Abstract
The evolutionary study of social systems in non-human primates has long been focused on ecological determinants. The predictive value of socio-ecological models remains quite low, however, in particular because such equilibrium models cannot integrate the course of history. The use of phylogenetic methods indicates that many patterns of primate societies have been conserved throughout evolutionary history. For example, the study of social relations in macaques revealed that their social systems are made of sets of correlated behavioural traits. Some macaque species are portrayed by marked social intolerance, a steep dominance gradient and strong nepotism, whereas others display a higher level of social tolerance, relaxed dominance and a weaker influence of kinship. Linkages between behavioural traits occur at different levels of organization, and act as constraints that limit evolutionary responses to external pressures. Whereas these constraints can exert strong stabilizing selection that opposes the potential changes required by the ecological environment, selective mechanisms may have the potential to switch the whole social system from one state to another by acting primarily on some key behavioural traits that could work as pacemakers.
Keywords: social system, socio-ecology, phylogeny, trade-off, primates
1. Introduction
To explain the diversity of life, mainstream evolutionary thinking considers directional selection as the pre-eminent driving force behind adaptive processes. It focuses on the mutability of livings beings to explain how they cope with the requirements of an ever-changing environment [1]. With the advent of the field of evolutionary developmental biology, however, a renewal of interest occurred for the other side of organisms, i.e. robustness: living beings preserve their state of adaptation by protecting the dynamics of developmental and functional systems against potentially disruptive factors. In order to make sense of variations, we must be able to specify the constraints that exert strong stabilizing selection by favouring organisms and organizations that better maintain balance between their components.
Since Gould & Lewontin [2] opened the debate on the adaptationist programme and the role of constraints versus natural selection in evolution, the term ‘constraint’ has been used with multiple if not contradictory meanings [3,4]. It is common to read about the effects of developmental, genetic, phylogenetic or ecological constraints in the literature. For the concept of constraint to remain useful, we have to specify a null model of evolution regarding the trait considered [3]. The null model states how traits would evolve in the absence of constraints, and we generally define it as the adaptation to external pressures. From this perspective, a constraint is a process that limits the evolutionary response of traits to external selective factors acting at a given level of organization [1,3,5]. A correlate is that selection has an internal component resulting from the fitness consequences of trait variation, as determined by the dynamics of biological systems. This maintains the robustness of systems by eliminating any disrupting variations that would lead to low payoffs for individuals.
The study of the allometric relationships and trade-offs binding the various components of organisms has a long tradition [6,7]. The interest for connections between life-history traits has recently extended to behavioural syndromes, i.e. sets of correlated behaviours characterizing the personality of individuals [8]. By contrast, although social systems are clearly complex wholes, it is still an open question whether they are mainly subjected to conditions of the external milieu, or tightly constrained by linkages internal to these systems. After all, individuals are autonomous reproductive agents pursuing their own strategies, and behaviours are often considered as especially plastic phenotypic traits.
In what follows, I will first review the evidence regarding the role of ecology and phylogeny in the evolution of non-human primate societies. Primates are long-living animals that form elaborated social relationships, and we could, therefore, expect strong interdependency in the patterns of their social systems. In a second step, I will examine how constraints and selective mechanisms shape social systems, basing my evaluation on the example of macaque societies which currently represents one of the best documented cases of covariation between behavioural traits.
2. Ecology and phylogeny
Adaptation to the environment and genealogical descent are the two main Darwinian processes, and both contribute to the evolution of living beings. The social systems in which individuals live are no exceptions, but the attention paid to the role of one or the other has varied to a surprising extent over the last half-century.
(a). Ecological factors
Consistent with the adaptationist perspective, a chief assumption of socio-ecology is that social systems are shaped by ecological factors. We do indeed know of many correlations between ecological and behavioural variables, and there is abundant proof that the behaviour of non-human primates and other animals is influenced by the characteristics of the environment in which they live [9–12]. For instance, individuals in larger groups face reduced predation risks, and as competition for resources increases with group size, so do foraging time and day range of group members [13–16].
As a matter of course, the models proposed so far to explain interspecific differences in the societies of primates were formulated in terms of ecological determinism [17–22]. For many years, a large part of primate ecology has been driven by the so-called synthetic socio-ecological model that aims to account for the diversity of female social relationships regarding patterns of dominance, nepotism, dispersal and coalitions, based on variations in the distribution of food resources and predation risks [22]. Recent reviews have, however, questioned the relevance of this model with three main arguments [23,24]. A first criticism bears upon the framing of the model. Because it is built on a verbal logic and variables related to food distribution are not accurately defined, it is difficult to either validate or invalidate it [25,26]. A second difficulty is the limited predictive value of the model. Numerous mismatches have been reported between the actual behaviour of animals and model expectations, even those most central to it. In particular, no convincing evidence has been found supporting the proposition that rates of competition vary according to the patchiness of food supplies, nor have any obvious correlations been seen between variations in diet and social relationships, dispersal or philopatry in females [23,24].
I will highlight a third concern pertaining to the assumptions of the model. The socio-ecological model is by design an equilibrium model postulating that the free play of adaptive trade-offs produces a state of organization adapted to the contemporary environment. In its first versions, the model stood as a genuine null model regarding the action of constraints because it involved no other factors than ecological determinants [18,19]. Sexual selection was introduced in the most recent synthesis, via the occurrence of infanticide by males, in an attempt to explain unresolved cases of group formation by the females’ need for protection [22]; but still the model did not include any concerns about the interdependency of phenotypic traits at the individual or social level. As such, it is understandable that the socio-ecological model fails to provide a realistic picture of primate societies (but see [27]). Models are heuristic tools designed to formalize hypotheses and guide testing; we should avoid turning the socio-ecological model into a unitary theory of primate social organization that would remain incomplete by nature.
