Abstract
Here, we review comparative studies of African mole-rats (family Bathyergidae) to explain how constraints acting at the ultimate (environmental) and proximate (organismal) levels have led to convergent gains and losses of sociality within this extensive adaptive radiation of subterranean rodents endemic to sub-Saharan Africa. At the ultimate level, living in environments that range from mesic through to arid has led to both variation and flexibility in social organization among species, culminating in the pinnacle of social evolution in the eusocial naked and Damaraland mole-rats (Heterocephalus glaber and Fukomys damarensis). The common mole-rat (Cryptomys hottentotus) provides a model example of how plasticity in social traits exists within a single species inhabiting areas with different ecological constraint. At the proximate level, reproductive strategies and cooperative breeding may be constrained by the correlated evolution of a suite of traits including physiological suppression of reproduction, the development of physiological and morphological castes, and the mode of ovulatory control and seasonality in breeding. Furthermore, recent neurobiological advances indicate that differential patterns of neurotransmitter expression within the forebrain may underpin (and limit) either a solitary or group living/cooperative lifestyle not only in mole-rats, but also more widely among disparate mammalian taxa.
Keywords: Bathyergidae, social evolution, cooperative breeding, African mole-rat, reproductive skew, eusociality
1. Introduction
Within Mammalia, rodents are an excellent taxon with which to investigate the dynamic nature of social behaviour; they are the largest mammalian order consisting of more than 2000 species, occurring in all the main habitats on every continent except Antarctica. Their diverse social and reproductive systems, but relative ease of studying in the wild and captivity often make them a model system of choice for hypothesis testing of the proximate mechanisms, and ultimate causation of a range of behavioural phenomenon [1].
African mole-rats of the family Bathyergidae form the largest of the extant African (Phiomorph) families in the rodent suborder Hystricomorpha. Since the discovery of eusociality in the naked mole-rat (Heterocephalus glaber) by Jarvis [2], there has been increasing interest in the family as a model mammalian system for understanding/unravelling the evolution and maintenance of vertebrate sociality and cooperative breeding. Jarvis adopted the definition of eusociality derived for social insects, i.e. species having a reproductive division of labour, overlapping generations and cooperative care of young. The unusual characteristics exhibited by naked mole-rats and other bathyergids result from adaptations to the extreme demands of the subterranean niche, culminating in the unique ‘insect-like’ social system of the former. A mole-rat living underground as part of a complex cooperatively breeding society requires a suite of neurobiological and physiological specializations imposed by the constraints that this lifestyle demands, while retaining some flexibility to respond to environmental changes (e.g. variation in rainfall and food supply). These specializations may differ markedly among mole-rat species, because some taxa have adopted a strictly solitary lifestyle, and lack social tolerance and the ability to form long-term social bonds. Thus, across the family Bathyergidae, at the species level plasticity in the response to exploiting the subterranean niche has resulted in a wide spectrum of social organization (from solitary to eusocial). However, at the extremes of this spectrum, specialization may restrict intraspecific flexibility in social structure, especially for the solitary species where neurobiological factors and the resultant behaviours may preclude sustained group living.
In this review, we will consider how living in environments that range from mesic through to arid has led to variation in social organization among African mole-rat species through convergent gains and losses of traits. We will discuss how, at the proximate level, reproductive strategies and cooperative breeding may be constrained not only by neural substrates, but also by the correlated evolution of a suite of other traits, including physiological suppression of reproduction, the development of physiological and morphological castes, and the mode of ovulatory control and seasonality in breeding.
2. Background: phylogeography and distribution
African mole-rats represent an extensive adaptive radiation of subterranean rodents across sub-Saharan Africa. A recently revised estimate of biodiversity in the family suggests 30 or more species comprising six genera: eusocial Heterocephalus (with the monotypic naked mole-rat), solitary dwelling Heliophobius, Bathyergus and Georychus, social Cryptomys and social/eusocial Fukomys (figure 1a) [3–8]. Mole-rats may be found from the Cape region of South Africa through to disjunct populations in Southern Sudan in the north, Somalia in the east and Ghana in West Africa. Over much of their range, speciation and diversity within the family appear to have been influenced by the physical, ecological and climatic changes associated with the formation of the African Rift Valley, with cladogenesis associated with major episodes of volcanism [4–6]. Additionally, shifting patterns of drainage evolution are of particular significance for populations of Fukomys in the Zambezian region of South-Central Africa, resulting in extensive vicariance and possible speciation events [8,9].
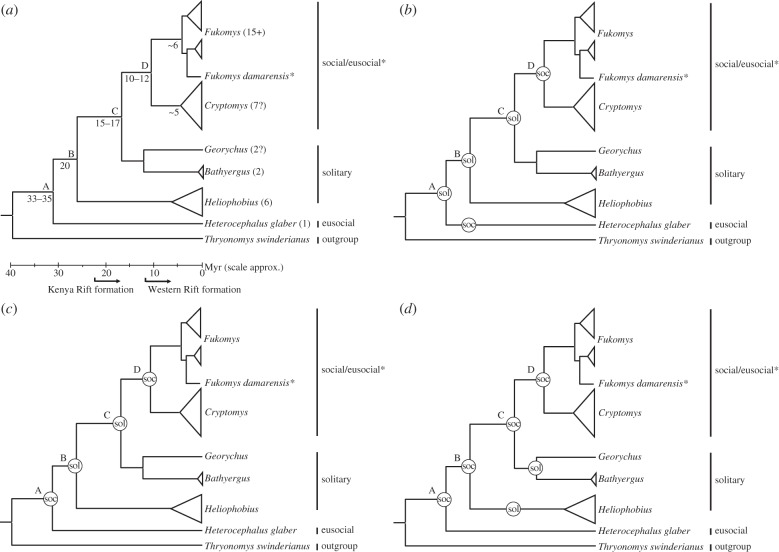
Figure 1.
(a) Simplified phylogeny for the Bathyergidae indicating main clades/genera, together with the closest extant outgroup, the cane rat Thryonomys swinderianus, and based on mitochondrial 12S rRNA and cytochrome b sequence data. Numbers on internal nodes represent divergence times in millions of years ago (Myr) estimated using a molecular clock approach, and using the bathyergid fossil Proheliophobius for calibration of genetic distances. Numbers in parentheses indicate current estimates of species numbers in each genus. (b–d) Represent alternative hypotheses for convergent gains and losses in solitarity/sociality, dependent on the status of the common ancestor of the family: (b) with a solitary common ancestor, sociality arises independently twice in Heterocephalus and the common ancestor of Fukomys/Cryptomys, with further elaboration to eusociality in extant H. glaber and F. damarensis; with a social common ancestor, either sociality is lost in the common ancestor of Heliophobius and descendent lineages to re-emerge in Fukomys/Cryptomys (c), or retained in common ancestors with loss and the evolution of a solitary lifestyle in lineages leading to Heliophobius, Bathyergus and Georychus (d). Other, less parsimonious explanations are also possible (data adapted from earlier studies [3–8]).
Over this wide distributional range mole-rats inhabit a variety of soil types in different biomes and climatic zones, and some general trends are evident. First, as they feed exclusively on underground roots and tubers, this is the overriding constraint on their distribution—for example, they are not found in heavily forested regions or in extreme deserts. Second, solitary mole-rats (Heliophobius, Bathyergus and Georychus) are mainly restricted to mesic regions of higher rainfall (with a moderate and predictable pattern of precipitation greater than 400 mm per annum). The social genera (Cryptomys and Fukomys) are found in both mesic and xeric (low and unpredictable rainfall of less than 400 mm per annum) regions. Heterocephalus occurs exclusively in the xeric regions of East Africa (parts of Kenya, Ethiopia and Somalia). On average, the arid regions inhabited by mole-rats may have only four months per year having more than 25 mm of rain (approximately the quantity required to soften the soil at the depth of foraging tunnels and thus facilitate burrowing) [10]. This association between the distribution of extant species with different social phenotypes and ecological constraints has led to formulation of the aridity food distribution hypothesis (AFDH) as an ultimate explanation for the evolution of sociality in the Bathyergidae (see §4 below).
