Abstract
The comprehensive understanding of individual variation in behavioural profiles is a current and timely topic not only in behavioural ecology, but also in biopsychological and biomedical research. This study focuses on the shaping of behavioural profiles by the social environment in mammals. We review evidence that the shaping of behavioural profiles occurs from the prenatal phase through adolescence and beyond. We focus specifically on adolescence, a sensitive phase during which environmental stimuli have distinctive effects on the modulation of behavioural profiles. We discuss causation, in particular, how behavioural profiles are shaped by social stimuli through behavioural and neuroendocrine processes. We postulate a central role for maternal hormones during the prenatal phase, for maternal behaviour during lactation and for the interaction of testosterone and stress hormones during adolescence. We refer to evolutionary history and attempt to place developmental shaping into broader evolutionary historical trends. Finally, we address survival value. We argue that the shaping of behavioural profiles by environmental stimuli from the prenatal phase through adolescence represents an effective mechanism for repeated and rapid adaptation during the lifetime. Notably, the adolescent phase may provide a last chance for correction if the future environment deviates from that predicted in earlier phases.
Keywords: adaptive behaviour, adolescence, behavioural development, hormones, maternal influences, prenatal
1. Introduction
The behavioural profile represents the whole array of an individual's characteristics related to behavioural traits, including social behaviours, cognitive abilities, emotions, as well as stress responses (cf. [1]). Behavioural profile may be considered synonymous with behavioural phenotype. This term encompasses personality, behavioural syndrome and temperament [2,3] and does not imply any specific underlying mechanisms or mediators (e.g. epigenetics, maternal effects). Behavioural profiles may vary conspicuously between members of the same species. Understanding such variation is of major importance because it is frequently related to differences in reproductive success, susceptibility to disease and quality of life [4].
Individual differences in behavioural profiles can be traced, on the one hand, to differences in genotype. On the other hand, they can be profoundly influenced by the environment in which the individual lives [5]. In this context, the social environment seems to be of particular importance: it can promote welfare and health (e.g. through effects of social support [6]), but it can also result in severe stress, eventually leading to disease and even death (e.g. in the case of social defeat, social instability or crowding [7]).
Most research on the development of behavioural profiles has focused on early phases or stages of life, in particular, the prenatal phase and the early postnatal phase, i.e. the time from birth until weaning. During this time, brain circuits are highly plastic, and the organism seems to be most susceptible to external influences [8]. For example, stressors that impinge on the maternal organism during pregnancy evoke high levels of anxiety in the offspring in later life [9], as does an adverse early postnatal environment [10]. However, circuits involving the prefrontal cortex and parts of the limbic system, such as the hippocampus and amygdala—which appear to mediate many of these effects—seem to retain their plasticity into adulthood. Presumably this would permit behavioural profiles also to be shaped by environmental influences during later phases of life. In particular, adolescence seems to provide an opportunity for substantial behaviour modulation [4].
The shaping of behavioural profiles during development has frequently been studied in biomedical and experimental psychological research that aims at understanding the pathological consequences of early adverse conditions. In this field, the characteristic traits of individuals exposed to environmental stressors during pregnancy and lactation are often regarded as deviations from the norm, or even as pathological. Indeed, studies in animals and humans clearly show that severe stressors acting either upon a pregnant female or directly on the offspring during early phases of life can have profoundly negative consequences for later development, including physical and mental health [11,12]. Nonetheless, recent experimental animal studies on the effects of moderate levels of stress on behavioural development, when considered from an evolutionary perspective, suggest an alternative perspective: that variation in behavioural phenotype brought about by stressors can represent an adaptation to the prevailing and/or future environmental situation for that individual (see review in Sachser et al. [4]). From this point of view, deviations from the behavioural and physiological standard (e.g. heightened levels of anxiety, increased cortisol responses to stressors, masculinization of daughters, feminization of sons; [13,14]) should not necessarily be regarded as pathological, but rather may be seen as representing adaptations to the offspring's likely environment. While the emphasis in these studies is often on stress, many of the stressors are social in nature. Moreover, effects of stress on later behavioural profiles are often mediated by social interactions, particularly with the mother.
Levels of anxiety, aggressiveness and stress responses can also be significantly influenced by stressful and social experiences in adolescence [15–17]. Very recently, it was argued that such shaping of behavioural profiles by social stress during adolescence might also be an instance of adaptive developmental plasticity [4,18]. From this perspective, for example, high aggressiveness brought about by a lack of social contact during adolescence would not be seen as a behavioural disorder or a behavioural by-product of some effect on neural systems. Rather it might be viewed as part of a resource defence strategy that would be successful under specific ecological conditions at low individual numbers.
This contribution is about the shaping of behavioural profiles during development in mammals. The focus will be on the effects of the social environment, that is, the presence of, and interaction with, members of the same species. In a first step, we will address the prenatal and early postnatal phases and briefly summarize, extend and discuss a recently developed model for the adaptive modulation of behavioural profile during these phases of life. In a second step, we will then focus on adolescence and, in particular, summarize recent experiments showing that variation in the social environment during this time can trigger a newly identified neuroendocrine mechanism that results in behavioural profiles and strategies that appear adaptive for specific environmental conditions. In a third step, we will suggest how these ideas might apply across species as well as across the lifespan. Finally, we will summarize how Tinbergen's four questions [19] provide the framework for a comprehensive understanding of developmental shaping of behavioural profiles.
2. The shaping of behavioural profiles during early phases of life
A schematic of the adaptive modulation of behavioural profiles during early phases of life is presented in figure 1. Stimuli from the social environment act on the pregnant/lactating female to influence maternal physiology and behaviour, and in this way shape foetal and infant brain development. As a consequence, differences in the environments experienced by mothers are translated into phenotypic variation in the offspring (see reviews in references [4,13]). Thereby, mothers try to maximize their own Darwinian fitness by adjusting their offspring to better meet current or future conditions [20]. There seems to be no fundamental difference between the prenatal and early postnatal phases in terms of the consequences of maternal effects for offspring behavioural profile. In both situations, the mother influences offspring development. The underlying mechanisms, however, vary considerably.
Figure 1.
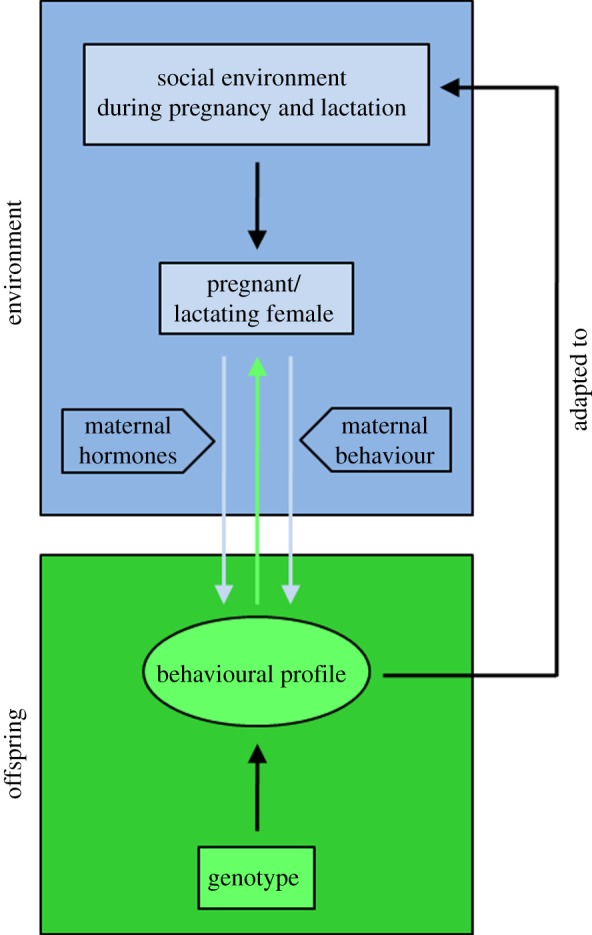
Adaptive modulation of behavioural profile during early phases of life. The behavioural profile of the offspring is affected by his/her genotype as well as the social environment in which the mother lives during pregnancy and lactation. Social environmental factors act on the pregnant and lactating female. The behavioural profile of offspring can be shaped via maternal hormones and/or maternal behaviour and thus be adapted to the environment in which the mother lives. The arrow from ‘offspring’ to ‘pregnant/lactating female’ indicates that the foetus/infant is not a passive recipient of the maternal signals, but rather signalling is bidirectional (for details see text). Redrawn after Sachser et al. [4] with permission from Elsevier.
