Abstract
Three agents have recently been approved to reduce the risk of stroke and embolism, and one agent is in phase 3 trials. These drugs cause less serious bleeding and are simpler to manage, compared with warfarin, but they are not without their risks.
INTRODUCTION
Atrial fibrillation (AF), the most commonly occurring cardiac arrhythmia, is associated with an increased risk of acute ischemic stroke.1 The magnitude of risk depends on the presence of certain patient characteristics—age and a history of hypertension, diabetes mellitus, moderately or severely impaired left ventricular systolic function, heart failure, prior ischemic stroke, transient ischemic attack, or systemic embolism—and varies across different patient groups. In patients with AF, long-term anticoagulation therapy decreases the future risk of stroke by preventing thrombus formation.2 Vitamin K antagonists (VKAs), such as warfarin (Coumadin, Bristol-Myers Squibb), are currently the standard of care for stroke prevention in these patients. Long-term warfarin thromboprophylaxis has been shown to reduce the risk of stroke by approximately 60% in patients with AF.3,4
Managing anticoagulation in patients with AF can be challenging, particularly when they are receiving a VKA such as warfarin. VKAs have a slow onset and offset of action, unpredictable pharmacokinetics, and multiple food and drug interactions. Their narrow therapeutic window, as defined by the International Normalized Ratio (INR) test, requires adequate anticoagulation to reduce thrombosis risk while avoiding excessive anticoagulation and bleeding.5–7 Patients receiving VKAs must be managed carefully to ensure that the required routine INR monitoring and subsequent dose adjustments are implemented. Despite its efficacy in stroke prevention, warfarin remains underprescribed because of concerns about bleeding risks and monitoring requirements.7,8
Newer oral anticoagulants have been developed to target specific components of the coagulation cascade—thrombin and Factor Xa (FXa)—and they share many practical benefits that make them easier to manage than warfarin.9 These advantages include predictable dose responses (fixed dosing), a rapid onset and offset of action, a wide therapeutic window (obviating the need for routine laboratory monitoring), and minimal drug and food interactions.9,10
Unlike warfarin, whose activity can be reversed with vitamin K, human fresh frozen plasma, or prothrombin complex concentrates, however, the newer agents currently have no proven reversal strategies.11 Antidotes to dabigatran and the direct factor Xa inhibitors are in different stages of development. These products, at best, will be clinically available in 2 to 3 years.
This article discusses oral anticoagulants that were either approved by the FDA or that have completed phase 3 trials for stroke prevention in patients with AF, as well as their potential impact on patients.
ORAL ANTICOAGULANTS IN STROKE PREVENTION
Direct Thrombin Inhibitors
The oral direct thrombin inhibitor (DTI) dabigatran (Pradaxa, Boehringer Ingelheim) was approved for reducing the risk of stroke and systemic embolism in patients with nonvalvular AF in October 2010. Dabigatran etexilate (a prodrug) is converted to active dabigatran by esterases after administration. Dabigatran has a low bioavailability, and approximately 80% of the orally administered dose is eliminated unchanged via renal excretion.12,13
Factor Xa Inhibitors
FXa inhibitors include rivaroxaban (Xarelto, Janssen), which was approved by the FDA for reducing the risk of stroke and systemic embolism in patients with nonvalvular AF in November 2011;14 apixaban (Eliquis, Bristol-Myers Squibb/Pfizer), approved in December 2012; and edoxaban tosilate hydrate (Lixiana, DU-176b, Daiichi Sankyo), which was approved in Japan in 2011 and is currently undergoing a phase 3 stroke-prevention trial.
Rivaroxaban has high oral bioavailability and is eliminated via both renal and fecal routes.14 Apixaban also has high oral bioavailability and multiple pathways of elimination, including the renal and intestinal routes.9 Edoxaban is an oral direct FXa inhibitor that reaches maximum concentration after 1 to 2 hours.15 It is eliminated through multiple pathways, but the majority of systemically absorbed drug is eliminated via renal excretion.
CLINICAL STUDIES
Trial designs and key clinical data for the following three studies are summarized in Table 1.
Table 1.
