Abstract
Previously, it was widely believed that each species has a specific social organization, but we know now that many species show intraspecific variation in their social organization. Four different processes can lead to intraspecific variation in social organization: (i) genetic variation between individuals owing to local adaptation (between populations) or evolutionarily stable strategies within populations; (ii) developmental plasticity evolved in long-term (more than one generation) unpredictable and short-term (one generation) predictable environments, which is mediated by organizational physiological effects during early ontogeny; (iii) social flexibility evolved in highly unpredictable environments, which is mediated by activational physiological effects in adults; (iv) entirely extrinsic factors such as the death of a dominant breeder. Variation in social behaviour occurs between individuals in the case of genetic variation and developmental plasticity, but within individuals in the case of social flexibility. It is important to study intraspecific variation in social organization to understand the social systems of species because it reveals the mechanisms by which species can adapt to changing environments, offers a useful tool to study the ultimate and proximate causes of sociality, and is an interesting phenomenon by itself that needs scientific explanation.
Keywords: social system, alternative reproductive tactics, population, frequency dependent selection, evolutionary stable strategy, striped mouse
1. Intraspecfic variation in social organization
Understanding variation in social systems between species has long been one of the main aims in evolutionary biology [1–5]. The social system of a species consists of the social organization (the composition of groups), the social structure (describing who interacts with whom) and the mating system [6]. Formerly, it was widely assumed that one species had one fixed form of social organization. Divergence of single individuals from the species-specific pattern of social behaviour was often treated as noise in the dataset. However, in the 1980s, it was realized that variation in social systems occurs in many species, either between or within populations [7,8].
Presently, the phylogenetic approach to understanding the evolution of different social systems is flourishing [9–11], but it requires each species to be categorized correctly with regard to its social organization. However, apart from the standard categories (e.g. solitary, pair-living, multi-male–multi-female species), there is no category for socially variable species. But to understand variation in social systems between species, it is important to understand the variation within species [7,12]. Here, I focus on the social organization, i.e. the composition of groups, because this is the parameter most easily measured in field studies, and because it influences the mating system, the social structure and thus the social system [6,13].
Intraspecific variation in social organization offers a unique opportunity to study the ultimate and proximate causes of social traits without any confounding phylogenetic effects [14,15]. Understanding the different mechanisms that underlie intraspecific variation in social organization will also help us to understand how and whether species can adapt to changing environments, which would also inform conservation efforts. Finally, intraspecific variation in social organization is itself an interesting phenomenon that needs scientific explanation both from the ultimate and the proximate perspective [7,15].
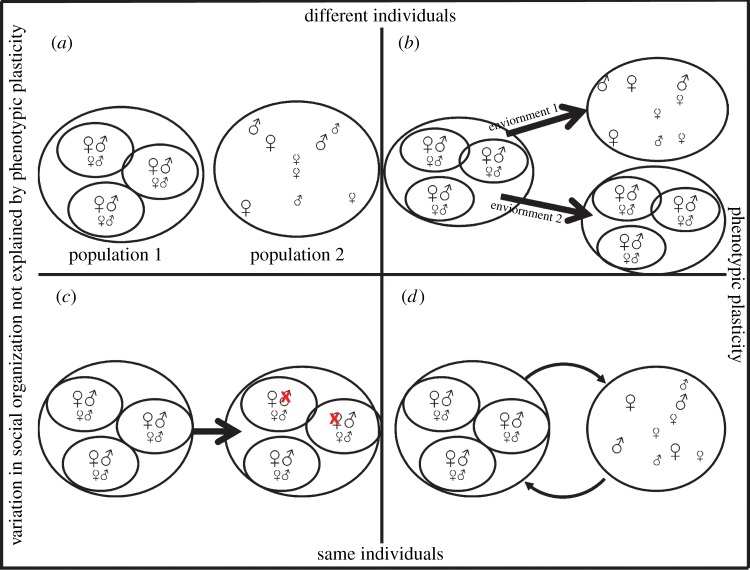
While the occurrence of intraspecific variation in social organization has received significant attention and acknowledgement recently, the focus has been on the associated ecological factors [13,16,17]. Additionally, we need to understand the different proximate and evolutionary mechanisms that arise from these ecological factors. While many examples of population differences in social organization exist [7], the underlying processes are typically not known. The aims of this review are to present an overview of the four processes that bring about intraspecific variation in social organization (figure 1), and to critically evaluate the ecological (extrinsic) and physiological (intrinsic) factors that bring about this variation.
Figure 1.
The four processes that can lead to intraspecific variation in social organization. To explain the variation observed at the population level, we need to consider variation at the individual level. Only the two processes in (b,d) represent phenotypic plasticity. (a,b) Different individuals differ in social behaviour. (a) Genetic variation: individuals of the same species but two different populations differ genetically, leading to differences in social organization. (b) Developmental plasticity: depending on the environment in which individuals grow up, environmental cues will activate developmental pathways for social behaviour that either leads to the same social organization as observed in their parent generation (environment 2) or to a different kind of social organization (environment 1). (c,d) The same individuals occur in different forms of social organization. (c) Entirely extrinsic factors: the unpredictable death/disappearance of some breeding individuals is the only cause of changes in social organization. This itself will cause behavioural flexibility in the survivors, such as mate searching, to return to the original state, but flexibility is the result, not the cause of the observed intraspecific variation in social organization. (d) Social flexibility: if the environment changes, social tactics of individuals change, which as a consequence will change the social organization of the population. This is reversible, as the same individuals can switch their tactic again, if the environment changes again. (Online version in colour.)
2. Genetic variation
Individuals of a species can differ genetically from each other in a way that influences their social behaviour [18,19], such as their ability to form pair-bonds and social bonds, and their propensity to show parental care [20,21]. These individual differences in genotype influencing social behaviour could contribute to intraspecific variation in social organization, both between and within populations.
If the environments of two populations of the same species differ in important aspects such as climate, availability of resources, pathogens or the occurrence of other competing species, then natural and sexual selection can differ between populations, leading to local adaptations. For example, if the carrying capacity and thus population density differs between populations owing to environmental factors, different social tactics might evolve in the two populations. Another example would be the benefits of maternal/parental and bi-parental care [22], which can vary with changes in food availability, availability of mating partners and ambient temperature [23]. Local adaptation might then result in genetic differences between populations regulating the expression of social behaviours, which can cause the social organization of the two populations to differ. For example, in prairie voles (Microtus ochrogaster) most individuals in some populations are pair-living, whereas the majority of individuals in other populations are solitary. Those two model types of social organization might represent genetic adaptations to different environments [24] (but see also [25]).
