Abstract
The multitude of variables associated with a battery of outcome measures presents a risk for spurious findings in clinical trials and observational studies of mild traumatic brain injury (mTBI). We have used principal components analysis (PCA) to facilitate data reduction by identifying components which represent subsets of neuropsychological measures that are selectively correlated with each other. By merging data from two concurrent mTBI studies using the same outcome measures, we obtained a cohort of 102 mTBI patients and 85 orthopedic injury (OI) comparison patients whom we recruited from 24 hours to 96 hours post-injury and evaluated at one week, 1 month, and 3 months post-injury. Cognitive domains included episodic memory, evaluated by both verbal and visual memory tasks, cognitive processing speed tests, and executive function. Post-concussion and stress-related symptoms were measured by rating scales. PCA identified four components, including cognitive processing speed, verbal memory, visual memory, and a symptom composite representing post-concussion and stress symptoms. mTBI patients older than the mean age of 18 years had slower cognitive processing than the OI patients, but there was no group difference in cognitive processing speed in younger patients. The symptom component score differed significantly as mTBI patients had more severe symptoms than the OI group at each occasion. Our results encourage replication with other cohorts using either the same outcome measures or at least similar domains. PCA is an approach to data reduction that could mitigate spurious findings and increase efficiency in mTBI research.
Key Words: mTBI, data reduction, principal components analysis
Introduction
Designing clinical trials and observational studies of traumatic brain injury (TBI) involves selecting appropriate outcome measures while considering the data management and statistical analysis of the data. Investigators frequently include several domains of outcome measures, each containing one or more tests that may have multiple variables. Consequently, it is advisable to develop a statistical analysis plan with a data reduction strategy to mitigate the risk of a Type 1 error due to multiple between group comparisons.
Reviews and meta-analyses of observational studies concerning outcome of mild TBI (mTBI) have identified domains of cognitive performance and symptoms that are sensitive to sequelae at least during the first week to 3 months post-injury.1–4 The cognitive domains represented in studies of mTBI often include episodic memory, cognitive processing speed, and executive functioning. However, the number of cognitive domains measured varies to some extent across studies. The cognitive domains are described below as are the symptoms.
Episodic memory is recall of information presented in a specific temporal and spatial context, as compared to general knowledge. This type of memory is typically evaluated by verbal learning tests in which word lists are presented for immediate recall on multiple ‘learning’ trials, followed by a delayed recall trial. Many studies also include visual memory for spatial location of targets or reproduction of a complex design, again followed by a delayed recall trial. Cognitive processing speed has been measured by various tasks which have in common the measurement of time to completion or a total correct score achieved within a time limit as the primary variable. Cognitive processing speed tasks include coding symbols with numbers according to a guide which the patient views while performing the test, simple and choice reaction time (RT), and visuomotor speed measured by the time taken to connect circles scattered on a paper in a numeric or alphanumeric sequence. Cognitive processing speed has also been measured by computer administered panels of timed measures which can be given using a handheld device or a portable computer.5,6 Other cognitive tests frequently used in studies of mTBI include cognitive interference that also depends on measurement of response time and tests of executive function that include flexibility in problem solving and the capability to shift strategy according to changes in the relevant conditions.
Post-concussion symptoms (PCS), including cognitive, somatic, and emotional symptoms, are generally measured by self-report forms, structured interview, or clinical examination. These symptoms often arise within hours after mTBI, and in some studies, persist after recovery of objectively measured cognitive deficits.4,7 Commonly reported cognitive symptoms include problems with memory and concentration (or attention). Somatic symptoms include headaches, fatigue, and dizziness. Frequently reported emotional symptoms include anxiety and depression. Symptoms of acute stress disorder may arise within the first month after mTBI even if it is premature to diagnose post-traumatic stress disorder (PTSD) which requires a symptom duration of at least 1 month. However, acute stress disorder symptoms may continue after 1 month, even if the patient does not meet diagnostic criteria for PTSD. PCS and PTSD symptoms may co-exist, and there is evidence for a positive correlation and partial overlap especially in military mTBI sustained during combat.3
With studies of mTBI measuring multiple cognitive variables, PCS, and both acute stress and PTSD-related symptoms, statistical analysis of these numerous outcome measures carries an increased risk of a Type 1 error. Consequently, we considered an approach to data reduction that could be useful in observational studies of recovery and in clinical trials.
Here we use a principal components analysis (PCA)8 approach to reduce the number of neuropsychological variables for the purpose of controlling Type I error in analyzing the outcome data of adolescent and adult patients after mTBI. We report prospective, longitudinal data of mTBI and orthopedically-injured (OI) patients whom we recruited in the emergency center and followed over a 3-month period, including an initial assessment within a few days after injury, at 1 month, and at 3 months post-injury. As in previous studies of mTBI outcome, we evaluated the cognitive domains of episodic memory, processing speed, and executive function. We also measured PCS and symptoms of post-traumatic stress. Following the PCA, we analyzed between-group differences in the component scores over occasions. In addition to the long term goal of developing an approach to data reduction for mTBI research, we hypothesized that a component representing symptoms would show the clearest differentiation of mTBI and OI patients over the three month follow-up.
Methods
Subjects
We obtained data from mTBI and OI patients in two concurrent, prospective projects that utilized similar protocols, including outcome measures and definitions of mTBI. The rationale for combining data for the two projects was to obtain a sample size sufficient for PCA. With the exception of the age range and the recruitment window, the eligibility criteria were identical for the two projects. Institutional approval was obtained by a full board review of the study protocol in both projects.
