Abstract
The Cry48Aa/Cry49Aa binary toxin of Bacillus sphaericus was recently discovered by its ability to kill Culex quinquefasciatus mosquito larvae through a novel interaction between its two components. We have investigated the target specificity of this toxin and show it to be non-toxic to coleopteran, lepidopteran and other dipteran insects, including closely related Aedes and Anopheles mosquitoes. This represents an unusually restricted target range for crystal toxins from either B. sphaericus or Bacillus thuringiensis. Gut extracts from Culex and Aedes larvae show differential processing of the Cry48Aa protein, with the location of cleavage sites in Culex reflecting those previously shown for the activation of Cry4 toxins in mosquitoes. Pre-activation of Cry48Aa/Cry49Aa with Culex extracts, however, fails to induce toxicity to Aedes larvae. Co-administration of Cry49Aa with Cry4Aa gives higher than predicted toxicity, perhaps suggesting weak synergism against Culex larvae between Cry49Aa and other three-domain Cry toxins.
Introduction
We have recently described a previously unknown toxin from Bacillus sphaericus strain IAB59 that is composed of two proteins, Cry48Aa and Cry49Aa (Jones et al., 2007). Cry49Aa forms part of a family with the BinA and BinB components of the mosquitocidal binary toxin of B. sphaericus, along with their relatives from Bacillus thuringiensis, Cry36 (Rupar et al., 2003) and Cry35, which itself is part of a binary toxin with the approximately 14 kDa Cry34 (Ellis et al., 2002; Baum et al., 2004). Cry48Aa is clearly a member of the family of three-domain Cry toxins of B. thuringiensis and shows approximately 30% identity with known mosquitocidal toxins such as Cry4Aa. Despite this, neither Cry48Aa nor Cry49Aa are toxic when fed individually to Culex quinquefasciatus mosquitoes. However, high-level toxicity to this insect is achieved when the individual proteins are co-administered at the optimum 1:1 ratio (with an LC50 that equates to 15.9 ng ml−1 Cry48Aa and 6.3 ng ml−1 Cry49Aa (Jones et al., 2007)). Cry48Aa/Cry49Aa therefore represent a new insecticidal combination that exploits an interaction between two previously un-associated toxin families. In this study, we investigate the insect target range of this toxin and demonstrate an apparent restriction of toxicity to the Culex pipiens complex of mosquitoes. We also investigate possible interactions between Cry49Aa and another related three-domain toxin, Cry4A.
Results
Bioassays
In bioassays using the individual recombinant B. thuringiensis producing Cry48Aa or Cry49Aa, no toxicity against Cx. quinquefasciatus was observed. However, when a combination of the two recombinants was used, 100% larval death occurred, confirming our previous findings (Jones et al., 2007). The Cry49Aa protein is a member of a family of proteins that includes Cry36A, which is reported to be somewhat toxic to coleopteran larvae (Rupar et al., 2003). However, the Cry48Aa- and Cry49Aa-producing strains showed no toxicity (individually or in combination) to the coleopteran Anthonomus grandis. Nor was any toxicity seen when cultures were assayed against the three lepidopteran targets Anticarsia gemmatalis, Spodoptera frugiperda or Plutella xylostella. In all cases, 100% mortality of target insects could be achieved by use of equivalent doses of appropriate B. thuringiensis strains (B. thuringiensis ssp. israelensis for Diptera; B. thuringiensis ssp. kurstaki HD-1 for Lepidoptera; B. thuringiensis ssp. tenebrionis T08 017 for Anth. grandis).
The genus Culex is in the taxonomic order Diptera, suborder Nematocera, so toxicity to more closely related insects from this suborder was tested. Chironomus riparius larvae were also refractory to the action of the toxins. Of particular note, however, was the complete absence of toxicity of the Cry48Aa and Cry49Aa toxins, individually or as a combination, to the mosquito species Aedes aegypti and Anopheles gambiae that share the same taxonomic family (Culicidae) with Culex.