Genetic variation could also contribute to variation in social organization within populations. If the variation in social organization occurs between generations, this could be due to frequency-dependent or fluctuating selection, such that different genotypes prevail at different times within the population. If variation in social organization occurs at the same time, this might be due to evolutionarily stable strategies (ESS) that are genetically determined [26]. In this case, two or more genetically determined strategies exist within the population that have the same fitness and a higher fitness than any alternative strategy [1]. Such ESS have been documented in the case of alternative reproductive tactics (ARTs). In side-blotched lizards (Uta stansburiana), three male tactics exist that are genetically different and maintained via frequency-dependent selection [27]. Genetically determined ARTs that could represent ESS occur in several species of fish [28], lizards [27], in ruffs (Philomachus pugnax) [29] and in the isopod Paracerceis sculpta [30]. In fire ants (Solenopsis invicta), the number of breeding queens per colony is determined by the single gene Gp-9, with one queen (monogynous social organization) when only the B-allele is present, but multiple queens (polygynous social organization) when the b-allele is present as well [31].
The existence of male ARTs alone might not change the social organization unless females also show ARTs [15], but only in a few species do both sexes show ARTs. The extent to which genetic differences can explain intraspecific variation in social organization is not well understood, perhaps because most studies focused on male ARTs within one population, which is easier than making comparisons between populations. Still, genetic differences between populations owing to local adaptation influencing social organization might be common, and this could initiate ecological speciation [32].
3. Phenotypic plasticity
Phenotypic plasticity is the capacity of a specific genotype to produce different phenotypes (in behaviour, physiology or morphology) in response to different environmental conditions, including the social and pre-natal environment [33]. Phenotypic plasticity evolves when (i) the environment of a population varies between or within generations, (ii) individuals can use reliable environmental cues indicating (iii) which phenotype has the highest fitness, meaning that (iv) different phenotypes have different fitness in different environments and (v) no phenotype exists that has the highest fitness in all environments (summarized by Chalambor [34]).
Phenotypic plasticity can occur early in development and lead to permanent changes of the phenotype, or it can be reversible, which often occurs in adults. The first phenomenon has been called developmental plasticity, the second one phenotypic flexibility [35]. Different physiological mechanisms exist for these two kinds of plasticity, which also suggest that different evolutionary forces are at work.
(a). Developmental plasticity
In the case of developmental plasticity, variation is due to environmental variation activating alternative developmental pathways of one genotype [35]. Developmental plasticity is non-reversible, making it empirically difficult to differentiate it from genetic variation between individuals. This can be best achieved by experimental studies in captivity testing for an influence of the environment on the phenotype. If environmental manipulation has no influence on the ratio of alternative phenotypes produced, genetic variation is likely to be the cause [36]. If the environment significantly influences the resulting phenotype, developmental plasticity is likely to be at play [37]. If in common garden experiments the observed differences in social organization between populations disappear in the common garden, then developmental plasticity would be the explanation for the differences observed in nature.
Developmental plasticity is a response to the early environment within which an individual develops and in which it later grows up. For this, some cues of the current environment must reliably predict the future environment in which the same individual will reproduce, and this predictability must have occurred in the past when developmental plasticity evolved. As such, developmental plasticity enables the development of alternative adaptive phenotypes that have a higher fitness on average than any fixed phenotype. Typically, the environmental influence is during early development (pre- and/or postnatal), later affecting the phenotype of adult breeding individuals. In rodents, maternal effects significantly influence stress response and social behaviour [38], which is an adaptive response to specific environments [39,40]. Developmental plasticity can also occur via environmentally induced changes in DNA methylation and as such represent epigenetic effects, causing phenotypic diversity [41].
If the environment of a population changes consistently, the development of the nervous system, and thus ultimately the social behaviour of this generation could significantly differ from that of other generations. Similarly, the environments of two different populations of the same species can differ significantly, inducing different developmental pathways, leading to intraspecific variation in social organization. However, I am not aware of any empirical studies demonstrating the importance of developmental plasticity for intraspecific variation in social organization, which could well be due to researchers not addressing the necessary research questions.
(b). Social flexibility
Social flexibility describes the phenomenon that the social organization of a species or population can change as a function of individuals reversibly changing their social tactics in response to short-term changes of the environment. Thus, social flexibility focuses on changes in the population that are a function of individuals of both sexes reversibly changing their reproductive and social tactics [15]. Individuals modify their interactions with other individuals (social structure), with whom they mate (mating system), and consequently the composition of groups (social organization) and thus the entire social system of the population. In the case of social flexibility, both males and females must have ARTs based on a single strategy (all individuals have the same decision rules) [42]. Ecological constraints are one of the most important factors for the evolution of social flexibility [15,17]. These constraints can vary between years, often due to changes in population density, such that in some years philopatry and the establishment of large groups are favoured, but in other years dispersal and solitary breeding [5,15,17]. Within a population, often two or more forms of social organization might exist, e.g. solitary- and group-living individuals, or monogamous and polygynous groups.
One problem with the term social flexibility is confusion arising from the fact that most behaviours are flexible (reversible). Many researchers in animal behaviour use the term ‘flexible’ to emphasize this characteristic feature of social behaviour. As such, ‘flexible’ is a vague term without scale (which behaviour is flexible, which is not?) and neither a scientific phenomenon nor a theoretical concept. In contrast, the value of the term ‘social flexibility’ is that it provides a conceptual framework that enables us to study ultimate and proximate factors of individual flexibility in social behaviour that lead to intraspecific variation in social organization [15].
Interestingly, species that are flexible in their social behaviour might not show social flexibility but rather social specialization. Primates are well known for their flexibility but have little social flexibility. This sounds contradictory, but means that their social behaviour shown towards other group members is flexible, but not the social organization of their group. In primates, social organization is most often species-specific and barely varies within species, even though many species have large distributions across ecologically variable habitats [43,44]. Baboons (Papio spp.), for example, always form multi-male, multi-female groups, even though they occur in a wide range of habitats [44], and gibbons (Hylobates spp.) always live in monogamous family groups [44]. Primates typically have a clear but flexible dominance hierarchy. Flexible social tactics enable them to respond to arising conflicts, environmental and social changes, stabilizing the social organization of the group. Thus, flexibility in social behaviour enables primates to maintain their species-specific social organization.
