Abstract
Osteoarthritis (OA) has traditionally been classified as a noninflammatory arthritis; however, the dichotomy between inflammatory and degenerative arthritis is becoming less clear with the recognition of a plethora of ongoing immune processes within the OA joint and synovium. Synovitis is defined as inflammation of the synovial membrane and is characteristic of classical inflammatory arthritidies. Increasingly recognized is the presence of synovitis in a significant proportion of patients with primary OA, and based on this observation, further studies have gone on to implicate joint inflammation and synovitis in the pathogenesis of OA. However, clinical OA is not one disease but a final common pathway secondary to many predisposing factors, most notably age, joint trauma, altered biomechanics, and obesity. How such biochemical and mechanical processes contribute to the progressive joint failure characteristic of OA is tightly linked to the interplay of joint damage, the immune response to perceived damage, and the subsequent state of chronic inflammation resulting in propagation and progression toward the phenotype recognized as clinical OA. This review will discuss a wide range of evolving data leading to our current hypotheses regarding the role of immune activation and inflammation in OA onset and progression. Although OA can affect any joint, most commonly the knee, hip, spine, and hands, this review will focus primarily on OA of the knee as this is the joint most well characterized by epidemiologic, imaging, and translational studies investigating the association of inflammation with OA.
Keywords: Osteoarthritis, inflammation, innate Immunity
Introduction
The classification of osteoarthritis (OA) as a noninflammatory arthritis is an unfortunate consequence of early observations noting fewer leukocytes in OA synovial fluid compared with that of rheumatoid arthritis (RA), reactive arthritis, and even septic arthritis. In spite of this classification, investigators decades ago observed synovial inflammation in so-called ‘post-traumatic’ synovitis [Soren et al. 1976], and similar histopathology has been described among at least a subset of patients with what is now designated primary OA. Given this greater appreciation for synovitis in patients with OA, inflammation has now been strongly implicated in the pathogenesis of OA [Scanzello and Goldring, 2012]. This is not to imply that all OA pathogenesis is related to the synovium. Rather, synovitis is likely a secondary process induced by innate immune activation following cartilage damage that provides a critical link in the chain of initiation and propagation of OA.
OA is associated with multiple risk factors, most notably age, joint trauma, altered biomechanics, and obesity [Felson, 2006]. Given its complex etiology, OA should not be thought of as a single disease, but rather as the clinical endpoint of numerous disorders leading to the eventual failure of one or more joints of the body. In fact, evidence suggests that the pathologic changes characteristic of OA share a common final pathway that operates to perpetuate joint destruction and eventual failure. The key features of this common final pathway linking biochemical and mechanical processes to progressive joint failure are the subject of this review. Focus will be devoted to the interaction between local tissue damage and the immune system, which ultimately leads to a state of low-grade, chronic joint inflammation that drives progression toward the phenotype recognized as clinical OA.
Unlike RA, in which disease-modifying antirheumatic drugs (DMARDs) have revolutionized treatment [Singh et al. 2012], therapies used to manage OA are limited to pain control, with no agent to date approved for the prevention or treatment of OA disease progression. Given the high disability burden among patients with OA, there is great need to develop disease-modifying osteoarthritic drugs (DMOADs), and increased understanding of the inflammatory pathways leading to the onset and progression of OA could enable development of targeted therapies.
Understanding osteoarthritis as an inflammatory disease
Perhaps the first step in understanding OA as an inflammatory disease is to acknowledge that inflammation is not exclusive to RA and the other classical inflammatory arthritidies. Early studies in RA, using OA tissues and fluids as a comparator, noted dramatically increased levels of inflammatory proteins in RA [Farahat et al. 1993; Nettelbladt and Sundblad, 1959; Smith et al. 1997]. Although largely overshadowed by more pronounced histologic and biochemical abnormalities in RA, even some of these historic studies from as early as 1959 revealed elevated levels of inflammatory plasma proteins in both the blood and synovial fluid of patients with OA [Nettelbladt and Sundblad, 1959: 148]. The authors suggested that, ‘This may indicate that the type of permeability change in the synovial tissue is similar in both diseases (OA and RA), although this change is much more marked in rheumatoid arthritis’ [Nettelbladt and Sundblad, 1959: 148]. The frequent use of OA fluids and tissues as controls for RA has arguably reinforced the pervasive notion of OA as a noninflammatory arthritis, perhaps leading to the oversight that OA tissue and synovial fluid, when compared with ‘normal’ fluid or tissue, is highly enriched for plasma proteins, complement components [Gobezie et al. 2007], and cytokines [Sohn et al. 2012]. In more recent years the field has come to appreciate that, at least in some cases, levels of synovitis between OA and RA tissue can appear nearly indistinguishable [Haraoui et al. 1991]. These paradigm-changing studies have freed OA from its reputation as anoninflammatory, ‘wear and tear’ arthritis, likely transforming the ways in which researchers and clinicians think about and treat the disease.
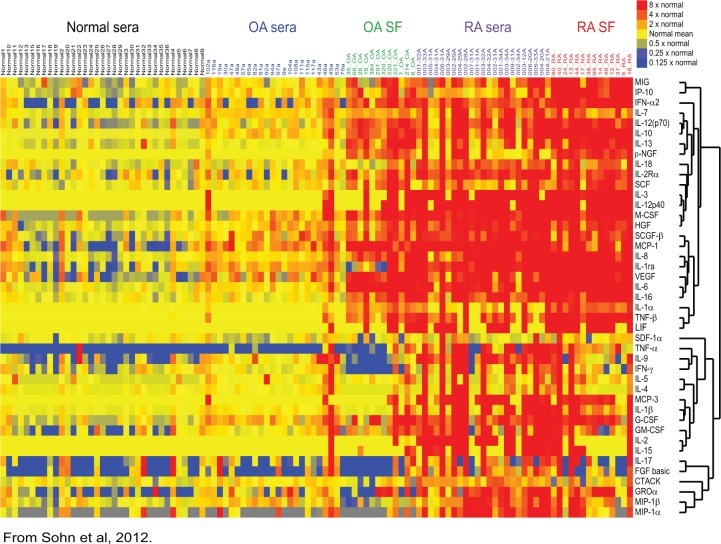
Using modern tools and techniques, our group has further characterized inflammatory mediators in OA and RA synovial fluid as well as OA, RA, and normal serum [Sohn et al. 2012]. Our findings mirrored those of Nettelbladt and Sundblad [Nettelbladt and Sundblad, 1959]. Figure 1 is a heatmap demonstrating a stepwise increase in levels of inflammatory cytokines from normal plasma, to OA plasma, to OA synovial fluid, to RA plasma, and finally to RA synovial fluid. Although RA is clearly associated with higher levels of inflammation, OA is by no means a ‘noninflammatory’ condition.
Figure 1.
Inflammatory cytokines are associated with osteoarthritis. Relative cytokine levels in serum and synovial fluid (SF) samples from patients with osteoarthritis (OA) or rheumatoid arthritis (RA) and in serum samples from healthy individuals (normal sera). Cytokine levels were measured with a multiplex bead-based immunoassay. Samples from individual patients are listed above the heatmap, and the individual cytokines are listed to the right of the heatmap. β-NGF, β nerve growth factor; CTACK, cutaneous T-cell attracting chemokine; FGF, fibroblast growth factor; GM-CSF, granulocyte macrophage colony-stimulating factor; GROα, growth-regulated oncogene α; G-CSF, granulocyte colony-stimulating factor; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; IL-1ra, interleukin-1 receptor antagonist; IL-2Rα, interleukin-2 receptor α chain; IP-10, interferon γ induced protein 10; LIF, leukemia inhibitory factor; MCP, monocyte chemotactic protein; M-CSF, macrophage colony-stimulating factor; MIG, monokine induced by interferon γ; MIP-1, macrophage inflammatory protein; SCF, stem cell factor; SCGF-β, stem cell growth factor β; SDF-1α, stromal cell-derived factor 1α; VEGF, vascular endothelial growth factor. (Reproduced from Sohn et al. [2012] with permission).
Several other studies lend additional support to the observation that systemic inflammation is associated with OA. These include key epidemiologic analyses demonstrating that serum levels of C-reactive protein (CRP) are strongly associated with the presence and progression of knee OA [Spector et al. 1997]. Additional studies have impressively demonstrated a positive correlation between levels of serum CRP and histologic evidence of synovitis and synovial fluid interleukin-6 (IL-6) at the time of joint replacement [Pearle et al. 2007]. These observations strongly suggest that the systemic inflammation observed in OA is at least partially reflective of local synovial inflammation.