Toxin synergism
As Cry48Aa is closely related to the Cry4 toxins but seems to require the presence of Cry49Aa for activity against Cx. quinquefasciatus, it was of interest to conduct a preliminary assessment of the potential of Cry49Aa to synergize with a Cry4 protein. In bioassays against the Syn-P colony, Cry49Aa crystals, as expected, produced no mortality at up to 200 μg ml−1. In contrast, Cry4Aa caused mortality that exhibited a fluctuating plateau from 5 to 200 μg ml−1, averaging 59.6%, similar to previous assays with this material against other Cx. quinquefasciatus colonies (Wirth and Georghiou, 1997). This behaviour indicates that the Cry4Aa is poorly active and does not fit the assumption of a normal distribution, hence precluding Probit analysis. The Cry4Aa preparation was then co-administered in a 3:1 ratio with Cry49Aa crystals. At 200 μg ml−1 (150 μg ml−1 Cry4Aa plus 50 μg ml−1 Cry49Aa), the observed mortality was 81.3% (standard deviation, sigma = 7.5), 1.7 times higher than the 47% (sigma = 11.5) seen for 150 μg of Cry4Aa alone. Although these preliminary assays do not establish that this apparent increase in activity in the presence of Cry49Aa is statistically significant, the consistently higher mortality caused by the mixture may be indicative of a weak synergistic interaction. However, synergism was not evident at combined toxin concentrations of 20 or 2 μg ml−1 and further experiments would be required to definitively establish synergy. Mortality following exposure to Cry4Aa (150 μg ml−1) plus Cry49Aa (50 μg ml−1) in the Cry4A + Cry4B-resistant colony Cq4AB was 25%. This is again higher than predicted for the mixture, but cannot be statistically distinguished from it because of the high standard deviation (sigma = 18).
Molecular model of Cry48Aa
blast database searches (Altschul et al., 1990) and amino acid sequence analysis of Cry48Aa have revealed that it shows homology to the three-domain Cry toxins for which crystal structures are available. The homology-modelling server, swiss-model (Schwede et al., 2003), was used to generate a first-approximation three-dimensional model of Cry48Aa.
While care must be taken when considering three-dimensional models, the general features of the Cry48Aa model show a typical three-domain Cry toxin structure (Fig. 1). Domain I is predicted to contain the seven α-helical bundle, thought to be involved in pore formation, and domain III is modelled to have a β-sheet ‘jelly roll’ topology as seen in the Cry toxin structures in the PDB database. Domain II exhibits the anti-parallel β-sheets that are found in the other Cry toxin structures and the regions predicted to correspond to the exposed loops thought to be involved in receptor binding (Li et al., 1991; Grochulski et al., 1995; Galitsky et al., 2001; Morse et al., 2001; Boonserm et al., 2005). The domain II loops of the Cry48Aa model align to the region of Cry4Ba containing exposed loops and, as in Cry4Ba, these loops are smaller than in other toxins such as Cry3Aa and Cry1Aa. It has been shown that mutations in loop 3 of Cry4Ba can result in the introduction of toxicity to Cx. quinquefasciatus and Cx. pipiens larvae (Delécluse et al., 1993; Abdullah et al., 2006), towards which wild-type Cry4Ba shows no significant natural toxicity (Delécluse et al., 1993). Mutations in loops 1 and 2 also resulted in loss of toxicity towards Aedes and Anopheles, with no increase in toxicity towards Culex, confirming the importance of these regions in determination of specificity.
Fig. 1.
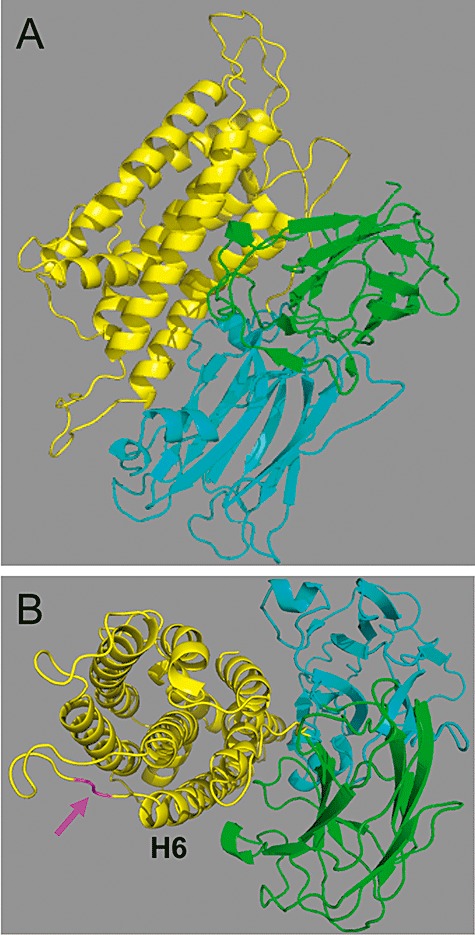
Molecular model of Cry48Aa. Model of Cry48Aa showing domain I (yellow), domain II (blue), domain III (green). The cleavage site for Culex gut extracts between the central helices 5 and 6 (H6) is shown in magenta in B. Image created using Pymol software.
