Abstract
Adult mesenchymal stem cells secrete a variety of angiogenic cytokines and growth factors, so we proposed that these paracrine mechanisms may be used to promote vascularization and growth for tissue engineering in vivo. We tested whether or not human adipose-derived stem cells (ASCs) promote tissue formation in rats. ASCs were evaluated in vitro for mRNA expression of angiogenic factors, including the vascular endothelial growth factor, basic fibroblast growth factor, interleukin-8 (IL-8), and stromal cell-derived factor-1 (SDF-1) and proliferative activity on human microvascular endothelial cells. For in vivo analysis, CM-DiI-labeled ASCs were implanted with a rat cardiac extracellular matrix (ECM) extract-derived hydrogel into a chamber with a femoral arteriovenous loop in the groin of male nude rats for 7 days. Vascularization in newly generated tissue was estimated by histomorphometry after endothelial nitric oxide synthase (eNOS) immunostaining. ASCs expressed growth factor mRNA and produced an angiogenic activity in vitro. After implantation, ASCs survived, but remained suspended in the ECM and relatively few were incorporated into the newly formed tissue. The volume of newly generated tissue was significantly higher in chambers containing ASCs and it was enriched with vasculature when compared with the ECM alone. We conclude that human ASCs promote tissue growth and angiogenesis in the rat vascularized chamber, thereby showing promise for tissue-engineering applications for regenerative therapy.
Introduction
Aparacrine mechanism has been postulated for some of the beneficial effects of mesenchymal stem cell (MSC) therapy in cardiac tissue regeneration settings,1–3 in part, through promotion of angiogenesis.4,5 MSCs from a range of sources have been shown to express and release several important angiogenic growth factors and cytokines, including the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).6 Moreover, the conditioned medium from MSCs, which contains these angiogenic factors, promotes angiogenesis in vitro and in vivo.4,5,7 MSCs may also differentiate8–10 or integrate into vascular components and potentially stabilize their structure during network development.8–10
Regulation of the paracrine activity in adult stem cells is not well understood, but may represent an important element of endogenous repair mechanisms. Defining how local factors regulate stem cell paracrine function will clearly be important for optimizing cell therapy applications and manipulation of this capacity of stem cells in cell therapies could optimize healing and survival of damaged tissue. In particular, stem cell therapy applications may provide a suitable means for continuous local delivery of angiogenic factors over extended periods.11 The delivery of stem cells and their angiogenic activities to regions of tissue repair may result in angiogenesis and improvement of repair, or it may prove useful to harness these beneficial effects of stem cells for in vivo tissue-engineering applications. The advancement of tissue engineering as a regenerative therapy relies on rapid vascularization of ex vivo formed tissue constructs by the host vasculature,12 or as we have shown previously in various tissue settings, assembly of a large construct with vasculature in situ13–15 or ectopically for later transplantation.16 MSCs may prove beneficial to promote rapid assembly and growth of a vasculature to support tissue-engineering outcomes in vivo.
The aim of this study was to test the hypothesis that implanting MSCs regulates angiogenesis in tissue engineering in vivo. For this purpose, we have used a previously characterized17 adipose-derived stem cell (ASC) population. We have confirmed that endothelial growth is stimulated by their conditioned medium in vitro, and then examined their role in promoting vascularization and tissue growth during in vivo tissue engineering. In a previous study, we identified the angiogenic activity when ASCs were implanted in a growth factor-rich hydrogel scaffold (Matrigel) together with cardiomyocytes.9 In the current study, we used an alginate scaffold with no added growth factors, as well as a novel cardiac muscle-derived hydrogel based on alginate to assess whether cell–matrix interactions influence MSC angiogenic activity and tissue formation.
Materials and Methods
Cell culture
ASCs were isolated from human subcutaneous adipose tissue as described previously.6,9,17 Fat tissue samples were collected from female donors aged between 43 and 52 years, with approval of the St. Vincent's Health Human Research Ethics Committee and with informed consent. Once isolated, ASCs were maintained in the complete medium (low-glucose Dulbecco's modified Eagle's medium [DMEM-LG] containing L-glutamine; Invitrogen), 10% fetal calf serum (FCS; Sigma), 1% penicillin/streptomycin/amphotericin (Invitrogen), at 37°C in a humidified incubator with 5% CO2. The cells have been previously characterized as MSCs by their multipotency (osteogenic, chondrogenic, and adipogenic differentiation) and their expression using flow cytometry of CD73, CD90, and CD105, but not hemopoietic lineage markers CD34 and CD45.6,17
To determine the effect of the matrix substrate on ASC expression of angiogenic factors, six-well culture plates were coated with extracellular matrix (ECM) solutions overnight; either cardiogel, a rat cardiac matrix extract (10, 30, 100, 300 μg/mL; see below for extraction method), fibronectin (10 μg/mL; Sigma), or uncoated tissue culture plastic as a control. ASCs (passages 2–6) were seeded at 1×105 cells per well and incubated for 24 h before extracting RNA from the cells. Four independent experiments were conducted in duplicate.
