Abstract
Epidermal growth factor receptor (EGFR) is overexpressed in a variety of human malignancies, including pancreatic cancer, breast cancer, colon cancer, and non-small cell lung cancer. Overexpression of EGFR is a predictive marker of therapeutic response and several lines of evidence suggest that EGFR is an excellent target for tumor therapy. However, the effective antitumor capacity of EGFR-specific T cells against EGFR-overexpressing tumor cells has not been fully elucidated. In our previous study, we identified an anti-EGFR single-chain variable fragment (scFv) with specific and high affinity after screening by ribosome display. In this study, the anticancer potential of anti-EGFR scFv was investigated on the basis of cell-targeted therapy. A chimeric antigen receptor (CAR) targeting EGFR was constructed and expressed on the cell membrane of T lymphocytes. These CAR-modified T cells demonstrated antitumor efficacy both in vitro and in vivo. In addition, the safety evaluation showed that CAR-modified lymphocytes have no or very minimal acute systemic toxicity. Taken together, our study provided the experimental basis for clinical application of genetically engineered lymphocytes; moreover, we also evaluate a new and interesting cell therapy protocol.
Introduction
The epidermal growth factor receptor (EGFR) is overexpressed on the surface of the cell membranes in a wide variety of solid tumors, particularly in non-small cell lung cancer (NSCLC). Overexpression of EGFR has marked effects on various aspects of carcinogenesis, like cell growth and invasion, angiogenesis, and metastasis. The potential value of EGFR as a target for the diagnosis and therapy of human tumors has been recognized for several years [1–4]. A number of targeting agents directed at tumors that express high levels of EGFR have been approved for clinical use of treating NSCLC, and antibody therapy plays an important role in these agents [5,6]. Monoclonal antibodies (mAbs), such as cetuximab, have shown promising results in providing a survival advantage for advanced-stage NSCLC patients [7–10]. However, due to EGFR mutations and the poor penetration of antibodies into the tumor tissue, these agents are not able to kill a sufficient number of malignant cells and cause tumor regression [11,12]. It is necessary to direct the antibody with a cytotoxic agent to the target to enhance the efficacy of its antitumor activity.
Adoptive cell therapy (ACT) is a new cancer therapy strategy that is usually approached through infusion of antitumor immune effector cells to obtain or enhance antitumor responses in patients. Dr Rosenberg initiated the prelude of ACT in 1985 when he treated melanoma with lymphocyte-activated killer cells for the first time [13]. Many tumor-specific antigen T cell receptor (TCR) genes, such as the melanoma antigen tumor-specific TCR gene, have been isolated and identified along with the rapid development of genetic engineering technology in recently years [14–18]. Furthermore, chimeric antigen receptor (CAR) technology has combined both the specificity of mAbs and the efficient cytotoxic of T lymphocytes. The biggest advantage of CAR is that it is not subject to major histocompatibility complex (MHC) restriction, as natural TCR is. Nonspecific lymphocytes can be endowed with the ability to specifically recognize tumor antigens and to release cytokines by CAR gene transfection [19,20]. Using this approach in the clinical treatment of a variety of tumors, such as lymphoma [21,22], neuroblastoma [23], and metastatic renal cell carcinoma [24], has yielded encouraging results.
In our previous study, after screening by ribosome display, we generated an anti-EGFR single-chain variable fragment (scFv) with high antigen specificity and affinity [25]. An rGel-based EGFR-specific immunotoxin was constructed, expressed, and purified; this immunotoxin resulted in significant antigen specificity and antitumor effect both in vitro and in vivo. Here, we aimed to further investigate the antitumor potential of the anti-EGFR scFv using the cell-targeted therapy strategy, with a view to establishing whether the CAR-modified T lymphocytes have potential for application in the treatment of EGFR-positive carcinoma. To this end, the CAR was constructed to consist of the human interleukin-2 (IL-2; hIL-2) signal peptide, anti-EGFR scFv, hemagglutinin (HA) tag, IgG2 Fc, and the transmembrane region and part of the cytoplasmic region of the human CD28 signaling chain fused to the cytoplasmic region of CD3ζ chain. Then, the CAR gene was cloned into the NheI and BglII sites of a CMV promoter-based nonviral vector, pmaxCloning. After verification of the expression of the CAR on the cell membrane of T lymphocytes, the antitumor effects of CAR-modified T cells were evaluated both in vitro and in vivo. The systemic acute toxicity of this strategy was also evaluated.
Materials and Methods
Cell Lines and T Cells
Human epithelial carcinoma cell line A431, human NSCLC cell line A549, human Burkitt's lymphoma cell line Raji, and human ovarian cancer cell line A2780 were obtained from American Type Culture Collection (Manassas, VA). A549, A2780, and Raji cell lines were cultured in RPMI 1640 (Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated FBS. The A431 cell line was maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FBS.
Human T cells were isolated from peripheral blood of healthy donors under an Institutional Review Board-approved protocol (95-054), using BD Vacutainer CPT tubes (Becton Dickinson, Franklin Lakes, NJ) following the manufacturer's instructions. T cells were cultured in RPMI 1640 supplemented with 300 IU/ml IL-2 (Novartis Pharmaceuticals, Shanghai, China) and stimulated with anti-CD3 and anti-CD28 mAbs.
Mice
Five- to six-week-old female nonobese diabetic/severe combined immunodeficiency (NOD/SCID) CB-17 mice were purchased from Beijing HFK Bio-Technology Co, Ltd (Beijing, China). Animals were kept in a specific pathogen-free facility at the Animal Experimental Center of Sichuan University (Sichuan, China). All animal studies were performed in accordance with the Institutional Animal Care guidelines.
