Abstract
As a neurotransmitter, serotonin (5-HT) is widely used throughout the brain and known to play a role in many processes including emotion and brain development. Of the 15 subtypes of 5-HT receptors, the 1A receptor (5-HT1A) has been implicated in depression and suicide. Using the [carbonyl-11C]WAY100635 ([11C]WAY) ligand and positron emission tomography, we have studied the 5-HT1A receptor, first in a group of healthy controls, then in two separate groups of subjects with major depressive disorder (MDD) (antidepressant exposed and not recently medicated), and, lastly, in a group of subjects remitted from MDD. All MDD subjects were medication-free at the time of scan. We found higher 5-HT1A binding potential (BPF) in MDD subjects not recently exposed to an antidepressant compared with controls and recently medicated MDD subjects; and higher BPF in subjects with the C(-1019)G promoter polymorphism. We replicated these findings in a novel cohort and reconciled our discrepant findings with other groups using alternate quantification techniques. We also reported higher BPF in subjects remitted from a major depressive episode than in controls. From this work, we proposed a temporal model in which 5-HT1A BPF may be a trait abnormality of MDD. To further explore the genetic components of MDD and utility of 5-HT1A imaging as a potential tool for biomarker or treatment response prediction, these findings should be replicated in a larger cohort using the [11C]CUMI-101 agonist tracer.
Keywords: serotonin 1A, positron emission tomography, depression, genotype
1. Introduction
Serotonin is one of the most widely recognized neurotransmitters. To date, 15 subtypes of serotonin receptors have been identified in the brain and throughout the body [1]. The serotonergic system has been implicated in various psychiatric disorders and conditions such as anxiety [2], chronic stress [3], schizophrenia, major depressive disorder (MDD) and suicide [4]. Major depression is particularly pervasive in that estimates approximate a 6.7 per cent prevalence rate in adults over a twelve month period [5] and 13 per cent prevalence over the lifetime [6]. Moreover, MDD is associated with an increased risk in suicide and overall poorer health. Economic estimates suggest the burden of MDD is greater than 10 billion dollars owing to disability, loss of productivity and treatment [7–9], whereas projections indicate MDD will become the second greatest contributor to disability across all age groups by the year 2020 [10].
There is great utility in the quantification of neuroreceptors in understanding and possibly treating major depressive disorder. Characterizing the function or dysfunction of specific receptors in MDD will lead to better understanding of the disorder and plausible utility of these receptors as biomarkers, which could further improve treatment paradigms or help in predicting treatment response. Positron emission tomography (PET) is one modality through which serotonin receptors can be imaged in various disorders, including MDD.
Animal studies [11,12], antidepressant treatment response [13] and post-mortem [14] studies have implicated the serotonin 1A receptor (5-HT1A) in MDD, which can be imaged using the [carbonyl-11C]WAY100635 ([11C]WAY) ligand. Pike et al. [15] previously described the selectivity and sensitivity of [11C-WAY] as a PET ligand, and the characteristics which make this ligand more amenable to quantification and modelling than its predecessors [15]. Using the [11C]WAY ligand, we have carefully characterized its modelling and binding in healthy controls, taken the ligand into clinical populations, determined human dosimetry and replicated our initial findings in a second cohort. This work has led us to develop a temporal model in which we purport alterations in 5-HT1A binding may be a trait abnormality in subjects with MDD compared with controls (figure 1; [16]).
Figure 1.
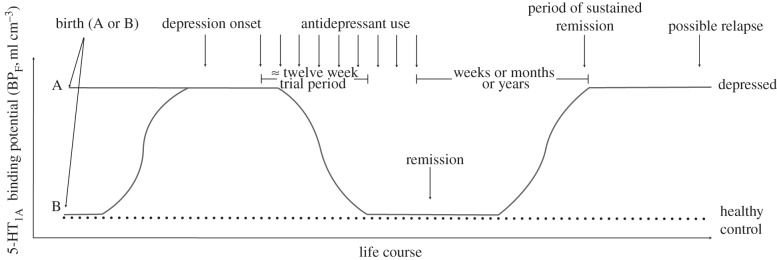
Temporal model of serotonin 1A receptor binding potential over the lifetime of subjects with major depression compared with controls. Subjects may be born with a preexisting vulnerability towards major depression (position A) or not (position B).