Clinical Trials of New Anticoagulants for Stroke Prevention in Patients With Atrial Fibrillation
| RE-LY | ROCKET-AF | ARISTOTLE | ENGAGE-AF | |||||
|---|---|---|---|---|---|---|---|---|
| Drug | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | ||||
| Dose | 150 mg twice daily 110 mg twice daily |
20 mg once daily | 5 mg twice daily | 30 or 60 mg twice daily | ||||
| Population (sample size) | Atrial fibrillation with one additional risk factor (n = 18,113) | Atrial fibrillation with moderate to high risk (n = 14,264) | Atrial fibrillation with one additional risk factor (n =18,201) | Atrial fibrillation with moderate to high risk (n = 20,500) | ||||
| Primary efficacy endpoint | Stroke or systemic embolism | Stroke or systemic embolism | Ischemic or hemorrhagic stroke or systemic embolism | Composite of stroke and systemic embolic events | ||||
| Dabigatran | Warfarin | Rivaroxaban | Warfarin | Apixaban | Warfarin | |||
| 150 mg | 110 mg | |||||||
| Primary efficacy endpoint | ||||||||
| Percent/year | 1.11 | 1.53 | 1.69 | 1.7 | 2.2 | 1.27 | 1.60 | NA |
| RR/HRa | 0.66 | 0.91 | 0.79 | 0.79 | ||||
| 95% CIa | 0.53–0.82 | 0.74–1.11 | 0.66–0.96 | 0.66–0.95 | ||||
| P valuea | P < 0.001b | P < 0.001c | P < 0.001c | P < 0.001c,d | ||||
| Major bleeding | ||||||||
| Percent/year | 3.11 | 2.71 | 3.36 | 14.9 | 14.5 | 2.13 | 3.09 | NA |
| RR/HRa | 0.93 | 0.80 | 1.03 | 0.69 | ||||
| 95% CIa | 0.81–1.07 | 0.69–0.93 | 0.96–1.11 | 0.60–0.80 | ||||
| P valuea | P = 0.31 | P = 0.003 | P = 0.44 | P < 0.001 | ||||
Trials: ARISTOTLE = Apixaban for Reduction in Stroke and Other Thromboembolic events in Atrial Fibrillation; ENGAGE-AF TIMI 48 = Global Study to Assess the Safety and Effectiveness of DU-176b vs. Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation; RE-LY = Randomized Evaluation of Long-term Anticoagulation Therapy; ROCKET-AF = Rivaroxaban Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation.
CI = confidence interval; HR = hazard ratio; NA = not applicable; RR = relative risk.
Versus warfarin.
For superiority.
For non-inferiority.
P = 0.01 for superiority.
RE-LY
Dabigatran (Pradaxa)
In the RE-LY trial (Randomized Evaluation of Long-term Anticoagulation Therapy), 18,113 patients with AF and at least one additional risk factor for stroke were randomly assigned to receive double-blinded dabigatran 110 mg or 150 mg twice daily or open-label warfarin (with a target INR of 2.0–3.0).16 The dabigatran 150-mg twice-daily dose significantly lowered the rates of stroke and systemic embolism compared with warfarin: 1.11% per year for dabigatran 150 mg and 1.69% per year for warfarin. The relative risk (RR) was 0.66, and the 95% confidence interval (CI) was 0.53 to 0.82 (P < 0.001), meeting the criteria for superiority. The dabigatran 110-mg twice-daily dose produced a decreased rate of stroke and systemic embolism similar to that for warfarin: 1.53% per year for dabigatran 110 mg (RR with dabigatran = 0.91; 95% CI, 0.74–1.11; P < 0.001), meeting the criteria for non-inferiority.
Patients receiving dabigatran also had lower rates of major bleeding compared with those who received warfarin. Rates of major bleeding were 3.36% per year with warfarin, 2.71% per year in patients with dabigatran 110 mg (P = 0.003), and 3.11% per year with dabigatran 150 mg (P = 0.31).16 However, there was a significantly higher rate of major gastrointestinal (GI) bleeding with dabigatran 150 mg than with warfarin (1.51% per year with dabigatran 150 mg vs. 1.02% per year with warfarin; P < 0.001). Patients who received dabigatran also had lower risks of intracranial bleeding (0.3% per year vs. 0.74% per year; P ≤ 0.001), life-threatening bleeding (1.45% per year vs. 1.8% per year; P = 0.04), and hemorrhagic strokes (0.1% per year vs. 0.38% per year; P < 0.001).