Proteolytic processing of the toxin
Cry insecticidal toxins are produced during sporulation as parasporal crystals, which are solubilized in the insect gut and undergo proteolytic processing before receptor binding and membrane pore formation occurs. The major gut proteinases involved in Cry toxin processing are trypsin-like and chymotrypsin-like enzymes, while thermolysin-like and elastase-like enzymes have also been reported (Dai and Gill, 1993; Rukmini et al., 2000). Differential processing of the Cry pro-toxins by different larvae has also been shown to determine target insect toxicity (Haider et al., 1986; Haider and Ellar, 1987a, b).
To determine whether the processing of the Cry48Aa/Cry49Aa toxin in Ae. aegypti and Cx. quinquefasciatus larvae might be responsible for the differential toxicity to these two mosquitoes, both Cry48Aa and Cry49Aa were incubated in vitro with larval gut extracts from these insects, as well as the enzymes trypsin, chymotrypsin and proteinase K (Fig. 2). Cry49Aa processing (Fig. 2A) produces bands of similar size on incubation with both gut extracts and with trypsin, chymotrypsin or proteinase K. N-terminal sequencing of the protein activated by Cx. quinquefasciatus gut extract identified the site of processing to be between F48 and N49, a chymotrypsin-like cleavage site.
Fig. 2.
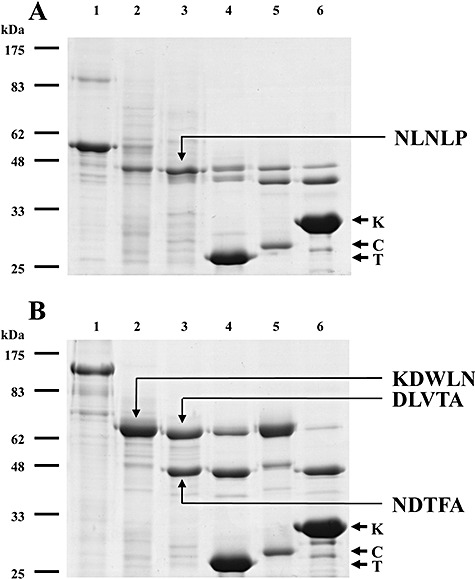
Digestion of Cry48 and Cry49 with gut extracts. Processing of Cry49Aa (A) and Cry48Aa (B) by Ae. aegypti gut extract (lane 2), Cx. quinquefasciatus gut extract (lane 3), trypsin (lane 4), chymotrypsin (lane 5) or proteinase K (lane 6). Undigested Cry protein is shown in lane 1. N-terminal sequences derived from the bands marked are given. Bands corresponding to the purified proteinases added in lanes 4–6 are marked T (trypsin), C (chymotrypsin) and K (proteinase K).
In contrast to the apparently similar processing of Cry49Aa under all conditions, different patterns were seen (Fig. 2B) after incubation of Cry48Aa with Ae. aegypti gut extract and Cx. quinquefasciatus extracts. N-terminal sequencing of the product of Cry48Aa activated by Ae. aegypti extract identified a cleavage site between Y35 and K36, corresponding to a chymotrypsin-like cleavage on the carboxyl side of an aromatic amino acid. This is consistent with the similar protein bands observed after processing by Ae. aegypti gut extract and chymotrypsin (Fig. 2, lanes 2 and 5). Cry48Aa processed by Cx. quinquefasciatus gut extract yields two major products. Edman degradation revealed that the higher molecular mass product (∼60 kDa) was typical of chymotrypsin-like cleavage between Y52 and D53, while the lower molecular mass (∼46 kDa) product is a result of a trypsin-like cleavage between R238 and N239 (consistent with a similar banding pattern of trypsin incubation, Fig. 2B, lanes 2 and 5).
Effect of activation on target range
As the processing of Cry48Aa by Cx. quinquefasciatus gut extracts produced smaller products than those from Ae. aegypti extracts, experiments were undertaken to determine whether differential processing of Cry48Aa and Cry49Aa in the two mosquito species might be responsible for the pattern of toxicity observed against the larvae of each species. Purified Cry48Aa and Cry49Aa were incubated with Cx. quinquefasciatus gut extract to allow processing as before. Following incubation, both samples were combined in a selective bioassay against Ae. aegypti larvae to test the possibility that the Culex activation of the proteins might confer toxicity to Ae. aegypti. Control bioassays were also prepared containing no toxin, and an un-processed Cry49Aa/Cry48Aa crystal protein mixture added directly to the bioassay. A bioassay using Cx. quinquefasciatus larvae, exposed to the Cry48Aa/Cry49Aa toxin pre-incubated with Cx. quinquefasciatus gut extract, was also prepared, as above, to confirm that the processed toxin retained toxicity when fed to susceptible larvae. No toxicity was observed against Ae. aegypti larvae, using either the activated Cry48Aa/Cry49Aa toxin or the crystal proteins. Bioassay of the activated toxin against Cx. quinquefasciatus larvae resulted in 100% mortality while no mortality was observed in the control bioassays containing no toxins.