(b). Phylogenetic relatedness
Despite the emphasis by early ethologists on phylogeny [28], and the statement that ‘each species brings a different phylogenetic heritage into a particular ecological scene’ [29], the search for environmental determinants has long overshadowed the role of evolutionary history in the study of behaviour [30]. Similar ecological problems were considered to produce similar solutions, i.e. convergence was assumed to have occurred regardless of the phylogenetic past of species. For several decades, this stance could not be countered on quantitative grounds because of the lack of analytic tools, such as the comparative phylogenetic methods [30,31].
Through an analysis conducted at the scale of the primate order, Di Fiore & Rendall [32] first showed in the 1990s that social traits cluster according to taxonomic groups; they show strong phylogenetic signal, meaning that greater similarity is found in more closely related species [33,34]. Old World monkeys display a high level of uniformity in the basic patterns of their social systems although they live in a diverse range of habitats. Moreover, patterns of social relationships in females including dominance, nepotism, dispersal and coalition formation appear to be particularly conservative throughout evolutionary history. This contrasts with the assumptions of the socio-ecological model, which regards these patterns as the main variables liable to match ecological diversity [22].
A different set of evidence came from the comparison of primate communities belonging to different ecosystems, which revealed that similarities in dietary and locomotor characteristics between communities are most likely when species are closely related [35]. Furthermore, a comparative review of social systems in lemurs led Kappeler [36] to conclude that their social relationships and demographic structures have no equivalent among other primates; a lack of convergence indicated that lemurs followed their own distinct evolutionary path.
In a next step, studies were carried out at the genus level. Cross-species comparisons in macaques (Macaca) demonstrated that patterns of dominance, aggression, submission and reconciliation vary in a consistent way with phylogeny [37–40]. Comparisons in the genus Eulemur also showed that the demographic structure of social groups correlates with phylogenetic distance, but not with ecological variables [41]. It is worth adding that phylogenetic relatedness can also predict a fair amount of behavioural diversity in other vertebrate taxa (e.g. equids [42] and birds [43,44]).
Recently, Shultz et al. [45] built on the strong phylogenetic signal found in the basic demographic structures within the primate order to reconstruct the evolutionary pathways leading to different types of social organization. Their tests support a stepwise model composed of several transitions, mainly unilateral, where the development of sociality proceeds from solitary life to loose aggregations of several males and females, to stable multi-male–multi-female groups, and then to pairs or unimale groups. The initial switch to sociality coincides with a shift from nocturnal to diurnal activity, which supports the hypothesis that group-living represents a protective strategy against predators. If these conclusions were to be confirmed, it would mean that the evolution of some aspects of primate social systems follow directional patterns. Once again, this challenges the assumptions of the socio-ecological model postulating—as any equilibrium model—that all transitions from one state to another are equally possible.
3. Constraints and selective changes
We should be careful not to oppose phylogeny and adaptation; history is a pattern rather than a factor, and, therefore, cannot be regarded as a constraint [46]. The term ‘phylogenetic constraint’ is commonly used as shorthand to mean constraints induced by mechanisms responsible for the occurrence of a phylogenetic signal, but these mechanisms may be adaptive or not depending on the level of organization considered (see §1), which makes the concept of phylogenetic constraint too vague to be useful. Correlations stem from nothing other than the genetic and epigenetic processes involved in the renewed development of phenotypes in each generation [5]. I will address these processes by examining the social organization of macaques. The genus Macaca is characterized by a wide range of social styles, i.e. sets of behavioural traits that remain relatively stable despite environmental and demographical changes. Some species are portrayed by marked social intolerance, a steep dominance gradient and strong nepotism, whereas others display a higher level of social tolerance, relaxed dominance and a weaker influence of kinship [47,48]. Moreover, a number of traits appear to covary in a consistent way (table 1), meaning that social styles belong to a single family of forms. In other words, the social relationships of macaques range within a socio-space that is defined by linkages between traits. Such coupling can occur at the level of the organism or the society, and channels evolutionary changes.
Table 1.
Covarying traits in macaque societies. A number of behavioural traits appear to vary in a correlated way along a scale ranging from low to high social tolerance [39,47–55].
| behavioural traits | lower social tolerance | higher social tolerance |
|---|---|---|
| hierarchical steepness | + | – |
| proportion of conflicts involving counter-aggression | – | + |
| proportion of conflicts involving biting | + | – |
| development of formal submission signals | + | – |
| appropriating resources from subordinates | + | – |
| development of cooperative behaviours | – | + |
| conciliatory tendency | – | + |
| rate of affiliative contacts | – | + |
| degree of mother permissiveness | – | + |
| amount of allomothering behaviour | – | + |
| rate of immature interference in matings | – | + |
| rate and cooperative patterning of social play | – | + |
| degree of kin preference among females | + | – |
| rules of female rank inheritance | + | – |
| rate of male dispersal | + | – |
| coalitions between males | – | + |
| centrality of top-ranking males | + | – |
| reactive temperament | + | – |
(a). Organism linkages
The null model regarding the role of constraints assumes that organisms change exclusively in response to requirements of the environment, as if internal linkages were not influential enough to limit their ability to evolve. This stands in sharp contrast to the well-established fact that genetic and phenotypic correlations constitute a prevalent feature of organisms [5,56].
Coupling between traits can arise from the architecture and regulation processes of the genome. The proximity of two gene loci on a chromosome reduces their rates of recombination, leading certain allelic effects to be correlated, several genes can also share the same regulatory circuits, making it harder for characters to shift independently, and most genes have pleiotropic effects, thus producing multiple correlations [5,57]. As an example, aggressiveness is linked to reduced serotonin activity. Serotonin is a neurotransmitter involved in the control of the neurohormonal stress axis, and polymorphism occurs at the promoter region of a gene encoding the serotonin transporter protein. Two main alleles of this gene, S and L, have been identified; by conferring decreased efficiency to the serotonin gene promoter the S allele is responsible for impaired serotonin function [58]. Studies in rhesus macaques (Macaca mulatta) have shown that low levels of serotonin are associated with a cascade of behavioural effects including impulsive risk-taking and unrestrained aggression; low-level males have tendencies for social isolation, early dispersal, frequent wounding and increased mortality rates, whereas low-level females are more protective mothers yet experience higher rates of infant loss [59]. Moreover, there are hints that genetic variation in serotoninergic transmission is related to inter-species differences in the behaviours of macaques [60,61].