3. Inferring convergent gains and losses of sociality
Before embarking on a detailed discussion of the AFDH and ultimate drivers of social evolution in the Bathyergidae, it is important to consider the occurrence of sociality and cooperative breeding within an evolutionary context, and thus enable a comparative phylogenetic approach to understanding their social evolution. Figure 1b–d illustrates three possible scenarios that would explain the observed pattern of sociality across the genera, varying according to what the social status of the common ancestor of the family was (node A in figure 1). Inferences may be made by extending the phylogeny to also consider the status of the extant Phiomorph outgroups of the Bathyergidae, which are dassie rats (Petromuridae), cane rats (Thryonomyidae) and Old World porcupines (Hystricidae). While detailed studies on the sociobiology of these taxa are sparse, the close outgroup Petromus typicus (the dassie rat) is reported to exhibit social monogamy forming strong social bonds, low levels of inter-generational aggression and paternal care [11]. Phylogenetic character mapping to reconstruct ancestral states within the Bathyergidae gives ambiguous results, with likelihood and maximum-parsimony approaches producing equivocal states for the ancestors at nodes A, B and C in figure 1. What is unequivocal is that irrespective of the status of the bathyergid common ancestor at node A, there have been convergent gains and losses of sociality within the family (figure 1b,c), presumably in response to changing environmental pressures (ultimate factors), such as climate change and tectonics.
4. Ultimate factors: an ecological constraints model for social evolution
The subterranean niche confers a number of advantages, such as protection from predators and buffering of thermal extremes. In terms of predation and predator avoidance itself being a driver for social evolution in the Bathyergidae, it is not thought that these play a major role as causative factors because predators (‘mole-snakes’ and birds of prey) are prevalent across all mole-rat habitats, yet levels of sociality differ. Despite the advantages, there are also considerable costs and constraints associated with living underground: dispersal and new burrow formation may be costly as digging through soil is energetically expensive, perhaps up to 3600 times that of surface locomotion [12]. Costs of burrowing may vary with soil hardness and moisture content [13], which may in turn vary among different habitats, or within a habitat between the seasons (e.g. before and after rains). It is these ultimate constraints that are hypothesized as drivers of social evolution in African mole-rats.
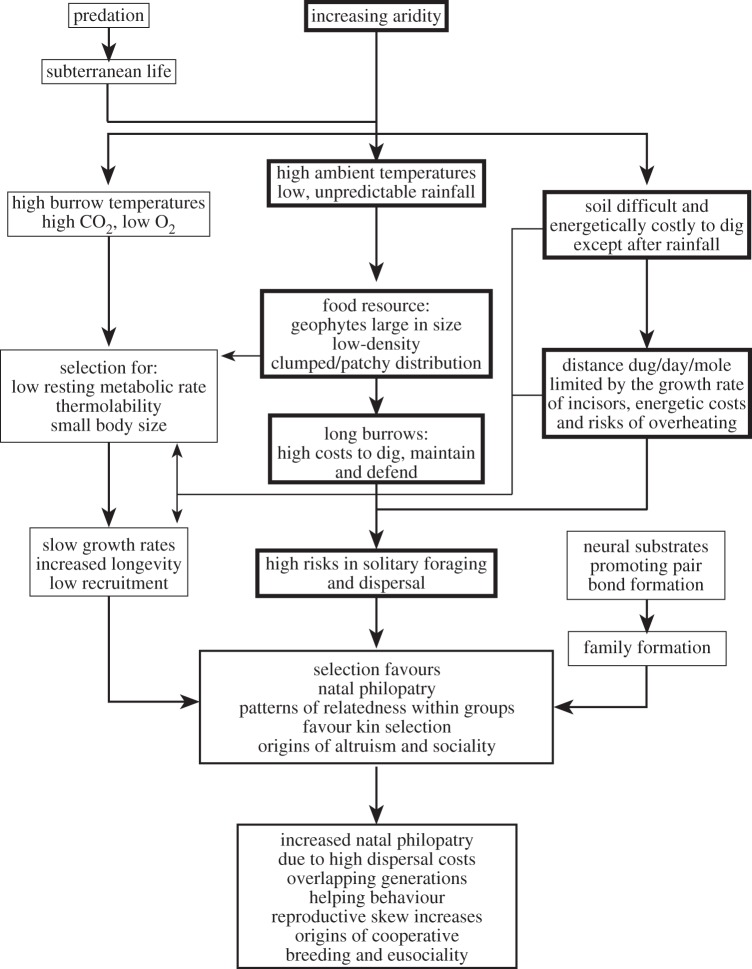
All African mole-rats feed on underground roots and tubers (geophytes) and Bathyergus may also supplement its diet with above-ground forbes and grasses, which are encountered by digging foraging tunnels from the central core of the burrow system (for review see [14]). The distribution of these roots and tubers eaten by mole-rats varies with habitat. In mesic regions, these food resources are more uniformly distributed, whereas in xeric regions of low or unpredictable rainfall, the plants are arid-adapted and more widely dispersed, or occur in high-density clumps that are widely dispersed and patchily distributed. The AFDH brings together these environmental constraints as an explanation for social evolution in the Bathergidae (figure 2). It posits that increased natal philopatry leading to cooperative breeding, and ultimately eusocial behaviour in African mole-rats may have evolved in response to the unpredictable rainfall patterns of the habitat, its effects on their food distribution, and the resulting costs and associated risks of unsuccessful foraging, dispersal and new colony formation [3,10,15–18]. In such a scenario, sociality would be adaptive, as cooperative foraging and burrow maintenance distributes the energetic costs of burrowing among colony members and increases the probability of finding food. The clumped nature of the geophytes and, in some cases, their large size, ensures that these resources are potentially sufficient to sustain large groups of animals. The AFDH is dependent on individuals having all the necessary neurological substrates in place to facilitate social tolerance, extended periods of philopatry and thus group living and ultimately cooperative behaviour. Thus, populations of mole-rats that are subject to changing climate (for example aridification) or that are expanding from mesic into more arid environments will be under strong selection for genotypes that give rise to social phenotypes. An increasing body of work is now identifying candidate genes and the mutations that may underpin differences in the expression of social behaviour within and among species (see §5 below).
Figure 2.
Summary of the ultimate and proximate factors, and their interrelationships, thought to be important in the evolution of sociality and cooperative breeding in the Bathyergidae. Boxes outlined with bold lines indicate the principal components of the aridity food distribution hypothesis (AFDH). Modified from Bennett & Faulkes [14].
Support for the AFDH playing at least a role in the evolution of sociality comes from both inter- and intraspecific studies across the Bathyergidae. A phylogenetically controlled comparative analysis of data from across the family revealed significant relationships as predicted by the AFDH between social group size and geophyte density, the coefficient of rainfall variation and the mean number of months per year where rainfall exceeded 25 mm. Interspecific analyses of burrow geometry using calculations of fractal dimension clearly demonstrate that in arid habitats, colonies containing larger numbers of individuals are able to explore their habitat more effectively with a greater degree of complexity of the foraging tunnels than smaller groups [19]. In addition, studies of burrow geometry within a single social species (Fukomys mechowii) further support the AFDH, where burrow fractal dimension increased with colony size and was higher during the wet season than during the dry season [20]. Furthermore, a long-term field study of eusocial Damaraland mole-rats has shown that larger colonies are more likely to survive when environmental conditions are at their most extreme (as observed during an extended period of drought), again emphasizing the adaptive advantage of sociality [18].