Discussion
The results presented here confirm our previous finding that both components of the Cry48Aa/Cry49Aa pair are necessary to cause toxicity in Cx. quinquefasciatus larvae. Synergism between the Bin toxins of B. sphaericus, which are related to Cry49Aa, and Cry toxins of B. thuringiensis has been reported previously (Wirth et al., 2004b). In our assays, the toxicity of a Cry49Aa/Cry4Aa combination was higher than predicted for this mixture against both susceptible and Cry4-resistant larvae, indicating possible synergy between the two proteins. Interactions of Cry49Aa with three-domain proteins other than Cry48Aa, thus appear to be a possibility.
The target range for the Cry48Aa/Cry49Aa toxin pair from B. sphaericus is apparently very narrowly limited to the genus Culex. The processing of the proteins by gut extracts was, thus, investigated as a possible source of toxicity differences with Culex and Aedes targets. Processing of Cry48Aa is significantly different with extracts from the two mosquitoes, with Culex extracts producing fragments akin to those produced by the cleavage of Cry4Aa and Cry4Ba by mosquito gut extracts or trypsin, which is thought to involve cleavage to a 60–68 kDa protein before further processing into two fragments of 46–48 and 16–18 kDa (Angsuthanasombat et al., 1991, 1992). Inspection of the homology model of Cry48Aa shows the processing site for the Cx. quinquefasciatus gut extract after R238 to lie in domain I, which comprises an α-helical bundle, known to be important for toxicity, involved in lysis of midgut epithelial cells by formation of pores. Processing at this site would result in cleavage in the long predicted inter-helical loop between the central helices 5 and 6, as shown in Fig. 1B. Processing between helices 5 and 6 is also known to occur in Cry4Aa and Cry4Ba, and it has been suggested that this may assist the Cry4Ba toxin to undergo a conformational change, facilitating the insertion of domain I into the membrane (Angsuthanasombat et al., 1993; Boonserm et al., 2005). However, removal of the inter-helical cleavage sites in Cry4Ba and Cry4Aa is not detrimental to toxicity, suggesting that processing at this site is not essential for the conformational change required for pore formation (Angsuthanasombat et al., 1993; Boonserm et al., 2004; Boonserm et al., 2006). Perhaps, consistent with this observation, differential processing of the Cry48Aa/Cry49Aa proteins in Ae. aegypti mosquitoes does not account for its non-toxicity to this insect as pre-processing with Culex extracts does not reveal Aedes toxicity. This may indicate that the specificity of this toxin is mediated by receptors in Cx. quinquefasciatus that may be absent from the Ae. aegypti larval gut. Previously described toxins do not necessarily kill all related insect species: for instance, Cry4A and Cry10A of B. thuringiensis ssp. israelensis are toxic to Ae. aegypti, but show negligible toxicity to Chironomus tepperi (Crickmore et al., 1995; Hughes et al., 2005); and the Bin toxin variants of B. sphaericus are highly active against Cx. quinquefasciatus but have little to no activity against Ae. aegypti (Berry et al., 1993), but most toxins are active against more than one species in a closely related group of insects. This makes the non-toxicity of the Cry48Aa/Cry49Aa combination against Ae. aegypti and An. gambiae particularly interesting.
Culex quinquefasciatus (more correctly Cx. pipiens quinquefasciatus) is one of four subspecies in the Cx. pipiens complex that also includes Cx. pipiens pipiens. The Cry48Aa/Cry49Aa toxin pair is able to overcome resistance in Cx. quinquefasciatus to the Bin toxin of B. sphaericus (Jones et al., 2007). Strains producing Cry48Aa/Cry49Aa, such as B. sphaericus IAB59, LP1G and 47-6B, can also overcome Bin resistance in Cx. quinquefasciatus larvae (Wirth et al., 2000; Pei et al., 2002; Yuan et al., 2003). These strains have also been observed to overcome resistance in Cx. pipiens pipiens larvae (Silva-Filha et al., 2004), indicating probable Cry48Aa/Cry49Aa toxicity to this related mosquito. Therefore, the Cry48Aa/Cry49Aa toxin should be considered as toxic to Cx. pipiens complex mosquitoes but, at least at present, this must be designated as the only known target for these proteins. This toxin therefore remains something of an enigma: an obligate binary toxin comprising members of two previously separate toxin families, capable of high-level toxicity in purified form but severely limited by low-level production of the Cry48Aa component in vivo and with a severely limited target range. It forms part of the arsenal of minor toxins of B. sphaericus that include the Mtx1, Mtx2, Mtx3 and predicted Mtx4 (Hu et al., 2008), vegetative toxins that may play minor roles in toxicity in the field but have significant potential for exploitation in strain improvement in this bacterium.