The fact that different personality traits of individuals cluster in stable combinations has attracted much attention in recent years [8]. While linkages can proceed from genetic determinants, they may also be underpinned by epigenetic mechanisms involving trade-offs at the phenotypic level. Comparative studies show that macaque species consistently differ in their behavioural reactivity to the environment. As a general rule, more tolerant macaques appear less easily aroused by psychological stressors and are more explorative than their less tolerant counterparts [62,63].
In view of their common neurobiological, psychological and developmental bases, it is understandable that many behavioural characters are linked, while incompatibilities between traits can also occur, thus producing negative correlations [8]. One system can be involved in multiple behaviours. For example, anxiety may be activated by the management of the risks incurred by various social interactions such as social conflicts, reconciliations and the retrieval of infants by mothers [64,65]; we may expect that two populations differing in baseline anxiety will show systematic variations in their behavioural responses in these three types of interactions.
(b). Social linkages
When considering the evolution of individuals within the social context, the null model centred on organisms is no longer relevant. Social interactions generate linkages between individuals, and a null model including the social phenotype becomes necessary to conceive adaptation in the absence of constraints. I therefore propose a second null model stating that organisms change exclusively in response to the requirements of the ecological environment; the external component of selection comes from the ecological environment, whereas the internal component comes from both the organism and the social system. From this perspective, a constraint is any mechanism originating from the organism or the social system that limits adaptation to the ecological environment. This perspective should not look that unfamiliar. When we assert that body size partly results from a trade-off between competition between or within sexes (sexual selection) and resource availability (natural selection), this amounts to saying that social mechanisms act as constraints with respect to ecological adaptations.
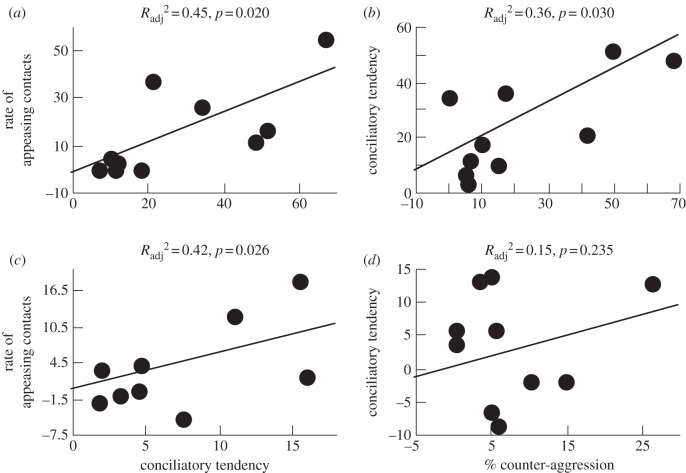
The study of the covariation of traits in macaque social relationships (table 1) points at a number of trade-offs liable to constrain their evolution by reducing their societies to a subset of possible social styles. Tactical considerations foster the idea that if the risk of being wounded in a conflict is elevated, the better tactic for the weaker individual is to submit or flee rather than to retaliate, which corresponds to a strong dominance gradient between group members [48]. Conversely, when the dominance gradient is low as in more tolerant species, the threatened individual can protest or retaliate, forcing the opponent to avoid potentially dangerous attacks. In animals capable of using graded threats, a high proportion of counter-aggression in conflicts is not compatible with frequent biting and wounding, as it would represent poor tactics. As an outcome, highly intensive aggression is associated with strong contest asymmetry, a steep dominance gradient and conspicuous submission signals (figures 1 and 2). The rates of reconciliations occurring between previous opponents after a conflict vary accordingly. In intolerant macaque species, asymmetric conflicts and increased risk of injury can inhibit the occurrence of affiliative contacts between opponents. In more tolerant species, a low dominance gradient creates room for negotiation, while conciliatory behaviours reduce the probability of conflict escalation by facilitating information exchange between adversaries [66–69].
Figure 1.
Testing cross-species relationships between conciliatory tendency, appeasing contact and counter-aggression in macaques. Regression analyses used either (a,b) raw data on social groups or (c,d) independent contrasts computed from the phylogeny provided in Purvis (adapted from Thierry et al. [39]).
Figure 2.
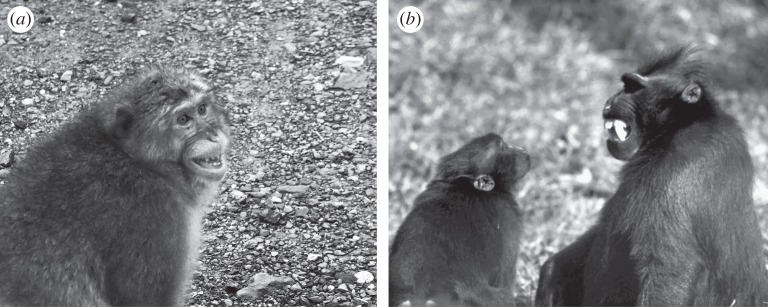
Function of the silent bared-teeth display in macaques: (a) in a quite intolerant species such as Japanese macaques (Macaca fuscata), subordinates use this facial expression to submit, i.e. to formally acknowledge their lower status relative to higher-ranking conspecifics (photograph by B. Thierry); (b) in a tolerant species such as crested macaques (Macaca nigra), the same display does not communicate social status, instead it signals the sender's peaceful intentions like a smile (photograph by O. Petit).
