Abstract
Significance: The physiological mechanism(s) for recognition and removal of red blood cells (RBCs) from circulation after 120 days of its lifespan is not fully understood. Many of the processes thought to be associated with the removal of RBCs involve oxidative stress. We have focused on hemoglobin (Hb) redox reactions, which is the major source of RBC oxidative stress. Recent Advances: The importance of Hb redox reactions have been shown to originate in large parts from the continuous slow autoxidation of Hb producing superoxide and its dramatic increase under hypoxic conditions. In addition, oxidative stress has been shown to be associated with redox reactions that originate from Hb reactions with nitrite and nitric oxide (NO) and the resultant formation of highly toxic peroxynitrite when NO reacts with superoxide released during Hb autoxidation. Critical Issues: The interaction of Hb, particularly under hypoxic conditions with band 3 of the RBC membrane is critical for the generating the RBC membrane changes that trigger the removal of cells from circulation. These changes include exposure of antigenic sites, increased calcium leakage into the RBC, and the resultant leakage of potassium out of the RBC causing cell shrinkage and impaired deformability. Future Directions: The need to understand the oxidative damage to specific membrane proteins that result from redox reactions occurring when Hb is bound to the membrane. Proteomic studies that can pinpoint the specific proteins damaged under different conditions will help elucidate the cellular aging processes that result in cells being removed from circulation. Antioxid. Redox Signal. 18, 2274–2283.
Introduction
The formation of red blood cells (RBCs) begins with the pluripotent stem cells of the bone marrow (48, 81). These stem cells undergo proliferation and differentiation into progenitor committed cells and then into progressively maturing precursors and finally mature RBCs, white cells, and platelets. The earliest progenitor cell committed to the erythroid is the BFU-E, which forms large bursts of erythroblast colonies. Further differentiation results in the first morphologically recognizable erythroid precursors, the proerythroblasts, which eventually develop into reticulocytes. The reticulocytes eject the nucleus before being released into the circulation, but retain the mitochondria, ribosomes, and ribosomal RNA necessary for the synthesis of hemoglobin (Hb) and other proteins. It has been estimated that ∼20%–30% of the total Hb is synthesized in reticulocytes. The reticulocytes in the circulation have a half-life of ∼30 h during which time they transform to mature RBCs with complete elimination of traces of DNA, mitochondria, endoplasmic reticulum, and ribosomes.
Recent proteomics studies indicate that Hb accounts for 95 to 97% of the cytosolic protein of RBCs. Most of the other cytosolic proteins are involved in neutralizing oxidants (peroxiredoxin2 [PRDX2], catalase, superoxide dismutase (SOD) and glutathione peroxidase [GPx]), and in the metabolism of glucose and synthesis of ATP (both anaerobic glycolysis and the hexose monophosphate shunt) (14, 74). The RBC membrane consists of a lipid layer with phospholipids and cholesterol. There are glycol proteins and glycol lipids on the exterior surface. The inner surface is secured through transmembrane proteins to a cytoskeleton consisting of an elastic network of skeletal protein that enables the RBC to deform and flow through the narrow capillaries of the microcirculation to deliver oxygen to the tissues. The membrane also contains proteins that provide channels for the uptake and release of ions and low molecular weight hydrophilic compounds necessary for maintaining ionic homeostasis.
The RBC is, thus, especially designed for the delivery of oxygen to the tissues by the reversible binding of oxygen to the very large pool of Hb contained in the cell. The other properties of RBC are necessary to facilitate this process. The synthesis of ATP facilitates ionic homeostasis, which retains an excess surface area, and the cytoskeleton provides the elastic properties. Both the biconcave shape with excess surface area and elasticity are necessary for the cell to deform and deliver oxygen to the tissues (Fig. 1). The extensive antioxidant system is designed to neutralize the harmful reactive oxygen species (ROS) generated as a result of the constant exposure to variable oxygen tensions.
FIG. 1.
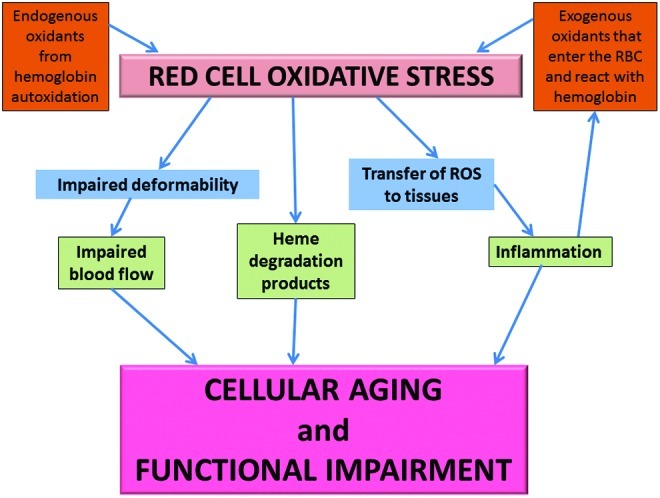
Red cell oxidative stress. Scheme showing how both endogenous hemoglobinautoxidation and exogenous oxidants generate oxidative stress that can result in cellular aging and functional impairment. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
To optimize the amount of oxygen the cell can carry, by increasing the Hb concentration, and the ability to transport this oxygen, by increasing the cellular deformability, the mature RBC has ejected all the nonessential components of the RBC that prevent this optimization (29, 52).
The Removal of RBCs from the Circulation
The mature RBC without a nucleus to regulate regeneration also lacks many of the features of other cells that are responsible for their survival and proper functioning. They have no mitochondria required for efficient oxidative metabolism, no DNA or ribosomes necessary for protein synthesis to replace damaged proteins, and the de novo synthesis of lipids is also metabolically precluded. The RBC continuously undergoing normoxic and hypoxic cycling is constantly exposed to oxidative insults during its 120-day lifespan that results in continuous biochemical, physical, and immunological changes. These changes impair the ability of the RBC to transport oxygen and eventually trigger removal from circulation by the reticuloendothelial system. The reticuloendothelial system involves the mononuclear phagocytic cells primarily in the spleen, and also in the liver and lymph nodes. The processes responsible for the actual triggering of the removal have been extensively studied (5, 6, 13, 88).
Membrane microvesiculation is a regulated process that accelerates in older cells (104). It is responsible for the increase in cell density coupled with a decrease in deformability and flexibility (10, 101). These changes limit the ability of the RBC to maintain the highly deformable biconcave shape necessary to pass through narrow pores, thus contributing to their removal from circulation (Fig. 1). It has, however, been shown that the vesicles formed contain elevated levels of phosphatidylserine (PS), IgG, and breakdown products of band 3 that have exposed antigenic sites, which have been shown to trigger the removal of RBCs from circulation (see below). Based on this finding, it was suggested that vesiculation may actually be a self-protective mechanism that increases the RBC lifespan (103, 104).