In contrast, social flexibility (population level) might arise in species that have little flexibility in social behaviour (individual level) towards other group members. If conflict arises in these species, they might have to change their social organization to resolve it. This is evident in the socially flexible African striped mouse (Rhabdomys pumilio) that can live solitarily, in single family groups or extended family groups [45]. In this species, one can distinguish between non-breeding philopatric males and females, one breeding male and up to four breeding females per group [46]. Surprisingly, no measurable dominance hierarchy exists and groups are highly egalitarian [47]. If conflict occurs, no changes in dominance hierarchy are possible nor do submissive behaviours exist to resolve conflict. Instead, striped mice switch to a solitary lifestyle if conflict increases [45,48]. Social flexibility might thus arise from the absence of flexibility in social behaviour, making solitary-living the only adaptive alternative to group-living when inter-individual conflict increases to a certain threshold level. In contrast, species with pronounced dominance hierarchies are able to adjust their social interactions with other individuals (social structure), enabling them to maintain their social organization.
Social flexibility is predicted to be an adaptation to unpredictably changing environments, selecting for high phenotypic flexibility that is based on a broad reaction norm, not on genetic polymorphisms for specific tactics [15]. The environment in which it evolved had to be less predictable than in the case of developmental plasticity, such that the environment in which an individual grows up does not provide significant information about the environment in which the same individual will reproduce. Thus, selection favours individuals that can change their social behaviour as adults. If this occurs in both sexes, the entire social organization of a population can change. For example, both male and female striped mice can be highly sociable and form extended family groups, but the same individuals can switch to solitary-living with very few if any social interactions [15,45,48]. The fact that groups can exist even under conditions of low population density, but split at the beginning of the breeding season (individuals leave the group) indicates that the individuals have a choice between group- and solitary-living. Thus, the change in social organization is a consequence of individual decisions [49] and not entirely owing to extrinsic environmental factors (see §4).
Social flexibility occurs in several species, including insects, birds and mammals (examples in table 1). The most important factor selecting for social flexibility seems to be unpredictable fluctuations in population density influencing the extent of intraspecific competition. For example, in African striped mice, population density declined from one generation to the next from 32.4 to 1.5 mice ha−1 (factor of 21) owing to an unexpected drought, and only a few generations (i.e. years) later owing to an unexpected and local increase of predation pressure (presence of a single wild cat) from 30.5 to 6.5 mice ha−1 (factor of 5; [45]). In both cases, striped mice grew up under high population densities, when group-living and communal breeding were the best tactic, but they reproduced when population density was low and thus solitary breeding was the best tactic. Under high population density, inter-group competition in the form of territoriality is very strong [62] leading to small territories [63] and constrained dispersal, such that breeding in groups is favoured [45]. However, living in groups can lead to female–female aggression and female infanticide [45] as well as sexual suppression of males by the dominant male [61], resulting in significant fitness costs that can be avoided by becoming solitary [48]. Thus, under low population density, individual costs of inter-group competition are lower than costs of intragroup competition, making the switch from group- to solitary-living adaptive.
Table 1.
Species showing social flexibility.
| species | male tactics | female tactics | social organizations | fluctuation in population density? | importance of intraspecific competition | references |
|---|---|---|---|---|---|---|
| burying beetle, Nicrophorus vespilloides | —attract females via pheromones —attract females to carcass —satellite |
—single breeding —communal breeding —parasite |
—solitary —pair-living —one male, multi-female |
yes | carrion size determines number of females that can breed together | [50–52] |
| pied kingfisher, Ceryle rudis | —breeder —non-reproducing helper —reproducing helper |
breeder | —pair-living —family group —two males, one female |
yes | availability of good nesting sites influences whether unrelated helpers are accepted | [53,54] |
| prairie vole, Microtus ochrogaster | —philopatric helper —solitary wanderer —breeder |
—philopatric helper —single breeder —communal breeder |
—solitary —pair-living —multi-male, multi-female |
yes (breeding seasons versus winter) | Female–female competition in communally breeding groups: higher reproductive success if only one female breeds per group | [55–58] |
| house mouse, Mus musculus | —philopatric helper —solitary roamer —breeder |
—philopatric helper —single breeder —communal breeder |
—solitary —pair-living —multi-male, multi-female |
yes | —intrasexual aggression —female infanticide |
[59,60] |
| striped mouse, Rhabdomys pumilio | —philopatric helper —solitary roamer —breeder |
—philopatric helper —single breeder —communal breeder |
—solitary —pair-living —multi-male, multi-female |
yes | —intrasexual aggression —female infanticide —male reproductive suppression |
[45,46,48,61] |
4. Entirely extrinsic factors
Intraspecific variation in social organization can also result solely from extrinsic factors (stochastic processes) leading to non-adaptive changes in social organization. As such there can be no ultimate or proximate explanation for it. In this case the observed intraspecific variation in social organization is a direct consequence of a demographic interruption, and this variation is not due to an adaptive response of individuals to environmental change. Hereby the individuals will be forced to show flexibility in their behaviour to respond adaptively to the change (such as starting mate searching, dispersal), but this flexibility is not the reason for the observed intraspecific variation in social organization that we want to explain but its consequence (figure 1).
In socially monogamous species, the natural death of one of the dominant breeders will change the social organization not because the remaining family members chose this new social organization, but simply because the disappearance of one breeder changes group composition. For example, Callitrichids, small New World monkeys, have been reported to show the highest degree of social flexibility in primates [44,64], but in several species this might be due to social disruption (mortality of breeder) of the default social organization of pair-living [12]. Nevertheless, if such disruption occurs regularly and has significant fitness effects [65–67], it could function as an important selection pressure for social flexibility. On the other hand, the significant fitness costs associated with a deviation from social monogamy, especially female infanticide [65,66], might explain the absence of social flexibility and instead a disposition to re-arrange social monogamy after social disruption. Thus, instead of the evolution of social flexibility, evolution of flexibility in social behaviour that promotes a return to a socially monogamous situation might have been favoured.
Individuals may be constrained to live solitarily when population density is very low, and constrained to be group-living when population density is very high, without giving individuals the choice to remain in a group or to become solitary. In some species, it is well known that the social organization is rather inflexible and not influenced by population density. Obligate social species form groups even under very low population densities, for example lions (Panthera leo) in the Kalahari [68]. On the other hand, some species like whistling rats (Parotomys brantsii) live solitarily even under very high population densities [69]. Thus, while ecological factors are important in explaining intraspecific variation in social organization, it is not clear whether these extrinsic factors alone are sufficient to explain differences between species.
