Abstract
The expansion and intensification of soya bean agriculture in southeastern Amazonia can alter watershed hydrology and biogeochemistry by changing the land cover, water balance and nutrient inputs. Several new insights on the responses of watershed hydrology and biogeochemistry to deforestation in Mato Grosso have emerged from recent intensive field campaigns in this region. Because of reduced evapotranspiration, total water export increases threefold to fourfold in soya bean watersheds compared with forest. However, the deep and highly permeable soils on the broad plateaus on which much of the soya bean cultivation has expanded buffer small soya bean watersheds against increased stormflows. Concentrations of nitrate and phosphate do not differ between forest or soya bean watersheds because fixation of phosphorus fertilizer by iron and aluminium oxides and anion exchange of nitrate in deep soils restrict nutrient movement. Despite resistance to biogeochemical change, streams in soya bean watersheds have higher temperatures caused by impoundments and reduction of bordering riparian forest. In larger rivers, increased water flow, current velocities and sediment flux following deforestation can reshape stream morphology, suggesting that cumulative impacts of deforestation in small watersheds will occur at larger scales.
Keywords: soya beans, watersheds, nitrogen, phosphorus, soil
1. Introduction
The expansion of agricultural land and the intensification of agricultural production are among the most profound of human alterations of the Earth's environment. Global production of cereal crops has doubled since 1960, and agriculture now provides food for more than seven billion people, largely because of increases in yield achieved through increased applications of water, fertilizer, pesticides and new crop strains [1,2]. Globalization and rising incomes worldwide have created soaring demands for energy, meat, animal feeds and other agricultural products and have increased the demand for more intensive farming on existing lands and for more total agricultural area. The growing demand for food and the availability of new arable land in the tropics have now pushed intensive cropland farming deep into the rural lands of many tropical countries that are the Earth's last frontier for agricultural expansion [3].
The expansion and intensification of tropical agriculture threatens the services provided by native ecosystems. Structuring agricultural production to meet human demands for food while maintaining ecosystem functions and minimizing environmental impacts are one of humanity's great challenges [4]. Meeting this challenge requires a better understanding of the environmental impacts of cropland expansion and intensification in the tropics, the vulnerability or resilience of the tropical landscapes where croplands are expanding, and how environmental consequences can be predicted from environmental variables such as soils and climate.
The impacts of agriculture on hydrology and biogeochemistry fall in three main areas: (i) alterations of water budgets and streamflows, (ii) changes to nutrient movement in soils and delivery to surface waters that degrade water quality and (iii) changes to stream habitats and stream biota. There is widespread evidence that the removal of forest cover preceding cropland expansion shifts water balances by reducing evapotranspiration and increasing stream runoff [5,6]. How clearing and post-clearing agricultural land use influences streamflow dynamics is more variable and depends on the infiltrability and hydraulic conductivity of soils [7], and the extent to which agricultural practices cause compaction of surface and subsurface soils [8]. In regions with predominantly vertical water flow pathways, groundwater-driven baseflows predominate in streams, and stormflows contribute only a small percentage (less than 5%) of total streamflow [9–11]. By contrast, regions in which soil infiltrability and hydraulic conductivity are lower and exceeded by intense rainfalls, overland flows or subsurface lateral flows develop and produce a much higher percentage (up to greater than 50%) of total streamflow in quick stormflows [5]. The effects of land clearing on stream baseflows, particularly during the dry season, are less clear and probably depend on the extent of distribution of rainfall and its intensity and the extent to which quickflows develop [6].
Intensive cropland agriculture is associated with increased delivery of nitrogen (N) and phosphorus (P) to surface waters and the development of eutrophic conditions in many regions around the world [12–16]. While these associations are well established in temperate landscapes, much less is known about how intensive agriculture will influence water quality in tropical regions. Waters in tropical regions may be particularly sensitive to increases in N fertilizer application, because many tropical soils cycle large amounts of N and contain high concentrations of nitrate in soil solution [17–19]. Thus, they have been predicted to leak large amounts of N to surface waters with increasing rates of N application [20]. Large losses of N have been reported from semi-tropical intensive croplands [21]. Nitrogen-fixing soya beans, which are expanding rapidly in tropical regions, may contribute a substantial but currently poorly known amount of new N to croplands [22]. In addition, double-cropping of soya beans with corn, with higher N application rates, is replacing single cropping of soya beans over large areas, particularly in the Amazon [23].
Removal of riparian vegetation can degrade stream habitat structure [24,25] and alter stream biota [26–28]. It can also raise streamwater temperatures [26–28], an effect that is amplified when conversion is accompanied by the construction of water impoundments [29]. Riparian buffers designed to mitigate effects of agriculture on streams are mandated in some tropical countries, but many, such as those provided by the Brazilian Forest Code, often come under political pressure [30,31].
Brazil's Amazon region contains the Earth's largest remaining tropical forest, and it is the location of the largest absolute extent of forest clearing each year [3]. For many decades, cattle pasture was the predominant fate of cleared forest in Brazil's Amazon region. In the early 2000s, the nature of agriculture that replaced tropical forest along a wide swathe of the Amazon's southern boundary shifted from cattle pasture to soya bean cropland [32]. During this time, Amazon soya bean production became a global industry and Brazil is now the world's second-largest soya bean exporter after the USA [33]. Much expansion of soya beans in the Amazon has occurred in the state of Mato Grosso [32,34] and has been concentrated in areas of low relief covered by highly weathered, acidic soils that are highly deficient in P [35]. Recently, soya bean cultivation has intensified and double-cropping of soya beans, mostly with corn, increased from 35 to 65 per cent of Mato Grosso's cropland between 2001 and 2010 [36]. Several general analyses of both the scope of potential environmental impacts [37] and opportunities for impact management [38] in the Amazon soya bean region have been made. But there have been few studies that examine ecosystem-scale changes of this ongoing conversion and offer insights into its environmental impacts or sustainability.