Dabigatran was associated with higher rates of dyspepsia (P < 0.001) and myocardial infarction (MI) with the 110-mg dose (P = 0.07) and with the 150-mg dose (P = 0.048) compared with warfarin.
Based primarily on the results of the RE-LY study, the FDA approved the 150-mg twice-daily dose of dabigatran for stroke prevention in patients with AF. However, the FDA did not approve the 110-mg dose, instead opting to recommend a lower 75-mg twice-daily dabigatran dose (which was not studied in RE-LY) for patients with severe renal impairment, as defined by a creatinine clearance (CrCl) of 15 to 30 mL/minute.
ROCKET-AF
Rivaroxaban (Xarelto)
In the double-blind, randomized ROCKET-AF trial (Rivaroxaban Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation), 14,264 patients with nonvalvular AF and additional risk factors for stroke received either rivaroxaban 20 mg once daily or dose-adjusted warfarin.17 The 15-mg once-daily dose was administered to patients with a CrCl of 30 to 49 mL/minute.
Rivaroxaban was non-inferior to warfarin for preventing stroke or systemic embolism in the intent-to-treat (ITT) population.17 Stroke or systemic embolism occurred at rates of 1.7% per year in patients receiving rivaroxaban and 2.2% per year in patients receiving warfarin. The hazard ratio (HR) in the rivaroxaban group was 0.79 (95% CI, 0.66–0.96; P < 0.001), meeting the criteria for non-inferiority.
There was no significant difference between the two groups in major bleeding. Intracranial and fatal bleeding occurred significantly less frequently with rivaroxaban. Intracranial hemorrhage occurred at rates of 0.5% with rivaroxaban and 0.7% with warfarin per year, respectively (P = 0.02). Fatal bleeding occurred at rates of 0.2% and 0.5% per year, respectively (P = 0.003). Patients in the rivaroxaban group had higher bleeding rates from GI tract sites compared with those in the warfarin group (3.2% vs. 2.2%, respectively; P < 0.001).17
In ROCKET-AF, patients taking rivaroxaban experienced a higher rate of thrombotic events after discontinuing the study drug compared with patients taking warfarin (4.7% vs. 4.3%, respectively; P = 0.58).17 For patients who were assigned to rivaroxaban and then switched to warfarin, the median time to the first therapeutic INR value was 13 days after cessation of rivaroxaban, with no bridging anticoagulant used during this time.
The increased rate of thrombotic events was probably related to the increased difficulty of making the transition from rivaroxaban to open-label warfarin and of achieving a therapeutic INR compared with the transition from on-study warfarin to open-label warfarin.17
AVERROES and ARISTOTLE
Apixaban (Eliquis)
In the AVERROES trial (Apixaban versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who have Failed or are Unsuitable for Vitamin K Antagonist Treatment), 5,599 patients with AF and one or more additional risk factors for stroke were randomly assigned to receive apixaban 5 mg twice daily or acetylsalicylic acid (ASA) 81 to 324 mg daily.18 The trial was terminated early when an interim analysis showed a clear efficacy advantage for apixaban over ASA. Stroke and systemic embolism rates were 1.6% per year with apixaban and 3.7% per year with ASA, respectively (P < 0.001).
In the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic events in Atrial Fibrillation), 18,201 patients with AF and additional risk factors for stroke received either apixaban 5 mg twice daily or dose-adjusted warfarin (target INR, 2.0–3.0). A lower dose of apixaban (2.5 mg twice daily) was used for patients with two or more of the following criteria: 80 years of age or older, body weight less than 60 kg, and a serum creatinine level of 1.5 mg/dL or greater.19 Stroke and systemic embolism rates were lower in patients receiving apixaban, at 1.27% per year, than in those receiving warfarin, at 1.60% per year (HR with apixaban = 0.79; 95% CI, 0.66–0.95; P < 0.001 for non-inferiority; P = 0.01, meeting the criteria for superiority).
