Abstract
Ecological factors explain variation in sociality both within and between species of marmots—large alpine ground squirrels. Fifty years of study, by me and my colleagues, of the yellow-bellied marmots (Marmota flaviventris) at the Rocky Mountain Biological Laboratory, near Crested Butte, CO, USA, has created opportunities to see how sociality changes with population and group size. Over the past decades, we have witnessed a natural experiment whereby the population tripled in size. If we view sociality as an emergent process, then demography acts as a constraint on interactions between individuals, and the threefold increase in population size should have consequences for group structure. We have used social network statistics to study the causes and consequences of social interactions by capitalizing on this demographic variation. Such an emergent view is ideally studied in an integrative Tinbergian way that focuses on both mechanism and function. We have determined that some social attributes are heritable, that social cohesion is established through age and kin structure, that well-embedded females (but not males) are less likely to disperse, and that there are fitness consequences of social attributes. Together, this integrative relationship-centred view expands on the traditional ecological model of sociality and offers a framework that can be applied to other systems.
Keywords: sociality, embeddedness, social network, social attributes, yellow-bellied marmots
1. Introduction
A core assumption of the primate socioecological model is that local ecology, identified by competition for resources and predation risk [1–6], influences social interactions within groups because individuals try to manage the negative effects of competition while still attempting to reap antipredator benefits. But, while not all taxa compete directly for access to food, all species, for at least some point in their lives, experience some risk of predation [7], and many face infanticide risks [8,9]. Thus, some aspects of the socioecological model are certainly applicable to many non-primate species [10].
While there have been attempts to apply the primate socioecological model to understand social variation in carnivores [11] and ungulates [12], rodents form another potentially interesting out-group for study for several reasons. First, there are many species of rodents, and these species vary in the degree to which they form stable groups and other attributes of complex social behaviour [13]. Second, some species exhibit intraspecific variation in social systems and, in some cases, it is possible to manipulate ecological factors in ways to gain insights into mechanisms underlying social variation [14–16]. Third, it is often possible to study multiple groups over time and tease apart sources of social variation [17].
Here, I review 50 years of insights from on-going work with yellow-bellied marmot (Marmota flaviventris) social behaviour. I adopt the definition of social behaviour used throughout this Theme Issue that comes from Kappeler & van Schaik [18] and that focuses on describing the size, composition, cohesion and genetic structure of a social unit. Social mating system is also important but somewhat distinct. Rather than focusing exclusively on evaluating the primate socioecological model in marmots, I adopt a Tinbergian approach [19,20] that integrates mechanism with function [20] and that has identified remarkable flexibility as well as some constraints on social organization, mating system and social structure (see Blumstein et al. [21] for definitions). Nevertheless, many factors considered by the socioecological model may influence marmot behaviour. For instance, ecological variation clearly influences marmot sociality through creating the demographic opportunity for social groups to form. All 14 species of marmots hibernate and ecological factors influence the probability of surviving hibernation and the rate at which individuals can gain body mass—two factors that are likely to explain variation among marmot species in their degree of sociality [22–24]. My work is predicated on an emergent view of social behaviour that first requires a sufficient number of animals cohabiting an area (sensu [25]), and then seeks to understand the development and maintenance of social relationships (sensu [26]). I will first review what Armitage [27,28] discovered over the first four decades of study at the Rocky Mountain Biological Laboratory (RMBL) near Crested Butte, Colorado, USA, and then highlight some recent insights that have emerged during the past decade of study.
2. Yellow-bellied marmots: highlights from first four decades
The yellow-bellied marmots at the RMBL have been under continuous study since 1962. Armitage [27] began the study to understand the role social behaviour plays in population dynamics. Armitage's work was stimulated by both Calhoun [29] and Errington [30], who both showed that social tolerance and intolerance may have profound effects on vertebrate population biology. In part, based on thinking about Calhoun's results where more rats could be raised in the same space if females were housed alone than together, Armitage wished to decouple the roles of social behaviour and the more well-studied environmental influences on population biology in a natural population [27].
Yellow-bellied marmots are one of 14 species of marmots. Because they hibernate for seven to eight months per year, much of their summer active season focuses around gaining sufficient body mass to survive the winter. They are very efficient hibernators and lose about 1 g per day during deep torpor [31]. However, not all winter is spent in deep torpor and in years where the winter is long, spring mortality for underweight animals is high [32].
Marmots live in 0.15–7.24 ha habitat patches [33]. Armitage described the marmots as living in a harem-polygynous society—one male defended one or more females [34]. Females form matrilines that started when a mother recruited one or more of her daughters to her territory [35]. However, one important early insight was that matriline size varies considerably, and female amicable relationships are essential to create a matriline [35]. Importantly, there is considerable social variation; many females live alone in relatively small habitat patches, whereas only the larger habitat patches support multiple females [36]. Using Kappeler & van Schaik's [18] terminology, marmots live in mixed sex aggregations, where one or more males and one or more females reproduce.
Additionally, relationships between females are the glue that holds the society together, because the nature of female relationships, as described below, influences the probability of female dispersal. The decision to remain in the natal group may lead to reproductive suppression [37]. When an older female was present, 2- and 3-year olds were less likely to reproduce than in situations where there was no older female present.
Armitage recognized the importance of kinship in determining the pattern of agonistic and affiliative behaviour. Armitage [36] noted that males behaved uniformly aggressively towards other males, regardless of relationship. For females, kinship is important. Those females who were related with an r < 0.25 typically behaved aggressively towards each other, while females with r > 0.25 typically behaved amicably towards each other. Amicable behaviour (which both sexes engage in) includes behavioural elements such as greeting, allogrooming, sitting in body contact, play (which is restricted to juveniles and yearlings) and foraging together. It is possible that animals cooperatively thermoregulate (sensu [38]); but yellow-bellied marmots are the most efficient marmot hibernators [31], and such cooperation may not be required. That said, most social behaviour is much more subtle than that which one might observe in social primates, and (for unknown adaptive reasons) rates of social interactions drop as the season progresses (in a proximate sense, social behaviour declines after the breeding season).
3. What are the ecological factors that explain this variation?
High elevation populations of yellow-bellied marmots may be food-limited [39,40], but marmots living in the sub-alpine meadows around the RMBL do not appear food-limited in non-drought years (marmots fail to gain sufficient body mass to survive hibernation in drought years [41]). Indeed, a cross-sectional habitat analysis found that factors associated with predation risk, not with food, explained substantial variation in whether marmots were present in an area and, if so, whether they persisted or periodically went extinct [42]. However, consistent with an expectation of the socioecological model, patch size is positively correlated with population size. Indeed, patch size is a good predictor of both the number of adult females (r = 0.86) and the number of adults of both sexes (r = 0.83) [39,40].