The RBC membrane band 3 is the dominant integral transmembrane protein. It has several crucial functions: (i) the maintenance of anion homeostasis, (ii) providing a link between the membrane and the cytoskeleton responsible for maintaining the cell shape, and (iii) providing for the interaction of a number of cytosolic proteins with the membrane via the amino terminal region that protrudes into the cytosol. This region of band 3 competitively binds both Hb, and a number of glycolytic enzymes (60). The changes in Hb binding to band 3 as a function of the Hb oxygenation, therefore, couple Hb oxygenation to glycolysis and ATP production.
Damage to band 3 has been linked to RBC aging including the exposure of senescent specific neo-antigens that bind autologous IgG triggering RBC removal (42). IgG binding has also been linked to band 3 clusters, which is triggered by the binding of denatured oxidized Hb (hemichromes) to band 3 (30, 54, 80).
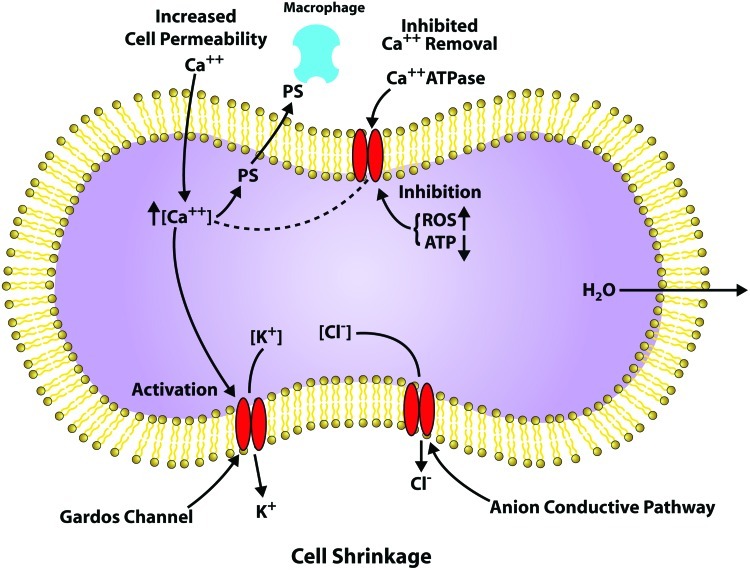
The RBC membrane has a Ca-ATPase, which maintains a low intracellular concentration of free calcium (Fig. 2) (51). During aging, this calcium homeostasis is disrupted and there is a gradual increase in intracellular calcium (44, 93). Increased intra-cellular calcium activates the Gordo's potassium channel causing the leakage of potassium from the cell, resulting in cell shrinkage and impaired deformability (15, 32). Calcium also activates u-calpain, transglutaminase-2, and some caspases that can degrade/cross-link cytoskeleton proteins (79). It also inhibits phosphotyrosine phosphatase increasing band 3 phosphorylation (108).
FIG. 2.
Leakage of calcium into red blood cells (RBCs). Calcium induced reactions in the RBC that contribute to cell shrinkage. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
The RBC lipid bilayer contains an asymmetric distribution of phospholipids with PS being maintained on the inner surface by the competition between scramblase that randomizes the distribution and flippase that internalizes the PS. Coupled with an increase in sphingomyelinase that increases ceramide, increased intracellular calcium (Fig. 2) has been linked to the exposure of PS, which triggers interaction with macrophages and eryptosis (24, 31, 102). Caspase-3 activation, which modifies the band 3 linkage to ankyrin and the cytoskeleton, and flippase activity, has been shown to induce PS exposure.
RBC Oxidative Stress
As outlined above, there are a number of processes that damage the RBC as it ages. The relative role of each of these processes in RBC aging and the removal of the RBC from circulation is, however, not always clear. Since many of these processes are directly affected by oxidative stress, a significant role for oxidative stress in determining RBC aging must exist (Fig. 1). Examples of the contribution of oxidative stress to these processes include the following: (i) distorted calcium homeostasis (Fig. 2), which has been attributed to oxidative stress (6, 50); (ii) caspase-3 activation is clearly triggered by oxidative stress (42, 99); (iii) exposure of the neo-antigen on band 3 and band 3 clustering are triggered by oxidative stress (42, 54).
The predominant factor that determines oxidative stress in the RBC is Hb (Figs. 1 and 3). The reactive free radical species generated by Hb reactions and the interactions of Hb with membrane and cytoskeleton proteins both induce oxidative stress and are involved in RBC aging.
FIG. 3.
Redox reactions associated with increased autoxidation of partially oxygenated hemoglobin (Hb) bound to the red cell membrane. The formation of superoxide and hydrogen peroxide result in formation of hemichromes and degradation of the heme releasing free iron and fluorescent degradation products that interact with the membrane. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
Oxygen transport by the RBC involves the reversible binding of oxygen to the 5 mM tetrameric Hb. Reversible oxygenation requires that the heme iron be in the Fe(II) reduced form. However, Fe(II) is readily oxidized to Fe(III). Although oxidation of Fe(II) is slower when the iron is incorporated into a protein, the Fe(II) heme of Hb also, although at a much slower rate, continuously undergoes a redox reaction involving the oxygen bound to Hb that oxidizes Hb producing superoxide (Fig. 3).
Although this reaction is slow under normoxic conditions (k=0.0115 h−1) (66), it is dramatically enhanced under hypoxic conditions (2). Appreciable concentrations of superoxide are, therefore, generated by high concentration of Hb, especially under hypoxic conditions found in the microcirculation (2, 4, 8, 45, 82, 86, 87). This superoxide dismutates to produce hydrogen peroxide (H2O2), a process that spontaneously occurs at a rate of 3.9×107 M−1s−1, but even more rapidly in the presence of RBC SOD. Both of these reduced oxygen species (superoxide and H2O2) are reactive and can react with both Hb and other cellular constituents.
The autoxidation reaction produces Fe(III) methemoglobin (metHb) at the same time that it produces superoxide. MetHb does not bind oxygen and cannot transport oxygen. To maintain functional Hb, most of the metHb is reduced back to Fe(II) Hb by metHb reductase. The residual non-reduced metHb also has a lower affinity for the heme prosthetic group resulting in free hemin (38). This hemin interacts with the red cell membrane disrupting skeletal protein interactions (94, 105) and reacts with membrane lipid hydroperoxides releasing lipophilic radicals (25). In the presence of hydrogen peroxide it can also induce lipid peroxidation. In the red cell glutathione, which is present in the mM concentration range scavenges hemin, inhibiting hemin induced damage (96).
In addition to the autoxidation reaction, the RBC in circulation is continuously exposed to extracellular oxidants that can be taken up by the RBC (Fig. 1) and react with various groups including Hb (7, 72).