Rates of major bleeding were lower with apixaban (2.13% per year) than with warfarin (3.09% per year) (P < 0.001), as were mortality rates (P = 0.047) and hemorrhagic stroke rates (P < 0.001).
Summary
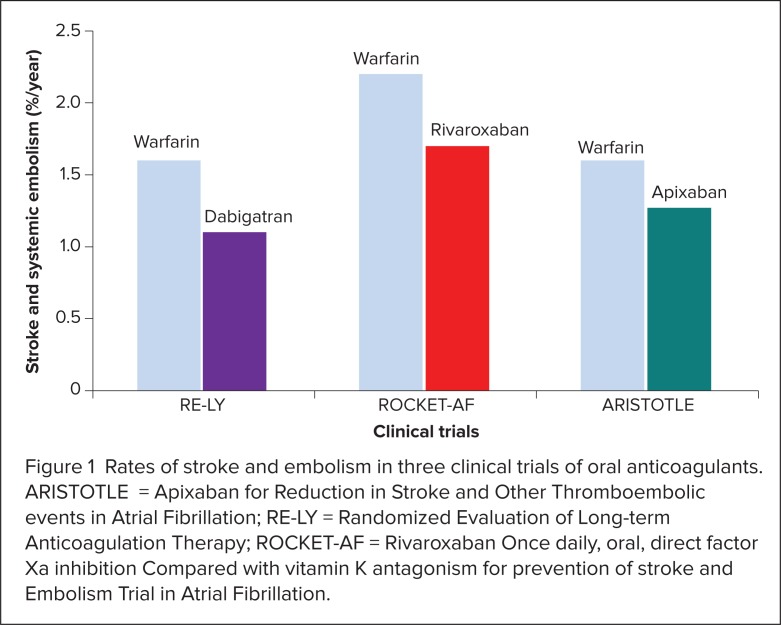
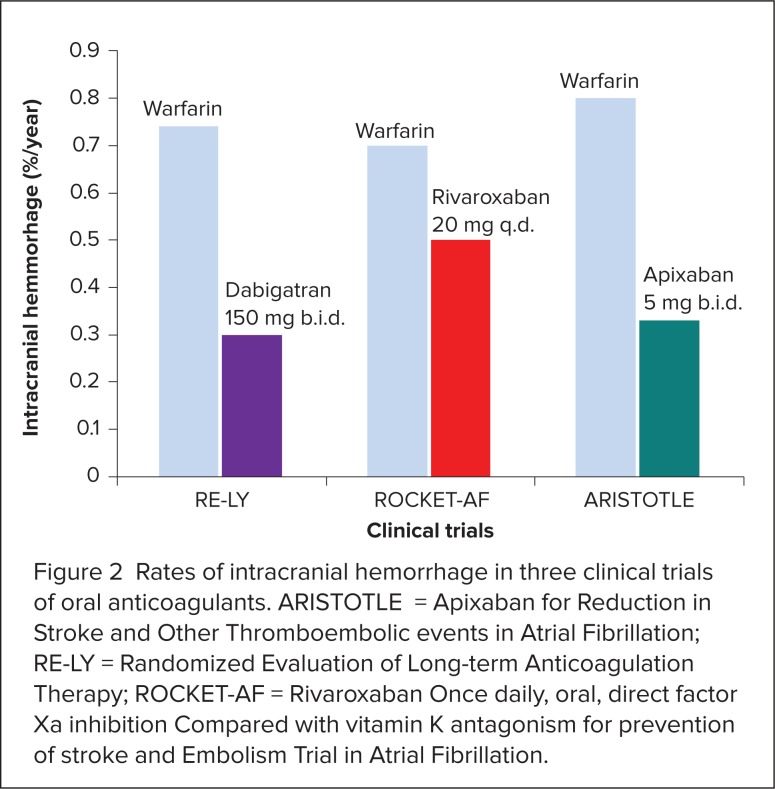
Stroke and systemic embolism rates in RE-LY, ROCKET-AF, and ARISTOTLE are shown in Figure 1. Intracranial hemorrhage rates in the three trials are presented in Figure 2.16,17,19 Given significant differences in study design, which included differences in patient risk factors and warfarin management, the efficacy and safety results for each new agent should not be directly compared with each other. Head-to-head trials are required to determine whether one agent is superior to another.