Predators are a constant threat. While Armitage initially discounted their importance because direct predation is rarely observed, several lines of evidence suggest that they play a very important role in behaviour and population persistence. First, marmots have a rich repertoire of antipredator behaviour and predator-detection abilities. They allocate time to antipredator vigilance (a heritable trait [43]) and modify it based on a variety of factors [44], including visibility [45]. They are able to recognize the sight [46], sounds [47] and smells [48] of their predators (including canid, felid and raptorial predators). They emit alarm calls in seemingly adaptive situations [49]. They dig burrows [50] into which they escape [51]. Flight initiation distance is influenced by the costs and benefits of fleeing [44,51]. Once they retreat into their burrows, the time they remain in their burrows is sensitive to both the benefits and costs of hiding, and these decisions are likely to have fitness consequences [52,53]. Second, we can see an ecological effect of predators: populations of marmots living in patches with rocks and good visibility persist while those living in areas with fewer rocks and worse visibility periodically go extinct [42]. Finally, in some years, all of the young at certain social groups and colonies are killed by predators [33,54]. Thus, predators directly influence the opportunity for social interactions and social behaviour by influencing the population size. From this perspective, we can view predators as being both an important constraint on solitary living and one that maintains variation in group size and social flexibility.
4. A natural experiment
Over the past decades, the average spring air temperature at the RMBL has increased, [55] the snow has melted sooner [55,56] and marmots at the well-studied RMBL town site location have emerged earlier [55,56]. Because the snow has melted sooner, the growing season has begun earlier, and thus the duration of food availability for marmots has lengthened [55]. As a consequence of increased food availability and a longer active season, marmot body mass in summer has increased, resulting in better condition before hibernation, and higher over-winter survival of adult females [56]. Taken together, these environmental changes have resulted in an unprecedented threefold increase in the population size over the past decades (figure 1). Such a rapid increase in population size has released a constraint and created the opportunity for different kinds of social relationships to emerge.
Figure 1.
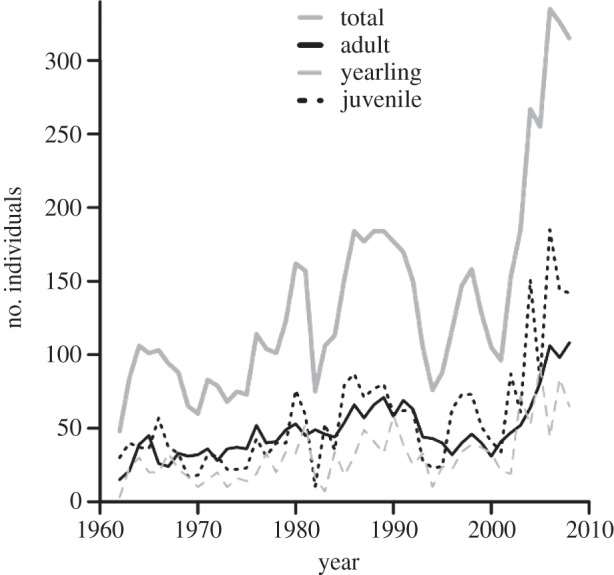
Variation in the population size of yellow-bellied marmots at the RMBL, 1962–2010. Juveniles are pups of the year, yearlings are 1-year olds, and adults are 2 years and older.
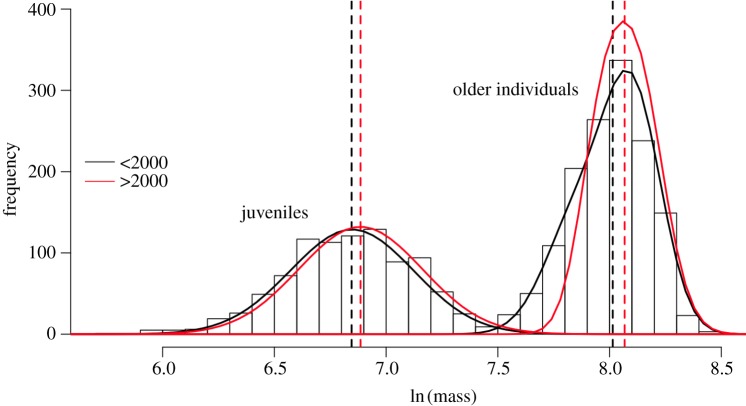
Earlier snowmelt and earlier emergence not only increased the length of the food availability period, but also decreased hibernation duration. Pre-hibernation body mass is strongly related to winter survival and to reproductive success [32,57]. We thus expected that selection pressure on mass and mass-related parameters has decreased with climatic changes. Furthermore, we already know that there was no selection on the overall rate of mass gain for marmots between 2003 and 2009 [58]. Given the dramatic growth of the population since 2000, without immigration, we expected density-dependence effects would soon appear in the population. It should be noted that we already observed a slight decrease in mean August body mass of females (figure 2), suggesting that density-dependent effects started to appear, at least at the lower elevation site.
Figure 2.
The distribution of August body mass for juveniles and older individuals that has shifted slightly up, for both age classes, since 2000. Adapted from Ozgul et al. [56]. (Online version in colour.)
Observed changes in environment and population dynamics are expected to affect not only mass and mass-related parameters, but also important traits that emerge from greater population densities. In marmots, one might expect that as group size increased, more females would be recruited to colonies in larger habitat patches, and with more females, one might expect more males. Thus, increased population size might influence the ability of males to monopolize females and potentially influence multiple paternity. However, this need not be true; if one male could successfully defend large groups of females, then we might not expect an increase in the number of males. Animals are constrained in the number of, and time allocated to, social interactions [59,60]. It was not known whether or where those limits were for marmots, or what the emergent consequences might be, but traits, such as the rate of agonistic or affiliative interactions and social network structure, were expected to vary.
We had predicted that selection pressure on social traits in marmots would increase in the next decade because of increased density dependence, but a major population crash occurred during an exceptionally long winter in 2011. This crash will ultimately provide a priceless opportunity to study social dynamics when individuals, previously living at higher densities, find themselves suddenly surrounded by many fewer conspecifics.
Focusing on such intraspecific variation in sociality is not new; Lott [61,62] called attention to this variation in two reviews, and a number of primatologists have highlighted the fact that demography influences the opportunity for sociality to emerge as well as the importance of intraspecific variation in sociality [25,63].
5. Sociality: an emergent view
If we view social structure as emerging from demographic opportunities (also see Barrett & Henzi [25]), then there are a number of natural questions that follow. These questions focus on the emergent consequences of animals interacting. Indeed, sociality as an emergent process is a long-held view that dates back at least to Hinde's [26,64] view of social behaviour, which focused on how interactions among and between individuals form the basis for bonds and relationships. Such a relationship-focused view of sociality lends itself very nicely to social network analysis [65–69]. And, in contrast to the traditional socioecological model that focuses mostly on the ultimate consequences of social behaviour and one that treats mechanism largely as a search for identifying the key ecological factors that explain variation, an emergent view of sociality lends itself to both proximate and ultimate studies.
In the following sections, I will discuss several recent studies that we have conducted. I will review studies that asked whether male–male cooperation emerged from increased male density, and studies that examined the heritability and development of social relationships, as well as studies that evaluated the adaptive value of social relationships. Together, these studies could serve as a template for integrative Tinbergian studies of social variation and plasticity.