Project 1
Sixty-three patients were recruited within 96 hours after sustaining mTBI as defined by an injury to the head from blunt trauma, acceleration, or deceleration forces that resulted in loss or other alteration of consciousness due to verified head trauma according to an entry in the medical record based on a report by emergency medical service personnel or another witness. The rationale was to ensure that all mTBI patients enrolled actually sustained head trauma and to mitigate the possibility that OI patients had occult head trauma that was overlooked in the emergency center. In addition, all mTBI patients had one or more of the following: (1) observed or self-reported confusion, disorientation, or otherwise impaired consciousness; (2) loss of consciousness lasting less than 30 min; (3) PCS;9 (4) Glasgow Coma Scale (GCS)10 score of 13–15 on examination by an emergency medicine physician or resident in the emergency center; and (5) post-traumatic amnesia (PTA) duration less than 24 h.11 Other eligibility criteria were absence of parenchymal abnormalities on computed tomographic (CT) scan of the head within 24 h after injury and mild or no concomitant extra cranial injury as measured by the Abbreviated Injury Scale (AIS).12 For comparison, 61 patients who had a mild OI that did not necessitate surgery or hospitalization were enrolled within the same time window.12 Eligibility criteria for the mTBI and OI groups included age 12–30 years, right handedness, no previous neurological disorder that could affect cognition, no history of schizophrenia or bipolar disorder, and no evidence of substance dependence on screening tests of substance use.13–15 All patients also completed a Litigation and Compensation Questionnaire so the investigators could determine whether positive responses differed for the mTBI and OI groups, and whether positive responses were associated with outcome data. Informed consent was obtained from the patient or, in the case of a minor, from the parent or other legally authorized representative. Patients in both groups were recruited from the emergency centers of Ben Taub General Hospital (BTGH), Memorial Hermann Hospital (MHH), or Texas Children's Hospital (TCH).
Project 2
Thirty-nine patients with mTBI as defined above were recruited within the first 24 hours post-injury from the emergency centers of BTGH and MHH. Eligibility criteria were identical to Project 1 with the exception of an age range of 18–50 years and a 24-hour window for recruitment. Twenty-four patients with OI were also recruited in Project 2 using criteria similar to Project 1 except for the age range (18–50 years) and the post-injury evaluation window (24–48 h). Socioeconomic level was measured for all patients.16 As in Project 1, patients completed a Litigation and Compensation Questionnaire.
Study design
Projects 1 and 2 were prospective, longitudinal observational studies with follow-up intervals of 1 month and 3 months. Although the first assessment in Project 1 was scheduled at the earliest opportunity after enrollment within 96 h, and Project 2 had the first assessment scheduled at 1 week post-injury, the actual post-injury intervals for the first cognitive assessment in both projects approximated 1 week. Consequently, we combined data for the earliest assessment from the two projects and refer to this occasion as the one-week assessment.
Methods of measurement
The outcome measures given on each occasion included the following:
Automated Neuropsychological Assessment Metrics (ANAM).17,18
Administered on a hand-held Palm Digital Assistant, the ANAM subtests selected for this project emphasized processing speed and general cognition. Reaction time (RT) and/or errors were recorded for all subtests.
Simple Reaction Time (SimRT)
The participant quickly tapped an asterisk, which appeared at various locations across trials (20 trials).
Code Substitution Learning (CodeSub)
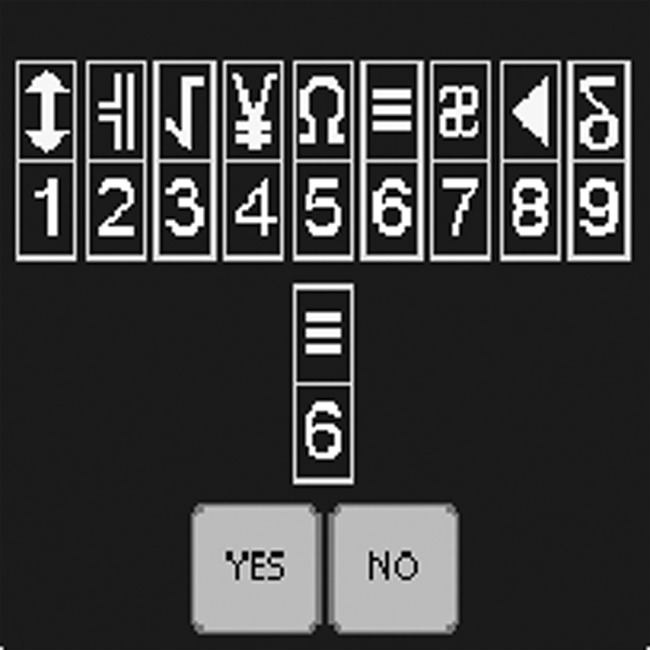
Subject responded by key press whether a symbol-digit pair at center was one of nine symbol-digit pairs shown at top of screen (Fig. 1), (72 trials).
FIG. 1.
Screenshot of CodeSub test of the ANAM.
Code Substitution Recognition (CodeSubRec)
Recognition memory test for the symbol-number pairs used in CodeSub test (18 trials).
Matching to Sample (Match)
A different 4×4 checkerboard matrix was presented on each trial. After a 5-second delay, two matrices were presented and the subject selected the one which matched the sample (16 trials).