Figure 1.
Rates of stroke and embolism in three clinical trials of oral anticoagulants. ARISTOTLE = Apixaban for Reduction in Stroke and Other Thromboembolic events in Atrial Fibrillation; RE-LY = Randomized Evaluation of Long-term Anticoagulation Therapy; ROCKET-AF = Rivaroxaban Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation.
Figure 2.
Rates of intracranial hemorrhage in three clinical trials of oral anticoagulants. ARISTOTLE = Apixaban for Reduction in Stroke and Other Thromboembolic events in Atrial Fibrillation; RE-LY = Randomized Evaluation of Long-term Anticoagulation Therapy; ROCKET-AF = Rivaroxaban Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation.
ENGAGE-AF TIMI
Edoxaban (Lixiana)
In the randomized phase 3 ENGAGE-AF TIMI 48 trial (Global Study to Assess the Safety and Effectiveness of DU-176b vs. Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation), approximately 20,500 patients with AF and additional risk factors for stroke received edoxaban 30 mg daily, edoxaban 60 mg daily, or warfarin (INR range, 2.0–3.0).20 The follow-up period is 24 months. Completion of the trial is expected by March 2013, and results are due to be reported in the fall of 2013.21
COST CONCERNS
In the U.S. and around the world, both dabigatran and rivaroxaban are more expensive than warfarin, and the other new oral anticoagulants are also anticipated to be more expensive than warfarin. However, when warfarin’s requirement for routine coagulation monitoring and its associated nonmedical costs (lost work productivity, laboratory visits, and transportation time) are calculated, the new agents may prove more cost-effective than warfarin.22
In addition, in clinical practice, an anticoagulant without routine coagulation monitoring and frequent dose adjustments may free up time for physicians, support staff, and patients. Simplifying anticoagulation management may also improve patients’ compliance, with consequent patient benefits and cost savings. On the other hand, with the loss of constant oversight by a health care practitioner and routine monitoring that are associated with warfarin, patients will require extensive instruction on the importance of adherence with their anticoagulation regimen. Patients need to be informed of the thromboembolic risks associated with missed doses and should be advised against doubling doses, which may increase the risk of hemorrhagic events.
A cost-effectiveness study using results from RE-LY found that dabigatran 150 mg twice daily was more cost-effective in AF patients at high risk of hemorrhage or stroke with a score of 3 or higher according to CHADS2 (Congestive heart failure, Hypertension, Age ≥ 75, Diabetes and Stroke) but was less cost-effective when INR control with warfarin was excellent (time in therapeutic range above 72.6%).23 In this analysis, neither dabigatran 110 mg nor dual therapy, consisting of ASA plus clopidogrel (Plavix, Bristol-Myers Squibb/Sanofi) was cost-effective. Conversely, warfarin was more likely to be cost-effective in moderate-risk populations with AF unless INR control was poor or the risk of hemorrhage was high.
ADDITIONAL CONSIDERATIONS
In emergency situations, bleeding episodes are usually managed with direct compression at the bleeding site and with aggressive replacement of volume and blood products until hemostasis is restored.24,25
Non-emergency management of bleeding includes withholding further doses of the anticoagulant to reduce the effect and administering fresh frozen plasma or prothrombin complex concentrates, which promote blood clotting, although data supporting this practice are limited.26
The effects of warfarin can be reversed by administering vitamin K1 (although full reversal takes up to 24 hours) and, when necessary, coadministration of fresh frozen plasma or prothrombin complex concentrates.11 Protamine sulfate can counteract the effects of heparin, and the antihemostatic effect of aspirin and other antiplatelet drugs can be corrected by administering platelet transfusions, desmopressin, or both if needed.27
The newer oral anticoagulants have relatively short half-lives (7–17 hours); therefore, drug discontinuation is appropriate for patients with bleeding that is not imminently life-threatening. Although no commercially available antidotes are available for the newer generation of anticoagulants, recombinant activated Factor VII (rFVIIa) and prothrombin complex concentrates, which are potent procoagulants, have been proposed as potential reversal agents. Studies in healthy volunteers have shown conflicting results with rFVIIa, whereas in vitro and animal studies with prothrombin complex concentrates show some promise.12 Further study is needed to determine the clinical applicability of these findings to humans.