6. Consequences for social structure
While multi-male groups were relatively uncommon in the past (typical groups had a single dominant male [37]), between 2001 and 2008, 45 per cent of groups had more than a single male [70]. Olson and I capitalized on this unusual pattern to study the origin and maintenance of these groups. We were interested in determining which of several coalitionary traits male marmots possessed and whether they cooperated when living socially.
The coalitionary traits hypothesis [71] suggests that it is profitable to view complex coalitionary behaviour as comprising several constituent traits: mutual tolerance; collaboration; and partner preference. Mutual tolerance occurs when animals share space. Collaboration is seen when males work together to achieve an outcome they could not obtain individually—ousting dominant males, defence against predators, territorial defence, etc. Partner preference is seen when males prefer to associate with each other. Rudimentary coalitions only contain one or a few of these traits, whereas more complex coalitionary behaviour contains more traits.
We found that social groups contained one to several adult males, who did or did not monopolize reproduction [70]. Indeed, we found a mix of reproductive strategies that included one male in multi-male groups monopolizing all reproduction, males monopolizing reproduction with a subset of females and females mating with multiple males. Males did not discriminate among each other's perioral olfactory secretions, suggesting that they did not show partner preference for males within their group. We exposed males to a mounted American badger (Taxidea taxus), a very effective marmot predator [33], to see whether they were more likely to emit alarm calls to warn others when in multi-male groups. We found that they did not adjust alarm-calling behaviour in a way consistent with male–male collaboration. Finally, males did not enhance their reproductive success by forming multi-male groups.
Multi-male marmot groups are formed in habitat areas of sufficient size when the population (i.e. number of females) expands. However, simply because males are environmentally forced to live together, this constraint has not seemingly selected for complex social organization. We therefore concluded that male yellow-bellied marmots have rudimentary social organization and that, somewhat such as European badgers (Meles meles), they are social but not cooperative [72]. Thus, while the socioecological model emphasizes the role of oestrous synchrony and the inability of males to monopolize large groups of females as key factors in driving the formation of multi-male groups [73,74], we found that apparent ecological constraints (including, perhaps, the inability of males to monopolize a group) were important factors that explained the formation of multi-male groups.
7. Novel insights from using social network statistics
We have been engaged in a series of studies [68,75–78] that explore the use of social network statistics in marmot behaviour. As other recent studies of mammals have shown [79–82], formal network statistics provide an opportunity to study not only direct relationships between individuals but also indirect relationships between individuals. And, while previous studies have shed important insights into the structure and function of social variation, few have tested key assumptions required to see whether social traits quantified using network analyses are biologically and evolutionarily meaningful. I believe that if these traits are meaningful they should be heritable, should explain variation in biologically important behaviours, could be under selection, and some should have fitness consequences.
A network trait, by definition, is the result of an interaction between two (or more) individuals, and these interactions may only be under partial control of a focal subject. Despite this constraint, documenting heritable variation in network traits (particularly if other potentially important variables are properly controlled for statistically) would suggest that these traits may be evolutionarily salient.
I shall review several of our recent studies here. It is important to point out that because we follow the fate of virtually all marmots, and because they live in known geographical locations, we are not required to assume ‘the gambit of the group’ (i.e. that each individual in a group interacts with all other members in that group) [67,83]. Thus, we can effectively study the impacts of sampling on parameter values, without having to simultaneously worry about group composition varying much as a function of sampling. This is a strength of the marmot study because, typically, the gambit of the group is not a problem with other studies of individually marked animals whose behaviour is intensively studied (e.g. habituated primates, social carnivores, many rodents).
There are many possible social network statistics that can be calculated [65]: each describes what I shall refer to as an attribute of sociality. Lehman & Ross [82] note that a complex set of networked relationships are required for describing an individual's social environment. In any dataset, some attributes are correlated with each other, whereas others are orthogonal. In our case, all attributes were calculated from association matrices of observed interactions (affiliative or agonistic), but others have focused on different sorts of networks (e.g. grooming, dominance, foraging, etc. [81,82]). Networks can be directional (i.e. individual A could initiate or receive a bout of social behaviour, where a bout is defined as a unique interaction), and because affiliative and agonistic relationships may have different causes and consequences, we typically calculate separate analyses for each. Networks can also be binary, where one is interested in the mere presence of any interaction between individuals that is then scored as 0 or 1, or weighted, where the number of interactions between individuals becomes an important parameter. Because we fit many models, we have used a variety of model fitting techniques.
Ultimately, the results from any single study are suggestive and warrant additional studies in other systems to search for general trends. However, at this point, I believe, as will be illustrated below, that we have collectively demonstrated there is great use in adopting a social network approach to studying social behaviour and that a social network approach will provide novel insights not otherwise captured by evaluating a traditional socioecological approach.
(a). Network position is heritable
Social attributes can be heritable. This is an essential finding that suggests that the propensity to form relationships can respond to selection and evolve. We capitalized on a well-supported genetically based genealogy [43] and our ability to calculate a variety of social network statistics on the marmots. Using the quantitative genetic methods of ‘the animal model’ [84], we partitioned out components of variation and estimated additive genetic variation in social attributes [76].
We hypothesized that social attributes under control of an individual (i.e. those that an animal initiates or was directly involved in) would be more heritable than those based largely on the behaviour of other group members. Indeed, variation in these indirect measures was largely explained by non-genetic variation. However, the heritability of received agonistic behaviour was significant, whereas there was no significant heritable variation in initiated behaviour. This ‘heritable victimization’ is fascinating and suggests that being able to receive and tolerate aggression is an important social attribute. In retrospect, this makes some sense in that in hierarchically structured systems there is only one alpha male/female. Marmots maintain dominance hierarchies [85], and as long as there are benefits to being in a social group there should be benefits to tolerating aggression from others. Somewhat analogously, spotted hyaenas (Crocuta crocuta) maintain strong social bonds with kin despite considerable competition for food, in part because there are benefits from being in a social group [79]. Our finding in marmots is also a call for a search for mechanism: what is it that explains variation—in a proximate sense—in the propensity to receive aggression? Future studies will have to address this.
(b). Social cohesion is established through age and kin structuring
While the cause of social relationships is studied using the socioecological model [5], the ontogeny of social relationships is a question not immediately asked under a traditional socioecological approach. By contrast, the development of relationships is a question that emerges nicely from an approach that seeks to understand the causes and consequences of social relationships. For this study, Wey & Blumstein [77] evaluated network measures that captured both direct interactions and indirect interactions.