The same rationale may account for the coupling of dominance and maternal styles. Any association between intense aggression and a high degree of maternal permissiveness is unlikely because females living in an intolerant social environment have to protect their infants from potential dangers, and they limit their offsprings' social contacts by retrieving them frequently. By contrast, in more tolerant macaques, mothers may safely allow others to take their infants away, thus promoting the development of allomothering behaviour [47,70].
Much our knowledge remains correlational, and we generally do not know to what extent the behaviours that we study are heritable. We should be cautious not to extrapolate too quickly from cross-species correlations to evolutionary processes. When we correct for phylogenetic relatedness in comparative analyses, some correlations persist but other disappear. For example, the association found between conciliatory tendencies and rates of appeasing contacts such as clasps and mounts remains after controlling for phylogeny (figure 1), supporting the hypothesis that appeasement behaviours are instrumental in promoting reconciliation between previous opponents [39]. This functional dependency between variables may act as a constraint internal to the social system. Quite to the contrary, the positive relationship observed between conciliatory tendencies and rates of counter-aggression vanishes when phylogenetic effects are removed (figure 1), indicating that their association may arise from common ancestry because of connections, possibly coincidental, with other unidentified traits [39].
As for allometric scaling of organisms, we are still far from disentangling the many processes responsible for the clustering of behavioural traits. It is all the more true that behaviours include a learned component capable of reinforcing inter-individual linkages. In an experiment where young rhesus macaques were co-housed with slightly older stump-tailed macaques (Macaca arctoides), the standard conciliatory tendencies of the former were multiplied threefold, stabilizing at levels comparable with those typical of the latter [71]. In another study in wild olive baboons (Papio anubis), the more competitive resident males died following tuberculosis outbreak, thus leaving a cohort of less aggressive males in the troop. One decade after the disappearance of the latter, newly immigrant males still displayed atypically high levels of social tolerance, indicating that this style of relation could have been transmitted socially [72]. Such effects are especially important in societies founded upon overlapping generations. Offspring are influenced by both the acquired and inherited characters of adults, reducing the variation range of behaviours through mechanisms of social inheritance [73].
(c). Selective changes
Adaptive value is usually appraised from the fitness advantage conferred by a particular behavioural trait, but this is an oversimplification. Correlational selection is the rule rather than the exception since selection on one trait not only produces a direct effect on the distribution of that trait in a population, but also produces indirect effects on the distribution of correlated traits; this is a main operating mechanism for stabilizing selection [74,75]. With regard to macaque societies, any shift in social style can entail a series of advantages and disadvantages. In theory, all of them should be taken into account to assess the net selective outcome of a given set of traits. I have considered the relationships between traits until now, but each trait alone may have further consequences. For example, when comparing the consequences of a less tolerant and a more tolerant social style in relation to the behavioural strategies of individuals, we may speculate that: (i) a reactive temperament allows better resistance to stressful conditions, whereas a more tractable one is physiologically less costly, (ii) a higher rate of male dispersal favours gene flow, but a lower rate decreases mortality in bachelors, (iii) clear-cut contests reduce the number of potential conflicts and shorten their duration, but elaborated negotiation skills favour the resolution of conflicts and diminish the occurrence of wounds, (iv) higher maternal restrictiveness protects an infant against short-term dangers, but allowing alloparental care increases the number of potential protectors, (v) a lower level of tolerance corresponds to an appropriate cautiousness when facing the unknown, but a higher level enhances social contacts and information transmission between group-mates. It should be stressed that some of these strategies can be adaptive when considered from the ecological environment standpoint (first null model), whereas others can be adaptive from the social environment standpoint, but act as constraints from the ecological environment standpoint (second null model).
The reconstruction of the ancestral social style of macaques using phylogenetic methods indicates that some species underwent limited changes over several million years despite the occurrence of wide climatic and ecological variations [38,40]. If this reconstruction is accurate, the occurrence of evolutionary stasis, therefore, supports the belief that multiple interconnections can maintain traits in an entrenched condition over long periods [76]. We cannot exclude the possibility that social styles are neutral with regard to the ecological environment, and that such an evolution is a mere outcome of genetic drift. Such a hypothesis appears unlikely, however. While the ancestral social style of macaques was rather tolerant, four species—rhesus, Japanese, long-tailed (Macaca fascicularis) and pigtailed macaques (Macaca nemestrina)—have evolved towards a highly intolerant style [38]. The drift hypothesis does not suffice to explain why these species show impoverished appeasement behaviours compared with more tolerant species; it is generally held that the high levels of competition and cooperation engendered by group-living promote the development of elaborated negotiating skills, and there should be a reason why some species followed regressive paths as it may occur whenever biological traits no longer bring benefits to individuals.
Selective mechanisms have the potential to switch a social system from one state to another by targeting pacemaker characters, i.e. key behavioural traits able to pull the whole suite of correlated traits [47]. The preferred candidates for playing this role are often the traits related to dominance relationships and agonistic interactions—their asymmetry, intensity and nepotistic character [22,37]—but so far we have not found any relationships between dominance style and present-day ecological conditions [77]. This does not exclude the possibility that some past events could have driven changes in social styles, however. For instance, the colonization of new environments can involve non-random gene flow and induce phenotypic rearrangements through spatial sorting [78,79]. The four macaque species cited above proved to be the most successful colonizers of their genus in recent geological times. As this happening hinges on sustained rates of dispersal, it could sort individuals whose temperament prompts them to emigrate earlier and frequently, thus pulling species towards an intolerant social style [47].
4. Conclusions
In the study of social behaviour, the focus on evolutionary changes has perpetuated an atomistic perspective of phenotypes for a longer period than in other subfields of biology. Although equilibrium models are useful to formalize hypotheses about the action of ultimate factors, none of them can provide a unitary theory of animal societies, as they are not designed to integrate historical events. The use of phylogenetic methods in the last two decades taught us that many patterns of primate societies were quite conservative throughout evolutionary history, which may be due to the action of constraints occurring at various levels of the individual and social phenotype.
