs may transdifferentiate into ECs (Ricci-Vitiani et al., 2010; Soda et al., 2011; Wang et al., 2010). These reports have been controversial, as the frequency of GSC-EC conversion was not defined, and ECs do not contain cancer genetic alterations in human GBMs (Kulla et al., 2003; Rodriguez et al. 2012). As pericytes are physically proximal to ECs on vessels, distinguishing ECs and pericytes by location alone poses challenge. A complementary or competing hypothesis would be a lineage commitment of GSCs to vascular pericytes. There are important reasons to consider GSCs as potential pericyte progenitors. GSCs have the ability to undergo mesenchymal differentiation (deCarvalho et al., 2010; Ricci-Vitiani et al., 2008). GSCs share properties with neural stem cells (NSCs) that display the potential to transdifferentiate into pericytes (Ii et al., 2009; Morishita et al., 2007). Further, pericytes are similar to mensenchymal stem cells (MSCs) (Crisan et al., 2008). Thus, we interrogated the potential of GSCs to generate vascular pericytes and contribute to the remodeling of perivascular niches, and determined the significance of GSC-derived pericytes in maintaining functional vessels to support GBM tumor growth.
RESULTS
GSCs Are Able to Assume a Pericyte Lineage in Vitro
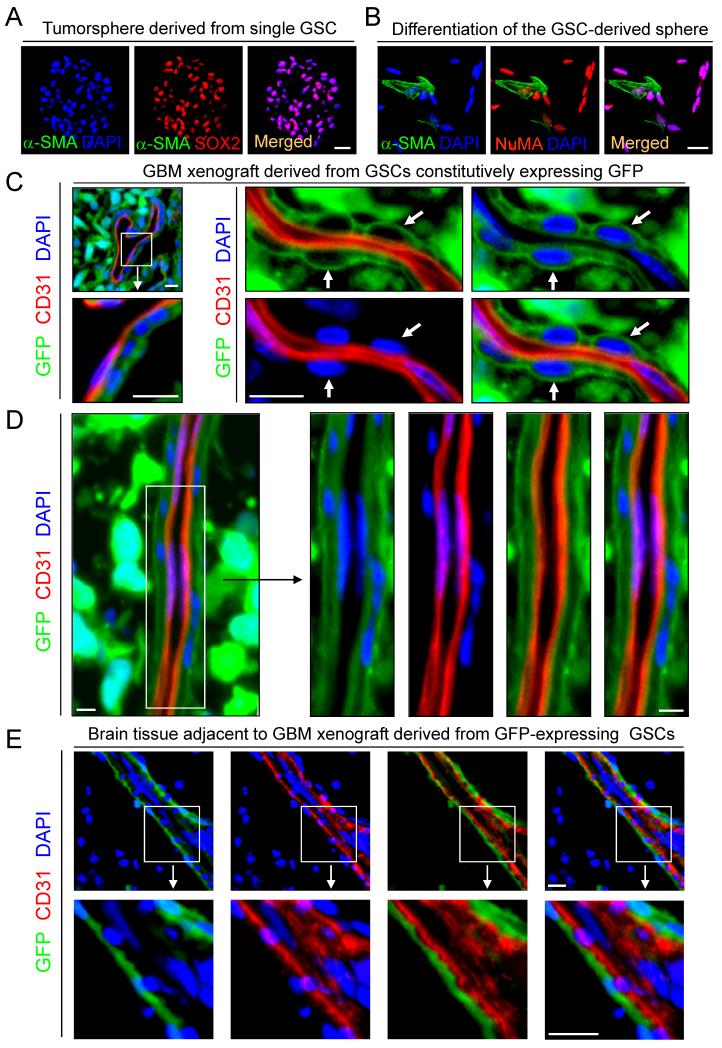
To interrogate a potential lineage link between GSCs and pericytes, we initially examined GSC capacity to differentiate into pericytes in vitro. GSCs were isolated from GBM tumors and validated through functional assays (self-renewal, multipotency and tumor formation) as previously described (Bao et al., 2006a; Guryanova et al., 2011). Immunofluorescent (Imf) staining of freshly sorted GSCs from primary GBMs and the GSC-generated tumorspheres demonstrated SOX2 expression but complete absence of pericyte markers α-SMA and NG2 (Figure S1A and S1B), supporting a lack of contamination of GSC populations by pericytes. After GSCs or tumorspheres were induced for differentiation, the differentiated cells contained a fraction (4-11%) of cells expressing multiple pericyte markers (α-SMA, NG2, CD248 and CD146) (Figure S1C-S1E). To further determine GSC ability to assume a pericyte lineage, we examined the cellular fate of single GSC-derived tumorsphere that did not contain any cell expressing pericyte markers (Figure 1A). Upon differentiation, cells derived from the single GSC-derived tumorsphere contained a fraction of cells expressing pericyte markers (Figure 1B). To rule out potential contamination of host-derived pericyte progenitors in xenograft-derived GSCs, we performed secondary sorting of enriched GSCs with positive selection for the human cell-specific surface antigen TRA-1-85 and negative selection for the pericyte marker CD146. We confirmed that the single GSC-generated spheres derived from the resorted GSCs (SOX2+) did not contain any cell expressing pericyte markers (Figure S1F), while the differentiated cells derived from the single GSC-derived sphere contain pericyte marker-expressing cells (Figure S1G). These pericyte marker-positive cells also expressed the human cell-specific nuclear antigen NuMA (Figure S1G), confirming that these pericytes were derived from human GSCs but not from murine pericytes or their progenitors. Collectively, these data demonstrate that GSCs have the capacity to assume a pericyte lineage in vitro.
Figure 1. GSCs Have the Potential to Assume a Pericyte Lineage.
(A) Immunofluorescent (Imf) staining of SOX2 (a GSC marker) and α-SMA (a pericyte marker) in the single GSC-derived tumorsphere. Nuclei were stained with DAPI.
(B) Imf staining of α-SMA and NuMA (a human cell-specific nuclear antigen) in differentiated cells derived from the single GSC-derived tumorsphere.
(C and D) In vivo lineage tracing of GSCs with GFP constitutive expression. Sections of GBM tumors derived from the GFP-labeled GSCs (D456 or CCF2170) were immunostained for CD31 to mark ECs and counterstained with DAPI. Arrows indicate GFP+ cells with pericytic location.
(E) ImF staining of CD31 in peritumoral brain adjacent to the GBM tumor derived from GFP-labeled GSCs (CCF2170).
All scale bars represent 25 μm. See also Figure S1.