CAR Construction
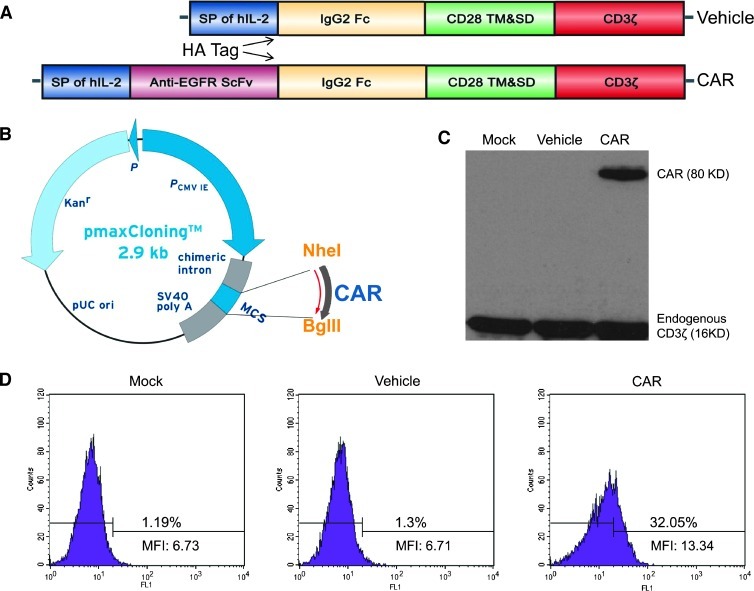
The CAR constructs used in this study are schematically illustrated in Figure 1A, using an assembly technique as follows. Generation of the anti-EGFR-scFv DNA has recently been described in detail [25]. The hIgG2-Fc fragment was subcloned by polymerase chain reaction (PCR) from pFUSE-hIgG2-Fc1 plasmid (InvivoGen, San Diego, CA). The fused CD28 transmembrane region and a part of its intracellular region and CD3-ζ molecules were synthesized by the GenScript company. Several genetic fragments were assembled by overlapping PCR, in which the signal peptide of hIL-2 and an HA tag was also introduced through the forward primer. The expression cassettes were subcloned into the NheI and BglII sites of pmaxCloning vector. The sequence of the whole genetic fragment was confirmed by direct sequencing. The vehicle construct contained the same components in similar configuration and order, except that it lacked the anti-EGFR scFv sequence.
Figure 1.
Construction and expression of the EGFR-specific CAR in transduced human T lymphocytes. (A and B) Schematic representation of the construction of EGFR-specific CAR. This CAR consisted of the hIL-2 signal peptide, anti-EGFR scFv, HA tag, IgG2 Fc, the transmembrane region, and part of the cytoplasmic region of the human CD28 signaling chain, fused to the cytoplasmic region of the CD3ζ chain. This CAR gene was cloned into NheI and BglII sites of a CMV promoter-based nonviral vector, pmaxCloning. (C) Western blot analysis of CAR expression in T lymphocytes at 16 hours after transduction. PVDF membranes were labeled with an anti-human CD3ζ antibody and a goat anti-rabbit IgG HRP-conjugated second antibody. (D) Flow cytometric analysis of CAR expression in T lymphocytes at 16 hours after transduction. The transduced cells were stained with anti-EGFR scFv antiserum and goat anti-rabbit IgG FITC-conjugated secondary antibody and were then detected by a BD FACSCalibur flow cytometer. MFI is the abbreviation of mean fluorescence intensity. Results are representative of three experiments.
Antigen-specific Cytotoxicity and Cytokine Secretion by CAR-Modified T Cells
The cytolytic activity of CAR-modified T cells was measured by assays of lactate dehydrogenase (LDH) release after 6 hours using a Cytotoxicity Detection Kit (Promega, Madison, WI) according to the manufacturer's instructions. The capacity of modified T cells to produce cytokines [interferon-γ (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, and IL-4] was determined by ELISA Kit in supernatants after 24 hours of incubation with EGFR-positive cells (Neobioscience, Shenzhen, China).
Mouse Models
Tumorigenicity model. CAR-transfected lymphocytes and A549 tumor cells were washed with serum-free, antibiotic-free medium and the cell concentrations were adjusted to 2 x 108/ml and 2 x 107/ml, respectively. Ten mice per group were used and each mouse was inoculated subcutaneously (s.c.) in the scapular region with lymphocyte/tumor cell mixture in 100 µl of serum-free, antibiotic-free medium at a ratio of 10:1. All animals were monitored for a total of 60 days; animals were weighed and observed for clinical signs every 3 days. The tumor volume was estimated using two measurements of the nodules, with the formula V = 0.52 x length x width2.
S.c. model. Log-phase A431 or A2780 cells (5 x 106 cells per mouse) were injected s.c. into the scapular region. Once tumors were grown to 200 to 500 mm3 in size, animals were administered CAR-modified T cells [1 x 107 cells per mouse; intravenously (i.v.) through the tail vein] at 24, 48, and 72 hours after mice were grouped.
Lung metastasis model. A549 cells (2 x 106 cells) were administered i.v. to mice on day 0. Then, tumor-bearing mice were randomly assigned to one of the four groups: 1) mice treated with normal saline (NS), 2) mice treated with Mock T cells, 3) mice treated with Vehicle T cells, and 4) mice treated with CAR T cells. CAR-modified T cells (2 x 106) were administered i.v. at days 3, 6, 9, 12, 15, and 18 after tumor injection. Five mice per group were sacrificed at day 30, and the lung metastasis index of each group was statistically determined as described in our previous report [26].
Analysis of Toxic Effects Induced by Systemic Administration in Mice
NOD/SCID mice (five mice per group) were studied for evidence of acute toxic effects induced by a single i.v. injection of CAR-modified T cells (2 x 107 cells per mouse). Forty-eight hours later, the mice were sacrificed and serum was separated. Some of the mice from the lung metastasis model were also sacrificed 48 hours after the last cell administration, and serum was separated. Levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured at the Biochemistry Laboratory in the National Chengdu Center for Safety Evaluation of Drugs (Chengdu, China). After sacrifice of the mice in the lung metastasis model at 30 days, the heart, liver, spleen, and kidney tissues were used for morphologic analysis and hematoxylin and eosin (H&E) staining.
Statistical Analysis
All statistical analyses were performed using SPSS 19.0 software. Data are presented as means ± SD. Statistical analysis was performed using Student's t test for comparing two groups and by analysis of variance for multiple group comparisons. P values less than .05 were considered statistically significant.