Logical Relation (Logic)
On each trial, a single symbol pair “# &” or “& #” was displayed along with a written statement that correctly or incorrectly described the order of the symbols. Subject responded whether the statement was correct (24 trials).
Stroop Tasks
Color words (e.g., “Red”) were presented and the subject identified color of the print under Congruent (the color of letters corresponded to color name) and Incongruent (e.g., “blue” printed in red letters conditions (30 trials).
Go-No-Go
Participant responded to asterisk and withheld response to a plus sign (40 trials, including 12 no-go trials).
Symbol-Digit Modalities Test (SDMT).19
Referring to a key at top of the page to translate nonverbal symbols to an alpha-numeric digit, participant filled in boxes (written version) or verbalized the correct digit for each symbol on this timed test. Total correct responses within 90 seconds were measured.
Verbal Selective Reminding Test (VSRT).20
Episodic verbal memory was tested for 12 words that the examiner selectively presented over six trials, depending on the number of words the subject recalled on the previous trial. Delayed recall was tested 30 min after the sixth trial. Variables analyzed were total number of words consistently recalled on trials 1-6 (CLTR) and number of words recalled after a 30 min delay.
Brief Visuospatial Memory Test-Revised (BVMT-R).21
The participant reproduced six geometric figures after they were presented simultaneously. This was repeated on two additional learning trials. Scores for the three learning trials were obtained for accuracy of drawing the figures and for the spatial locations. Delayed recall after 25 minutes was scored similarly.
Verbal Fluency Test.22
Participants were presented a single letter (e.g., “S”) and asked to verbally produce as many words as possible that start with that letter. Total number of correct words was summed across three trials.
Rivermead Post-Concussion Symptoms Questionnaire (RPSQ).23
This 16-item self-report form measured severity of postconcussion symptoms using a 5-point scale.23,24 Higher score denotes worse severity of symptom relative to pre-injury.
Post-Traumatic Stress Checklist-Civilian Form (PCL-C).25
This 17-item self-report scale measured severity of PTSD symptoms with higher scores reflecting more severe PTSD symptoms.
Statistical analysis
Since the variables of interest were correlated with each other, PCA was used to reduce the redundancies and Type I error for multiple between-group comparisons and to aid data reduction. The PCA was followed by a rotation in an attempt to achieve a “simple structure.”26–28 Since the correlations between the components using oblique rotation were not high and less meaningful than those obtained from using orthogonal rotation, orthogonal rotation was used to extract the principal components. Absolute loading of 0.4 or higher was used as criterion for variables to be loaded on the corresponding components. Interpretability, accounting for at least 10% of total variance, and the combined components accounting for at least 80% of the cumulative variance were used as criteria to select components.
To check for the appropriateness of analyzing the change of principal components across post-injury intervals, the correlation matrices across the three occasions were compared, which did not reveal a substantially different pattern of variable relations (p value=0.275). In addition, PCA was done for each occasion, and results showed that the structure of the principal components was consistent across the initial (1 week), 1 month, and 3 month assessments. Finally, PCA was done for the entire data set and changes of the rotated principal components across the post-injury intervals were analyzed using linear mixed models. The actual post-injury interval in days was used as a time variable, and was centered at 30 days. Linear and quadratic patterns were checked for the unconditional (level 1) model; the variances for both linear and quadratic terms of interval were significant (see Table 3). Then group, age, gender, and socioeconomic level were entered to the level 2 model to evaluate their effects on the intercept, slope, and the change of slope. Only significant effects (below a threshold of 0.05) were included in the model. For the polynomial function of time, if the quadratic term was significant, the linear term was included automatically. Figures 2–4 were created using fitted values, and the y axes are scores of the components, which are weighted sums of the product of scoring coefficients and the subject's standardized scores of the original variables. So the y axes of Figures 2–4 are unitless. However, higher scores denote better performance and negative numbers reflect a relatively worse performance than the average.
Table 3.