2. Quantifying and characterizing [11C]WAY binding
In order to develop this model, it was necessary to quantify PET imaging data across subjects. In this quantification, the primary outcome measures of interest are binding potentials: BPF ((VT − VND)/fP), BPP (VT − VND) and BPND ((VT − VND)/VND); where VT is the total volume of distribution in a region of interest, VND is the total non-specific binding usually measured in reference tissue, and fP is the plasma free fraction [17]. While BPND may be the easiest outcome measure to obtain as it does not require an arterial input function, it does require the most assumptions: namely that there is a reliable reference region that is devoid of specific binding, does not differ between groups and can be fit with a one-tissue compartment (1-TC) model [18]. On the other hand, BPF requires full arterial sampling, but makes the fewest assumptions and provides the measure closest in vivo to Bmax/KD, a measure of receptor density. With some tracers but not all, BPF obtained through arterial sampling also outperforms BPND quantified via reference tissue methods [19,20] with regard to reproducibility and bias [21].
As previously mentioned, a good reference region is imperative to performing quantitative PET. Both reference tissue and kinetic analysis methods require an estimate of non-specific binding (VND) to calculate any of the three outcome measures. With low levels of 5-HT1A receptors, the cerebellum is commonly used as the reference region. Yet, we estimated specific binding (VT) in the cerebellum to be approximately 48 per cent [22], which in vitro data suggest is concentrated in the cerebellar vermis and grey matter. Therefore, to avoid biasing the VND estimate, we used and recommend using a region most devoid of receptors, such as cerebellar white matter (CWM), as reference tissue for in vivo quantification. As compared with a total cerebellar reference region, CWM is better fit with a 1-TC model and improves reproducibility and identifiability [22]. Hirvonen et al. [23] also assert that CWM may be used as an optimal reference region for kinetic analysis of [11C]WAY [24].
Choosing appropriate modelling methods and reference regions is imperative to data analysis, as different techniques may effect the interpretation of the results. While reference region methods may be desirable in clinical settings because they do not require an arterial line and, thus, are less physically demanding on the subject, they consistently underestimate [11C]WAY binding [18,21]. Similarly, using the total cerebellum as reference region would underestimate binding and possibly obfuscate the direction of any hypothesized group differences, because VND, which is subtracted from the VT of the region of interest, contains measurable specific binding. Taken together, these findings suggest that kinetic modelling with an arterial input function and CWM as reference region provides the best quantitative estimates of BPF when using [11C]WAY.
Satisfied with the optimal modelling methods, we proceeded to characterize the ligand in a group of healthy control subjects. Previous studies reported age [25], sex [26,27] and aggression [28,29] dependencies on 5-HT1A estimates, although not all in consistent directions. Consistent with Rabiner et al. [30] we did not find an age dependency in our group of healthy controls. However, we did find lower 5-HT1A BPF in males compared with females, consistent with post-mortem findings [26], but only partially consistent with BPND findings from Moses-Kolko et al. [27]. We also reported an inverse correlation between lifetime aggression and BPF [31], consistent with pre-clinical data [28,29]. Having identified these covariates in our subject group, future studies using [11C]WAY should therefore incorporate both sex and aggression as covariates in the statistical model.
Our primary statistical analyses consist of linear mixed effects models with the subject as the random effect and region, and diagnostic group as fixed effects. Including all regions of interest in the model simultaneously increases the power and accounts for the correlation between regions within a subject, while decreasing the issue of multiple comparisons. All analyses including more than one region of interest are done on log-transformed data to reduce variance and alleviate any skewness [32]. Additionally, our observations are weighted with standard errors estimated using a bootstrap algorithm which takes into account errors in the plasma, metabolite and brain data [33]. Once the model is built, individual variables like sex and aggression can be incorporated into the model as covariates when appropriate.
3. [11C]WAY binding in major depression: first cohort, replication and reconciliation
Following previous publications reporting lower 5-HT1A binding in depressed subjects compared with controls [34,35], we hypothesized similar group differences in our cohort. However, using the optimal modelling and reference region methods we previously described, we found higher BPF in not recently medicated (NRM) subjects in the midst of a depressive episode compared with both currently depressed subjects with prior antidepressant exposure within the past 4 years and healthy controls [36]. The findings remained significant when we included sex and aggression in the model. Also, consistent with Lemonde et al. [37], in this same subject group, we found a significant effect of the 5-HT1A C(-1019)G promoter polymorphism in the dorsal raphe region of interest: specifically, BPF increased with number of G alleles. These findings raised several questions relating to the genetic predisposition of depression, the effect of antidepressant exposure, possible treatment resistance and major depression as a state or trait phenomenon.