An assumption implicit in the AFDH is that increased aridity should constrain and reduce dispersal and increase within-group relatedness (R), and the limited empirical data available from appropriate intra-specific comparisons support this. Hess [21] used microsatellite genotyping to estimate relatedness in colonies of the naked mole-rat across an aridity gradient in Kenya. Although the sample size was small, a weak but statistically significant correlation was found in support of aridity constraining dispersal. Similarly, comparison of intra-colony relatedness in two populations of Damaraland mole-rats revealed a lower R value at Hotazel, South Africa, compared with a more arid site at Dordabis, Namibia [22]. Within-species comparisons of philopatry and dispersal in arid and mesic-dwelling populations of the common mole-rat (Cryptomys hottentotus hottentotus) in South Africa have also shown that immigration and emigration were lower at an arid site than at a mesic one, indicating that constraints on dispersal are higher in areas of low and unpredictable rainfall [23]. Furthermore, behavioural tests on animals from the arid population revealed substantially higher levels of rejection of foreign conspecifics in dyadic encounters than the mesic population, suggesting increased xenophobia in the former [24]. These differences in dispersal indicate adaptive variation in social behaviour between the regions, and strongly suggest that delayed dispersal, sociality and cooperation may be more crucial to individual survival in arid than in mesic areas. As such, these findings provide persuasive support for the underlying contention of the AFDH, that ecological constraints on successful dispersal and/or colony formation in arid areas have promoted a greater degree of social cohesion in mole-rats occurring in these regions. Confirmation that sociality and group size are adaptive is difficult to determine empirically. However, in field studies of Damaraland mole-rats, there are significant positive effects of group size on offspring recruitment and survival (A. J. Young, N. C. Bennett and J. U. M. Jarvis 1987–1999, unpublished data), and colonies of Damaraland mole-rats with larger group sizes have a higher survival [18]. Burda et al. [25] argue against a causal relationship between aridity, cooperative foraging for dispersed food resources and the evolution of sociality in mole-rats. They suggest instead that the social behaviour of mole-rats is a result of an ancestral tendency for ‘cooperative monogamy’ reinforced by a subterranean lifestyle that constrains dispersal (as contended by the AFDH). Clearly, at any particular point in time, an organism's phenotype is phylogenetically constrained; an ancestral mole-rat would need to either have a predisposition to family formation to facilitate cooperative behaviour, or in a changing environment selection may have favoured individuals with genotypes that enable expression of a more socially tolerant phenotype. Such a mutation would be highly adaptive in the right conditions, e.g. where ecological constraints were high, and rapidly spread through the population. Gains and losses of social phenotype appear to have occurred more than once in the Bathyergidae, and the proximate factors and mechanisms that underlie these changes will be discussed below.
5. Proximate factors
(a). Neurobiology of social behaviour
African mole-rats are extraordinary among mammals in the diversity and range of social strategies adopted by the member species, and as such, they offer a unique model system with which to address the neurobiology of sociality, together with the genetics underlying different neurobiological phenotypes. In mammals, the evolution of cooperative breeding is restricted to socially monogamous species [26], which are comparatively uncommon among mammals (5% of all mammalian species) [27]. Pair bonding, the selective and enduring attachment of a male and female, is the defining feature of monogamy. Recent studies indicate that the expression of such behaviour is dependent on particular neurobiological phenotypes, and, therefore, possession of such a phenotype is a prerequisite to pair bonding, monogamy and sociality. Key insights into the neurobiological substrates underlying the formation of monogamous and/or parent-offspring bonds have been obtained from comparative studies of voles (genus Microtus) that are either monogamous and social, with bi- and alloparental care, or promiscuous and solitary [28]. In this context, the neuropeptides oxytocin (OXT) and vasopressin (AVP) have been shown to be involved in modulating sociality, pair bonding and aggression [29]. Specifically, the pattern and density of receptors for OXT and AVP (the V1a receptor) differ significantly between promiscuous and monogamous voles (Microtus spp.) and mice (Peromyscus spp.) [30]. Social functions depend on whether OXT or AVP receptors are expressed at certain forebrain sites, including the nucleus accumbens and ventral pallidum; these sites incorporate dopaminergic projections from the ventral tegmental area, which signal rewards associated with cues for identifying mate, offspring, or kin [28]. Levels of both OXT receptors and OXT receptor transcript expression in the nucleus accumbens are higher in monogamous prairie voles than in promiscuous, solitary montane voles [31]. Recently, it has also been determined that the eusocial naked mole-rat exhibits higher levels of OXT receptor binding than the solitary Cape mole-rat (Georychus capensis) in several significant regions of the forebrain. As with social voles, OXT receptor levels in naked mole-rats are intense and extensive in the nucleus accumbens, whereas OXT receptors are not detectable in the Cape mole-rat in this area [32]. This abundance of OXT receptor levels in the nucleus accumbens of naked mole-rats reflects their high levels of sociality, alloparenting behaviour and potential for reproductive attachments, whereas the reduced oxytocinergic signalling at this site in Cape mole-rats reflects a paucity of prosocial behaviours. Furthermore, there is a remarkable correspondence between the OXT receptor levels in some areas of the brain in the eusocial naked mole-rat and the monogamous and pair-bonding prairie vole at one end of the spectrum, and the asocial Cape mole-rat and the solitary, non-pair-bonding and promiscuous voles at the other [28,32]. Recent studies have confirmed that the Damaraland mole-rat also resembles the naked mole-rat (and social voles) with respect to OXT receptor binding being present in the nucleus accumbens. These observations suggest convergent evolution across different rodent suborders for this particular neurobiological phenotype underpinning aspects of social behaviour.
AVP has also been found to facilitate social behaviours in a number of species, including voles, and in the formation of affiliations by males AVP acting through the V1a receptor may play a more significant role than OXT, with its critical actions occurring within the ventral pallidum rather than the nucleus accumbens [28]. Comparisons between naked and Cape mole-rats have found that different distributions of V1a in several brain areas between these two species suggest an association between the vasopressinergic system and prosocial behaviours [33]. However, the patterns of receptor distribution are different to that seen in the monogamous/social and promiscuous/asocial vole model. Conversely, the high V1a receptor binding seen in the ventral pallidum of monogamous/social voles is not seen in eusocial mole-rats, but it is apparent in this region in solitary Cape mole-rats although this is not matched by presence of V1a peptide. Further, the Damaraland mole-rat unexpectedly has strong V1a receptor and peptide (AVP) binding in the nucleus accumbens, which is not seen in either social voles or naked mole-rats. Collectively, these studies suggest a combination of the retention of a particular neurological substrate (OXT receptor expression in the nucleus accumbens) and the emergence of a new one (AVP receptor expression in the nucleus accumbens) in Damaraland mole-rats, leading to the convergent evolution of social behaviour (arguing for a scenario as in figure 1b or c). Investigation of other mole-rat species will be informative in understanding the evolutionary gains and losses of these neural substrates within the family. A recent study on another social species, Ansell's mole-rat Fukomys anselli [34], examined the distribution of OXT and AVP immunoreactive neurons, but not receptor distributions, thus preventing a full comparison across the family. Looking beyond the Bathyergidae at other Hystricognath rodents in the family Ctenomyidae, Beery et al. [35] also found marked differences in OXT and V1a receptor distributions between a social and a solitary dwelling species of tuco-tuco (Ctenomys sociabilis and Ctenomys haigi, respectively). However, they exhibited a pattern of nucleus accumbens OXT and ventral pallidum V1a receptor binding that was different from that associated with the formation of opposite-sex pair bonds in voles; in particular, binding was completely absent in the nucleus accumbens. Thus, in the Ctenomyidae, it would appear that different neural mechanisms may underpin the proximate maintenance of pair bond formation and sociality.
(b). Colony composition, cooperative behaviour and divisions of labour
At one end of the social continuum seen in African mole-rats are the asocial, strictly solitary and highly xenophobic species, where mating couples only pair up for the briefest of periods during a defined breeding season, and offspring leave the natal burrow soon after weaning [36–39]. At the opposite end of the spectrum, the eusocial species exhibit extreme reproductive skew; reproduction is confined to a single female per colony (the queen) and one to three breeding male consorts, with offspring remaining philopatric and undertaking cooperative behaviours of one form or another [2,25,40–42]. There is inter- and intraspecific variability in colony composition and reproductive and behavioural divisions of labour within the social mole-rat species (e.g. group size, skew in lifetime reproductive success and behavioural polyethism) [43,44]; hence, sociality seems to offer greater flexibility in response to the prevailing environmental constraints. The breeders constitute a clearly defined caste (i.e. a distinct group performing a specialized function) within colonies of all social mole-rats and can be discerned by their morphology and physiology [45–48]. However, extra-colony copulations and paternities may also occur to a greater or lesser extent, producing variance in lifetime reproductive success depending on the species, as discussed below [22,43,49,50].