In this article, we synthesize results from field studies of multiple headwater streams in watersheds with either forest or soya bean land use to identify ways in which soya bean farming can affect local and regional watersheds. We also report the ways in which soya bean farming in southern Amazonia is likely to be resilient to changes from cropland expansion. We examine these changes in three main areas: water budgets and streamflows, nutrient movement and water quality and stream habitats.
2. Study area
This study was conducted at Tanguro Ranch in eastern Mato Grosso, Brazil (figure 1). Tanguro Ranch lies in a region of high land conversion rates and is representative in terms of physical and climate environment of the areas on which soya beans are expanding. Roughly half of the farm's 80 000 ha were cleared from forest for cattle pasture in the early 1980s and converted to soya beans between 2003 and 2008. More than 50 small impoundments created to provide water for cattle in the dry season remain as a legacy of former pasture use. Tanguro Ranch lies in an area of low relief on the Brazilian Shield on Precambrian gneisses of the Xingu Complex [39]. The dominant soils of this large region are very deep (greater than 10 m), acidic (pH of native forest soil = 3.9) Oxisols (Haplustox), with a sandy clay texture (mean 43% clay) [39,40]. Mean annual rainfall derived from Tropical Rainfall Measuring Mission data is 1770 mm for the period 2000–2011. There is a dry season from May to September in which little rain falls. During the period of study, fields were single-cropped with soya beans and were left fallow with little green vegetation during the dry season. Tanguro Ranch applies 50 kg P yr−1 as rock phosphate [35] but no N to single-cropped soya beans. After 2010, an additional approximately 60 kg N yr−1 as urea and ammonium sulfate was applied to some fields where soya beans were double-cropped with corn (T. Ranch, personal communication).
Figure 1.
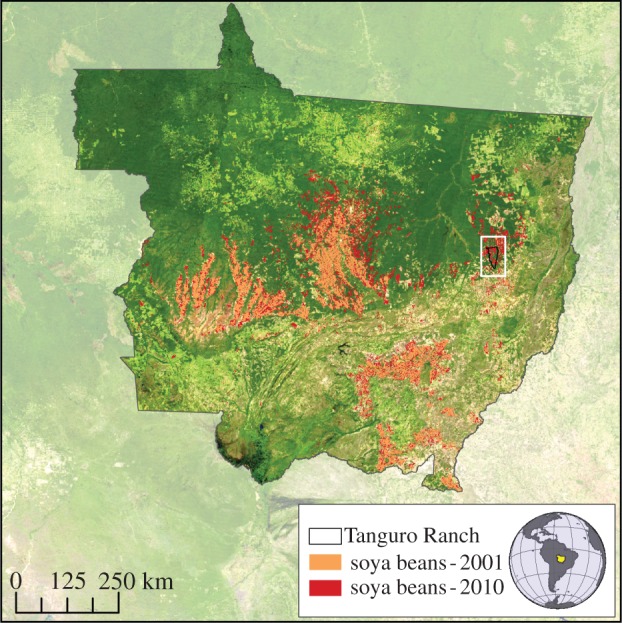
The Brazilian state of Mato Grosso showing the extent of soya bean agriculture in 2001 and 2010 and the location of Tanguro Ranch. Soya bean areas were calculated using methodology in [34].
3. Results and discussion
(a). Water balance and streamflows
The surface infiltrability and subsurface hydraulic conductivity of forest soils at Tanguro Ranch are high [41]. Soil infiltrability and hydraulic conductivity decrease by more than half in soya bean fields but remains sufficiently high to make the generation of Horton overland flow (surface flow derived from rainfall rate in excess of soil surface infiltration rate) extremely rare (figure 2). Lower hydraulic conductivity in the deeper soil layers of soya bean fields compared with forests increases the possibility of subsurface stormflows, but these also occur rarely [41]. These properties create a landscape that is resistant to rill erosion in cultivated fields. Field management in the region reflects the absence of erosion-generating overland flows under the current distribution of rainfall intensities. For example, at Tanguro Ranch earthen berms that were constructed at the initiation of soya bean farming in the mid-2000s to prevent erosion were removed in 2011, because they were found to be unnecessary and impeded equipment movement. We measured infiltration 4 years after pastures were converted to soya bean fields. While fields are managed with no-till practices that should reduce soil degradation, the long-term effects of soya bean cultivation on soil infiltrability and hydraulic conductivity are not known. There is still widespread potential for soil erosion from roadsides and other point sources, but these effects have not been quantified.
Figure 2.
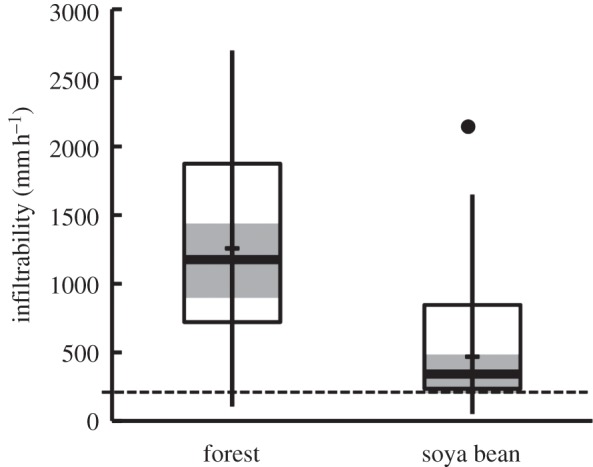
Surface soil infiltrability in forest and soya beans. Horizontal bar is the median of 25 measurements in three separate 1 ha forest and soya bean plots. Shaded area is the 95% CI. Horizontal bar is the data range. The dashed line is the 5-min maximum intensity based on 1 year of rainfall data collected at Tanguro Ranch. Dot is a single outlier. Data from [41].