5. Physiological mechanisms underlying intraspecific variation in social organization
We know that the gene–environment interaction determines phenotypes (including behavioural phenotypes). Thus, changes in both genotypes and in the environment can induce changes in social behaviours. The four different processes that can explain intraspecific variation in social organization are predicted to have evolved in different environments with different selection pressures (table 2). The underlying physiological factors, themselves being the result of evolution, differ accordingly (table 2). Here, the concept of organizational versus activational effects is important [70]. Organizational effects occur early in development and are non-reversible, as in the case of sex determination in mammals [70]. Activational effects typically occur in adulthood and are reversible, for example the activation of sexual behaviour by testosterone [70].
Table 2.
Comparison of the four processes that can cause intraspecific variation in social organization.
| process | genetics | influence of environment on behaviourb | variability within individuals? | environment in which it evolved | physiological mechanisms |
|---|---|---|---|---|---|
| (i) genetic variation | —polymorphism —narrow reaction norm |
no | no | predictable | organizational |
| (ii) developmental plasticity | —monomorphisma
—broad reaction norm |
non-reversible | no | short-term: predictable long-term: unpredictable |
organizational |
| (iii) social flexibility | —monomorphisma
—broad reaction norm |
reversible | yes | unpredictable | activational |
| (iv) entirely extrinsic factors | —monomorphisma
—narrow reaction norm |
no | yes | predictable | none that leads to variation in social organization |
aThe term genetic monomorphism does not imply that genetic variation is absent, only that the major part of variation observed in social behaviour is not due to genetic but to environmental factors.
bOther behaviours can be influenced during early environment, but this cannot explain the variation in social organization.
In species where genetic variation explains the variation in social organization, two or more genotypes occur that cause different developmental pathways characterized by different organizational effects, such as in the case of genetically determined ARTs [36]. In the case of developmental plasticity only one genotype occurs, but depending on the environment this can lead to alternative developmental pathways that will rely on different organizational effects, resulting in a determined, non-flexible adult phenotype [35].
Physiological mechanisms of social flexibility are expected to consist of activational, reversible effects such as environmentally induced neural activation or secretion of specific hormones or neuropeptides. The effects of many endocrine parameters on changes in social behaviour have been demonstrated, such as those of prolactin and steroid hormones on parental care [71], and those of testosterone on reproductive behaviour [72]. Neuroendocrine changes, e.g. in the production and secretion of neuropeptides (oxytocin and vasopressin), have been demonstrated to be important too, but have been shown only in a few species because of the difficulties of assessing neuropeptides [73]. Finally, activation of existing neuronal pathways and neural mechanisms of learning can be expected to be important, but are difficult to study and poorly understood. In sum, social flexibility is regulated by activational physiological mechanisms, enabling an adaptive and reversible response to unpredictable environments.
Genetically or developmentally determined neuronal pathways can be superior to flexible ones, because flexibility requires energy, time and the opportunity to change [74]. In the neurosciences, it is widely held that genetically fixed neuronal patterns are favoured under long-term environmental stability, but that flexibility will be favoured when there is significant environmental instability [75]. Developmental plasticity is more advantageous than genetic determinism when change occurs predictably. Flexibility, by learning or by physiological activational effects, is favoured over genetically or developmentally determined pathways when change happens unpredictably. This is related to the Baldwin effect which states that while acquired traits cannot be inherited, the tendency to acquire traits can be inherited [76]. Thus, the Baldwin effect describes the evolution of the ability to respond optimally to a particular environment. This is the result of selection for genes for plasticity and flexibility enabling adaptation to the current environment, rather than genes for a fixed phenotype [77]. However, while the idea of the Baldwin effect is more than 100 years old and many studies have demonstrated how organisms rapidly respond both physiologically and behaviourally to changing environments, we still cannot easily link functional importance and inheritance of novel accommodations, i.e. the idea that natural selection sorts among developmental variants for genes enabling plasticity and flexibility [78]. In other words: we do not know whether flexible genes versus inflexible genes exist (but see [79]).
In the case of extrinsic factors alone causing intraspecific variation in social organization, no specific physiological mechanisms exist that lead to the change of social organization, which entirely depends on the environment. Instead, a stress response will be activated, leading to behavioural changes to return to the default social organization [80].
6. Future research
While the four suggested processes offer an approach to study ultimate and proximate explanations for intraspecific variation in social organization, many unanswered questions remain:
(1) What are the benefits of having a narrow reaction norm (environmental canalization reducing environmental influence on the phenotype) versus having a broad reaction norm? Constraints for the evolution of phenotypic plasticity have received significant theoretical attention, and many studies addressed the costs (same phenotype of plastic genotype has lower fitness than of specialized genotype) and limits (plastic phenotype cannot produce the trait as well as the specialized genotype) of phenotypic plasticity [34,81,82]. However, decades of research revealed that costs of phenotypic plasticity are surprisingly low [81,83] and we still do not understand why so many species are specialists instead of being plastic generalists. Few if any empirical studies directly compared specialized versus plastic species, asking under which circumstances flexible species can outcompete specialized ones and vice versa.
(2) What environmental selection pressures lead to the evolution of developmental plasticity, and which ones to the evolution of social flexibility? Theoretical models exist to explain the evolution of plasticity (developmental plasticity or flexibility) [34] as well as its costs, limits and constraints [81]. If the current environment reliably predicts the future environment, developmental plasticity is predicted to evolve, but with increasing unpredictability social flexibility might evolve (table 2), enabling a quick response that can be reversed if the environment changes again. This might be especially important in (i) highly unpredictable environments and (ii) in long-lived species, as predictability tends to decrease with increasing time in the future. In contrast, developmental plasticity resulting in an inflexible phenotype might reduce specific costs of plasticity, especially maintenance and information-acquisition costs: for developmental plasticity, individuals only need to acquire information about the environment once in early development, when deciding which developmental trajectory to follow.
All four processes mentioned here are possible explanations for intraspecific variation in social organization, but few studies have identified which one explains the pattern found in a specific population or species. In table 3, I provide several predictions for the four different processes that can be used to determine which one explains the pattern of intraspecific variation in social organization observed in a particular species. While writing this review, it became evident to me how little we know about the processes underlying intraspecific variation in social organization. I wanted to include many empirical examples for the four possible processes, but apart from my own research topic—social flexibility—not much literature exists for the other processes. Of course, flexibility might be the most common process for intraspecific variation in social organization and the other processes might be less important. Genetic variation and developmental plasticity might be important in the expression of individual differences in social behaviour (for example ARTs), but rarely explain intraspecific variation in social organization. Entirely extrinsic factors might be the most overlooked process, because even in this case ecological factors do exist (stochastic mortality by a predator is ultimately also owing to an ecological factor), and researchers in behavioural ecology are prone to see intraspecific variation as an adaptive response to a changing environment, without taking non-adaptive alternatives into account.