During the prenatal phase, the maternal organism responds with hormonal changes to environmental stimuli. These hormones circulate within the maternal bloodstream, and can at least, in part, cross the placenta. In this way, foetal endocrine state and brain development can be affected (see review in Kaiser & Sachser [13]). But while it is generally accepted that effects of the social environment in which the mother lives during pregnancy on offspring behavioural profile are mediated by maternal hormones, the details are far from being understood. Different hypotheses exist concerning the specific neuroendocrine pathways. Most authors favour a role for the maternal hypothalamic–pituitary–adrenal (HPA) system in mediating the effects of stress during pregnancy on offspring development [21,22]. It is argued that stressors activate the maternal HPA axis, resulting in elevated plasma glucocorticoid concentrations [21]. These then affect foetal brain development by, for example, permanently altering offspring glucocorticoid receptor expression in the amygdala [23,24], which, in turn, is associated with an anxiogenic behavioural profile [25]. Elevated glucocorticoid concentrations can also exert their effects in different ways, for instance, by eliminating or attenuating the testosterone (T) surge in the male foetus [26]. As a consequence, less pronounced male-typical traits are expressed. Others have suggested alternative pathways that may not involve the HPA system. One hypothesis is that social stress during pregnancy activates the maternal sympathetic–adrenomedullary system, which suppresses maternal androgen secretion to lead to disrupted steroid activity in the foetus [27,28]. In summary, the data at present suggest different neuroendocrine pathways that may underlie the modulation of behavioural profiles during the prenatal phase.
During the early postnatal phase, the mother shapes the phenotype of her young through different mechanisms. Maternal hormones no longer pass directly from mother to foetus, though hormones and other constituents in the milk are now potential means of shaping the infant's behavioural profile [29]. However, the biggest change is that the mother is now truly a social stimulus and the most dominant, crucial, stable and predictable element of the infant's social environment. As a result, the nature of the infant's relation with the mother, specific behaviours she directs towards the young, as well as periods of separation from her, represent potential means by which the mother can and does modulate later behavioural profiles of her offspring. The central feature of the infant–mother relation in many species is an attachment process, through which the young develops a powerful attraction to the mother, and by which she comes to provide security for her infant [30]. It has long been known that the security provided by the mother can influence the offspring's behaviour when alone [31]. In humans, social behaviour in a variety of domains appears to be shaped by the relative security provided by the early attachment relation [32]. Greater relative security, in turn, appears to stem from a caring, sensitive mothering style [33].
Some of the most detailed descriptions of the mechanisms by which early postnatal social stimulation shapes the offspring are from the study of the effects of variation in the specific maternal behaviours provided by lactating rats. This variation appears to arise both from individual differences and as a result of environmental challenge [34]. Simple differences in the amount of maternal licking and other stimulation, together with the form of the nursing posture, appear to produce widespread and stable changes in DNA methylation and gene expression that alter development of an assortment of neural, neurochemical and endocrine outcomes that underlie HPA responsiveness as well as defensive behaviour and female reproductive function [35,36]. Although maternal influences are pervasive, other animals may also shape the development of behavioural profiles. Depending on the species, additional social influences exerted on the infant can emanate from littermates, other siblings, fathers and other related and unrelated adults and juveniles [37–39]. While we know much less about how these additional social partners might shape later behavioural profiles, studies of the mechanisms activated by maternal care provide a template for future research.
A critical link in our model is the correspondence between the nature of the environment experienced by the mother and the information she provides her young. In other words, how is information about the environment translated into appropriate signals. In a broad way, this connection seems very understandable. As described earlier, the maternal organism responds with hormonal changes to environmental stimuli during the prenatal phase. Thus, more aversive environments should be associated with repeated or prolonged elevations of maternal stress hormones, which can influence foetal endocrine state and brain development (see review in [13]). Furthermore, there are several studies that show a clear connection between the environment in which the mother lives and the quality of maternal care. In bighorn sheep, high population density results in reduced maternal care, which is associated with reduced body mass of the offspring [40]. In mice, the presence of odour cues from potentially infanticidal males decreases specific aspects of maternal behaviour [41]. Moreover, exposing female mice to unfamiliar male bedding at various points during pregnancy and lactation both increases maternal corticosterone levels prenatally and, reduces maternal care (licking, grooming, arched back nursing) during the postnatal phase [42]. Notably, at least, in one preceding study exposure to male bedding led to a more anxious and less explorative behavioural profile in the offspring in later life [1]. Other work has shown that exposing females to stressors just during pregnancy can affect maternal behaviour displayed during lactation: in rats, for example, restraint during pregnancy reduced the licking and grooming that a subset of the females subsequently exhibited towards their pups [43].
The model of early shaping, as depicted in our previous paper [4], may give the impression that the foetus/infant is a passive recipient of the signals shaping its future behavioural profile. It has long been appreciated, however, that care provided by the mother can be elicited by signals or cues from the young [44]. Further, the notion of conflict between infant and mother for nutritional and other resources has been widely recognized since Trivers' [45] classic treatise. While it is in the best interests of the offspring to receive the most accurate prediction of future conditions, and thereby optimize its own survival and reproductive fitness, the mother's best interests are determined by her lifetime reproductive success, rather than by the survival and breeding of any particular offspring. If providing totally veridical information to the young has potential costs to the mother's future reproductive success (e.g. reduced metabolic resources, increased risk of predation), then one might expect the mother to furnish information that is biased towards her optimum. These differences in relative advantage for mother and offspring are hypothesized to play out in conflict beginning prenatally between the mother and foetus [46], with each member of the dyad exerting countermeasures to shift the information pertaining to the offspring phenotype towards their own optimal value. After birth, the conflict between mother and offspring continues into postnatal life [47]. Accordingly, we have modified our model with an arrow from ‘offspring’ to ‘pregnant/lactating female’ in figure 1 to indicate an active role of the foetus/infant in this conflict between the shaping influences of the mother and the eliciting of shaping influences by the young to produce a future behavioural phenotype. In this competition, the infant appears able to potently elicit beneficial responses even from unrelated individuals who have nothing to gain biologically from the interaction [48,49].
Our model to this point may suggest a certain uniformity in outcome. That is, all mothers in a particular environment would perceive comparable information and provide similar information to their young. Likewise, all offspring would have similar interests, and so would seemingly exert similar countermeasures to the mother's influences. The result would seem to be a population of young with very similar behavioural profiles. Empirical data, however, show an opposite picture; that is, offspring expressing a range of behavioural profiles not only in their natural habitats but also under highly standardized laboratory conditions [50]. Recent studies with specific genetic polymorphisms illustrate examples of gene by environment interactions through which such variation might be generated.
For example, a polymorphism in the serotonin transporter (5-HTT) gene regulatory region results in allelic variation of 5-HTT expression and function [51]. The 5-HTT affects the availability of the neurotransmitter serotonin in the synapse by returning serotonin to the presynaptic terminal, and is a key element in the regulation of social behaviours, cognitive abilities, emotional traits and stress responses (see review in Canli & Lesch [52]). In humans, the 5-HTT gene appears in two length variants that differ in their efficiency (‘short’—less efficient; ‘long’—more efficient). Carriers of at least one short 5-HTT gene variant display higher levels of neuroticism and harm-avoidance [51], as well as higher trait anxiety [53], than do homozygous carriers of the long variant. Of particular interest here is that phenotypic consequences of the 5-HTT polymorphism in humans seem to depend critically on environmental influences, including influences during early development. In a widely cited study [54], the impact of stressors varied systematically across subgroups of individuals possessing different alleles of the 5-HTT gene. Stressors increased depressive symptoms when individuals possessed the short allele, and especially when they were homozygous. Studies of either monkeys possessing orthologues of the 5-HTT gene [55], or mice in which this gene has been deleted (see reviews in references [1,56]) support these findings. Both short alleles/deleted genes and early adversity, especially when they occurred in combination, promoted depressive-like and anxiety-like behaviour. Carola et al. [57] reported, for example, that heterozygous 5-HTT knockout mice showed a more pronounced increase in anxiety-related behaviour after the experience of low maternal care than did wild-type mice. Further, when lactating mice lived in a threatening environment during pregnancy and lactation, offspring showed increased anxiety-like behaviour and reduced exploratory locomotion compared with offspring of mothers who lived in a safe environment, and these effects were most pronounced in homozygous 5-HTT knockout mice when compared with wild-types [58]. Thus, such gene by environment interactions clearly illustrate how social influences might shape a range of behavioural profiles. Given that other such environmentally sensitive polymorphisms are now coming to light (e.g. for monoamine oxidase A, catechol-O-methyltransferase, neuropeptide S receptor [59,60]) and the likelihood that many more remain to be identified, the variation that could be generated would seem to be substantial.
According to the evolutionary argument, behavioural profiles shaped early in life have adaptive consequences under conditions in which the adult environment matches that experienced during the early phases. In rats, reduced licking and grooming of pups can occur in challenging environments [43]. Pups receiving such reduced care from their mother exhibit increased HPA activation to stressors and defensive behaviour responses in adulthood [36,61]. The females, in addition, show enhanced reproductive activity [62]. It is hypothesized that increased HPA activity may allow animals of both sexes to better cope with the ‘predicted’ challenging environment in adulthood [36]. Similarly, greater fecundity (as opposed to greater investment in few offspring) is hypothesized to favour females in these circumstances [62]. In guinea pigs, daughters whose mothers lived in an unstable social environment during pregnancy and lactation show a masculinized pattern that comprises behaviour, endocrine system as well as brain development, whereas sons showed a delayed development and a less-pronounced expression of male-typical traits (see review in [13]). Based on the behavioural ecology of the wild cavy, the progenitor of the guinea pig, it was argued that these distinct reproductive strategies—masculinization of females and biobehavioural developmental delay of males—may best serve their respective needs when populations expand and competition among species members increases. At low individual numbers, more-typical ‘male’ and ‘female’ patterns would be of advantage [13,14].