GSCs Give Rise to Vascular Pericytes in GBM Xenografts in Vivo
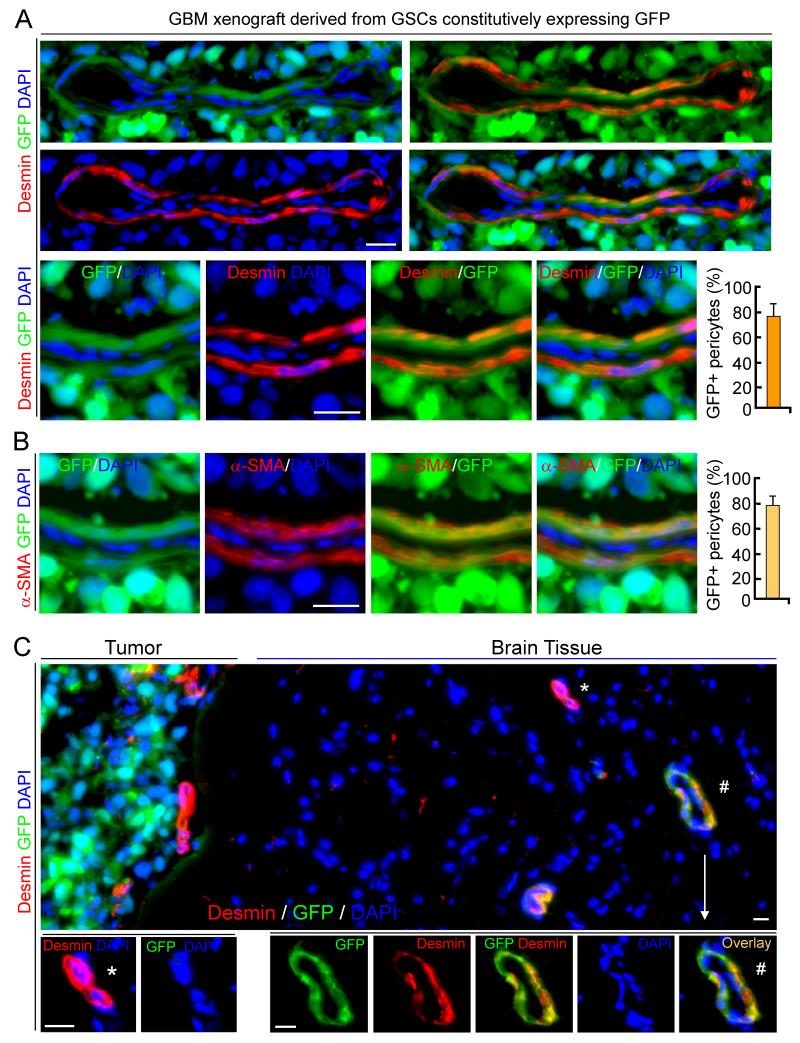
To extend the lineage analysis of GSCs in vivo, we examined the origin of pericytes in GBM xenografts and found that pericytes (CD146+CD248+; 2.63-6.14% of total cells) sorted from the xenografts were largely positive for human NuMA and TRA-1-85 (Figure S1H, S1I, S1L). In contrast, purified ECs (CD31+CD105+) from GBM xenografts were completely negative for human NuMA and TRA-1-85 (Figure S1J-S1L). We then performed a lineage tracing study by transducing GSCs with GFP constitutive expression and implanted the GSCs orthotopically to establish xenografts. Tumor sections of the xenografts derived from the GFP-labeled GSCs was immunostained for an EC marker (CD31) and several pericyte markers (α-SMA, Desmin, NG2, CD146, CD248, Ang1, CD13 and PDGFRβ), as these pericyte markers are expressed in normal brain and primary GBMs (Figure S2A-S2C). No tumor showed GFP-positive ECs, but most tumor vessels were adorned with GFP-positive cells with typical pericytic location and morphology on the vascular external surface (Figure 1C and 1D). Imf analyses of pericyte markers further confirmed expression of pericyte markers (Desmin, α-SMA, NG2, PDGFRβ, CD248 and CD146) overlapped with GFP in the majority (mean 78%, range 57-89%) of pericytes (Figures 2A, 2B, S2D and S2E), indicating that the majority of vascular pericytes were derived from GSCs. We validated this result in 21 GBM xenografts using GFP-labeled GSCs isolated from 12 primary GBMs and 9 GBM xenografts, suggesting that the contribution of GSCs to pericytes is a common event during GBM growth. Notably, a minor fraction (mean 22%, range 11-43%) of vascular pericytes in the GBM xenografts did not overlap with GFP expression, indicating that these pericytes were host-derived. Most tumor vessels had a mixture of GSC- and host-derived pericytes (Figure 2A). Taken together, these data demonstrate that GSCs have the capacity to generate the majority of vascular pericytes in GBM xenografts.
Figure 2. GSCs Generate Pericytes Expressing Specific Markers in Vivo.
(A and B) In vivo lineage tracing of GSCs and Imf staining of pericyte marker Desmin (A) or α-SMA (B) in GBM tumors derived from GFP-labeled GSCs (D456). Quantifications show frations of GSC-derived pericytes (GFP+ and Desmin+/α-SMA+).
(C) Imf staining of Desmin in peritumoral brain adjacent to GBM tumor derived from GFP-labeled GSCs (CCF1468). A vessel containing GSC-derived pericytes (Desmin+ and GFP+) in peritumoral brain was marked (#) and enlarged.
All scale bars represent 20 μm. The error bars represent SD. See also Figure S2.
Peritumoral Brain Vessels Contain GSC-derived Pericytes
As GBMs commonly invade into normal brain, we examined whether GSCs contribute to vascular pericytes in peritumoral brain. We found that a subset of vessels in peritumoral brain adjacent to the GFP-labeled GSC xenograft also contain GFP-positive pericytes (Figure 1E). Imf analyses validated a fraction of vessels coexpressing pericyte markers and GFP and in brain tissue near the GFP tumor (Figure 2C). These data indicate that GSCs can also give rise to pericytes in the peritumoral brain. Notably, GSC-derived pericytes (GFP+) were detectable not only in peritumoral brain but also in tumor-free brain up to 0.86 mm distant from the tumor edge, suggesting that GSCs were recruited by ECs in the peritumoral brain to generate pericytes. Thus, GSCs also generate vascular pericytes in the peritumoral brain.
Validation of GSC-derived Pericytes by Lineage Specific Fluorescent Reporters
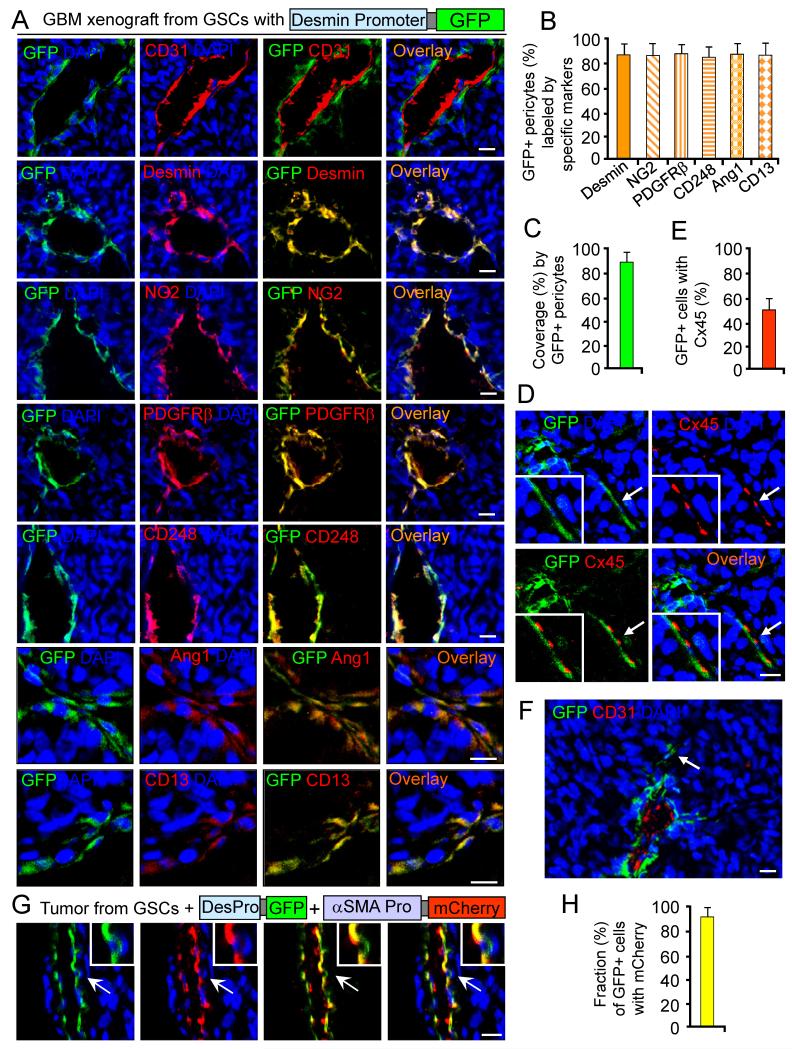
To provide direct evidence validating GSC capacity to generate pericytes in vivo, we performed in vivo cell lineage tracing of GSCs with a pericyte marker (Desmin or α-SMA) promoter-driven expression of GFP or mCherry, which served as fluorescent reporters of pericyte lineage. We cloned the human Desmin promoter (Li and Paulin, 1991) and α-SMA core promoter (Keogh et al., 1999; Nakano et al., 1991), and then generated lentiviral constructs for the Desmin promoter-driven GFP expression (DesPro-GFP) or α-SMA promoter-driven mCherry expression (αSMAPro-mCherry). We confirmed that the cloned Desmin and α-SMA promoters were functional and pericyte specific, as GFP or mCherry expression occurred specifically in human brain vascular pericytes (HBVPs) (Figure S3A, left panels). We then implanted DesPro-GFP-transduced GSCs into mouse brains and examined tumor vessels by Imf analysis. DesPro-driven GFP expression specifically marked perivascular cells that expressed pericyte markers, including Desmin, NG2, PDGFRβ, CD248, Ang1 and CD13 (Figure 3A-3C), validating that GSCs generated vascular pericytes in the GBM xenografts. The GSC-derived pericytes also expressed the gap junction protein Connexin45 (Cx45) that is often localized at pericyte-EC contacts (Figure 3D and 3E). Notably, GFP-positive cells were mainly located in perivascular regions close to vessels but rarely detected in regions distant from vessels in tumors (Figure 3F). We further performed an additional pericyte lineage tracing of GSCs cotransduced with DesPro-GFP and αSMAPro-mCherry, and detected coexpression of mCherry and GFP in perivascular cells (Figure 3G and 3H). GFP+ perivascular cells were abundant around vessels and the majority of pericyte marker-positive cells (>83%) expressed GFP (Figure 3A-3F), confirming that GSCs generated the majority of pericytes in these tumors. Tumor pericytes are often exhibit abnormal morphologies, sometimes extending their processes away from the endothelium (Morikawa et al., 2002). The GSC-derived pericytes often displayed such irregular morphology (Figure 3A and 3F). Recent appreciation of intertumoral heterogeneity of GBMs has informed a mesenchymal subtype in contrast to proneural and classical subtypes (Verhaak et al., 2010). Interestingly, in vivo lineage tracing showed that mesenchymal GSCs have significantly greater ability to generate pericytes than classic and proneural GSCs in xenografts (Figure S3B, S3C and Table S1). Collectively, these data provide direct evidence demonstrating that GSCs have the capacity to generate pericytes in vivo.