Results
Efficient Generation of CAR-Modified T Cells Using a Nucleofection Gene Transfer System
To investigate the therapeutic potential of primary human T lymphocytes genetically modified to recognize and kill tumors that express EGFR, an EGFR-specific single-chain antibody sequence was selected due to its excellent specificity and binding affinity, as shown in our previous report [25]. In this study, an EGFR-based CAR, consisting of scFv of mAb 108 linked to CD28 and CD3 signaling moieties, was constructed and cloned into a nonviral vector, pmaxCloning (Figure 1, A and B). On the basis of the protocol of the Nucleofector System (Lonza, Walkersville, MD), optimized nuclear transfer of lymphocytes was performed in a preliminary experiment. We found that the best balance between transfection efficiency and cell survival could be achieved using 8 x 106 T cells and 3 µg of plasmid per electroporation cuvette and the T-23 program (data not shown). This method was thus used in the subsequent experiments.
The expression of the CAR was first confirmed by staining with anti-CD3ζ antibody in Western blot analysis. As shown in Figure 1C, a protein of the expected molecular mass (80 kDa) was detected. The surface expression of the CAR on T cells was further identified by staining with anti-EGFR scFv serum. Cells were then analyzed by flow cytometry; we found that only 1.19% and 1.3% of the cells showed binding of anti-EGFR scFv serum, while 32.05% of T cells expressed the CAR (Figure 1D).
Functional Characterization of Anti-EGFR CAR-Expressing Primary Human T Cells
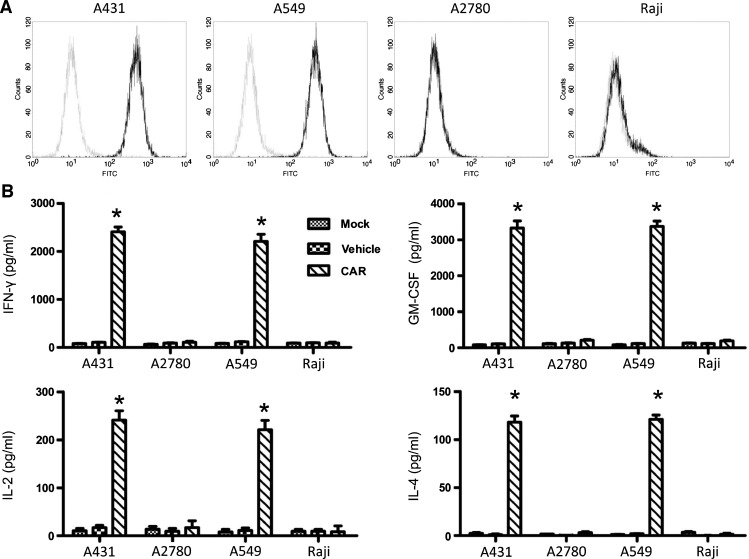
To determine whether CAR-modified lymphocytes could specifically target and kill tumor cells, we first determined the expression of EGFR in several cell lines using flow cytometry, employing staining with the anti-EGFR antibody. Two EGFR overexpression cell lines (A431 and A549) and two EGFR-negative cell lines (A2780 and Raji) were selected for use in the subsequent experiments (Figure 2A). To investigate whether the CAR-modified T cells were capable of specifically recognizing tumor cells, a panel of tumor cell lines and the amount of secreted effector cytokines, IFN-γ, GM-CSF, IL-2, and IL-4, were determined. The CAR-modified T cells produced high levels of Tc1 cytokines IFN-γ, GM-CSF, and IL-2 as well as the Tc2 cytokine IL-4 when they were co-cultured with EGFR-overexpressing tumor cells, whereas the CAR-modified T cells cocultured with EGFR-negative tumor cells showed no significant cytokine secretion. In addition, the mock-treated (Mock) and vehicle-treated (Vehicle) T cells co-cultured with either EGFR-overexpressing or EGFR-negative tumor cell lines secreted no measurable cytokines (Figure 2B).
Figure 2.
Cytokine production triggered by EGFR-specific CAR. (A) The expression of EGFR of four tumor cells was detected by flow cytometric analysis. A431, A549, A2780, and Raji cells were washed once by phosphate-buffered saline and then incubated with anti-EGFR primary antibody and goat anti-rabbit IgG FITC-conjugated antibody. (B) CAR-modified T lymphocytes were cultured with A431, A549, A2780, and Raji tumor cells for 24 hours. Supernatants were harvested and the production of IFN-γ, GM-CSF, IL-2, and IL-4 were detected by ELISA. Results were expressed as means ± SD of triplicate samples and representative of three experiments (*P < .01).
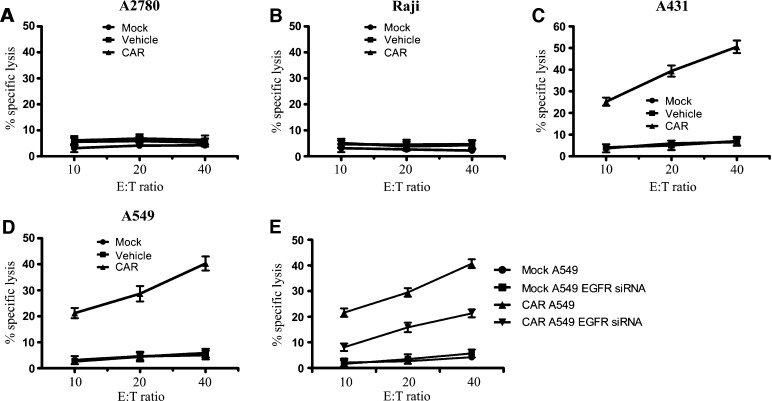
The cytotoxicity of CAR-modified lymphocytes was next evaluated by an LDH release assay. As shown in Figure 3, A to D, the CAR-modified T cells were able to lyse A431 and A549 tumor cells but not A2780 and Raji cells. Down-regulation of EGFR expression in A549 cells by siRNA was also performed to consequently verify the EGFR-mediated killing by CAR T cells. In addition, the results showed that cytotoxicity of CAR-modified lymphocytes was significantly decreased in EGFR silencing A549 cells (Figures W1 and 3E). The apoptosis staining was used to visualize the binding capacity of CAR-modified lymphocytes to tumor cells and the killing of tumor cells depending on EGFR expression. Sixteen hours after the coincubation with T lymphocytes, apoptosis or already dead A549 cells were identified by propidium iodide (PI) staining. The ratio of apoptotic and necrotic EGFR silencing A549 cells decreased significantly compared with normal A549 cells after the treatment of CAR T cells (Figure W2A). To quantitatively measure cell apoptosis, the cells are double stained with annexin V and PI. The annexin V-positive and PI-negative A549 cells were also obviously reduced when EGFR was silenced (Figure W2A). Taken together, the above data showed that CAR-modified T cells could effectively mediate antigen-specific cytokine release and cytolytic activity.