Although the notion that behavioural profiles can be shaped in earlier stages of development has become widespread and more broadly accepted, current studies—including our own—typically rely on conjectures concerning the adaptive value of the changes observed. Carefully designed experiments to test the reproductive fitness of adult animals in environments that have been hypothesized to either match or not match the early environment would go a long ways towards advancing our knowledge in this field. Only a few of these ‘match–mismatch’ experiments are to be found in the literature. In rats, for example, offspring of low maternal care dams (indicative of an adverse postnatal environment) generally show lower cognitive performance under basal (non-stress) conditions when compared with offspring of high-maternal care dams [63]. However, a different outcome is observed when these rats are tested under stressful conditions, i.e. a situation reminiscent of their early postnatal environment. Within a stressful context (e.g. fear conditioning task), adult offspring that received low maternal care during early life perform better than adult offspring of high-maternal-care dams [64,65].
3. The shaping of behavioural profiles during adolescence
There is increasing evidence that adolescence, that is, the gradual transition from childhood to adulthood, also represents an additional sensitive period (beyond the prenatal and early postnatal periods) in which behavioural profiles are routinely and profoundly shaped by social events. In rats and mice, social stressors, or even disruption of social activities at this time, have been found to produce a variety of later alterations in behaviour, central neural and neurochemical activity, as well as neuroendocrine stress responses [66–69]. In the golden hamster, exposure to aggressive adults during early adolescence accelerates the development of offensive aggression and results in a higher frequency of attacking conspecifics in adulthood. By contrast, exposure to aggressive adults in late adolescence inhibits offensive aggression [16,70]. Another important factor is social play, a major source of social experience during the adolescent period of many mammals [71,72]. In rats, for example, play is indispensable for an adequate development of coping with social challenges and it influences both behaviour [73] and neural organization [74]. In guinea pigs, a causal relationship exists between social experiences during adolescence, aggressive behaviour as adults, and the degree of social stress in chronic encounters. Males that have lived through adolescence in large mixed-sex colonies acquire the social skills to establish stable dominance relationships without displaying overt aggression and, with the help of this low-aggressive behavioural profile, they can integrate into groups of unfamiliar animals in a non-stressful way. A completely different picture emerges if males have lived in pairs consisting of one male and one female since early adolescence. They become increasingly incompatible with unfamiliar males and develop a behavioural profile of high aggression [4,15,75,76].
Recently, it was argued from an evolutionary point of view that the different behavioural profiles of colony- and pair-raised guinea pig males represent adjustments to different social situations [4,18]. Indeed, the social organization of guinea pigs is highly flexible and density-dependent male reproductive strategies have been described [77]. Under low-density conditions with only a few males of the same age and some females, the best strategy to maximize reproductive success appears to be to fight for dominance and defend the mating partner. The high aggressiveness of males living in male–female pairs might represent an adaptation to this situation. In the ancestor of the guinea pig, the wild cavy, a comparable situation exists in the natural habitat at low population densities [78]. A very different situation exists when animals live at high densities in large, age-graded populations: In the guinea pig, as in many species, high-ranking (alpha) males almost exclusively sire the offspring under these conditions. Remarkably, males usually do not attain an alpha position until well beyond the age of sexual maturity [77,79]. Thus, it would seem that a male born in a high-density population should avoid agonistic encounters at too early an age because they will not result in reproductive success. Rather a queuing strategy would seem promising. That is, males should minimize aggressiveness until their physical prowess relative to other males suggests the likelihood of success in dominance interactions. Accordingly, the low aggressiveness of colony-housed males might promote this strategy, which, incidentally, is also found at high populations in the wild cavy in its natural habitat [80]. Thus, social experiences during adolescence shape behavioural strategies that, indeed, might represent adaptations to the current and future social conditions.
Concerning underlying mechanisms, a key component of the age-related changes in behaviour may be the maturation of the HPA axis [81]. On the one hand, there is some evidence for increased physiological stress responsiveness in animal and human adolescents (see reviews in [82,83]). On the other hand, there are indicators that during this time stress responsiveness can actually be blunted: in periadolescent mice, the HPA response to novelty is lower than in adults [84], and periadolescent rats have a lower, although prolonged, corticosterone response compared with adult males [85]. Moreover, a period of cortisol (C) response suppression was discovered in maturing male guinea pigs [86]. This period coincides with the time when males begin to display social skills that allow them to negotiate potentially dangerous interactions with other males [15,76]; thus, a change in stress responsiveness might somehow facilitate the acquisition or expression of these behavioural skills. Of particular interest is a recent study showing that the magnitude of stress responsiveness during adolescence depends on social experiences during this phase of life [75]: late adolescent colony-housed guinea pig males display a significantly smaller increase in C levels in a novel environment than equally aged males kept only with a female since weaning. Thus, social experiences during adolescence are not only related to differential behavioural profiles of high and low aggressiveness, but also to differential C stress responses.
The social modulation of behavioural profile during adolescence also coincides with significant changes in T secretion. Male guinea pigs living in large mixed-sex colonies during adolescence are continually involved in social interactions and show relatively high circulating T concentrations [87,88]. Although they are sexually mature, they do not yet reproduce under these social conditions [77,89]. By contrast, males of the same age that live in male–female pairs are involved in fewer social interactions, have low T concentrations, and reproduce successfully [88]. These findings should not be surprising since it is well known and predicted by the ‘challenge hypothesis’ that social interactions—courting and particularly aggressive encounters—result in increased T levels [90–92].
Based on this research, we recently suggested a more general model of the adaptive modulation of behavioural profile in adolescent males in which we integrated mechanisms and function (figure 2). The model predicts that the frequency and intensity of social interactions during adolescence modulate T secretion. In turn, the amount of secreted T organizes the degree of C responsiveness during late adolescence, which controls the intensity of offensive aggressive behaviour towards competitors. As a consequence, the behavioural profiles of high and low aggressiveness that are shaped are part of behavioural/reproductive strategies that adjust the individual to different social situations: a fighting/resource defence (early reproduction) strategy in low-density situations and a queuing (later reproduction) strategy in high-density situations [4].
Figure 2.
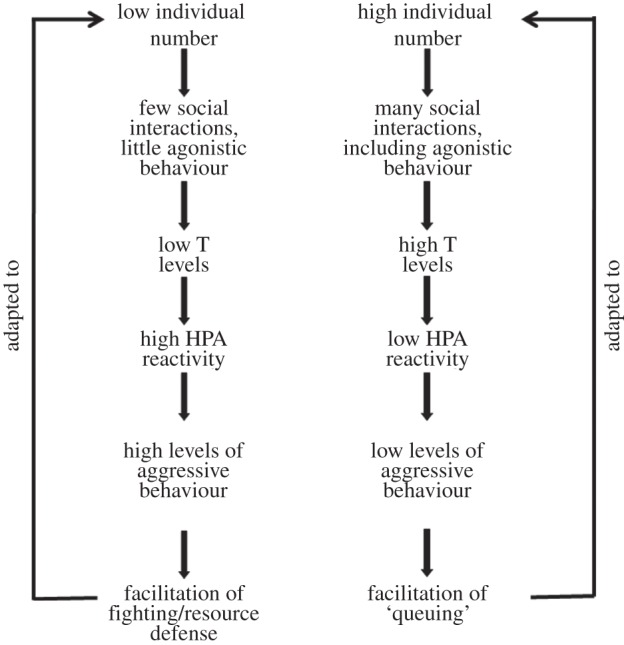
Adaptive modulation of behavioural profile in adolescent males. Variation in social environment (low versus high individual numbers) during this phase of life triggers endocrine pathways that result in behavioural profiles and strategies that match the current environmental situations. Note that concerning testosterone a specific organizational effect on HPA responsiveness is suggested that probably occurs at just this period of life. Possible activational effects of increased testosterone on aggression are not addressed. Reprinted from Sachser et al. [4] with permission from Elsevier.
During the past several years, portions of the hypotheses underlying this model have been tested in a series of experiments in the guinea pig. First, the social modulation of T concentrations during adolescence and its effects on C responsiveness were studied [93]. For this purpose, three groups of experimental animals were established that differed in the amount and kind of interaction they had with conspecifics during adolescence: (i) pair-housed males living with only one female, (ii) pair-housed males living with one female but having limited opportunities to interact with unfamiliar animals of both sexes and (iii) colony-housed males experiencing continuous social interactions with many males and females. In late adolescence, the C response was higher in totally pair-housed, than in colony-housed, males, whereas T concentrations showed the opposite pattern. Interestingly, social stimulation of pair-housed males—interaction with an unfamiliar animal twice a week for 10 min each for five weeks—caused a significant acute increase in T concentrations and reduced the C stress response to a level intermediate between that of colony- and pair-housed males. We hypothesized that with greater social stimulation the effects on stress responsiveness would intensify. Accordingly, in a second study, we compared pair-housed males without additional social stimulation with pair-housed males that received twice as much social stimulation during adolescence as in the previous study [94]. Indeed, C responses during late adolescence were significantly lower in pair-housed males that had received additional social stimulation than in pair-housed males living with one female and no further stimulation (figure 3a). In conclusion, these two experiments furnished conclusive evidence that the reduced C stress response observed in late adolescent male guinea pigs living in large mixed-sex colonies is causally related to social interactions. Furthermore, social interactions with adult animals of both sexes increase T secretion, which agrees with the idea that the reduced stress responsiveness may be mediated via elevated T levels over the course of adolescence.