Figure 3. In Vivo Lineage Tracing of GSCs with Pericyte Specific Promoter-driven Fluorescent Reporters.
(A-F) In vivo lineage tracing of GSCs with Desmin promoter-driven GFP (DesPro-GFP). Sections of GBM tumors derived from DesPro-GFP-GSCs were immunostained for an EC marker (CD31), the pericyte marker (Desmin, NG2, PDGFRβ, CD248, Ang1 or CD13) (A), or the pericyte-EC junction marker Connexin45 (Cx45) (D). Quantifications show fractions of GFP+ pericytes (B), coverage by GFP+ pericytes (C), or the fraction of GFP+ pericytes expressing Cx45 (E). An arrow indicates rare GFP+ pericytes away from vessels (F).
(G and H) In vivo lineage tracing of GSCs with coexpression of Desmin promoter-driven GFP and α-SMA promoter-driven mCherry in GBMs. Quantification shows the fraction of GFP+ cells with mCherry.
All scale bars represent 25 μm. The error bars represent SD. See also Figures S3 and S4, and Table S1.
As our in vivo cell fate tracing of GSCs with GFP constitutive expression failed to detect GSC-derived ECs (Figure 1C-1D), we performed the cell lineage tracing of GSCs with an EC marker (CD31 or CD105) promoter-driven GFP expression to directly address whether GSCs generate ECs. We cloned the human CD105 (Endoglin) promoter (Rius et al., 1998) and the CD31 (PECAM-1) promoter restricted to ECs (Almendro et al., 1996; Gumina et al., 1997), and then generated lentiviral constructs for conditional GFP expression driven by CD31 or CD105 promoter (CD31Pro-GFP; CD105Pro-GFP). We validated that the cloned CD31 and CD105 promoters were functional and EC specific, as CD31Pro- or CD105Pro-driven GFP expression specifically occurred in ECs (HBMECs) (Figure S3A, right panels). To perform EC lineage tracing of GSCs, GSCs with CD31Pro-GFP or CD105Pro-GFP were orthotopically implanted into mouse brains. In confirmation with our earlier studies, no GFP expression was detectable in tumor ECs marked by CD31 and Glut1 staining (Figure S3D and S3E), further ruling out the possibility of GSC-derived ECs in GBM xenografts.
To further characterize the GSC-derived pericytes (G-pericytes), we examined pericyte marker expression in G-pericytes and HBVP pericytes. We isolated G-pericytes by sorting GFP+CD146+ cells from GBM xenografts derived from the DesPro-GFP-GSCs. Comparative RT-PCR analyses of key pericyte markers (α-SMA, Desmin, CD248, NG2, CD146 and PDGFRβ) in the sorted G-pericytes and HBVPs confirmed similar marker expression in the GBM xenografts (Figure S4A). To address whether G-pericytes still express GSC markers after lineage switching, we examined expression of several putative GSC markers (SOX2, OLIG2, CD133 and Nestin) and pericyte markers in sorted GSCs (CD15+L1CAM+) and G-pericytes (GFP+CD146+). RT-PCR analyses showed that G-pericytes no longer express the GSC makers (Figure S4B). This result was confirmed by Imf staining of SOX2, OLIG2 or Nestin on frozen sections of the DesPro-GFP-GSC xenografts. Consistently, GFP expression was turned on specifically in perivascular cells that rarely (less than 0.8%) expressed SOX2, OLIG2 or Nestin (Figure S4C, S4D and S4F). In contrast, the SOX2, OLIG2, or Nestin-expressing cells (GSCs) are localized near perivascular niches (Figure S4C and S4D). The mutually exclusive expression of GSC and pericyte markers suggests that GSCs undergo differentiation to generate G-pericytes rather than being a GSC subpopulation adjacent to ECs in GBM tumors. In addition, G-pericytes do not express astrocyte markers such as GFAP and S100β (Figure S4A, S4E and S4F), indicating that G-pericytes are not a subpopulation of astrocytes. Consistently, pericytes and astrocytes are distinct cell populations without overlapping expression of specific markers in primary GBMs (Figure S4G). These data demonstrate that GSC-derived pericytes are unique cells expressing specific pericyte makers.