Experimental procedures
Strains and plasmids
Two recombinant B. thuringiensis ssp. israelensis strain 4Q7 derivatives containing either pSTABP135 or pHTP49 that direct production of Cry48Aa and Cry49Aa, respectively, were constructed in previous studies (Jones et al., 2007). These strains were grown in EMBRAPA medium (Monnerat et al., 2007) to > 98% sporulation (typically 48–72 h) as judged by phase contrast microscopy. At this time, they were harvested by centrifugation, washed in distilled water and lyophilized to produce powders for use in bioassays. For synergy studies, recombinant B. thuringiensis ssp. israelensis producing Cry4Aa (Delécluse et al., 1993) were also used.
Toxicity assays
Qualitative bioassays against a range of insect targets were carried out as follows. Bioassays against the mosquitoes Cx. quinquefasciatus, Ae. aegypti and An. gambiae used 10 second or third instar larvae in 10 ml of dechlorinated tap water to which was added 100 μl of a sporulated culture of B. sphaericus or recombinant B. thuringiensis. Mortality was assessed after 24 and 48 h at 28°C. Chironomus riparius assays were performed as previously described (Partridge and Berry, 2002) except that 100 μl of sporulated cultures was added to 10 second instar larvae in 10 ml of distilled water containing sediments of homogenized Whatman 3Mm paper. The Lepidoptera Ant. gemmatalis, S. frugiperda and P. xylostella were assayed by the methods of Monnerat and colleagues (2007) with the addition of 150 μl of bacterial culture to artificial diet for Ant. gemmatalis, 30 μl culture to S. frugiperda diet and the use of 1:100 dilutions of cultures for the dipping of leaves for the P. xylostella assay. The coleopteran Anth. grandis was challenged with artificial diet containing 200 μl of sporulated culture using the procedure of Soares Martins and colleagues (2007). In all cases, B. thuringiensis recombinants expressing Cry48Aa and Cry49Aa were assayed both independently and in combination. When combinations were used, the quantities of culture listed above were added to each bioassay for each of the recombinant B. thuringiensis strains.
For synergy studies, Cry49Aa crystals were prepared from B. thuringiensis ssp. israelensis 4Q7::pHTP49 following sucrose density gradient centrifugation as previously described (Jones et al., 2007). The Cry4Aa stock was prepared from the sporulated, lyophilized bacterial powders of B. thuringiensis producing this toxin by suspending weighed powder in deionized water with 20–25 glass beads to promote homogenization. Different concentrations of the Cry4Aa suspensions were fed to groups of 20 early fourth instar larvae of Cx. quinquefasciatus strain Syn-P (Wirth et al., 2004a) in 250 ml plastic cups containing 100 ml of deionized water. Eight or more concentrations that produced mortality between 0% and 100% plus an untreated control were used for each dose–response test and replicated five times on five different days. Stocks of Cry49Aa powder were similarly prepared and showed no activity at 200 μg ml−1. Consequently, testing was limited to five replicates of 20 larvae per cup over five different days. Similar tests were conducted with Cry4Aa against the Cq4AB colony, with high resistance to Cry4Aa (Wirth and Georghiou, 1997). No control mortality was observed. Powders were combined by weight in a 3:1 ratio of Cry4Aa and Cry49Aa and tested against groups of 20 larvae at 2, 20 and 200 μg ml−1, and replicated three to five times on three to five different days. Susceptible and resistant mosquito colonies were tested concurrently using the same test materials and stock suspensions. Larvae received a small amount of food at 24 h and mortality was determined at 48 h. Cry4Aa data were analysed using Probit analysis (Finney, 1971). Probit analysis was not possible on all Cry49Aa data and on Cry4Aa data against Cq4AB; therefore, average mortality and standard deviation were calculated.