Figure 3.
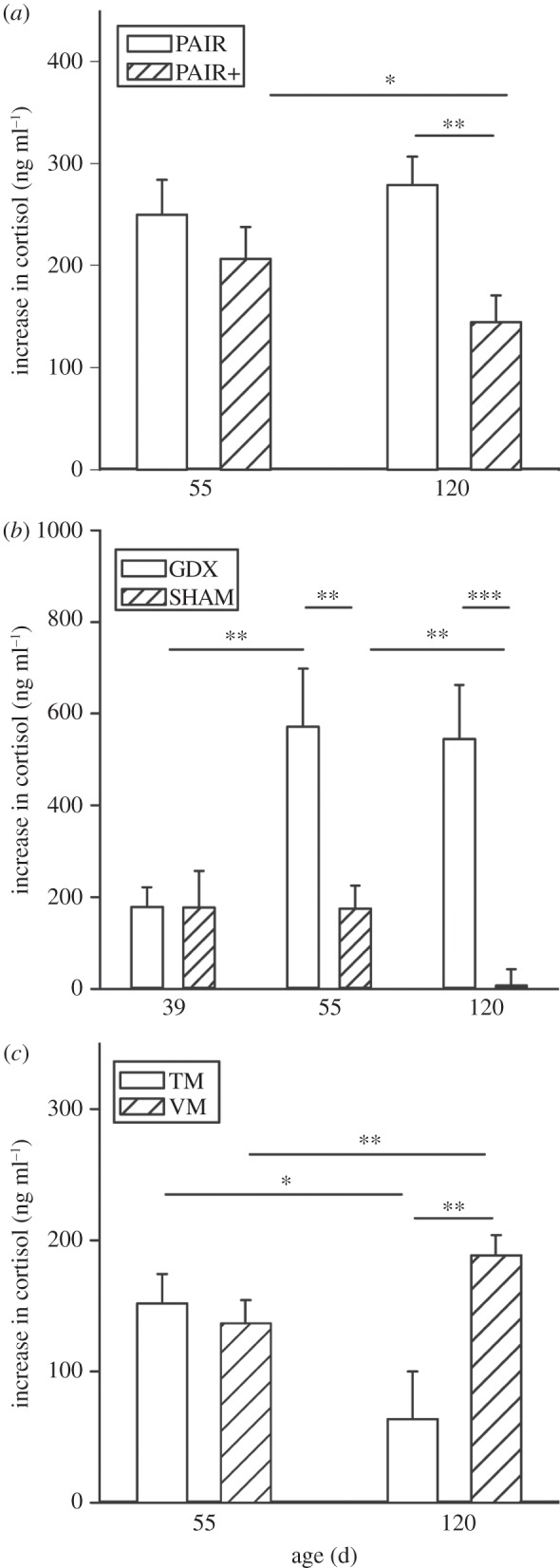
Increase in plasma cortisol levels (ng ml−1) 1 h after the onset of a psychological stressor (novel environment). Indicated are mean and s.e. of mean. Statistics: mixed factorial ANOVA, post hoc testing: Bonferroni–Holm corrected independent-samples as well as paired-samples t-tests. (a) PAIR, pair-housed males; PAIR+, pair-housed males with additional social stimulation. Statistics: main effect of group, p = 0.024; interaction of age × group, p = 0.049; n = 11. *p < 0.05; **p < 0.01; ***p < 0.001. (b) GDX, gonadectomized males; SHAM, sham-gonadectomized males. Statistics: main effect of age, p = 0.033; main effect of group, p = 0.007; interaction of age × group, p = 0.001. nGDX = 8, nSHAM = 7. **p ≤ 0.01; ***p ≤ 0.001. (c) TM, males treated with testosterone undecanoate; VM, males treated with vehicle. Statistics: interaction of age × group, p = 0.002. n = 12. *p ≤ 0.05; **p ≤ 0.01. Redrawn after [94,95] with permission from Elsevier.
To this point, the evidence for a possible relationship between high T levels and a decreased stress response was only correlative, although inhibiting effects of T on HPA function have been described [96,97]. Therefore, we next investigated whether T indeed had an inhibitory effect on C stress responsiveness during adolescence [95]. For this purpose, two independent experiments were conducted. In the first, colony-housed males—which usually have high T levels—were either gonadectomized (GDX) or sham-GDX (SHAM) during early adolescence, that is, before sexual maturity. In agreement with our hypothesis, GDX males showed a significantly increased C stress response in comparison to SHAM males (figure 3b). In the second experiment, pair-housed males—which usually have low T levels—were injected with either a T depot (T undecanoate) or with vehicle (VM). Indeed, T-injected males had a significantly lower C stress response than vehicle-injected males (figure 3c). Both experiments thus confirm an inhibiting effect of T on the C response during adolescence. Taken together, the findings of these experiments establish a causal chain, in which the frequency and intensity of social interactions during adolescence regulate T secretion, which, in turn, organizes the acute C response. Adolescence can thus be regarded as a crucial time window for the modulation of C responses by social interactions.
Reduced stress responsiveness has been hypothesized to facilitate the acquisition of behavioural patterns that are beneficial for life in a group [86]. Because a short-term increase in glucocorticoid concentrations can trigger enhanced aggressive behaviour (rats [98], hamsters [99]), a reduced C response might favour non-aggressive behavioural strategies. In preliminary work on the effects of acute elevations of C levels on aggressive behaviour, colony-housed males were treated with either ACTH or vehicle during late adolescence. Two hours after application, ACTH-treated males had significantly higher C values than vehicle-treated guinea pigs. In accordance with our hypothesis, significantly higher frequencies of aggressive behaviour also occurred in the group treated with ACTH. These findings agree with our general hypothesis, though they do not allow discrimination between the effects of ACTH and C [100].
In summary, the data presented here provide evidence for a heretofore unknown neuroendocrine mechanism that underlies the development of different behavioural profiles and stress responsiveness during adolescence: the quality/quantity of social interactions during adolescence seem to trigger T secretion, which, in turn, influences the C response and consequently the regulation of aggressive behaviour. Although the model has been established in the guinea pig, we predict that similar mechanisms exist in other group-living mammalian species. Because the number of social interactions will vary with population density, and because different strategies are likely to be adaptive at different densities, social interactions afford a useful cue for shaping behavioural profiles.
A major future aim is to better elucidate the predicted adaptive significance of this phenomenon. More specifically, we need to answer more fully the question of whether the behavioural alterations and canalizations that occur during adolescence are the by-products of physiological processes or whether they can represent adaptations to the environmental conditions in which the individuals live. As argued for the modulation of behavioural profiles during early phases of life, match–mismatch experiments, including the measurement of reproductive success are urgently needed if we are to draw firm conclusions regarding the true adaptiveness of shifts in social patterns during adolescence.
The findings reported here also further reinforce the idea that during adolescence, as is the case during the prenatal and early postnatal stages, developmental processes are particularly sensitive to environmental inputs. Owing to a lack of appropriate control studies, it is not clear, however, whether or not the environment can also shape behavioural profiles in the same way during later phases of life, that is, in full adulthood. Buwalda et al. [101], for example, conclude in a recent review that data do not suggest that adolescents are particularly vulnerable to the negative consequences of stress, because long-lasting effects of stress also occur in other phases of life. At the same time, they emphasize the high resilience of adolescent animals to developing psychopathological changes in behaviour after being exposed to stress. On the other hand, if animals are, in fact, more sensitive to environmental inputs during adolescence than at later ages, it is unclear whether adolescence represents a sensitive period that is distinct from that of the early phases. In the study of sexual differentiation, organizational effects of testosterone are now known to occur not only during the perinatal period, but also during adolescence. In that case, evidence suggests that the early sensitive period for testosterone continues, though diminishes in strength, as animals reach adolescence [102]. It is possible that a similar single, but diminishing, sensitive period underlies the fine tuning of behavioural profiles during adolescence, though this suggestion implies a commonality in the underlying substrate for the prenatal, early postnatal and adolescent periods. Such a commonality has yet to be clearly demonstrated.
Just as we expect behavioural profiles shaped during the perinatal period to be modulated by gene by environment interactions, so too do we expect such modulation during adolescence. For instance, in Caspi et al.'s [54] study, 5-HTT polymorphisms interacted with stressors to modulate later depressive symptoms, regardless of whether the stressors were experienced during early life or during the teenage years. As mentioned earlier, early-life adversity promotes anxiety-like behaviour, especially in mice containing a deletion for the 5-HTT gene [1,56]. In addition, evidence indicates that during adolescence the interaction between the 5-HTT genotype and social experience might again modulate behavioural profiles. Homozygous 5-HTT knockouts, but not heterozygote or wild-type mice, that had experienced social defeat at 68–70 days of age (which might be considered late adolescence in this species) exhibited increased anxiety-like behaviour and reduced exploration [103]. Moreover, when mice of about that age were confronted with a docile opponent in their own territory, the opponent's territory or in a neutral area, clear variation of behavioural strategies occurred: the amount of aggression shown by homozygous 5-HTT knockout mice was influenced by both the venue and the opponent's behaviour, whereas heterozygotes reacted only to the venue. Strikingly, wild-type mice always behaved the same way, irrespective of venue and opponent [104]. Thus, as illustrated by this single gene, interactions between environment and gene expression can have profound and pervasive modulating effects on the development of behavioural profiles and behavioural strategies during adolescence.
