Abstract
The world's increasingly voracious appetite for fossil fuels is driven by fast-growing populations and ever-rising aspirations for the lifestyles and standard of living exemplified in the developed world. Forecasts for higher electricity consumption, more comfortable living environments (via heating or cooling) and greater demand for transport fuels are well known. Similar growth in demand is projected for petrochemical-based products in the form of man-made fibres for clothing, ubiquitous plastic artefacts, cosmetics, etc. All drawing upon the same finite oil, gas and coal feedstocks. Biomass can, in principle, substitute for all of these feedstocks. Although ultimately finite, biomass resources can be expanded and renewed if this is a societal priority. This paper examines the projected growth of an energy-intensive international petrochemicals industry, considers its demand for both utilities and feedstocks, and considers the extent to which biomass can substitute for fossil fuels. The scope of this study includes biomass component extraction, direct chemical conversion, thermochemical conversion and biochemical conversion. Noting that the petrochemicals industry consumes around 10 per cent of the world's fossil fuels as feedstocks and almost as much again in utilities, various strategies for addressing future demand are considered. The need for long-term infrastructure and logistics planning is highlighted.
Keywords: biomass, petrochemical, infrastructure, resources, syngas
1. Introduction
Biomass offers a source of carbon from the biosphere as an alternative to fossilized carbon laid down tens of millions of years ago. Anything that grows and is available in non-fossilized form can be classified as biomass, including arable crops, trees, bushes, animal by-products, human and animal waste, waste food and any other waste stream that rots quickly—and it can be replenished on a rolling timeframe of years or decades. One of the attractions of biomass is its versatility: under the right circumstances, it can be used to provide a sustainable supply of electricity, heat, transport fuels or chemical feedstocks in addition to its many other uses. One of the drawbacks of biomass—especially in the face of so many potential end uses—is its limited availability, even though the precise limitation is the subject of debate. Compared with the level of attention given to biomass as a source of electricity or heat, relatively little attention has been paid to biomass as a chemical feedstock. However, in a world in which conventional feedstocks are becoming constrained and countries are endeavouring to meet targets for reducing carbon dioxide (CO2) emissions, there is a question as to whether biomass is too good to burn. While this paper does not set out to provide the definitive answer to that question, it aims to open up the discussion about how biomass might best contribute to addressing the feedstock supply and carbon footprint issues that are increasingly starting to tax the brains of those involved in the petrochemical industry.
The paper begins by examining the scale of the challenge, looking at the extent to which the petrochemical industry draws upon the world's limited fossil fuel reserves in order to produce the products and materials that developed and developing countries crave. There is, of course, a question about whether we should be trying to manage demand and expectations downward rather than looking for novel ways of perpetuating today's developed-world lifestyle preferences. It then goes on to compare the idea of a biorefinery with the more conventional crude oil refinery, mapping out the possible routes from biomass feedstocks to fuels and petrochemical-type products, drawing a distinction between thermochemical processes and biochemical processes.
Having outlined the technological possibilities, the paper then goes on to examine the thorny question of biomass availability, taking account of the multiplicity of other uses to which biomass can be put. For those who subscribe to the idea of a hierarchy of human needs, it is interesting to consider where the various man-made materials that the petrochemical industry has developed over recent decades and which are becoming ubiquitous in clothing and home construction materials sit alongside food and animal feed, for example. As might be expected, the precise limit on biomass availability depends also on what else our societies wish to use land, water and other resources for. Finally, the paper concludes by looking at the implications in terms of infrastructure development of setting off down a path towards cost-effective substitution of conventional petrochemical feedstocks with biomass.
2. Projected growth in petrochemicals
Petrochemicals are used to make many of the materials associated with modern-day living, as exemplified in table 1.
Table 1.
Examples of materials made from petrochemicals.
| petrochemical products | uses |
|---|---|
| polyester, nylon | textiles for clothing and carpets |
| polyurethane | adhesives and resins for particle board |
| PET | lightweight, unbreakable bottles |
| polyethylene | plastic bags |
| expanded polystyrene | packaging for fragile goods |
| various high-grade plastics | artificial limbs, food packaging/preservation materials, spectacle lenses, expensive consumer durables, crash helmets |
| uPVC | doors, window frames |
| glycols | surfactants for basic soaps and expensive cosmetics |
| ammonia-based fertilisers | increasing crop yields |
| insecticides and biocides | health protection |
| plastic composites | lightweight vehicles |
| synthetic rubber | tyres |
As populations in developing countries grow and expectations around lifestyle increase, the demand for these products is increasing.
Figure 1 illustrates some recent projections of refined product demand (principally gasoline and diesel) for both Organisation for Economic Cooperation and Development (OECD) and non-OECD countries, showing an aggregate rate of increase equivalent to 1.6 per cent year-on-year [1]. With current oil consumption standing at 4.4 billion tonnes of petroleum per year (or 90 million barrels per day [1]) of which approximately 10 per cent is used for petrochemical feedstocks [2,3], this equates to the equivalent of at least one billion tonnes of raw biomass per year, rising at 1.6 per cent per year, for full hydrocarbon feedstock substitution in the petrochemical industry excluding transport fuels.
Figure 1.