Pericytes in Primary GBMs Are Commonly Derived from Neoplastic Cells
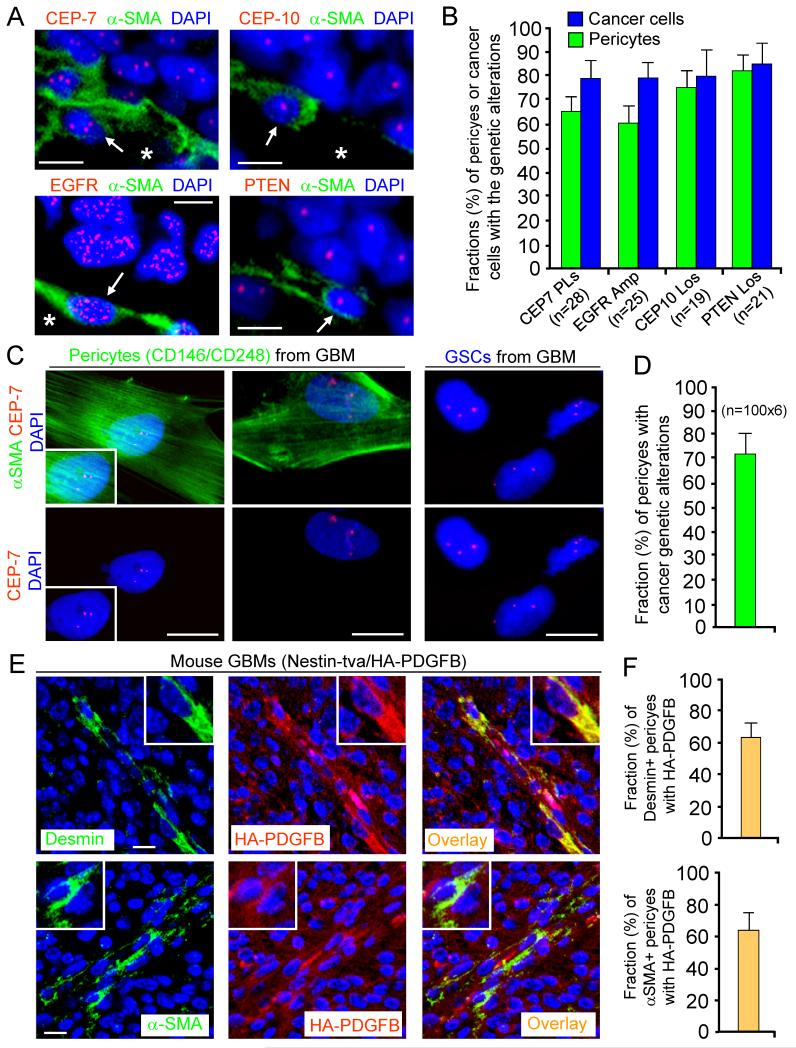
To examine whether pericytes are lineage related to cancer cells in human primary GBMs, we performed fluorescence in situ hybridization (FISH) analyses of common GBM genetic changes (Cancer Genome Atlas Research Network, 2008) in combination with Imf staining of a pericyte marker (α-SMA) to determine if pericytes carry cancer genetic alterations in GBMs. As gains of chromosome 7 (EGFR amplification) or losses of chromosome 10 (PTEN loss) are frequent in GBM cells permitting a lineage tracing to the neoplastic cells, we employed DNA probes for centromeres of chromosome 7 (CEP-7) and 10 (CEP-10), EGFR, and PTEN to detect cancer genetic alterations in pericytes, ECs and tumor cells in GBM tissue microarrays. FISH analyses showed the majority of tumor pericytes (mean 76%, range 58% to 83%) carried the same genetic alterations (CEP-7 polysomy, EGFR trisomy or amplification, CEP-10 loss or PTEN loss) as cancer cells in 49 GBMs (Figure 4A and 4B), indicating that tumor pericytes are commonly derived from cancer cells. In contrast, we rarely detected relevant genetic changes in ECs in these GBMs (Figure S5A and S5B). To further confirm these results, we isolated pericytes (CD146+CD248+; 2.18-5.26% of total cells) and ECs (CD31+CD105+) from primary GBMs and performed similar FISH analyses. The majority (>72%) of sorted tumor pericytes (α-SMA+) carried the same genetic alteration (CEP-7 polysomy) as matched GSCs (Figure 4C and 4D). In contrast, sorted ECs (CD31+CD105+) expressed Glut1 but did not share the GSC genetic alterations (Figure S5C and S5D). These results support a tumor source for pericytes but not for ECs in human primary GBMs.
Figure 4. Pericytes Are Commonly Derived from Neoplastic Cells in Primary GBMs.
(A and B) FISH analyses of genetic alterations with the CEP-7, CEP-10, EGFR or PTEN probe (in red) in pericytes (α-SMA+) in primary GBMs. Quantification shows average fractions of pericytes carrying the cancer genetic alterations (CEP-7 polysomy; EGFR amplification or trisomy; CEP-10 loss; or PTEN loss) in GBM tissue arrays (B).
(C and D) FISH analyses with CEP-7 probe in sorted pericytes (α-SMA+) and GSCs from primary GBMs. Quantification shows the fraction (mean 72%) of pericytes carrying the GSC genetic alterations (D).
(E and F) Imf staining of a pericyte marker (Desmin or α-SMA) and the HA-tagged PDGFB in the genetically engineered mouse GBMs (Nestin-tva/Ink4a/Arf−/−/HA-PDGFB model). Quantifications show fractions (mean 63%) of HA-PDGFB+ pericytes (F)
The scale bars represent 10 μm (A and C) and 25 μm (E). The error bars represent SD. See also Figure S5.
To further address if pericytes in endogenous GBMs are derived from cancer cells, we examined pericytes in the genetically engineered mouse GBMs (Nestin-tva/Ink4a/Arf−/−/HA-PDGFB models; Hambardzumyan et al., 2009). Imf staining of HA-tagged PDGFB (HA-PDGFB) and pericyte markers (Desmin, NG2, CD248 or α-SMA) showed that a significant fraction (mean 63%) of tumor pericytes expressed HA-PDGFB supporting a tumor origin (Figure 4E, 4F and data not shown). In contrast, staining of EC markers (CD31 or Glut1) and HA-PDGFB showed no tumor cell-derived ECs in these mouse GBMs (Figure S5E). These data demonstrate that pericytes in the genetically engineered mouse GBMs are also largely derived from neoplastic cells.
Selective Elimination of GSC-derived Pericytes Disrupts Tumor Vessels and Inhibits Tumor Growth
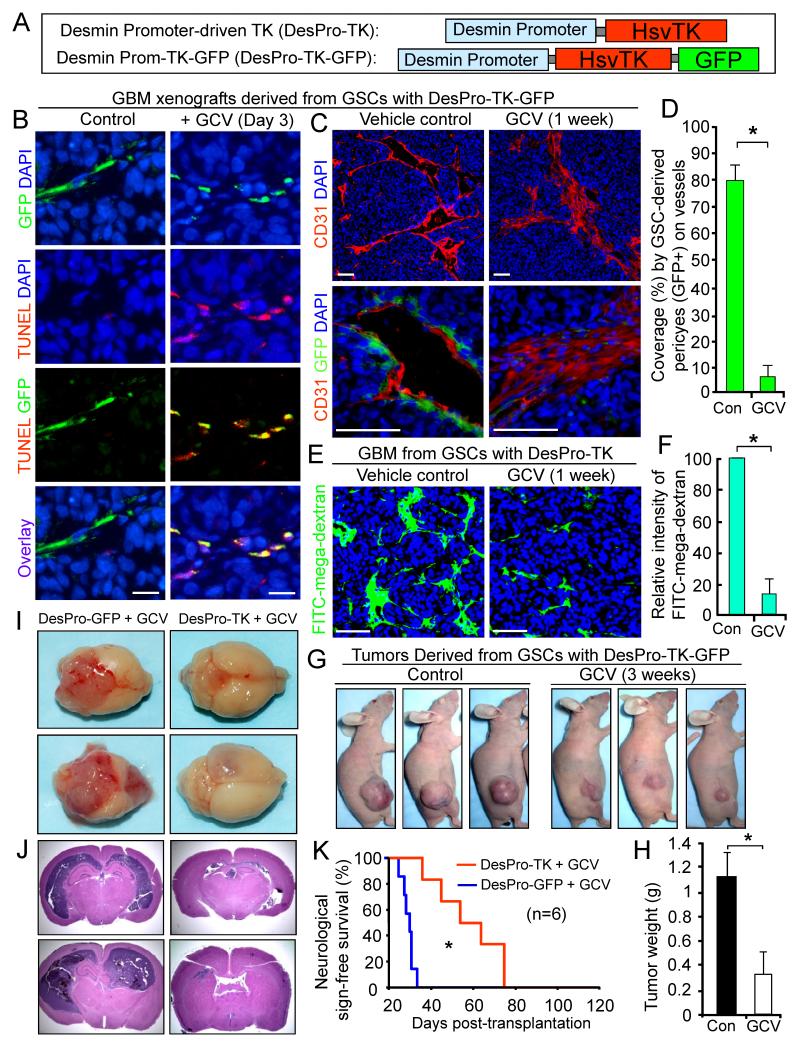
To determine the functional significance of G-pericytes, we examined effects of selective elimination of G-pericytes on vessels and tumor growth. GSCs were transduced with Desmin promoter-driven expression of herpes simplex virus thymidine kinase (HsvTK) (Figure 5A) to achieve conditional HsvTK expression in G-pericytes. As HsvTK metabolizes ganciclovir (GCV) into a toxic agent specifically in cells expressing HsvTK (Culver et al., 1992), G-pericytes expressing HsvTK should be sensitive to GCV and thus eliminated by GCV treatment. To confirm selective killing of G-pericytes expressing Desmin promoter-driven HsvTK by GCV treatment, we generated a construct for coexpression of HsvTK and GFP under the same promoter (DesPro-TK-GFP) (Figures 5A and S6A). As expected, after the DesPro-TK-GFP-transduced GSCs were induced to differentiate, GFP was expressed in a fraction of differentiated cells (G-pericytes) (Figure S6B). Apoptotic detection showed that GCV treatment selectively induced apoptosis in cells coexpressing GFP and HsvTK (Figure S6C). These data indicate that selective elimination of G-pericytes is achievable by using the Desmin promoter-driven HsvTK conditional expression with GCV treatment.