Figure 3.
Cytotoxicity of the EGFR-specific CAR to EGFR overexpression tumor cells. (A–D) The CAR-modified T cells were co-cultured with A431, A549, A2780, or Raji tumor cells at various effector-to-target (E:T) ratios at 37°C for 6 hours. The cytotoxicity of CAR-modified T cells to target cell lysis was assessed by LDH release assay. Results were expressed as means ± SD of triplicate samples and were representative of three experiments. (E) Decreased cytotoxicity of the CAR to EGFR silencing A549 cells. A549 cells were transfected with EGFR siRNA using Lipofectamine 2000 reagent following the manufacturer's instruction for 24 hours. The cytotoxicity of CAR-modified T cells to EGFR silencing A549 cells was performed similarly as in A to D.
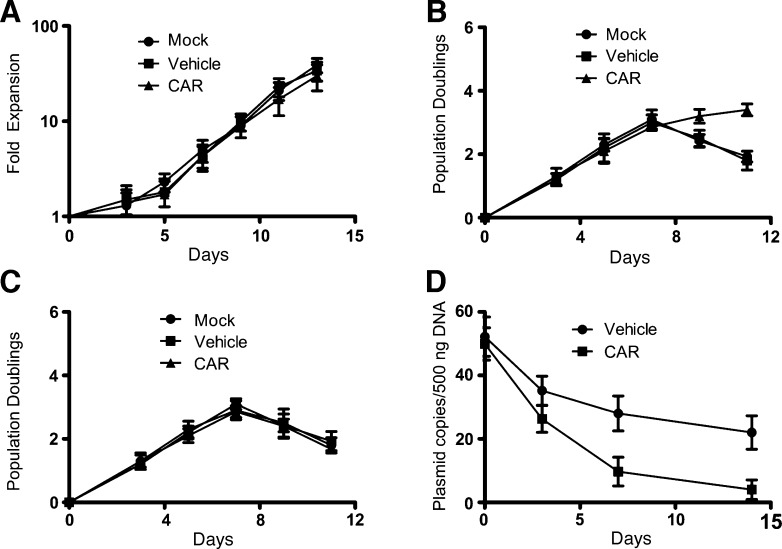
T cell proliferative ability is also a very important aspect for the antitumor immune response, in addition to their cytotoxicity and cytokine production. In vitro expansion of lymphocytes was performed to determine whether CAR-modified T cells retained the same cell proliferative ability as control T cells. About 50-fold expansion of CAR-modified T cells could be achieved under standard culture conditions on day 12 (Figure 4A). Next, we assessed whether the proliferation of CAR-modified T cells was an antigen-dependent or antigen-independent effect in vitro. As shown in Figure 4B, CAR-modified T cells exhibited significantly greater proliferation compared to Mock and Vehicle T cells after stimulation with A431 cells. However, no significant proliferation was observed when these different T cells were stimulated with EGFR-negative A2780 cells (Figure 4C). Another experiment was designed to verify whether CAR-modified T cells could also retain favorable proliferation in vivo. Three weeks after establishment of an A549 lung metastasis tumor model in NOD/SCID mice, the same numbers of Vehicle and CAR-modified T cells were i.v. co-injected. The sequences of the vector and anti-EGFR scFv were used to monitor the persistence of these cells. The results suggested that CAR-modified T cells had a higher in vivo persistence than Vehicle T cells (Figure 4D).
Figure 4.
Proliferation characteristics of EGFR-specific CAR-modified T cells. (A) In vitro expansion of human T lymphocytes following different transduction conditions. Twelve hours after nucleofection, exogenous IL-2 was added to the cultures, every other day, at 300 IU/ml. Data are representative of three separate experiments. (B and C) In vitro expansion of CAR-modified T lymphocytes with antigen-dependent effect. CAR-modified T lymphocytes were cultured under the same condition just as in A. Cultures were restimulated with either A431 cells (B) or A2780 cells (C) at 7 days. Positive T cells were stained with anti-HA tag antibody and counted by flow cytometry. Data are representative of at least two separate experiments. (D) In vivo persistence of EGFR-specific CAR-modified T cells. The same numbers of Vehicle and CAR T cells were co-injected (i.v.) into NOD/SCID mice bearing a A549 lung metastasis model. The number of copies of Vehicle and CAR vectors in the spleen DNA from mice was evaluated, at various times following T cell injection, by quantitative PCR. Bars, means ± SD.