The RBC is also exposed to nitric oxide (NO) and nitrite (Fig. 4), which can induce a new class of redox reactions involving reactive nitrogen species (RNS) (65, 83, 84, 91) that produce nitrosative stress. NO is produced by nitric oxide synthase (NOS) (47). While there is evidence of NOS in the RBC that can release NO in the RBC, most of the NO that the RBC is exposed to originates from endothelial e-NOS (49). NO in the RBC, rapidly reacts with oxyHb to form nitrate and metHb or deoxyHb to produce stable Hb(II) NO. Although these reactions are rapid (k=∼107 M−1 S−1) (21), any superoxide present due to autoxidation of oxyHb reacts with NO even faster than Hb (k=∼10 10 M−1 S−1) producing peroxynitrite (PN) (Fig. 4). PN is also generated in the interstitial spaces of the endothelium by the reaction of superoxide generated by NADPH oxidases, xanthine oxidase, and uncoupled e-NOS, which reacts with NO generated by e-NOS (33, 78). This PN generated in the vasculature spaces can diffuse into the circulation and then into RBCs. PN is a highly reactive species that can damage cellular constituents (73, 89, 90).
FIG. 4.
Red cell reactions involving nitric oxide (NO) released from the endothelium. NO can be oxidized to form nitrite that is taken up by the cell and reduced back to NO by deoxygenated Hb chains. NO can also react with superoxide in the cell and in the plasma to produce peroxynitrite, which is a highly toxic compound that reacts with Hb. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
An additional source for RNS in the RBC involves reactions involving nitrite (Fig. 4), which is produced by the oxidation of NO by oxygen or metalloproteins in plasma (95). Most of plasma nitrite originates from NO oxidation, explaining the use of plasma nitrite as a measure of e-NOS activity (71). However, some of the nitrite can also originate from the nitrite and nitrate in the diet. Nitrate also contributes to the pool of nitrite, because the nitrate is reduced to nitrite by bacteria in the saliva (36). Nitrite in the RBC reacts with oxyHb to produce nitrate, which is un-reactive, and metHb (43). However, nitrite also reacts with deoxyHb (22, 28, 65). Nitrite, which has a low affinity for Fe(II) heme, helps induce a conformational change that stabilizes the bound nitrite. Once the bound nitrite is protonated, a redox reaction results in the displacement of a hydroxyl ion from the nitrite producing a nitrosonium ion bound to Fe(II), which is in equilibrium with a Fe(III) NO complex. This hybrid intermediate in the β-chain was also shown to be in equilibrium with a thiyl radical on the β-93 sulfhydryl group. These metastable intermediates retain NO bioactivity, but are not quenched by reacting with Hb or superoxide. These intermediates, thus, provide a pool of potentially bioactive NO, which can be released to the vasculature when needed (84, 91, 92).
Reactions Associated with ROS and RNS Species Formed by Hb Redox Reactions
The superoxide, H2O2, hydroxyl radicals, ferrylhb, oxoferrylHb, and PN generated by these redox reactions can damage RBC membrane proteins, lipids, and the cytoskeleton (Figs. 1, 3, and 4), which are responsible for maintaining the RBC shape and deformability. They can trigger the uptake of calcium (Fig. 2), activation of caspase, and the disruption of lipid asymmetry resulting in the exposure of PS and damage to band 3, all of which can contribute to the triggering of the removal of RBCs from circulation by macrophages (6, 31, 42, 44, 50, 57, 60).
Although superoxide is a relatively unreactive free radical species, it does have some toxic effects on the RBC. This toxicity has been established using SOD1 knockout mice, where the lifetime of superoxide formed by Hb autoxidation is extended because of the slower spontaneous dismutation of the superoxide. These mice have been reported to develop Heinz bodies and result in cells with a shorter lifespan and a shift in glucose metabolism (37).
H2O2 initiates a cascade of oxidative reactions (Fig. 3). It reacts with Fe(II) Hb taking two electrons from the heme producing the highly reactive Fe(IV) ferrylhemoglobin. It will also react with any oxidized Fe(III) Hb producing Fe(IV) oxyferrylhemoglobin where a second electron is removed from the globin forming a reactive protein radical. Ferrylhemoglobin reacts with an additional molecule of H2O2 that is reduced to form a superoxide radical that is retained in the heme pocket long enough to react with the heme, eventually resulting in the degradation of the heme (Figs. 1 and 3) releasing iron and forming several fluorescent heme degradation products (34, 67–70). A relationship between the formation of these heme degradation products and cellular aging is indicated by the correlation between the level of heme degradation and the binding of autologous IgG to the RBC membrane (62). This effect can be due to the iron released during the formation of heme degradation products. The released iron can deposit on the membrane acting as a Fenton catalyst to oxidize lipids and proteins. An increase in redox active iron release has been observed during in vitro aging of RBCs and in cells treated with oxidizing agents. This release of iron was correlated to the binding of autologous IgG to the membrane of these cells, suggesting that iron-mediated redox reactions may be responsible for the expression of senescent antigens on the membrane (20). In addition to the effects of released iron, a possible direct effect of these degradation products cannot be ruled out.
PN (Fig. 4) has a short half-life (<10 S at neutral pH) and can directly react with various biological molecules to generate toxic species like •OH, •NO2 and •CO3 radicals (89, 90). In RBCs, PN is either scavenged by PRDX2, which is an abundant thiol protein, or reacts with Hb (58). Studies have shown that most of the PN present reacts with oxyHb to generate metHb and nitrate (90). Hence, oxyHb can act as a sink for PN. Because of the reaction of PN with oxyHb, the formation of 3-nitrotrosine, generally considered a marker for PN, is not a valid marker for PN in the RBC. However, it has been shown that PN also generates small quantities of ferrylHb and •NO2, in addition to metHb, during the reaction with oxyHb. These radical species can damage the RBC membrane and promote cellular aging (90). Recent studies have shown that the addition of PN to RBCs causes morphological alterations, decrease in cellular thiols, decrease in glycoporin A (a senescence marker), Band-3 clustering, PS exposure, and activation of caspase 2 and 3 that promotes programmed cell death in RBCs (17, 73, 76). Some of these changes are reversed by prior treatment with thiol compounds such as N-acetyl cysteine, antioxidants, and glucose (17). These results indicate that the effect of PN in RBCs involves disruption of the cellular redox system (73).
RBC Antioxidant System
To minimize the toxic effects of these redox reactions the RBC has an extensive antioxidant defense system including SOD1, which catalyzes the dismutation of superoxide to H2O2 and catalase, (GPx) and PRDX2, which scavenge peroxides and PN (53, 61). In addition, there are a number of low molecular weight antioxidants in the RBC that help to minimize oxidative stress (19).