Early osteoarthritis: synovial inflammation precedes structural change
The field of clinical rheumatology often considers OA to be the condition manifest by significant cartilage loss and joint space narrowing. In fact, the current American College of Rheumatology classification criteria for OA require the presence of radiographic changes of bony enlargement or osteophyte formation [Altman et al. 1986]. It is now clear that inflammation is present in OA joints well before the development of significant radiographic change. The combination of sensitive imaging modalities as well as direct arthroscopic visualization has suggested that, even at its earliest stages, before visible cartilage degeneration has occurred, OA is already an inflammatory disease. In one study, serial arthroscopies performed on knees with symptomatic but preradiographic OA revealed a clear association between the presence of synovitis and the future development of medial cartilage loss [Ayral et al. 2005]. Studies using magnetic resonance imaging (MRI) with or without contrast enhancement have similarly suggested an association between the presence of synovitis and OA progression [Krasnokutsky et al. 2011; Felson et al. 2003; Roemer et al. 2011].
Several additional studies support the observation of inflammation in the earliest phases of OA. In one study of 70 synovial tissues spanning a range of radiographic OA severity, severe synovial inflammation was observed in 31% of patients. Notably, synovial inflammation was present in many subjects with minimal radiographic disease [Haywood et al. 2003]. Benito and colleagues went on to compare early and late OA and demonstrated increased mononuclear cell infiltration and overexpression of inflammatory mediators in early compared with late disease [Benito et al. 2005]. Finally, in a cohort of patients without evidence of radiographic OA who were undergoing arthroscopic meniscectomy to repair traumatic meniscal injury, synovial inflammation was noted in 43% of patients and was associated with more severe preoperative pain and function scores [Scanzello et al. 2011]. These studies, demonstrating significant synovial inflammation in early OA, suggest a window of opportunity may exist in which disease-modifying interventions targeting inflammatory processes might be most efficacious for the prevention and treatment of OA.
Although the synovium is not the only tissue involved in OA-related inflammation, it is a major site of gross and microscopic inflammatory change [Sellam and Berenbaum, 2010] and is thus a major focus of this review. The synovium is normally two to three cell layers thick with notable lack of inflammatory cells. However, in the setting of inflammation there is often marked hyperplasia of the synovial lining cells with an infiltration of inflammatory cells consisting primarily of macrophages but also a smaller but quantifiable number of T and B cells [Bondeson et al. 2010], mast cells [Dean et al. 1993] and natural killer cells [Skrzeczynska-Moncznik et al. 2009]. Notably, the degree of infiltration is highly heterogeneous; some patients possess inflammation resembling that observed in RA, while others exhibit a minimally inflammatory and primarily degenerative histopathology [Benito et al. 2005].
It should be noted that the critical role of synovitis in no way excludes involvement of cartilage and the chondrocyte in the pathogenesis of either early or late OA. Cartilage breakdown products in synovial fluid as well as microfissures in articular cartilage are present long before any degeneration can be noted using current MRI technology or gross arthroscopic visualization [Mow et al. 1974; Pauli et al. 2011]. As discussed later, early cartilage degradation events may in fact play a driving role in the development of inflammation within the OA joint and specifically the OA synovium.
Chronic, low-grade inflammation sets the stage for chronic disease
Data are evolving to suggest that in a significant subset of patients with OA, chronic low-grade inflammation is a major driver of ongoing joint degeneration. It is additionally becoming clear that many of the physiologic maladies of aging are at least associated, if not directly related, to chronic low-grade inflammation. In atherosclerosis, chronic inflammation perpetuates and expands within the atherosclerotic plaque resulting in eventual plaque rupture and acute vascular events such as myocardial infarction or stroke [Libby, 2002]. Similarly, periodontitis is a chronic inflammatory process resulting not only in tooth loss but also in potentially increased rates of atherosclerotic cardiovascular disease, presumably related to locally initiated systemic inflammation [Hasturk et al. 2012]. Two additional degenerative diseases common with advancing age and associated with innate immune activation and low-grade inflammation are age-related macular degeneration [Telander, 2011] and Alzheimer’s disease [Wyss-Coray, 2006]. Given its role in numerous chronic diseases, chronic inflammation should be considered a key driver of progressive degeneration in OA joints.
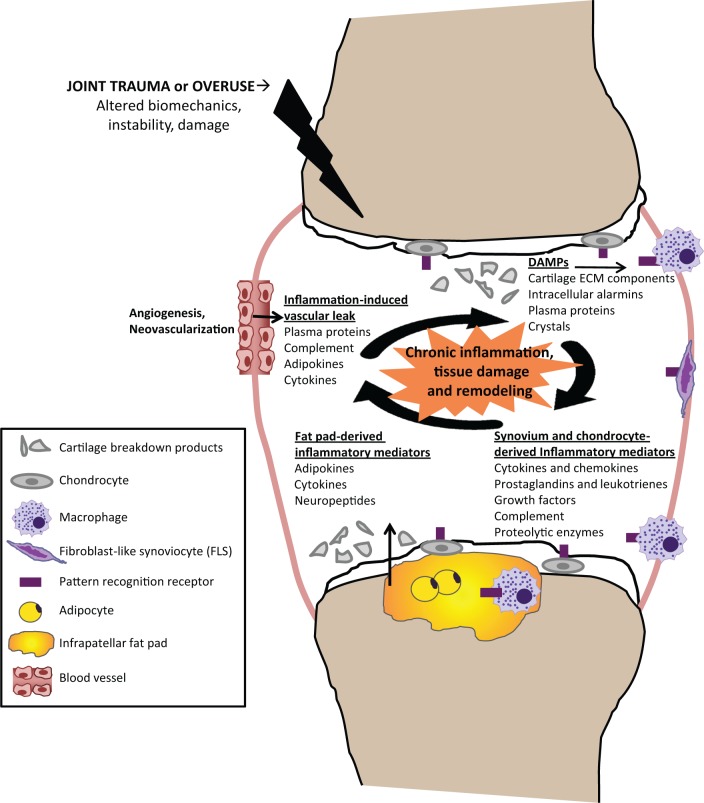
The development of chronic inflammation in OA following joint trauma or overuse can be understood as a vicious, self-perpetuating cycle of local tissue damage, inflammation, and repair, such that the OA joint has been likened to a chronic wound [Scanzello et al. 2008]. Initial damage, resulting from acute, subacute, or chronic injury, induces a local pathologic inflammatory response that results in further cartilage loss and progressive joint injury over time. This hypothesis is depicted in Figure 2. Biomechanics are central to the development of OA since chronic damage from prior mechanical derangement (such as a meniscal tear or extrusion), overuse, hypermobility, or anatomic misalignment can produce ongoing low-grade damage [Andriacchi et al. 2004; Englund et al. 2008]. However, in the current paradigm of chronic inflammation, mechanical derangement is the inducing factor.
Figure 2.
Schematic representation of chronic inflammation as a mediator of osteoarthritis. Following joint trauma or overuse, tissue damage results in the production of damage-associated molecular patterns (DAMPs), including cartilage extracellular matrix (ECM) breakdown products and intracellular alarmins that signal through pattern recognition receptors on synovial macrophages, fibroblast-like synoviocytes (FLS), or chondrocytes to induce the local production of inflammatory mediators. Inflammation-induced angiogenesis and increased vascular permeability results in the subsequent influx of plasma proteins also capable of functioning as DAMPs. Acute and chronic production of inflammatory mediators promote further cartilage degradation either directly or indirectly through their induction of proteolytic enzymes, amplifying a vicious cycle of innate immune activation in osteoarthritis.
How do mechanical processes induce and propagate inflammation? It seems unlikely that an isolated injury, even with resultant chronic mechanical instability, could alone result in the chronic inflammatory processes characteristic of OA. Thus, there must be perpetuating factors that can transduce mechanical events into inflammatory signals. These factors will be the focus of our next discussion.