4. The shaping of behavioural profile across species and the lifespan
In this review, we have suggested that behavioural profiles are constantly being shaped by social figures during development so as to be better adapted for present or future environments. Information correlated with the likely future environment is initially transmitted to the young by the mother's hormones, and later, by her behaviour. Once independent, the adolescent animal derives information directly from its social setting, and uses that information to help it further adapt to take fuller advantage of the opportunities that particular environments present, and to more effectively meet the challenges that they pose.
We are aware that this is an oversimplified view on the shaping of behavioural profiles and that important omissions exist. Sex, for example, needs to be more fully integrated into this conceptual framework.
So far, we have primarily used examples from laboratory rodents, in which most of the experimental results have been obtained. Yet, we imagine that the processes we describe hold more broadly across mammalian species. Certainly, the particulars are likely to vary along many dimensions. Perhaps most obviously, we would expect the processes to be more prominent in gregarious species than in those that are more solitary. Because the degree of competition among conspecifics would seem to be one critical aspect of the environment to which individuals need to adapt to reproduce successfully, we might also expect greater social shaping of behavioural profiles in those species subject to large yearly fluctuations in population density.
Differences also exist in when the most shaping occurs. As described earlier, guinea pigs appear to be shaped more by prenatal influences, whereas rats and mice appear to be shaped primarily by their mother postnatally. Such differences may often be explained simply by the relative lengths of gestation in the different species, or expressed differently, by the differences in the timing of birth relative to development. For the guinea pig, with a gestation period of about nine to 10 weeks, pups are highly developed at birth. They require little in the way of active maternal care, and so opportunities for maternal shaping of the infant's behaviour are relatively limited. On the other hand, the long gestation, during which most neural and other development occurs, is an opportune time for the endocrine signals of the mothers to influence the behavioural development of their young. Laboratory rats and mice are, by contrast, born after a short, three-week gestation. Much neural development occurs after birth, and the young require substantial active maternal care to ensure survival. So for the rat, mouse and similar altricial species, the early postnatal period is an especially favourable time for the mother to exert her influence over development of her young. This comparison of altricial and precocial species also can be seen as placing the social modulation of behavioural profiles during development into broader evolutionary historical trends. That is, it exemplifies how currently available mechanisms in particular species are likely to be influenced by their ancestry. However, systematic comparative studies on the shaping of behavioural profiles, which are needed to elucidate the evolutionary history of developmental shaping, are largely lacking to date.
The three stages discussed—the prenatal, early postnatal and adolescent—are clearly demarcated by the relative development and independence of the young and the completely different immediate environment characterizing each stage. Nonetheless, dividing the developmental process into well-defined phases is artificial in that it downplays the continuity and inter-dependence among the stages. For instance, much of prenatal shaping may increase postnatal plasticity, which then enhances opportunities for the mother to further shape behaviour after birth [105]. In addition, the timing of the onset of the adolescent stage may itself depend on the continued motivation for the mother and young to remain in proximity, which in turn, may depend on environmental conditions, such as the availability of mating partners or nutritional resources [106]. In this way, social cues influencing the timing of the transition from one phase to the next may represent a form of shaping that could potentially enhance future adaptation. Furthermore, adversity during the prenatal and early postnatal phases can make the individual more susceptible to the effects of adversity later in life, including the time of adolescence. (This so-called ‘double-hit hypothesis’ was first described by Bayer et al. [107]). In all these ways, information obtained during one stage may influence the shaping that occurs at later stages.
While most authors have focused on shaping during the prenatal and early postnatal phases, we have emphasized adolescence. At this developmental stage, the individual is receiving information about the environment directly, rather than indirectly through the mother. Moreover, the adolescent is entering, or about to enter, reproductive maturity and the environment in which reproduction will occur. Thus, adolescence appears to be a critical time for corrections and final adjustment before entering the fully adult stage. Specifically, adolescence offers an opportunity for the offspring to readjust in order to compensate for less than veridical information that may have been provided by the mother earlier [4,46]. Further adjustment during adolescence may also be necessary because the environment ‘predicted’ may now be in error: either owing to inaccurate information or its transmittal to the offspring, because environmental conditions have, in fact, changed unpredictably in some way, or because the offspring has emigrated to a new environment, which differs from the old. Moreover, it is possible that some aspects of the behavioural profile are primarily shaped during early phases of life (e.g. levels of anxiety, bold versus shy temperament), whereas others can only be modulated during adolescence (e.g. specific coping strategies related to reproduction). Thus, adolescence not only continues the shaping process that began in earlier phases, but may provide a critical, final opportunity for adjustment as reproductive maturity emerges.
The continuity in the processes by which social stimuli shape behavioural profiles from prenatal to adolescent life raises the question of possible shaping even beyond adolescence. One would imagine that once an individual reaches reproductive maturity that further behavioural adjustment to a more distant future would be superfluous. However, this need not be the case. The environment may change over successive reproductive seasons. Moreover, reproductive maturity alone does not ensure reproduction, and additional behavioural adjustment may be required for animals to achieve reproductive success. Thus, further adjusting of behavioural profile after adolescence appears to be likely.
5. Concluding remarks
Fifty years ago in his seminal publication ‘On aims and methods of ethology’, Tinbergen [19] argued that a comprehensive understanding of behavioural phenomena has to give equal attention to the questions of ontogeny, causation, survival value and evolution, and to their integration. The focus of this paper has been on the shaping of behavioural profiles in mammals. In the sense of Tinbergen, we reviewed when behavioural profiles are modulated during ontogeny and conclude that besides the prenatal and early postnatal phase, adolescence, though poorly studied as yet, may be a sensitive phase during which environmental stimuli have a distinctive effect on the modulation of behavioural profiles. We discussed causation, in particular, how behavioural profiles are shaped by environmental stimuli through mediating behavioural and neuroendocrine processes, and highlight the interaction of testosterone and stress hormones during one stage of ontogeny—adolescence. We addressed the survival value and asked why such a modulation occurs. We argued that the shaping of behavioural profiles by environmental stimuli from the prenatal phase through adolescence appears to represent an effective mechanism for repeated and rapid adaptation. We briefly treated the question of evolution but, so far, systematic comparative studies of the modulation of behavioural profiles during development are largely lacking as are studies that address the integration of the different questions [4]. In summary, we are still far from a comprehensive understanding of the developmental shaping of behavioural phenotypes. But the Tinbergen framework provides us with an excellent roadmap for successful future research on this critical and timely topic.
Acknowledgements
Our studies on the social modulation of behavioural development in wild cavies, guinea pigs and mice were supported continuously by the German Research Foundation (DFG) and are currently supplied by grants to Norbert Sachser (FOR 1232: Sa 389/11-1/11-2; SFB-TRR 58 project A1) and Sylvia Kaiser (FOR 1232: Ka1546/6-1/6-2/7-1/9-1). During preparation of this paper, Michael Hennessy was supported by a Research Visit grant from the DAAD (German Academic Exchange Service) and by a Professional Development Award from Wright State University.