Evaluating Antitumor Responses of CAR-Modified Human Primary T Cells In Vivo
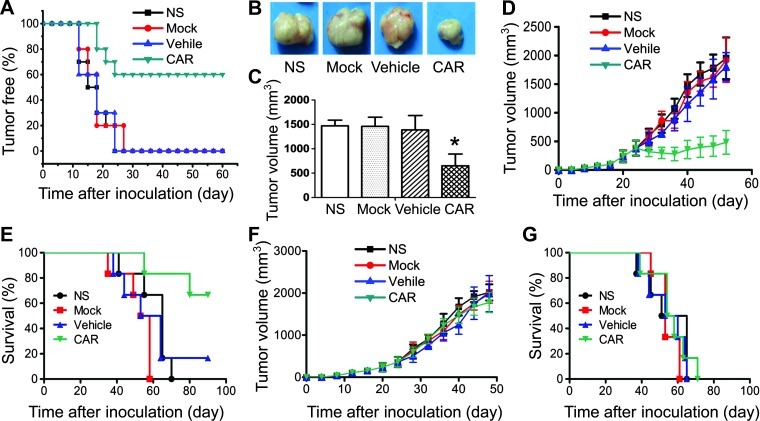
The in vivo antitumor efficacy of CAR-modified T cells was first evaluated in an A431 tumorigenicity model. Animals were monitored for a total of 60 days after co-inoculation of lymphocyte/tumor cell mixture. The mice infused with CAR-modified lymphocytes displayed a smaller tumorigenic proportion and lower tumor growth (Figure 5A). The tumor volume of the mice treated with CAR-modified lymphocytes was only half of that of the control groups on day 60 (Figure 5, B and C). The antitumor activity of CAR-modified T cells against an established A431 and A2780 s.c. tumor model was next evaluated. The mice were administered (i.v. through tail vein) CAR-modified T cells at days 23, 24, and 25, when tumors had grown to 200 to 500 mm3 in size. A431 tumor-bearing mice treated with CAR-modified T cells showed significant inhibition of tumor growth and prolonged survival of animals compared with mice treated with Mock and Vehicle T cells (Figure 5, D and E). In contrast, all of the mice treated with Mock, Vehicle, and CAR-modified T cells showed no specific antitumor effect above that observed with NS in A2780 tumor model (Figure 5, F and G).
Figure 5.
Efficient inhibition of s.c. tumor growth by CAR-modified T cells in vivo. (A–C) The suppression of tumorigenicity of A549 cells by CAR-modified T cells. CAR-transfected lymphocytes and A549 tumor cells were co-inoculated s.c. at a ratio of 10:1. Tumorigenicity of the animals was monitored and calculated every 3 days (A). All of the mice were sacrificed and the mean tumor volume was calculated. Bars, means ± SD (*P < .05) (B and C). (D–G) Inhibition of the tumor growth by CAR-modified T lymphocytes. A431 or A2780 tumor cells were implanted s.c. into NOD/SCID mice at day 0. Once tumors had grown to 200 to 500 mm3, animals were grouped and i.v. injected with CAR-modified T cells on days 23, 24, and 25. (D) Tumor volume of A431 tumors. Bars, means ± SD. (E) Survival of animals bearing A431 tumors. (F) Tumor volume of A2780 tumors. Bars, means ± SD. (G) Survival of animals bearing A2780 tumors.
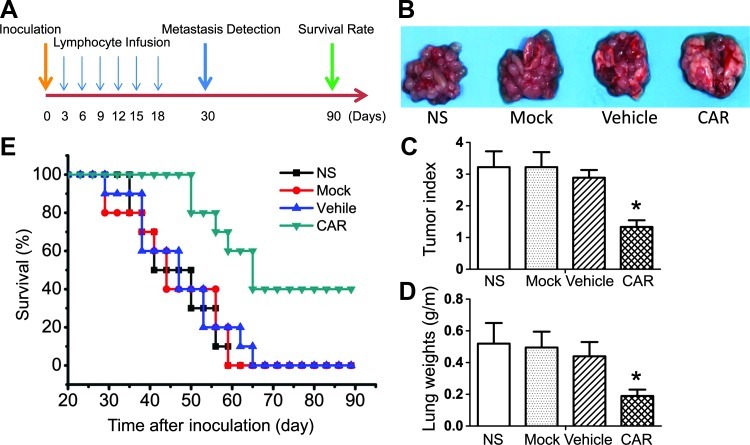
Advanced tumor is usually combined with occasional metastatic spread to the lung. A xenogeneic model of advanced lung metastatic A549 cancer was established through tail vein injection to evaluate the functional activity of CAR-modified T cells. CAR-modified T cells were administered at days 3, 6, 9, 12, 15, and 18 after tumor injection (Figure 6A). As shown in Figure 6, B and C, A549-derived tumor lung metastasis was significantly decreased when mice were treated with CAR-modified T cells. The mice treated with CARmodified T cells had a very low tumor metastasis index (P < .05 compared with all other groups), whereas the majority of mice treated with NS or Mock or Vehicle T cells had a higher tumor index (Figure 6C). Lung weights were also significantly different (P < .05) between mice treated with CAR-modified T lymphocytes and the other control groups (Figure 4D).
Figure 6.
CAR-modified T cells inhibit pulmonary metastases in A549 mouse model. NOD/SCID mice received i.v. injections of A549 tumor cells and were randomized into four groups before beginning therapy with CAR-modified T cells. Treatment strategy of A549 tumor-bearing mice as shown in A. (B) Kaplan-Meier survival of tumor-bearing mice. (C–E) Five mice per group were sacrificed at day 30, and the mean lung metastases index and lung weights of each group were calculated. Bars, means ± SD (*P < .05)
Analysis of Toxic Effects Induced by Systemic Administration in Mice
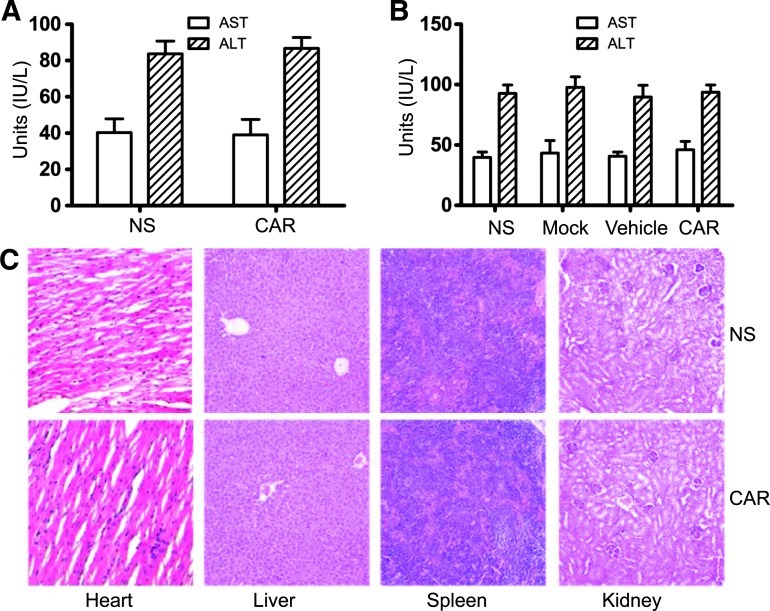
Systemic toxic effects in the mice were evaluated to characterize whether treatment with CAR-modified T lymphocytes is safe in preclinical experiments. Single doses of 2 x 107 CAR-modified T cells per mouse were given by tail vein injection. Blood samples were collected from the mice after treatment, and serum levels of liver AST and serum ALT were measured. However, treatment with CAR-modified T cells did not increase the levels of AST and ALT, indicating an absence of significant liver toxicity in the mice (Figure 7A). Some of the mice from the lung metastasis model experiment were also sacrificed 48 hours after the last T cell administration, and serum was separated; the same results as in Figure 7A were obtained (Figure 7B). The results of H&E staining showed that mice treated with CAR-modified T cells had no pathologic changes in major organs compared with NS mice (Figure 7C). Collectively, these data suggested that CAR-modified T cells have no or only minimal systemic acute toxicity.