Innate immunity in osteoarthritis: damage fueling the fire of inflammation
Unlike RA, OA does not appear to be associated with a robust adaptive immune response. However, activation of the innate immune system is a central feature of both diseases. Innate immunity refers to host immune responses induced by invariable pattern-recognition receptors (PRRs) that respond to conserved patterns in nature, including, but not limited to, those introduced by invading pathogens such as bacteria, viruses, and fungi [Kawai and Akira, 2010]. PRRs are composed of several families of cell surface, endosomal, and cytosolic receptors. One well characterized family is the toll-like receptors (TLRs) [Kawai and Akira, 2010]. Besides microbial patterns, PRRs also recognize multiple endogenous ‘danger signals’ resulting from tissue damage. Thus, in addition to so-called pathogen-associated molecular patterns (PAMPs), there exists another, potentially even more diverse, group of molecules known as damage (or danger) associated molecular patterns (DAMPs). Both PAMPs and DAMPs signal to the immune system a state of stress requiring a protective response to either combat infection or initiate repair processes.
Because the list of innate DAMPs is long and rapidly growing, a complete discussion is beyond the scope of this review. However, there exists a shorter, though again expanding, list of molecules implicated in the innate immune response within the damaged joint, each potentially contributing to the chronic inflammation observed in OA. Such molecules include breakdown products from damaged extracellular matrix (ECM) including fibronectin [Okamura et al. 2001] and hyaluronan [Termeer et al. 2002], as well as known [van Lent et al. 2012] and novel plasma DAMPs [Sohn et al. 2012], which become elevated in synovial fluid secondary to vascular exudation (Table 1).
Table 1.
Damage-associated molecular patterns (DAMPs) in osteoarthritis.
ECM, extracellular matrix; HMGB-1, high-mobility group box 1; LMW, low molecular weight; NLRP, nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing; PRR, pattern recognition receptor; TLR, toll-like receptor.
Extracellular matrix derived damage-associated molecular patterns
ECM breakdown is ubiquitous at sites of inflammation, including the OA joint [Evans et al. 1981]. An early observation by Homandberg and colleagues suggested that ECM breakdown products could promote inflammation and cartilage loss [Homandberg and Hui, 1996]. Even before the resurgence of interest in the innate immune system and the wide appreciation of PRRs, it was observed that fragments derived from the breakdown of fibronectin, when injected into knees of adolescent rabbits, resulted in cartilage damage as evidenced by loss of proteoglycans [Homandberg et al. 1993]. Further studies demonstrated that fibronectin fragments induced the production of proinflammatory cytokines, including tumor necrosis factor α (TNFα) and IL-1β, as well as matrix metalloproteinases MMP1 and MMP3, mediators now known to be implicated in chondrolysis [Homandberg and Hui, 1996]. The observations of Homandberg support a model in which damage resulting in ECM breakdown produces DAMPs capable of inciting local inflammatory responses resulting in further chondrolysis and release of additional ECM breakdown products. Notably, several additional ECM breakdown products have been implicated as DAMPs [Midwood et al. 2009; Okamura et al. 2001; Schaefer et al. 2005; Termeer et al. 2002] in mediating joint damage, including tenascin C [Midwood et al. 2009] and hyaluronic acid [Liu-Bryan and Terkeltaub, 2010].
Plasma protein damage-associated molecular patterns
In addition to ECM breakdown, sites of tissue damage and inflammation are characterized by vascular leak and exudation of plasma proteins [Stahel et al. 2007] capable of acting as DAMPs, including fibrinogen [Smiley et al. 2001]. Proteomic surveys of OA synovial fluid performed by our group [Sohn et al. 2012] and others [Gobezie et al. 2007] observed increased levels of many plasma proteins within OA synovial fluid. We evaluated the potential of a number of these plasma proteins to act as DAMPs in the elicitation of an inflammatory response. We observed that a select group of plasma proteins, including Gc-globulin, α1-microglobulin, and α2-macroglobulin, were capable of inducing TLR4-dependent macrophage production of inflammatory cytokines and growth factors implicated in OA, including TNFα, IL-6, IL-1β, and vascular endothelial growth factor (VEGF) [Sohn et al. 2012]. Thus, in addition to local production of DAMPs in the setting of joint injury, there appears to be a subsequent influx of inflammatory mediators resulting from inflammation- and damage-induced vascular leakage that further propagates the intra-articular inflammatory response and cartilage breakdown.
Intracellular alarmins
Intracellular proteins released from stressed, damaged, or necrotic cells can act as a third potential source of DAMPs. These ‘intracellular alarmins’, normally sequestered within the cell, can signal to the immune system when released from activated, stressed, or dying cells [Foell et al. 2007]. Intracellular alarmins implicated in OA include high-mobility group box 1 protein (HMGB-1) [Liu-Bryan and Terkeltaub, 2010] and the S100 family of proteins [van Lent et al. 2012]. One series of studies by Van Lent and colleagues demonstrated a potential role of the S100 family of proteins in OA-associated inflammation. Analyses of both human OA synovium as well as synovium derived from an animal model of OA revealed increased levels of S100A8 and S100A9 [van Lent et al. 2012]. In vitro studies further demonstrated the ability of these proteins to induce TLR4-dependent cartilage catabolism via upregulation of catabolic mediators, including MMPs 1, 3, 9, and 13, as well as the proinflammatory cytokine IL-6 with concomitant downregulation of the ECM components aggrecan and type II collagen [Schelbergen et al. 2012]. Moreover, disease severity was reduced in S100A9-deficient animals with collagenase-induced OA [van Lent et al. 2012].
Crystals as damage-associated molecular patterns
Microscopic inorganic crystals, including basic calcium phosphate (BCP) and calcium pyrophosphate dihydrate (CPPD) crystals, are frequently observed in osteoarthritic synovial fluids and tissues. At the time of joint replacement for severe OA, nearly all joints show cartilage deposition of calcium-containing crystals [Rosenthal, 2011]. Examination of 150 knees at autopsy by Gordon and colleagues found evidence of CPPD crystals in 93% of patients with severe OA, but only 24% of those with minimal or no OA. Additionally, they demonstrated an association of radiographic calcification with presence of pathologic synovitis [Gordon et al. 1984]. Although the presence of radiographic chondrocalcinosis was not associated with increased rates of cartilage loss by MRI [Neogi et al. 2006], the lack of precision in identification of chondrocalcinosis as well as the difficulty in radiographic identification of specific crystal types may inadequately represent the contribution of calcium-containing crystals to OA-associated inflammation.
Supporting the potential contributory role of calcium crystals to OA progression are numerous studies suggesting that calcium-containing crystals promote inflammation through their interaction with various components of the innate immune system. A role for both TLRs and Nod-like receptors (NLRs) in mediating the inflammatory properties of calcium-containing crystals has recently been demonstrated. CPPD crystals were able to induce chondrocyte production of nitric oxide in a TLR2-dependent manner [Liu-Bryan et al. 2005]. Furthermore, studies by Martinon and colleagues have demonstrated that CPPD crystals also engage the NLRP3 inflammasome in macrophages to induce the caspase-1-mediated activation and subsequent release of proinflammatory cytokines IL-1β and IL-18 [Martinon et al. 2006]. Similarly, the NLRP3 inflammasome has been shown to drive inflammation by BCP crystals [Pazar et al. 2011]. Additional inflammatory mediators induced by BCP include prostaglandins [Morgan et al. 2004], MMPs [McCarthy and Cheung, 1994], and S100A8 [Cunningham et al. 2012], a DAMP recently implicated in OA pathogenesis [van Lent et al. 2012].
In addition to calcium crystals, recent studies identified a strong association between synovial fluid uric acid levels and radiographic progression of OA [Denoble et al. 2011], providing a potential role for uric acid in contributing to inflammatory processes and cartilage degradation in OA. Supporting this hypothesis is the demonstration that monosodium urate (MSU) crystals are potent inducers of IL-1β by the NLRP3 inflammasome [Martinon et al. 2006]. Similarly, MSU crystals have been demonstrated to enhance TLR4-mediated production of IL1β through a caspase 1-mediated process [Giamarellos-Bourboulis et al. 2009]. Thus, these observations suggest that soluble or crystalline uric acid may prime innate immune responses to OA-associated DAMPs, many of which are known TLR agonists. Indirect support for the role of crystal-induced inflammation in OA was provided by a randomized trial of 60 women with OA in which treatment with colchicine was associated with symptomatic improvement in OA-associated pain [Aran et al. 2011].
Cellular mediators of innate immunity
Many cell types within the joint possess PRRs capable of responding to DAMPs. Although much of the innate immune activation and cytokine production in OA is attributed to the action of synovial macrophages [Bondeson et al. 2006, 2010], there is also a direct contributory role for other cells of the joint, including fibroblast-like syoviocytes (FLS) and chondrocytes.