References
- 1.Heiming RS, Sachser N. 2010. Consequences of serotonin transporter genotype and early adversity on behavioral profile: pathology or adaptation? Front. Neurosci. 4, 187. 10.3389/fnins.2010.00187 (doi:10.3389/fnins.2010.00187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sih A, Bell A, Johnson JC. 2004. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 3.Reale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 10.1111/j.1469-185X.2007.00010.x (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 4.Sachser N, Hennessy MB, Kaiser S. 2011. Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neurosci. Biobehav. Rev. 35, 1518–1533 10.1016/j.neubiorev.2010.09.002 (doi:10.1016/j.neubiorev.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 5.Gross C, Hen R. 2004. The developmental origins of anxiety. Nat. Rev. Neurosci. 5, 545–552 10.1038/nrn1429 (doi:10.1038/nrn1429) [DOI] [PubMed] [Google Scholar]
- 6.Hennessy MB, Kaiser S, Sachser N. 2009. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 30, 470–482 10.1016/j.yfrne.2009.06.001 (doi:10.1016/j.yfrne.2009.06.001) [DOI] [PubMed] [Google Scholar]
- 7.Von Holst D. 1998. The concept of stress and its relevance for animal behavior. In Advances in the study of behavior (eds Lehman DS, Hinde R, Shaw E.), pp. 1–131 London, UK: Academic Press [Google Scholar]
- 8.Champagne FA, Curley JP. 2005. How social experiences influence the brain. Curr. Opin. Neurobiol. 15, 704–709 10.1016/j.conb.2005.10.001 (doi:10.1016/j.conb.2005.10.001) [DOI] [PubMed] [Google Scholar]
- 9.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. 2003. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci. Biobehav. Rev. 27, 119–127 10.1016/S0149-7634(03)00014-9 (doi:10.1016/S0149-7634(03)00014-9) [DOI] [PubMed] [Google Scholar]
- 10.Meaney MJ. 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192 10.1146/annurev.neuro.24.1.1161 (doi:10.1146/annurev.neuro.24.1.1161) [DOI] [PubMed] [Google Scholar]
- 11.Harlow HF, Harlow MK. 1962. Social deprivation in monkeys. Sci. Am. 207, 136–146 10.1038/scientificamerican1162-136 (doi:10.1038/scientificamerican1162-136) [DOI] [PubMed] [Google Scholar]
- 12.Heim C, Nemeroff CB. 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49, 1023–1039 10.1016/S0006-3223(01)01157-X (doi:10.1016/S0006-3223(01)01157-X) [DOI] [PubMed] [Google Scholar]
- 13.Kaiser S, Sachser N. 2005. The effects of prenatal social stress on behaviour: mechanisms and function. Neurosci. Biobehav. Rev. 29, 283–294 10.1016/j.neubiorev.2004.09.015 (doi:10.1016/j.neubiorev.2004.09.015) [DOI] [PubMed] [Google Scholar]
- 14.Kaiser S, Sachser N. 2009. Effects of prenatal social stress on offspring development: pathology or adaptation? Curr. Dir. Psychol. Sci. 18, 118–121 10.1111/j.1467-8721.2009.01620.x (doi:10.1111/j.1467-8721.2009.01620.x) [DOI] [Google Scholar]
- 15.Sachser N, Lick C, Stanzel K. 1994. The environment, hormones and aggressive behaviour - a five-year-study in guinea pigs. Psychoneuroendocrinology 19, 697–707 10.1016/0306-4530(94)90051-5 (doi:10.1016/0306-4530(94)90051-5) [DOI] [PubMed] [Google Scholar]
- 16.Delville Y, Melloni RH, Ferris CF. 1998. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. J. Neurosci. 18, 2667–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terranova ML, Cirulli F, Laviola G. 1999. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology 24, 639–656 10.1016/S0306-4530(99)00019-0 (doi:10.1016/S0306-4530(99)00019-0) [DOI] [PubMed] [Google Scholar]
- 18.Sachser N, Kaiser S. 2010. The social modulation of behavioural development. In Animal behavior: evolution and mechanisms (ed. Kappeler P.), pp. 505–536 Berlin, Germany: Springer. [Google Scholar]
- 19.Tinbergen N. 1963. On aims and methods of ethology. Z. Tierpsychol. 20, 410–433 10.1111/j.1439-0310.1963.tb01161.x (doi:10.1111/j.1439-0310.1963.tb01161.x) [DOI] [Google Scholar]
- 20.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 10.1016/S0169-5347(98)01472-4 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 21.Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R. 1998. Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol. Behav. 64, 439–444 10.1016/S0031-9384(98)00056-0 (doi:10.1016/S0031-9384(98)00056-0) [DOI] [PubMed] [Google Scholar]
- 22.Cottrell EC, Seckl JR. 2009. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 3, 19. 10.3389/neuro.08.019.2009 (doi:10.3389/neuro.08.019.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welberg LAM, Seckl JR. 2001. Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 13, 113–128 10.1046/j.1365-2826.2001.00601.x (doi:10.1046/j.1365-2826.2001.00601.x) [DOI] [PubMed] [Google Scholar]
- 24.Bale TL. 2005. Is mom too sensitive? Impact of maternal stress during gestation. Front. Neuroendocrinol. 26, 41–49 10.1016/j.yfrne.2005.03.002 (doi:10.1016/j.yfrne.2005.03.002) [DOI] [PubMed] [Google Scholar]
- 25.Welberg LA, Seckl JR, Holmes MC. 2000. Inhibition of 11β-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur. J. Neurosci. 12, 1047–1054 10.1046/j.1460-9568.2000.00958.x (doi:10.1046/j.1460-9568.2000.00958.x) [DOI] [PubMed] [Google Scholar]
- 26.Harvey PW, Chevins PFD. 1984. Crowding or ACTH treatment of pregnant mice affects adult copulatory behavior of male offspring. Horm. Behav. 18, 101–110 10.1016/0018-506X(84)90035-7 (doi:10.1016/0018-506X(84)90035-7) [DOI] [PubMed] [Google Scholar]
- 27.Kaiser S, Kruijver FPM, Straub RH, Sachser N, Swaab DF. 2003. Early social stress in male guinea pigs changes social behaviour, and autonomic and neuroendocrine functions. J. Neuroendocrinol. 15, 761–769 10.1046/j.1365-2826.2003.01055.x (doi:10.1046/j.1365-2826.2003.01055.x) [DOI] [PubMed] [Google Scholar]
- 28.Kaiser S, Heemann K, Straub RH, Sachser N. 2003. The social environment affects behaviour and androgens, but not cortisol in pregnant female guinea pigs. Psychoneuroendocrinology 28, 67–83 10.1016/S0306-4530(02)00010-0 (doi:10.1016/S0306-4530(02)00010-0) [DOI] [PubMed] [Google Scholar]
- 29.Sullivan EC, Hinde K, Mendoza SP, Capitanio JP. 2011. Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperaments in sons, but not daughters. Dev. Psychobiol. 53, 96–104 10.1002/dev.20483 (doi:10.1002/dev.20483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowlby J. 1969. Attachment. In Attachment and loss, vol. 1 London, UK: Hogarth [Google Scholar]
- 31.Ainsworth MDS, Wittig BA. 1969. Attachment and the exploratory behavior of one-year-olds in a strange situation. In Determinants of infant behavior, vol. 4 (ed. Foss BM.), pp. 111–136 London, UK: Methuen [Google Scholar]
- 32.Carter CS, Ahnert L, Grossman GE, Hrdy SB, Lamb ME, Porges SW, Sachser N. (eds) 2005. Attachment and bonding: a new synthesis. Cambridge, MA: MIT Press [Google Scholar]
- 33.Egeland B, Farber EA. 1984. Infant-mother attachment: factors related to its development and changes over time. Child Dev. 55, 753–771 10.2307/1130127 (doi:10.2307/1130127) [DOI] [PubMed] [Google Scholar]
- 34.Champagne FA, Meaney MJ. 2001. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog. Brain Res. 133, 287–302 10.1016/S0079-6123(01)33022-4 (doi:10.1016/S0079-6123(01)33022-4) [DOI] [PubMed] [Google Scholar]
- 35.Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. 2008. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J. Neuroendocrinol. 20, 795–801 10.1111/j.1365-2826.2008.01725.x (doi:10.1111/j.1365-2826.2008.01725.x) [DOI] [PubMed] [Google Scholar]
- 36.Zhang T-Y, et al. 2006. Maternal programming of defensive responses through sustained effects on gene expression. Biol. Psychol. 73, 72–89 10.1016/j.biopsycho.2006.01.009 (doi:10.1016/j.biopsycho.2006.01.009) [DOI] [PubMed] [Google Scholar]
- 37.Solomon NG, French JA. (eds) 1997. Cooperative breeding in mammals. Cambridge, UK: Cambridge University Press [Google Scholar]
- 38.Mendoza SP, Mason WA. 1986. Parental division of labour and differentiation of attachments in a monogamous primate. Anim. Behav. 34, 1336–1347 10.1016/S0003-3472(86)80205-6 (doi:10.1016/S0003-3472(86)80205-6) [DOI] [Google Scholar]
- 39.Hudson R, Bautista A, Reyes-Meza V, Morales Motor J, Rödel H. 2011. The effect of siblings on early development: a potential contributor to personality differences in mammals. Dev. Psychobiol. 53, 564–574 10.1002/dev.20535 (doi:10.1002/dev.20535) [DOI] [PubMed] [Google Scholar]