The non-breeding colony members of both sexes may show varying patterns of task specialization and differences in the frequencies of cooperative ‘worker’ behaviour. While there are species-specific differences, there may also be variation within a species depending on colony age and size—this produces a complex relationship between body mass, age and the role of an individual within a colony. For example, in the genus Cryptomys, it appears that all colony members carry out such activities, performing work with a similar frequency [51,52]. These worker tasks include digging and maintaining foraging burrows, and provisioning food in both storage chambers and the nest chamber. By contrast, within the genus Fukomys, colonies of the eusocial Damaraland mole-rat (Fukomys damarensis) may have distinct groups of individuals that perform work-related tasks to differing degrees. In effect, there are smaller (but not necessarily younger) animals that perform a large proportion of the daily burrow maintenance activity and other larger (but not always older) individuals that perform little or no worker activities. This may in some colonies result in distinct groups of frequent workers, infrequent workers and non-workers [40,47]. In the Damaraland mole-rat, non-breeding workers may also carry out alloparental care such as pup grooming and retrieval of wandering pups to the nest chamber [40,47,53–55]. A similar work-related division of labour is also found in eusocial naked mole-rats which also results in a body size polyethism; all animals born enter a frequent worker group, then as they increase in body mass with age, work progressively less until they may enter a ‘non-worker’ group. Slower growing individuals may remain in the frequent worker caste, perhaps permanently, whereas faster growing animals become infrequent or non-workers or in a minority of cases, breeders. Conversely, ‘defence’-related behaviours, such as patrolling the burrow and guarding the nest chamber, are carried out with increasing frequency as individuals increase in body size. These larger individuals are also involved in defence against conspecifics and predators (snakes) [2,56,57].
In addition to the aforementioned and sometimes striking variation in growth rates observed among some individuals within litters, plasticity in growth rates is also seen between litters, particularly in newly establishing colonies. Bennett & Navarro [58] found that Damaraland mole-rats born as first and second litters to new pairs of breeding animals grew faster, reached higher absolute growth rates and attained greater body mass than subsequent litters. This phenomenon has also been observed in the naked mole-rat; the offspring from the first two litters in a newly founded colony are the only ones where age and body mass covary for all individuals, and they also attain the greatest body mass. Growth functions also vary significantly between litters and there is an inverse trend between asymptotic body mass and litter order, with many individuals remaining small within increasing orders of litters [59,60].
In addition to correlations between body mass and worker and defence behaviours, growth, body size, and dominance and reproductive status are also linked. Dominance rank position and breeding status correlate significantly with body size in both sexes, and growth responses in non-breeders may be triggered following the death or removal of breeding animals from a colony, especially among older litter members—attainment of reproductive status is almost always accompanied by a growth spurt in the individual [60–62]. The proximate physiological cause and control of the differential growth rates observed within and among litters in both Damaraland and naked mole-rats is intriguing, and the underlying mechanism not understood. One might speculate that it could be in part due to dominance/agonistic interactions among individuals, and the physiological consequences arising from these. It is unlikely that the greater growth rates recorded in first born litters may reflect less competition for parental care in those litters when compared with later born litters, as has been shown in some communally rearing rodents. Because mole-rats have long gestational periods and inter-birth intervals (the average is 80 and 90 days between litters in naked and Damaraland mole-rats respectively), by the time a new litter arrives previous pups are no longer receiving parental care and have been recruited into the workforce. Importantly, in both eusocial mole-rat species the variation in growth and body size polyethism enables a colony to rapidly and flexibly express a spectrum of behaviours essential for survival in response to high ecological constraints. In a remarkable example of convergent evolution, there are analogies in the allocation of behavioural roles and the flexible behavioural polyethism observed within colonies of eusocial mole-rats, and social Hymenoptera such as bees. Among social insects, behavioural division of labour is also characterized by a temporal polyethism, and, at least in bees, may also show variation among hives (or colonies) in the age of onset of particular behaviours. The division of labour in the honeybee is characterized by younger workers remaining in the hive and performing tasks there, whereas older workers perform more risky outside tasks, mainly foraging. Similarly in mole-rats, smaller animals undertake less risky foraging than the more risky defence behaviours seen in larger animals. Beshers et al. [63] suggest a model to explain this (that may also be appropriate to mole-rats) based on, first, an intrinsic process of behavioural development that is associated with physiological changes (with important roles played by juvenile hormone and octopamine). Second, an inhibition of development is dependent on the colony age demography, and mediated through social interactions among the workers in a honeybee colony, whereby the presence of foragers inhibits the maturation of younger bees to become foragers. This is analogous to the differential patterns of growth observed in successive litters of mole-rats.
Whether social mammals, like some social insects, also become so specialized that they form specific morphologically distinct castes is a contentious question and one that has generated much discussion, particularly in relation to what defines a eusocial species [64,65]. While the presence of fixed and distinct work-related behavioural castes is not unanimously supported in mole-rats, there is good evidence that both Damaraland and naked mole-rats have reproductively distinct castes. The first such discovery was the ‘disperser morph’ male phenotype in naked mole-rats, particular males that are behaviourally, hormonally and morphologically distinctive [45]. Subsequently, the morphological distinctiveness of the queen resulting from vertebral lengthening has been reported [46]. Similar adaptations have also been shown to occur in the Damaraland mole-rat, with larger infrequent workers thought to constitute a physiologically distinct dispersing caste who build up their own body reserves in preparation for dispersal and reproduction when environmental conditions are suitable (after periods of rainfall) [47]. Further, Young & Bennett [48] have recently shown a morphological divergence in body shape between breeders and non-breeding helpers, resulting from a modification of the growth trajectory of non-breeders upon on the acquisition of dominant breeding status. As with the naked mole-rat, the reproductive female Damaraland mole-rat develops a distinctly longer body relative to the skull size. Intriguingly, however, the change in shape among newly dominant Damaraland mole-rat females is achieved not through a sudden body length growth spurt via vertebral elongation as reported for the naked mole-rat [46,66]. This occurs as a result of a slight decline in body length growth, coupled with a large reduction in skull width growth. Young and Bennett speculate that this socially induced plasticity in growth, and resulting morphological divergence of dominants and subordinates may reflect a reproductive status-dependent switch in resource allocation towards maximizing reproductive output rather than investing in growing the tools of the workforce (i.e. the skull and associated incisors). These subtle differences in the proximate control of social factors between naked and Damaraland mole-rats reflect the independent origin of eusociality within the Bathyergidae and exemplify the flexibility in response to similar ecological constraints.
(c). Reproduction and reproductive suppression
In the subterranean niche, the costs of finding a mate and establishing a new colony are potentially much greater than those of surface dwelling species, owing to the high energetic costs of burrowing and other ecological constraints [12]. Both solitary and social species of mole-rats need to respond appropriately and flexibly to these constraints, and, not surprisingly given their geographical range, selection has favoured a number of physiological and behavioural adaptations. These include variation in the mode of ovulation, seasonality in breeding and, in social species, variation in reproductive skew. In some cases the latter is brought about by an extreme socially induced suppression of reproductive physiology in subordinate colony members. During the dry season or periods of prolonged drought, soils become hard and are generally not favourable for burrowing in [67]. However, the onset of rainfall softens the soil, increases food abundance and reduces the costs of mate searching in the solitary species, or dispersal and subsequent mate acquisition in social species. Thus, strong selective pressure for both seasonal reproduction and induced ovulation, where mating triggers ovulation, reducing the latency from pairing to conception, has led to the evolution of these traits in solitary species of mole-rats inhabiting regions with a predictable and seasonal rainfall (Georychus and Bathyergus) [38,40,68–70]. This is mirrored in Cryptomys, where induced ovulation occurs in the cooperatively breeding highveld mole-rat, Cryptomys hottentotus pretoriae, and the Natal mole-rat, Cryptomys hottentotus natalensis [71,72]. A common characteristic of these species, apart from the Natal mole-rat, is that they are all either seasonally breeding and or dependable on regular and predictable but short lived mating opportunities. Interestingly, the Natal mole-rat appears to have retained the trait of induced ovulation while exploiting higher altitude habitats where rainfall tends to be high throughout the year.