Figure 5. Selective Elimination of GSC-derived Pericytes Disrupts Tumor Vessels and Inhibits Tumor Growth.
(A) Schematic illustrations of Desmin promoter-driven expression of herpes simplex virus thymidine kinase (HsvTK) and coexpression of HsvTK and GFP.
(B) TUNEL assay detecting selective apoptosis (in red) of GSC-derived pericytes (G-pericytes, GFP+) induced by ganciclovir (GCV) in GBM tumors derived from DesPro-TK-GFP-GSCs (CCF2170).
(C and D) Imf staining of CD31 (in red) shows effects of selective elimination of G-pericytes (GFP+) by GCV on vessels in GBM tumors derived from DesPro-TK-GFP-GSCs. Quantification shows the reduced G-pericyte coverage by GCV treatment (D). *, p<0.001.
(E and F) Assessment of vascular function using the FITC-conjugated mega-dextran after selective elimination of G-pericytes in GBM tumors derived from DesPro-TK-GSCs. Quantification shows intensity of perfused FITC-mega-dextran into the control or GCV-treated tumors (F). *, p<0.001.
(G and H) The effect of targeting G-pericytes by GCV treatment on growth of subcutaneous tumors derived from DesPro-TK-GFP-GSCs. Quantification shows mean tumor weights in the control and GCV-treated mice. *, p < 0.001 (n=12).
(I and J) The effect of selective elimination of G-pericytes on GBM growth in mouse brains. Mice bearing tumors derived from DesPro-TK-GSCs or DesPro-GFP-GSCs (control) were treated with GCV for 3 weeks. Images of whole brains (I) and histological analysis (H&E staining) on brain sections (J) are shown.
(K) Kaplan-Meier survival curves of mice bearing GBM tumors derived from DesPro-TK-GSCs or DesPro-GFP-GSCs (control) after GCV treatment. *, p < 0.001 (n=6).
The scale bars represent 25 μm (B) and 100 μm (C and E). The error bars represent SD. See also Figure S6.
To examine the impact of selective targeting of G-pericytes on tumor vessels, we implanted the DesPro-TK-GFP-GSCs into mouse brains. Mice bearing the tumors were treated with vehicle control or GCV daily to induce the HsvTK-mediated toxicity to G-pericytes. Apoptotic detection by TUNEL staining demonstrated that GCV treatment for three days selectively induced cell death in G-pericytes (GFP+) in vivo (Figure 5B). Further, GCV treatment for one week caused almost a complete depletion of G-pericytes, collapse of vessel lumens, and disruption of endothelial walls in the GBM tumors (Figures 5C, 5D, S6D and S6E). Moreover, measurement of vascular function by FITC-conjugated mega-dextran showed that GCV treatment for one week to deplete G-pericytes severely attenuated vascular function in the DesPro-TK-GSC xenografts, as perfusion of FITC-mega-dextran into the tumors was dramatically reduced (Figures 5E, 5F, S6F and S6G). Collectively, these data demonstrate that selective elimination of G-pericytes potently disrupts vascular structure and function in GBM tumors.
To evaluate the impact of selective targeting of G-pericytes on tumor growth, we initially used subcutaneous tumor experiments to track sequential tumor volumes. The established subcutaneous tumors derived from the DesPro-TK-GFP-GSCs were treated with GCV or vehicle control for 3 weeks. GCV treatment caused significant regression of the tumors (Figure 5G and 5H), indicating that selective elimination of G-pericytes by HsvTK-induced GCV toxicity inhibited tumor growth. To further validate this result in orthotopic tumors, we transduced GSCs either with DesPro-GFP (control) or DesPro-TK, and implanted these GSCs into mouse brains to establish GBM xenografts. Both groups of mice bearing the tumors were administered with GCV to eliminate the G-pericytes expressing HsvTK. GCV treatment for two weeks caused extensive vessel regression in the GBM tumors derived from DesPro-TK-GSCs but not from DesPro-GFP-GSCs (Figure S6H and S6I). Moreover, GCV treatment for three weeks markedly inhibited intracranial tumor growth in GBM xenografts derived from DesPro-TK-GSCs but not in control tumors from DesPro-GFP-GSCs (Figure 5I and 5J). Alternatively, treatment by GCV but not vehicle control suppressed intracranial tumor growth in the GBM xenografts derived from DesPro-TK-GSCs (Figure S6J). As a consequence, GCV treatment significantly increased survival of animals implanted with the DesPro-TK-GSCs (Figure 5K). These data demonstrate that selective elimination of G-pericytes suppressed GBM tumor growth and malignant progression.
GSCs Are Recruited toward ECs via the SDF-1/CXCR4 Axis
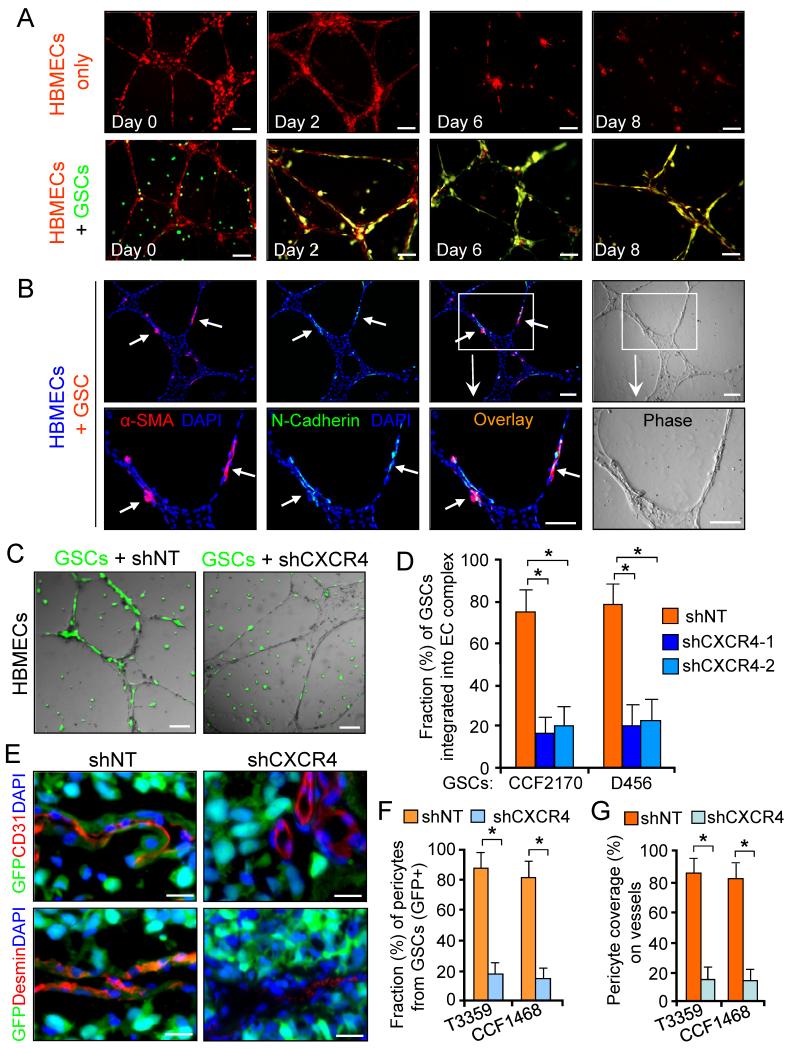
To understand the mechanisms underlying GSC recruitment toward ECs, we examined whether GSCs can be recruited by HBMECs to support the maintenance of EC complexes in vitro. GSCs labeled with the green fluorescent tracer CFSE were mixed with HBMEC complexes labeled with the red fluorescent tracer CMTRX. Integration of GSCs-derived cells into EC complexes was detected on day two after cell mixing, and the integration stabilized the EC complexes for extended periods (2.6 fold) relative to EC complex alone (Figure 6A). To address whether pericyte lineage specification of GSCs can be induced by EC complexes, we cocultured GSCs with HBMECs and detected integration of G-pericytes by α-SMA staining (Figure 6B). As the attachment of pericytes to ECs can be mediated through adherens junctions containing N-Cadherin (Gerhardt et al., 2000), we examined N-Cadherin expression and found that N-Cadherin was localized to the contact sites between G-pericytes (α-SMA+) and EC complexes (Figure 6B).