FLS contribute to OA pathogenesis by acting as intermediate mediators of local inflammation. Studies have demonstrated that OA FLS produce inflammatory cytokines such as TNFα, IL-1β, and chondrolytic mediators such as MMPs. Additionally, coculture experiments investigating the interaction between FLS and chrondrocytes have demonstrated the ability of FLS-derived IL-1β and TNFα to induce cartilage degradation [Steenvoorden et al. 2007]. Further support for the role of FLS as mediators of the innate immune response includes their ability to respond to both inflammatory cytokines as well as TLR ligands [Ospelt et al. 2004]. Moreover, a recent study demonstrated that synovial fluid from patients with early OA modulated the FLS response to TLR2 and TLR4 ligands via soluble CD14 (sCD14). This study also observed elevated levels of sCD14 in OA synovial fluid [Nair et al. 2012], suggesting that sCD14 may be another factor that promotes innate immune inflammation in the OA joint.
The involvement of chondrocytes as mediators of OA-associated inflammation is supported by studies demonstrating upregulation of TLRs, including TLR2 and TLR4 at sites of OA cartilage lesions [Kuroki et al. 2010]. Signaling through these receptors by their respective ligands strongly induces catabolic responses in chondrocytes [Kim et al. 2006; Liu-Bryan and Terkeltaub, 2010]. Furthermore, exposure of cartilage explant cultures to TLR-activating fibronectin fragments induces chondrolysis mediated by autocrine regulators of cartilage metabolism, including IL-1, IL-6, TNFα, and MMP3 [Homandberg and Wen, 1998; Homandberg et al. 1998].
Complement in osteoarthritis
In addition to the contribution of PRRs, activation of the complement system represents yet another innate immune mechanism by which OA inflammation and cartilage damage may be propagated. Proteomics analyses by our group [Sohn et al. 2012] and others [Gobezie et al. 2007] revealed increased levels of numerous complement components in osteoarthritic compared with healthy synovial fluids. Moreover, in OA synovium, upregulation of complement effector genes and downregulation of complement inhibitors was demonstrated relative to normal controls [Wang et al. 2011]. Further support for a role of complement in OA pathogenesis was provided by animal studies in which mice deficient in complement effectors C5 and C6 were protected in an experimental OA model, whereas disease was aggravated in mice deficient in CD59, a cell surface inhibitor of the membrane attack complex (MAC), C5b-9 [Wang et al. 2011]. Activation of complement by cartilage ECM components fibromodulin and aggrecan induced the assembly of MAC [Wang et al. 2011], which either through direct chondrolysis or induction of sublytic inflammatory signaling pathways promotes further cartilage damage and production of ECM breakdown products, perpetuating the cycle of complement activation. Notably, other ECM components have been demonstrated to activate complement including cartilage oligomeric matrix protein [Happonen et al. 2010], osteoadherin, and chondroadherin [Sjoberg et al. 2009].
Mechanical stress-induced immune activation
Another potential mechanism contributing to chronic inflammation, although not classically considered part of innate immunity, is the ability of mechanical forces to directly induce production of inflammatory mediators from cartilage and synovium [Loeser, 2006]. However, the presence of subtle cartilage damage at sites of mechanical forces could also be associated with the release of inflammation-inducing DAMPs from ECM damage or necrotic cell death.
Inflammatory mediators in osteoarthritis
Soluble inflammatory factors including cytokines, chemokines, adipokines, neuropeptides, and lipid inflammatory mediators have been implicated in OA pathogenesis. The following discussion and Table 2 provide an overview of these mediators.
Table 2.
Inflammatory mediators in osteoarthritis.
| Cytokines | Chemokines | Growth factors | Adipokines | Prostaglandins/leukotrienes |
|---|---|---|---|---|
| IL-1 [Dingle et al. 1979; Goldring et al. 1988; Pfander et al. 2004] | Chemerin [Huss et al. 2010] | TGFβ [Blaney Davidson et al. 2007] | Adiponectin [Conde et al. 2011] | PGE2 [Martel-Pelletier et al. 2003] |
| IL-6 [Kaneko et al. 2000] | IL-8 [Chauffier et al. 2012; Kaneko et al. 2000] | VEGF [Haywood et al. 2003] | Leptin [Dumond et al. 2003] | LTB4 [Wittenberg et al. 1993] |
| IL-15 [Scanzello et al. 2009] | MCP-1 [Vangsness et al. 2011] | Resistin [Choe et al. 2012] | ||
| IL-18 [Olee et al. 1999] | MIP-1α [Hsu et al. 2004; Vangsness et al. 2011] | Visfatin [Chen et al. 2010; Gosset et al. 2008] | ||
| TNFα [Saklatvala, 1986] | RANTES [Hsu et al. 2004] |
IL, interleukin; LTB4, leukotriene B4; MCP, monocyte chemotactic protein; MIP-1, macrophage inflammatory protein; PGE2, prostaglandin E2; RANTES, regulated and normal T-cell expressed and secreted; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Cytokines
Soluble inflammatory factors such as cytokines are central to most inflammatory processes, and several cytokines have been implicated in OA pathogenesis. Studies by Kaneko and colleagues observed increased levels of IL-6 and IL-8 in OA serum and synovial fluid [Kaneko et al. 2000], and work from our group [Sohn et al. 2012] (Figure 1) identified elevated levels of a large repertoire of cytokines in both blood and synovial fluid of patients with OA. Similarly, work by Scanzello and colleagues demonstrated elevated levels of synovial fluid IL-15 in early knee OA. Furthermore, they revealed a positive correlation between levels of synovial fluid IL-15 with numbers of CD8 T cells within the synovial membrane and levels of synovial fluid MMP1, MMP3, and IL-6 [Scanzello et al. 2009].
Although the contribution of these cytokines to OA pathogenesis is not always clear, numerous in vitro studies support an overall catabolic role for cytokines elevated in the OA joint. IL-1β and TNFα signaling, culminating in the activation of nuclear factor κB and activator protein 1 transcription factors, can induce autocrine production of IL-1β and TNFα as well as expression of other critical inflammatory and chrondrolytic mediators, including MMP1, MMP9, MMP13, nitric oxide, prostaglandin E2 (PGE2), and IL-6 [Attur et al. 1998]. The role of IL-6 in OA is controversial. Elevated at sites of inflammation, IL-6 has been shown to activate B cells, T cells, and mediate the recruitment of inflammatory cells to sites of inflammation [Gabay, 2006]. Additionally, IL-6 receptor blockade is efficacious in the treatment of RA [Jones et al. 2010]. However, in the zymosan-induced model of joint inflammation, IL-6 was observed to play a dual role by reducing proteoglycan loss in the acute phase but enhancing osteophyte formation in the chronic phase [van de Loo et al. 1997]. This same group also showed that mice deficient in IL-6 displayed increased levels of cartilage loss in a spontaneous aging model of OA [de Hooge et al. 2005], again suggesting a potential protective role for IL-6 in the development of OA.
In addition to the ability of inflammatory cytokines to induce cartilage catabolism, many cytokines can also promote OA progression by inhibiting anabolic processes critical to cartilage homeostasis [Goldring et al. 1994; Saklatvala, 1986]. As an example, IL-1β has been demonstrated to inhibit production of cartilage ECM components, including aggrecan [Pfander et al. 2004] and types II and IX collagen [Goldring et al. 1988].
Adipokines
In addition to traditional cytokines, a new class of soluble mediators known as adipokines has been associated with OA [Conde et al. 2011]. So-called because they are primarily (although not exclusively) derived from adipose tissue, adipokines, including leptin [Ku et al. 2009], have been associated with OA incidence and severity. The role of adiponectin is less clear. One study observed increased adiponectin levels in patients with erosive disease compared with nonerosive disease [Filkova et al. 2009], while others suggest a protective role for adiponectin in OA [Chen et al. 2006; Honsawek and Chayanupatkul, 2010]. Also supporting a role for adipokines in OA are several in vitro studies demonstrating the ability of adipokines including leptin, adiponectin, visfatin, and resistin to induce inflammatory mediators and chondrolysis [Conde et al. 2011]. Recent studies have shown a contributory role for adipokines in the development of obesity-related inflammation and the metabolic syndrome [Qatanani et al. 2009]. Thus, epidemiologic studies above demonstrating elevated adipokines in osteoarthritic serum may suggest one potential mechanism by which obesity increases the risk of OA [Felson et al. 1988]. However, the association between obesity and knee OA may be confounded by altered biomechanics also associated with obesity.