Concurrent with the aforementioned study, we questioned the acceptable limits of the radiation dose associated with [11C]WAY. A closer look at the human dosimetry of the WAY ligand revealed that the renal system comprised the critical organs, which is discordant with the extrapolated data from rat studies identifying the liver as the critical organ [38]. From this novel in vivo data, we recommended the dose be limited to under 300 MBq in men and 227 MBq in women, and adjusted our studies accordingly.
With the new limits on injected dose, we replicated our finding of higher BPF in NRM subjects compared with controls in a novel cohort (figure 2; [32]). The finding remained significant when we combined the two cohorts and included covariates in the model: injected dose, injected mass, aggression and sex. We also replicated the genotype finding in the larger sample (figure 3).
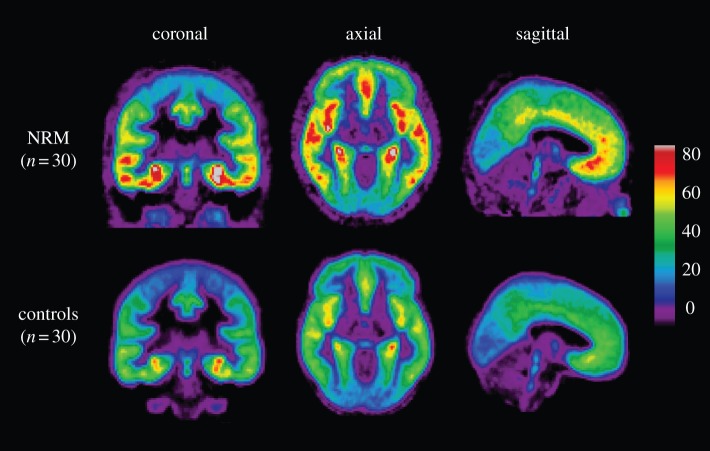
Figure 2.
Mean voxel BPF maps of 5-HT1A [carbonyl-11C]WAY100635 binding. Not recently medicated (NRM) subjects show visibly higher binding throughout the brain than do controls.
Figure 3.
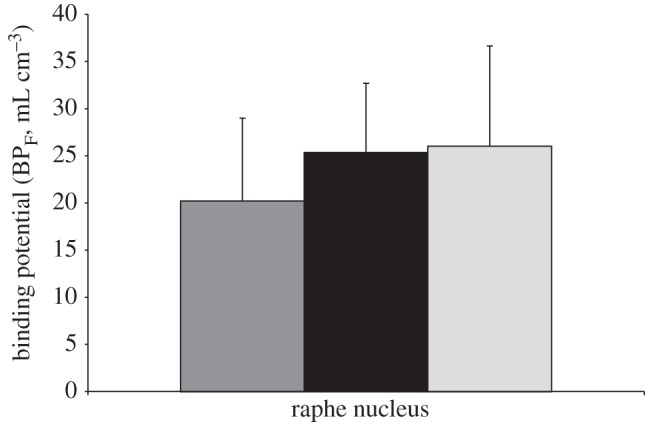
The C(-1019)G 5-HT1A promoter polymorphism in controls and NRM subjects. Binding potential (BPF) shows a stepwise increase in the raphe nuclei with G allele frequency: CC < CG < GG (d.f. = 1,78; F = 7.13; p = 0.009). The height of the bars indicates the weighted mean BPF, while the error bars represent the corresponding equivalent of the standard deviation of each weighted mean. Dark grey bar represents CC (n = 22), black bar represents CG (n = 47) and light grey bar represents GG (n = 11).
In addition to the replication, within this combined sample we were able to reconcile some of the discrepant findings between our group and others. Similar to Hirvonen et al. [23], we found no differences between NRM MDD and controls using BPND from a reference tissue model. However, using the same model but with cerebellar grey matter (CGM) as reference tissue, we found lower BPND in the NRM MDD subjects compared with controls. Although this is consistent with some previous reports [34,35,39], it is more likely to be reflective of differences in specific binding in the reference region, as we also found significantly different VT in CGM between NRM MDD and controls. To address the issue of specific binding in the reference tissue, we reanalysed pindolol blockade data [40]. We reported changes in CGM but not CWM following pindolol administration, which again demonstrates measureable in vivo specific binding in CGM, and further supports the use of CWM as a measure of non-specific binding for the reference region.