Figure 6. GSCs Are Recruited toward ECs through the SDF-1/CXCR4 Axis to Support Endothelial Complex.
(A) In vitro endothelial complex formation of HBMECs (labeled with red fluorescent tracer CMTRX) with or without GSCs (labeled with CFSE, in green).
(B) Imf staining of α-SMA and N-Cadherin in complexes of HBMECs and GSC-derived cells. Nuclei were stained with DAPI.
(C and D) Endothelial complexes of HBMECs with GFP-labeled GSCs expressing shCXCR4) or shNT. Quantification shows fractions of GSC-derived cells (GFP+) on HBMEC complexes (D). *, p < 0.001.
(E-G) In vivo lineage tracing of GSCs with GFP constitutive expression and Imf staining of CD31 or Desmin in tumors derived from GSCs expressing shCXCR4 or shNT. Quantifications show fractions of GSC-derived pericytes (GFP+) (F) and total pericyte coverage (G) on vessels. *, p < 0.001.
The scale bars represent 100 μm (A-C) and 25 μm (E). The error bars represent SD. See also Figure S7.
To define the molecular mechanisms underlying GSC recruitment by ECs, we analyzed the effect of several chemotactic factors (SDF-1α, PDGFB and TGF-β) secreted by HBMECs on GSC migration. We found that SDF-1 potently stimulated GSC migration (Figure S7A and S7B), while PDGFB only modestly attracted GSCs. To further address if HBMECs attract GSCs via SDF-1, we cocultured GSCs and HBMECs in separate chambers of transwells and detected that HBMECs potently attracted GSCs, an effect dependent on SDF-1 as an anti-SDF-1 antibody attenuated the effects (Figure S7C and S7D). As ECs in brain and GBMs constitutively express SDF-1 (Kokovay et al., 2010; Komatani et al., 2009), we confirmed that abundant SDF-1 formed a gradient around vessels with greater SDF-1 proximal to vessels in brain and GBMs (Figure S7E).
As SDF-1 is secreted by ECs and GSCs express the SDF-1 receptor, CXCR4 (Ehtesham et al., 2009; Folkins et al., 2009), we hypothesized that brain ECs may recruit GSCs at least in part through the SDF-1/CXCR4 axis. CXCR4 knockdown in GSCs reduced the recruitment of GFP-labeled GSCs to EC complexes (Figure 6C and 6D). In addition, a SDF-1 blocking antibody significantly reduced the integration of GFP-labeled GSCs into HBMEC complexes (Figure S7F and S7G). As a further confirmation, we examined the effect of a CXCR4 inhibitor (AMD3100) on GSC recruitment to EC complexes. AMD3100 treatment significantly reduced the integration of GSCs (CMTRX-labeled, in red) into CFSE-labeled HBMECs (in green) (Figure S7H and S7I).
To further determine whether GSC recruitment to ECs depends on the SDF-1/CXCR4 axis during tumor vascularization, GFP-labeled GSCs were transduced with shCXCR4 or non-targeting shRNA (shNT) and implanted into mouse brains. In shNT xenografts, tumor vessels were covered with abundant G-pericytes (GFP+ and Desmin+), while G-pericytes and total pericyte coverage on vessels was significantly reduced in shCXCR4 xenografts (Figure 6E-6G). IHC staining confirmed that CXCR4 knockdown significantly decreased vessel density in the tumors (Figure S7J and S7K). Collectively, these data suggest ECs recruit GSCs via the SDF-1/CXCR4 axis and targeting this pathway reduces G-pericytes in GBMs.
TGF-β Induces Differentiation of GSCs into Pericytes
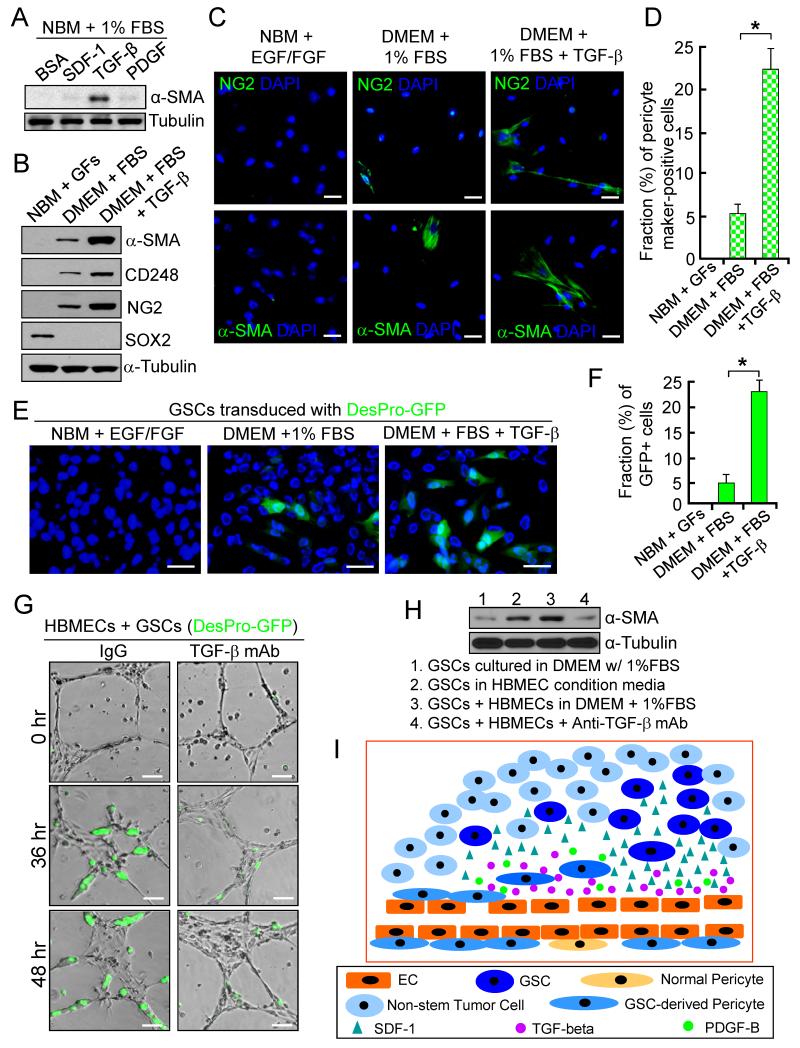
We next sought to understand the molecular mechanisms underlying the pericyte lineage specification of GSCs. To identify the potential factors inducing GSC differentiation into pericytes, we examined the effect of several EC-secreted cytokines (SDF-1, PDGFB, and TGF-β) on GSC differentiation into pericytes. Immunoblot analysis showed that TGF-β dominantly induced expression of α-SMA when GSCs were cultured in differentiation media (Figure 7A and 7B). Imf staining of multiple pericyte markers (NG2, α-SMA, CD146 and CD248) confirmed that TGF-β treatment increased the fraction of cells expressing pericyte markers in the differentiated cells (Figure 7C, 7D and data not shown). Further, TGF-β treatment induced GFP-expressing cells in differentiated cells derived from DesPro-GFP-GSCs (Figure 7E and 7F). To address whether ECs induce GSC differentiation into pericytes through TGF-β, we cocultured DesPro-GFP-GSCs and HBMEC complexes and monitored GFP-expressing cells (G-pericytes) over time. GFP+ cells were induced and integrated into EC complexes, an effect was attenuated by incubation of the EC complexes with an anti-TGF-β antibody (Figure 7G). Immunoblot analysis validated that coculture of GSCs with HBMECs or their conditioned media induced expression of pericyte marker α-SMA in differentiated cells, an effect was reduced by a TGF-β neutralizing antibody (Figure 7H). Collectively, these data demonstrate that HBMECs induce pericyte lineage specification of GSCs at least in part through TGF-β. Thus, the recruitment of GSCs toward ECs via the SDF-1/CXCR4 axis and the induction of GSC differentiation into pericytes by TGF-β are two events controlled by different molecular mechanisms (Figure 7I).