Although the exact contribution of fat and adipokines to OA pathophysiology remains to be clarified, several recent studies have investigated the role of the infrapatellar fat pad as a local mediator of pain and inflammation in OA. Infrapatellar fat pads derived from knees of patients with OA have been observed to contain not only adipocytes but also increased numbers of macrophages, lymphocytes, and granulocytes [Clockaerts et al. 2010]. Studies of fat pad explants have demonstrated the ability of this tissue to produce and secrete large amounts of leptin and adiponectin [Hui et al. 2012] as well as inflammatory mediators such as VEGF, TNFα, and IL-6 [Ushiyama et al. 2003]. Additionally, the OA fat pad is highly innervated by small C-fiber neurons containing the neuroinflammatory mediator and vasodilator Substance P that mediates not only pain sensation, but also directly acts on a variety of immune cells and the vascular system to induce proinflammatory cytokine (IL-1β and TNFα) production and vascular leak respectively [Bohnsack et al. 2005]. Thus, local fatty tissues within the joint including the infrapatellar fat pad may provide an additional source of inflammatory mediators such as adipokines and neuropeptides, as well as more classic soluble mediators of inflammation such as IL-1β, TNFα, and IL-6.
Prostaglandins, leukotrienes, and other lipid mediators
The enzyme cyclooxygenase-2 (COX-2) is upregulated in inflamed joint tissues and is responsible for elevated production of lipid mediators including prostaglandins such as PGE2 in the OA joint [Martel-Pelletier et al. 2003]. Studies have suggested that overexpression of COX-2 is likely induced by proinflammatory mediators such as IL-1β, TNFα, and IL-6, as well as via TLR4 stimulation [Geng et al. 1995]. There is in fact an extensive literature beyond the scope of this review suggesting that PGE2 is involved in inflammation, apoptosis, angiogenesis, and possibly structural changes that characterize arthritic diseases, which is well reviewed elsewhere by Martel-Pelletier and colleagues [Martel-Pelletier et al. 2003].
The biosynthetic pathway producing prostaglandin begins with production of arachidonic acid by the enzyme phospholipase A2. In addition to the generation of prostaglandins by COX enzymes, arachadonic acid can be converted to another class of lipid mediators known as leukotrienes through the action of the lipoxygenase family of enzymes. These mediators, primarily leukotriene B4 (LTB4) and its metabolite LTC4, are produced by OA synovium and to a lesser extent OA bone and cartilage [Wittenberg et al. 1993]. In addition to its role as a powerful leukocyte chemoattractant [Casale et al. 1992], LTB4 has been demonstrated to stimulate TNFα and IL-1β production from human OA synovial explants [He et al. 2002].
Imaging to identify inflammation: epidemiologic tool and clinical potential
Although pathologic evaluation may remain the gold standard for identifying the presence of inflammation in OA, the invasiveness of the synovial biopsy (or the need to wait until time of surgical intervention such as joint arthroplasty) limits the opportunity to study potential new therapies for OA. However, imaging technologies have advanced significantly and now provide a useful surrogate for identification and quantitation of synovial inflammation. The mainstay of synovial imaging has been MRI, with early studies demonstrating the presence of bone marrow edema along the medial or lateral knee predicting progression of OA in their respective compartments [Felson et al. 2003]. It should be noted that medial bone marrow lesions were seen mostly in patients with varus limbs while lateral lesions were seen mostly in those with valgus limbs, and risks were attenuated 37–52% after adjustment for limb alignment. Thus, malalignment and mechanical derangement, although perhaps not the effectors of inflammation, are clearly the harbingers of both development of inflammation and propagation of OA pathology.
Bone marrow edema may be a useful marker of joint inflammation, but whether it is itself associated with joint inflammation or local damage resulting in propagation is unclear. Some have suggested that bone marrow edema represents a local inflammatory process at the bone–cartilage interface and that this interaction forms a functional unit which, when deranged by damage or inflammation, is involved in the initiation of OA [Lories and Luyten, 2011]. Thus the potential primary role of excessive stress at the bone– cartilage interface may yet be an initiating, if not a driving mechanism in the development of OA. The observation of increased levels of inflammatory cytokines within the bone–cartilage unit at sites of early OA support this hypothesis [Lories and Luyten, 2011]. Interestingly, one small study directly investigating the histopathology of MRI bone marrow lesions at the time of total joint arthroplasty did observe low-grade inflammation; however, the predominant findings were more consistent with localized infarction as demonstrated by vascular leak and local fibrinoid reactions with thrombus inclusions [Hunter et al. 2009]. One obvious caveat is the late stage at which these lesions were obtained. Notably, however, both cellular necrosis and vascular leak may provide a source of DAMPs capable of contributing to ongoing inflammation.
Further work in MRI has now directly evaluated the joint synovium for pathologic changes of inflammation. Earlier studies of MRI in which gadolinium contrast was not utilized concluded that the presence of synovitis at a limited number of sites in the joint (infrapatellar fat pad, suprapatellar and intercondylar regions) was associated with increased pain. Notably, the presence of synovitis by these criteria was not associated with future cartilage loss on follow-up MRI [Hill et al. 2007]. The same group performed another study in which the presence of joint effusion synovitis, identified without the use of contrast, was associated with risk of subsequent cartilage loss at 30 months. Interestingly, another study by Pelletier and colleagues also observed a small but statistically significant association of noncontrast-enhanced MRI synovitis with loss of cartilage volume only 60 days later by repeat MRI [Pelletier et al. 2008].
More recently, imaging has moved toward the use of contrast-enhanced MRI (CE-MRI). A recent translational study comparing non-CE-MRI and CE-MRI demonstrated that although noncontrast T2-weighted sequences could identify fluid and effusion volume, only CE-MRI was able to reliably detect synovitis according to a reference synovial biopsy [Loeuille et al. 2011].
It should be noted that MRI is not the only imaging methodology being developed for the evaluation and quantitation of synovitis in OA. A recent study comparing CE-MRI and power Doppler ultrasound (PD-US) noted that PD-US has superior sensitivity compared with non-CE-MRI but not that of CE-MRI. Notably, the use of contrast enhancement for PD-US was potentially even more sensitive than CE-MRI for the identification of effusion synovitis as defined in this study [Song et al. 2008]. Additionally, Conaghan and colleagues demonstrated that ultrasound-observed effusion, in combination with radiographic and clinical symptoms, was associated with increased progression to joint replacement at 3-year follow up [Conaghan et al. 2010].
Overall, it is clear that advances in imaging by MRI and/or ultrasound to assess inflammation will provide the ability to perform large-scale epidemiologic and translational studies of inflammation in OA. Although the earliest stages of synovial inflammation likely occur before synovitis is detectable by current imaging, the ability to identify radiographic features predictive of future cartilage loss will undoubtedly facilitate the development of therapeutic strategies targeting early OA with the presence of synovitis and visualization of cartilage denudation [Hunter et al. 2008] as both an entry point and surrogate endpoint to identify promising pathways and therapeutics.
Inflammation as a target for disease modification in osteoarthritis
To date, no agent has been shown to have disease-modifying effects on the structural progression of OA. Current therapies, including nonsteroidal anti-inflammatory drugs, COX-2 selective agents, intra-articular hyaluronic acid injections, and opioids offer only symptomatic relief. Agents that have demonstrated potential efficacy for disease modification include the MMP inhibitor doxycycline [Brandt et al. 2005] and the combined lipoxygenase/cyclooxygenase inhibitor licofelone [Raynauld et al. 2009]. Despite the clear role of inflammation in OA, recent trials of potent anti-inflammatory therapies, including use of systemic and intra-articular biologic agents to inhibit TNFα and IL-1β, proved disappointing [Hunter, 2008]. Major challenges for the development of DMOADs include the need for improved measures of structural damage (beyond the use of X-rays) as well as improved understanding of the appropriate population and disease stage for intervention. OA is a final common pathway following many predisposing factors and thus therapeutics may have limited, if any, efficacy in those with pre-existing joint damage, biomechanical predisposition, or obesity [Felson and Kim, 2007]. The requirement of radiographic change, a finding observed in those with relatively advanced OA, likely identifies a population less amenable to anti-inflammatory intervention. No studies to date have targeted very early OA at a time when anti-inflammatory intervention might be most effective. Although synovitis is more frequently observed in those with end stage OA [Smith et al. 1997], the ability to identify and quantitate synovitis (and vis à vis inflammation) before the onset of irreversible joint failure, provides great promise to the targeting of preradiographic inflammation akin to the paradigm now well accepted for the early aggressive treatment of RA [Knevel et al. 2010]. Thus, the increasing appreciation of clinical risk factors for the development of OA as well as the advent of highly sensitive imaging modalities capable of visualizing early synovitis and cartilage change holds great promise for the identification of the at-risk population most suitable for very early anti-inflammatory interventions.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Jeremy Sokolove, Department of Medicine/ Immunology, VA Palo Alto Health Care System, 3801 Miranda Ave, Mail Stop 154R, Palo Alto, CA 94034 Stanford University, Palo Alto, CA, USA.