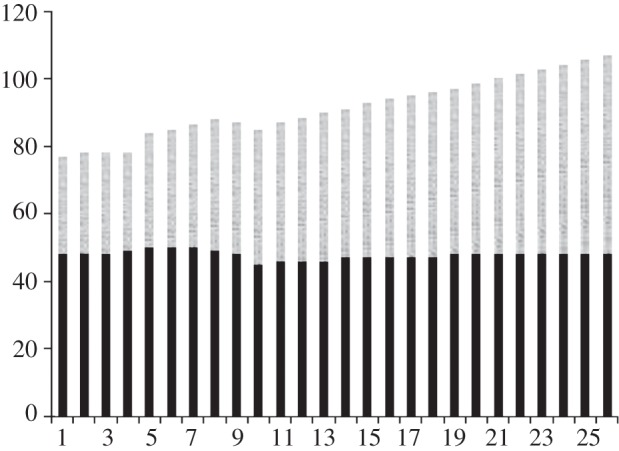
World refined product demand outlook (millions of barrels per day), adapted from Jacobs Consulting [1]. Grey bars, non-OECD; black bars, OECD.
One issue with the projected growth in demand for petrochemicals—assuming a business-as-usual approach to production—is that feedstock prices (and therefore product prices) will rise as demand begins to outstrip ability to supply from conventional sources and more difficult feedstocks have to be brought into play (e.g. highly acidic crude oils, very deep oil wells, shale gas). Another issue is the carbon footprint associated with converting fossil fuels into materials whose production and end-of-life disposal leads to CO2 emissions—effectively releasing within (say) a hundred years CO2 that was captured by animal and plant life forms over a period of tens of millions of years. According to the social policy think tank Civitas, the green taxes proposed recently by the UK government in the face of such a prospect could spell the end for Britain's chemical industry [4].
Approximately 40 per cent of global CO2 emissions come from industry whether direct from the factories (7.6 Gte per year) or from their proportion of power station emissions (3.9 Gte per year) [5]. The industries considered to contribute most to these figures are petrochemicals, oil refining, cement, glass and metals processing—with the first of these (petrochemicals) being the subject of this paper. The main petrochemical feedstocks are crude oil fractions such as naphtha and heavier oils along with natural gas. The challenge in respect of biomass is to find a cost-effective way of synthesizing these feedstocks for use with existing petrochemical technology or to develop alternative feedstocks (viz. sources of carbon), which would need to be processed in a slightly different way. The climate change benefit derives from biomass' short carbon cycle namely most of the CO2 associated with biomass conversion and use is counterbalanced by the CO2 absorbed during the recent growth of that biomass.
3. The biorefinery concept
The biorefinery concept is analogous to an oil refinery. Just as an oil refinery enables the conversion of a range of crude oils into high-specification fuels and chemical feedstocks, a biorefinery enables the conversion of a range of biomass feedstocks into fuels and chemical feedstocks [6]. As with the increasingly expensive commodity of crude oil, the aim is to extract maximum value from the wide range of components contained within biomass [7]. This can be done by using thermochemical or biochemical conversion processes as described below to produce the necessary chemical intermediates. For resource-efficiency reasons, thermochemical processes are often used for dry feedstocks and biochemical processes for wet feedstocks. Both of these processes disrupt complex molecular chains, some of which pertain to highly valuable components. Where raw biomass contains viable quantities of valuable components, it is often cost-effective to extract those prior to thermochemical or biochemical conversion [8].
A key challenge for the biorefinery concept overall is scale-up: building processes at sufficient scale to be of relevance to industrial facilities that operate on a petrochemicals scale. This is starting to happen in Brazil now [9]. Generally speaking, a biorefinery that could produce a total output across its product slate of (say) one million tonnes per year would be considered relevant as part of the supply chain for petrochemicals production.
As with an oil refinery, there are numerous opportunities for heat integration within the overall biorefinery. Thus, heat removed in one of the thermochemical or biochemical-processing units as part of a cooling process can often be used to supply heat elsewhere in the complex. Waste heat can also be supplied to adjacent downstream petrochemical facilities in order to maximize the overall profitability and efficiency of the biorefinery complex and reduce the utilities demand of the other plants. Some biorefinery designs include a combined heat and power (CHP) unit that supplies both heat and electricity through combustion of otherwise unusable biomass wastes. A good chemical engineering design will maximize the deployment of cost-effective heat integration.
4. Thermochemical routes to converting biomass
4.1. Gasification
Thermochemical conversion routes are based on biomass gasification [10–12]. Gasification is a partial combustion process in which biomass reacts at a high temperature with oxygen, which is supplied at levels insufficient to support full combustion. The outputs are a gas (the desired product) and a solid ash residue whose composition depends on the type of biomass. Continuous gasification processes for various feedstocks have been under development since the early 1930s. Ideally, the gas produced would be a mixture of hydrogen and carbon monoxide, but, in practice, it also contains methane, CO2 and a range of contaminants. The gas produced tends to be called syngas (or synthesis gas) although some people prefer to reserve that term for a blend of hydrogen and carbon monoxide alone. If, for example, the biomass is derived from coppiced wood, then the syngas is almost carbon-neutral, because the CO2 emitted if the syngas or its derived products are burnt is effectively absorbed again during the photosynthetic growth of the next crop.