In contrast, all species studied so far from the social genera Fukomys and Heterocephalus are spontaneous ovulators and may breed throughout the year [73–76]. In some species this appears to be in response to a reduction in seasonality at lower latitudes in habitats where rainfall is relatively high (e.g. F. mechowii and F. anselli) [20,77]. Comparative phylogenetic analysis has revealed a positive correlation between seasonality in breeding and induced ovulation. A likelihood-based reconstruction suggests that the ancestral state is induced ovulation in the Bathyergidae and that this trait has been convergently lost in at least these two lineages of cooperatively breeding mole-rats exhibiting spontaneous ovulation [75].
Eusocial naked and Damaraland mole-rats, although phylogenetically divergent, are found in similar harsh habitats where rainfall is sporadic and unpredictable, with high costs to dispersal. The resultant high degree of natal philopatry and cooperative behaviour required to exploit this niche necessitates a high reproductive skew, as predicted by theories of optimal reproductive skew [43]. There are differences in the proximate maintenance of optimal skew, dependent on the species, with key factors being inbreeding avoidance and the level of environmental constraint limiting dispersal. In the extreme, naked mole-rats are unique in that skew is maintained by a suppression of reproductive physiology, resulting in the disrupted production of mature gametes in non-breeding animals of both sexes [73,78]. This socially induced reproductive suppression is mediated by dominance and interactions with the breeding queen. It is necessary because naked mole-rats are facultative inbreeders, and have no hesitation in mating with close relatives should there be no opportunities for outbreeding—although the latter is the preferred option [79]. The Damaraland mole-rat is enigmatic in that it has both components of incest avoidance and a suppression of female reproductive physiology [80]. Because they are strict outbreeders, incest avoidance alone could be sufficient to maintain high reproductive skew if their colonies were founded by unrelated opposite-sexed conspecifics, and contained just their offspring. However, this is not always the case, and although, like the naked mole-rat, the Damaraland mole-rat shows a high degree of natal philopatry, during periods of good rain unrelated dispersing individuals may enter functionally complete colonies. In such instances, an immigrating unrelated male could potentially mate with non-reproductive females, if the latter were not physiologically suppressed by the breeding female. Thus, by enforcing suppression on the non-reproductive females, the reproductive female ensures that plural breeding does not arise within the colony, high skew is maintained and non-breeders are available to work cooperatively [22,49,80].
In the obligate outbreeding social species of Cryptomys and Fukomys inhabiting mesic environments, where regular opportunities for dispersal and establishing independent colonies are available, the chances of establishing a new colony and to accrue reproductive success are relatively high. These mesic-adapted species have not evolved a physiological suppression, and rely solely on incest avoidance to maintain reproductive skew in colonies. Thus an immigrating unrelated male to the colony may enable the reproductive female opportunity for extra-pair copulations, but also potentially mate with subordinate females. This produces flexibility in reproductive strategy within the cooperative breeding framework, and variation in lifetime reproductive success. For example, in a study by Bishop et al. [50], paternity was not always assigned to the phenotypically distinguishable breeding male—both extra-colony males and subordinate males within colonies, hitherto classed as ‘non-breeders’, were also responsible for paternities. Further, there were significant differences in the proportions of these assignments to different males, depending on the habitat. At a mesic site where ecological constraints on dispersal were lower, only 19 per cent of within-colony paternities were assigned to the ‘breeding male’, with 81 per cent owing to a different within-colony male. Overall, 29 per cent of paternities were assigned to extra-colony males. Conversely, at an arid site where dispersal costs were higher, 79 per cent of within-colony paternities were assigned to the ‘breeding male’; overall, 18 per cent of paternities at the site were assigned to extra-colony males. Although suppression of reproductive physiology is not seen in this species, plural breeding among females is extremely rare, despite opportunities for mating with unrelated individuals. These disparate results are a clear exemplar of alternative reproductive strategies for males of this species, depending on the environmental conditions and the ease of movement of males between colonies, and it is likely that similar strategies will be found in other as yet unstudied mole-rat species.
6. Conclusions
Molecular phylogenies have enabled robust comparative analyses of the Bathyergidae that can estimate gains and losses of sociality, and the interplay between the ultimate and proximate factors driving the evolution and maintenance of social behaviour and cooperative breeding. These studies show that sociality and cooperative breeding requires the correlated evolution of a mosaic of traits, including, first, the appropriate neurobiological phenotype (i.e. the ability to form social bonds), followed by a raft of behavioural (e.g. mate choice/inbreeding avoidance, division of labour) and reproductive adaptations (control of ovulation and seasonality of breeding, suppression of reproduction). Given that sociality in the context of the subterranean niche appears to be highly adaptive—social mole-rats in both Fukomys and Cryptomys successfully exploit both arid and mesic habitats—we are faced with a conundrum regarding asocial species: why do they lose the neural phenotype for social tolerance and pair bond formation when it is apparently such a successful and flexible trait allowing a range of lifestyle options? Another major challenge now is to understand the genetic basis underlying the variance in neural phenotype that facilitates long-term pair bond formation, monogamy and ultimately sociality. Previous studies have shown that the V1a receptor gene differs between monogamous and social (prairie) and promiscuous and asocial (montane) voles, and this difference has been shown to determine its expression pattern and, thus, variation in male behaviour [15,16]. Whether the differential expression of OXT and V1a receptors in asocial and social African mole-rats is determined genetically in a similar way to that seen in voles remains a fascinating question to be answered. Furthermore, does genetic variance in any way account for the different developmental pathways followed by some mole-rats, for example whether they remain a worker, or become a disperser or a breeder? A role for epigenetic effects in this context remains to be investigated. The application of newly emerging genomic data should begin to shed light on some of these issues [81].
Acknowledgements
We acknowledge research grants from the National Research Foundation, the University of Pretoria as well as a DST-NRF South African Research Chair of Mammal Behavioural Ecology and Physiology award to N.C.B. Neurobiological work was supported by BBSRC project grant no. BBD5231861 (C.G.F.).