We focused on expansiveness—the tendency to initiate interactions relative to other individuals in a network; and attractiveness—the tendency to receive interactions relative to other nodes in the network. Both of these reflected direct interactions between individuals. In addition, we quantified two metrics that described both direct and indirect social interactions: out-closeness—which quantifies the ability to reach all other individuals in a network through short path lengths (i.e. by going through few intermediate individuals); and in-closeness—which quantifies the ability of others to reach an actor through short path lengths. A challenge in interpreting these metrics is understanding the consequences of such indirect emergent relationships because they are slightly divorced from the immediate consequences of individual interactions. By this, I mean that there is a potential for an immediate consequence of a direct interaction (e.g. a sexually transmitted disease might be directly acquired from an individual), but the consequences of an indirect relationship may be present but might be less pronounced (of course, a sexually transmitted disease might be more likely when having sex with an individual who has had sex with more individuals than with one who has had sex with fewer individuals). Nonetheless, I suggest that indirect relationships are demonstrably important if their variation can be explained by biologically important factors—in this case, age and kinship.
We found that interactions between yearlings are particularly important in structuring marmot social groups. Yearlings engage in affiliative behaviour (play and greetings) with other yearlings and adults, whereas older animals (often adult males) initiate aggressive interactions that are mostly directed at yearlings. Thus, interactions initiated by yearlings form important ties between individuals. We also found that social cohesion, as measured by these variables, is maintained by a preference for interacting with similar-aged relatives. Thus, it is the relationships between individuals, not the entire group, that are seemingly important. We found no support for the hypothesis that direct measures of social attributes would show stronger age-related changes than those attributes that also quantified indirect relationships.
(c). Dispersal: the importance of social relationships are sex-specific
Bekoff's [86] long-standing but not well-supported hypothesis about the role that social cohesion plays in dispersal was ripe for testing using social network statistics because these statistics provide the tools for formally quantifying cohesion. Blumstein et al. [75] focused on degree—the number of individuals one interacts with; and embeddedness—a statistic that describes how well integrated to the rest of the group an individual is. Because about half of our population of female yearlings disperse whereas almost all male yearlings disperse, we expected that social relationships would be relatively more important for females than for males. We found that female yearlings that interacted with more individuals (i.e. had a greater degree) and those that were more embedded in their group were less likely to disperse (figure 3). Importantly, embeddedness was relatively more important than degree in explaining variation in female dispersal, and models focusing on affiliative interactions explained more variation than models focusing on agonistic interactions. Thus, the pattern of affiliative social relationships may be an important factor that influences the structure of marmot groups. And, because philopatry logically precedes any social benefits from philopatry [87], affiliative social relationships may be an important determinant of more complex social benefits. Potential proximate drivers of social variation include variation in the hypothalamic pituitary axis and variation in personality. Previous analyses found no relationship between measures of circulating stress hormones and network traits [78], and current studies examine the degree of individual consistency in network traits.
Figure 3.
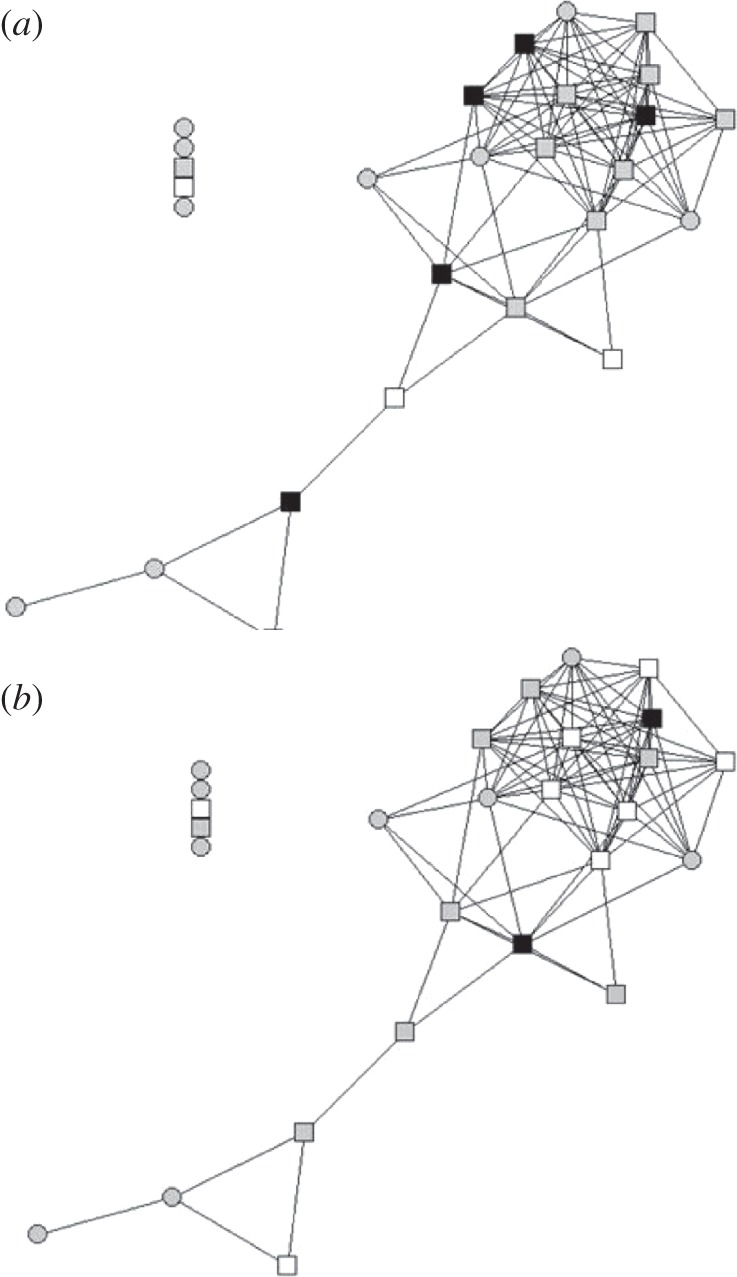
Socially embedded yearling females (a), but not males (b), are less likely to disperse. Adapted from Blumstein et al. [75]. (a,b) square, yearling; circle, adult; white, dispersed; and black, not dispersed.
(d). Network position has fitness consequences
One of the more exciting recent studies that illustrate our relationship-based approach to studying sociality demonstrated that different social attributes differentially affect reproductive success [78]. In this study, we used factor analysis to reduce correlated attributes to four factors that we interpreted as those that measure connectivity, affiliation strength, victimization and bullying. Bullying males had higher reproductive success, a finding consistent with our studies of dominance [85]. Interestingly, we found that affiliation strength was negatively associated with reproductive success in adult females, but connectivity (on which embeddedness loaded) was not. This suggests that there is a cost of direct social cohesion to adult females, and the finding provides additional support for our impression that yellow-bellied marmots are social but not cooperative, for if females were cooperative we would have expected fitness to increase, not decrease, with embeddedness (sensu [88,89]). Finally, these results are consistent with the hypothesis that marmots live in groups because of demographic chance and ecological constraints. Recent results in other taxa also show that network position and strength may have fitness consequences [79,80].