Macaque social styles appear as stable clusters of behavioural traits connected by numerous links, and we should ask to what extent behavioural traits are similarly covariant in other taxa. Some data support the view that the degree of entrenchment depends on the level of integration of social systems. In species forming small and loosely structured groups such as certain lemurs (Eulemur fulvus and Eulemur macaco), no association appears between levels of conflict asymmetry and rates of reconciliation [80]. By contrast, the comparison of the more complex social relationships of chimpanzees (Pan troglodytes) and bonobos (Pan paniscus) shows an association similar to those found in macaques between social tolerance, dominance gradient, intensity of physical aggression, development of cooperative behaviours and individual reactivity [81]. Interestingly, cross-cultural comparisons in humans again yield the same kind of correlations. Differences between tolerant and intolerant societies have been documented for a long time in anthropology [82], and a recent investigation demonstrated that tightness of social order, severity of sanctions, strictness of rearing practices and levels of individual self-control are related patterns; moreover, the degree of social intolerance appears to increase with population density and environmental threats [83]. It would be worth extending the scope of comparative studies to non-primate animals in order to assess how far social traits may be internally linked in species forming stable social groups.
It appears necessary to explore causal links between variables using agent-based models [84], and to develop the analysis of evolutionary transitions in relation to variations in ecology and life-history traits [24,45]. I have advocated the role of internal constraints, but it should not be forgotten that the external milieu may be also responsible for the clustering of traits. Adaptation to the same set of ecological conditions can lead different species to converge on similar social systems. Furthermore, the filling of a new niche by ecologically similar species has the potential to generate phylogenetic signal when these species come to diverge [85]. A main goal for future research will be to disentangle the different sources of correlations between behavioural traits, and to investigate the ability of selective processes to break up linkages.
Lastly, given the pervasive nature of the pleiotropic effects of the individual upon the social system [47], we cannot do without widening our knowledge of the mechanisms through which individual phenotypes produce social phenotypes. Experiments in rhesus macaques showed for instance that the personality profile of individuals predicts the social relationships and networks they build when they are subsequently gathered into social groups [86,87]. Unravelling the heritable and acquired components of the individual behaviours responsible for the clustering of social traits would enable understanding of how constraints work within social systems.
Acknowledgements
I am grateful to Peter Kappeler for the invitation to take part in the Göttinger Freilandtage, and to two anonymous referees for constructive comments.
References
- 1.Schwenk K, Wagner GP. 2004. The relativism of constraints on phenotypic evolution. In Phenotypic integration (eds Pigliucci M, Preston K.), pp. 391–408 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Gould SJ, Lewontin RC. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B 205, 581–598 10.1098/rspb.1979.0086 (doi:10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- 3.Antonovics J, van Tienderen PH. 1991. Ontoecogenophyloconstraints? The chaos of constraint terminology. Trends Ecol. Evol. 6, 166–168 10.1016/0169-5347(91)90059-7 (doi:10.1016/0169-5347(91)90059-7) [DOI] [PubMed] [Google Scholar]
- 4.Olson ME. 2012. The developmental renaissance in adaptationism. Trends Ecol. Evol. 27, 278–287 10.1016/j.tree.2011.12.005 (doi:10.1016/j.tree.2011.12.005) [DOI] [PubMed] [Google Scholar]
- 5.Schlichting CD, Pigliucci M. 1998. Phenotypic evolution. Sunderland, MA: Sinauer [Google Scholar]
- 6.Schmidt-Nielsen K. 1984. Scaling: why is animal size so important? Cambridge, UK: Cambridge University Press [Google Scholar]
- 7.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 8.Sih A, Bell AM, Chadwick Johnson J, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 9.Clutton-Brock TH, Harvey PH. 1977. Primate ecology and social organisation. J. Zool. 183, 1–39 10.1111/j.1469-7998.1977.tb04171.x (doi:10.1111/j.1469-7998.1977.tb04171.x) [DOI] [Google Scholar]
- 10.Terborgh JW, Janson CH. 1986. Sociobiology of primates. Annu. Rev. Ecol. Syst. 17, 111–135 10.1146/annurev.es.17.110186.000551 (doi:10.1146/annurev.es.17.110186.000551) [DOI] [Google Scholar]
- 11.Kappeler PM, van Schaik CP. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707–740 10.1023/A:1015520830318 (doi:10.1023/A:1015520830318) [DOI] [Google Scholar]
- 12.Silk JB. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559 10.1098/rstb.2006.1994 (doi:10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Schaik CP, van Hooff JARAM. 1983. On the ultimate causes of primate social systems. Behaviour 85, 91–117 10.1163/156853983X00057 (doi:10.1163/156853983X00057) [DOI] [Google Scholar]