Parameter Estimates from Growth Curve Model
| Parameter | Estimates | t value | p value |
|---|---|---|---|
| Component 1—Speed Processing | |||
| Intercept | 0.4828 | 1.99 | 0.0487 |
| OI vs. mTBI | −1.0690 | −2.65 | 0.0097 |
| Age at injury | −.02079 | −2.09 | 0.0403 |
| Age at injury*Group (OI vs. mTBI) | .05793 | 3.31 | 0.0014 |
| SCI | .2660 | 3.32 | 0.0014 |
| Interval (slope) | .01327 | 10.85 | <0.0001 |
| Interval2 (quadratic) | −.00017 | −7.03 | <0.0001 |
| Component 2—Visual Memory | |||
| Intercept | 1.0500 | 5.46 | <0.0001 |
| OI vs. mTBI | .06925 | .57 | 0.5688 |
| Age at injury | −.05143 | −6.77 | <0.0001 |
| Interval (slope) | .003086 | 3.93 | 0.0001 |
| Component 3—Verbal Memory | |||
| Intercept | −.1023 | −1.21 | 0.2289 |
| OI vs. mTBI | .1854 | 1.53 | 0.1300 |
| SCI | .1888 | 2.51 | 0.0141 |
| Interval (slope) | .000489 | .49 | 0.6227 |
| Component 3—PTSD Symptoms | |||
| Intercept | .2282 | 2.37 | 0.0187 |
| OI vs. mTBI | −.7330 | −5.74 | <0.0001 |
| Gender (F vs. M) | .4692 | 3.36 | 0.0012 |
| Interval (Slope) | −.00385 | −4.24 | <0.0001 |
| Interval*group (OI vs. mTBI) | .00295 | 2.19 | 0.0316 |
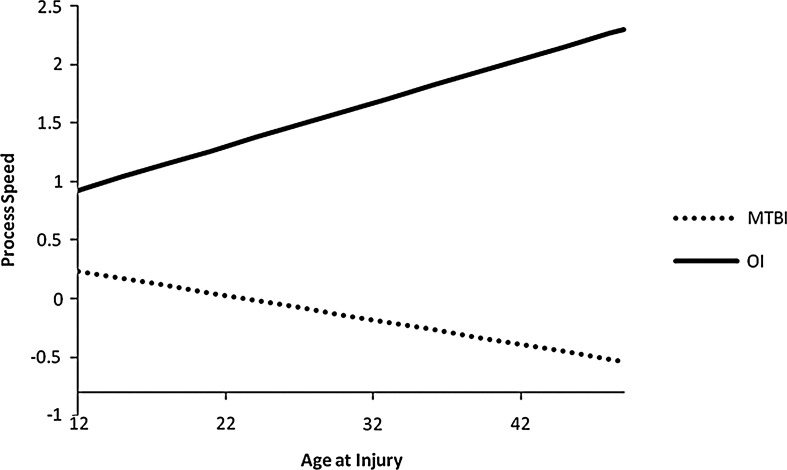
FIG. 2.
Processing speed component score by age at injury for mTBI and OI groups.
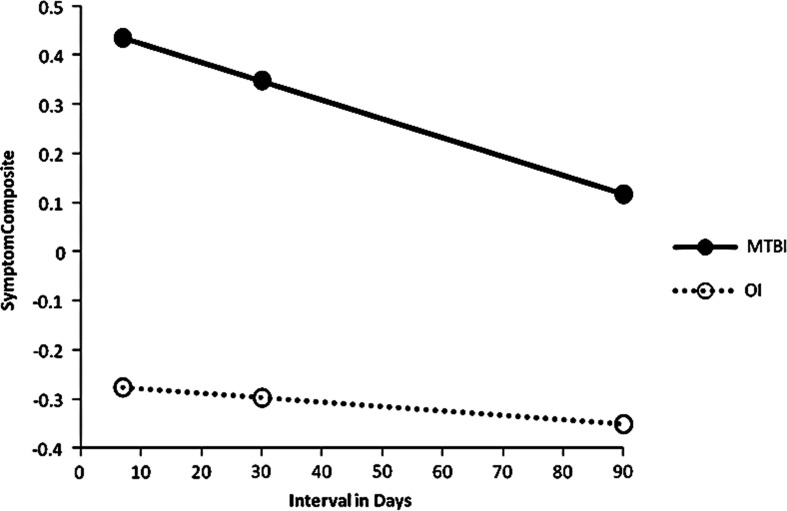
FIG. 4.
Scatter plot of symptom composite score by post-injury interval for mTBI and OI patients at 1 week, 1 month, and 3 months.
For the ANAM, only the Codesub RT was included in the PCA because no significant group differences were found for the other subtests, and including all of the ANAM variables would have added more noise to the model, possibly resulting in less interpretable components, and reducing the power for the current sample size. The correlations of the verbal fluency test scores with other outcome measures were very low, so it was not included in the PCA and between-group analyses.
Demographic characteristics were compared for the mTBI and OI groups using Chi-square or Fisher's exact test for categorical variables and t-test for continuous variables.
Results
Demographic and clinical features of the mTBI and OI groups
Table 1 shows that within each project, there were no significant differences in age at injury, gender, or socioeconomic level between the mTBI and OI groups. As seen in Table 1, motor vehicle crashes were over-represented in the mTBI groups, whereas sports-related injuries were more frequent in the OI groups. Using the overall mean age of 18 years as a cutoff, the mechanism of injury tended to differ depending on whether the patients were older or younger than 18 years and whether they had an mTBI or OI. Within the mTBI group, motor vehicle crashes were a more frequent cause of injury in patients older than 18 years (63.1% of this subgroup) as compared with OI patients older than 18 years (14.04%) and younger mTBI (29.7%) and OI (3.6%) patients. In contrast, sports-related injuries were more common in young OI patients (64.3%) as compared with young mTBI (32.4%), older OI (19.3%) and older mTBI (4.6%) patients. Table 1 also shows that positive responses to the questions about applying for compensation and pursuing litigation did not differ between the mTBI and OI groups.
Table 1.