Figure 7. TGF-β Induces Differentiation of GSCs into Pericytes.
(A) Immunoblot (IB) analysis of pericyte marker (α-SMA) expression in differentiated cells from GSCs (CCF1992) in the presence of indicated cytokines (1 ng/ml) in culture media.
(B) IB analysis of pericyte markers (α-SMA, CD248 and NG2) and a GSC marker (SOX2) in GSCs and differentiated cells with or without treatment of TGF-β (2 ng/ml).
(C and D) Imf staining of pericyte markers (NG2 and α-SMA) in GSCs (CW1217) and differentiated cells induced by serum or TGF-β. Quantification shows pericyte fractions. *, p<0.001.
(E and F) In vitro pericyte lineage tracing of GSCs with Desmin promoter-driven GFP induced by serum or TGF-β (2 ng/ml). Quantification shows frations of GFP+ cells in the differentiated cells (F). *, p<0.001.
(G) In vitro HBMEC complex formation with DesPro-GFP-GSCs in the presence of anti-TGF-β antibody (mAb) or IgG.
(H) IB analysis of α-SMA expression after coculture of GSCs with HBMECs or their conditioned media in the presence of anti-TGF-β antibody or IgG.
(I) A schematic illustration showing the recruitment of GSCs toward ECs and the differentiation of GSCs into pericytes in GBMs. GSCs expressing CXCR4 are recruited toward ECs by SDF-1, and induced predominantly by TGF-β to become pericytes to support vessel function and tumor growth.
The scale bars represent 25 μm (C and E) and 100 μm (G). The error bars represent SD.
DISCUSSION
Pericytes play essential roles to maintain functional vessels to support tumor growth. Tumor pericytes are thought to be derived from their progenitors from the surrounding normal tissue or from the bone marrow-derived cells homing in tumors after treatments (De Palma et al., 2005; Du et al., 2008). In this study, we demonstrate that the majority of vascular pericytes in GBMs are derived from GSCs. As GSC-derived pericytes (G-pericytes) express similar pericyte markers as normal brain vascular pericytes, GSCs function as pericyte progenitors and contribute to vasculature formation in GBMs. The ability of GSCs to generate vascular pericytes in vivo suggests that GSCs may actively remodel their microenvironment and create a supportive niche, permitting functional vessels to augment tumor growth without depending on the limited source of normal pericyte progenitors from surrounding tissues.
As NSCs can transdifferentiate into pericytes (Ii et al., 2009; Morishita et al., 2007), a lineage link between NSCs and pericytes is present in normal tissues. Since GSCs share regulatory programs with NSCs, the plasticity of GSCs toward a pericyte lineage may be a product of aberrant developmental biology. Although previous reports suggest that GSCs may give rise to ECs in GBMs (Ricci-Vitiani et al., 2010; Soda et al., 2011; Wang et al., 2010), such event may be very rare as ECs in GBMs rarely carry the cancer genetic mutations as demonstrated in our study and others (Kulla et al., 2003; Rodriguez et al., 2012). Moreover, our complementary lineage tracing studies failed to demonstrate GSC-derived ECs in vivo, although in the culture condition we occasionally observed rare EC marker-expressing cells (<0.6%) in differentiated cells from GSCs. As vascular pericytes closely attach to ECs and both cells appear very thin, prior studies may have missed the true identity of tumor-derived cells on vessels. Since both ECs and pericytes express Tie2 (De Palma et al., 2005), the use of Tie2 promoter-driven HsvTK expression for targeting “GSC-derived ECs” (Ricci-Vitiani et al., 2010) might actually eliminate the GSC-derived pericytes. Our in vivo lineage tracing with pericyte- or EC-specific promoter-driven fluorescent reporters directly demonstrated that GSCs give rise to pericytes rather than ECs in vivo.
The contribution of GSCs to vascular pericytes requires GSC recruitment toward ECs. As ECs in brain and GBMs express abundant SDF-1 forming chemoattractant gradient, the expression of CXCR4 (the receptor for SDF-1) in GSCs (Ehtesham et al., 2009; Folkins et al., 2009) may provide a paracrine loop for recruitment of GSCs toward ECs. A recent study showed that NSCs can be recruited to perivascular niches in normal brain through the CXCR4/SDF-1 axis (Kokovay et al., 2010). The recruitment of pericyte progenitors to ECs in normal tissues also depends on the SDF-1/CXCR4 signaling (Song et al., 2009). SDF-1 expression has been proposed as one of mechanisms underlying the resistance to the anti-angiogenic therapy in GBM trials (Batchelor et al., 2007). Elevated SDF-1 signaling may enhance GSC recruitment toward ECs and increase G-pericyte coverage to protect tumor vessels, leading to resistance to the anti-angiogenic therapy.
The potent capacity of GSCs to generate vascular pericytes allows active vascularization in GBMs to support tumor growth. Since GSCs contribute to the majority of vascular pericytes in GBMs, G-pericytes may have a crucial role in mediating therapeutic resistance in GBMs. As pericytes juxtacrine to ECs express significant levels of VEGF and other factors to support EC survival (Franco et al., 2012; Song et al., 2005; Winkler et al., 2011), G-pericytes may protect ECs and render ECs less responsive to anti-angiogenic agents in GBMs. Thus, targeting G-pericytes may synergize with current therapies targeting ECs to achieve more effective outcome. As cancer stem cells (CSCs) are present in other solid cancers (Magee et al., 2012), it is important to determine whether CSCs can generate vascular pericytes in other malignant tumors with florid angiogenesis. Our studies demonstrate that GSCs not only interact with perivascular niches but also have the capacity to remodulate their microenvironment by contributing pericyte compartments of the neovasculature. As selective elimination of G-pericytes potently disrupted vessels and inhibited tumor growth, therapeutic targeting of GSC-derived pericytes may have a significant impact on improving GBM treatment efficacy.
EXPERIMENTAL PROCEDURES
Isolation of GSCs and Non-stem Tumor Cells from GBMs
GBM surgical specimens were collected in accordance with a Cleveland Clinic Institutional Review Board-approved protocol. GSCs and non-stem tumor cells were derived from GBM tumors and functionally validated as described (Bao et al., 2006a; Guayronva et al., 2011). For the detailed procedure, please see the supplemental methods.