- 14.Wrangham RW, Gittleman JL, Chapman CA. 1993. Constraints on group size in primates and carnivores: population density and day range as assays of exploitation competition. Behav. Ecol. Sociobiol. 32, 99–209 [Google Scholar]
- 15.Janson CH, Goldsmith MC. 1995. Predicting group size in primates: foraging costs and predation risks. Behav. Ecol. 6, 326–336 10.1093/beheco/6.3.326 (doi:10.1093/beheco/6.3.326) [DOI] [Google Scholar]
- 16.Pollard KA, Blumstein DT. 2008. Time allocation and the evolution of group size. Anim. Behav. 76, 1683–1699 10.1016/j.anbehav.2008.08.006 (doi:10.1016/j.anbehav.2008.08.006) [DOI] [Google Scholar]
- 17.Crook JH, Gartlan JS. 1966. Evolution of primate societies. Nature 210, 1200–1203 10.1038/2101200a0 (doi:10.1038/2101200a0) [DOI] [PubMed] [Google Scholar]
- 18.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262–300 10.1163/156853980X00447 (doi:10.1163/156853980X00447) [DOI] [Google Scholar]
- 19.van Schaik CP. 1989. The ecology of social relationships amongst female primates. In Comparative socioecology (eds Standen V, Foley RA.), pp. 195–218 Oxford, UK: Blackwell [Google Scholar]
- 20.Isbell LA. 1991. Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behav. Ecol. 2, 143–155 10.1093/beheco/2.2.143 (doi:10.1093/beheco/2.2.143) [DOI] [Google Scholar]
- 21.Isbell LA, Van Vuren D. 1996. Differential costs of locational and social dispersal and their consequences for female group-living primates. Behaviour 133, 1–36 10.1163/156853996X00017 (doi:10.1163/156853996X00017) [DOI] [Google Scholar]
- 22.Sterck EHM, Watts DW, van Schaik CP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 29–309 [Google Scholar]
- 23.Thierry B. 2008. Primate socioecology, the lost dream of ecological determinism. Evol. Anthropol. 17, 93–96 10.1002/evan.20168 (doi:10.1002/evan.20168) [DOI] [Google Scholar]
- 24.Clutton-Brock TH, Janson CH. 2012. Primate socioecology at the crossroads: past, present and future. Evol. Anthropol. 21, 136–150 10.1002/evan.21316 (doi:10.1002/evan.21316) [DOI] [PubMed] [Google Scholar]
- 25.Isbell LA, Young TP. 2002. Ecological models of female social relationships in primates: similarities, disparities, and some directions for future clarity. Behaviour 139, 177–202 10.1163/156853902760102645 (doi:10.1163/156853902760102645) [DOI] [Google Scholar]
- 26.Koenig A, Borries C. 2006. The predictive power of socioecological models: a reconsideration of resource characteristics, agonism, and dominance hierarchies. In Feeding ecology in apes and other primates (eds Hohmann G, Robbins MM, Boesch C.), pp. 263–284 Cambridge, UK: Cambridge University Press [Google Scholar]
- 27.Koenig A, Borries C. 2009. The lost dream of ecological determinism: time to say goodbye? Or a white queen's proposal? Evol. Anthropol. 18, 166–174 10.1002/evan.20225 (doi:10.1002/evan.20225) [DOI] [Google Scholar]
- 28.Lorenz K. 1941. Vergleichende Bewegungsstudien an Anatiden. J. Ornithol. 89, 194–293 [Google Scholar]
- 29.Struhsaker TT. 1969. Correlates of ecology and social organization among African cercopithecines. Folia Primatol. 11, 80–118 10.1159/000155259 (doi:10.1159/000155259) [DOI] [PubMed] [Google Scholar]
- 30.Brooks DR, McLennan DA. 1991. Phylogeny, ecology, and behavior. Chicago, IL: University of Chicago Press [Google Scholar]
- 31.Nunn CL, Barton RA. 2001. Comparative methods for studying primate adaptation and allometry. Evol. Anthropol. 10, 81–98 10.1002/evan.1019 (doi:10.1002/evan.1019) [DOI] [Google Scholar]
- 32.Di Fiore A, Rendall D. 1994. Evolution of social organization: a reappraisal for primates by using phylogenetic methods. Proc. Natl Acad. Sci. USA 91, 9941–9945 10.1073/pnas.91.21.9941 (doi:10.1073/pnas.91.21.9941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blomberg SP, Garland T. 2002. Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. J. Evol. Biol. 15, 899–910 10.1046/j.1420-9101.2002.00472.x (doi:10.1046/j.1420-9101.2002.00472.x) [DOI] [Google Scholar]
- 34.Revell L, Harmon LJ, Collar DC. 2008. Phylogenetic signal, evolutionary process, and rate. Syst. Biol. 57, 591–601 10.1080/10635150802302427 (doi:10.1080/10635150802302427) [DOI] [PubMed] [Google Scholar]
- 35.Fleagle JG, Reed KE. 1996. Comparing primate communities: a multivariate approach. J. Hum. Evol. 30, 489–510 10.1006/jhev.1996.0039 (doi:10.1006/jhev.1996.0039) [DOI] [Google Scholar]
- 36.Kappeler PM. 1997. Determinants of primate social organization: comparative evidence and new insights from Malagasy lemurs. Biol. Rev. 72, 111–151 10.1017/S0006323196004999 (doi:10.1017/S0006323196004999) [DOI] [PubMed] [Google Scholar]
- 37.Matsumura S. 1999. The evolution of ‘egalitarian’ and ‘despotic’ social systems among macaques. Primates 43, 23–31 10.1007/BF02557699 (doi:10.1007/BF02557699) [DOI] [PubMed] [Google Scholar]
- 38.Thierry B, Iwaniuk AN, Pellis SM. 2000. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca). Ethology 106, 713–728 10.1046/j.1439-0310.2000.00583.x (doi:10.1046/j.1439-0310.2000.00583.x) [DOI] [Google Scholar]
- 39.Thierry B, Aureli F, Nunn C, Petit O, Abegg C, de Waal FBM. 2008. A comparative study of conflict resolution in macaques: insights into the nature of trait covariation. Anim. Behav. 75, 847–860 10.1016/j.anbehav.2007.07.006 (doi:10.1016/j.anbehav.2007.07.006) [DOI] [Google Scholar]