Figure 7.
Systemic toxicity evaluation of CAR-modified T cells. (A) Acute toxic effects induced by a single i.v. injection of CAR-modified T cells (2 x 107 cells per mouse). Forty-eight hours later, the mice were sacrificed and serum was separated. Levels of serum ALT and AST were measured. Bars, means ± SD. (B and C) Acute toxic effects induced by multiple i.v. injections of CAR-modified T cells. Some of the mice from the lung metastasis model were sacrificed 48 hours after the last cell administration, and several organs and serum were separated. (B) Levels of ALT and AST were measured using the same method as in A. Bars, means ± SD. (C) The heart, liver, spleen, and kidney tissues were used for morphologic analysis and H&E staining (original magnification, x100).
Discussion
The long-term goal of tumor immunotherapy is to enhance the targeting ability of the immune system to the cancer antigen. However, this is very challenging, because most tumor-specific antigens are autologous proteins and have a low immunogenicity. Moreover, tumors can also downregulate the expression of the MHC and immunosuppressive molecules and cytokines to escape the attacks of the immune system [27]. To overcome this difficulty, many researchers have tried a variety of immunotherapeutic strategies, including tumor vaccine [28,29], mAb [30,31], and ACT [15,32]. Genetically modified T lymphocytes, which can express a CAR to recognize tumor antigens, hold great potential as a treatment strategy in the field of cancer immunotherapy [33].
In contrast to tumor infiltrating lymphocytes (TIL) or cytokine-induced killer (CIK), CAR is used to overcome MHC restriction on the inherent antigen presentation of T lymphocytes. The core part of this strategy is the generation of genetically modified T lymphocytes. An effective T lymphocyte must meet the following rigorous conditions: capacity to specifically recognize tumor targets, long time in vivo persistence, low immunogenicity risk, and it should be easily obtained. In the present study, we have generated a new kind of EGFR-specific CAR gene-modified T lymphocytes using a nonviral vector delivered by nucleofection technology. The results of Western blot and flow cytometry experiments showed that the CAR was efficiently expressed on the cell membrane. Moreover, this CAR-modified T cells demonstrated good antitumor effects both in vitro and in vivo, without MHC restriction. Moreover, no marked systemic toxicity was detected during the treatment process.
The selection of the target antigen is an important aspect for CAR gene-mediated cell therapy. EGFR is overexpressed on the cell surface of a wide variety of solid tumors [34]. It plays crucial roles in cell signaling, cell proliferation, regulation of apoptosis and angiogenesis, and tumor occurrence and development. Several targeting agents directed at tumors that express high levels of EGFR have been approved for clinical treatment [5,6,35]. Prerequisites for effective CAR-modified lymphocytes are the target specificity and antigen affinity of the scFv. An anti-EGFR single-chain antibody gene was previously obtained by ribosome display technology, and an anti-EGFR immunotoxin was generated in our previous study [25]. The recombinant anti-EGFR immunotoxin showed high specificity, affinity, and effective cytotoxicity to EGFR-positive cells, both in vitro and in vivo. However, higher affinity is not always advantageous because of the potential toxicity (on target/off tumor) to normal tissue. The EGFR antigen is also weakly expressed in some normal tissues. If the affinity of the anti-EGFR scFv is too high, CAR-modified lymphocytes may bind to normal tissues before it arrives at the tumor tissue and thus cause serious side effects [22,36,37]. Therefore, we have examined the potential systemic toxicity of CAR-modified lymphocytes; our results showed that it may be a safe tumor therapy strategy.
In vivo persistence of CAR-modified lymphocytes has also great impact on therapy efficacy, in addition to targeting specificity. Injection or expressions of cytokines, immune clearance, and addition of gene fragments that can extend the life of CAR-modified T lymphocytes to the intracellular domain of CAR have been used extensively. Several researchers have reported that IL-2, IL-7, IL-15, and IL-21 could improve in vivo persistence and antitumor effects [38–41]. However, expression of these cytokines will be regulated by many factors, such as Treg cells [42]. It is hard to judge to what extent these cytokines will promote tumor therapeutic efficacy. In the present study, IL15 was designed to be co-expressed with the CAR in the T lymphocytes. However, this strategy did not significantly improve the therapeutic effectiveness of CAR-modified lymphocytes. In addition, some researchers found that Treg cells soon have a marked negative regulatory effect following injection of therapeutic lymphocytes [43]. Rosenberg et al. have improved the therapeutic response, to more than 70%, using a sublethal dose of radiation before applying ACT. However, many older patients are not able to tolerate sublethal doses of immune clearance strategies, and high-dose IL-2 can cause significant side effects, such as renal toxicity [44]. CAR construction has improved from the first to the third generation in recent years. Some studies have shown that the first-generation CARs only had basic antitumor effects, and there was insufficient evidence to prove that the CAR-modified lymphocytes could significantly improve antigen-mediated cell proliferation. The main design of the second generation of CARs was the insertion of the CD28 or 4-1BB intracellular signaling domains between the transmembrane region and the CD3ζ domain. Experimental results have proven that second generation CARs can significantly enhance lymphocyte proliferation, while still maintaining the same antitumor effects as first-generation CARs [45,46]. Third-generation CARs were produced by connecting a third intracellular signal component to the second-generation CAR. These modified CARs can further amplify the signal transduction triggered by antigen binding by the CAR, thus markedly enhancing its antitumor effects. However, it also increases the risk in clinical treatment because of the potential of producing a high level of inflammatory cytokines in a short time, termed a “cytokine storm” [47,48]. On the basis of this concern, we designed the CAR used in this study following a second-generation approach, hoping in so doing to keep the balance between sufficient antitumor effects and low systemic toxicity.