Proteolytic processing of toxins
Extracts were prepared from the guts of fourth instar larvae of Cx. quinquefasciatus and Ae. aegypti as described by Thanabalu and colleagues (1992). Guts were dissected from 20 larvae and placed into ice-cold microfuge tubes containing 200 μl of PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3) following removal of the peritrophic membranes. The guts were homogenized in 1.5 ml microfuge tubes using a pestle that exactly fits the tube. The particulate material was then removed by centrifugation (17 000 g, 5 min), and the supernatant fraction was used fresh in proteolytic incubations.
Individual crystal proteins were purified from the recombinant B. thuringiensis on sucrose gradients (Jones et al., 2007), and ∼10 μg of each was solubilized in 50 mM NaOH for 1 h at 30°C and any insoluble material was removed by centrifugation (17 000 g, 5 min). The solution was then adjusted to 20 mM Tris-HCl, 150 mM NaCl, 2.5 mM CaCl2, pH 8.4 in a final volume of 100 μl. This solubilized toxin was treated with 10 μl of mosquito larval gut extract or 1 μg of proteolytic enzyme [trypsin, α-chymotrypsin or proteinase K (Sigma, Poole Dorset, UK)] and incubated at 30°C for 1 h. Control reactions were prepared in an identical manner to the test reactions, except that no gut extract or proteolytic enzymes were added prior to the incubation step. The digest products were then precipitated by the addition of trichloro acetic acid to 10% (w/v) final concentration and incubation on ice for 20 min. The precipitated protein was harvested by centrifugation (17 000 g, 15 min) and the pellet was washed with acetone, pre-cooled to −20°C. The samples were centrifuged (17 000 g, 5 min, 4°C), the supernatant discarded and the protein pellets allowed to air-dry before re-suspension in SDS-PAGE protein sample buffer and analysis of the digest products by SDS-PAGE in a 10% acrylamide gel, stained with Coomassie blue. The N-terminal sequences of processed products were determined by automated Edman degradation (Alta Bioscience, Birmingham, UK) of bands transferred to PVDF membrane from SDS-PAGE gels run with tricine in place of glycine in the running buffer.
Molecular modelling of Cry48Aa
The homology-modelling server, swiss-model (Schwede et al., 2003), was used to generate a first-approximation three-dimensional model of Cry48Aa. This automated procedure involves submission of a primary amino acid sequence to the server, allowing selection of templates based on protein homology. An alignment of the submitted sequence and the templates is generated, followed by the building of the model backbone, side-chain modelling and energy minimization. Templates used in this case were Cry1Aa, Cry3Aa, Cry3Bb, Cry4Aa and Cry4Ba (ExPDB codes 1ciy, 1dLc, 1ji6A, 2c9kA and 1w99A respectively).
References
- Abdullah MA, Valaitis AP, Dean DH. Identification of a Bacillus thuringiensis Cry11Ba toxin-binding aminopeptidase from the mosquito, Anopheles quadrimaculatus. BMC Biochem. 2006;7:16. doi: 10.1186/1471-2091-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Angsuthanasombat C, Crickmore N, Ellar DJ. Cytotoxicity of a cloned Bacillus thuringiensis subsp. israelensis CryIVB toxin to an Aedes aegypti cell line. FEMS Microbiol Lett. 1991;67:273–276. doi: 10.1016/0378-1097(91)90488-v. [DOI] [PubMed] [Google Scholar]
- Angsuthanasombat C, Crickmore N, Ellar DJ. Comparison of Bacillus thuringiensis subsp. israelensis CryIVA and CryIVB cloned toxins reveals synergism in vivo. FEMS Microbiol Lett. 1992;73:63–68. doi: 10.1016/0378-1097(92)90584-b. [DOI] [PubMed] [Google Scholar]
- Angsuthanasombat C, Crickmore N, Ellar DJ. Effects on toxicity of eliminating a cleavage site in a predicted interhelical loop in Bacillus thuringiensis CryIVB delta-endotoxin. FEMS Microbiol Lett. 1993;111:255–261. doi: 10.1111/j.1574-6968.1993.tb06395.x. [DOI] [PubMed] [Google Scholar]