4. Future directions and implications
Our finding of higher 5-HT1A in NRM MDD is replicable and also consistent with post-mortem data [26], as well as data from other modalities [29,37,41]. Furthermore, it lends additional support to the hypothesis of higher 5-HT1A in depression and agrees with the purported model underlying selective serotonin reuptake inhibitors (SSRIs) [42,43]. Although it is known that not all patients will respond to SSRIs, it is often the first line of treatment sought and used [44]. Under naturalistic treatment conditions, we found that subjects with higher 5-HT1A were less likely to remit after 1 year of treatment [45]. Genetic [46] and animal [47] studies also suggest higher 5-HT1A may be associated with poorer treatment response. Similar to Spindelegger et al. [48], we have also found decreases in 5-HT1A BPF in NRM subjects following acute SSRI treatment [49], which again is consistent with the underlying mechanism of action of SSRIs. Lastly, we reported higher 5-HT1A within a cohort of subjects who were currently remitted from a major depressive episode, suggesting higher 5-HT1A expression could be a trait feature of MDD [16]. Thus, if future prospective studies are able to replicate these findings, 5-HT1A imaging could aide in treatment development, approach and planning.
Having characterized many aspects of the [11C]WAY ligand over several years, it is our assertion that modelling and reference region choices can significantly influence the results and interpretations of studies. For [11C]WAY and other ligands, it is imperative to conduct and evaluate full kinetic modelling prior to using reference tissue methods, which require several assumptions. In the case of [11C]WAY, the reference tissue assumptions cannot be made and full arterial sampling or comparable estimates of input function are necessary to describe the data optimally. However, a new agonist 5-HT1A ligand, [11C]CUMI-101, may be more amenable to reference tissue modelling [50]. Using reference tissue methods or simulated annealing [51,52] to estimate input function, the need for arterial sampling would be eliminated, thereby reducing the subject burden. Ideally, this would make it easier to obtain quantitative data in larger samples, which are needed to better explore genetic and treatment effects on 5-HT1A expression.
Various subtypes of MDD could also be better classified and described in larger samples. Under the current Diagnostic and Statistical Manual-IV description, MDD can be diagnosed by meeting five of nine criteria [53], which creates 1099 ways to meet criteria for the diagnosis; this implies that there is a great deal of individual variation within a MDD cohort. A dimensional approach to symptom categorization, as that proposed under the Research Domain Criteria [54,55] initiative, may help better describe subject groups in future studies. Focusing on symptom clusters across diagnostic groups may benefit treatment prediction studies and identification of biomarkers by decreasing the variance inherent in studying diagnostic groups. Eventually, with the application of careful methodology, quantification and hope of identifying treatment predictors, it may be possible to bring serotonergic imaging of the 5-HT1A and other receptors into clinical use.
Acknowledgements
The authors gratefully acknowledge Drs J. John Mann, R. Todd Ogden, Francesca Zanderigo and Christine DeLorenzo for their work with the [11C]WAY ligand over the years, and assistance with this manuscript.
References
- 1.Berger M, Gray JA, Roth BL. 2009. The expanded biology of serotonin. Annu. Rev. Med. 60, 355–366 10.1146/annurev.med.60.042307.110802 (doi:10.1146/annurev.med.60.042307.110802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan GM, Oquendo MA, Simpson N, Van Heertum RL, Mann JJ, Parsey RV. 2005. Brain serotonin 1A receptor binding in major depression is related to psychic and somatic anxiety. Biol. Psychiat. 58, 947–954 10.1016/j.biopsych.2005.05.006 (doi:10.1016/j.biopsych.2005.05.006) [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic H, Perski A, Berglund H, Savic I. 2011. Chronic stress is linked to 5-HT(1A) receptor changes and functional disintegration of the limbic networks. NeuroImage 55, 1178–1188 10.1016/j.neuroimage.2010.12.060 (doi:10.1016/j.neuroimage.2010.12.060) [DOI] [PubMed] [Google Scholar]