8. Discussion and conclusions
Ecological constraints [90] and demographic chance [25] may force animals to live socially and can modulate group size. What individuals do once they are living socially, however, is profitably studied by examining their social interactions from which sociality emerges. The network- and interaction-based approach, which I refer to as an attribute-based approach, that we have adopted in our studies of yellow-bellied marmots can be profitably adopted by those studying other species and I encourage others to quantify the social networks of their species. I believe that, unlike traditional socioecological studies of sociality, this attribute-based approach will give us complementary insights into which specific social attributes influence and are influenced by specific social behaviours.
For instance, and by analogy, if we look at social drivers of complex communication [91], we find that demographic role variation drives the evolution of repertoire size in sciurid rodents [92], but social group size drives the evolution of individuality [93]. Indeed, in other taxa, variation in the complexity of reproductive roles is associated with the individuality of facial markings [94], and colony size was associated with both bird and bat contact call individuality [95–98]. In primates, group size is associated with vocal repertoire size [99] and the ability to produce variable facial expressions [100].
Importantly, a series of Tinbergian questions [19,20] naturally emerges from an attribute-based approach to study sociality that does not necessarily emerge if one is focused on evaluating a more traditional socioecological model. For instance, as discussed earlier, it is important to ask questions about genetic and environmental mechanisms underlying the formation of social relationships, as has also been done in hyaenas [79] and meerkats (Suricata suricatta) [80], and it is important to understand both the current adaptive use and the evolution of these relationships. By asking all four Tinbergian questions, we develop a rich understanding of a phenomenon. For all of these reasons, an attribute-based approach to studying sociality will become increasingly important in the future and will help us develop a rich and nuanced view of the tremendous amount of social variation seen in nature. Tinbergen would be pleased.
Acknowledgements
I am grateful to Peter Kappeler, Louise Barrett, Stephen Dobson, Jenn Smith and two anonymous reviewers for many constructive comments on previous versions of this review. My work was supported by the U.C.L.A. Academic Senate and Division of Life Sciences, The National Geographic Society, and the N.S.F. (IDBR-0754247 and DEB-1119660 to D.T.B.; DBI 0242960, 0731346 to the Rocky Mountain Biological Laboratory). Marmots were studied under research protocol ARC 2001-191-01 as well as permits issued by the Colorado Division of Wildlife. The research protocol was approved by the UCLA Animal Care Committee on 13 May 2002 and renewed annually.
References
- 1.Crook JH, Gartlan JS. 1966. Evolution of primate societies. Nature 210, 1200–1203 10.1038/2101200a0 (doi:10.1038/2101200a0) [DOI] [PubMed] [Google Scholar]
- 2.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262–300 10.1163/156853980X00447 (doi:10.1163/156853980X00447) [DOI] [Google Scholar]
- 3.van Schaik CP. 1989. The ecology of social relationships amongst female primates. In Comparative socioecology of mammals and humans (eds Standen V, Foley R.), pp. 195–218 Oxford, UK: Blackwell [Google Scholar]
- 4.Sterck EHM, Watts DP, van Schaik CP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291–309 10.1007/s002650050390 (doi:10.1007/s002650050390) [DOI] [Google Scholar]
- 5.Janson CH. 2000. Primate socioecology: the end of a golden age. Evol. Anthropol. 9, 73–86 (doi:10.1002/(SICI)1520-6505(2000)9:2<73::AID-EVAN2>3.0.CO;2-X) [DOI] [Google Scholar]
- 6.Isbell LA, Young TP. 2002. Ecological models of female social relationships in primates: similarities, disparities, and some directions for future clarity. Behaviour 139, 177–202 10.1163/156853902760102645 (doi:10.1163/156853902760102645) [DOI] [Google Scholar]
- 7.Caro T. 2005. Antipredator defenses in birds and mammals. Chicago, IL: University of Chicago Press [Google Scholar]
- 8.van Schaik CP, Janson CH. (eds) 2000. Infanticide by males and its implications. Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Ebensperger LA, Blumstein DT. 2007. Functions of non-parental infanticide in rodents. In Rodent societies: an ecological and evolutionary perspective (eds Wolff JO, Sherman PW.), pp. 267–279 Chicago, IL: University of Chicago Press [Google Scholar]
- 10.Clutton-Brock T, Janson C. 2012. Primate socioecology at the crossroads: past, present, and future. Evol. Anthropol. 21, 136–150 10.1002/evan.21316 (doi:10.1002/evan.21316) [DOI] [PubMed] [Google Scholar]
- 11.Macdonald DW. 1983. The ecology of carnivore social behaviour. Nature 301, 379–384 10.1038/301379a0 (doi:10.1038/301379a0) [DOI] [Google Scholar]
- 12.Rubenstein DI. 1986. Ecology of sociality in horses and zebras. In Ecological aspects of social evolution (eds Rubenstein DI, Wrangham RW.), pp. 282–302 Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Wolff JO, Sherman PW. (eds) 2007. Rodent societies: an ecological and evolutionary perspective. Chicago, IL: University of Chicago Press [Google Scholar]
- 14.Calhoun JB. 1950. The study of wild animals under controlled conditions. Ann. NY Acad. Sci. 51, 1113–1122 10.1111/j.1749-6632.1950.tb27339.x (doi:10.1111/j.1749-6632.1950.tb27339.x) [DOI] [Google Scholar]
- 15.Lucia KE, Keane B, Hayes LD, Lin YK, Schaefer R, Solomon NG. 2008. Philopatry in prairie voles: an evaluation of the habitat saturation hypothesis. Behav. Ecol. 19, 774–783 10.1093/beheco/arn028 (doi:10.1093/beheco/arn028) [DOI] [Google Scholar]
- 16.Schoepf I, Schradin C. 2012. Better off alone! Reproductive competition and ecological constraints determine sociality in the African striped mouse (Rhabdomys pumilio). J. Anim. Ecol. 81, 649–656 10.1111/j.1365-2656.2011.01939.x (doi:10.1111/j.1365-2656.2011.01939.x) [DOI] [PubMed] [Google Scholar]