- Baum JA, Chu CR, Rupar M, Brown GR, Donovan WP, Huesing JE, et al. Binary toxins from Bacillus thuringiensis active against the western corn rootworm, Diabrotica virgifera virgifera LeConte. Appl Environ Microbiol. 2004;70:4889–4898. doi: 10.1128/AEM.70.8.4889-4898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, Hindley J, Ehrhardt AF, Grounds T, De Souza I, Davidson EW. Genetic determinants of the host range of the Bacillus sphaericus mosquito larvicidal toxins. J Bacteriol. 1993;175:510–518. doi: 10.1128/jb.175.2.510-518.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonserm P, Angsuthanasombat C, Lescar J. Crystallization and preliminary crystallographic study of the functional form of the Bacillus thuringiensis mosquito-larvicidal Cry4Aa mutant toxin. Acta Crystallogr. 2004;60:1315–1318. doi: 10.1107/S0907444904011205. [DOI] [PubMed] [Google Scholar]
- Boonserm P, Davis P, Ellar DJ, Li J. Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J Mol Biol. 2005;348:363–382. doi: 10.1016/j.jmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Boonserm P, Mo M, Angsuthanasombat C, Lescar J. Structure of the functional form of the mosquito larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.8-angstrom resolution. J Bacteriol. 2006;188:3391–3401. doi: 10.1128/JB.188.9.3391-3401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore N, Bone EJ, Williams JA, Ellar DJ. Contribution of the individual components of the delta-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. Israelensis. FEMS Microbiol Lett. 1995;131:249–254. [Google Scholar]
- Dai SM, Gill SS. In vitro and in vivo proteolysis of the Bacillus thuringiensis subsp. israelensis CryIVD protein by Culex quinquefasciatus larval midgut proteases. Insect Biochem Mol Biol. 1993;23:273–283. doi: 10.1016/0965-1748(93)90008-g. [DOI] [PubMed] [Google Scholar]
- Delécluse A, Poncet S, Klier A, Rapoport G. Expression of cryIVA and cryIVB genes, independently or in combination, in a crystal-negative strain of Bacillus thuringiensis subsp. Israelensis. Appl Environ Microbiol. 1993;59:3922–3937. doi: 10.1128/aem.59.11.3922-3927.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RT, Stockhoff BA, Stamp L, Schnepf HE, Schwab GE, Knuth M, et al. Novel Bacillus thuringiensis binary insecticidal crystal proteins active on western corn rootworm, Diabrotica virgifera virgifera LeConte. Appl Environ Microbiol. 2002;68:1137–1145. doi: 10.1128/AEM.68.3.1137-1145.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. Probit Analysis – A Statistical Treatment of the Sigmoid Response Curve. Cambridge, UK: Cambridge University Press; 1971. [Google Scholar]
- Galitsky N, Cody V, Wojtczak A, Ghosh D, Luft JR, Pangborn W, English L. Structure of the insecticidal bacterial delta-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta Crystallogr D Biol Crystallogr. 2001;D57:1101–1109. doi: 10.1107/s0907444901008186. [DOI] [PubMed] [Google Scholar]
- Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz J-L, Brousseau R, Cygler M. Bacillus thuringiensis Cry1A(a) insecticidal toxin: crystal structure and channel formation. J Mol Biol. 1995;254:447–464. doi: 10.1006/jmbi.1995.0630. [DOI] [PubMed] [Google Scholar]
- Haider MZ, Ellar DJ. Analysis of the molecular basis of insecticidal specificity of Bacillus thuringiensis crystal delta-endotoxin. Biochem J. 1987a;248:197–201. doi: 10.1042/bj2480197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider MZ, Ellar DJ. Characterization of the toxicity and cytopathic specificity of a cloned Bacillus thuringiensis crystal protein using insect cell culture. Mol Microbiol. 1987b;1:59–66. doi: 10.1111/j.1365-2958.1987.tb00527.x. [DOI] [PubMed] [Google Scholar]
- Haider MZ, Knowles BH, Ellar DJ. Specificity of Bacillus thuringiensis var. colmeri insecticidal delta-endotoxin is determined by differential proteolytic processing of the protoxin by larval gut proteases. Eur J Biochem. 1986;156:531–540. doi: 10.1111/j.1432-1033.1986.tb09612.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Fan W, Han B, Liu H, Zheng D, Li Q, et al. Complete genome sequences of the mosquitocidal bacterium Bacillus sphaericus C3–41 and comparisons with closely related Bacillus species. J Bacteriol. 2008;190:2892–2902. doi: 10.1128/JB.01652-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PA, Stevens MM, Park H-W, Federici BA, Dennis ES, Akhurst R. Response of larval Chironomus tepperiDipteraChironomidae) to individual Bacillus thuringiensis var. israelensis toxins and toxin mixtures. J Invertebr Pathol. 2005;88:34–39. doi: 10.1016/j.jip.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Jones GW, Nielsen-Leroux C, Yang Y, Yuan Z, Dumas VF, Monnerat RG, Berry C. A new Cry toxin with a unique two-component dependency from Bacillus sphaericus. FASEB J. 2007;21:4112–4120. doi: 10.1096/fj.07-8913com. [DOI] [PubMed] [Google Scholar]