- 4.Nikolaus S, Antke C, Muller HW. 2009. In vivo imaging of synaptic function in the central nervous system. II. Mental and affective disorders. Behav. Brain Res. 204, 32–66 10.1016/j.bbr.2009.06.009 (doi:10.1016/j.bbr.2009.06.009) [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiat. 62, 617–627 10.1001/archpsyc.62.6.617 (doi:10.1001/archpsyc.62.6.617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasin DS, Goodwin RD, Stinson FS, Grant BF. 2005. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch. Gen. Psychiat. 62, 1097–1106 10.1001/archpsyc.62.10.1097 (doi:10.1001/archpsyc.62.10.1097) [DOI] [PubMed] [Google Scholar]
- 7.Pincus HA, Pettit AR. 2001. The societal costs of chronic major depression. J. Clin. Psychiat. 62, 5–9 [PubMed] [Google Scholar]
- 8.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. 2003. Cost of lost productive work time among US workers with depression. J. Am. Med. Assoc. 289, 3135–3144 10.1001/jama.289.23.3135 (doi:10.1001/jama.289.23.3135) [DOI] [PubMed] [Google Scholar]
- 9.Bender A, Farvolden P. 2008. Depression and the workplace: a progress report. Curr. Psychiat. Rep. 10, 73–79 10.1007/s11920-008-0013-6 (doi:10.1007/s11920-008-0013-6) [DOI] [PubMed] [Google Scholar]
- 10.WHO 2011. Depression. Geneva, Switzerland: World Health Organization; See http://www.who.int/mental_health/management/depression/definition/en/ (updated on 18 August 2011; accessed on 15 November 2011). [Google Scholar]
- 11.Naudon L, El Yacoubi M, Vaugeois JM, Leroux-Nicollet I, Costentin J. 2002. A chronic treatment with fluoxetine decreases 5-HT(1A) receptors labeling in mice selected as a genetic model of helplessness. Brain Res. 936, 68–75 10.1016/S0006-8993(02)02548-9 (doi:10.1016/S0006-8993(02)02548-9) [DOI] [PubMed] [Google Scholar]
- 12.Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. 1998. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc. Natl Acad. Sci. USA 95, 15 049–15 054 10.1073/pnas.95.25.15049 (doi:10.1073/pnas.95.25.15049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddjeri N, Blier P, de Montigny C. 1998. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J. Neurosci. 18, 10 150–10 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. 2001. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 25, 892–903 10.1016/S0893-133X(01)00310-4 (doi:10.1016/S0893-133X(01)00310-4) [DOI] [PubMed] [Google Scholar]
- 15.Pike VW, et al. 1996. Exquisite delineation of 5-HT1A receptors in human brain with PET and carbonyl-11 C]WAY-100635. Eur. J. Pharmacol. 301, R5–R7 10.1016/0014-2999(96)00079-9 (doi:10.1016/0014-2999(96)00079-9) [DOI] [PubMed] [Google Scholar]
- 16.Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, Parsey RV. 2009. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology 34, 2275–2284 10.1038/npp.2009.54 (doi:10.1038/npp.2009.54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innis RB, et al. 2007. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 27, 1533–1539 10.1038/sj.jcbfm.9600493 (doi:10.1038/sj.jcbfm.9600493) [DOI] [PubMed] [Google Scholar]
- 18.Slifstein M, Parsey RV, Laruelle M. 2000. Derivation of [11C]WAY-100635 binding parameters with reference tissue models: effect of violations of model assumptions. Nucl. Med. Biol. 27, 487–492 10.1016/S0969-8051(00)00117-7 (doi:10.1016/S0969-8051(00)00117-7) [DOI] [PubMed] [Google Scholar]
- 19.Hume SP, Myers R, Bloomfield PM, Opacka-Juffry J, Cremer JE, Ahier RG, Luthra SK, Brooks DJ, Lammertsma AA. 1992. Quantitation of carbon-11-labeled raclopride in rat striatum using positron emission tomography. Synapse 12, 47–54 10.1002/syn.890120106 (doi:10.1002/syn.890120106) [DOI] [PubMed] [Google Scholar]
- 20.Lammertsma AA, Hume SP. 1996. Simplified reference tissue model for PET receptor studies. NeuroImage 4, 153–158 10.1006/nimg.1996.0066 (doi:10.1006/nimg.1996.0066) [DOI] [PubMed] [Google Scholar]
- 21.Parsey RV, et al. 2000. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. J. Cereb. Blood Flow Metab. 20, 1111–1133 10.1097/00004647-200007000-00011 (doi:10.1097/00004647-200007000-00011) [DOI] [PubMed] [Google Scholar]