- 17.Hoogland JL. 1995. The black-tailed prairie dog: social life of a burrowing mammal. Chicago, IL: University of Chicago Press [Google Scholar]
- 18.Kappeler PM, van Schaik CP. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707–740 10.1023/A:1015520830318 (doi:10.1023/A:1015520830318) [DOI] [Google Scholar]
- 19.Tinbergen N. 1963. On aims and methods of ethology. Z. Tierpsychol. 20, 410–433 10.1111/j.1439-0310.1963.tb01161.x (doi:10.1111/j.1439-0310.1963.tb01161.x) [DOI] [Google Scholar]
- 20.Blumstein DT. 2010. Tinbergen's four questions. In The encyclopedia of applied animal behaviour and welfare (ed. Mills D.), pp. 605–606 Wallingford, UK: CAB International [Google Scholar]
- 21.Blumstein DT, et al. 2010. Towards an integrative understanding of social behavior: new models and new opportunities. Front. Behav. Neurosci. 4, 34. 10.3389/fnbeh.2010.00034 (doi:10.3389/fnbeh.2010.00034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armitage KB. 1999. Evolution of sociality in marmots. J. Mammal 80, 1–10 [Google Scholar]
- 23.Armitage KB. 2000. The evolution, ecology, and systematics of marmots. Oecologia Mont. 9, 1–18 [Google Scholar]
- 24.Armitage KB, Blumstein DT. 2002. Body-mass diversity in marmots. In Holarctic marmots as a factor of biodiversity. Proc. 3rd Int Conf. Marmots, Cheboksary, Russia, 25–30 August 1997 (eds Armitage KB, Rumiantsev VY.), pp. 22–40 Moscow: ABF Publishing House [Google Scholar]
- 25.Barrett L, Henzi SP. 2002. Constraints on relationship formation among female primates. Behaviour 139, 263–289 10.1163/156853902760102672 (doi:10.1163/156853902760102672) [DOI] [Google Scholar]
- 26.Hinde RA. 1976. Interactions, relationships and social structure. Man 11, 1–17 10.2307/2800384 (doi:10.2307/2800384) [DOI] [Google Scholar]
- 27.Armitage KB. 2010. Individual fitness, social behavior, and population dynamics of yellow-bellied marmots. In The ecology of place: contributions of place-based research to ecological understanding (eds Billick I, Price MV.), pp. 132–154 Chicago, IL: University of Chicago Press [Google Scholar]
- 28.Armitage KB. 2012. Sociality, individual fitness and population dynamics of yellow-bellied marmots. Mol. Ecol. 21, 532–540 10.1111/j.1365-294X.2011.05323.x (doi:10.1111/j.1365-294X.2011.05323.x) [DOI] [PubMed] [Google Scholar]
- 29.Calhoun JB. 1952. The social aspects of population dynamics. J. Mammal 33, 139–159 10.2307/1375923 (doi:10.2307/1375923) [DOI] [Google Scholar]
- 30.Errington PL. 1956. Factors limiting higher vertebrate populations. Science 124, 304–307 10.1126/science.124.3216.304 (doi:10.1126/science.124.3216.304) [DOI] [PubMed] [Google Scholar]
- 31.Armitage KB, Blumstein DT, Woods BC. 2003. Energetics of hibernating yellow-bellied marmots (Marmota flaviventris). Comp. Biochem. Physiol. A 134, 101–114 10.1016/S1095-6433(02)00219-2 (doi:10.1016/S1095-6433(02)00219-2) [DOI] [PubMed] [Google Scholar]
- 32.Lenihan C, Van Vuren D. 1996. Growth and survival of juvenile yellow-bellied marmots (Marmota flaviventris). Can. J. Zool. 74, 297–302 10.1139/z96-037 (doi:10.1139/z96-037) [DOI] [Google Scholar]
- 33.Armitage KB. 2004. Badger predation on yellow-bellied marmots. Am. Midl. Nat. 151, 378–387 10.1674/0003-0031(2004)151[0378:BPOYM]2.0.CO;2 (doi:10.1674/0003-0031(2004)151[0378:BPOYM]2.0.CO;2) [DOI] [Google Scholar]
- 34.Armitage KB. 1986. Marmot polygyny revisited: determinants of male and female reproductive strategies. In Ecological aspects of social evolution (eds Rubenstein DI, Wrangham RW.), pp. 303–331 Princeton, NJ: Princeton University Press [Google Scholar]
- 35.Armitage KB. 1984. Recruitment in yellow-bellied marmot populations: kinship, philopatry, and individual variability. In The biology of ground-dwelling squirrels (eds Murie JO, Michener GR.), pp. 377–403 Lincoln, NB: University of Nebraska Press [Google Scholar]
- 36.Armitage KB. 1974. Male behaviour and territoriality in the yellow-bellied marmot. J. Zool. Lond. 172, 233–265 10.1111/j.1469-7998.1974.tb04104.x (doi:10.1111/j.1469-7998.1974.tb04104.x) [DOI] [Google Scholar]
- 37.Armitage KB. 2003. Reproductive competition in female yellow-bellied marmots. In Adaptive strategies and diversity in marmots (eds Ramousse R, Allaine D, Le M. Berre), pp. 133–142 Lyon, France: International Marmot Network [Google Scholar]
- 38.Arnold W. 1993. Social evolution in marmots and the adaptive value of joint hibernation. Verh. Dtsch. Zool. Ges. 86, 79–93 [Google Scholar]
- 39.Svendsen GE. 1974. Behavioral and environmental factors in the spatial distribution and population dynamics of a yellow-bellied marmot population. Ecology 55, 760–771 10.2307/1934412 (doi:10.2307/1934412) [DOI] [Google Scholar]
- 40.Armitage KB. 1991. Social and population dynamics of yellow-bellied marmots: results from long-term research. Ann. Rev. Ecol. Syst. 22, 379–407 10.1146/annurev.es.22.110191.002115 (doi:10.1146/annurev.es.22.110191.002115) [DOI] [Google Scholar]
- 41.Armitage KB. 2003. Recovery of a yellow-bellied marmot population following a weather-induced decline. In Adaptive strategies and diversity in marmots (eds Ramousse R, Allaine D, Le M. Berre), pp. 215–222 Lyon, France: International Marmot Network [Google Scholar]
- 42.Blumstein DT, Ozgul A, Yovovitch V, Van Vuren DH, Armitage KB. 2006. Effect of predation risk on the presence and persistence of yellow-bellied marmot (Marmota flaviventris) colonies. J. Zool. Lond. 270, 132–138 10.1111/j.1469-7998.2006.00098.x (doi:10.1111/j.1469-7998.2006.00098.x) [DOI] [Google Scholar]
- 43.Blumstein DT, Lea AJ, Olson LE, Martin JGA. 2010. Heritability of anti-predatory traits: vigilance and locomotor performance in marmots. J. Evol. Biol. 23, 879–887 10.1111/j.1420-9101.2010.01967.x (doi:10.1111/j.1420-9101.2010.01967.x) [DOI] [PubMed] [Google Scholar]
- 44.Blumstein DT, et al. 2004. Locomotor ability and wariness in yellow-bellied marmots. Ethology 110, 615–634 10.1111/j.1439-0310.2004.01000.x (doi:10.1111/j.1439-0310.2004.01000.x) [DOI] [Google Scholar]