The flow regimes in streams in small watersheds reflect both the soil characteristics and land cover changes. The high infiltrability and permeability of the terrestrial land surface results in total water export being dominated by baseflow in forest and soya bean watersheds [42] (figure 3). The proportion of baseflow (greater than 95%) is similar in both environments and changes by less than a factor of two between wet and dry season. The lower leaf area, rooting depth and shorter growing season of soya bean compared with forest decreases evapotranspiration and increases streamflow. Evaluation of monthly mean evapotranspiration data available from the MODIS MOD16 product [43] for Tanguro Ranch shows a 30 per cent reduction in annual mean evapotranspiration in soya bean compared with forest (approx. 1 mm d−1 decrease; M. T. Coe 2013, unpublished data). The greatest difference in evapotranspiration occurs in the dry season when the soya bean fields are fallow, while peak values during the wet season are comparable in forest and soya bean (M. T. Coe 2013, unpublished data). As a result, there is greater soil moisture through the soil profile in soya bean fields compared with forest (figure 4) and the total streamwater export is threefold to fourfold greater from soya bean watersheds [42]. Because increased total runoff of water arrives to small streams nearly exclusively by infiltration into the soil and subsequent lateral transport through the groundwater, increased water delivery to the streams is spread out over time and increased flows are not accompanied by significant stream channel restructuring. Increased water delivery to small streams appears to be accompanied by increased riparian groundwater levels and increased wetting of near-stream hillslopes (C. Neill 2012, personal observation), though this has not been quantified.
Figure 3.
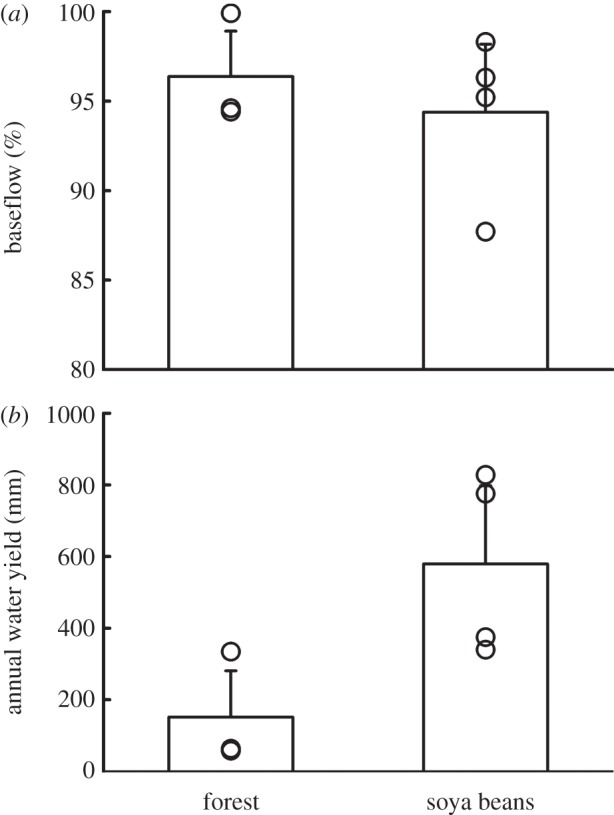
(a) Percent baseflow and (b) annual water yield in three forest watersheds and four soya bean watersheds at Tanguro Ranch. Bars are means+s.e. Symbols are individual watersheds. Data from [42].
Figure 4.
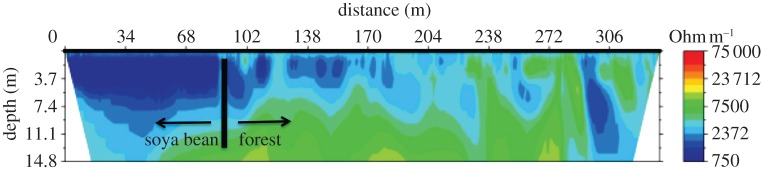
Soil resistivity across a forest–soya bean field boundary at Tanguro Ranch during the wet season in January 2012 showing lower resistivity (higher soil moisture) at 0–7 m depth in the soya bean field. Resistivity was measured using a Sting Resistivity Imaging System (Advance Geosciences, Inc.) using methods outlined in [43].
(b). Nutrient movement and water quality
Soils of soya bean fields retain large amounts of added fertilizer P in surface horizons. Plant-available P in surface soils of soya bean fields exceeds that in the original forest, but there is no evidence that this P moves below 20 cm depth after 1–6 years of soya bean cultivation (figure 5). The well-known mechanism for this retention is the high iron and aluminium oxides content of Oxisols that imparts a high capacity for binding fertilizer P into forms that are not immediately available for uptake by plants [45]. This rapid P fixation causes these Oxisols to require higher amounts of P fertilizer than non-P-binding soils to attain equivalent soya bean yields [35]. The combination of very deep soils (often exceeding 10 m), nearly exclusively vertical hydrological flowpaths and high P binding creates a landscape in which the potential for leaching of P from soya bean fields into groundwater and into streams is relatively low.
Figure 5.