Christin M. Lepus, Department of Medicine/Rheumatology, VA Palo Alto Health Care System and Stanford University, Palo Alto, CA, USA
References
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., et al. (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29: 1039–1049 [DOI] [PubMed] [Google Scholar]
- Andriacchi T., Mundermann A., Smith R., Alexander E., Dyrby C., Koo S. (2004) A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng 32: 447–457 [DOI] [PubMed] [Google Scholar]
- Aran S., Malekzadeh S., Seifirad S. (2011) A double-blind randomized controlled trial appraising the symptom-modifying effects of colchicine on osteoarthritis of the knee. Clin Exp Rheumatol 29: 513–518 [PubMed] [Google Scholar]
- Attur M., Patel I., Patel R., Abramson S., Amin A. (1998) Autocrine production of IL-1 beta by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proc Assoc Am Physicians 110: 65–72 [PubMed] [Google Scholar]
- Ayral X., Pickering E., Woodworth T., Mackillop N., Dougados M. (2005) Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13: 361–367 [DOI] [PubMed] [Google Scholar]
- Babelova A., Moreth K., Tsalastra-Greul W., Zeng-Brouwers J., Eickelberg O., Young M., et al. (2009) Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem 284: 24035–24048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito M., Veale D., FitzGerald O., van den Berg W., Bresnihan B. (2005) Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 64: 1263–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson E., van der Kraan P., van den Berg W. (2007) TGF-beta and osteoarthritis. Osteoarthritis Cartilage 15: 597–604 [DOI] [PubMed] [Google Scholar]
- Bohnsack M., Meier F., Walter G., Hurschler C., Schmolke S., Wirth C., et al. (2005) Distribution of substance-P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: a neurohistological approach to anterior knee pain syndrome. Arch Orthop Trauma Surg 125: 592–597 [DOI] [PubMed] [Google Scholar]
- Bondeson J., Blom A., Wainwright S., Hughes C., Caterson B., van den Berg W. (2010) The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum 62: 647–657 [DOI] [PubMed] [Google Scholar]
- Bondeson J., Wainwright S., Lauder S., Amos N., Hughes C. (2006) The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 8: R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K., Mazzuca S., Katz B., Lane K., Buckwalter K., Yocum D., et al. (2005) Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum 52: 2015–2025 [DOI] [PubMed] [Google Scholar]
- Casale T., Abbas M., Carolan E. (1992) Degree of neutrophil chemotaxis is dependent upon the chemoattractant and barrier. Am J Respir Cell Mol Biol 7: 112–117 [DOI] [PubMed] [Google Scholar]
- Chauffier K., Laiguillon M., Bougault C., Gosset M., Priam S., Salvat C., et al. (2012) Induction of the chemokine IL-8/Kc by the articular cartilage: possible influence on osteoarthritis. Joint Bone Spine 15 February (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Chen T., Chen L., Hsieh M., Chang C., Chou D., Tsai S. (2006) Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta 1762: 711–718 [DOI] [PubMed] [Google Scholar]
- Chen W., Bao J., Feng J., Hu P., Shi Z., Wu L. (2010) Increased serum concentrations of visfatin and its production by different joint tissues in patients with osteoarthritis. Clin Chem Lab Med 48: 1141–1145 [DOI] [PubMed] [Google Scholar]
- Chevalier X., Claudepierre P., Groult N., Zardi L., Hornebeck W. (1996) Presence of ED-A containing fibronectin in human articular cartilage from patients with osteoarthritis and rheumatoid arthritis. J Rheumatol 23: 1022–1030 [PubMed] [Google Scholar]
- Choe J., Bae J., Jung H., Park S., Lee H., Kim S. (2012) Serum resistin level is associated with radiographic changes in hand osteoarthritis: cross-sectional study. Joint Bone Spine 79: 160–165 [DOI] [PubMed] [Google Scholar]
- Clockaerts S., Bastiaansen-Jenniskens Y., Runhaar J., Van Osch G., Van Offel J., Verhaar J., et al. (2010) The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage 18: 876–882 [DOI] [PubMed] [Google Scholar]
- Conaghan P., D’Agostino M., Le Bars M., Baron G., Schmidely N., Wakefield R., et al. (2010) Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: results from a large, 3-year, prospective EULAR study. Ann Rheum Dis 69: 644–647 [DOI] [PubMed] [Google Scholar]
- Conde J., Scotece M., Gomez R., Lopez V., Gomez-Reino J., Gualillo O. (2011) Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis 2011: 203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C., Mills E., Mielke L., O’Farrell L., Lavelle E., Mori A., et al. (2012) Osteoarthritis-associated basic calcium phosphate crystals induce pro-inflammatory cytokines and damage-associated molecules via activation of Syk and PI3 kinase. Clin Immunol 144: 228–236 [DOI] [PubMed] [Google Scholar]
- Dahl I., Husby G. (1985) Hyaluronic acid production in vitro by synovial lining cells from normal and rheumatoid joints. Ann Rheum Dis 44: 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G., Hoyland J., Denton J., Donn R., Freemont A. (1993) Mast cells in the synovium and synovial fluid in osteoarthritis. Br J Rheumatol 32: 671–675 [DOI] [PubMed] [Google Scholar]
- de Hooge A., van de Loo F., Bennink M., Arntz O., de Hooge P., van den Berg W. (2005) Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage 13: 66–73 [DOI] [PubMed] [Google Scholar]
- Denoble A., Huffman K., Stabler T., Kelly S., Hershfield M., McDaniel G., et al. (2011) Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A 108: 2088–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J., Saklatvala J., Hembry R., Tyler J., Fell H., Jubb R. (1979) A cartilage catabolic factor from synovium. Biochem J 184: 177–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumond H., Presle N., Terlain B., Mainard D., Loeuille D., Netter P., et al. (2003) Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum 48: 3118–3129 [DOI] [PubMed] [Google Scholar]
- Englund M., Guermazi A., Gale D., Hunter D., Aliabadi P., Clancy M., et al. (2008) Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 359: 1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C., Mears D., McKnight J. (1981) A preliminary ferrographic survey of the wear particles in human synovial fluid. Arthritis Rheum 24: 912–918 [DOI] [PubMed] [Google Scholar]
- Farahat M., Yanni G., Poston R., Panayi G. (1993) Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis 52: 870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D. (2006) Clinical practice. Osteoarthritis of the knee. N Engl J Med 354: 841–848 [DOI] [PubMed] [Google Scholar]
- Felson D., Anderson J., Naimark A., Walker A., Meenan R. (1988) Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med 109: 18–24 [DOI] [PubMed] [Google Scholar]
- Felson D., Kim Y. (2007) The futility of current approaches to chondroprotection. Arthritis Rheum 56: 1378–1383 [DOI] [PubMed] [Google Scholar]
- Felson D., McLaughlin S., Goggins J., LaValley M., Gale M., Totterman S., et al. (2003) Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med 139: 330–336 [DOI] [PubMed] [Google Scholar]
- Filkova M., Liskova M., Hulejova H., Haluzik M., Gatterova J., Pavelkova A., et al. (2009) Increased serum adiponectin levels in female patients with erosive compared with non-erosive osteoarthritis. Ann Rheum Dis 68: 295–296 [DOI] [PubMed] [Google Scholar]
- Foell D., Wittkowski H., Roth J. (2007) Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol 3: 382–390 [DOI] [PubMed] [Google Scholar]
- Gabay C. (2006) Interleukin-6 and chronic inflammation. Arthritis Res Ther 8(Suppl. 2): S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Blanco F., Cornelisson M., Lotz M. (1995) Regulation of cyclooxygenase-2 expression in normal human articular chondrocytes. J Immunol 155: 796–801 [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E., Mouktaroudi M., Bodar E., van der Ven J., Kullberg B., Netea M., et al. (2009) Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 beta by mononuclear cells through a caspase 1-mediated process. Ann Rheum Dis 68: 273–278 [DOI] [PubMed] [Google Scholar]
- Gobezie R., Kho A., Krastins B., Sarracino D., Thornhill T., Chase M., et al. (2007) High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther 9: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M., Birkhead J., Sandell L., Kimura T., Krane S.M. (1988) Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest 82: 2026–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M., Fukuo K., Birkhead J., Dudek E., Sandell L. (1994) Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem 54: 85–99 [DOI] [PubMed] [Google Scholar]