- 45.Bednekoff PA, Blumstein DT. 2009. Peripheral visibility influences marmot vigilance: integrating observational and experimental results. Behav. Ecol. 20, 1111–1117 10.1093/beheco/arp104 (doi:10.1093/beheco/arp104) [DOI] [Google Scholar]
- 46.Blumstein DT, Ferando E, Stankowich T. 2009. A test of the multipredator hypothesis: yellow-bellied marmots respond fearfully to the sight of novel and extinct predators. Anim. Behav. 78, 873–878 10.1016/j.anbehav.2009.07.010 (doi:10.1016/j.anbehav.2009.07.010) [DOI] [Google Scholar]
- 47.Blumstein DT, Cooley L, Winternitz J, Daniel JC. 2008. Do yellow-bellied marmots respond to predator vocalizations? Behav. Ecol. Sociobiol. 62, 457–468 10.1007/s00265-007-0473-4 (doi:10.1007/s00265-007-0473-4) [DOI] [Google Scholar]
- 48.Blumstein DT, Barrow L, Luterra M. 2008. Olfactory predator discrimination in yellow-bellied marmots. Ethology 114, 1135–1143 10.1111/j.1439-0310.2008.01563.x (doi:10.1111/j.1439-0310.2008.01563.x) [DOI] [Google Scholar]
- 49.Blumstein DT. 2007. The evolution, function, and meaning of marmot alarm communication. Adv. Study Behav. 37, 371–401 10.1016/S0065-3454(07)37008-3 (doi:10.1016/S0065-3454(07)37008-3) [DOI] [Google Scholar]
- 50.Svendsen GE. 1976. Structure and location of burrows of yellow-bellied marmot. Southwest Nat. 20, 487–494 10.2307/3669865 (doi:10.2307/3669865) [DOI] [Google Scholar]
- 51.Li C, Monclús R, Maul TL, Jiang Z, Blumstein DT. 2011. Quantifying human disturbance on antipredator behaviour and flush initiation distance in yellow-bellied marmots. Appl. Anim. Behav. Sci. 129, 146–152 10.1016/j.applanim.2010.11.013 (doi:10.1016/j.applanim.2010.11.013) [DOI] [Google Scholar]
- 52.Blumstein DT, Pelletier D. 2005. Yellow-bellied marmot hiding time is sensitive to variation in costs. Can. J. Zool. 83, 363–367 10.1139/z05-020 (doi:10.1139/z05-020) [DOI] [Google Scholar]
- 53.Rhoades E, Blumstein DT. 2007. Predicted fitness consequences of threat-sensitive hiding behavior. Behav. Ecol. 18, 937–943 10.1093/beheco/arm064 (doi:10.1093/beheco/arm064) [DOI] [Google Scholar]
- 54.Blumstein DT. 2008. A localized ecological disaster. Denver Post July 1 See http://www.denverpost.com/headlines/ci_9756521.
- 55.Inouye DW, Barr B, Armitage KB, Inouye BD. 2000. Climate change is affecting altitudinal migrants and hibernating species. Proc. Natl Acad. Sci. USA 97, 1630–1633 10.1073/pnas.97.4.1630 (doi:10.1073/pnas.97.4.1630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485 10.1038/nature09210 (doi:10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woods BC, Armitage KB. 2003. Effects of food addition on life history of yellow-bellied marmots. Oecol. Montana 12, 1–8 [Google Scholar]
- 58.Lea AJ, Martin JGA, Olson LE, Blumstein DT. In preparation Evolutionary potential of growth-related traits in a changing alpine environment. [Google Scholar]
- 59.Dunbar RIM. 1992. Time: a hidden constraint on the behavioral ecology of baboons. Behav. Ecol. Sociobiol. 31, 35–49 10.1007/BF00167814 (doi:10.1007/BF00167814) [DOI] [Google Scholar]
- 60.Pollard KA, Blumstein DT. 2008. Time allocation and the evolution of group size. Anim. Behav. 76, 1683–1699 10.1016/j.anbehav.2008.08.006 (doi:10.1016/j.anbehav.2008.08.006) [DOI] [Google Scholar]
- 61.Lott DF. 1984. Intraspecific variation in the social systems of wild vertebrates. Behaviour 88, 266–325 10.1163/156853984X00353 (doi:10.1163/156853984X00353) [DOI] [Google Scholar]
- 62.Lott DF. 1991. Intraspecific variation in the social systems of wild vertebrates. Cambridge, UK: Cambridge University Press [Google Scholar]
- 63.Strier KB. 2009. Seeing the forest through the seeds: mechanisms of primate behavioral diversity from individuals to populations and beyond. Curr. Anthropol. 50, 213–228 10.1086/592026 (doi:10.1086/592026) [DOI] [PubMed] [Google Scholar]
- 64.Hinde RA. 1979. Towards understanding relationships. London, UK: Academic Press [Google Scholar]
- 65.Wasserman S, Faust K. 1994. Social network analysis. Cambridge, UK: Cambridge University Press [Google Scholar]
- 66.Krause J, Croft DP, James R. 2007. Social network theory in the behavioural sciences: potential applications. Behav. Ecol. Sociobiol. 62, 15–27 10.1007/s00265-007-0445-8 (doi:10.1007/s00265-007-0445-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Croft DP, James R, Krause J. 2008. Exploring animal social networks. Princeton, NJ: Princeton University Press [Google Scholar]
- 68.Wey T, Blumstein DT, Shen W, Jordán F. 2008. Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim. Behav. 75, 333–344 10.1016/j.anbehav.2007.06.020 (doi:10.1016/j.anbehav.2007.06.020) [DOI] [Google Scholar]
- 69.Barrett L, Henzi SP, Lusseau D. 2012. Taking sociality seriously: the structure of multi-dimensional social networks as a source of information for individuals. Phil. Trans. R. Soc. B 367, 2108–2118 10.1098/rstb.2012.0113 (doi:10.1098/rstb.2012.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olson LE, Blumstein DT. 2010. Applying the coalitionary-traits metric: sociality without complex cooperation in male yellow-bellied marmots. Behav. Ecol. 21, 957–965 10.1093/beheco/arq094 (doi:10.1093/beheco/arq094) [DOI] [Google Scholar]
- 71.Olson LE, Blumstein DT. 2009. A trait-based approach to understand the evolution of complex coalitions in male mammals. Behav. Ecol. 20, 624–632 10.1093/beheco/arp040 (doi:10.1093/beheco/arp040) [DOI] [Google Scholar]
- 72.da Silva J, Macdonald DW, Evans PGH. 1994. Net costs of group living in a solitary forager, the Eurasian badger (Meles meles). Behav. Ecol. 5, 151–158 10.1093/beheco/5.2.151 (doi:10.1093/beheco/5.2.151) [DOI] [Google Scholar]
- 73.Kappeler PM. 1999. Primate socioecology: new insights from males. Naturwissenschaften 85, 18–29 10.1007/s001140050563 (doi:10.1007/s001140050563) [DOI] [PubMed] [Google Scholar]
- 74.Nunn CL. 1999. The number of males in primate social groups: a comparative test of the socioecological model. Behav. Ecol. Sociobiol. 46, 1–13 10.1007/s002650050586 (doi:10.1007/s002650050586) [DOI] [Google Scholar]