A variety of gasification technologies is available across a range of sizes, from small updraft and downdraft gasifiers through a range of fluidized bed gasifiers at an intermediate scale and on to larger entrained flow and plasma gasifiers [10,13]. (In an updraft gasifier, the oxidant is blown up through the fixed gasifier bed with the syngas exiting at the top; in a downdraft gasifier, the oxidant is blown down through the reactor with the syngas exiting at the bottom.) Gasification processes tend to operate either above the ash melting temperature (typically >1200°C) or below the ash melting temperature (typically <1000°C). In the higher-temperature processes, there is little methane or tar formation. There is a choice between oxygen or air as the oxidant, and some choice over operating pressure. E4Tech [14] have published a report on the experimental and commercial deployment of gasification technology. From this, it can be seen that the question of which gasification technology is ‘best’ depends on whether the priority is to minimize cost, maximize reliability, produce a very pure syngas, handle a wide range of feedstock types, avoid pre-processing of biomass or operate at a large scale.
Several demonstration projects have been implemented around the world to show how gasifiers can, when designed right, operate reliably either in isolation or in conjunction with a downstream conversion process. A well-known example is the plant in Güssing, Austria, which gasifies 40 oven-dry tonnes per day of local wood chip and wood industry waste in a dual fluidized bed gasifier linked to a Jenbacher CHP engine designed to provide 2 MW of electricity and 4.5 MW of heat [15]. While most of the syngas is used to provide heat and power to the town of Güssing, a slipstream is diverted for development work on Fischer–Tropsch synthesis, methanation of syngas (methane being a major petrochemicals feedstock) and fuelling a solid oxide fuel cell [15]. By contrast, the largest gasifiers in the world (which are more than 300 times bigger than the Güssing demonstration plant) tend to be found in more straightforward applications such as large-scale conversion of low-value streams on oil refineries into raw syngas for power generation [16].
If the scale of interest is around one million tonnes of refined product per year, the biomass feedstock required will be of the order of six million tonnes per year, which equates to 8000 oven-dry tonnes per day or 2500 MWth. At the current state of gasifier technology development, this tends to imply entrained flow gasifiers or possibly circulating fluidized bed gasifiers depending on the number of gasification units desired as part of a cost-versus-reliability optimization [10]. The entrained flow gasifier offers economies of scale because it can run at feed rates of up to 10 000 oven-dry tonnes per day, but it requires a highly processed feedstock to compensate for the very short residence time in the gasification reactor—a matter of seconds. One option is to grind the biomass to achieve a particle size of less than 1 mm, which, for a fibrous-vegetable-based feedstock, may require an upstream torrefaction step to make it grindable [10]. (Torrefaction is a heat-treatment process carried out in the absence of added oxygen at temperatures around 250°C, converting biomass into a charcoal-like material which retains 90% of its original energy content.)
Another option for feedstock processing is to convert the biomass into pyrolysis oil via a fast pyrolysis step and inject it as a fine spray into the gasifier. Pyrolysis is a thermal conversion process in which no oxygen is added [17]. Entrained flow gasifiers operate at high temperature (1200–1600°C), usually on oxygen, and at pressure of up to 100 bar [18]. The circulating fluidized bed gasifier can handle a more variable feedstock slate provided the particle size is kept below 20 mm. It operates below the ash melting point and offers the option of pressurized operation to facilitate running at up to 1800 oven-dry tonnes per day. It is usually blown by air rather than oxygen because of the large volumes of gas required to keep the biomass and the inert bed material in a fluidized state.
4.2. Gas conversion
A major attraction of syngas is the very wide range of potential uses [19]. Broadly speaking, there are four options for converting syngas into useful downstream products: Fischer–Tropsch synthesis of hydrocarbon chains, methanol synthesis, mixed alcohol synthesis and syngas fermentation [14]. The Fischer–Tropsch process was developed in the 1920s to produce an alternative to conventional diesel fuel [20]. It has been deployed in South Africa, Germany and elsewhere for synthesizing transport fuels and petrochemical feedstocks, often in circumstances of economic or political necessity [21]. By choosing an appropriate catalyst (usually based on iron or cobalt) and appropriate reaction conditions (usually 200–350°C and 20–40 bar), the process with its associated cracking and separation stages can be optimized to produce heavy waxes for conversion to diesel, light olefins for gasoline, naphtha for petrochemicals production or methane as a replacement for natural gas [22]. The ideal Fischer–Tropsch feedstock is a syngas consisting of a mixture of hydrogen and carbon monoxide with a molar ratio of 2 : 1 [23].
Methanol synthesis is another attractive conversion route, because methanol is one of the top 10 petrochemical commodities traded internationally [22]. Syngas can be converted into methanol over a copper–zinc oxide catalyst at 220–300°C and 50–100 bar [14]. Methanol, in turn, can be used to make acetic acid, formaldehyde for resins, petrol additives and petrochemical building blocks such as ethylene and propylene [22].
Under slightly more aggressive process conditions (up to 425°C and 300 bar), a wider range of mixed alcohols can be produced [14], which opens up a wider potential product slate. The processes use catalysts modified from either Fischer–Tropsch synthesis or methanol synthesis, by addition of alkali metals. Finally, the syngas fermentation route uses biochemical processes and reaction conditions that are close to ambient temperature and pressure to make ethanol or other alcohols. Biochemical processes are addressed below.
The National Renewable Energy Laboratory has listed 16 different end-uses for syngas, which (in addition to those listed above) include producing hydrogen, ammonia (which is the second largest synthetic chemical in the world), dimethyl ether (for use as a refrigerant or a chemical feedstock), acetic acid, formaldehyde, methyl tertiary butyl ether, olefins and gasoline [22].