- 40.Festa-Bianchet M, Jorgenson JT, Réale D. 2000. Early development, adult mass, and reproductive success in bighorn sheep. Behav. Ecol. 11, 633–639 10.1093/beheco/11.6.633 (doi:10.1093/beheco/11.6.633) [DOI] [Google Scholar]
- 41.Mandillo S, D'Amato FR. 1999. Male olfactory cues affect mothers’ behavior in mice: effects of benzodiazepines. Psychopharmacology 146, 297–302 10.1007/s002130051120 (doi:10.1007/s002130051120) [DOI] [PubMed] [Google Scholar]
- 42.Heiming RS, Bodden C, Jansen F, Lewejohann L, Kaiser S, Lesch K-P, Palme R, Sachser N. 2011. Living in a dangerous world decreases maternal care: a study in serotonin transporter knockout mice. Horm. Behav. 60, 397–407 10.1016/j.yhbeh.2011.07.006 (doi:10.1016/j.yhbeh.2011.07.006) [DOI] [PubMed] [Google Scholar]
- 43.Champagne FA, Meaney MJ. 2006. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol. Psychiatry 59, 1227–1235 10.1016/j.biopsych.2005.10.016 (doi:10.1016/j.biopsych.2005.10.016) [DOI] [PubMed] [Google Scholar]
- 44.Noirot E. 1970. Priming of maternal responses by auditory and olfactory cues from mouse pups. Dev. Psychobiol. 2, 273–276 10.1002/dev.420020413 (doi:10.1002/dev.420020413) [DOI] [PubMed] [Google Scholar]
- 45.Trivers RL. 1974. Parent–offspring conflict. Am. Zool. 14, 249–264 [Google Scholar]
- 46.Del Giudice M. 2012. Fetal programming by maternal stress: insights from a conflict perspective. Psychoneuroendocrinology 37, 1614–1629 10.1016/j.psyneuen.2012.05.014 (doi:10.1016/j.psyneuen.2012.05.014) [DOI] [PubMed] [Google Scholar]
- 47.Trillmich F, Wolf JBW. 2008. Parent–offspring and sibling conflict in Galapagos fur seals and sea lions. Behav. Ecol. Sociobiol. 62, 363–375 10.1007/s00265-007-0423-1 (doi:10.1007/s00265-007-0423-1) [DOI] [Google Scholar]
- 48.Glocker ML, Langleben DD, Ruparel K, Loughead JW, Gur RC, Sachser N. 2009. Baby schema in infant faces induces cuteness perception and motivation for caretaking in adults. Ethology 115, 257–263 10.1111/j.1439-0310.2008.01603.x (doi:10.1111/j.1439-0310.2008.01603.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, Sachser N, Gur RC. 2009. Baby schema modulates the brain reward system in nulliparous women. Proc. Natl Acad. Sci. USA 106, 9115–9119 10.1073/pnas.0811620106 (doi:10.1073/pnas.0811620106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richter SH, et al. 2011. Effect of population heterogenization on the reproducibility of mouse behavior: a multi-laboratory study. PLoS ONE 6, e16461. 10.1371/journal.pone.0016461 (doi:10.1371/journal.pone.0016461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lesch K-P, et al. 1996. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531 10.1126/science.274.5292.1527 (doi:10.1126/science.274.5292.1527) [DOI] [PubMed] [Google Scholar]
- 52.Canli T, Lesch K-P. 2007. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 10, 1103–1109 10.1038/nn1964 (doi:10.1038/nn1964) [DOI] [PubMed] [Google Scholar]
- 53.Schinka JA, Busch RM, Robichaux-Keene N. 2004. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol. Psychiatry 9, 197–202 10.1038/sj.mp.4001405 (doi:10.1038/sj.mp.4001405) [DOI] [PubMed] [Google Scholar]
- 54.Caspi A, et al. 2003. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389 10.1126/science.1083968 (doi:10.1126/science.1083968) [DOI] [PubMed] [Google Scholar]
- 55.Suomi SJ. 2006. Risk, resilience, and gene × environment interactions in rhesus monkeys. Ann. NY Acad. Sci. 1094, 52–62 10.1196/annals.1376.006 (doi:10.1196/annals.1376.006) [DOI] [PubMed] [Google Scholar]
- 56.Homberg JR, van den Hove DL. 2012. The serotonin transporter gene and functional and pathological adaptation to environmental variation across the life span. Prog. Neurobiol. 99, 117–127 10.1016/j.pneurobio.2012.08.003 (doi:10.1016/j.pneurobio.2012.08.003) [DOI] [PubMed] [Google Scholar]
- 57.Carola V, Frazzetto G, Pascucci T, Audero E, Puglisi-Allegra S, Cabib S, Lesch K-P, Gross C. 2008. Identifying molecular substrates in a mouse model of the serotonin transporter × environment risk factor for anxiety and depression. Biol. Psychiatry 63, 840–846 10.1016/j.biopsych.2007.08.013 (doi:10.1016/j.biopsych.2007.08.013) [DOI] [PubMed] [Google Scholar]
- 58.Heiming RS, Jansen F, Lewejohann L, Kaiser S, Schmitt A, Lesch K-P, Sachser N. 2009. Living in a dangerous world: the shaping of behavioral profile by early environment and 5-HTT genotype. Front. Behav. Neurosci. 3, 26. 10.3389/neuro.08.026.2009 (doi:10.3389/neuro.08.026.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klauke B, Deckert J, Zwanzger P, Baumann C, Arolt V, Pauli P, Reif A, Domschke K. 2012. Neuropeptide S receptor gene (NPSR) and life events: G × E effects on anxiety sensitivity and its subdimensions. World J. Biol. Psychiatry. 10.3109/15622975.2011.646302 (doi:10.3109/15622975.2011.646302) [DOI] [PubMed] [Google Scholar]
- 60.Nemoda Z, Szekely A, Sasvari-Szekely M. 2011. Psychological aspects of dopaminergic gene polymorphisms in adolescence and young adulthood. Neurosci. Biobehav. Rev. 35, 1665–1686 10.1016/j.neubiorev.2011.04.002 (doi:10.1016/j.neubiorev.2011.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu D, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. 1997. Maternal care, hippocampal glucocorticoid receptor gene expression and hypothalamic–pituitary–adrenal responses to stress. Science 277, 1659–1662 10.1126/science.277.5332.1659 (doi:10.1126/science.277.5332.1659) [DOI] [PubMed] [Google Scholar]
- 62.Cameron NM, Fish EW, Meaney MJ. 2008. Maternal influences on the sexual behavior and reproductive success of the female. Horm. Behav. 54, 178–184 10.1016/j.yhbeh.2008.02.013 (doi:10.1016/j.yhbeh.2008.02.013) [DOI] [PubMed] [Google Scholar]
- 63.Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. 2003. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience 118, 571–576 10.1016/S0306-4522(02)00918-1 (doi:10.1016/S0306-4522(02)00918-1) [DOI] [PubMed] [Google Scholar]
- 64.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. 2008. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 28, 6037–6045 10.1523/JNEUROSCI.0526-08.2008 (doi:10.1523/JNEUROSCI.0526-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. 2010. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci. Biobehav. Rev. 34, 853–866 10.1016/j.neubiorev.2009.07.006 (doi:10.1016/j.neubiorev.2009.07.006) [DOI] [PubMed] [Google Scholar]
- 66.Terranova ML, Laviola G, Alleva E. 1993. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev. Psychobiol. 26, 467–481 10.1002/dev.420260805 (doi:10.1002/dev.420260805) [DOI] [PubMed] [Google Scholar]
- 67.Burke AR, Renner KJ, Forster GL, Watt MJ. 2010. Adolescent social defeat alters neural, endocrine, and behavioral responses to amphetamine in adult rats. Brain Res. 1352, 147–156 10.1016/j.brainres.2010.06.062 (doi:10.1016/j.brainres.2010.06.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCormick CM, Nixon F, Thomas C, Lowie B, Dyck J. 2010. Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats. Behav. Brain Res. 208, 23–29 10.1016/j.bbr.2009.11.003 (doi:10.1016/j.bbr.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 69.Schmidt MV, Scharf SH, Liebl C, Harbich D, Mayer B, Holsboer F, Müller MB. 2010. A novel chronic social stress paradigm in female mice. Horm. Behav. 57, 415–420 10.1016/j.yhbeh.2010.01.010 (doi:10.1016/j.yhbeh.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 70.Delville Y, David JT, Taravosh-Lahn K, Wommack JC. 2003. Stress and the development of agonistic behaviour in golden hamsters. Horm. Behav. 44, 263–270 10.1016/S0018-506X(03)00130-2 (doi:10.1016/S0018-506X(03)00130-2) [DOI] [PubMed] [Google Scholar]
- 71.Burghardt GM. 2005. The genesis of animal play: testing the limits. Cambridge, MA: MIT Press [Google Scholar]
- 72.Held S, Spinka M. 2011. Animal play and animal welfare. Anim. Behav. 81, 891–899 10.1016/j.anbehav.2011.01.007 (doi:10.1016/j.anbehav.2011.01.007) [DOI] [Google Scholar]
- 73.van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. 1999. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 34, 129–138 (doi:10.1002/(SICI)1098-2302(199903)34:2<129::AID-DEV6>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 74.Bell HC, Pellis SM, Kolb BB. 2010. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav. Brain Res. 207 7–13 10.1016/j.bbr.2009.09.029 (doi:10.1016/j.bbr.2009.09.029) [DOI] [PubMed] [Google Scholar]