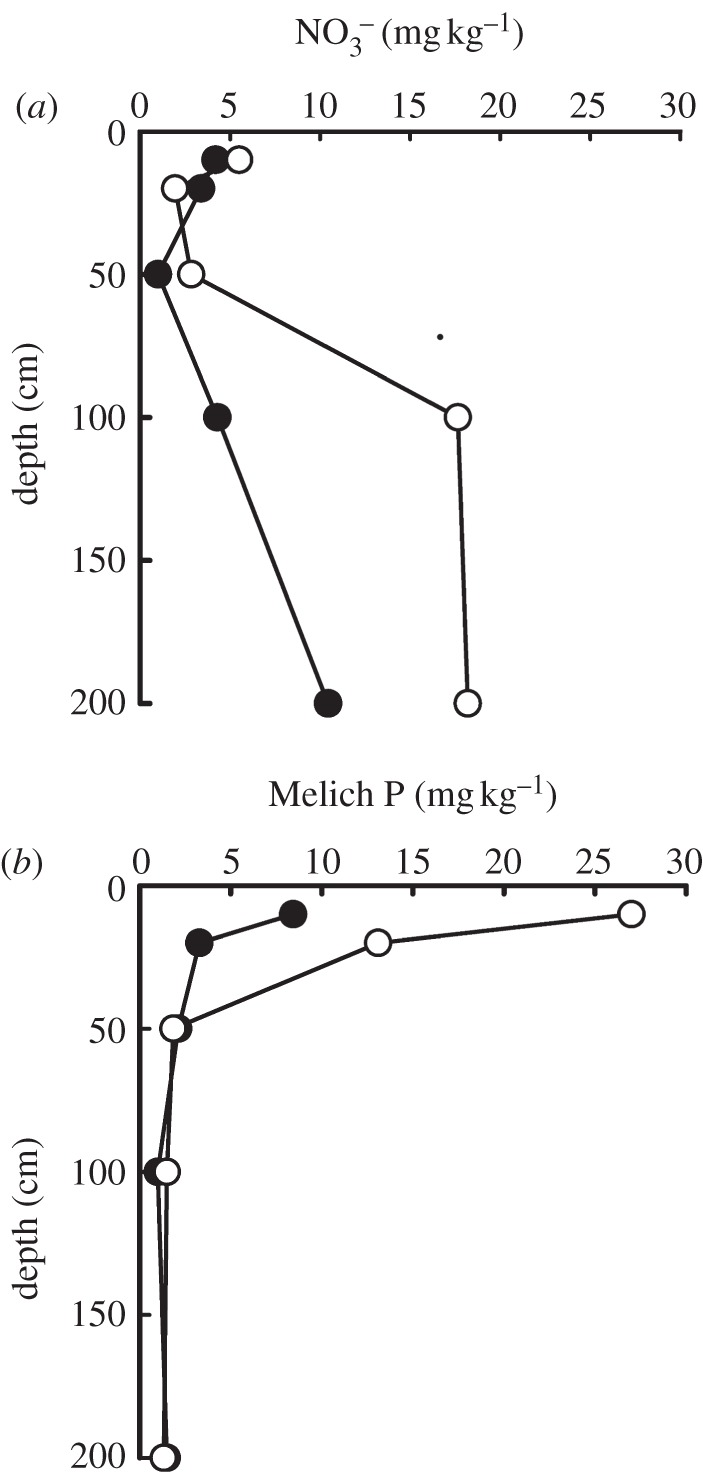
(a) Extractable nitrate and (b) extractable (Melich) P in forest (solid circles) and soya bean (open circles) soil profiles at Tanguro Ranch. Nitrate data are unpublished from A. M. Figueira. Phosphorus data are from [44].
Nitrogen is generally more mobile than P, and inorganic N in the form of nitrate moves readily through soils and groundwaters into the surface waters in many intensively farmed regions [15]. Concentrations of soil extractable nitrate are higher in the deep soils of forest than soya bean watersheds (figure 5). This suggests the presence of soil anion exchange capacity that could retard downward nitrate leaching [46] and act as a brake on N losses at the watershed scale [47]. Nitrate concentrations at depth increase in soya bean fields (figure 5), indicating that soils could have significant capacity to retain N that is fixed by the soya bean crop. Soya bean rates of N fixation have not been quantified directly at Tanguro Ranch but have been estimated at up to 170 kg N ha−1 under similar cropping systems [48].
There is no evidence to date that phosphate and nitrate move from soils into groundwater under the current practices at Tanguro Ranch. Concentrations of phosphate in groundwater and streams are low and similar between forest and soya bean watersheds (table 1). Concentrations of nitrate in groundwater and streams are variable and slightly higher in soya bean watersheds (table 1), but none of the differences in nitrate, phosphate or total suspended solids between land uses were significant. However, similar concentrations combined with greater water export lead to greater watershed nitrate and phosphate export from soya bean compared with forest watersheds.
Table 1.
Mean nitrate, phosphate and total suspended solids (TSS) in groundwater and streamwater in three forest and four soya bean watersheds at Tanguro Ranch (± s.e.) [48]. Mean concentrations in each land use were derived from mean concentrations from multiple measurements in each watershed. Groundwater sample sizes were: forest (8, 6 and 4) and soya bean (6, 6, 3 and 12). Streamwater sample sizes were: forest (112, 65 and 14) and soya bean (141, 86, 15 and 76). None of the differences between land uses were significant.
| groundwater |
streamwater |
|||
|---|---|---|---|---|
| forest (n = 3) | soya beans (n = 4) | forest (n = 3) | soya beans (n = 4) | |
| NO3− (µM) | 5.6 ± 3.9 | 14.0 ± 6.5 | 8.6 ± 5.5 | 12.9 ± 8.0 |
| PO4− (µM) | 0.4 ± 0.2 | 0.5 ± 0.2 | 1.2 ± 0.7 | 0.8 ± 0.3 |
| TSS (mg l−1) | — | — | 12.8 ± 1.6 | 9.6 ± 1.3 |
Several factors could influence the streamwater quality both now and in the future. The large capacity of soils to adsorb P would appear to buffer groundwater and streamwater from changes to phosphate concentrations for many decades [35,45] in the absence of erosion. However, the extent of erosion is not known in the region, nor is it known if the stream hydrologic regimes and the potential for erosion will increase in the future if cultivation over many years leads to increased soil compaction. The magnitude of any buffering of ground- and streamwater against increases in nitrate concentration by soil anion exchange is also unknown. The long-term capacity of soils to continue to adsorb N is also unknown. Double-cropping with N fertilizer is very recent at Tanguro Ranch and the approximately 60 kg N ha−1 yr−1 applied to double-cropped soya beans and corn is low compared with more than 150 kg N ha−1 yr−1 applied to intensive row crops in many other places in the world [49]. The effect on stream nitrate concentrations of longer periods of N fertilizer use or higher fertilization rates are unknown. Currently, forested riparian zones play little role in buffering streamwater against increased nitrate concentrations either in forest or in soya bean watersheds, because groundwater arrives at streams already low in nitrate concentration (table 1). This role could change in the future if greater use of N fertilizer exceeds soil nitrate adsorption capacity and groundwater nitrate concentrations increase, and the extent to which forested buffers could intercept increased groundwater nitrate is not known.