References
- 1.Wolff JO, Sherman PW. 2007. Rodent societies. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Jarvis JUM. 1981. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science 212, 571–573 10.1126/science.7209555 (doi:10.1126/science.7209555) [DOI] [PubMed] [Google Scholar]
- 3.Faulkes CG, Bennett NC, Bruford MW, O'Brien HP, Aguilar GH, Jarvis JUM. 1997. Ecological constraints drive social evolution in the African mole-rats. Proc. R. Soc. Lond. B 264, 1619–1627 10.1098/rspb.1997.0226 (doi:10.1098/rspb.1997.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faulkes CG, Verheyen E, Verheyen W, Jarvis JUM, Bennett NC. 2004. Phylogeographical patterns of genetic divergence and speciation in African mole-rats (Family: Bathyergidae). Mol. Ecol. 13, 613–629 10.1046/j.1365-294X.2004.02099.x (doi:10.1046/j.1365-294X.2004.02099.x) [DOI] [PubMed] [Google Scholar]
- 5.Faulkes C, Mgode GF, Le Comber SC, Bennett NC. 2010. Cladogenesis and endemism in Tanzanian mole-rats, genus Fukomys: (Rodentia Bathyergidae): a role for tectonics? Biol. J. Linn. Soc. 100, 337–352 10.1111/j.1095-8312.2010.01418.x (doi:10.1111/j.1095-8312.2010.01418.x) [DOI] [Google Scholar]
- 6.Faulkes CG, Bennett NC, Cotterill FPD, Stanley W, Mgode GF, Verheyen E. 2011. Phylogeography and cryptic diversity of the solitary-dwelling silvery mole-rat, genus Heliophobius (family: Bathyergidae). J. Zool. 285, 324–338 10.1111/j.1469-7998.2011.00863.x (doi:10.1111/j.1469-7998.2011.00863.x) [DOI] [Google Scholar]
- 7.Ingram CM, Burda H, Honeycutt RL. 2004. Molecular phylogenetics and taxonomy of the African mole-rats, genus Cryptomys and the new genus Coetomys Gray, 1864. Mol. Phylogenet. Evol. 31, 997–1014 10.1016/j.ympev.2003.11.004 (doi:10.1016/j.ympev.2003.11.004) [DOI] [PubMed] [Google Scholar]
- 8.Van Daele PAAG, Verheyen E, Brunain M, Adriaens D. 2007. Cytochrome b sequence analysis reveals differential molecular evolution in African mole-rats of the chromosomally hyperdiverse genus Fukomys (Bathyergidae, Rodentia) from the Zambezian region. Mol. Phylogenet. Evol. 45, 142–157 10.1016/j.ympev.2007.04.008 (doi:10.1016/j.ympev.2007.04.008) [DOI] [PubMed] [Google Scholar]
- 9.Van Daele PAAG, Dammann P, Meier JL, Kawalika M, Van De Woestijne C, Burda H. 2004. Chromosomal diversity in mole-rats of the genus Cryptomys (Rodentia: Bathyergidae) from the Zambezian region: with descriptions of new karyotypes. J. Zool. 264, 317–326 10.1017/S0952836904005825 (doi:10.1017/S0952836904005825) [DOI] [Google Scholar]
- 10.Bennett NC, Faulkes CG, Jarvis JUM. 1999. Socially induced infertility, incest avoidance and the monopoly of reproduction in cooperatively breeding African mole-rats, family Bathyergidae. Adv. Stud. Behav. 28, 75–114 10.1016/S0065-3454(08)60216-8 (doi:10.1016/S0065-3454(08)60216-8) [DOI] [Google Scholar]
- 11.Rathbun G, Rathbun C. 2006. Social monogamy in the noki or dassie-rat (Petromus typicus) in Namibia. Mamm. Biol. 71, 203–213 10.1016/j.mambio.2006.01.008 (doi:10.1016/j.mambio.2006.01.008) [DOI] [Google Scholar]
- 12.Vleck D. 1979. The energy cost of burrowing by the pocket gopher Thomomys bottae. Physiol. Zool. 52, 122–136 [Google Scholar]
- 13.Lovegrove BG. 1989. The cost of burrowing by the social mole rats (Bathyergidae) Cryptomys damarensis and Heterocephalus glaber: the role of soil moisture. Physiol. Zool. 62, 449–469 [Google Scholar]
- 14.Bennett NC, Faulkes CG. 2000. African mole-rats: ecology and eusociality. Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Jarvis JUM. 1978. Energetics of survival in Heterocephalus glaber (Ruppell), the naked mole-rat (Rodentia: Bathyergidae). Bull. Carnegie Mus. Nat. Hist. 6, 81–87 [Google Scholar]
- 16.Lovegrove BG, Wissel C. 1988. Sociality in molerats. Oecologia 74, 600–606 10.1007/BF00380059 (doi:10.1007/BF00380059) [DOI] [PubMed] [Google Scholar]
- 17.Lovegrove BG. 1991. The evolution of eusociality in molerats (Bathyergidae): a question of risks, numbers, and costs. Behav. Ecol. Sociobiol. 28, 37–45 10.1007/BF00172137 (doi:10.1007/BF00172137) [DOI] [Google Scholar]
- 18.Jarvis JUM, Bennett NC, Spinks AC. 1998. Food availability and foraging by wild colonies of Damaraland mole-rats (Cryptomys damarensis): implications for sociality. Oecologia 113, 290–298 10.1007/s004420050380 (doi:10.1007/s004420050380) [DOI] [PubMed] [Google Scholar]
- 19.Le Comber SC, Spinks AC, Bennett NC, Jarvis JUM, Faulkes CG. 2002. Fractal dimension of African mole-rat burrows. Can. J. Zool. 80, 436–441 10.1139/Z02-026 (doi:10.1139/Z02-026) [DOI] [Google Scholar]
- 20.Sichilima AM, Bennett NC, Faulkes CG, Le Comber SC. 2008. Evolution of African mole-rat sociality: burrow architecture, rainfall and foraging in colonies of the cooperatively breeding Fukomys mechowii. J. Zool. 275, 276–282 10.1111/j.1469-7998.2008.00439.x (doi:10.1111/j.1469-7998.2008.00439.x) [DOI] [Google Scholar]
- 21.Hess J. 2004. A population genetic study of the eusocial naked mole-rat (Heterocephalus glaber). PhD Thesis University of Washington, Washington, DC, USA [Google Scholar]
- 22.Burland TM, Bennett NC, Jarvis JUM, Faulkes CG. 2002. Eusociality in African mole-rats: new insights from patterns of genetic relatedness in the Damaraland mole-rat (Cryptomys damarensis). Proc. R. Soc. Lond. B 269, 1025–1030 10.1098/rspb.2002.1978 (doi:10.1098/rspb.2002.1978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinks AC, Jarvis JUM, Bennett NC. 2000. Comparative patterns of philopatry and dispersal in two common mole rat populations: implications for the evolution of mole rat sociality. J. Anim. Ecol. 69, 224–234 10.1046/j.1365-2656.2000.00388.x (doi:10.1046/j.1365-2656.2000.00388.x) [DOI] [Google Scholar]
- 24.Spinks AC, Bennett NC, Jarvis JUM. 2000. A comparison of the ecology of two populations of the common mole-rat, Cryptomys hottentotus hottentotus: the effect of aridity on food, foraging and body mass. Oecologia 125, 341–349 10.1007/S004420000460 (doi:10.1007/S004420000460) [DOI] [PubMed] [Google Scholar]
- 25.Burda H, Honeycutt RL, Begall S, Locker-Grütjen O, Scharff A. 2000. Are naked and common mole-rats eusocial and if so, why? Behav. Ecol. Sociobiol. 47, 293–303 10.1007/s002650050669 (doi:10.1007/s002650050669) [DOI] [Google Scholar]
- 26.Lukas D, Clutton-Brock T. 2012. Cooperative breeding and monogamy in mammalian societies. Proc. R. Soc. B 279, 2151–2156 10.1098/rspb.2011.2468 (doi:10.1098/rspb.2011.2468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleiman DG. 1977. Monogamy in mammals. Q. Rev. Biol. 52, 39–69 10.1086/409721 (doi:10.1086/409721) [DOI] [PubMed] [Google Scholar]
- 28.Young LJ, Wang Z. 2004. The neurobiology of pair bonding. Nat. Neurosci. 7, 1048–1054 10.1038/nn1327 (doi:10.1038/nn1327) [DOI] [PubMed] [Google Scholar]
- 29.Lim MM, Young LJ. 2006. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav. 50, 506–517 10.1016/j.yhbeh.2006.06.018 (doi:10.1016/j.yhbeh.2006.06.018) [DOI] [PubMed] [Google Scholar]