Table 3.
The predictions to differentiate between the four processes that bring about intraspecific variation in social organization.
| study | trait investigated | yes (trait exists) | no (trait does not exist) |
|---|---|---|---|
| 1 | variability within individuals | social flexibility or extrinsic factors | genetic variation or developmental plasticity |
| 2 | genetic polymorphism | genetic variation | developmental plasticity, social flexibility or extrinsic factors |
| 3 | early environment can induce changes in individual behaviour | developmental plasticity or social flexibility | genetic variation, extrinsic factors |
| 4 | alternative forms of social organization are stable | genetic variation, developmental plasticity, or social flexibility | extrinsic factors |
| 5 | physiological mechanisms organizational | genetic variation or developmental plasticity | social flexibility |
| 6 | predictability of environment in which it evolved | this factor can change significantly between generations (thus between years, decades or centuries) and is difficult to use for categorization; it is best used for comparative studies | |
Environmental factors are important regulators of intraspecific variation in social organization, and these factors have been studied extensively [1,15,17,84,85]. For all four processes, the environment is a significant factor, either as a selection pressure (genetic variation), because the current environment determines the best tactic for the next generation but not for more distant generations (developmental plasticity), or because only the current environment determines the best tactic for the currently breeding individuals (social flexibility). Thus, to really understand both the ultimate reasons and the proximate causes of intraspecific variation in social organization, we have to go further than focusing on the environmental factors. By a combination of only two studies, one can differentiate between the four processes (table 4).
Table 4.
Key to determine the process leading to intraspecific variation in social organization, based on the predictions from table 3. Study 5 from table 3 should be used to confirm the results obtained from this key.
| no. | question | result | go to |
|---|---|---|---|
| 1 | (a) variability occurs within individuals | 3 | |
| (b) variability does not occur within individuals | 2 | ||
| 2 | (a) genetic polymorphism | genetic variation | |
| (b) genetic monomorphism | developmental plasticity | ||
| 3 | (a) environment induces changes in individual behaviour and alternative forms of social organization are stable | social flexibility | |
| (b) environment induces no changes in individual behaviour and alternative forms of social organization are not stable | entirely extrinsic factors |
The resilience of a species, population or an individual to environmental change depends on its ability to respond adaptively. To understand how species are able to adapt to changing environments, we need to understand the different mechanisms of intraspecific variation in social organization, which are the consequence of individual adaptive responses. If environmental change happens slowly, fluctuates predictably or if environments differ between populations, then genetic variation might be the evolutionarily most stable response. If changes happen faster than genetic adaptation, phenotypic plasticity or flexibility can enable an adaptive response. Developmental plasticity provides animals with opportunity to mount a response during their growth phase that is adaptive when they reproduce later as adults. Social flexibility results from the immediate response of individuals to current changing environmental conditions. In contrast to developmental plasticity, social flexibility is reversible and evolves in highly unpredictable environments. In highly predictable environments, plasticity and flexibility are not favoured by natural selection and instead specialization evolves. Understanding these different processes of intraspecific variation in social organization will help us to understand the evolution of sociality and adaptability of species.
Acknowledgements
Important comments by Loren Hayes, Neville Pillay, Nancy Solomon, Josh van Buskirk and three referees significantly improved this manuscript.
References
- 1.Krebs JR, Davies NB. 1993. An introduction to behavioural ecology, 3rd edn Oxford, UK: Blackwell Science [Google Scholar]
- 2.Crook JH. 1964. Evolution of social organisation and visual communication in the weaver birds (Ploceinae). Behav. Suppl. 10, 1–178 [Google Scholar]
- 3.Jarman PJ. 1974. The social-organization of antelope in relation to their ecology. Behaviour 48, 215–267 10.1163/156853974X00345 (doi:10.1163/156853974X00345) [DOI] [Google Scholar]
- 4.Goldizen W. 1990. A comparative perspective on the evolution of tamarin and marmoset social systems. Int. J. Primatol. 11, 63–83 10.1007/BF02193696 (doi:10.1007/BF02193696) [DOI] [Google Scholar]
- 5.Hayes LD. 2000. To nest communally or not to nest communally: a review of rodent communal nesting and nursing. Anim. Behav. 59, 677–688 10.1006/anbe.1999.1390 (doi:10.1006/anbe.1999.1390) [DOI] [PubMed] [Google Scholar]
- 6.Kappeler PM, Schaik CPv. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707–740 10.1023/A:1015520830318 (doi:10.1023/A:1015520830318) [DOI] [Google Scholar]
- 7.Lott DF. 1991. Intraspecific variation in the social systems of wild vertebrates. New York, NY: Cambridge University Press [Google Scholar]
- 8.Lott DF. 1984. Intraspecific variation in the social systems of wild vertebrates. Behaviour 88, 266–325 10.1163/156853984X00353 (doi:10.1163/156853984X00353) [DOI] [Google Scholar]