Gene transfection of lymphocytes is also a major problem in immunology research. The most popular strategy used in transfection of lymphocytes employs an integrated virus, such as yielded by lentiviral and retroviral vectors. These viruses can randomly integrate into the genome of lymphocytes, which may lead to the activation of some unknown cancer genes and other disease genes, resulting in a latent hazard [49]. In this study, we designed the CAR construct using a nonviral vector and achieved efficient transfection efficiency using the Nucleofector System after optimizing the electric transfer parameters. Cell viability could be maintained at more than 85%, and transfection efficiency of lymphocytes achieved was 25% to 35%. Although there is still a big gap of the transfection efficiency of nucleofector compared with viral vectors, it is rare in the nonviral vector system and has a higher safety. The nonviral vector-based CAR in our study could be continually expressed in vivo in 2 weeks, which ensured a sufficient time for its antitumor effect (Figure 4D).
Taken together, we have investigated the anticancer ability of the EGFR-targeting scFv in genetically modified EGFR-targeting lymphocytes. The EGFR-targeted CAR-modified T lymphocytes demonstrated specific cytotoxicity and expressed cytokines efficiently after stimulation with the tumor antigen. Through the recognition of EGFR, our ACT method directly inhibited tumor formation, growth, and metastasis, and the CAR-modified lymphocyte treatment strategy demonstrated good safety. Our study provides an experimental basis for the clinical application of genetically engineered EGFR-targeted lymphocytes; moreover, we also evaluated a new and interesting therapy protocol.
Supplementary Materials and Methods
Antibody
Anti-EGFR and anti-CD3ζ antibodies were purchased from Cell Signaling Technology (Danvers, MA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was purchased from Trevigen (Gaithersburg, MD). To prepare anti-EGFR scFv serum, New Zealand white rabbits (Laboratory Animal Center of Sichuan University, Chengdu, China) were immunized s.c. three times with a mixture of EGFR scFv protein/complete and incomplete Freud's adjuvant; then, the rabbits were sacrificed and serum collected and stored at -80°C.
CAR Gene Transduction of Human T Cells
After stimulating with anti-CD3 and anti-CD28 mAbs for 5 days, human primary T cells were washed once in RPMI 1640 medium without FBS and were resuspended in 100 µl of nucleofector solution; 3 µg of plasmid DNA (viz., EGFR-specific CAR vector or an equal amount of the vehicle vector, in moles) was immediately added and mixed well with the cell solution. The cell/DNA mixture was subsequently transferred to an electroporation cuvette and placed in the Nucleofector device (Lonza). Nucleofection of the cells was accomplished using the T-23 program, and samples were immediately transferred to six-well plates containing 2 ml of prewarmed media without hIL-2. Cells were incubated in a humidified 37°C/5% CO2 incubator. Medium that contained IL-2 was changed 12 hours after the transfection.
Detection of CAR Expression on Human T Cells
For Western blot analysis, human T cells transfected with the CAR construct were lysed in RIPA buffer and the cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% acrylamide gel. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, which was then incubated first with a rabbit anti-human CD3 zeta antibody and then with a goat anti-rabbit IgG HRP-conjugated antibody. Antibody binding was revealed using an Enhanced Chemiluminescent Kit (Santa Cruz Biotechnology Inc, Santa Cruz, CA).
For flow cytometric analysis, CAR construct-transfected human T cells were washed once with phosphate-buffered saline and then incubated with anti-EGFR scFv serum and subsequently with a goat anti-rabbit IgG fluorescein isothiocyanate (FITC)-conjugated antibody. Positive cells were detected using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Competitive In Vivo Persistence Experiment with CAR T Cells and Vehicle T Cells
Mice were engrafted with 2 x 106 A549 cells, followed 3 weeks later by escalating doses of a 1:1 mixture of CAR T cells and Vehicle T cells, after adjusting according to the transfection efficiency. Some of the animals were sacrificed and spleen samples were collected 0, 7, 14, and 28 days after T cell injection. The biodistribution of the CAR T cells and Vehicle T cells were investigated using quantitative PCR for the vector and anti-EGFR scFv sequence.
Supplementary Material
Abbreviations
- EGFR
epidermal growth factor receptor
- ACT
adoptive cell therapy
- CAR
chimeric antigen receptor
Footnotes
This work is supported by China National Science and Technology Programs of Significant New Drugs to Create (No. 2013ZX09301304-003), National Key Basic Research Program (973 Program) of China (No. 2010CB529900), China Postdoctoral Science Foundation, and the National Natural Science Foundation of China (No. NSFC81202324). The authors declare that they have no conflict of interest.