- 75.Blumstein DT, Wey TW, Tang K. 2009. A test of the social cohesion hypothesis: interactive female marmots remain at home. Proc. R. Soc. B 276, 3007–3012 10.1098/rspb.2009.0703 (doi:10.1098/rspb.2009.0703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lea AJ, Blumstein DT, Wey TW, Martin JGA. 2010. Heritable victimization and the benefits of agonistic relationships. Proc. Natl Acad. Sci. USA 107, 21587–21592 10.1073/pnas.1009882107 (doi:10.1073/pnas.1009882107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wey TW, Blumstein DT. 2010. Social cohesion in yellow-bellied marmots is established through age and kin structuring. Anim. Behav. 79, 1343–1352 10.1016/j.anbehav.2010.03.008 (doi:10.1016/j.anbehav.2010.03.008) [DOI] [Google Scholar]
- 78.Wey TW, Blumstein DT. 2012. Social attributes and associated performance measures in marmots: bigger male bullies and weakly affiliating females have higher annual reproductive success. Behav. Ecol. Sociobiol. 66, 1075–1085 10.1007/s00265-012-1358-8 (doi:10.1007/s00265-012-1358-8) [DOI] [Google Scholar]
- 79.Holekamp KE, Smith JE, Strelioff CC, Van Horn RC, Watts HE. 2012. Society, demography and genetic structure in the spotted hyena. Mol. Ecol. 21, 613–632 10.1111/j.1365-294X.2011.05240.x (doi:10.1111/j.1365-294X.2011.05240.x) [DOI] [PubMed] [Google Scholar]
- 80.Madden JR, Drewe JA, Pearce GP, Clutton-Brock TH. 2009. The social network structure of a wild meerkat population: 2. Intragroup interactions. Behav. Ecol. Sociobiol. 64, 81–95 10.1007/s00265-009-0820-8 (doi:10.1007/s00265-009-0820-8) [DOI] [Google Scholar]
- 81.Madden JR, Drewe JA, Pearce GP, Clutton-Brock TH. 2011. The social network structure of a wild meerkat population: 3. Position of individuals within networks. Behav. Ecol. Sociobiol. 65, 1857–1871 10.1007/s00265-011-1194-2 (doi:10.1007/s00265-011-1194-2) [DOI] [Google Scholar]
- 82.Lehman J, Ross C. 2011. Baboon (Papio anubis) social complexity: a network approach. Am. J. Primatol. 73, 775–789 10.1002/ajp.20967 (doi:10.1002/ajp.20967) [DOI] [PubMed] [Google Scholar]
- 83.Whitehead H, Dufault S. 1999. Techniques for analyzing vertebrate social structure using identified individuals: review and recommendations. Adv. Study Behav. 28, 33–74 10.1016/S0065-3454(08)60215-6 (doi:10.1016/S0065-3454(08)60215-6) [DOI] [Google Scholar]
- 84.Kruuk LEB. 2004. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890 10.1098/rstb.2003.1437 (doi:10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang B, Wey TW, Blumstein DT. 2011. Correlates and consequences of dominance in a social rodent. Ethology 117, 573–585 10.1111/j.1439-0310.2011.01909.x (doi:10.1111/j.1439-0310.2011.01909.x) [DOI] [Google Scholar]
- 86.Bekoff M. 1977. Mammalian dispersal and the ontogeny of individual behavioral phenotypes. Am. Nat. 111, 715–732 10.1086/283201 (doi:10.1086/283201) [DOI] [Google Scholar]
- 87.Cahan SH, Blumstein DT, Sundström L, Liebig J, Griffin A. 2002. Social trajectories and the evolution of social behavior. Oikos 96, 206–216 10.1034/j.1600-0706.2002.960202.x (doi:10.1034/j.1600-0706.2002.960202.x) [DOI] [Google Scholar]
- 88.Silk JB. 2007. Social components of fitness in primate groups. Science 317, 1347–1351 10.1126/science.1140734 (doi:10.1126/science.1140734) [DOI] [PubMed] [Google Scholar]
- 89.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Female chacma baboons form strong, equitable, and enduring social bonds. Behav. Ecol. Sociobiol. 64, 1733–1747 10.1007/s00265-010-0986-0 (doi:10.1007/s00265-010-0986-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson DDP, Kayes R, Blackwell PG, Macdonald DW. 2002. Does the resource dispersion hypothesis explain group living? Trends Ecol. Evol. 17, 563–570 10.1016/S0169-5347(02)02619-8 (doi:10.1016/S0169-5347(02)02619-8) [DOI] [Google Scholar]
- 91.Pollard KA, Blumstein DT. 2012. Evolving communicative complexity: insights from rodents and beyond. Phil. Trans. R. Soc. B 367, 1869–1878 10.1098/rstb.2011.0221 (doi:10.1098/rstb.2011.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blumstein DT, Armitage KB. 1997. Does sociality drive the evolution of communicative complexity? A comparative test with ground-dwelling sciurid alarm calls. Am. Nat. 150, 179–200 10.1086/286062 (doi:10.1086/286062) [DOI] [PubMed] [Google Scholar]
- 93.Pollard KA, Blumstein DT. 2011. Social group size predicts the evolution of individuality. Curr. Biol. 21, 413–417 10.1016/j.cub.2011.01.051 (doi:10.1016/j.cub.2011.01.051) [DOI] [PubMed] [Google Scholar]
- 94.Tibbetts EA. 2004. Complex social behaviour can select for variable visual features: a case study in Polistes wasps. Proc. R. Soc. Lond. B 271, 1955–1960 10.1098/rspb.2004.2784 (doi:10.1098/rspb.2004.2784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Medvin MB, Stoddard PK, Beecher MD. 1993. Signals for parent–offspring recognition: a comparative analysis of the begging calls of cliff swallows and barn swallows. Anim. Behav. 45, 841–850 10.1006/anbe.1993.1105 (doi:10.1006/anbe.1993.1105) [DOI] [Google Scholar]
- 96.Mathevon N, Charrier I, Jouventin P. 2003. Potential for individual recognition in acoustic signals: a comparative study of two gulls with different nesting patterns. Curr. Biol. 326, 329–337 10.1016/S1631-0691(03)00072-6 (doi:10.1016/S1631-0691(03)00072-6) [DOI] [PubMed] [Google Scholar]
- 97.Freeberg TM. 2006. Social complexity can drive vocal complexity. Psychol. Sci. 17, 557–561 10.1111/j.1467-9280.2006.01743.x (doi:10.1111/j.1467-9280.2006.01743.x) [DOI] [PubMed] [Google Scholar]
- 98.Wilkinson GS. 2003. Social and vocal complexity in bats. In Animal social complexity: intelligence, culture, and individualized societies (eds de Waal FBM, Tyack PL.), pp. 322–341 Cambridge, MA: Harvard University Press [Google Scholar]
- 99.McComb K, Semple S. 2005. Co-evolution of vocal communication and sociality in primates. Biol. Lett. 1, 381–385 10.1098/rsbl.2005.0366 (doi:10.1098/rsbl.2005.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dobson SD. 2009. Socioecological correlates of facial mobility in non-human anthropods. Am. J. Phys. Anthropol. 139, 413–420 10.1002/ajpa.21007 (doi:10.1002/ajpa.21007) [DOI] [PubMed] [Google Scholar]