There are many options for converting biomass syngas into petrochemical feedstocks. Consider, for example, the olefins conversion chain in which ethylene and propylene are converted into polymers (polyethylene, polypropylene, PVC), glycols (ethylene glycol, propylene glycol) and a range of familiar materials such as acetone, acetic acid, petrol additives and surfactants. The olefins can be produced by synthesizing naphtha in a Fischer–Tropsch process and then cracking it in a conventional naphtha cracker to make ethylene and propylene. Alternatively, a methanol-to-olefins (MTO) process can be used, based on methanol from syngas [22]. Much remains to be done to identify the optimal conversion routes from syngas through to the various end products.
One of the factors that impacts on the optimization of conversion routes is syngas purity [24, ch. 8.2]. Depending on the source of biomass feedstock and the choice of gasifier technology, the raw syngas can contain varying amounts of particulates (e.g. ash or char, which can lead to erosion, plugging or fouling), alkali metals (which can cause hot corrosion and catalyst poisoning), water-soluble trace components (e.g. halides, ammonia), light oils or tars (e.g. benzene, toluene, xylene or naphthalene, which can lead to catalyst carbonization and fouling), polyaromatic compounds, sulfur components, phosphorus components as well as methane and CO2 [10]. Many of these can be removed (if required)—at a cost—either using standard chemical-industry equipment such as cyclones, filters, electrostatic precipitators, water scrubbers, oil scrubbers, activated carbon and adsorbents, or via clean-up processes such as hydrolysis and various CO2 capture processes [25,26]. Some of the more intractable areas such as tar removal continue to attract considerable research interest [27,28].
Another important factor is the ratio of H2 to CO in the syngas. Different conversion routes require different ratios, e.g. 1.7 : 1 and 2.15 : 1 for producing Fischer–Tropsch gasoline and diesel, respectively, or 3 : 1 for methanol synthesis [22]. Because biomass molecules contain oxygen within their structure, biomass-derived syngas often needs to have its H2 to CO ratio boosted. One option for achieving this is to react some of the syngas with steam over a catalyst to produce H2 and CO2 in the water–gas shift reaction [19], accepting a CO2 removal cost unless there is a by-product H2 source readily available.
The extent to which gas clean-up is required depends on the choice of syngas conversion route. Generally, the level of particulates will need to be reduced to 0.001–0.01 mg Nm−3 for any chemical synthesis process, but the precise extent to which (say) sulfur or halide levels need to be reduced depends on the catalysts that are going to be used. For methanol synthesis process, for example, the sulfur content of the syngas has to be below 100 ppbv [24]. For ammonia synthesis process, there is a similar sulfur constraint, and the CO2 content must be below 10 ppmv.
5. Biochemical routes to converting biomass
A range of biochemical processes is under development. Fermentation is one of the basic processes used to produce ethanol for transport and general industrial use. While fermentation of sugars is relatively easy, much of the available biomass contains both cellulose and the more difficult hemicellulose encapsulated within a lignin-based structure [29]. Cellulose is a linear polymer of glucose molecules. Hemicellulose is a branched polymer of both six-carbon sugars such as glucose and five-carbon sugars. Lignin is a polymeric structure of aromatic units [30]. The structure of lignocellulosic biomass can be described as a skeleton of cellulose chains embedded in a cross-linked matrix of hemicellulose surrounded by a crust of lignin in an intricate structure that is resistant to attack by chemicals or enzymes [31]. Processes that manage to penetrate that structure often degrade the carbohydrates, making their subsequent conversion more difficult [30]. Dilute acid treatment processes, steam explosion processes and an ammonia fibre explosion process have been developed for breaking through the lignin and making the cellulose accessible for yeast-based fermentation [30]. Much work is focused on the development of suitable enzymes for handling biomass derived from a variety of waste streams and on assessing the overall viability and carbon footprint of various pathways [31–34].
The process that is attracting most interest at scale is anaerobic digestion (AD). AD is a microbial process in which complex organic materials are broken down into their simpler chemical components by various enzymes [35]. The reactions take place in the absence of oxygen and produce biogas and a digestate. A typical biogas composition would be approximately 60 per cent methane 40 per cent CO2, with carbohydrate-based feedstocks tending to produce more CO2 and lipid-based feedstocks tending to produce more methane. Feedstocks for AD reactors often consist of animal slurries, energy crops and other agricultural, retail and industrial wastes. The four steps involved are hydrolysis, acidogenesis, acetogenesis and methanogenesis [36]. The different bacteria involved in the various stages (fermentive bacteria, acetogens and methanogens) have different requirements in terms of temperature and pH in order to produce high yields [37]. It is therefore common to see the AD process being carried out in two steps: one in the mesophilic range (20–40°C) and the other in the thermophilic range (40–60°C) [38]. Tight temperature control within the reactors is important when local outdoor temperatures are variable [39].
Given that natural gas is a major chemical feedstock for the petrochemical industry (as well as a fuel), one approach to increasing the use of biomass in petrochemicals is to purify and condition the gas produced in an AD process to the point where it can be injected into a country's natural gas grid, and then use that existing grid to supply petrochemical facilities [40]. Table 2 shows some of the requirements for a German system.
Table 2.