There is extensive literature on the metabolic changes that occur during cell aging (1, 11, 39, 55, 56, 75, 77, 98, 107). Changes have been reported for the antioxidant enzymes SOD1, catalase, and GPx (35). There are, however, uncertainties regarding many of the specific changes that occur. A number of studies indicate changes in glutathione metabolism as a function of cellular age including changes in enzymes involved in the hexose monophosphate shunt (1, 35, 39, 40). This includes changes in glutathione, glutathione reductase, and glucose-6-phosphate dehydrogenase, which produces the NADPH required for glutathione reduction. However, other studies indicate that there is no change in the hexose monophosphate shunt with no change in glutathione (55). These studies do, nevertheless, indicate metabolic changes that result in a decrease in glucose metabolism. However, the change is attributed to anaerobic glycolysis (The Embden–Myerhoff pathway). This reduced glucose metabolism is primarily caused by a decrease in hexokinase activity. The decreased glucose metabolism has been shown to result in a decrease in ATP (11, 55). The reduced ATP affects many cellular processes. It can also contribute to the removal of RBCs from circulation by reducing the activity of Ca-ATPase, which limits the intracellular calcium concentration. ATP is also required for flippase activity, which retains the phospholipid asymmetry that prevents the exposure of PS (27).
The uncertainty in much of these data is attributed to several issues. (i) Many of these studies have been performed with animals and may not be directly related to humans. (ii) Most of the earlier studies made use of the separation of cells by density. While it has been shown that older cells are more dense, there does not seem to be a linear relationship between cell age and density (23). (iii) In dealing with the time-dependent changes in RBCs it is necessary to distinguish between changes attributed to reticulocytes converting into mature RBCs and the subsequent aging of mature RBCs. Since relatively large changes in many enzymes occur as the reticulocyte matures and the less dense fractions of RBCs will tend to have higher levels of reticulocytes, this can make it difficult to distinguish the two processes. It is however possible to determine the actual concentration of reticulocytes in any fraction (97). Further, these levels are usually very low in the denser fractions of RBCs. Thus, although density fractionation does not provide an accurate measure of the time-dependent changes, it can provide a measure of the changes that occur during aging.
The uncertainty involving the density fractionation was addressed (107) by transfusing biotinylated young RBCs into rabbits and following the biotinylated cells as a function of time. In this way it was possible to follow changes in activity of 19 enzymes during cellular aging. In this study, the changes occurring during reticulocyte maturing and during the aging of mature RBCs could be separated by the time dependence of the changes observed. Other recent studies have also used biotinylated cells to monitor RBC aging (100, 106).
Although there are some uncertainties about the exact changes that occur during cellular aging, it is, however, clear that as the RBC ages the cell's ability to neutralize ROS is impaired (35). This was confirmed by studies (35) where trypan blue uptake after treating cells with xanthine oxidase was determined. They found that the older cells were more readily damaged. This enhanced susceptibility to oxidative damage will produce a synergistic process where the RBC oxidative processes described above are more pronounced in older cells, further exacerbation the aging process.
The Role of Interaction of Hb with the RBC Membrane
Hb, and a number of glycolytic enzymes, binds to the amino terminus of the portion of band 3 that protrudes into the cytosol on the cytosolic side of the membrane (Figs. 3 and 4). OxyHb has a relatively low affinity for band 3, with deoxyHb having an appreciably higher affinity (18). Recent data suggest that partially oxygenated Hb, which is the dominant form of Hb in the microcirculation, may actually have a higher affinity for band 3 than deoxyHb (16). This can be attributed to the greater flexibility at the interface between Hb subunits where band 3 binding occurs. Even though band 3 accounts for a major fraction of the RBC membrane proteins, the total number of band 3 sites (1.2×106/cell) correspond to<1% of the Hb molecules and most of the redox reactions involving Hb occur in the cytosol. However, since the antioxidant enzymes are predominantly cytosolic, the reactive species that are not neutralized by the antioxidant enzymes are predominantly produced by the redox reactions of Hb that is bound to the membrane (26, 64, 85).
Partial Hb oxygenation (Fig. 3) not only increases the affinity for the RBC membrane, but also dramatically increases the rate of Hb autoxidation (2). This increase in rate of autoxidation has been attributed to increased fluctuations across the α1β1 interface that alters the distal heme pocket of the oxygenated chains increasing the probability for a nucleophilic displacement of oxygen as superoxide by the distal histidine (8). This superoxide can damage the RBC band 3 and/or leak out of the RBC through the anion channel. Once out of the cell, it will dismutate to H2O2. The diffusion of this H2O2 into capillary venules was demonstrated by perfusing lungs with hypoxic RBCs (45).
As a result of the increased affinity of partially oxygenated Hb with the membrane, hypoxia not only increases the production of ROS, but makes it more difficult to protect the cell from the ROS by antioxidant defense enzymes. This is confirmed by the elevated oxidative stress observed in RBCs of severe anemic mice, which usually experience greater hypoxia than normal RBCs (63).
The nitrite reductase activity (Fig. 4) of deoxyHb requires unliganded chains and is favored by the liganded R quaternary conformation. This reaction is, therefore, also most efficient for partially oxygenated Hb. We have also suggested that during the nitrite reaction there is a contribution from the nucleophilic interaction with the heme iron that is responsible for Hb autoxidation. In this case an analogous interaction releases NO from an Hb(II) NO+ intermediate formed during the nitrite reaction (84, 91). Redox reactions involving ROS and RNS species are, therefore, most pronounced as the cells pass through the microcirculation and begin to release oxygen.
In addition to the reversible binding to band 3 associated with the oxygenation of Hb, it has been shown that oxidized Hb and particularly hemichromes, formed by the denaturation of Hb, have a much higher affinity for the RBC membrane producing irreversible cross-linking involving both band 3 (54, 80) and spectrin (44). The formation of membrane-bound denatured Hb does not increase linearly as the cells age, but form in the oldest, dehydrated most dense cells. The in vivo band 3 association in dog RBCs was studied where the cells were completely biotinylated and the remaining biotinylated cells were separated at later times. This provided a much more reliable measure of how long the cells are in the circulation. In this study, the binding of hemichrome to band 3 was found to begin and increase exponentially after 85 days in the circulation. The formation of this denatured Hb would reflect the gradual decrease in the antioxidant systems, which impair the ability of the cells to neutralize ROS and to reduce oxidized Hb. It is perhaps only after appreciable aging that the impairment reaches the point that hemichromes and denatured Hb form (80).
The band 3 interactions with hemichromes, unlike the interaction with deoxyHb, involve a stoichiometry of 2.5:1 (Hb to band 3). This binding is also thought to disrupt the interactions of band 3 with ankyrin weakening the linkage of band 3 to the cytoskeleton triggering the clustering of band 3. Band 3 clustering has been shown to increase the binding to band 3 of IgG, which contributes to the uptake of RBCs by macrophages (30, 41).