The results described here are derived from Tanguro Ranch, which represents a small portion of the southern Amazon cropland frontier region. However, the red-yellow latosols that occur at Tanguro Ranch are typical of a large portion of this Mato Grosso and cover 262 000 km2 with relatively similar topography [50]. While no similar information exists from other locations in this region, to the extent that soils and topography exert strong controls on watershed hydrology and biogeochemistry, these results may be applicable over wide areas.
(c). Stream habitat quality
In contrast to the current high degree of buffering against biogeochemical changes, stream temperature is modified by soya bean cropland in the watershed. The temperature of streamwater is 2°C–6°C higher in soya bean than forest watersheds and varies more both diurnally and seasonally than in forest watersheds (figure 6). This temperature effect is caused both by the presence of small impoundments and by loss of riparian forest cover. These changes are widespread, and impoundment-induced warming alone is estimated to affect 45 per cent of the stream network in the upper Xingu watershed [29]. While temperature is only one aspect of stream habitat, it is a particularly important one because higher temperature affects the development time, growth rates and energetic requirements of aquatic organisms [51,52]. Recent research on ectotherms has found that species living in stable warm climates have narrow thermal tolerances and live in climates that are closer to their physiological limits, thus tropical stream-dwelling animals may be disproportionally vulnerable to the metabolic effects of small increases in temperature [53]. An additional impact is the higher energy demands associated with higher current velocities caused by higher discharge [54,55]. The direct metabolic effects of increasing temperatures combined with higher energy expenditures for swimming may exceed food availability and lead to declining productivity. Although not widely studied in tropical streams, the loss or deterioration of streamside riparian forest buffers and higher stream temperatures may also be associated with changes to macroinvertebrate communities and degradation of channel habitats [25,56].
Figure 6.
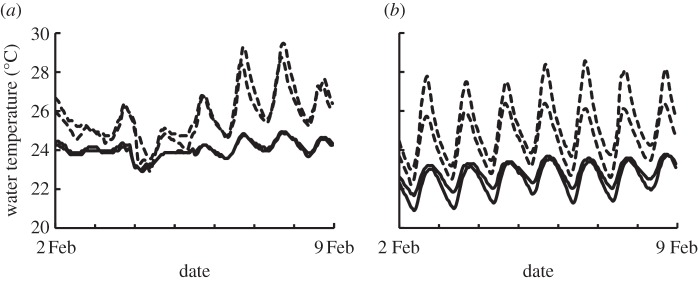
Streamwater temperatures in two forested (solid lines) and soya bean (dashed lines) watersheds over 7 days in the (a) wet and (b) dry season. Patterns in the first few days of the wet season record coincides with rain events. Data from [29].
(d). Effects at larger scales
Observations in the southern Amazon region now link deforestation and agriculture land use to changes in the hydrology of large river networks. During the last 40 years, deforestation has been accompanied by greater discharge in the Tocantins [57] and Araguaia [58] rivers. In the Aruaguaia River, greater discharge increased sediment flux in the main river channel 31 per cent between the 1970s and 2000 [59]. This has caused accumulation of sediments in side channels, accretion of lateral sand bars and a 30 per cent reduction in the number of small islands [59]. Compared with the 1960s, the river now has a larger central corridor to accommodate the increased bedload [58]. These responses are consistent with hydrological and hydromorphic changes induced by deforestation and conversion to agriculture in other locations [60,61]. Almost nothing is known about sediment or solute concentrations in the wide range of smaller to medium-sized rivers between the scale of small streams at Tanguro Ranch and the Araguaia and Tocantins rivers.
4. Conclusions
Deep, permeable soils, coupled with a high capacity to fix P and potential to retain N deep in the soil profile, buffer headwater watersheds at Tanguro Ranch against changes to surface runoff, stream stormflows and stream solute and sediment concentrations. By contrast, headwater streams are vulnerable to changes in temperature caused by loss of riparian forest cover and the presence of small impoundments in most headwater watersheds. Increased discharge from headwater watersheds causes little erosion or sediment transport in small streams but leads to extensive sediment transport and sediment infilling in larger river basins. This suggests that many of the most important impacts of expanding soya bean cultivation caused by changes to water balance and sediment transport will occur at larger scales in river networks.
Acknowledgements
We thank Paulo Brando, Wanderley Rocha and workers at the Amazon Environmental Research Institute (IPAM) for field and logistical support for the variety of field studies reported on here. Grupo A. Maggi allowed access to Tanguro Ranch. This work was conducted under a formal research agreement between IPAM and Grupo A. Maggi that allows scientific freedom and unencumbered publication of results. Thanks to Daniel Nepstad for the original invitation to C.N., A.V.K. and L.A.D. to work at Tanguro. This study was supported by grants from NSF (DEB-0640661, DEB-0949996, DEB-0743703) the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 08/58089-9), the Packard Foundation, the Gordon and Betty Moore Foundation and the Brazilian Council for Scientific and Technological Development (CNPq).