Demographic and Injury Characteristics of Mild TBI (mTBI) and Orthopedic Injury (OI) Groups
| |
MTBI (N=102) |
OI (N=85) |
|
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | p value | |
| Age at injury | 22.91 | 8.96 | 12.16–49.00 | 21.48 | 6.48 | 12.06–46.00 | 0.2065 |
| SCI1 | 0.03 | 0.82 | −1.7–2.05 | 0.02 | 0.78 | −1.47–2.10 | 0.9272 |
| TBI (n, %) | OI (n, %) | ||||||
| Gender | |||||||
| Male | 71 (69.61) | 64 (75.29) | 0.3875 | ||||
| Mechanism of injury | |||||||
| MV3 | 52 (50.98) | 9 (10.59) | <0.0001 | ||||
| Non-MV3 | 25 (24.51) | 32 (37.65) | |||||
| Sports | 15 (14.71) | 29 (34.12) | |||||
| Assault | 10 (9.80) | 15 (17.65) | |||||
| Race | |||||||
| Black | 34 (33.33) | 31 (36.47) | 0.6537 | ||||
| Non-Black | 68 (66.67) | 54 (63.53) | |||||
| GCS2 | |||||||
| 13 | 3 (2.97) | N/A | N/A | ||||
| 14 | 14 (13.86) | ||||||
| 15 | 84 (83.17) | ||||||
| Litigation | |||||||
| Compensation | |||||||
| Yes | 19 (18.63%) | 10 (11.76%) | 0.1774 | ||||
| Yes | 14 (13.73%) | 11 (12.94%) | 0.8337 | ||||
SCI, Socioeconomic Composite Index; 2GCS, Glasgow Coma Scale; 3MV, motor vehicle; SD, standard deviation.
Principal components
PCA with orthogonal rotation disclosed a four-component solution, accounting for 83.5% of the variance (Table 2). Component 1 consists of the ANAM CodeSub test, and both the written and oral versions of the SDMT, which explained 24.4% of the total variance. Based on the loadings shown, we labeled Component 1 as Cognitive Processing Speed. Component 2, which is comprised by the BVMT-R recall and delayed recall and explained 20.2% of the total variance, can be described as representing Visual Memory, whereas Component 3 is comprised by Verbal Memory (Verbal Selective Reminding Test) and explained 19.5% of the total variance, including the number of words consistently recalled over trials (CLTR) and the number of words recalled after a delay. Component 4, which comprises the Rivermead and PCL-C and explained 19.4% of the total variance, represents symptoms, including PCS and symptoms of post-traumatic stress.
Table 2.
Factor Pattern Coefficients
| Variables | Component 1 | Component 2 | Component 3 | Component 4 |
|---|---|---|---|---|
| SDMTW | 0.86 | 0.08 | 0.20 | −.05 |
| SDMTO | 0.85 | 0.09 | 0.29 | −.02 |
| CodeSubRT | −.75 | −.25 | −.06 | 0.15 |
| BVMT1 _DELAY_R | 0.13 | 0.93 | 0.13 | −.09 |
| BVMT1 _RECALL_R | 0.20 | 0.91 | 0.19 | −.04 |
| VSR2_Delay | 0.19 | 0.15 | 0.90 | 0.08 |
| VSR2_CLTR | 0.27 | 0.17 | 0.88 | −.01 |
| RiverMPCS3 _16 | −.06 | −.05 | 0.03 | 0.92 |
| PCLS4 _Tot | −.10 | −.07 | 0.04 | 0.92 |
BVMT-R, Brief Visual Memory Test-Revised; 2VSR,Verbal Selective Reminding Test; 3River MPCS-Rivermead Postconcussion Symptoms Questionnaire; 4PCL-C-Posttraumatic Stress Checklist-Civilian Form.
For Cognitive Processing Speed, there was an age x group interaction for the intercept as the difference in performance between the mTBI and OI groups was greater for older patients, F(1,77)=10.96, p=0.0014. Figure 2 shows the difference on processing speed in the OI group as compared with the mTBI patients by age at 1 month (30 days). This interaction did not differ by post-injury interval (i.e., it was present in the data obtained across the three assessments). Also, there was a quadratic pattern for the recovery in both groups, indicating that there was a larger slope for improvement in processing speed at 1 month than between 1 and 3 months post-injury.
Visual Memory (Component 2) improved across occasions in both groups (F (1,142)=15.46, p=0.0001) and was inversely related to age (F (1, 79)=45.85, p<0.0001). However, these effects did not vary by group and there was no difference in performance between the mTBI and OI groups. Similar to Visual Memory, Verbal Memory (Component 3) did not differ between the mTBI and OI groups. In contrast to Visual Memory, Verbal Memory did not significantly change over the post-injury intervals F (1,141)=0.24, p=0.6227). Verbal Memory was better in patients who had a higher socioeconomic level than in those with a lower socioeconomic level (F (1, 78)=6.31, p=0.0141).
The Symptom Composite (Component 4), including PCS and PTSD symptoms, was significantly higher in the mTBI patients than in the OI group. Figure 3 represents the group x interval interaction (F (1, 79)=4.79, p=0.0316) indicating each group had a different recovery pattern (slope). Figure 3 also shows partial resolution of symptoms in the mTBI group at 3 months, as the group difference decreased with time. However the group difference was still significant at 3 months, t (79)=− 4.03, p=0.0001. The “floor effect” seen in Figure 3 reflects a generally low level of symptoms in the OI patients. There was also a significant decrease in the Symptom Composite over occasions. There was no significant interaction of group or occasion with age.
FIG. 3.
Symptom composite score by post-injury interval for mTBI and OI groups.