Quality requirements for biogas injection in Germany (adapted from Graf & Klaas [40]).
| parameter | value |
|---|---|
| propane | 6% v/v |
| butane | 2% v/v |
| H2 | 5% v/v |
| CO2 | 6% v/v |
| H2S | 5 mg m−3 |
| mercaptan sulfur | 6 mg m−3 |
Biological scrubbing (with alkaline water) or chemical desulfurization (using iron oxides and iron salts) followed by treatment with activated carbon or zinc oxide can be used to achieve the hydrogen sulfide specification [40]. Siloxanes can be removed using activated carbon [41]. There is also a requirement to add propane and butane in order to mimic the composition of natural gas (because natural gas is not pure methane).
6. Is biomass available on a petrochemicals scale?
The range of possible feedstocks available is very wide. Some are derived from crops that are also used to feed people or animals, leading to a question about the scope for growing more. Where ultimate limits on availability are envisaged and prioritization is required, questions arise about the rights of the poor and disadvantaged as compared with the hard economics of investment [42]. Developing countries may view increases in demand for biomass as a predictable market upon which they can profitably develop their agriculture to reach its potential—or they may view it as a route to exploitation of poor people, threatening land rights and workers' rights. From a sustainability perspective, it is important to consider both the direct effects and the indirect effects of any land-use change prompted by new markets for biomass [42]. Agricultural wastes such as wheat straw, rice husks and empty fruit bunches avoid many of the issues, but there remains a question about how much land should be given over to agriculture in the first place, especially in areas of water stress [43]. Proposals to use wood from rainforests attract regular criticism, but there is considerable interest in sustainable forestry in less-sensitive areas and particularly in finding viable end uses for small roundwood [44]. There are considerable volumes of waste wood available from construction and demolition activity. Other relevant waste-based feedstocks are the putrescible fraction of municipal solid waste and the fats, oils and greases from catering outlets. In the UK, municipal solid waste arisings are predicted to reach 50 million tonnes per year in 2020 [45].
Waste plastic presents an intriguing opportunity. While there is a strong case for recycling plastics, their properties tend to degrade after multiple cycles. If at that point they are diverted for use as a gasification feedstock, then they can re-enter the supply chain for high-grade polymer production. A closed loop from (for example) plastic bottle to plastic bottle can thereby envisaged. This is appealing in a material-constrained world, especially if the starting point is a biomass-derived plastic [46,47].
Much has been written about where the limit on biomass availability might lie. In some ways, this is an impossible question to answer because it depends so much on the choices that societies make and the priorities that they set. The world's land mass in its current form of usage divides roughly into 10 per cent arable crops, 30 per cent pasture (including raising animals for food), 30 per cent forest (some of which is ecologically very sensitive) and 30 per cent barren (including urban and desert). Societies can make choices about diet, with a meat-based diet requiring about seven times more land than a vegetable-based diet [48]. Countries can decide whether to promote population growth at the present rate, growth in personal transport at the present rate, growth in the use of petrochemical-based materials, and whether to switch to more intensive forms of crop cultivation and animal husbandry. Their approach to forestry can range from unused/undermanaged through to intensive/sustainable forestry and right on to unsustainable forest clearances. Slade et al. [49] have examined the question of biomass availability from the opposite direction, effectively saying: you can have any level of biomass availability that you care to aim for if you are prepared to live with the following consequences. By considering choices such as those listed above, they cover a range that equates to more than two times the total global primary energy demand.
Beyond all of these land-based sources of feedstock, there is also the option of using algae as a source of biomass [50,51]. The options include macro-algae (seaweed) and micro-algae (e.g. pond scum), grown in fresh water or salt water. Algae use the sun's energy to convert CO2, water and inorganic salts into biomolecules. Algae can be a source of both lipids (oil-based) and residual biomass. The Botryococcus braunii strain produces a hydrocarbon oil similar to crude oil, but unfortunately it grows very slowly—unlike many other algal strains. Although the aquaculture industry is still at an early stage of development compared with agriculture, it is making rapid progress [52].
There is also the question of how to prioritize the various demands for biomass that arise from primitive heating, more advanced renewable heating, renewable electricity, renewable transport fuels of various types and sustainable production of chemicals and polymers. For some of these, there are alternatives to biomass: for others, there are not [53]. For the 10 per cent of oil and gas that is currently consumed as petrochemicals feedstocks, it could be argued that there are relatively few options for substitution and therefore that biomass for petrochemicals substitution should be prioritized. The economic question about how best to balance biomass valorization for energy against conversion into high-value chemicals warrants careful consideration [8]. There is also a case for looking much more closely at how much biomass is wasted or lost along the various supply chains.
7. The implications for developing infrastructure
Petrochemical industry tends to develop in clusters, with feedstocks arriving by ship, rail, pipeline, etc., and various plants supplying one another with chemical intermediates and sharing utilities. Markets for products are both national and international. There is a huge body of existing infrastructure in terms of deep ports and storage facilities for liquids, gases and solid materials. Biomass supply, by contrast, is highly dispersed. Questions therefore arise as to whether biomass should be transported in its raw (usually solid) form, or mechanically processed into chips or pellets, and whether it should be dried in order to reduce the moisture content (often 50%) in the interests of minimizing transportation costs and carbon footprint. Biomass can be torrefied into a charcoal-like material in order to minimize its weight without sacrificing too much of the energy content—but some potentially valuable components are destroyed in that process. Alternatively, biomass can be converted into an energy-dense liquid (pyrolysis oil) and transported in that form [17].