- 22.Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, Mann JJ. 2005. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J. Cereb. Blood Flow Metab. 25, 785–793 10.1038/sj.jcbfm.9600072 (doi:10.1038/sj.jcbfm.9600072) [DOI] [PubMed] [Google Scholar]
- 23.Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, Nagren K, Salminen JK, Hietala J. 2008. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int. J. Neuropsychopharmacol. 11, 465–476 10.1017/s1461145707008140 (doi:10.1017/s1461145707008140) [DOI] [PubMed] [Google Scholar]
- 24.Hirvonen J, Kajander J, Allonen T, Oikonen V, Nagren K, Hietala J. 2007. Measurement of serotonin 5-HT1A receptor binding using positron emission tomography and [carbonyl-(11)C]WAY-100635: considerations on the validity of cerebellum as a reference region. J. Cereb. Blood Flow Metab. 27, 185–195 10.1038/sj.jcbfm.9600326 (doi:10.1038/sj.jcbfm.9600326) [DOI] [PubMed] [Google Scholar]
- 25.Cidis Meltzer C, et al. 2001. Gender-specific aging effects on the serotonin 1A receptor. Brain Res. 895, 9–17 10.1016/S0006-8993(00)03211-X (doi:10.1016/S0006-8993(00)03211-X) [DOI] [PubMed] [Google Scholar]
- 26.Arango V, Underwood MD, Gubbi AV, Mann JJ. 1995. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 688, 121–133 10.1016/0006-8993(95)00523-S (doi:10.1016/0006-8993(95)00523-S) [DOI] [PubMed] [Google Scholar]
- 27.Moses-Kolko EL, et al. 2011. Age, sex, and reproductive hormone effects on brain serotonin-1A and serotonin-2A receptor binding in a healthy population. Neuropsychopharmacology 36, 2729–2740 10.1038/npp.2011.163 (doi:10.1038/npp.2011.163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleare AJ, Bond AJ. 2000. Ipsapirone challenge in aggressive men shows an inverse correlation between 5-HT1A receptor function and aggression. Psychopharmacology (Berl.) 148, 344–349 10.1007/s002130050061 (doi:10.1007/s002130050061) [DOI] [PubMed] [Google Scholar]
- 29.Cleare AJ, Bond AJ. 2000. Experimental evidence that the aggressive effect of tryptophan depletion is mediated via the 5-HT1A receptor. Psychopharmacology (Berl.) 147, 439–441 10.1007/s002130050014 (doi:10.1007/s002130050014) [DOI] [PubMed] [Google Scholar]
- 30.Rabiner EA, et al. 2002. A database of [(11)C]WAY-100635 binding to 5-HT1A receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. NeuroImage 15, 620–632 10.1006/nimg.2001.0984 (doi:10.1006/nimg.2001.0984) [DOI] [PubMed] [Google Scholar]
- 31.Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. 2002. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 954, 173–182 10.1016/S0006-8993(02)03243-2 (doi:10.1016/S0006-8993(02)03243-2) [DOI] [PubMed] [Google Scholar]
- 32.Parsey RV, et al. 2010. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol. Psychiat. 68, 170–178 10.1016/j.biopsych.2010.03.023 (doi:10.1016/j.biopsych.2010.03.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogden RT, Tarpey T. 2006. Estimation in regression models with externally estimated parameters. Biostatistics 7, 115–129 10.1093/biostatistics/kxi044 (doi:10.1093/biostatistics/kxi044) [DOI] [PubMed] [Google Scholar]
- 34.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. 1999. PET imaging of serotonin 1A receptor binding in depression. Biol. Psychiat. 46, 1375–1387 10.1016/S0006-3223(99)00189-4 (doi:10.1016/S0006-3223(99)00189-4) [DOI] [PubMed] [Google Scholar]
- 35.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. 2000. Brain serotonin 1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch. Gen. Psychiat. 57, 174–180 10.1001/archpsyc.57.2.174 (doi:10.1001/archpsyc.57.2.174) [DOI] [PubMed] [Google Scholar]
- 36.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. 2006. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol. Psychiat. 59, 106–113 10.1016/j.biopsych.2005.06.016 (doi:10.1016/j.biopsych.2005.06.016) [DOI] [PubMed] [Google Scholar]
- 37.Lemonde S, et al. 2003. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J. Neurosci. 23, 8788–8799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsey RV, Belanger MJ, Sullivan GM, Simpson NR, Stabin MG, Van Heertum R, Mann JJ. 2005. Biodistribution and radiation dosimetry of 11C-WAY100,635 in humans. J. Nucl. Med. 46, 614–619 [PubMed] [Google Scholar]