- 30.Curtis JT, Liu Y, Aragona BJ, Wang Z. 2007. Neural regulation of social behavior in rodents. In Rodent societies: an ecological and evolutionary perspective (eds Wolff JO, Sherman PW.), pp. 185–194 Chicago, IL: University of Chicago Press [Google Scholar]
- 31.Young LJ, Huot B, Nilsen R, Wang Z, Insel TR. 1996. Differences in central oxytocin receptor gene expression: comparative analysis of promoter sequences. J. Neuroendocrinol. 8, 777–783 10.1046/j.1365-2826.1996.05188.x (doi:10.1046/j.1365-2826.1996.05188.x) [DOI] [PubMed] [Google Scholar]
- 32.Kalamatianos T, Faulkes CG, Oosthuizen MK, Poorun R, Bennett NC, Coen CW. 2010. Telencephalic binding sites for oxytocin and social organization: a comparative study of eusocial naked mole-rats and solitary Cape mole-rats. J. Comp. Neurol. 518, 1792–1813 10.1002/cne.22302 (doi:10.1002/cne.22302) [DOI] [PubMed] [Google Scholar]
- 33.Coen CW, Zhou S, Kalamatianos T, Faulkes CG, Bennett NC. 2011. Telencephalic distribution of oxytocin and vasopressin and their binding sites in Damaraland mole-rats: implications for eusocial behavior. In Society for Neuroscience Annual Meeting, Washington, DC, 12–16 November 2011. 186.11/SS10. Society for Neuroscience. [Google Scholar]
- 34.Valesky EM, Burda H, Kaufmann R, Oelschläger HHA. 2012. Distribution of oxytocin- and vasopressin-immunoreactive neurons in the brain of the eusocial mole rat (Fukomys anselli). Anat. Rec. 295, 474–480 10.1002/ar.22414 (doi:10.1002/ar.22414) [DOI] [PubMed] [Google Scholar]
- 35.Beery AK, Lacey EA, Francis DD. 2008. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis). J. Comp. Neurol. 507, 1847–1859 10.1002/cne.21638 (doi:10.1002/cne.21638) [DOI] [PubMed] [Google Scholar]
- 36.Bennett NC, Jarvis JUM. 1988. The reproductive biology of the Cape mole rat, Georychus capensis (Rodentia, Bathyergidae). J. Zool. 214, 95–106 10.1111/j.1469-7998.1988.tb04989.x (doi:10.1111/j.1469-7998.1988.tb04989.x) [DOI] [Google Scholar]
- 37.Bennett NC, Jarvis JUM, Aguilar GH, McDaid EJ. 1991. Growth and development in six species of African mole-rats (Rodentia: Bathyergidae). J. Zool. 225, 13–26 10.1111/j.1469-7998.1991.tb03798.x (doi:10.1111/j.1469-7998.1991.tb03798.x) [DOI] [Google Scholar]
- 38.Herbst M, Jarvis JUM, Bennett NC. 2004. A field assessment of reproductive seasonality in the threatened wild Namaqua dune mole rat (Bathyergus janetta). J. Zool. 263, 259–268 10.1017/S0952836904005114 (doi:10.1017/S0952836904005114) [DOI] [Google Scholar]
- 39.Sumbera R, Burda H, Chitaukali W. 2003. Reproductive biology of a solitary subterranean bathyergid rodent, the silvery mole-rat (Heliophobius argenteocinereus). J. Mammalogy 84, 278–287 10.1644/1545-1542(2003)084 (doi:10.1644/1545-1542(2003)084) [DOI] [Google Scholar]
- 40.Bennett NC, Jarvis JUM. 1988. The social structure and reproductive biology of colonies of the mole-rat, Cryptomys damarensis (Rodentia, Bathyergidae). J. Mammalogy 69, 293–302 10.2307/1381379 (doi:10.2307/1381379) [DOI] [Google Scholar]
- 41.Burda H, Kawalika M. 1993. Evolution of eusociality in the Bathyergidae. The case of the giant mole rats (Cryptomys mechowi). Naturwissenschaften 80, 235–237 10.1007/BF01175742 (doi:10.1007/BF01175742) [DOI] [PubMed] [Google Scholar]
- 42.Janse van Rensburg L, Bennett NC, van der Merwe M, Schoeman AS. 2002. Seasonal reproduction in the highveld mole-rat, Cryptomys hottentotus pretoriae (Rodentia: Bathyergidae). Can. J. Zool. 80, 810–820 10.1139/z02-051 (doi:10.1139/z02-051) [DOI] [Google Scholar]
- 43.Faulkes CG, Bennett NC. 2009. Reproductive skew in African mole-rats: behavioural and physiological mechanisms to maintain high skew. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R, Jones CB.), pp. 369–396 Cambridge, UK: Cambridge University Press [Google Scholar]
- 44.O'Riain M, Faulkes CG. 2008. African mole-rats: eusociality, relatedness and ecological constraints. In Ecology of social evolution (eds Heinze J, Korb J.), pp. 205–220 Berlin, Germany: Springer [Google Scholar]
- 45.O'Riain MJ, Jarvis JUM, Faulkes CG. 1996. A dispersive morph in the naked mole-rat. Nature 380, 619–621 10.1038/380619a0 (doi:10.1038/380619a0) [DOI] [PubMed] [Google Scholar]
- 46.O'Riain MJ, Jarvis JUM, Alexander R, Buffenstein R, Peeters C. 2000. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA 97, 13 194–13 197 10.1073/pnas.97.24.13194 (doi:10.1073/pnas.97.24.13194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scantlebury M, Speakman JR, Oosthuizen MK, Roper TJ, Bennett NC. 2006. Energetics reveals physiologically distinct castes in a eusocial mammal. Nature 440, 795–797 10.1038/nature04578 (doi:10.1038/nature04578) [DOI] [PubMed] [Google Scholar]
- 48.Young AJ, Bennett NC. 2010. Morphological divergence of breeders and helpers in wild Damaraland mole-rat societies. Evolution 64, 3190–3197 10.1111/j.1558-5646.2010.01066.x (doi:10.1111/j.1558-5646.2010.01066.x) [DOI] [PubMed] [Google Scholar]
- 49.Burland TM, Bennett NC, Jarvis JUM, Faulkes CG. 2004. Colony structure and parentage in wild colonies of co-operatively breeding Damaraland mole-rats suggest incest avoidance alone may not maintain reproductive skew. Mol. Ecol. 13, 2371–2379 10.1111/j.1365-294X.2004.02233.x (doi:10.1111/j.1365-294X.2004.02233.x) [DOI] [PubMed] [Google Scholar]
- 50.Bishop JM, Jarvis JUM, Spinks AC, Bennett NC, O'Ryan C. 2004. Molecular insight into patterns of colony composition and paternity in the common mole-rat Cryptomys hottentotus hottentotus. Mol. Ecol. 13, 1217–1229 10.1111/mec.2004.13.issue-5 (doi:10.1111/mec.2004.13.issue-5) [DOI] [PubMed] [Google Scholar]
- 51.Bennett NC. 1989. The social structure and reproductive biology of the common mole rat, Cryptomys h. hottentotus and remarks on the trends in reproduction and sociality in the family. J. Zool. 219, 45–59 10.1111/j.1469-7998.1989.tb02564.x (doi:10.1111/j.1469-7998.1989.tb02564.x) [DOI] [Google Scholar]
- 52.Moolman M, Bennett NC, Schoeman AS. 1998. The social structure and dominance hierarchy of the highveld mole rat Cryptomys hottentotus pretoriae (Rodentia: Bathyergidae). J. Zool. 246, 193–201 10.1111/j.1469-7998.1998.tb00148.x (doi:10.1111/j.1469-7998.1998.tb00148.x) [DOI] [Google Scholar]
- 53.Bennett NC, Jarvis JUM, Wallace DB. 1990. The relative age structure and body masses of complete wild captured colonies of two social mole rats, the common mole rat, Cryptomys hottentotus hottentotus and the Damaraland mole-rat Cryptomys damarensis. J. Zool. 220, 469–485 10.1111/j.1469-7998.1990.tb04319.x (doi:10.1111/j.1469-7998.1990.tb04319.x) [DOI] [Google Scholar]
- 54.Jacobs DS, Bennett NC, Jarvis JUM. 1991. The colony structure and dominance hierarchy of the Damaraland mole rat, Cryptomys damarensis (Rodentia: Bathyergidae), from Namibia. J. Zool. 224, 553–576 10.1111/j.1469-7998.1991.tb03785.x (doi:10.1111/j.1469-7998.1991.tb03785.x) [DOI] [Google Scholar]