Where large concentrations of biomass feedstock exist, it may be cost-effective to gasify the biomass close to its source and then build a network of syngas pipelines [19]. It could be argued that national gas grids used to carry a form of syngas before they were converted to carry natural gas instead in the 1970s. Long-distance pipelines tend to cost around a million pounds per mile to build, but can be economically viable at large scale [54,55]. The AD industry is already exploring that path for biogas in natural gas pipelines.
Where companies are interested in using biomass in solid or liquid form, they tend to set up their own separate supply chains and then seek to use shared infrastructure for storage and transport logistics. Where companies are interested in pipeline supplies of syngas, there is a need to create the pipeline infrastructure. There is little evidence of this happening at the moment, but the relevant issues are being explored in analogous discussions about creating new CO2 pipelines to carry captured CO2 from large point sources to one or more geological storage locations [56–59]. The issues that need to be dealt with relate to defining an entry specification for gas into a shared network, deciding how to fund an oversized pipeline network that can handle higher throughputs in future, agreeing how early users and later users pay for access in a way that provides equitable reward to those who invested the capital, and finding a workable approach to securing access rights over land owned by multiple parties along with the relevant planning approvals [19].
8. Conclusions
If societies around the world conclude that the comforts and lifestyles associated with petrochemical products represent a priority in a resource-constrained world, it is possible to secure access to sufficient biomass resource to support a large-scale petrochemical-style industry given the size of its feedstock demand relative to energy and transport. There are clear trade-offs against other priorities such as mobility, diet and visual amenity. The building blocks for the necessary technologies have been considerably developed over recent decades but have seen only limited deployment in a chemicals production context to-date. The precise manner in which they need to be assembled and further refined depends on societal choices regarding which products society ‘must’ have and the end uses to which limited feedstocks can legitimately be put. Given the long lead time involved in developing large-scale infrastructure for transporting syngas and for managing biomass logistics, it is time to start laying the foundations.
Acknowledgements
Funding from the Regional Development Agency for North East England (One Northeast) and from the Tees Valley Industrial Programme for exploring the regional implications of this work is acknowledged.
References
- 1.Jacobs Consulting 2011. Trends in the hydrocarbon industry: demand, cost and competition. See www.gnobr.com/presentations/ (accessed 24 July 2012). [Google Scholar]
- 2.Glass SJ. 2007. Sharing perspectives on the global petrochemical industry. See http://www.touchbriefings.com/pdf/2779/Glass.pdf (accessed 29 August 12). [Google Scholar]
- 3.BP 2012. BP statistical review of world energy June 2012. See http://www.bp.com/statisticalreview (accessed 29 August 12). [Google Scholar]
- 4.Merlin-Jones D. 2011. Chain reactions: how the chemical industry can shrink our carbon footprint. London, UK: Civitas [Google Scholar]
- 5.IEA 2010. Energy technology perspectives 2010. Paris, France: International Energy Agency [Google Scholar]
- 6.Smith W. 2007. Mapping the development of UK biorefinery complexes (NFC 07/008). See http://www.nnfcc.co.uk National Non Food Crop Centre. [Google Scholar]
- 7.Aksew MF. 2012. Extracting additional value from biomass. In Comprehensive renewable energy, vol. 5: biomass and biofuels (ed. Roddy DJ.). Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 8.Kazmi A, Clark J. 2012. Expanding the envelope: biomass to chemicals. In Comprehensive renewable energy, vol. 5: biomass and biofuels (ed. Roddy DJ.). Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 9.Altieri A. 2012. Bioethanol development in Brazil. In Comprehensive renewable energy, vol. 5: biomass and biofuels (ed. Roddy DJ.). Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 10.Roddy DJ, Manson-Whitton C. 2012. Biomass gasification and pyrolysis. In Comprehensive renewable energy, vol. 5: biomass and biofuels (ed. DJ Roddy), pp. 133–154 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 11.Kwant KW, Knoef H. 2004. Status of biomass gasification in countries participating in the IEA bioenergy task 33 biomass gasification and EU gasnet. International Energy Agency Report 2004. See www.nachhaltigwirtschaften.at/english (accessed 3/2/2012)
- 12.Ciferno JP, Marano JJ. 2002. Benchmarking biomass gasification technologies for fuels, chemicals and hydrogen production, National Energy Technology Laboratory (NETL) report, 2002. See www.seca.doe.gov/technologies/coalpower/gasification/pubs/pdf/BMassGasFinal.pdf (accessed 2 March 2012).
- 13.Bridgwater AV. 2003. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 91, 87–102 10.1016/S1385-8947(02)00142-0 (doi:10.1016/S1385-8947(02)00142-0) [DOI] [Google Scholar]
- 14.E4Tech 2009. Review of technologies for gasification of biomass and wastes. NNFCC project 09/008. See www.nnfcc.co.uk.
- 15.Knoef HAM. 2005. Handbook of biomass gasification, BTG. See www.btgworld.com. [Google Scholar]
- 16.Worldwide Gasification Database 2010. See http://www.netl.doe.gov/technologies/coalpower/gasification/worlddatabase/index.html (accessed 10 January 2011).