- 40.Balasubramaniam KN, et al. 2012. Hierarchical steepness and phylogenetic models: phylogenetic signals in Macaca. Anim. Behav. 83, 1207–1218 10.1016/j.anbehav.2012.02.012 (doi:10.1016/j.anbehav.2012.02.012) [DOI] [Google Scholar]
- 41.Ossi K, Kamilar JM. 2006. Environmental and phylogenetic correlates of Eulemur behavior and ecology (Primates: Lemuridae). Behav. Ecol. Sociobiol. 61, 53–64 10.1007/s00265-006-0236-7 (doi:10.1007/s00265-006-0236-7) [DOI] [Google Scholar]
- 42.Linklater WL. 2000. Adaptive explanation in socio-ecology: lessons from the Equidae. Biol. Rev. 75, 1–20 10.1017/S0006323199005411 (doi:10.1017/S0006323199005411) [DOI] [PubMed] [Google Scholar]
- 43.Prum RO. 1994. Phylogenetic analysis of the evolution of alternative social behavior in the manakins (Aves: Pipridae). Evolution 48, 1657–1675 10.2307/2410255 (doi:10.2307/2410255) [DOI] [PubMed] [Google Scholar]
- 44.Johnson KP, McKinney F, Sorenson MD. 1999. Phylogenetic constraint on male parental care in the dabbling ducks. Proc. R. Soc. B 266, 759–763 10.1098/rspb.1999.0702 (doi:10.1098/rspb.1999.0702) [DOI] [Google Scholar]
- 45.Shultz S, Opie C, Atkinson QD. 2011. Stepwise evolution of stable sociality in primates. Nature 479, 219–222 10.1038/nature10601 (doi:10.1038/nature10601) [DOI] [PubMed] [Google Scholar]
- 46.Losos JB, Miles DB. 1994. Adaptation, constraint, and the comparative method: phylogenetic issues and methods. In Ecological morphology (eds Wainwright PC, Reilly S.), pp. 60–98 Chicago, IL: University of Chicago Press [Google Scholar]
- 47.Thierry B. 2004. Social epigenesis. In Macaque societies: a model for the study of social organization (eds Thierry B, Singh M, Kaumanns W.), pp. 267–290 Cambridge, UK: Cambridge University Press [Google Scholar]
- 48.Thierry B. 2007. Unity in diversity: lessons from macaque societies. Evol. Anthropol. 16, 224–238 10.1002/evan.20147 (doi:10.1002/evan.20147) [DOI] [Google Scholar]
- 49.Petit O, Desportes C, Thierry B. 1992. Differential probability of ‘coproduction’ in two species of macaque (Macaca tonkeana, M. mulattai). Ethology 90, 107–120 10.1111/j.1439-0310.1992.tb00825.x (doi:10.1111/j.1439-0310.1992.tb00825.x) [DOI] [Google Scholar]
- 50.Thierry B. 2010. The macaques: a double-layered social organization. In Primates in perspective (eds Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK.), pp. 229–241 Oxford, UK: Oxford University Press [Google Scholar]
- 51.Sueur C, Petit O. 2008. Shared or unshared consensus decision in macaques? Behav. Proc. 78, 84–92 10.1016/j.beproc.2008.01.004 (doi:10.1016/j.beproc.2008.01.004) [DOI] [PubMed] [Google Scholar]
- 52.Sueur C, Petit O, De Marco A, Jacobs A, Watanabe K, Thierry B. 2011. A comparative network analysis of social style in macaques. Anim. Behav. 82, 845–852 10.1016/j.anbehav.2011.07.020 (doi:10.1016/j.anbehav.2011.07.020) [DOI] [Google Scholar]
- 53.Bélisle P, Prud'homme J, Dubuc C. 2012. The impact of kinship, defence cost and priority of access on food competition. In The monkeys of Stormy Mountain: 60 years of primatological research on the Japanese macaques of Arashiyama (eds Leca JB, Huffman MA, Vasey PL.), pp. 331–355 Cambridge, UK: Cambridge University Press [Google Scholar]
- 54.Ciani F, Dall'Olio S, Stanyon R, Palagi E. 2012. Social tolerance and adult play in macaque societies: a comparison with different human cultures. Anim. Behav. 84, 1313–1322 10.1016/j.anbehav.2012.09.002 (doi:10.1016/j.anbehav.2012.09.002) [DOI] [Google Scholar]
- 55.Dobson SD. 2012. Coevolution of facial expression and social tolerance in macaques. Am. J. Primatol. 74, 229–235 10.1002/ajp.21991 (doi:10.1002/ajp.21991) [DOI] [PubMed] [Google Scholar]
- 56.West-Eberhard M. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 57.Price T, Langen T. 1992. Evolution of correlated characters. Trends Ecol. Evol. 7, 307–310 10.1016/0169-5347(92)90229-5 (doi:10.1016/0169-5347(92)90229-5) [DOI] [PubMed] [Google Scholar]
- 58.Canli T, Lesch KP. 2007. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 10, 1103–1109 10.1038/nn1964 (doi:10.1038/nn1964) [DOI] [PubMed] [Google Scholar]
- 59.Higley JD, Suomi SJ, Chaffin AC. 2011. Impulsivity and aggression as personality traits in nonhuman primates. In Personality and behavioral syndromes in nonhuman primates (eds Weiss A, King JE, Murray L.), pp. 257–263 New York, NY: Springer [Google Scholar]
- 60.Westergaard GC, Suomi SJ, Higley JD, Mehlman PT. 1999. CSF 5-HIAA and aggression in female macaque monkeys: species and interindividual differences. Psychopharmacology 146, 440–446 10.1007/PL00005489 (doi:10.1007/PL00005489) [DOI] [PubMed] [Google Scholar]
- 61.Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi S. 2006. Differential functional variability of serotonin transporter and monoamine oxidase A genes in macaque species displaying contrasting levels of aggression-related behavior. Behav. Gen. 36, 163–172 10.1007/s10519-005-9017-8 (doi:10.1007/s10519-005-9017-8) [DOI] [PubMed] [Google Scholar]
- 62.Thierry B, Anderson JR, Demaria C, Desportes C, Petit O. 1994. Tonkean macaque behaviour from the perspective of the evolution of Sulawesi macaques. In Current primatology, vol. 2: social development, learning and behaviour (eds Roeder JJ, Thierry B, Anderson JR, Herrenschmidt N.), pp. 103–117 Strasbourg, France: Université Louis Pasteur [Google Scholar]