- 75.Kaiser S, Haderthauer S, Sachser N, Hennessy MB. 2007. Social housing conditions around puberty determine later changes in plasma cortisol levels and behavior. Physiol. Behav. 90, 405–411 10.1016/j.physbeh.2006.10.002 (doi:10.1016/j.physbeh.2006.10.002) [DOI] [PubMed] [Google Scholar]
- 76.Sachser N, Dürschlag M, Hirzel D. 1998. Social relationships and the management of stress. Psychoneuroendocrinology 23, 891–904 10.1016/S0306-4530(98)00059-6 (doi:10.1016/S0306-4530(98)00059-6) [DOI] [PubMed] [Google Scholar]
- 77.Sachser N. 1986. Different forms of social organization at high and low population densities in guinea pigs. Behaviour 97, 253–272 10.1163/156853986X00630 (doi:10.1163/156853986X00630) [DOI] [Google Scholar]
- 78.Asher M, Oliveira ES, Sachser N. 2004. Social system and spatial organization of wild guinea pigs (Cavia aperea) in a natural low density population. J. Mammal 85, 788–796 10.1644/BNS-012 (doi:10.1644/BNS-012) [DOI] [Google Scholar]
- 79.Setchell JM, Charpentier M, Wickings EJ. 2005. Sexual selection and reproductive careers in mandrills (Mandrillus sphinx). Behav. Ecol. Sociobiol. 58, 474–485 10.1007/s00265-005-0946-2 (doi:10.1007/s00265-005-0946-2) [DOI] [Google Scholar]
- 80.Asher M, Lippmann T, Epplen JT, Kraus C, Trillmich F, Sachser N. 2008. Large males dominate: ecology, social organization, and mating system of wild cavies, the ancestors of the guinea pig. Behav. Ecol. Sociobiol. 62, 1509–1521 10.1007/s00265-008-0580-x (doi:10.1007/s00265-008-0580-x) [DOI] [Google Scholar]
- 81.Romeo RD. 2010. Pubertal maturation and programming of hypothalamic–pituitary–adrenal reactivity. Front. Neuroendocrinol 31, 232–240 10.1016/j.yfrne.2010.02.004 (doi:10.1016/j.yfrne.2010.02.004) [DOI] [PubMed] [Google Scholar]
- 82.Spear LP. 2000. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463 10.1016/S0149-7634(00)00014-2 (doi:10.1016/S0149-7634(00)00014-2) [DOI] [PubMed] [Google Scholar]
- 83.McCormick CM, Mathews IZ. 2010. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog. Neuro-Psychopharmocol. 34, 756–765 10.1016/j.pnpbp.2009.09.019 (doi:10.1016/j.pnpbp.2009.09.019) [DOI] [PubMed] [Google Scholar]
- 84.Adriani W, Laviola G. 2000. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology 39, 334–346 10.1016/S0028-3908(99)00115-X (doi:10.1016/S0028-3908(99)00115-X) [DOI] [PubMed] [Google Scholar]
- 85.McCormick CM, Smith C, Mathews IZ. 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav. Brain Res. 187, 228–238 10.1016/j.bbr.2007.09.005 (doi:10.1016/j.bbr.2007.09.005) [DOI] [PubMed] [Google Scholar]
- 86.Hennessy MB, Hornschuh G, Kaiser S, Sachser N. 2006. Cortisol responses and social buffering: a study throughout the life span. Horm. Behav. 49, 383–390 10.1016/j.yhbeh.2005.08.006 (doi:10.1016/j.yhbeh.2005.08.006) [DOI] [PubMed] [Google Scholar]
- 87.Sachser N, Pröve E. 1988. Plasma-testosterone development in colony and individually housed male guinea pigs. Ethology 79, 62–70 10.1111/j.1439-0310.1988.tb00699.x (doi:10.1111/j.1439-0310.1988.tb00699.x) [DOI] [Google Scholar]
- 88.Sachser N. 1990. Social organization, social status, behavioural strategies and endocrine responses in male guinea pigs. In Hormones, brain and behaviour in vertebrates. II. Behavioural activation in males and females—social interaction and reproductive endocrinology Comp. Physiol., vol. 9 (ed. Balthazart J.), pp. 176–187 Basel, Switzerland: Karger [Google Scholar]
- 89.Sachser N. 1998. Of domestic and wild guinea pigs: studies in sociophysiology, domestication, and social evolution. Naturwissenschaften 85, 307–317 10.1007/s001140050507 (doi:10.1007/s001140050507) [DOI] [PubMed] [Google Scholar]
- 90.Sachser N, Pröve E. 1984. Short-term effects of residence on the testosterone response to fighting in alpha male guinea pigs. Aggress. Behav. 10, 285–292 (doi:10.1002/1098-2337(1984)10:4<285::AID-AB2480100402>3.0.CO;2-8) [DOI] [Google Scholar]
- 91.Hirschenhauser K, Oliveira RF. 2006. Social modulation of androgens in vertebrates: meta-analysis of the challenge hypothesis. Anim. Behav. 71, 265–277 10.1016/j.anbehav.2005.04.014 (doi:10.1016/j.anbehav.2005.04.014) [DOI] [Google Scholar]
- 92.Goymann W, Landys MM, Wingfield JC. 2007. Distinguishing seasonal androgen responses from male-male andogen responsiveness: revisiting the challenge hypothesis. Horm. Behav. 51, 463–476 10.1016/j.yhbeh.2007.01.007 (doi:10.1016/j.yhbeh.2007.01.007) [DOI] [PubMed] [Google Scholar]
- 93.Lürzel S, Kaiser S, Sachser N. 2010. Social interaction, testosterone, and stress responsiveness during adolescence. Physiol. Behav. 99, 40–46 10.1016/j.physbeh.2009.10.005 (doi:10.1016/j.physbeh.2009.10.005) [DOI] [PubMed] [Google Scholar]
- 94.Lürzel S, Kaiser S, Sachser N. 2011. Social interaction decreases stress responsiveness during adolescence. Psychoneuroendocrinology 36, 1370–1377 10.1016/j.psyneuen.2011.03.010 (doi:10.1016/j.psyneuen.2011.03.010) [DOI] [PubMed] [Google Scholar]
- 95.Lürzel S, Kaiser S, Krüger C, Sachser N. 2011. Inhibiting influence of testosterone on stress responsiveness during adolescence. Horm. Behav. 60, 691–698 10.1016/j.yhbeh.2011.09.007 (doi:10.1016/j.yhbeh.2011.09.007) [DOI] [PubMed] [Google Scholar]
- 96.El Hani A, Dalle M, Delost P. 1980. Role of testosterone in the sexual dimorphism of adrenal activity at puberty in the guinea-pig. J. Endocrinol. 87, 455–461 10.1677/joe.0.0870455 (doi:10.1677/joe.0.0870455) [DOI] [PubMed] [Google Scholar]
- 97.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lithman SL. 2004. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J. Neuroendocrinol. 16, 989–998 10.1111/j.1365-2826.2004.01258.x (doi:10.1111/j.1365-2826.2004.01258.x) [DOI] [PubMed] [Google Scholar]
- 98.Mikics E, Kruk MR, Haller J. 2004. Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology 29, 618–635 10.1016/S0306-4530(03)00090-8 (doi:10.1016/S0306-4530(03)00090-8) [DOI] [PubMed] [Google Scholar]
- 99.Hayden-Hixson DM, Ferris CF. 1991. Steroid-specific regulation of agonistic responding in the anterior hypothalamus of male hamsters. Physiol. Behav. 50, 793–799 10.1016/0031-9384(91)90020-O (doi:10.1016/0031-9384(91)90020-O) [DOI] [PubMed] [Google Scholar]
- 100.Tiedtke T. 2012. Acute effects of glucocorticoids on agonistic behaviour in male guinea pigs. Master Thesis, University of Muenster, Muenster, Germany [Google Scholar]
- 101.Buwalda B, Geerdink M, Vidal J, Koolhaas JM. 2011. Social behavior and social stress in adolescence: a focus on animal models. Neurosci. Biobehav. Rev. 35, 1713–1721 10.1016/j.neubiorev.2010.10.004 (doi:10.1016/j.neubiorev.2010.10.004) [DOI] [PubMed] [Google Scholar]
- 102.Schulz KM, Molenda-Figueira HA, Sisk CL. 2009. Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 55, 597–604 10.1016/j.yhbeh.2009.03.010 (doi:10.1016/j.yhbeh.2009.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jansen F, Heiming RS, Lewejohann L, Touma C, Palme R, Schmitt A, Lesch K-P, Sachser N. 2010. Modulation of behavioural profile and stress response by 5-HTT genotype and social experience in adulthood. Behav. Brain Res. 207, 21–29 10.1016/j.bbr.2009.09.033 (doi:10.1016/j.bbr.2009.09.033) [DOI] [PubMed] [Google Scholar]
- 104.Jansen F, Heiming RS, Kloke V, Kaiser S, Palme R, Lesch K-P, Sachser N. 2011. Away game or home match: the influence of venue and serotonin transporter genotype on the display of offensive aggression. Behav. Brain Res. 219, 291–301 10.1016/j.bbr.2011.01.029 (doi:10.1016/j.bbr.2011.01.029) [DOI] [PubMed] [Google Scholar]
- 105.Pluess M, Belsky J. 2011. Prenatal programming of postnatal plasticity? Dev. Psychpathol. 23, 29–38 10.1017/S0954579410000623 (doi:10.1017/S0954579410000623) [DOI] [PubMed] [Google Scholar]
- 106.Hennessy MB. 2003. Enduring maternal influences in a precocial rodent. Dev. Psychobiol. 42, 225–236 10.1002/dev.10095 (doi:10.1002/dev.10095) [DOI] [PubMed] [Google Scholar]
- 107.Bayer TA, Falkai P, Maier W. 1999. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the ‘two hit hypothesis’. J. Psychiatr. Res. 33, 543–548 10.1016/S0022-3956(99)00039-4 (doi:10.1016/S0022-3956(99)00039-4) [DOI] [PubMed] [Google Scholar]