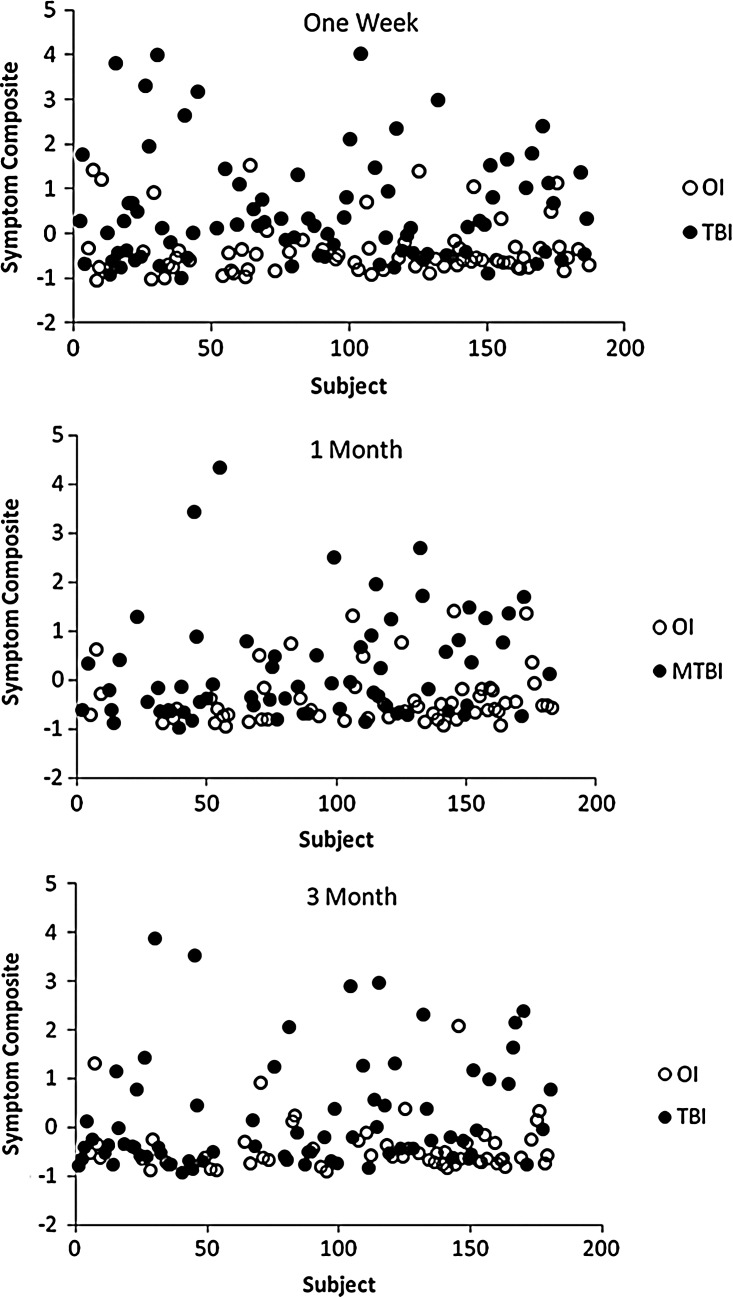
The scatterplots in Figure 4A-C of the Symptom Composite display the individual patient data for the 1 week, 1 month, and 3 month occasions in the mTBI and OI groups. Reduction in symptom severity over 3 months is seen for both groups. However, there is still a clear discrimination between mTBI and OI groups at three months.
Table 3 shows the parameter estimates (the estimated change per unit value) and significance testing from the models for the four principal components.
We compared the component scores of patients who reported that they were engaged in litigation to those who were not by adding this variable in the current models. Involvement in litigation was not related to Cognitive Processing Speed, F(1,60)=0.06, p=0.8000, Visual Memory F(1,60)<0.01, p=0.9441, Verbal Memory F(1,60)<0.01, p=0.9526, or the Symptom Composite, F(1,61)=3.63, p=0.0615. We did a similar analysis for compensation. Pursuing compensation was not related to Cognitive Processing, F(1,59)=0.62, p=0.4340, Verbal Memory, F(1,60)=0.13, p=0.7173, Visual Memory F (1,60)=3.73, p=0.0583 or the Symptom Composite, F(1,61)=1. 51, p=0.2231.
Discussion
Observational studies and clinical trials of interventions for mTBI which include several domains of outcome measures may increase the Type 1 error associated with multiple between-group statistical comparisons. Outcome domains including episodic memory, cognitive processing speed, and PCS have been commonly used in the mTBI literature and frequently supplemented by other cognitive domains and measurement of PTSD symptoms.1,11 With analysis of multiple variables for at least a subset of the frequently used outcome measures, there is a risk of spurious findings. As an initial step toward developing a data reduction strategy, we carried out a PCA of the outcome data in two ongoing prospective, longitudinal studies of mTBI which use similar measures of cognition and symptoms in different cohorts of patients. We found that the outcome status of mTBI patients during the first week post-injury, at 1 month, and at 3 months could be characterized by four components, including Cognitive Processing Speed, Verbal Memory, Visual Memory, and a Symptom Composite representing PCS and PTSD symptoms.
The components identified by PCA differed in their sensitivity to between group differences and interactions. We found an interaction of group with age on Cognitive Processing Speed, indicating that the effect of mTBI on slowing cognitive performance was accentuated in older patients (i.e., adults who ranged in age up to 50 years). In contrast, there was a negligible between-group difference in Cognitive Processing Speed in adolescents. With an association between motor vehicle crashes in adults who sustained mTBI as compared with young adolescents whose injuries were frequently sports-related, it is plausible that the older mTBI patients were subjected to greater traumatic forces, resulting in more extensive axonal injury. However, it is difficult to differentiate effects of age from injury mechanism to account for the observed interaction of group with age. Visual episodic memory generally improved over the 3 month follow-up interval, but there was no differential gain in performance between the mTBI and OI groups. Although age was inversely related to Visual Memory performance, we did not find an interaction with group. In contrast, Verbal Memory did not significantly improve over time. Although Verbal Memory was not related to age, performance was better in patients from higher than lower socioeconomic backgrounds. Overall, we did not find a memory deficit in the mTBI group as compared with orthopedic patients who had similar demographic characteristics.