- 63.Clarke SA, Boinski S. 1995. Temperament in nonhuman primates. Am. J. Primatol. 37, 103–125 10.1002/ajp.1350370205 (doi:10.1002/ajp.1350370205) [DOI] [PubMed] [Google Scholar]
- 64.Maestripieri D. 1993. Maternal anxiety in rhesus macaques (Macaca mulatta). II. Emotional bases of individual differences in mothering style. Ethology 95, 32–42 10.1111/j.1439-0310.1993.tb00454.x (doi:10.1111/j.1439-0310.1993.tb00454.x) [DOI] [Google Scholar]
- 65.Aureli F, Schino G. 2004. The role of emotions in social relationships. In Macaque societies: a model for the study of social organization (eds Thierry B, Singh M, Kaumanns W.), pp. 38–55 Cambridge, UK: Cambridge University Press [Google Scholar]
- 66.de Waal FBM. 1986. The integration of dominance and social bonding in primates. Q. Rev. Biol. 61, 459–479 10.1086/415144 (doi:10.1086/415144) [DOI] [PubMed] [Google Scholar]
- 67.Preuschoft S. 1995. ‘Laughter’ and ‘smiling’ in macaques. PhD Thesis, Rijksuniversiteit Utrecht, Utrecht, The Netherlands [Google Scholar]
- 68.Aureli F, Cords M, van Schaik CP. 2002. Conflict resolution following aggression in gregarious animals: a predictive framework. Anim. Behav. 64, 325–343 10.1006/anbe.2002.3071 (doi:10.1006/anbe.2002.3071) [DOI] [Google Scholar]
- 69.Silk JB. 1997. The function of peaceful post-conflict contacts among primates. Primates 38, 265–279 10.1007/BF02381614 (doi:10.1007/BF02381614) [DOI] [Google Scholar]
- 70.McKenna JJ. 1979. The evolution of allomothering among colobine monkeys: function and opportunism in evolution. Am. Anthropol. 81, 818–840 10.1525/aa.1979.81.4.02a00040 (doi:10.1525/aa.1979.81.4.02a00040) [DOI] [Google Scholar]
- 71.de Waal FBM, Johanowicz DL. 1993. Modification of reconciliation behavior through social experience: an experiment with two macaque species. Child Dev. 64, 897–908 10.2307/1131225 (doi:10.2307/1131225) [DOI] [PubMed] [Google Scholar]
- 72.Sapolsky RM, Share LJ. 2004. A pacific culture among wild baboons: its emergence and transmission. PLoS Biol. 3, 534–541 10.1371/journal.pbio.0020106 (doi:10.1371/journal.pbio.0020106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf JB, Brodie ED, Cheverud JM, Moore AJ, Wade MJ. 1998. Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69 10.1016/S0169-5347(97)01233-0 (doi:10.1016/S0169-5347(97)01233-0) [DOI] [PubMed] [Google Scholar]
- 74.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226 10.2307/2408842 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 75.Blows MW, Brooks R. 2003. Measuring non-linear selection. Am. Nat. 162, 815–820 10.1086/378905 (doi:10.1086/378905) [DOI] [PubMed] [Google Scholar]
- 76.Wimsatt WC, Schank JC. 2004. Generative entrenchment, modularity and evolvability: when genic selection meets the whole organism. In Modularity in development and evolution (eds Schlosser G, Wagner G.), pp. 359–394 Chicago, IL: University of Chicago Press [Google Scholar]
- 77.Ménard N. 2004. Do ecological factors explain variation in social organization? In Macaque societies: a model for the study of social organization (eds Thierry B, Singh M, Kaumanns W.), pp. 237–262 Cambridge, UK: Cambridge University Press [Google Scholar]
- 78.Shine R, Brown GP, Phillips BL. 2011. An evolutionary process that assembles phenotypes through space rather than through time. Proc. Natl Acad. Sci. USA 108, 5708–5711 10.1073/pnas.1018989108 (doi:10.1073/pnas.1018989108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edelaar P, Bolnick DI. 2012. Non-random gene flow: and underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665 10.1016/j.tree.2012.07.009 (doi:10.1016/j.tree.2012.07.009) [DOI] [PubMed] [Google Scholar]
- 80.Roeder JJ, Fornasieri I, Gosset D. 2002. Conflict and postconflict behaviour in two lemur species with different social organizations (Eulemur fulvus and Eulemur macaco): a study of captive groups. Aggr. Behav. 28, 62–74 10.1002/ab.90006 (doi:10.1002/ab.90006) [DOI] [Google Scholar]
- 81.Hare B, Wobber V, Wrangham R. 2012. The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Anim. Behav. 83, 573–578 10.1016/j.anbehav.2011.12.007 (doi:10.1016/j.anbehav.2011.12.007) [DOI] [Google Scholar]
- 82.Pelto PJ. 1968. The differences between ‘tight’ and ‘loose’ societies. Trans. Action 5, 37–40 [Google Scholar]
- 83.Gelfand MJ, et al. 2011. Differences between tight and loose cultures: a 33-nation study. Science 332, 1100–1104 10.1126/science.1197754 (doi:10.1126/science.1197754) [DOI] [PubMed] [Google Scholar]
- 84.Hemelrijk CK, Wantia J. 2005. Individual variation by self-organisation. Neurosci. Biobehav. Rev. 29, 125–136 10.1016/j.neubiorev.2004.07.003 (doi:10.1016/j.neubiorev.2004.07.003) [DOI] [PubMed] [Google Scholar]
- 85.Price T. 1997. Correlated evolution and independent contrasts. Phil. Trans. R. Soc. Lond. B 352, 519–529 10.1098/rstb.1997.0036 (doi:10.1098/rstb.1997.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Capitanio JP. 2004. Personality factors between and within species. In Macaque societies: a model for the study of social organization (eds Thierry B, Singh M, Kaumans W.), pp. 13–37 Cambridge, UK: Cambridge University Press [Google Scholar]
- 87.Weinstein TAR, Capitanio JP. 2008. Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Anim. Behav. 76, 455–465 10.1016/j.anbehav.2008.01.024 (doi:10.1016/j.anbehav.2008.01.024) [DOI] [PMC free article] [PubMed] [Google Scholar]