- 9.Kamilar JM, Cooper N. 2013. Phylogenetic signal in primate behaviour, ecology and life history. Phil Trans. R. Soc. B. 368, 20120341 10.1098/rstb.2012.0341 (doi:10.1098/rstb.2012.0341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kappeler PM, Barrett L, Blumstein DT, Clutton-Brock TH. 2013. Constraints and flexibility in mammalian social behaviour: introduction and synthesis. Phil. Trans. R. Soc. B 368, 20120337 10.1098/rstb.2012.0337 (doi:10.1098/rstb.2012.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thierry B. 2013. Identifying constraints in the evolution of primate societies. Phil. Trans. R. Soc. B 368, 20120342 10.1098/rstb.2012.0342 (doi:10.1098/rstb.2012.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anzenberger G, Falk B. 2012. Monogamy and family life in callitrichid monkeys: deviations, social dynamics and captive management. Int. Zoo Yearbook 46, 109–122 10.1111/j.1748-1090.2012.00176.x (doi:10.1111/j.1748-1090.2012.00176.x) [DOI] [Google Scholar]
- 13.Silk JB. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559 10.1098/rstb.2006.1994 (doi:10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brashares JS, Garland T, Arcese P. 2000. Phylogenetic analysis of coadaption in behavior, diet, and body size in the African antelope. Behav. Ecol. 4, 452–463 10.1093/beheco/11.4.452 (doi:10.1093/beheco/11.4.452) [DOI] [Google Scholar]
- 15.Schradin C, Lindholm AK, Johannesen J, Schoepf I, Yuen C-H, König B, Pillay N. 2012. Social flexibility and social evolution in mammals: a case study of the African striped mouse (Rhabdomys pumilio). Mol. Ecol. 21, 541–553 10.1111/j.1365-294X.2011.05256.x (doi:10.1111/j.1365-294X.2011.05256.x) [DOI] [PubMed] [Google Scholar]
- 16.Maher CR, Burger JR. 2011. Intraspecific variation in space use, group size, and mating systems of caviomorph rodents. J. Mammal 92, 54–64 10.1644/09-MAMM-S-317.1 (doi:10.1644/09-MAMM-S-317.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koenig WD, Pitelka FA, Carmen WJ, Mumme RL, Stanback MT. 1992. The evolution of delayed dispersal in cooperative breeders. Q. Rev. Biol. 67, 111–150 10.1086/417552 (doi:10.1086/417552) [DOI] [PubMed] [Google Scholar]
- 18.Stirling DG, Réale D, Roff DA. 2002. Selection, structure and the heritability of behaviour. J. Evol. Biol. 15, 277–289 10.1046/j.1420-9101.2002.00389.x (doi:10.1046/j.1420-9101.2002.00389.x) [DOI] [Google Scholar]
- 19.Anholt RRH, Mackay TFC. 2010. Genetics of social interactions. Principles of behavioral genetics, pp. 185–206 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 20.Rymer T, Pillay N. 2011. Transmission of parental care behavior in African striped mice, Rhabdomys pumilio. J. Exp. Zool. A Ecol. Gen. Physiol. 315, 631–638 10.1002/jez.712 (doi:10.1002/jez.712) [DOI] [PubMed] [Google Scholar]
- 21.Hammock EAD, Young LJ. 2005. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 308, 1630–1634 10.1126/science.1111427 (doi:10.1126/science.1111427) [DOI] [PubMed] [Google Scholar]
- 22.Maynard SJ. 1977. Parental investment: prospective analysis. Anim. Behav. 25, 1–9 10.1016/0003-3472(77)90062-8 (doi:10.1016/0003-3472(77)90062-8) [DOI] [Google Scholar]
- 23.Schradin C, Pillay N. 2005. The influence of the father on offspring development in the striped mouse. Behav. Ecol. 16, 450–455 10.1093/beheco/ari015 (doi:10.1093/beheco/ari015) [DOI] [Google Scholar]
- 24.Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le W, Hoffman GE. 2004. Intraspesific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res. 1016, 247–254 10.1016/j.brainres.2004.05.010 (doi:10.1016/j.brainres.2004.05.010) [DOI] [PubMed] [Google Scholar]
- 25.Ophir AG, Phelps SM, Sorin AB, Wolff JO. 2007. Morphological, genetic and behavioral comparisons of two prairie vole populations in the field and laboratory. J. Mammal 88, 989–999 10.1644/06-MAMM-A-250R.1 (doi:10.1644/06-MAMM-A-250R.1) [DOI] [Google Scholar]
- 26.Maynard Smith J. 1974. The theory of games and the evolution of animal conflicts. J. Theor. Biol. 47, 209–221 10.1016/0022-5193(74)90110-6 (doi:10.1016/0022-5193(74)90110-6) [DOI] [PubMed] [Google Scholar]
- 27.Sinervo B, Zamudio KR. 2001. The evolution of alternative reproductive strategies: fitness differential, heritability, and genetic correlation between the sexes. J. Hered. 92, 198–205 10.1093/jhered/92.2.198 (doi:10.1093/jhered/92.2.198) [DOI] [PubMed] [Google Scholar]
- 28.Taborsky M. 2008. Alternative reproductive tactics in fish. In Alternative reproductive tactics (eds Oliveira RF, Taborsky M, Brockmann HJ.), pp. 251–299 Cambridge, UK: Cambridge University Press [Google Scholar]
- 29.Lank DB, Smith CM, Hanotte O, Burke T, Cooke F. 1995. Genetic polymorphism for alternative mating behaviour in lekking male ruff Philomachus pugnax. Nature 378, 59–62 10.1038/378059a0 (doi:10.1038/378059a0) [DOI] [Google Scholar]
- 30.Shuster SM, Sassaman C. 1997. Genetic interaction between male mating strategy and sex ratio in a marine isopod. Nature 388, 373–376 10.1038/41089 (doi:10.1038/41089) [DOI] [Google Scholar]
- 31.Gotzek D, Ross KG. 2007. Genetic regulation of colony social organization in fire ants: an integrative overview. Q. Rev. Biol. 82, 201–226 10.1086/519965 (doi:10.1086/519965) [DOI] [PubMed] [Google Scholar]
- 32.Simpson GG. 1953. The Baldwin effect. Evolution 7, 110–117 10.2307/2405746 (doi:10.2307/2405746) [DOI] [Google Scholar]
- 33.Bradshaw AD. 1965. Evolutionary significance of phenotypic plasticity in plants. In Advances in genetics (eds Caspari EW, Thoday JM.), pp. 115–155 New York, NY: Academic Press [Google Scholar]
- 34.Chalambor CK, Angeloni LM, Carroll SP. 2010. Behavior as phenotypic plasticity. In Evolutionary behavioral ecology (eds Westneat DF, Fox CW.), pp. 90–107 Oxford, UK: Oxford University Press [Google Scholar]