- Gordon G., Villanueva T., Schumacher H., Gohel V. (1984) Autopsy study correlating degree of osteoarthritis, synovitis and evidence of articular calcification. J Rheumatol 11: 681–686 [PubMed] [Google Scholar]
- Gosset M., Berenbaum F., Salvat C., Sautet A., Pigenet A., Tahiri K., et al. (2008) Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis Rheum 58: 1399–1409 [DOI] [PubMed] [Google Scholar]
- Happonen K., Saxne T., Aspberg A., Morgelin M., Heinegard D., Blom A. (2010) Regulation of complement by cartilage oligomeric matrix protein allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum 62: 3574–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraoui B., Pelletier J., Cloutier J., Faure M., Martel-Pelletier J. (1991) Synovial membrane histology and immunopathology in rheumatoid arthritis and osteoarthritis. In vivo effects of antirheumatic drugs. Arthritis Rheum 34: 153–163 [DOI] [PubMed] [Google Scholar]
- Hasturk H., Kantarci A., Van Dyke T. (2012) Oral inflammatory diseases and systemic inflammation: role of the macrophage. Front Immunol 3: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood L., McWilliams D., Pearson C., Gill S., Ganesan A., Wilson D., et al. (2003) Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum 48: 2173–2177 [DOI] [PubMed] [Google Scholar]
- He W., Pelletier J., Martel-Pelletier J., Laufer S., Di Battista J. (2002) Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with antiinflammatory cytokines. J Rheumatol 29: 546–553 [PubMed] [Google Scholar]
- Hill C., Hunter D., Niu J., Clancy M., Guermazi A., Genant H., et al. (2007) Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 66: 1599–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homandberg G., Hui F. (1996) Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage cultured with fibronectin fragments. Arch Biochem Biophys 334: 325–331 [DOI] [PubMed] [Google Scholar]
- Homandberg G., Meyers R., Williams J. (1993) Intraarticular injection of fibronectin fragments causes severe depletion of cartilage proteoglycans in vivo. J Rheumatol 20: 1378–1382 [PubMed] [Google Scholar]
- Homandberg G., Wen C. (1998) Exposure of cartilage to a fibronectin fragment amplifies catabolic processes while also enhancing anabolic processes to limit damage. J Orthop Res 16: 237–246 [DOI] [PubMed] [Google Scholar]
- Homandberg G., Wen C., Hui F. (1998) Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage 6: 231–244 [DOI] [PubMed] [Google Scholar]
- Honsawek S., Chayanupatkul M. (2010) Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res 41: 593–598 [DOI] [PubMed] [Google Scholar]
- Hori O., Brett J., Slattery T., Cao R., Zhang J., Chen J., et al. (1995) The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270: 25752–25761 [DOI] [PubMed] [Google Scholar]
- Hsu Y., Hsieh M., Liang Y., Li C., Sheu M., Chou D., et al. (2004) Production of the chemokine eotaxin-1 in osteoarthritis and its role in cartilage degradation. J Cell Biochem 93: 929–939 [DOI] [PubMed] [Google Scholar]
- Hui W., Litherland G., Elias M., Kitson G., Cawston T., Rowan A., et al. (2012) Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis 71: 455–462 [DOI] [PubMed] [Google Scholar]
- Hunter D. (2008) Are there promising biologic therapies for osteoarthritis? Curr Rheumatol Rep 10: 19–25 [DOI] [PubMed] [Google Scholar]
- Hunter D., Gerstenfeld L., Bishop G., Davis A., Mason Z., Einhorn T., et al. (2009) Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther 11: R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D., Niu J., Zhang Y., LaValley M., McLennan C., Hudelmaier M., et al. (2008) Premorbid knee osteoarthritis is not characterised by diffuse thinness: the Framingham Osteoarthritis Study. Ann Rheum Dis 67: 1545–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss R., Huddleston J., Goodman S., Butcher E., Zabel B. (2010) Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum 62: 3799–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., et al. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179 [DOI] [PubMed] [Google Scholar]
- Jones G., Sebba A., Gu J., Lowenstein M., Calvo A., Gomez-Reino J., et al. (2010) Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 69: 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Satoh T., Chiba J., Ju C., Inoue K., Kagawa J. (2000) Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther 6: 71–79 [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384 [DOI] [PubMed] [Google Scholar]
- Kim H., Cho M., Choi H., Yoon C., Jhun J., Oh H., et al. (2006) The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum 54: 2152–2163 [DOI] [PubMed] [Google Scholar]
- Knevel R., Schoels M., Huizinga T., Aletaha D., Burmester G., Combe B., et al. (2010) Current evidence for a strategic approach to the management of rheumatoid arthritis with disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 69: 987–994 [DOI] [PubMed] [Google Scholar]
- Krasnokutsky S., Belitskaya-Levy I., Bencardino J., Samuels J., Attur M., Regatte R., et al. (2011) Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum 63: 2983–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku J., Lee C., Joo B., An B., Choi S., Wang T., et al. (2009) Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol 28: 1431–1435 [DOI] [PubMed] [Google Scholar]
- Kuroki K., Stoker A., Sims H., Cook J. (2010) Expression of toll-like receptors 2 and 4 in stifle joint synovial tissues of dogs with or without osteoarthritis. Am J Vet Res 71: 750–754 [DOI] [PubMed] [Google Scholar]
- Li Z., Cheng G., Hu K., Li M., Zang W., Dong Y., et al. (2011) Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clin Invest Med 34: E298. [DOI] [PubMed] [Google Scholar]
- Libby P. (2002) Inflammation in atherosclerosis. Nature 420: 868–874 [DOI] [PubMed] [Google Scholar]
- Liu-Bryan R., Pritzker K., Firestein G., Terkeltaub R. (2005) TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol 174: 5016–5023 [DOI] [PubMed] [Google Scholar]
- Liu-Bryan R., Terkeltaub R. (2010) Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous toll-like receptor 2/toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum 62: 2004–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. (2006) Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum 54: 1357–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeuille D., Sauliere N., Champigneulle J., Rat A., Blum A., Chary-Valckenaere I. (2011) Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage 19: 1433–1439 [DOI] [PubMed] [Google Scholar]
- Lories R., Luyten F. (2011) The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 7: 43–49 [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J., Fahmi H. (2003) Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum 33: 155–167 [DOI] [PubMed] [Google Scholar]
- Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241 [DOI] [PubMed] [Google Scholar]
- McCarthy G., Cheung H. (1994) The role of cyclic-3′,5′-adenosine monophosphate in prostaglandin-mediated inhibition of basic calcium phosphate crystal-induced mitogenesis and collagenase induction in cultured human fibroblasts. Biochim Biophys Acta 1226: 97–104 [DOI] [PubMed] [Google Scholar]
- Melrose J., Fuller E., Roughley P., Smith M., Kerr B., Hughes C., et al. (2008) Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res Ther 10: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood K., Sacre S., Piccinini A., Inglis J., Trebaul A., Chan E., et al. (2009) Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 15: 774–780 [DOI] [PubMed] [Google Scholar]
- Morgan M., Whelan L., Sallis J., McCarthy C., Fitzgerald D., McCarthy G. (2004) Basic calcium phosphate crystal-induced prostaglandin E2 production in human fibroblasts: role of cyclooxygenase 1, cyclooxygenase 2, and interleukin-1beta. Arthritis Rheum 50: 1642–1649 [DOI] [PubMed] [Google Scholar]
- Mow V., Lai W., Eisenfeld J., Redler I. (1974) Some surface characteristics of articular cartilage. II. On the stability of articular surface and a possible biomechanical factor in etiology of chondrodegeneration. J Biomech 7: 457–468 [DOI] [PubMed] [Google Scholar]