The spectrin Hb cross-linking found in vivo is similar to that generated by H2O2 and may be related to the heme degradation products that have been found to be generated by non-neutralized H2O2 (Fig. 3). It is, thus, interesting that both spectrin-Hb binding and heme degradation formation, both of which are induced by H2O2, also induce an increase in IgG binding to the membrane (12, 102). The Hb spectrin interaction involves interactions with the spectrin head group that inhibits the dimer to tetramer association of spectrin. This disrupts the cytoskeleton and decreases RBC deformability. It has also been suggested that the spectrin globin association, which affects the spectrin cytoskeleton, disrupts band 3 interactions with the cytoskeleton and can also contribute to band 3 clustering.
Conclusion
Hb redox reactions associated, both with the endogenous autoxidation of Hb and exogenous reactive species that enter the RBC, are a source for producing ROS and RNS species that can damage RBC protein and phospholipids (59). This continuous source of oxidative stress causes accumulative damage in RBCs that have no DNA or ribosomes necessary for protein synthesis to replace damaged proteins. This accumulated damage causes metabolic impairment that exacerbates the situation by reducing the cells ability to neutralize toxic compounds and to reduce oxidized Hb. Enhanced rates for the formation of ROS and RNS occur under hypoxic conditions, where an increased fraction of Hb is bound to the RBC membrane. This facilitates damage to membrane proteins and the cytoskeleton that regulated the shape, deformability (Fig. 1), and eventual recognition for removal by macrophages.
The linkage between Hb-membrane interactions and RBC oxidative processes provides a unique way to specifically turn off RBC-induced oxidative stress. Instead of requiring large concentrations of antioxidants to neutralize the ROS species formed, blocking the interaction of Hb with the membrane will make it possible for the large supply of RBC antioxidants and antioxidant enzymes to eliminate the Hb-generated oxidants and, thereby, prevent RBC oxidative stress. Such a reduction in RBC oxidative stress will slow down RBC aging and ameliorate pathological effects associated with RBC oxidative stress.
Abbreviations Used
- 2PS
phosphatidylserine
- GPx
glutathione peroxidase
- Hb
hemoglobin
- NO
nitric oxide
- NOS
nitric oxide synthase
- PN
peroxynitrite
- PRDX2
peroxiredoxin
- RBCs
red blood cells
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Acknowledgment
This research was supported by the Intramural Research Program of National Institute on Aging, National Institutes of Health.
References
- 1.Abraham EC. Taylor JF. Lang CA. Influence of mouse age and erythrocyte age on glutathione metabolism. Biochem J. 1978;174:819–825. doi: 10.1042/bj1740819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abugo OO. Rifkind JM. Oxidation of hemoglobin and the enhancement produced by nitroblue tetrazolium. J Biol Chem. 1994;269:24845–24853. [PubMed] [Google Scholar]
- 3. This reference has been deleted.
- 4.Ajmani RS. Fleg JL. Demehin AA. Wright JG. O'Connor F. Heim JM. Tarien E. Rifkind JM. Oxidative stress and hemorheological changes induced by acute treadmill exercise. Clin Hemorheol Microcirc. 2003;28:29–40. [PubMed] [Google Scholar]
- 5.Ajmani RS. Rifkind JM. Hemorheological changes during human aging. Gerontology. 1998;44:111–120. doi: 10.1159/000021993. [DOI] [PubMed] [Google Scholar]
- 6.Antonelou MH. Kriebardis AG. Papassideri IS. Aging and death signalling in mature red cells: from basic science to transfusion practice. Blood Transfus. 2010;8(Suppl 3):s39–s47. doi: 10.2450/2010.007S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoshiba K. Nakajima Y. Yasui S. Tamaoki J. Nagai A. Red blood cells inhibit apoptosis of human neutrophils. Blood. 1999;93:4006–4010. [PubMed] [Google Scholar]
- 8.Balagopalakrishna C. Manoharan PT. Abugo OO. Rifkind JM. Production of superoxide from hemoglobin-bound oxygen under hypoxic conditions. Biochemistry. 1996;35:6393–6398. doi: 10.1021/bi952875+. [DOI] [PubMed] [Google Scholar]
- 9. This reference has been deleted.
- 10.Bartosz G. Erythrocyte aging: physical and chemical membrane changes. Gerontology. 1991;37:33–67. doi: 10.1159/000213251. [DOI] [PubMed] [Google Scholar]
- 11.Bartosz G. Grzelinska E. Wagner J. Aging of the erythrocyte. XIV. ATP content does decrease. Experientia. 1982;38:575. doi: 10.1007/BF02327057. [DOI] [PubMed] [Google Scholar]
- 12.Bartosz G. Soszynski M. Wasilewski A. Aging of the erythrocyte. XVII. Binding of autologous immunoglobulin G. Mech Ageing Dev. 1982;20:223–232. doi: 10.1016/0047-6374(82)90089-6. [DOI] [PubMed] [Google Scholar]
- 13.Barvitenko NN. Adragna NC. Weber RE. Erythrocyte signal transduction pathways, their oxygenation dependence and functional significance. Cell Physiol Biochem. 2005;15:1–18. doi: 10.1159/000083634. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharya D. Mukhopadhyay D. Chakrabarti A. Hemoglobin depletion from red blood cell cytosol reveals new proteins in 2-D gel-based proteomics study. Proteomics Clin Appl. 2007;1:561–564. doi: 10.1002/prca.200700178. [DOI] [PubMed] [Google Scholar]
- 15.Brugnara C. Membrane transport of Na, and K and cell dehydration in sickle erythrocytes. Experientia. 1993;49:100–109. doi: 10.1007/BF01989413. [DOI] [PubMed] [Google Scholar]
- 16.Cao Z. Bell JB. Mohanty JG. Nagababu E. Rifkind JM. Nitrite enhances RBC hypoxic ATP synthesis and the release of ATP into the vasculature: a new mechanism for nitrite-induced vasodilation. Am J Physiol Heart Circ Physiol. 2009;297:H1494–H1503. doi: 10.1152/ajpheart.01233.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celedon G. Gonzalez G. Pino J. Lissi EA. Peroxynitrite oxidizes erythrocyte membrane band 3 protein and diminishes its anion transport capacity. Free Radic Res. 2007;41:316–323. doi: 10.1080/10715760601090305. [DOI] [PubMed] [Google Scholar]