- 35.Piersma T, Drent J. 2003. Phenotypic flexibility and the evolution of organismal design. Tree 18, 228–233 [Google Scholar]
- 36.Moore MC, Hews DK, Knapp R. 1998. Hormonal control and evolution of alternative male phenotypes: generalizations of models for sexual differentiation. Am. Zool. 38, 133–151 [Google Scholar]
- 37.Ellers J, Stuefer J. 2010. Frontiers in phenotypic plasticity research: new questions about mechanisms, induced responses and ecological impacts. Evol. Ecol. 24, 523–526 10.1007/s10682-010-9375-4 (doi:10.1007/s10682-010-9375-4) [DOI] [Google Scholar]
- 38.Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. 2004. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 10.1038/nn1276 (doi:10.1038/nn1276) [DOI] [PubMed] [Google Scholar]
- 39.Siegeler K, Sachser N, Kaiser S. 2011. The social environment during pregnancy and lactation shapes the behavioral and hormonal profile of male offspring in wild cavies. Dev. Psychobiol. 53, 575–584 10.1002/dev.20585 (doi:10.1002/dev.20585) [DOI] [PubMed] [Google Scholar]
- 40.Heiming RS, Bodden C, Jansen F, Lewejohann L, Kaiser S, Lesch K-P, Palme R, Sachser N. 2011. Living in a dangerous world decreases maternal care: a study in serotonin transporter knockout mice. Horm. Behav. 60, 397–407 10.1016/j.yhbeh.2011.07.006 (doi:10.1016/j.yhbeh.2011.07.006) [DOI] [PubMed] [Google Scholar]
- 41.Champagne FA. 2012. Epigenetics and developmental plasticity across species. Dev. Psychobiol. 10.1002/dev.21036 (doi:10.1002/dev.21036). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schradin C, Lindholm AK. 2011. Relative fitness of alternative male reproductive tactics in a mammal varies between years. J. Anim. Ecol. 80, 908–917 10.1111/j.1365-2656.2011.01831.x (doi:10.1111/j.1365-2656.2011.01831.x) [DOI] [PubMed] [Google Scholar]
- 43.Thierry B, Iwaniuk AN, Pellis SM. 2000. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca). Ethology 106, 713–728 10.1046/j.1439-0310.2000.00583.x (doi:10.1046/j.1439-0310.2000.00583.x) [DOI] [Google Scholar]
- 44.Shultz S, Opie C, Atkinson QD. 2011. Stepwise evolution of stable sociality in primates. Nature 479, 219–222 10.1038/nature10601 (doi:10.1038/nature10601) [DOI] [PubMed] [Google Scholar]
- 45.Schradin C, König B, Pillay N. 2010. Reproductive competition favours solitary living while ecological constraints impose group-living in African striped mice. J. Anim. Ecol. 79, 15–21 [DOI] [PubMed] [Google Scholar]
- 46.Schradin C, Pillay N. 2004. The striped mouse (Rhabdomys pumilio) from the succulent karoo of South Africa: a territorial group living solitary forager with communal breeding and helpers at the nest. J. Comp. Psychol. 118, 37–47 10.1037/0735-7036.118.1.37 (doi:10.1037/0735-7036.118.1.37) [DOI] [PubMed] [Google Scholar]
- 47.Schubert M, Pillay N, Schradin C. 2009. Parental and allo-parental care in a polygynous mammal. J. Mammal 90, 724–731 10.1644/08-MAMM-A-175R1.1 (doi:10.1644/08-MAMM-A-175R1.1) [DOI] [Google Scholar]
- 48.Schoepf I, Schradin C. 2012. Better off alone! Reproductive competition and ecological constraints determine sociality in the African striped mouse (Rhabdomys pumilio). J. Anim. Ecol. 81, 649–656 10.1111/j.1365-2656.2011.01939.x (doi:10.1111/j.1365-2656.2011.01939.x) [DOI] [PubMed] [Google Scholar]
- 49.Schradin C, Schubert M, Pillay N. 2006. Winter huddling groups in the striped mouse. Can. J. Zool. 117, 317–324 [Google Scholar]
- 50.Eggert A-K. 1992. Alternative male mate-finding tactics in burying beetles. Behav. Ecol. 3, 243–254 10.1093/beheco/3.3.243 (doi:10.1093/beheco/3.3.243) [DOI] [Google Scholar]
- 51.Müller JF, Braunisch V, Hwang W, Eggert A-K. 2006. Alternative tactics and individual reproductive success in natural associations of the burying beetle, Nicrophorus vespilloides. Behav. Ecol. 18, 196–203 10.1093/beheco/arl073 (doi:10.1093/beheco/arl073) [DOI] [Google Scholar]
- 52.Eggert A-K, Müller JK. 2000. Timing of oviposition and reproductive skew in cobreeding female burying beetles (Nicrophorus vespilloides). Behav. Ecol. 11, 357–366 10.1093/beheco/11.4.357 (doi:10.1093/beheco/11.4.357) [DOI] [Google Scholar]
- 53.Reyer HU. 1980. Flexible helper structure as an ecological adaptation in the pied kingfisher (Ceryle rudis rudis L.). Behav. Ecol. Sociobiol. 6, 219–227 10.1007/BF00569203 (doi:10.1007/BF00569203) [DOI] [Google Scholar]
- 54.Reyer H-U. 1984. Investment and relatedness: a cost/benefit analysis of breeding and helping in the pied kingfisher (Ceryle rudis). Anim. Behav. 32, 1163–1178 10.1016/S0003-3472(84)80233-X (doi:10.1016/S0003-3472(84)80233-X) [DOI] [Google Scholar]
- 55.McGuire B, Getz LL. 1998. The nature and frequency of social interactions among free-living prairie voles (Microtus ochrogaster). Behav. Ecol. Sociobiol. 43, 271–279 10.1007/s002650050491 (doi:10.1007/s002650050491) [DOI] [Google Scholar]
- 56.Lucia KE, Keane B, Hayes LD, Lin YK, Schaefer RL, Solomon NG. 2008. Philopatry in prairie voles: an evaluation of the habitat saturation hypothesis. Behav. Ecol. 19, 774–783 10.1093/beheco/arn028 (doi:10.1093/beheco/arn028) [DOI] [Google Scholar]
- 57.Ophir AG, Phelps SM, Sorin AB, Wolff JO. 2008. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim. Behav. 75, 1143–1154 10.1016/j.anbehav.2007.09.022 (doi:10.1016/j.anbehav.2007.09.022) [DOI] [Google Scholar]
- 58.Solomon NG, Crist TO. 2008. Estimates of reproductive success for group-living prairie voles, Microtus ochrogaster, in high-density populations. Anim. Behav. 76, 881–892 10.1016/j.anbehav.2008.01.028 (doi:10.1016/j.anbehav.2008.01.028) [DOI] [Google Scholar]
- 59.Berry RJ, Tattersall FH, Hurst J. 2008. Genus Mus. In Mammals of the British Isles handbook, 4th edn. (eds Harris S, Yalden DW.). Southampton, UK: The Mammal Society [Google Scholar]