Of the four principal components identified by the PCA, the Symptom Composite representing PCS and PTSD symptoms showed the most consistent between-group difference. Patients who sustained mTBI had more severe symptoms than OI patients and the between-group difference during the first week post-injury was confirmed at 1 and 3 months post-injury. Figure 4a-c, which displays the Symptom Composite scores of individual patients, shows a pattern of consistent, distinct separation of the mTBI and OI groups at 3 months but smaller in magnitude as compared with the data obtained during the first week or at 1 month after injury. We infer from these data that symptoms resulting from mild brain insult were more persistent in the mTBI patients than following mild orthopedic injury. This interpretation is consistent with previous studies indicating that PCS often persist longer than cognitive deficits following mTBI.4,7
Our findings support the potential application of PCA as a data reduction strategy in clinical trials of interventions for mTBI. We recognize that the PCA reported here necessitated that we merge data from two concurrent studies to obtain a sufficient sample size. Although this is an inherent limitation of PCA, it is possible that replication in other samples of mTBI patients using a broader range of outcome measures will identify theoretically derived component measures which could be applied to new samples without the necessity of repeating the PCA. The components identified in the two prospective, longitudinal investigations described here are consistent with the results of previous studies using similar designs which found that subacute mTBI patients had slowed cognitive processing speed, impaired episodic memory, and post-concussion symptoms such as headaches, dizziness, and fatigue.7,29 In the present pair of studies, we also measured acute stress disorder and PTSD symptoms that were present and highly correlated with PCS. Consequently, the fourth component identified in the PCA was a composite of PCS and PTSD symptoms.
The principal components identified here appear to be valid over the first 3 months post-injury. Provided that the results are confirmed in new samples of mTBI and OI patients using an expanded range of outcome measures, utilization of principal components could potentially streamline the number of outcome measures and mitigate Type 1 error in between-group comparisons, possibly permitting smaller sample sizes for clinical trials and more targeted assessment batteries. We also acknowledge that the PCA results may differ for data obtained using different cognitive tests even if they represent the same domains that we included in this study.
Based on our results, administration of a single measure of cognitive processing speed appears to be sufficient. In the present study, performance on the CodeSub test of the ANAM was highly correlated with SDMT scores, thus obviating any advantage of administering both tests. Although the device that we used to present the ANAM is no longer widely available, the ANAM can be administered using other platforms.30 From these results, it would also appear that investigators could utilize an abbreviated battery of tests while retaining sensitivity to the effects of mTBI.
Since it was not possible for us to obtain pre-injury data on the outcome measures, we are not able to determine whether the mTBI and OI groups differed before injury on the component scores. However, we investigated all possible factors that may affect these components and controlled those significant effects in the models to look at the group differences and their changes across post-injury intervals.
Other caveats include our exclusion of patients with substance dependence, those who had brain lesions on CT within 24 hours after injury, and patients with prior hospitalization for TBI. Consequently, caution is advised in extrapolating these results to the general mTBI population. Although our merging of data from two concurrent projects may be an issue, there were relatively minor differences in the eligibility criteria for enrollment. Combining the samples provided a wider age range and enabled us to include additional patients who were recruited within a window ranging from less than 24 hours to 96 hours after injury. With prospective, longitudinal investigation of mTBI patients beginning with recruitment in the emergency center, we did not administer measures of effort or symptom validity. However, we did find that the proportions of patients who became involved in litigation or pursuing compensation did not differ between the mTBI and OI groups. Positive responses to a questionnaire about these activities were also not related to component scores. Although including a measure of effort is advisable, there is no indication that secondary gain biased one group more than the other in this study. Cognitive performance also improved over time in the mTBI group, suggesting that patients provided valid data.
In conclusion, the results of PCA support the feasibility of using component scores in observational and intervention studies of mTBI. This approach could reduce the number of variables and mitigate Type I errors associated with numerous between-group comparisons.
Acknowledgments
This research was supported by Grant 5P01NS056202 from the National Institute of Neurological Disorders and Stroke and by award W81XWH-08-2-0133 PT074693P2 from the Department of Defense (Post-Traumatic Stress Disorder and Traumatic Brain Injury (PTSD/TBI) Research Program). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, the National Institutes of Health, the US Army, or the Department of Defense.
Author Disclosure Statement
None of the authors have any financial or other relationship(s) that could be construed as a conflict of interest with respect to the content of this article.