References
- 1.Cassman KG. 1999. Ecological intensification of cereal production systems: yield potential, soil quality, and precision agriculture. Proc. Natl Acad. Sci. USA 96, 5952–5959 10.1073/pnas.96.11.5952 (doi:10.1073/pnas.96.11.5952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilman D, Cassman KG, Matson PA, Naylor R, Polansky S. 2002. Agricultural sustainability and intensive production practices. Nature 418, 671–677 10.1038/nature01014 (doi:10.1038/nature01014) [DOI] [PubMed] [Google Scholar]
- 3.FAO (Food and Agriculture Organization) 2011. The state of the world‘s forests. Rome, Italy: Food and Agriculture Organization [Google Scholar]
- 4.Robertson GP, Swinton SM. 2005. Reconciling agricultural productivity and environmental integrity: a grand challenge for agriculture. Front. Ecol. Environ. 3, 557–560 10.1890/1540-9295(2005)003[0557:LDACAE]2.0.CO;2 (doi:10.1890/1540-9295(2005)003[0557:LDACAE]2.0.CO;2) [DOI] [Google Scholar]
- 5.Bonell M. 2005. Runoff generation in tropical forests. In Forests, water and people in the humid tropics (eds Bonell M, Bruijnzeel LA.), pp. 314–406 Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Brown AE, Zhang L, McMahon TA, Western AW, Vertessy RA. 2005. A review of paired catchment studies for determining changes in water yield resulting from alterations in vegetation. J. Hydrol. 310, 28–61 10.1016/j.jhydrol.2004.12.010 (doi:10.1016/j.jhydrol.2004.12.010) [DOI] [Google Scholar]
- 7.Elsenbeer H, Vertessy RH. 2000. Stormflow generation and flowpath characteristics in an Amazonian rainforest catchment. Hydrol. Process. 14, 2367–2381 (doi:10.1002/1099-1085(20001015)14:14<2367::AID-HYP107>3.0.CO;2-H) [DOI] [Google Scholar]
- 8.Zimmermann B, Elsenbeer H, de Moraes JM. 2006. The influence of land-use changes on soil hydraulic properties: implications for runoff generation. Forest Ecol. Manag. 222, 29–38 10.1016/j.foreco.2005.10.070 (doi:10.1016/j.foreco.2005.10.070) [DOI] [Google Scholar]
- 9.Nortcliff S, Thornes JB. 1984. Floodplain response of a small tropical stream. In Catchment experiments in fluvial hydrology (eds Burt TP, Walling DE.), pp. 73–85 Norwich, UK: Geo-Books [Google Scholar]
- 10.Leopoldo PR, Franken WK, Villa Nova NA. 1995. Real evapotranspiration and transpiration through a tropical rainforest in central Amazonia as estimated by the water balance method. Forest Ecol. Manag. 73, 185–195 10.1016/0378-1127(94)03487-H (doi:10.1016/0378-1127(94)03487-H) [DOI] [Google Scholar]
- 11.Hodnett MG, Vendrome I, de O. Marques Filho A, Oyama MD, Tomasella J. 1997. Soil water storage and groundwater behavior in a catenary sequence beneath forest in central Amazonia. II. Floodplain water table behavior and implications for streamflow generation. Hydrol. Earth Syst. Sci. 1, 279–290 10.5194/hess-1-279-1997 (doi:10.5194/hess-1-279-1997) [DOI] [Google Scholar]
- 12.Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH. 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 8, 559–568 10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2 (doi:10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2) [DOI] [Google Scholar]
- 13.Turner RE, Rabalais NN. 2003. Linking landscape and water quality in the Mississippi River basin for 200 years. BioScience 53, 563–572 10.1641/0006-3568(2003)053[0563:LLAWQI]2.0.CO;2 (doi:10.1641/0006-3568(2003)053[0563:LLAWQI]2.0.CO;2) [DOI] [Google Scholar]
- 14.Daoji L, Daler D. 2004. Ocean pollution from land-based sources: East China Sea, China. Ambio 33, 107–113 10.1639/0044-7447(2004)033[0107:OPFLSE]2.0.CO;2 (doi:10.1639/0044-7447(2004)033[0107:OPFLSE]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 15.Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ. 2003. The nitrogen cascade. BioScience 53, 341–356 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2 (doi:10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2) [DOI] [Google Scholar]
- 16.Billen G, et al. 2011. Nitrogen flows from European regional watersheds to coastal marine waters. In European nitrogen assessment (eds Sutton MA, Howard CM, Erisman JW, Billen ABG, Grennfelt P, van Griinsven H, Grizzetti B.), pp. 271–297 Cambridge, UK: Cambridge University Press [Google Scholar]
- 17.Neill C, Piccolo MC, Steudler PA, Melillo JM, Feigl BJ, Cerri CC. 1995. Nitrogen dynamics in soils of forests and active pastures in the western Brazilian Amazon Basin. Soil Biol. Biochem. 27, 1167–1175 10.1016/0038-0717(95)00036-E (doi:10.1016/0038-0717(95)00036-E) [DOI] [Google Scholar]
- 18.Markewitz D, Davidson E, Moutinho P, Nepstad D. 2004. Nutrient loss and redistribution after forest clearing on a highly weathered soil in Amazônia. Ecol. Appl. 14, S177–S199 10.1890/01-6016 (doi:10.1890/01-6016) [DOI] [Google Scholar]
- 19.Hedin LO, Brookshiire ENJ, Menge DNL, Barron A. 2009. The nitrogen paradox in tropical forest ecosystems. Annu. Rev. Ecol. Evol. Syst. 40, 613–635 10.1146/annurev.ecolsys.37.091305.110246 (doi:10.1146/annurev.ecolsys.37.091305.110246) [DOI] [Google Scholar]