- 60.Latham N, Mason G. 2004. From house mouse to mouse house: the behavioural biology of free-living Mus musculus and its implications in the laboratory. Appl. Anim. Behav. Sci. 86, 261–289 10.1016/j.applanim.2004.02.006 (doi:10.1016/j.applanim.2004.02.006) [DOI] [Google Scholar]
- 61.Schradin C, Schneider C, Yuen CH. 2009. Age at puberty in male African striped mice: the impact of food, population density and the presence of the father. Funct. Ecol. 23, 1004–1013 10.1111/j.1365-2435.2009.01569.x (doi:10.1111/j.1365-2435.2009.01569.x) [DOI] [Google Scholar]
- 62.Schradin C. 2004. Territorial defense in a group living solitary forager: who, where, against whom? Behav. Ecol. Sociobiol. 55, 439–446 10.1007/s00265-003-0733-x (doi:10.1007/s00265-003-0733-x) [DOI] [Google Scholar]
- 63.Schradin C, Pillay N. 2006. Female striped mice (Rhabdomys pumilio) change their home ranges in response to seasonal variation in food availability. Behav. Ecol. 17, 452–458 10.1093/beheco/arj047 (doi:10.1093/beheco/arj047) [DOI] [Google Scholar]
- 64.Garber PA. 1997. One for all and breeding for one: cooperation and competition as a tamarin reproductive strategy. Evol. Anthropol. 3, 187–199 (doi:10.1002/(SICI)1520-6505(1997)5:6<187::AID-EVAN1>3.0.CO;2-A) [DOI] [Google Scholar]
- 65.Bezerra BM, Souto ADS, Schiel N. 2007. Infanticide and cannabalism in a free-ranging plurally breeding group of common marmosets (Callithrix jacchus). Am. J. Primatol. 69, 945–952 10.1002/ajp.20394 (doi:10.1002/ajp.20394) [DOI] [PubMed] [Google Scholar]
- 66.Digby LJ. 1995. Infant care, infanticide, and female reproductive strategies in polygynous groups of common marmosets (Callithrix jacchus). Behav. Ecol. Sociobiol. 37, 51–61 10.1007/BF00173899 (doi:10.1007/BF00173899) [DOI] [Google Scholar]
- 67.Huck M, Löttker P, Böhle UR, Heymann EW. 2004. Paternity and kinship patterns in polyandrous moustached tamarins (Saguinus mystax). Am. J. Phys. Anthropol. 127, 449–464 10.1002/ajpa.20136 (doi:10.1002/ajpa.20136) [DOI] [PubMed] [Google Scholar]
- 68.Bothma JdP, Walker C. 1999. Larger carnivores of the African savannas. Pretoria, South Africa: van Schaik [Google Scholar]
- 69.Jackson TP. 1999. The social organisation and breeding system of Brantś whistling rat (Parotomys brantsii). J. Zool. Lond. 247, 323–331 10.1111/j.1469-7998.1999.tb00995.x (doi:10.1111/j.1469-7998.1999.tb00995.x) [DOI] [Google Scholar]
- 70.Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissue mediating mating behavior in the female guniea pig. Endocrinology 65, 369–382 10.1210/endo-65-3-369 (doi:10.1210/endo-65-3-369) [DOI] [PubMed] [Google Scholar]
- 71.Schradin C, Anzenberger G. 1999. Prolactin, the hormone of paternity. News Physiol. Sci. 14, 223–231 [DOI] [PubMed] [Google Scholar]
- 72.Wingfield JC. 2009. Hormone-behavior interrelationships in a changing environment. In Endocrinology of social relationships (eds Ellison PT, Gray PB.), pp. 74–94 Cambridge, MA: Harvard University Press [Google Scholar]
- 73.Porges SW, Carter CS. 2011. Mechanisms, mediators, and adaptive consequences of caregiving. In Perspectives from evolutionary biology, neuroscience, and the social sciences: moving beyond self-interest (eds Brown SL, Brown RM, Penner LA.), pp. 53–74 Oxford, UK: Oxford University Press [Google Scholar]
- 74.Gazzinga MS. 2011. Who`s in charge? New York, NY: HarperCollins [Google Scholar]
- 75.Papineau D. 2005. Social learning and the Baldwin effect. In Evolution, rationality and cognition: a cognitive science for the twenty-first century (ed. Zilao A.), pp. 40–60 New York, NY: Routledge [Google Scholar]
- 76.Baldwin JM. 1896. A new factor in evolution. Am. Nat. 30, 441–451 10.1086/276408 (doi:10.1086/276408) [DOI] [Google Scholar]
- 77.Krubitzer L, Kaas L. 2005. The evolution of the neocotex in mammals: how is phenotypic diversity generated? Curr. Opt. Neurobiol. 15, 444–453 10.1016/j.conb.2005.07.003 (doi:10.1016/j.conb.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 78.Badyaev AV. 2009. Evolutionary significance of phenotypic accommodation in novel environments: an empirical test of the Baldwin effect. Phil. Trans. R. Soc. B 364, 1125–1141 10.1098/rstb.2008.0285 (doi:10.1098/rstb.2008.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silander OK, Nikolic N, Zaslaver A, Bren A, Kikoin I, Alon U, Ackermann M. 2012. A genome-wide analysis of promoter-mediated phenotypic noise in Escherichia coli. PLoS Genet. 8, e1002443. 10.1371/journal.pgen.1002443 (doi:10.1371/journal.pgen.1002443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mendoza SP. 1991. Behavioural and physiological indices of social relationships: comparative studies of New World monkeys. In Primate responses to environmental change (ed. Box HO.), pp. 311–335 London, UK: Chapman and Hall [Google Scholar]
- 81.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511 10.1098/rspb.2009.1355 (doi:10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Givnish TJ. 2002. Ecological constraints on the evolution of plasticity in plants. Evol. Ecol. 16, 213–242 10.1023/A:1019676410041 (doi:10.1023/A:1019676410041) [DOI] [Google Scholar]
- 83.VanBuskirk J, Steiner UK. 2009. The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 22, 852–860 10.1111/j.1420-9101.2009.01685.x (doi:10.1111/j.1420-9101.2009.01685.x) [DOI] [PubMed] [Google Scholar]
- 84.Lacey EA, Wieczorek JR. 2003. Ecology of sociality in rodents: a Ctenomyid perspective. J. Mammal 84, 1198–1211 10.1644/BLe-014 (doi:10.1644/BLe-014) [DOI] [Google Scholar]
- 85.Solomon NG, Keane B. 2012. Make space enough between you: Intraspecific variation in animal spacing. In Animal behaviour, vol. 3 (ed. Yasukawa K.). Westport, CT: Praeger [Google Scholar]