- 17.McKendry P. 2001. Energy production form biomass (part 2): conversion technologies. Bioresour. Technol. 83, 47–54 10.1016/S0960-8524(01)00119-5 (doi:10.1016/S0960-8524(01)00119-5) [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss R. 2003. Coal gasification technologies. Proc. Inst. Mech. Eng. J. Power Energy 217, 27–33 10.1243/095765003321148664 (doi:10.1243/095765003321148664) [DOI] [Google Scholar]
- 19.Roddy DJ. In press. A syngas network for reducing industrial carbon footprint and energy use. Appl. Thermal Energy. 10.1016/j.applthermaleng.2012.02.032 (doi:10.1016/j.applthermaleng.2012.02.032) [DOI] [Google Scholar]
- 20.Fischer F. 1925. Liquid fuels from water gas. Ind. Eng. Chem. 17, 574–576 10.1021/ie50186a009 (doi:10.1021/ie50186a009) [DOI] [Google Scholar]
- 21.van Dyk JC, Keyser MJ, Coertzen M. 2006. Syngas production from South African coal sources using Sasol–Lurgi gasifiers. Intern. J. Coal Geol. 65, 243–253 10.1016/j.coal.2005.05.007 (doi:10.1016/j.coal.2005.05.007) [DOI] [Google Scholar]
- 22.Spath PL, Dayton DC. 2003. Preliminary screening: technical and economic assessment of synthesis gas to fuels and chemicals with emphasis on the potential for biomass-derived syngas. TP-510–34929. Golden, CO: National Renewable Energy Laboratory. [Google Scholar]
- 23.Evans G, Smith C. 2012. Biomass To Liquids technology. In Comprehensive renewable energy, vol. 5: biomass and biofuels (ed. Roddy DJ.), pp. 155–204 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 24.Higman C, van der Burgt M. 2008. Gasification. London, UK: Elsevier/GPP [Google Scholar]
- 25.Stevens D. 2001. Hot gas conditioning: recent progress with larger-scale biomass gasification systems. Report no. NREL/SR-510-29952 Golden, CO: National Renewable Energy Laboratory. [Google Scholar]
- 26.Boerrigter H, den Uil H, Calis HP. 2002. Green diesel from biomass via Fischer-Tropsch synthesis: new insights in gas cleaning and process design In Paper presented at Pyrolysis and Gasification of Biomass and Waste Expert Meeting, Strasbourg, France, 30 September–1 October 2002 See http://www.scribd.com/iiser1024/d/57909109-Fischer-Tropsch02 (accessed 2 March 2012). [Google Scholar]
- 27.Sutton D, Kelleher B, Ross RH. 2001. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 73, 155–173 10.1016/S0378-3820(01)00208-9 (doi:10.1016/S0378-3820(01)00208-9) [DOI] [Google Scholar]
- 28.Devi L, Ptasinski KJ, Janssen FJJG. 2003. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 24, 125–140 10.1016/S0961-9534(02)00102-2 (doi:10.1016/S0961-9534(02)00102-2) [DOI] [Google Scholar]
- 29.Black, Veatch 2008. Lignocellulosic ethanol plant in the UK: feasibility study. NNFCC 08-007. See http://nnfcc.co.uk. [Google Scholar]
- 30.Arshadi M. 2011. Biochemical production of bioethanol. In Handbook of biofuels production (eds Luque R, Campelo J, Clark J.), pp. 199–257 Cambridge, UK: Woodhead Publishing [Google Scholar]
- 31.Refaat A. 2012. Biofuels from waste materials. In Comprehensive renewable energy, vol. 5: biomass and biofuels (ed. Roddy DJ.), pp. 217–262 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 32.Taherzadeh MJ, Karimi K. 2008. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 9, 1621–1651 10.3390/ijms9091621 (doi:10.3390/ijms9091621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ. 2010. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 101, 4851–4861 10.1016/j.biortech.2009.11.093 (doi:10.1016/j.biortech.2009.11.093) [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Cheng J. 2002. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 83, 1–11 10.1016/S0960-8524(01)00212-7 (doi:10.1016/S0960-8524(01)00212-7) [DOI] [PubMed] [Google Scholar]
- 35.Boyle G. 2004. Renewable energy. Oxford, UK: Oxford University Press in association with the Open University [Google Scholar]
- 36.Macias-Corral M, Samani Z, Hanson A, Smith G, Funk P, Yu H, Longworth J. 2008. Anaerobic digestion of municipal solid waste and agricultural waste and the effect of co-digestion with dairy cow manure. Bioresour. Technol. 99, 8288–8293 10.1016/j.biortech.2008.03.057 (doi:10.1016/j.biortech.2008.03.057) [DOI] [PubMed] [Google Scholar]
- 37.Cavinato C, Fatone F, Bolzonella D, Pavan P. 2010. Thermophilic anaerobic co-digestion of cattle manure with agro-wastes and energy crops: comparison of pilot and full scale experiences. Bioresour. Technol. 101, 545–550 10.1016/j.biortech.2009.08.043 (doi:10.1016/j.biortech.2009.08.043) [DOI] [PubMed] [Google Scholar]
- 38.Deublein D, Steinhauser A. 2008. Biogas from waste and renewable resources: an introduction. Weinheim, Germany: Wiley-VCH [Google Scholar]