- Nair A., Kanda V., Bush-Joseph C., Verma N., Chubinskaya S., Mikecz K., et al. (2012) Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to TLR-4 and TLR-2 ligands via soluble CD14. Arthritis Rheum 64: 2268–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogi T., Nevitt M., Niu J., LaValley M., Hunter D., Terkeltaub R., et al. (2006) Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis: results of two prospective longitudinal magnetic resonance imaging studies. Arthritis Rheum 54: 1822–1828 [DOI] [PubMed] [Google Scholar]
- Nettelbladt E., Sundblad L. (1959) Protein patterns in synovial fluid and serum in rheumatoid arthritis and osteoarthritis. Arthritis Rheum 2: 144–151 [DOI] [PubMed] [Google Scholar]
- Okamura Y., Watari M., Jerud E., Young D., Ishizaka S., Rose J., et al. (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276: 10229–10233 [DOI] [PubMed] [Google Scholar]
- Olee T., Hashimoto S., Quach J., Lotz M. (1999) IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol 162: 1096–1100 [PubMed] [Google Scholar]
- Ospelt C., Neidhart M., Gay R., Gay S. (2004) Synovial activation in rheumatoid arthritis. Front Biosci 9: 2323–2334 [DOI] [PubMed] [Google Scholar]
- Park J., Svetkauskaite D., He Q., Kim J., Strassheim D., Ishizaka A., et al. (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370–7377 [DOI] [PubMed] [Google Scholar]
- Pauli C., Grogan S., Patil S., Otsuki S., Hasegawa A., Koziol J., et al. (2011) Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage 19: 1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazar B., Ea H., Narayan S., Kolly L., Bagnoud N., Chobaz V., et al. (2011) Basic calcium phosphate crystals induce monocyte/macrophage IL-1beta secretion through the NLRP3 inflammasome in vitro. J Immunol 186: 2495–2502 [DOI] [PubMed] [Google Scholar]
- Pearle A., Scanzello C., George S., Mandl L., DiCarlo E., Peterson M., et al. (2007) Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage 15: 516–523 [DOI] [PubMed] [Google Scholar]
- Pelletier J., Raynauld J., Abram F., Haraoui B., Choquette D., Martel-Pelletier J. (2008) A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage 16(Suppl. 3): S8–S13 [DOI] [PubMed] [Google Scholar]
- Pfander D., Heinz N., Rothe P., Carl H., Swoboda B. (2004) Tenascin and aggrecan expression by articular chondrocytes is influenced by interleukin 1beta: a possible explanation for the changes in matrix synthesis during osteoarthritis. Ann Rheum Dis 63: 240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qatanani M., Szwergold N., Greaves D., Ahima R., Lazar M. (2009) Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest 119: 531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynauld J., Martel-Pelletier J., Bias P., Laufer S., Haraoui B., Choquette D., et al. (2009) Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann Rheum Dis 68: 938–947 [DOI] [PubMed] [Google Scholar]
- Roemer F., Guermazi A., Felson D., Niu J., Nevitt M., Crema M., et al. (2011) Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis 70: 1804–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. (2011) Crystals, inflammation, and osteoarthritis. Curr Opin Rheumatol 23: 170–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. (1986) Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 322: 547–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello C., Goldring S. (2012) The role of synovitis in osteoarthritis pathogenesis. Bone 51: 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello C., McKeon B., Swaim B., DiCarlo E., Asomugha E., Kanda V., et al. (2011) Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum 63: 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello C., Plaas A., Crow M. (2008) Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol 20: 565–572 [DOI] [PubMed] [Google Scholar]
- Scanzello C., Umoh E., Pessler F., Diaz-Torne C., Miles T., Dicarlo E., et al. (2009) Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage 17: 1040–1048 [DOI] [PubMed] [Google Scholar]
- Schaefer L., Babelova A., Kiss E., Hausser H., Baliova M., Krzyzankova M., et al. (2005) The matrix component biglycan is proinflammatory and signals through toll-like receptors 4 and 2 in macrophages. J Clin Invest 115: 2223–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelbergen R., Blom A., van den Bosch M., Sloetjes A., Abdollahi-Roodsaz S., Schreurs B., et al. (2012) Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on toll-like receptor 4. Arthritis Rheum 64: 1477–1487 [DOI] [PubMed] [Google Scholar]
- Sellam J., Berenbaum F. (2010) The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 6: 625–635 [DOI] [PubMed] [Google Scholar]
- Singh J., Furst D., Bharat A., Curtis J., Kavanaugh A., Kremer J., et al. (2012) 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 64: 625–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoberg A., Manderson G., Morgelin M., Day A., Heinegard D., Blom A. (2009) Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol 46: 830–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzeczynska-Moncznik J., Stefanska A., Zabel B., Kapinska-Mrowiecka M., Butcher E., Cichy J. (2009) Chemerin and the recruitment of NK cells to diseased skin. Acta Biochim Pol 56: 355–360 [PMC free article] [PubMed] [Google Scholar]
- Smiley S., King J., Hancock W. (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167: 2887–2894 [DOI] [PubMed] [Google Scholar]
- Smith M., Triantafillou S., Parker A., Youssef P., Coleman M. (1997) Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 24: 365–371 [PubMed] [Google Scholar]
- Sohn D., Sokolove J., Sharpe O., Erhart J., Chandra P., Lahey L., et al. (2012) Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via toll-like receptor 4. Arthritis Res Ther 14: R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I., Althoff C., Hermann K., Scheel A., Knetsch T., Schoenharting M., et al. (2008) Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann Rheum Dis 67: 19–25 [DOI] [PubMed] [Google Scholar]
- Soren A., Klein W., Huth F. (1976) Microscopic comparison of the synovial changes in posttraumatic synovitis and osteoarthritis. Clin Orthop Relat Res (121): 191–195 [PubMed] [Google Scholar]
- Spector T., Hart D., Nandra D., Doyle D., Mackillop N., Gallimore J., et al. (1997) Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum 40: 723–727 [DOI] [PubMed] [Google Scholar]
- Stahel P., Smith W., Moore E. (2007) Role of biological modifiers regulating the immune response after trauma. Injury 38: 1409–1422 [DOI] [PubMed] [Google Scholar]
- Steenvoorden M., Bank R., Ronday H., Toes R., Huizinga T., DeGroot J. (2007) Fibroblast-like synoviocyte-chondrocyte interaction in cartilage degradation. Clin Exp Rheumatol 25: 239–245 [PubMed] [Google Scholar]
- Su S., Tsai C., Lee C., Salter D., Lee H. (2005) Expression and regulation of toll-like receptor 2 by IL-1beta and fibronectin fragments in human articular chondrocytes. Osteoarthritis Cartilage 13: 879–886 [DOI] [PubMed] [Google Scholar]
- Telander D. (2011) Inflammation and age-related macular degeneration (AMD). Semin Ophthalmol 26: 192–197 [DOI] [PubMed] [Google Scholar]
- Termeer C., Benedix F., Sleeman J., Fieber C., Voith U., Ahrens T., et al. (2002) Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195: 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama T., Chano T., Inoue K., Matsusue Y. (2003) Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis 62: 108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo F., Kuiper S., van Enckevort F., Arntz O., van den Berg W. (1997) Interleukin-6 reduces cartilage destruction during experimental arthritis. A study in interleukin-6-deficient mice. Am J Pathol 151: 177–191 [PMC free article] [PubMed] [Google Scholar]
- Vangsness C., Jr, Burke W., Narvy S., MacPhee R., Fedenko A. (2011) Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis – a pilot study. Bull NYU Hosp Jt Dis 69: 122–127 [PubMed] [Google Scholar]
- van Lent P., Blom A., Schelbergen R., Sloetjes A., Lafeber F., Lems W., et al. (2012) Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum 64: 1466–1476 [DOI] [PubMed] [Google Scholar]
- Wang Q., Rozelle A., Lepus C., Scanzello C., Song J., Larsen D., et al. (2011) Identification of a central role for complement in osteoarthritis. Nat Med 17: 1674–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg R., Willburger R., Kleemeyer K., Peskar B. (1993) In vitro release of prostaglandins and leukotrienes from synovial tissue, cartilage, and bone in degenerative joint diseases. Arthritis Rheum 36: 1444–1450 [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 12: 1005–1015 [DOI] [PubMed] [Google Scholar]