- 20.Downing JA, et al. 1999. The impact of accelerating land-use change on the N-cycle of tropical aquatic ecosystems: current conditions and projected changes. Biogeochemistry 46, 109–148 10.1007/BF01007576 (doi:10.1007/BF01007576) [DOI] [Google Scholar]
- 21.Ahrens TD, Beman JM, Harrison JA, Jewett PK, Matson PA. 2008. A synthesis of nitrogen transformations from land to sea in the Yaqui Valley agricultural region of northwest Mexico. Water Resour. Res. 44, W00A05. 10.1029/2007WR006661 (doi:10.1029/2007WR006661) [DOI] [Google Scholar]
- 22.Filoso S, Martinelli LA, Howarth RW, Boyer E, Dentener F. 2006. Human activities changing the nitrogen cycle in Brazil. Biogeochemistry 79, 61–89 10.1007/s10533-006-9003-0 (doi:10.1007/s10533-006-9003-0) [DOI] [Google Scholar]
- 23.Galford GL, Mustard JF, Melillo JM, Gendrin A, Cerri CC, Cerri CEP. 2008. Wavelet analysis of MODIS time series to detect expansion and intensification of row-crop agriculture in Brazil. Remote Sens. Environ. 112, 576–587 10.1016/j.rse.2007.05.017 (doi:10.1016/j.rse.2007.05.017) [DOI] [Google Scholar]
- 24.Sweeney BW, Bott TL, Jackson JK, Kaplan LA, Newbold JD, Standley LJ, Hession WC, Horwitz RJ. 2004. Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proc. Natl Acad. Sci. USA 101, 14 132–14 137 10.1073/pnas.0307955100 (doi:10.1073/pnas.0307955100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deegan L, Neill C, Haupert C, Ballester M, Krusche A, Victoria R, Thomas SM, Moor E. 2011. Amazon deforestation alters small stream structure, nitrogen biogeochemistry and connectivity to larger rivers. Biogeochemistry 105, 53–74 10.1007/s10533-010-9540-4 (doi:10.1007/s10533-010-9540-4) [DOI] [Google Scholar]
- 26.Pringle CM, Scatena FN. 1999. Aquatic ecosystem deterioration in Latin America and the Caribbean. In Managed ecosystems: the Mesoamerican experience (eds Hatch LU, Swisher ME.), pp. 104–113 New York, NY: Oxford University Press [Google Scholar]
- 27.Benstead JP, Douglas MM, Pringle CM. 2003. Relationships of stream invertebrate communities to deforestation in eastern Madagascar. Ecol. Appl. 13, 1473–1490 10.1899/07-171.1 (doi:10.1899/07-171.1) [DOI] [Google Scholar]
- 28.Iwata T, Nakano S, Inoue M. 2003. Impacts of past riparian deforestation on stream communities in a tropical rainforest in Borneo. Ecol. Appl. 13, 461–473 10.1890/1051-0761(2003)013[0461:IOPRDO]2.0.CO;2 (doi:10.1890/1051-0761(2003)013[0461:IOPRDO]2.0.CO;2) [DOI] [Google Scholar]
- 29.Macedo MN, Coe MT, DeFries R, Uriarte M, Brando PM, Neill C, Walker WS. 2013. Land-use-driven stream warming in southeastern Amazonia. Phil. Trans. R. Soc. B 368, 20120153. 10.1098/rstb.2012.0153 (doi:10.1098/rstb.2012.0153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez A, Pringle CM, Wantzen KM. 2008. Tropical stream conservation. In Tropical stream ecology (ed. Dudgeon D.), pp. 285–304 New York, NY: Academic Press [Google Scholar]
- 31.Tollefson J. 2012. President prunes forest reforms: Rousseff rejects elements of Brazil's revised forest code. Nature 486, 13. 10.1038/486013a (doi:10.1038/486013a) [DOI] [PubMed] [Google Scholar]
- 32.Morton DC, DeFries R, Shimbukuro YE, Anderson LO, Arai E, Espirito-Santo d B, Freitas R, Morisette J. 2006. Cropland expansion changes deforestation dynamics in the Southern Brazilian Amazon. Proc. Natl Acad. Sci. USA 103, 14 637–14 641 10.1073/pnas.0606377103 (doi:10.1073/pnas.0606377103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FAO (Food and Agriculture Organiztion) 2012. FAOSTAT–agriculture database. Rome, Italy: FAO; See http://faostat.fao.org/site/339/default.aspx (accessed 15 August 2012) [Google Scholar]
- 34.Macedo M, DeFries R, Morton D, Stickler C, Galford G, Shimabukuro Y. 2012. Decoupling of deforestation and soy production in the Southern Amazon during the late 2000s. Proc. Natl Acad. Sci. USA 109, 1341–1346 10.1073/pnas.1111374109 (doi:10.1073/pnas.1111374109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riskin SH, Porder S, Schipanski ME, Bennett EM, Neill C. 2013. Soils mediate environmental consequences of intensive soybean agriculture. BioScience 63, 49–54 10.1525/bio.2013.63.1.10 (doi:10.1525/bio.2013.63.1.10) [DOI] [Google Scholar]
- 36.Mahr DE. 2011. Drivers of land-use change in Mato Grosso: a ten-year MODIS analysis. BS Honors thesis, Center for Environmental Studies, Brown University, Providence, RI, USA [Google Scholar]
- 37.Fearnside PM. 2001. Soybean cultivation as a threat to the environment in Brazil. Environ. Conserv. 28, 23–38 10.1017/S0376892901000030 (doi:10.1017/S0376892901000030) [DOI] [Google Scholar]
- 38.Nepstad DC, Striklet CM, Almeida OT. 2006. Globalization of the Amazon soy and beef industries: opportunities for conservation. Conserv. Biol. 20, 1595–1603 10.1111/j.1523-1739.2006.00510.x (doi:10.1111/j.1523-1739.2006.00510.x) [DOI] [PubMed] [Google Scholar]
- 39.Radambrasil 1981. Geologia, geomorphologia, pedologia, vegetacão, uso potencial da terra. Folha SD 22 Goiàs, Rio de Janeiro, Brazil: Departamento Nacional de Produção Mineral [Google Scholar]