- 39.Alvarez R, Liden G. 2008. The effect of temperature variation on biomethanation at high altitude. Bioresour. Technol. 99, 7278–7284 10.1016/j.biortech.2007.12.055 (doi:10.1016/j.biortech.2007.12.055) [DOI] [PubMed] [Google Scholar]
- 40.Graf F, Klaas U. 2009. State of biogas injection to the gas grid in Germany In 24th World Gas Conf. 2009 Buenos Aires. See http://www.igu.org/html/wgc2009/papers/docs/wgcFinal00259.pdf (accessed 10 March 2011). [Google Scholar]
- 41.Accettola F, Guebitz GM, Schoeftner R. 2008. Siloxane removal from biogas by biofiltration: biodegradation studies. Clean Technol. Environ. Policy 10, 211–218 10.1007/s10098-007-0141-4 (doi:10.1007/s10098-007-0141-4) [DOI] [Google Scholar]
- 42.Roddy DJ. 2009. Biofuels: environmental friend or foe? Proc. Instit. Civil Eng. Energy 162, 121–130 [Google Scholar]
- 43.Juniper Consultancy Services Ltd 2000. Technology and business review: pyrolysis and gasification of waste: a worldwide technology and business review, vols 1 and 2. Stroud, UK: Juniper Consultancy Services [Google Scholar]
- 44.Wright LL, Eaton LM, Perlack RD, Stokes BJ. 2012. Woody biomass for energy applications: state of technology. In Comprehensive renewable energy, vol. 5: biomass and biofuels (ed. Roddy DJ.). Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 45.Lee P, et al. Quantification of the potential energy from residuals (EfR) in the UK. Oakdene Hollins Ltd.; 2005. Commissioned by The Institution of Civil Engineers. Aylesbury, UK: [Google Scholar]
- 46.Ahmed II, Nipattummakul N, Gupta AK. 2011. Characteristics of syngas from co-gasification of polyethylene and woodchips. Appl. Energy 88, 165–174 10.1016/j.apenergy.2010.07.007 (doi:10.1016/j.apenergy.2010.07.007) [DOI] [Google Scholar]
- 47.Kuppens T, Cornelissen T, Carleer R, Yperman J, Schreurs S, Jans M, Theway T. 2010. Economic assessment of flash co-pyrolysis of short rotation coppice and biopolymer waste streams. J. Environ. Manage. 91, 2736–2747 10.1016/j.jenvman.2010.07.022 (doi:10.1016/j.jenvman.2010.07.022). [DOI] [PubMed] [Google Scholar]
- 48.Pimental D, Pimental M. 2003. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 78, 660–663 [DOI] [PubMed] [Google Scholar]
- 49.Slade R, Saunders R, Gross R, Bauen A. 2011. Energy from biomass: the size of the global resource. London, UK: Imperial College Centre for Energy Policy and Technology and UK Energy Research Centre [Google Scholar]
- 50.Benemann JR, Goebel RP, Weissman JC, Augenstein DC. 1982. Microalgae as a source of liquid fuels. Final technical Report to U.S. DOE BER. See http://www.osti.gov/bridge/ (accessed 7th March 2011).
- 51.Darzins A, Pienkos P, Edye L. 2010. Current status and potential for algal biofuels production. Report T39-T2, IEA Bioenergy Task 39. See http://www.task39.org (accessed 7th March 2011)
- 52.Rowbotham JS, Dyer PW, Greenwell HC, Theodorou MK. 2012. Thermochemical processing of macroalgae: a late bloomer in the development of third-generation biofuels? Biofuels 3, 441–461 10.4155/bfs.12.29 (doi:10.4155/bfs.12.29) [DOI] [Google Scholar]
- 53.MacKay DJC. 2009. Sustainable energy: without the hot air. London, UK: UIT Cambridge [Google Scholar]
- 54.AMEC 2010. Engineering design and capture technologies for carbon capture and storage in the tees valley. See http://nepic.co.uk (accessed 28 June 2011) [Google Scholar]
- 55.Dooley JJ, Dahowski RT, Davidson CL. 2008. Comparing existing pipeline networks with the potential scale of future US CO2 pipeline networks. Energy Procedia 1, 1595–1602 10.1016/j.egypro.2009.01.209 (doi:10.1016/j.egypro.2009.01.209) [DOI] [Google Scholar]
- 56.Roddy DJ. 2012. Development of a CO2 network for industrial emissions. Appl. Energy 90, 459–465 10.1016/j.apenergy.2011.10.016 (doi:10.1016/j.apenergy.2011.10.016) [DOI] [Google Scholar]
- 57.Fish JR, Martin EL. 2010. Carbon dioxide pipelines, California carbon capture and storage review panel technical advisory committee report. See http://www.climatechange.ca.gov/carbon_capture_review_panel/meetings/2010-08-18/white_papers/Carbon_Dioxide_Pipelines.pdf (accessed 11 May 2011)
- 58.Parfomak PW, Folger P, Vann A. 2009. Carbon dioxide (CO2) pipelines for carbon sequestration: emerging policy issues, congressional research service. See http://nepinstitute.org/get/CRS_Reports/CRS_Climate_and_Environment/Carbon_and_CO2/CO2_Pipelines_for_Carbon_Sequestration.pdf (accessed 5 May 2011). CRS Order Code RL33971, Washington, DC, July 31 2009
- 59.Esposito RA, Monroe LS, Friedman JS. 2011. Deployment models for commercialised carbon capture and storage. Environ. Sci. Technol. 45, 139–146 10.1021/es101441a (doi:10.1021/es101441a) [DOI] [PubMed] [Google Scholar]