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Sridhar SS, Seymour L, Shepherd FA. Inhibitors of epidermal-growth-factor receptors: a review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol. 2003;4:397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt M, Reiser P, Hills D, Gullick WJ, Wels W. Expression of an oncogenic mutant EGF receptor markedly increases the sensitivity of cells to an EGF-receptor-specific antibody-toxin. Int J Cancer. 1998;75:878–884. doi: 10.1002/(sici)1097-0215(19980316)75:6<878::aid-ijc10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593–1611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- 4.Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer. 2001;8:3–9. doi: 10.1677/erc.0.0080003. [DOI] [PubMed] [Google Scholar]
- 5.Fish-Steagall A, Searcy P, Sipples R. Clinical experience with anti-EGFR therapy. Semin Oncol Nurs. 2006;22:10–19. doi: 10.1016/j.soncn.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007;298:70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- 7.Bush T, Freeman D, Ogbagabriel S, Belmontes B, Kozlosky C, Baher A, Johnson C, Van G, Cerretti D, Radinsky R. Activity of panitumumab alone and in combination with chemotherapy against mutant epidermal growth factor receptor (EGFr)-expressing non-small cell lung carcinoma (NSCLC) cell lines and xenografts. Clin Cancer Res. 2005;11:9047s. [Google Scholar]
- 8.Freeman DJ, Bush T, Ogbagabriel S, Belmontes B, Juan T, Plewa C, Van G, Johnson C, Radinsky R. Activity of panitumumab alone or with chemotherapy in non-small cell lung carcinoma cell lines expressing mutant epidermal growth factor receptor. Mol Cancer Ther. 2009;8:1536–1546. doi: 10.1158/1535-7163.MCT-08-0978. [DOI] [PubMed] [Google Scholar]
- 9.Rossi A, Maione P, Gridelli C. Cetuximab in advanced non-small cell lung cancer. Crit Rev Oncol Hematol. 2006;59:139–149. doi: 10.1016/j.critrevonc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Borghaei H, Langer CJ, Millenson M, Ruth KJ, Litwin S, Tuttle H, Seldomridge JS, Rovito M, Mintzer D, Cohen R, et al. Phase II study of paclitaxel, carboplatin, and cetuximab as first line treatment, for patients with advanced non-small cell lung cancer (NSCLC): results of OPN-017. J Thorac Oncol. 2008;3:1286–1292. doi: 10.1097/JTO.0b013e318189f50e. [DOI] [PubMed] [Google Scholar]
- 11.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 14.Morgan RA, Dudley ME, Rosenberg SA. Adoptive cell therapy: genetic modification to redirect effector cell specificity. Cancer J. 2010;16:336–341. doi: 10.1097/PPO.0b013e3181eb3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yee C. Adoptive therapy using antigen-specific T-cell clones. Cancer J. 2010;16:367–373. doi: 10.1097/PPO.0b013e3181eacba8. [DOI] [PubMed] [Google Scholar]
- 17.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coccoris M, Straetemans T, Govers C, Lamers C, Sleijfer S, Debets R. T cell receptor (TCR) gene therapy to treat melanoma: lessons from clinical and preclinical studies. Expert Opin Biol Ther. 2010;10:547–562. doi: 10.1517/14712591003614756. [DOI] [PubMed] [Google Scholar]
- 19.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross G, Gorochov G, Waks T, Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc. 1989;21:127–130. [PubMed] [Google Scholar]
- 21.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Qiu J, Wang Z, Huang N, Li X, Li Q, Zhang Y, Zhao C, Luo C, Zhang N, et al. In vitro and in vivo anti-tumor activities of anti-EGFR single-chain variable fragment fused with recombinant gelonin toxin. J Cancer Res Clin Oncol. 2012;138:1081–1090. doi: 10.1007/s00432-012-1181-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Li X, Gou M, Qiu J, Li J, Yu C, Zhang Y, Zhang N, Teng X, Chen Z, et al. Antitumoral efficacy by systemic delivery of heparin conjugated polyethylenimine-plasmid interleukin-15 complexes in murine models of lung metastasis. Cancer Sci. 2011;102:1403–1409. doi: 10.1111/j.1349-7006.2011.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Majumder N, Lin H, Chen J, Falo LD, Jr, You Z. Enhanced immunity by NeuEDhsp70 DNA vaccine is needed to combat an aggressive spontaneous metastatic breast cancer. Mol Ther. 2005;11:941–949. doi: 10.1016/j.ymthe.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Tian S, Liu Z, Donahue C, Falo LD, Jr, You Z. Genetic targeting of the active transcription factor XBP1s to dendritic cells potentiates vaccine-induced prophylactic and therapeutic antitumor immunity. Mol Ther. 2012;20:432–442. doi: 10.1038/mt.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 31.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, Rosenberg SA, Morgan RA. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 2011;19:751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarden Y. The EGFR family and its ligands in human cancer signalling-mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(suppl 4):S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 35.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serrano LM, Pfeiffer T, Olivares S, Numbenjapon T, Bennitt J, Kim D, Smith D, McNamara G, Al-Kadhimi Z, Rosenthal J, et al. Differentiation of naive cord-blood T cells into CD19-specific cytolytic effectors for posttransplantation adoptive immunotherapy. Blood. 2006;107:2643–2652. doi: 10.1182/blood-2005-09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu C, Jones SA, Cohen CJ, Zheng Z, Kerstann K, Zhou J, Robbins PF, Peng PD, Shen X, Gomes TJ, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109:5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagaraj S, Ziske C, Schmidt-Wolf IG. Human cytokine-induced killer cells have enhanced in vitro cytolytic activity via non-viral interleukin-2 gene transfer. Genet Vaccines Ther. 2004;2:12. doi: 10.1186/1479-0556-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markley JC, Sadelain M. IL-7 and IL-21 are superiorto IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–3519. doi: 10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu C, Hughes MS, Zheng Z, Bray RB, Rosenberg SA, Morgan RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005;175:7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez-Vallina L, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol. 1996;26:2304–2309. doi: 10.1002/eji.1830261006. [DOI] [PubMed] [Google Scholar]
- 46.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buning H, Uckert W, Cichutek K, Hawkins RE, Abken H. Do CARs need a driver's license? Adoptive cell therapy with chimeric antigen receptor-redirected T cells has caused serious adverse events. Hum Gene Ther. 2010;21:1039–1042. doi: 10.1089/hum.2010.131. [DOI] [PubMed] [Google Scholar]
- 49.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.