- 18.Chu H. Breite A. Ciraolo P. Franco RS. Low PS. Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: implications for O2 regulation of erythrocyte properties. Blood. 2008;111:932–938. doi: 10.1182/blood-2007-07-100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cimen MY. Free radical metabolism in human erythrocytes. Clin Chim Acta. 2008;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Comporti M. Signorini C. Buonocore G. Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free Radic Biol Med. 2002;32:568–576. doi: 10.1016/s0891-5849(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 21.Cooper CE. Nitric oxide and iron proteins. Biochim Biophys Acta. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 22.Cosby K. Partovi KS. Crawford JH. Patel RP. Reiter CD. Martyr S. Yang BK. Waclawiw MA. Zalos G. Xu X. Huang KT. Shields H. Kim-Shapiro DB. Schechter AN. Cannon RO., III Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 23.Dale GL. Norenberg SL. Density fractionation of erythrocytes by Percoll/hypaque results in only a slight enrichment for aged cells. Biochim Biophys Acta. 1990;1036:183–187. doi: 10.1016/0304-4165(90)90032-r. [DOI] [PubMed] [Google Scholar]
- 24.Daleke DL. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr Opin Hematol. 2008;15:191–195. doi: 10.1097/MOH.0b013e3282f97af7. [DOI] [PubMed] [Google Scholar]
- 25.Delcarte J. Jacques P. Fauconnier ML. Hoyaux P. Matsui K. Marlier M. Thonart P. The homolytic and heterolytic fatty acid hydroperoxide lyase-like activities of hematin. Biochem Biophys Res Commun. 2001;286:28–32. doi: 10.1006/bbrc.2001.5334. [DOI] [PubMed] [Google Scholar]
- 26.Demehin AA. Abugo OO. Jayakumar R. Lakowicz JR. Rifkind JM. Binding of hemoglobin to red cell membranes with eosin-5-maleimide-labeled band 3: analysis of centrifugation and fluorescence data. Biochemistry. 2002;41:8630–8637. doi: 10.1021/bi012007e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devaux PF. Herrmann A. Ohlwein N. Kozlov MM. How lipid flippases can modulate membrane structure. Biochim Biophys Acta. 2008;1778:1591–1600. doi: 10.1016/j.bbamem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Doyle MP. Pickering RA. DeWeert TM. Hoekstra JW. Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 29.Farooqui SM. Wali RK. Baker RF. Kalra VK. Effect of cell shape, membrane deformability and phospholipid organization on phosphate-calcium-induced fusion of erythrocytes. Biochim Biophys Acta. 1987;904:239–250. doi: 10.1016/0005-2736(87)90373-7. [DOI] [PubMed] [Google Scholar]
- 30.Ferru E. Giger K. Pantaleo A. Campanella E. Grey J. Ritchie K. Vono R. Turrini F. Low PS. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117:5998–6006. doi: 10.1182/blood-2010-11-317024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foller M. Huber SM. Lang F. Erythrocyte programmed cell death. IUBMB Life. 2008;60:661–668. doi: 10.1002/iub.106. [DOI] [PubMed] [Google Scholar]
- 32.Foller M. Kasinathan RS. Koka S. Lang C. Shumilina E. Birnbaumer L. Lang F. Huber SM. TRPC6 contributes to the Ca(2+) leak of human erythrocytes. Cell Physiol Biochem. 2008;21:183–192. doi: 10.1159/000113760. [DOI] [PubMed] [Google Scholar]
- 33.Forstermann U. Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 34.Giulivi C. Davies KJ. Hydrogen peroxide-mediated ferrylhemoglobin generation in vitro and in red blood cells. Methods Enzymol. 1994;231:490–496. doi: 10.1016/0076-6879(94)31032-7. [DOI] [PubMed] [Google Scholar]
- 35.Glass GA. Gershon D. Decreased enzymic protection and increased sensitivity to oxidative damage in erythrocytes as a function of cell and donor aging. Biochem J. 1984;218:531–537. doi: 10.1042/bj2180531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govoni M. Jansson EA. Weitzberg E. Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Grzelak A. Kruszewski M. Macierzynska E. Piotrowski L. Pulaski L. Rychlik B. Bartosz G. The effects of superoxide dismutase knockout on the oxidative stress parameters and survival of mouse erythrocytes. Cell Mol Biol Lett. 2009;14:23–34. doi: 10.2478/s11658-008-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hargrove MS. Singleton EW. Quillin ML. Ortiz LA. Phillips GN., Jr. Olson JS. Mathews AJ. His64(E7)→Tyr apomyoglobin as a reagent for measuring rates of hemin dissociation. J Biol Chem. 1994;269:4207–4214. doi: 10.2210/pdb1mgn/pdb. [DOI] [PubMed] [Google Scholar]
- 39.Imanishi H. Nakai T. Abe T. Takino T. Glutathione metabolism in red cell aging. Mech Ageing Dev. 1985;32:57–62. doi: 10.1016/0047-6374(85)90035-1. [DOI] [PubMed] [Google Scholar]
- 40.Imanishi H. Nakai T. Abe T. Takino T. Glutathione-linked enzyme activities in red cell aging. Clin Chim Acta. 1986;159:73–76. doi: 10.1016/0009-8981(86)90168-3. [DOI] [PubMed] [Google Scholar]
- 41.Kannan R. Labotka R. Low PS. Isolation and characterization of the hemichrome-stabilized membrane protein aggregates from sickle erythrocytes. Major site of autologous antibody binding. J Biol Chem. 1988;263:13766–13773. [PubMed] [Google Scholar]
- 42.Kay MM. Generation of senescent cell antigen on old cells initiates IgG binding to a neoantigen. Cell Mol Biol (Noisy-le-grand) 1993;39:131–153. [PubMed] [Google Scholar]
- 43.Keszler A. Piknova B. Schechter AN. Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiefer CR. Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7:113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Kiefmann R. Rifkind JM. Nagababu E. Bhattacharya J. Red blood cells induce hypoxic lung inflammation. Blood. 2008;111:5205–5214. doi: 10.1182/blood-2007-09-113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. This reference has been deleted.