- 40.Oliviera J, Jacomine P, Camargo M. 1992. Classes gerais de solos do Brasil. São Paulo, Brazil: Jaboticabal [Google Scholar]
- 41.Scheffler R, Neill C, Krusche AV, Elsenbeer H. 2011. Soil hydraulic response to land-use change associated with the recent soybean expansion at the Amazon agricultural frontier. Agric. Ecosyst. Environ. 144, 281–289 10.1016/j.agee.2011.08.016 (doi:10.1016/j.agee.2011.08.016) [DOI] [Google Scholar]
- 42.Hayhoe SJ, Neill C, Porder S, McHorney R, Lebebvre P, Coe MT, Elsenbeer H, Krusche AV. 2011. Conversion to soy on the Amazonian agricultural frontier increases streamflow without affecting stormflow dynamics. Glob. Change Biol. 17, 1821–1833 (doi:10.1111/j.1365–2486.2011.02392.x) [DOI] [Google Scholar]
- 43.Mu Q, Zhao M, Runing SW. 2011. Improvements to a MODIS global terrestrial evapotranspiration algorithm. Remote Sens. Environ. 115, 1781–1800 10.1016/j.rse.2011.02.019 (doi:10.1016/j.rse.2011.02.019) [DOI] [Google Scholar]
- 44.Ferreira JN, Bustamante M, Garcia-Montiel DC, Caylor KK, Davidson EA. 2007. Spatial variation in vegetation structure coupled to plant available water determined by two-dimensional soil resistivity profiling in a Brazilian savanna. Oecologia 153, 417–430 10.1007/s00442-007-0747-6 (doi:10.1007/s00442-007-0747-6) [DOI] [PubMed] [Google Scholar]
- 45.Riskin SH, et al. 2013. The fate of phosphorus fertilizer in Amazon soya bean fields. Phil. Trans. R. Soc. B 368, 20120154. 10.1098/rstb.2012.0154 (doi:10.1098/rstb.2012.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez P. 1977. Properties and management of soils in the tropics. New York, NY: Wiley [Google Scholar]
- 47.Lohse KA, Matson PA. 2005. Consequences of nitrogen additions for soil losses from wet tropical forests. Ecol. Appl. 15, 1629–1648 10.1890/03-5421 (doi:10.1890/03-5421) [DOI] [Google Scholar]
- 48.Bustamante M, Medina E, Asner GP, Nardoto GB, Garcia-Montiel DC. 2006. Nitrogen cycling in tropical and temperate savannas. Biogeochemistry 79, 209–237 10.1007/s10533-006-9006-x (doi:10.1007/s10533-006-9006-x) [DOI] [Google Scholar]
- 49.Liu J, Liangzhi Y, Amini M, Obersteiner M, Herrero M, Zehnde AJB, Yang H. 2010. A high-resolution assessment of global nitrogen flows in cropland. Proc. Natl Acad. Sci. USA 107, 8035–8040 10.1073/pnas.091358107 (doi:10.1073/pnas.091358107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IBGE—EMBRAPA. 2001. Mapa de solos do Brasil. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatísticas. Scale 1:5 000 000.
- 51.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 10.1126/science.1061967 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 52.Neuheimer AB, Thresher RE, Lyle JM, Semmens JM. 2011. Tolerance limit for fish growth exceeded by warming waters. Nat. Clim. Change 1, 110–113 10.1038/nclimate1084 (doi:10.1038/nclimate1084) [DOI] [Google Scholar]
- 53.Tewksbury JJ, Huey RB, Deutsch CA. 2008. Putting the heat on tropical animals. Science 320, 1296–1297 10.1126/science.1159328 (doi:10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 54.Enders EC, Boisclair D, Roy AG. 2003. The effect of turbulence on the cost of swimming for juvenile Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 60, 1149–1160 10.1139/f03-101 (doi:10.1139/f03-101) [DOI] [Google Scholar]
- 55.Deegan L, Golden H, Harrison J, Kracko K. 2005. Swimming ability and metabolism of 0+ Arctic grayling (Thymallus arcticus). J. Fish Biol. 67, 910–918 10.1111/j.0022-1112.2005.00784.x (doi:10.1111/j.0022-1112.2005.00784.x) [DOI] [Google Scholar]
- 56.Lorion CM, Kennedy BP. 2009. Relationships between deforestation, riparian forest buffers and benthic macroinvertebrates in neotropical headwater streams. Freshwater Biol. 54, 165–180 10.1111/j.1365-2427.2008.02092.x (doi:10.1111/j.1365-2427.2008.02092.x) [DOI] [Google Scholar]
- 57.Costa MH, Botta A, Cardille JA. 2003. Effects of large-scale changes in land cover on the discharge of the Tocantins River, southeastern Amazonia. J. Hydrol. 283, 206–217 10.1016/S0022-1694(03)00267-1 (doi:10.1016/S0022-1694(03)00267-1) [DOI] [Google Scholar]
- 58.Coe MT, Latrubesse EM, Ferreira ME, Amsler ML. 2011. The effects of deforestation and climate variability on the streamflow of the Araguaia River, Brazil. Biogeochemistry 105, 119–131 10.1007/s10533-011-9582-2 (doi:10.1007/s10533-011-9582-2) [DOI] [Google Scholar]
- 59.Latrubesse EM, Amsler ML, de Moraes RP, Aquino S. 2009. The geomorphologic response of a large pristine alluvial river to tremendous deforestation in the South American tropics: the case of the Araguaia River. Geomorphology 113, 239–252 10.1016/j.geomorph.2009.03.014 (doi:10.1016/j.geomorph.2009.03.014) [DOI] [Google Scholar]
- 60.Bruijnzeel LA. 1990. Hydrology of moist tropical forests and effects of conversion: a state of knowledge review. Paris, France: UNESCO [Google Scholar]
- 61.Knox JC. 2006. Floodplain sedimentation in the Upper Mississippi Valley: natural versus human accelerated. Geomorphology 79, 286–310 10.1016/j.geomorph.2006.06.031 (doi:10.1016/j.geomorph.2006.06.031) [DOI] [Google Scholar]