- Li JD, Carroll J, Ellar DJ. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature. 1991;353:815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- Monnerat RG, Batista AC, Telles de Medeiros P, Soares Martins E, Melatti VM, Praça LB, et al. Screening of Brazilian Bacillus thuringiensis isolates active against Spodoptera frugiperdaPlutella xylostella and Anticarsia gemmatalis. Biol Control. 2007;41:291–295. [Google Scholar]
- Morse RJ, Yamamoto T, Stroud RM. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure. 2001;9:409–417. doi: 10.1016/s0969-2126(01)00601-3. [DOI] [PubMed] [Google Scholar]
- Partridge MR, Berry C. Insecticidal activity of the Bacillus sphaericus Mtx1 toxin against Chironomus riparus. J Invertebr Pathol. 2002;79:135–136. doi: 10.1016/s0022-2011(02)00025-3. [DOI] [PubMed] [Google Scholar]
- Pei G, Oliveira CMF, Yuan Z, Nielsen-Leroux C, Silva-Filha MH, Yan J, Regis L. A strain of Bacillus sphaericus causes a slower development of resistance in Culex quinquefasciatus. Appl Environ Microbiol. 2002;68:3003–3009. doi: 10.1128/AEM.68.6.3003-3009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukmini V, Reddy CY, Venkateswerlu G. Bacillus thuringiensis crystal delta-endotoxin: role of proteases in the conversion of protoxin to toxin. Biochimie. 2000;82:109–116. doi: 10.1016/s0300-9084(00)00355-2. [DOI] [PubMed] [Google Scholar]
- Rupar MJ, Donovan WP, Chu C-R, Pease E, Tan Y, Slaney AC, et al. Nucleic Acids Encoding Coleopteran-Toxic Polypeptides and Insect-Resistant Transgenic Plants Comprising Them. St. Louis, MO, USA: Monsanto Technology LLC; 2003. [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucl Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Filha MH, Oliveira CM, Regis L, Yuan Z, Rico CM, Nielsen-LeRoux C. Two Bacillus sphaericus binary toxins share the midgut receptor binding site: implications for resistance of Culex pipiens complex (DipteraCulicidae) larvae. FEMS Microbiol Lett. 2004;241:185–191. doi: 10.1016/j.femsle.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Soares Martins E, Praça LB, Dumas VF, Silva-Werneck JO, Sone EH, Waga IC, et al. Characterization of Bacillus thuringiensis isolates toxic to cotton boll weevil (Anthonomus grandis. Biol Control. 2007;40:65–68. [Google Scholar]
- Thanabalu T, Hindley J, Berry C. Proteolytic processing of the mosquitocidal toxin from Bacillus sphaericus SSII-1. J Bacteriol. 1992;174:5051–5056. doi: 10.1128/jb.174.15.5051-5056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MC, Delecluse A, Walton WE. Laboratory selection for resistance to Bacillus thuringiensis subsp. jegathesan or a component toxin, Cry11B. Culex quinquefasciatusDipteraCulicidae. J Med Entomol. 2004a;41:435–441. doi: 10.1603/0022-2585-41.3.435. [DOI] [PubMed] [Google Scholar]
- Wirth MC, Georghiou GP. Cross-resistance among CryIV toxins of Bacillus thuringiensis subsp. israelensis in Culex quinquefasciatusDipteraCulicidae. J Econ Entomol. 1997;90:1471–1477. [Google Scholar]
- Wirth MC, Georghiou GP, Malik JI, Abro GH. Laboratory selection for resistance to Bacillus sphaericusCulex quinquefasciatusDipteraCulicidae) from California, USA. J Med Entomol. 2000;37:534–540. doi: 10.1603/0022-2585-37.4.534. [DOI] [PubMed] [Google Scholar]
- Wirth MC, Jiannino JA, Federici BA, Walton WE. Synergy between toxins of Bacillus thuringiensis subsp. israelensis and Bacillus sphaericus. J Med Entomol. 2004b;41:935–941. doi: 10.1603/0022-2585-41.5.935. [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Pei GF, Regis L, Nielsen-Leroux C, Cai QX. Cross-resistance between strains of Bacillus sphaericus but not B. thuringiensis israelensis in colonies of the mosquito Culex quinquefasciatus. Med Entomol. 2003;17:251–256. doi: 10.1046/j.1365-2915.2003.00429.x. [DOI] [PubMed] [Google Scholar]