- 47.Kleinbongard P. Schulz R. Rassaf T. Lauer T. Dejam A. Jax T. Kumara I. Gharini P. Kabanova S. Ozuyaman B. Schnurch HG. Godecke A. Weber AA. Robenek M. Robenek H. Bloch W. Rosen P. Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 48.Koury MJ. Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24:105–131. doi: 10.1146/annurev.nutr.24.012003.132306. [DOI] [PubMed] [Google Scholar]
- 49.Lancaster JR., Jr. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang KS. Duranton C. Poehlmann H. Myssina S. Bauer C. Lang F. Wieder T. Huber SM. Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 2003;10:249–256. doi: 10.1038/sj.cdd.4401144. [DOI] [PubMed] [Google Scholar]
- 51.Larsen FL. Katz S. Roufogalis BD. Calmodulin regulation of Ca2+ transport in human erythrocytes. Biochem J. 1981;200:185–191. doi: 10.1042/bj2000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J. Guo X. Mohandas N. Chasis JA. An X. Membrane remodeling during reticulocyte maturation. Blood. 2010;115:2021–2027. doi: 10.1182/blood-2009-08-241182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Low FM. Hampton MB. Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–1630. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- 54.Low PS. Waugh SM. Zinke K. Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985;227:531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- 55.Magnani M. Piatti E. Serafini N. Palma F. Dacha M. Fornaini G. The age-dependent metabolic decline of the red blood cell. Mech Ageing Dev. 1983;22:295–308. doi: 10.1016/0047-6374(83)90084-2. [DOI] [PubMed] [Google Scholar]
- 56.Magnani M. Rossi L. Stocchi V. Cucchiarini L. Piacentini G. Fornaini G. Effect of age on some properties of mice erythrocytes. Mech Ageing Dev. 1988;42:37–47. doi: 10.1016/0047-6374(88)90061-9. [DOI] [PubMed] [Google Scholar]
- 57.Mandal D. Moitra PK. Saha S. Basu J. Caspase 3 regulates phosphatidylserine externalization and phagocytosis of oxidatively stressed erythrocytes. FEBS Lett. 2002;513:184–188. doi: 10.1016/s0014-5793(02)02294-9. [DOI] [PubMed] [Google Scholar]
- 58.Manta B. Hugo M. Ortiz C. Ferrer-Sueta G. Trujillo M. Denicola A. The peroxidase and peroxynitrite reductase activity of human erythrocyte peroxiredoxin 2. Arch Biochem Biophys. 2009;484:146–154. doi: 10.1016/j.abb.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 59.Miyazawa T. Suzuki T. Fujimoto K. Kinoshita M. Age-related change of phosphatidylcholine hydroperoxide and phosphatidylethanolamine hydroperoxide levels in normal human red blood cells. Mech Ageing Dev. 1996;86:145–150. doi: 10.1016/0047-6374(95)01687-2. [DOI] [PubMed] [Google Scholar]
- 60.Mohandas N. Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagababu E. Chrest FJ. Rifkind JM. Hydrogen-peroxide-induced heme degradation in red blood cells: the protective roles of catalase and glutathione peroxidase. Biochim Biophys Acta. 2003;1620:211–217. doi: 10.1016/s0304-4165(02)00537-8. [DOI] [PubMed] [Google Scholar]
- 62.Nagababu E. Fabry ME. Nagel RL. Rifkind JM. Heme degradation and oxidative stress in murine models for hemoglobinopathies: thalassemia, sickle cell disease and hemoglobin C disease. Blood Cells Mol Dis. 2008;41:60–66. doi: 10.1016/j.bcmd.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagababu E. Gulyani S. Earley CJ. Cutler RG. Mattson MP. Rifkind JM. Iron-deficiency anaemia enhances red blood cell oxidative stress. Free Radic Res. 2008;42:824–829. doi: 10.1080/10715760802459879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagababu E. Mohanty JG. Bhamidipaty S. Ostera GR. Rifkind JM. Role of the membrane in the formation of heme degradation products in red blood cells. Life Sci. 2010;86:133–138. doi: 10.1016/j.lfs.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagababu E. Ramasamy S. Abernethy DR. Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 66.Nagababu E. Ramasamy S. Rifkind JM. Jia Y. Alayash AI. Site-specific cross-linking of human and bovine hemoglobins differentially alters oxygen binding and redox side reactions producing rhombic heme and heme degradation. Biochemistry. 2002;41:7407–7415. doi: 10.1021/bi0121048. [DOI] [PubMed] [Google Scholar]
- 67.Nagababu E. Rifkind JM. Formation of fluorescent heme degradation products during the oxidation of hemoglobin by hydrogen peroxide. Biochem Biophys Res Commun. 1998;247:592–596. doi: 10.1006/bbrc.1998.8846. [DOI] [PubMed] [Google Scholar]
- 68.Nagababu E. Rifkind JM. Heme degradation during autoxidation of oxyhemoglobin. Biochem Biophys Res Commun. 2000;273:839–845. doi: 10.1006/bbrc.2000.3025. [DOI] [PubMed] [Google Scholar]
- 69.Nagababu E. Rifkind JM. Reaction of hydrogen peroxide with ferrylhemoglobin: superoxide production and heme degradation. Biochemistry. 2000;39:12503–12511. doi: 10.1021/bi992170y. [DOI] [PubMed] [Google Scholar]
- 70.Nagababu E. Rifkind JM. Heme degradation by reactive oxygen species. Antioxid Redox Signal. 2004;6:967–978. doi: 10.1089/ars.2004.6.967. [DOI] [PubMed] [Google Scholar]
- 71.Nagababu E. Rifkind JM. Measurement of plasma nitrite by chemiluminescence without interference of S-, N-nitroso and nitrated species. Free Radic Biol Med. 2007;42:1146–1154. doi: 10.1016/j.freeradbiomed.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nohl H. Stolze K. The effects of xenobiotics on erythrocytes. Gen Pharmacol. 1998;31:343–347. doi: 10.1016/s0306-3623(97)00457-6. [DOI] [PubMed] [Google Scholar]
- 73.Pacher P. Beckman JS. Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasini EM. Mann M. Thomas AW. Red blood cell proteomics. Transfus Clin Biol. 2010;17:151–164. doi: 10.1016/j.tracli.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 75.Piccinini G. Minetti G. Balduini C. Brovelli A. Oxidation state of glutathione and membrane proteins in human red cells of different age. Mech Ageing Dev. 1995;78:15–26. doi: 10.1016/0047-6374(94)01511-j. [DOI] [PubMed] [Google Scholar]
- 76.Pietraforte D. Salzano AM. Scorza G. Marino G. Minetti M. Mechanism of peroxynitrite interaction with ferric hemoglobin and identification of nitrated tyrosine residues. CO(2) inhibits heme-catalyzed scavenging and isomerization. Biochemistry. 2001;40:15300–15309. doi: 10.1021/bi010998q. [DOI] [PubMed] [Google Scholar]
- 77.Piomelli S. Seaman C. Mechanism of red blood cell aging: relationship of cell density and cell age. Am J Hematol. 1993;42:46–52. doi: 10.1002/ajh.2830420110. [DOI] [PubMed] [Google Scholar]
- 78.Ray R. Shah AM. NADPH oxidase and endothelial cell function. Clin Sci (Lond) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 79.Redding GS. Record DM. Raess BU. Calcium-stressed erythrocyte membrane structure and function for assessing glipizide effects on transglutaminase activation. Proc Soc Exp Biol Med. 1991;196:76–82. doi: 10.3181/00379727-196-43166a. [DOI] [PubMed] [Google Scholar]