References
- 1.Iverson GL, et al. Mild TBI, in: Brain Injury Medicine: Principles and Practice. In: Zasler ND, editor; Katz DI, editor; Zafonte RD, editor. Demos Medical Publishing, LLC; New York: 2007. [Google Scholar]
- 2.IOM, Gulf War and Health: Volume 7. IOM (Institute of Medicine); Washington, DC: 2009. Long-term Consequences of Traumatic Brain Injury. [PubMed] [Google Scholar]
- 3.Stein MB. McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry. 2009;166:768–776. doi: 10.1176/appi.ajp.2009.08101604. [DOI] [PubMed] [Google Scholar]
- 4.Carroll LJ. Cassidy JD. Peloso PM. Borg J. von Holst H. Holm L. Paniak C. Pepin M. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehab Med. 2004;43:84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 5.Bleiberg J. Kane RL. Reeves DL. Garmoe WS. Halpern E. Factor analysis of computerized and traditional tests used in mild brain injury research. Clin Neuropsychol. 2000;14:287–294. doi: 10.1076/1385-4046(200008)14:3;1-P;FT287. [DOI] [PubMed] [Google Scholar]
- 6.Lovell MR. Collins MW. Iverson GL. Field M. Maroon JC. Cantu R. Podell K. Powell JW. Belza M. Fu FH. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98:296–301. doi: 10.3171/jns.2003.98.2.0296. [DOI] [PubMed] [Google Scholar]
- 7.Levin HS. Mattis S. Ruff RM. Eisenberg HM. Marshall LF. Tabaddor K. High WM., Jr Frankowski RF. Neurobehavioral outcome following minor head injury: A three-center study. J Neurosurg. 1987;66:234–243. doi: 10.3171/jns.1987.66.2.0234. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RA. Wichern DW. 6th. Upper Saddle River, N.J.: Pearson Prentice Hall; 2007. Applied multivariate statistical analysis; pp. xviii–773. [Google Scholar]
- 9.National Center for Injury Prevention and Control. Steps to Prevent a Serious Public Health Problem, Centers for Disease Control and Prevention; Atlanta: 2003. Report to Congress on Mild Traumatic Brain Injury in the United States. [Google Scholar]
- 10.Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 11.Carroll LJ. Cassidy JD. Holm L. Kraus J. Coronado VG. Methodological issues and research recommendations for mild traumatic brain injury: The WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehab Med. 2004;43:113–125. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Injury Scaling, Abbreviated Injury Scale. Arlington Heights, IL: Association for the Advancement of Automotive Medicine; 1998. [Google Scholar]
- 13.Saunders JB. Aasland OG. Babor TF. de la Fuente JR. Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 14.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 15.Maisto SA. Carey MP. Carey KB. Gordon CM. Gleason JR. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess. 2000;12:186–192. doi: 10.1037//1040-3590.12.2.186. [DOI] [PubMed] [Google Scholar]
- 16.Yeates KO. Taylor HG. Drotar D. Wade SL. Klein S. Stancin T. Schatschneider C. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J Intl Neuropsychol Soc. 1997;3:617–630. [PubMed] [Google Scholar]
- 17.Cernich A. Reeves D. Sun W. Bleiberg J. Automated Neuropsychological Assessment Metrics sports medicine battery. Arch Clin Neuropsychol. 2007;22:101–114. doi: 10.1016/j.acn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Reeves DL. ANAM 2001. Clinical and Research Modules, National Cognitive Recovery Foundation; San Diego, CA: 2002. User's Manual. [Google Scholar]
- 19.Smith A. Symbol Digit Modalities Test (SDMT) Manual (Revised) Los Angeles: Western Psychological Services 1982 [Google Scholar]
- 20.Hannay HJ. Levin HS. Selective reminding test: An examination of the equivalence of four forms. J Clin Exp Neuropsychol. 1985;7:251–263. doi: 10.1080/01688638508401258. [DOI] [PubMed] [Google Scholar]
- 21.Benedict RH. Psychological Assesment Resources, Inc.; 1997. Brief Visuospatial Memory Test-Revised, in Odessa. [Google Scholar]
- 22.Delis DC. Kramer JH. Kaplan E. Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: An update. J Intl Neuropscyhol Soc. 2004;10:301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- 23.King NS. Crawford S. Wenden FJ. Moss NE. Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 24.Crawford S. Wenden FJ. Wade DT. The Rivermead head injury follow up questionnaire: A study of a new rating scale and other measures to evaluate outcome after head injury. J Neurol Neurosurg Psychiatry. 1996;60:510–514. doi: 10.1136/jnnp.60.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanchard EB. Jones-Alexander J. Buckley TC. Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 26.Hoteling AJ. Owens KG. Improved PSD and CID on a MALDI TOFMS. J Am Soc Mass Spectrom. 2004;15:523–535. doi: 10.1016/j.jasms.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Jolliffe L. Rotation of principal components: Choice of normal constraints. J App Stat. 1995;22:29–35. [Google Scholar]
- 28.SAS, SAS/Stat Software User's Guide. SAS Institute; Carey, NC: 2003. [Google Scholar]
- 29.Ponsford J. Cameron P. Fitzgerald M. Grant M. Mikocka-Walus A. Long-term outcomes after uncomplicated mild traumatic brain injury: A comparison with trauma controls. J Neurotrauma. 2011;28:937–946. doi: 10.1089/neu.2010.1516. [DOI] [PubMed] [Google Scholar]
- 30.Roebuck-Spencer TM. Vincent AS. Twillie DA. Logan BW. Lopez M. Friedl KE. Grate SJ. Schlegel RE. Gilliland K. Cognitive change associated with self-reported mild traumatic brain injury sustained during the OEF/OIF conflicts. Clin Neuropsychol. 2012;26:473–489. doi: 10.1080/13854046.2011.650214. [DOI] [PubMed] [Google Scholar]