- 55.Gaylard A, Harrison Y, Bennett NC. 1998. Temporal changes in the social structure of a captive colony of the Damaraland mole rat, Cryptomys damarensis: the relationship of sex and age to dominance and burrow maintenance activity. J. Zool. 244, 313–321 10.1111/j.1469-7998.1998.tb00035.x (doi:10.1111/j.1469-7998.1998.tb00035.x) [DOI] [Google Scholar]
- 56.Lacey EA, Sherman PW. 1991. Social organization of naked mole-rat colonies: evidence for divisions of labor. In the biology of the naked mole-rat (eds Sherman PW, Jarvis JUM, Alexander RD.), pp. 275–336 New York, NY: Princeton University Press [Google Scholar]
- 57.Faulkes CG, Abbott DH, Liddell CE, George LM, Jarvis JUM. 1991. Hormonal and behavioral aspects of reproductive suppression in female naked mole-rats. In The biology of the naked mole-rat (eds Sherman P, Jarvis J, Alexander RD.), pp. 426–444 Princeton, NJ: Princeton University Press [Google Scholar]
- 58.Bennett NC, Navarro R. 1997. Differential growth patterns between successive litters of the eusocial Damaraland mole rat, Cryptomys damarensis, from Namibia. J. Zool. 241, 185–202 10.1111/j.1469-7998.1997.tb05508.x (doi:10.1111/j.1469-7998.1997.tb05508.x) [DOI] [Google Scholar]
- 59.Jarvis JUM, O'Riain MJ, McDaid EJ. 1991. Growth and factors affecting body size in naked mole-rats. In The biology of the naked mole-rat (eds Sherman P, Jarvis J, Alexander RD.), pp. 358–383 Princeton, NJ: Princeton University Press [Google Scholar]
- 60.O'Riain MJ, Jarvis JUM. 1998. The dynamics of growth in naked mole rats: the effects of litter order and changes in social structure. J. Zool. 246, 49–60 10.1111/j.1469-7998.1998.tb00131.x (doi:10.1111/j.1469-7998.1998.tb00131.x) [DOI] [Google Scholar]
- 61.Clarke FM, Faulkes CG. 1997. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc. R. Soc. Lond. B 264, 993–1000 10.1098/rspb.1997.0137 (doi:10.1098/rspb.1997.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clarke FM, Faulkes CG. 1998. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc. R. Soc. Lond. B 265, 1391–1399 10.1098/rspb.1998.0447 (doi:10.1098/rspb.1998.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beshers SN, Huang ZY, Oono Y, Robinson G. 2001. Social inhibition and the regulation of temporal polyethism in honey bees. J. Theor. Biol. 213, 461–479 10.1006/jtbi.2001.2427 (doi:10.1006/jtbi.2001.2427) [DOI] [PubMed] [Google Scholar]
- 64.Crespi BJ, Yanega D. 1995. The definition of eusociality. Behav. Ecol. 6, 109–115 10.1093/beheco/6.1.109 (doi:10.1093/beheco/6.1.109) [DOI] [Google Scholar]
- 65.Costa JT, Fitzgerald TD. 1996. Developments in social terminology: semantic battles in a conceptual war. Trends Ecol. Evol. 11, 285–289 10.1016/0169-5347(96)10035-5 (doi:10.1016/0169-5347(96)10035-5) [DOI] [PubMed] [Google Scholar]
- 66.Dengler-Crish CM, Catania KC. 2007. Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J. Exp. Biol. 210, 4351–4358 10.1242/jeb.009399 (doi:10.1242/jeb.009399) [DOI] [PubMed] [Google Scholar]
- 67.Jarvis JUM, Bennett NC. 1993. Eusociality has evolved independently in two genera of bathyergid mole-rats—but occurs in no other subterranean mammal. Behav. Ecol. Sociobiol. 33, 253–260 10.1007/BF02027122 (doi:10.1007/BF02027122) [DOI] [Google Scholar]
- 68.van Sandwyk JHDT, Bennett NC. 2005. Do solitary, seismic signalling Cape mole-rats (Georychus capensis) demonstrate spontaneous or induced ovulation? J. Zool. 267, 75. 10.1017/S0952836905007302 (doi:10.1017/S0952836905007302) [DOI] [Google Scholar]
- 69.Hart L, O'Riain MJ, Jarvis JUM, Bennett NC. 2006. Is the Cape dune mole-rat, Bathyergus suillus (Rodentia: Bathyergidae), a seasonal or aseasonal breeder? J. Mammalogy 87, 1078–1085 10.1644/05-MAMM-A-411R2.1 (doi:10.1644/05-MAMM-A-411R2.1) [DOI] [Google Scholar]
- 70.Parag A, Bennett NC, Faulkes CG, Bateman PW. 2006. Penile morphology of African mole rats (Bathyergidae): structural modification in relation to mode of ovulation and degree of sociality. J. Zool. 270, 323–329 10.1111/j.1469-7998.2006.00141.x (doi:10.1111/j.1469-7998.2006.00141.x) [DOI] [Google Scholar]
- 71.Jackson CR, Bennett NC. 2005. Is the Natal mole-rat (Cryptomys hottentotus natalensis) a spontaneous or induced ovulator? J. Mammalogy 86, 1–6 10.1644/1545-1542(2005)086 (doi:10.1644/1545-1542(2005)086) [DOI] [Google Scholar]
- 72.Malherbe GP, Schoeman AS, Bennett NC. 2004. Is the highveld mole-rat Cryptomys hottentotus pretoriae (Rodentia: Bathyergidae) an induced or spontaneous ovulator? J. Zool. 263, 159–165 10.1017/S0952836904004996 (doi:10.1017/S0952836904004996) [DOI] [Google Scholar]
- 73.Faulkes CG, Abbott DH, Jarvis JUM. 1990. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J. Reprod. Fertil. 88, 559–568 10.1530/jrf.0.0880559 (doi:10.1530/jrf.0.0880559) [DOI] [PubMed] [Google Scholar]
- 74.Snyman P, Jackson C, Bennett NC. 2006. Do dispersing non-reproductive female Damaraland mole-rats, Cryptomys damarensis (Rodentia: Bathyergidae) exhibit spontaneous or induced ovulation? Physiol. Behav. 87, 88–94 10.1016/j.physbeh.2005.09.003 (doi:10.1016/j.physbeh.2005.09.003) [DOI] [PubMed] [Google Scholar]
- 75.Faulkes CG, Sichilima AM, van Sandwyk J, Lutermann H, Bennett NC. 2010. Control of ovulation in female giant mole-rats Fukomys mechowii (Rodentia: Bathyergidae), and phylogenetic trends within the family. J. Zool. 282, 64–74 10.1111/j.1469-7998.2010.00713.x (doi:10.1111/j.1469-7998.2010.00713.x) [DOI] [Google Scholar]
- 76.Bennett NC, van Sandwyk J, Lutermann H. 2010. The pattern of ovulation in Ansell's mole-rat: phylogenetic or ecological constraints? J. Zool. 281, 66–73 10.1111/j.1469-7998.2009.00685.x (doi:10.1111/j.1469-7998.2009.00685.x) [DOI] [Google Scholar]
- 77.Sichilima A, Bennett NC, Faulkes CG. 2011. Field evidence for colony size and aseasonality of breeding and in Ansell's Mole-Rat, Fukomys anselli (Rodentia: Bathyergidae). Afr. Zool. 46, 334–339 10.3377/004.046.0212 (doi:10.3377/004.046.0212) [DOI] [Google Scholar]
- 78.Faulkes CG, Abbott DH, Jarvis JUM. 1991. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J. Reprod. Fertil. 91, 593–604 10.1530/jrf.0.0910593 (doi:10.1530/jrf.0.0910593) [DOI] [PubMed] [Google Scholar]
- 79.Clarke FM, Faulkes CG. 1999. Kin discrimination and female mate choice in the naked mole-rat Heterocephalus glaber. Proc. R. Soc. Lond. B 266, 1995–2002 10.1098/rspb.1999.0877 (doi:10.1098/rspb.1999.0877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bennett NC, Faulkes CG, Molteno AJ. 1996. Reproductive suppression in subordinate, non-breeding female Damaraland mole-rats: two components to a lifetime of socially induced infertility. Proc. R. Soc. Lond. B 263, 1599–1603 10.1098/rspb.1996.0234 (doi:10.1098/rspb.1996.0234) [DOI] [PubMed] [Google Scholar]
- 81.Kim EB, et al. 2011. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature 479, 223–227 10.1038/nature10533 (doi:10.1038/nature10533) [DOI] [PMC free article] [PubMed] [Google Scholar]