Products such as recombinant factor X, with a mutation that prevents catalytic activity, but allows binding of FXa inhibitors, and a monoclonal antibody fragment to dabigatran are being developed to reverse the anticoagulant effects of these new agents, but they have not yet been tested in people. Prothrombin complex concentrates, derived from plasma and initially developed for patients with hemophilia, are now being studied to determine efficacy in treating new oral anticoagulant-related bleeding.25,28–32
Because the novel agents have different mechanisms of action, health care institutions should consider developing new drug-specific protocols for the prevention and management of bleeding in patients receiving these new anticoagulants.
Another fact to consider in clinical practice is that the efficacy and safety of these newer agents remain to be fully investigated in certain groups of patients. Pregnant women and patients with severe heart-valve disorders, uncontrolled hypertension, active liver disease, and severe renal impairment (an estimated CrCl of 30 mL/minute or lower in RE-LY; below 30 mL/minute in ROCKET-AF, and less than 25 mL/minute in ARISTOTLE) were excluded from phase 3 trials.16,17,19 In addition, patients who required more than 100 mg/day of ASA in ROCKET-AF or more than 165 mg/day in ARISTOTLE and those who were receiving dual antiplatelet therapy were also excluded from the rivaroxaban and apixaban trials.17,19
Finally, the dosing frequency of newer oral anticoagulants should be taken into account, because this may affect patient adherence. Dabigatran and apixaban are given twice daily, whereas rivaroxaban, like warfarin, is administered once daily. Although no data are available about the effect of dosing frequency for these agents in patients with AF, a systematic review of adherence in other chronic disease states suggests that patients are more compliant with once-daily treatment regimens compared with twice-daily regimens.33
CONCLUSION
In phase 3 trials, the oral DTI dabigatran and the oral FXa inhibitors rivaroxaban and apixaban were superior and non-inferior to dose-adjusted warfarin in preventing thromboembolic stroke and systemic embolism in patients with AF.16,17 Moreover, these drugs did not increase the risk of bleeding and they were well tolerated. Apixaban was superior to dose-adjusted warfarin or ASA alone in reducing the risk of thromboembolic stroke.18
A reduction in thrombosis or bleeding events with these newer oral anticoagulants, compared with warfarin, as well as the possibility of simpler management, may improve patient outcomes and provide significant cost benefits.
Footnotes
Disclosure: The authors report no financial or commercial relationships in regard to this article. Isabelle Leach, MB ChB, provided editorial support with funding from Janssen Scientific Affairs, LLC.
REFERENCES
- 1.You JJ, Singer DE, Howard PA, et al. Antithrombotic Therapy for Atrial Fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133(6 Suppl):546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 3.European Atrial Fibrillation Trial: Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke: EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342(8882):1255–1262. [PubMed] [Google Scholar]
- 4.Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA. 1999;281(19):1830–1835. doi: 10.1001/jama.281.19.1830. [DOI] [PubMed] [Google Scholar]
- 5.Siguret V, Pautas E, Gouin-Thibault I. Warfarin therapy: Influence of pharmacogenetic and environmental factors on the anticoagulant response to warfarin. Vitam Horm. 2008;78:247–264. doi: 10.1016/S0083-6729(07)00012-X. [DOI] [PubMed] [Google Scholar]
- 6.van Walraven C, Jennings A, Oake N, et al. Effect of study setting on anticoagulation control: A systematic review and meta-regression. Chest. 2006;129(5):1155–1166. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 7.Hylek EM, D’Antonio J, Evans-Molina C, et al. Translating the results of randomized trials into clinical practice: The challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37(4):1075–1080. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 8.Waldo AL, Becker RC, Tapson VF, Colgan KJ. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46(9):1729–1736. doi: 10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 9.Turpie AG. New oral anticoagulants in atrial fibrillation. Eur Heart J. 2008;29(2):155–165. doi: 10.1093/eurheartj/ehm575. [DOI] [PubMed] [Google Scholar]