- 80.Rettig MP. Low PS. Gimm JA. Mohandas N. Wang J. Christian JA. Evaluation of biochemical changes during in vivo erythrocyte senescence in the dog. Blood. 1999;93:376–384. [PubMed] [Google Scholar]
- 81.Rifkind JM. Heme iron in hemoglobin and myoglobin. In: Fuchs H, editor; Mohanty JG, editor; Nagababu E, editor; Ramasamy S, editor; Ravi L, editor. Iron Metabolism and Diseases. Trivandrum, Kerala, India: Research Signpost; 2008. pp. 365–392. [Google Scholar]
- 82.Rifkind JM. Abugo OO. Alterations in erythrocyte deformability under hypoxia: implications for impaired oxygen transport. Adv Exp Med Biol. 1994;361:345–351. doi: 10.1007/978-1-4615-1875-4_54. [DOI] [PubMed] [Google Scholar]
- 83.Rifkind JM. Nagababu E. Ramasamy S. Nitric oxide redox reactions and red cell biology. Antioxid Redox Signal. 2006;8:1193–1203. doi: 10.1089/ars.2006.8.1193. [DOI] [PubMed] [Google Scholar]
- 84.Rifkind JM. Nagababu E. Ramasamy S. The quaternary hemoglobin conformation regulates the formation of the nitrite-induced bioactive intermediate and the dissociation of nitric oxide from this intermediate. Nitric Oxide. 2011;24:102–109. doi: 10.1016/j.niox.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rifkind JM. Ramasamy S. Manoharan PT. Nagababu E. Mohanty JG. Redox reactions of hemoglobin. Antioxid Redox Signal. 2004;6:657–666. doi: 10.1089/152308604773934422. [DOI] [PubMed] [Google Scholar]
- 86.Rifkind JM. Salgado MT. Cao Z. Regulation of oxygen delivery by the reaction of nitrite with RBCs under hypoxic conditions. Adv Exp Med Biol. 2012;737:183–189. doi: 10.1007/978-1-4614-1566-4_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rifkind JM. Zhang L. Levy A. Manoharan PT. The hypoxic stress on erythrocytes associated with superoxide formation. Free Radic Res Commun. 1991;12–13(Pt 2):645–652. doi: 10.3109/10715769109145842. [DOI] [PubMed] [Google Scholar]
- 88.Rogers SC. Said A. Corcuera D. McLaughlin D. Kell P. Doctor A. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J. 2009;23:3159–3170. doi: 10.1096/fj.09-130666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romero N. Denicola A. Radi R. Red blood cells in the metabolism of nitric oxide-derived peroxynitrite. IUBMB Life. 2006;58:572–580. doi: 10.1080/15216540600936549. [DOI] [PubMed] [Google Scholar]
- 90.Romero N. Radi R. Hemoglobin and red blood cells as tools for studying peroxynitrite biochemistry. Methods Enzymol. 2005;396:229–245. doi: 10.1016/S0076-6879(05)96021-7. [DOI] [PubMed] [Google Scholar]
- 91.Salgado MT. Nagababu E. Rifkind JM. Quantification of intermediates formed during the reduction of nitrite by deoxyhemoglobin. J Biol Chem. 2009;284:12710–12718. doi: 10.1074/jbc.M808647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salgado MT. Ramasamy S. Tsuneshige A. Manoharan PT. Rifkind JM. A new paramagnetic intermediate formed during the reaction of nitrite with deoxyhemoglobin. J Am Chem Soc. 2011;133:13010–13022. doi: 10.1021/ja1115088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samaja M. Rubinacci A. Motterlini R. De PA. Portinaro N. Red cell aging and active calcium transport. Exp Gerontol. 1990;25:279–286. doi: 10.1016/0531-5565(90)90063-8. [DOI] [PubMed] [Google Scholar]
- 94.Shaklai N. Avissar N. Rabizadeh E. Shaklai M. Disintegration of red cell membrane cytoskeleton by hemin. Biochem Int. 1986;13:467–477. [PubMed] [Google Scholar]
- 95.Shiva S. Wang X. Ringwood LA. Xu X. Yuditskaya S. Annavajjhala V. Miyajima H. Hogg N. Harris ZL. Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 96.Shviro Y. Shaklai N. Glutathione as a scavenger of free hemin. A mechanism of preventing red cell membrane damage. Biochem Pharmacol. 1987;36:3801–3807. doi: 10.1016/0006-2952(87)90441-2. [DOI] [PubMed] [Google Scholar]
- 97.Siekmeier R. Bierlich A. Jaross W. Determination of reticulocytes: three methods compared. Clin Chem Lab Med. 2000;38:245–249. doi: 10.1515/CCLM.2000.036. [DOI] [PubMed] [Google Scholar]
- 98.Stocchi V. Kolb N. Cucchiarini L. Segni M. Magnani M. Fornaini G. Adenine, and pyridine nucleotides during rabbit reticulocyte maturation and cell aging. Mech Ageing Dev. 1987;39:29–44. doi: 10.1016/0047-6374(87)90084-4. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki Y. Ohkubo N. Aoto M. Maeda N. Cicha I. Miki T. Mitsuda N. Participation of caspase-3-like protease in oxidation-induced impairment of erythrocyte membrane properties. Biorheology. 2007;44:179–190. [PubMed] [Google Scholar]
- 100.Wang S. Dale GL. Song P. Viollet B. Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem. 2010;285:19976–19985. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Waugh RE. Narla M. Jackson CW. Mueller TJ. Suzuki T. Dale GL. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–1358. [PubMed] [Google Scholar]
- 102.Weiss E. Rees DC. Gibson JS. Role of calcium in phosphatidylserine externalisation in red blood cells from sickle cell patients. Anemia. 2011;2011:379894. doi: 10.1155/2011/379894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Willekens FL. Roerdinkholder-Stoelwinder B. Groenen-Dopp YA. Bos HJ. Bosman GJ. van den Bos AG. Verkleij AJ. Werre JM. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–751. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]
- 104.Willekens FL. Werre JM. Groenen-Dopp YA. Roerdinkholder-Stoelwinder B. de PB. Bosman GJ. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–556. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 105.Wyse JW. Butterfield DA. Interaction of hemin with erythrocyte membranes: alterations in the physical state of the major sialoglycoprotein. Biochim Biophys Acta. 1989;979:121–126. doi: 10.1016/0005-2736(89)90531-2. [DOI] [PubMed] [Google Scholar]
- 106.Xu X. von LK. Soldau K. Noack D. Vu A. Friedman JS. A novel approach for in vivo measurement of mouse red cell redox status. Blood. 2011;118:3694–3697. doi: 10.1182/blood-2011-03-342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zimran A. Forman L. Suzuki T. Dale GL. Beutler E. In vivo aging of red cell enzymes: study of biotinylated red blood cells in rabbits. Am J Hematol. 1990;33:249–254. doi: 10.1002/ajh.2830330407. [DOI] [PubMed] [Google Scholar]
- 108.Zipser Y. Piade A. Barbul A. Korenstein R. Kosower NS. Ca2+ promotes erythrocyte band 3 tyrosine phosphorylation via dissociation of phosphotyrosine phosphatase from band 3. Biochem J. 2002;368:137–144. doi: 10.1042/BJ20020359. [DOI] [PMC free article] [PubMed] [Google Scholar]