Pericyte or EC-specific Promoter-driven Expression of GFP or mCherry
Human Desmin promoter (312 bp) with an enhancer (284 bp) (Li and Paulin, 1991), α-SMA promoter (262 bp) with an enhancer (123 bp) (Keogh et al., 1999; Nakano et al., 1991), CD105 promoter plus enhancer (955 bp) (Rius et al., 1998), and CD31 promoter plus enhancer (887bp) restricted to ECs (Almendro et al., 1996; Gumina et al., 1997) were cloned by PCR and confirmed by sequencing. The specific promoter with enhancer was inserted into pCDH-CMV-EF1-Puro lentiviral vector (System Biosciences) to replace the original CMV promoter. Then the ORF of GFP or mCherry was inserted into the vector to generate lentiviral constructs. Lentiviruses were produced and tittered as described (Guryanova et al., 2011).
Cell Lineage Tracing of GSCs
To perform cell lineage tracing, GSCs were transduced with GFP or mCherry constitutive expression or conditional expression driven by the pericyte or EC-specific promoter through lentiviral infection, and then transplanted into brains of athymic BALB/c nu/nu mice to establish xenografts as described (Guryanova et al., 2011). To trace cell lineage of GSCs in vivo, sections of mouse brains bearing the xenografts were immunostained for pericyte or EC markers and analyzed for GFP or mCherry expression. Immunofluorescent (Imf) and Immunohistochemical (IHC) stainings were performed as described (Guryanova et al., 2011). Tumor sections of the genetically engineered mouse GBMs were provided by Dr. Dolores Hambardzumyan. For detailed methods and the antibody information, please see the supplemental methods.
Selective Targeting of GSC-derived Pericytes in GBM Xenografts
GSCs were transduced with Desmin or CD31 promoter-driven expression of HsvTK (herpes simplex virus 1 thymidine kinase), GFP or HsvTK plus GFP through lentiviral infection, and then transplanted into brains of athymic mice. Mice bearing the xenografts received ganciclovir (GCV, Sigma-Aldrich) at 75 mg/kg/day or vehicle control daily through intraperitoneal injection. The xenografts were collected for Imf and IHC staining and fluorescent analysis. To evaluate the targeting effect on animal survival, mice were maintained until the development of neurological signs.
HBVPs, HBMECs, and EC Complex Formation
Human brain vascular pericytes (HBVPs) and human brain microvessel endothelial cells (HBMECs) were obtained from ScienCell. HBMECs with low passage were used for coculture and endothelial complex formation assays as described (Bao et al., 2006b). For the detailed procedure and the labeling of GSCs and HBMECs, please see the supplemental methods.
Statistical Analysis
All quantified data were statistically analyzed. Grouped data are presented as mean ± standard deviation (SD). The difference between experimental groups was assessed by one-way ANOVA or one-way ANOVA on Ranks testing. For the animal survival experiments, log-rank survival analysis was performed.
(Please find other experimental procedures in the Supplemental Information).
Supplementary Material
Highlights.
GBM stem cells (GSCs) generate vascular pericytes to maintain tumor vessels
Targeting GSC-derived pericytes disrupts vessel function and inhibits GBM growth
GSCs are recruited to the perivascular niche via SDF-1-CXCR4 signaling
TGF-β predominantly promotes GSCs to assume a pericyte lineage
ACKNOWLEDGEMENTS
We thank Brain Tumor and Neuro-Oncology Centers at Cleveland Clinic, University Hospitals, and Duke Medical Center for providing GBM specimens. We also thank Dr. Dolores Hambardzumyan for providing the genetically engineered mouse GBMs. We are grateful to Cathy Shemo at the Flow Cytometry Core and Judith Drazba at the Imaging Core for their help. This work was supported by the Cleveland Clinic Foundation and a NIH R01 grant (NS070315) to S. Bao.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest.
REFERENCES
- Almendro N, Bellón T, Rius C, Lastres P, Langa C, Corbí A, Bernabéu C. Cloning of the human platelet endothelial cell adhesion molecule-1 promoter and its tissue-specific expression. Structural and functional characterization. J Immunol. 1996;157:5411–5421. [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006a;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006b;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- deCarvalho AC, Nelson K, Lemke N, Lehman NL, Arbab AS, Kalkanis S, Mikkelsen T. Gliosarcoma stem cells undergo glial and mesenchymal differentiation in vivo. Stem Cells. 2010;28:181–190. doi: 10.1002/stem.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtesham M, Mapara KY, Stevenson CB, Thompson RC. CXCR4 mediates the proliferation of glioblastoma progenitor cells. Cancer Lett. 2009;274:305–312. doi: 10.1016/j.canlet.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev. Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, Kirschbaum NE, Piotrowski K, Newman PJ. Characterization of the human platelet/endothelial cell adhesion molecule-1 promoter: identification of a GATA-2 binding element required for optimal transcriptional activity. Blood. 1997;89:1260–1269. [PubMed] [Google Scholar]
- Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. Modeling adult gliomas using RCAS/t-va technology. Transl. Oncol. 2009;2:89–95. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Nishimura H, Sekiguchi H, Kamei N, Yokoyama A, Horii M, Asahara T. Concurrent vasculogenesis and neurogenesis from adult neural stem cells. Circ Res. 2009;105:860–868. doi: 10.1161/CIRCRESAHA.109.199299. [DOI] [PubMed] [Google Scholar]
- Keogh MC, Chen D, Schmitt JF, Dennehy U, Kakkar VV, Lemoine NR. Design of a muscle cell-specific expression vector utilising human vascular smooth muscle alpha-actin regulatory elements. Gene Ther. 1999;6:616–628. doi: 10.1038/sj.gt.3300866. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatani H, Sugita Y, Arakawa F, Ohshima K, Shigemori M. Expression of CXCL12 on pseudopalisading cells and proliferating microvessels in glioblastomas: an accelerated growth factor in glioblastomas. Int. J. Oncol. 2009;34:665–672. doi: 10.3892/ijo_00000192. [DOI] [PubMed] [Google Scholar]
- Kulla A, Burkhardt K, Meyer-Puttlitz B, Teesalu T, Asser T, Wiestler OD, Becker AJ. Analysis of the TP53 gene in laser-microdissected glioblastoma vasculature. Acta Neuropathol. 2003;105:328–332. doi: 10.1007/s00401-003-0681-6. [DOI] [PubMed] [Google Scholar]
- Li ZL, Paulin D. High level desmin expression depends on a muscle-specific enhancer. J. Biol. Chem. 1991;266:6562–6570. [PubMed] [Google Scholar]
- Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita R, Nagata K, Ito H, Ueda H, Asano M, Shinohara H, Kato K, Asano T. Expression of smooth muscle cell-specific proteins in neural progenitor cells induced by agonists of G protein-coupled receptors and transforming growth factor-beta. J. Neurochem. 2007;101:1031–1040. doi: 10.1111/j.1471-4159.2006.04405.x. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Nishihara T, Sasayama S, Miwa T, Kamada S, Kakunaga T. Transcriptional regulatory elements in the 5′ upstream and first intron regions of the human smooth muscle (aortic type) alpha-actin-encoding gene. Gene. 1991;99:285–289. doi: 10.1016/0378-1119(91)90140-7. [DOI] [PubMed] [Google Scholar]
- Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat. Rev. Neurol. 2009;5:610–620. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Pallini R, Larocca LM, Lombardi DG, Signore M, Pierconti F, Petrucci G, Montano N, Maira G, De Maria R. Mesenchymal differentiation of glioblastoma stem cells. Cell Death Differ. 2008;15:1491–1498. doi: 10.1038/cdd.2008.72. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- Ríus C, Smith JD, Almendro N, Langa C, Botella LM, Marchuk DA, Vary CP, Bernabéu C. Cloning of the promoter region of human endoglin, the target gene for hereditary hemorrhagic telangiectasia type 1. Blood. 1998;92:4677–4690. [PubMed] [Google Scholar]
- Rodriguez FJ, Orr BA, Ligon KL, Eberhart CG. Neoplastic cells are a rare component in human glioblastoma microvasculature. Oncotarget. 2012;3:98–106. doi: 10.18632/oncotarget.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari S, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat. Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, Fu Y, Luo Y. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res. 2009;69:6057–6064. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumor-initiating cells: challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.