- 10.Bereznicki LR, Peterson GM. New antithrombotics for atrial fibrillation. Cardiovasc Ther. 2010;28(5):278–286. doi: 10.1111/j.1755-5922.2010.00209.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation. 2003;107(12):1692–1711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- 12.Pradaxa (dabigatran), package insert. Ridgefield, Conn.: Boehringer Ingelheim; 2011. [Google Scholar]
- 13.van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate: A novel, reversible, oral direct thrombin inhibitor: Interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116–1127. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 14.Xarelto (rivaroxaban), package insert. Titusville, N.J.: Janssen; 2011. [Google Scholar]
- 15.Ogata K, Mendell-Harary J, Tachibana M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel Factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50(7):743–753. doi: 10.1177/0091270009351883. [DOI] [PubMed] [Google Scholar]
- 16.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 18.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 19.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 20.Ruff CT, Giugliano RP, Antman EM, et al. Evaluation of the novel Factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: Design and rationale for the Effective aNticoaGulation with Factor x. A next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48) Am Heart J. 2010;160(4):635–641. doi: 10.1016/j.ahj.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 21.Global Study to Assess the Safety and Effectiveness of DU-176b vs. Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation (ENGAGE AF TIMI 48) 2011. Available at: www.clinicaltrials.gov/ct2/show/NCT00781391. Accessed January 21, 2013.
- 22.Guanella R, Ducruet T, Johri MMMJ, et al. Economic burden and cost determinants of deep vein thrombisis during 2 years following diagnosis: A prospective evaluation. J Thromb Haemost. 2011;9(12):2397–2405. doi: 10.1111/j.1538-7836.2011.04516.x. [DOI] [PubMed] [Google Scholar]
- 23.Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123(22):2562–2570. doi: 10.1161/CIRCULATIONAHA.110.985655. [DOI] [PubMed] [Google Scholar]
- 24.Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost. 2009;7(Suppl 1):107–110. doi: 10.1111/j.1538-7836.2009.03429.x. [DOI] [PubMed] [Google Scholar]
- 25.Fishman PE, Drumheller BC, Dubon ME, Slesinger TL. Recombinant activated Factor VII use in the emergency department. Emerg Med J. 2008;25(10):625–630. doi: 10.1136/emj.2007.057158. [DOI] [PubMed] [Google Scholar]
- 26.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 27.Levi MM, Eerenberg E, Lowenberg E, Kamphuisen PW. Bleeding in patients using new anticoagulants or antiplatelet agents: Risk factors and management. Neth J Med. 2010;68(2):68–76. [PubMed] [Google Scholar]
- 28.Lu G, Luan P, Hollenbach S, et al. Reconstructed recombinant Factor Xa as an antidote to reverse anticoagulation by Factor Xa inhibitors (Abstract OC-TH-107) J Thromb Haemost. 2009;7(Suppl 2) [Google Scholar]
- 29.Lu G, Luan P, Hollenbach S, et al. Recombinant antidote for reversal of anticoagulation by Factor Xa inhibitors (Abstract 983) Blood. 2008:112. [Google Scholar]
- 30.Gruber A, Marzec UM, Buetehorn U, et al. Potential of activated prothrombin complex concentrate and activated Factor VII to reverse the anticoagulant effects of rivaroxaban in primates (Abstract 3825) Blood. 2008;112(11) [Google Scholar]
- 31.Perzborn E, Harwardt M. Recombinant Factor VIIa partially reverses the effects of the Factor Xa inhibitor rivaroxaban on thrombin generation, but not the effects of thrombin inhibitors, in vitro(Abstract P-W-640) J Thromb Haemost. 2007;5(Suppl 2) [Google Scholar]
- 32.Perzborn E, Tinel H. FEIBA reverses the effects of a high dose of rivaroxaban in rats (Abstract P061) Pathophysiol Haemost Thromb. 2008;36:A40. [Google Scholar]
- 33.Saini SD, Schoenfeld P, Kaulback K, Dubinsky SC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22–e33. [PubMed] [Google Scholar]