- 39.Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. 2007. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl. Med. Biol. 34, 865–877 10.1016/j.nucmedbio.2007.06.008 (doi:10.1016/j.nucmedbio.2007.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez D, et al. 2001. Differential occupancy of somatodendritic and postsynaptic 5HT1A receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology 24, 209–229 10.1016/S0893-133X(00)00187-1 (doi:10.1016/S0893-133X(00)00187-1) [DOI] [PubMed] [Google Scholar]
- 41.O'Reilly KC, Trent S, Bailey SJ, Lane MA. 2007. 13-cis-Retinoic acid alters intracellular serotonin, increases 5-HT1A receptor, and serotonin reuptake transporter levels in vitro. Exp. Biol. Med. 232, 1195–1203 10.3181/0703-RM-83 (doi:10.3181/0703-RM-83) [DOI] [PubMed] [Google Scholar]
- 42.Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. 1998. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann. N Y Acad. Sci. 861, 204–216 10.1111/j.1749-6632.1998.tb10192.x (doi:10.1111/j.1749-6632.1998.tb10192.x) [DOI] [PubMed] [Google Scholar]
- 43.Gardier AM, Malagie I, Trillat AC, Jacquot C, Artigas F. 1996. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam. Clin. Pharmacol. 10, 16–27 10.1111/j.1472-8206.1996.tb00145.x (doi:10.1111/j.1472-8206.1996.tb00145.x) [DOI] [PubMed] [Google Scholar]
- 44.Rush AJ, et al. 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiat. 163, 1905–1917 10.1176/appi.ajp.163.11.1905 (doi:10.1176/appi.ajp.163.11.1905) [DOI] [PubMed] [Google Scholar]
- 45.Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. 2006. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology 31, 1745–1749 10.1038/sj.npp.1300992 (doi:10.1038/sj.npp.1300992) [DOI] [PubMed] [Google Scholar]
- 46.Le Francois B, Czesak M, Steubl D, Albert PR. 2008. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology 55, 977–985 10.1016/j.neuropharm.2008.06.046 (doi:10.1016/j.neuropharm.2008.06.046) [DOI] [PubMed] [Google Scholar]
- 47.Richardson-Jones JW, et al. 2010. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65, 40–52 10.1016/j.neuron.2009.12.003 (doi:10.1016/j.neuron.2009.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spindelegger C, et al. 2009. Influence of escitalopram treatment on 5-HT1A receptor binding in limbic regions in patients with anxiety disorders. Mol. Psychiat. 14, 1040–1050 10.1038/mp.2008.35 (doi:10.1038/mp.2008.35) [DOI] [PubMed] [Google Scholar]
- 49.Gray NA, Milak MS, Delorenzo C, Ogden RT, Huang Y, Mann JJ, Parsey RV. In press Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biol. Psychiat. [DOI] [PMC free article] [PubMed]
- 50.Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JS, Majo VJ, Mann JJ, Parsey RV. 2010. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. J. Nucl. Med. 51, 1892–1900 10.2967/jnumed.110.076257 (doi:10.2967/jnumed.110.076257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogden RT, Zanderigo F, Choy S, Mann JJ, Parsey RV. 2010. Simultaneous estimation of input functions: an empirical study. J. Cereb. Blood Flow Metab. 30, 816–826 10.1038/jcbfm.2009.245 (doi:10.1038/jcbfm.2009.245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanderigo F, Ogden RT, Mann JJ, Parsey RV. 2010. A voxel-based clustering approach for the automatic selection of testing regions in the simultaneous estimation of input functions in PET. NeuroImage 52, S176. 10.1016/j.neuroimage.2010.04.143 (doi:10.1016/j.neuroimage.2010.04.143) [DOI] [Google Scholar]
- 53.American Psychiatric Association 2000. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR, 4th edn Washington, DC: American Psychiatric Association [Google Scholar]
- 54.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiat. 167, 748–751 10.1176/appi.ajp.2010.09091379 (doi:10.1176/appi.ajp.2010.09091379) [DOI] [PubMed] [Google Scholar]
- 55.Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS, Cuthbert BN. 2010. Developing constructs for psychopathology research: research domain criteria. J. Abnorm. Psychol. 119, 631–639 10.1037/a0020909 (doi:10.1037/a0020909) [DOI] [PubMed] [Google Scholar]