Human microvascular endothelial cells were purchased from Lonza and were maintained in the endothelial growth medium (EGM; Lonza). Cells used in these experiments were passage 20 to 21.
Cardiac matrix preparation
Rat heart tissue weighing 22 g was homogenized in a 100 mL 3.4 M sodium chloride (NaCl) buffer and 1 mL protease inhibitors (0.5 mM phenylmethyl sulfonyl fluoride and 2 mM N-ethylmaleimide; Sigma) and centrifuged at 10,000 rpm for 10 min at 4°C. The pellet was suspended in 2 M urea in 0.05 M Tris/0.115 M NaCl buffer (TBS) containing a general protease inhibitor tablet (Sigma). The mixture was homogenized, mixed overnight at 4°C to solubilize the extracted proteins, and then centrifuged at 15,000 rpm for 30 min at 4°C. The supernatant was filtered and the insoluble phase discarded. About 1.5% w/v alginate (Pronova UP LVM; Novamatrix) was dissolved into the cardiogel followed by overnight dialysis against TBS with 0.5% w/v chloroform to sterilize the cardiogel. Cardiogel was then dialyzed against three changes of fresh TBS, followed by a final dialysis against Hank's balanced salt solution (Sigma) with 1% penicillin/streptomycin/amphotericin. A total protein concentration of 2.94 mg/mL was measured with a bicinchoninic acid protein assay (Pierce).
RNA isolation and cDNA synthesis
Nucleic acid isolation was performed with 0.5 mL Trizol (Invitrogen) per well, phase separation with chloroform and precipitation with isopropanol and glycogen (Ambion), washed with 75% ethanol, and then resuspended in nuclease-free water (Ambion). DNase (Promega) treatment of 600 ng of sample to remove contaminating genomic DNA was followed by reverse transcription using avian myeloblastosis virus reverse transcriptase (Roche Applied Science) in the presence of random primers (Invitrogen) and RNase inhibitor (Roche Applied Science).
Real-time polymerase chain reaction
Expression of four candidate angiogenic factors, interleukin-8 (IL-8), vascular endothelial growth factor, basic fibroblast growth factor, and stromal cell derived factor-1 (SDF-1), were analyzed using real-time polymerase chain reaction (RT-PCR). TaqMan technology and Assay-on-Demand primer/probe sets were used (Hs00174103_m1, Hs00900055_m1, Hs00266645_m1, and Hs00171022_m1, respectively; Applied Biosystems). RT-PCR was conducted using the ABI Prism 7300 Real-Time PCR System (Applied Biosystems), with 1 μL cDNA per replicate well. Reactions were carried out in triplicate for each sample, and included at least one reverse transcriptase negative control and no-template control per run. The machine ran at 50°C for 2 min for activation of AmpErase UNG, 95°C for 10 min for activation of AmpliTaq Gold polymerase, and then 50 cycles of 95°C for 15 s followed by 60°C for 1 min for primer/probe dissociation and annealing/elongation, respectively. Relative fold change in expression was calculated by normalizing to the housekeeping gene, 18S rRNA (Hs99999901_s1; Applied Biosystems) to adjust for loading variation, and to the experimental control, untreated ASCs.
Endothelial cell proliferation assay
To investigate the effect of the ASC-conditioned medium on endothelial cell proliferation, human microvascular endothelial cells (HMECs) were cultured for 3 days in the conditioned or control medium, and the cell number determined using 3-[4,5-dimethylthiazol-2-yl] 2,5-diphenyltetrazolium bromide (MTT) assay. HMECs in the EGM bullet kit (5% FCS) were seeded into 96-well plates at a density of 2000 cells/well and allowed to adhere overnight. Media were replaced with equal parts of the EGM bullet kit (2% FCS) and the test (or control) medium. Positive and negative control media were the DMEM-LG supplemented with 10% FCS or 0% FCS, respectively. Test media were conditioned for 48 h by 106 ASCs on one of plastic, cardiogel (300 μg/mL), or fibronectin (10 μg/mL). Plates were incubated for 3 days at 37°C, 5% CO2, and then MTT assay performed (Sigma). Four independent experiments were conducted in triplicate.
In vivo study
To test the effects of ASCs on angiogenesis and tissue formation in tissue engineering, an alginate-based hydrogel scaffold containing ASCs (with or without cardiogel) was implanted into the rat tissue-engineering chamber, incubated for 7 days, and the tissue construct in the chamber evaluated histologically.
Preparation of cells and hydrogel scaffold
Passage 4–10 human ASCs were prepared as described above and labeled with CM-DiI (Molecular Probes; 3 μL of CM-DiI for 2.0×106 cells for 5 min, and then 15 min on ice). After washing with phosphate-buffered saline, cells were suspended in 200 μL of cardiogel. Controls were cells suspended in alginate without cardiac extract, or hydrogels implanted without cells.
Preparation of vascularized tissue-engineering chamber
An arteriovenous loop was constructed in the groin region of male CBH/rnu/rnu (nude) rats (ARC) that weighed 250–320 g as previously described14,15 by interposing a femoral vein graft between the femoral artery and the femoral vein (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). The loop was laid inside the base of a polycarbonate chamber (0.5-mL internal volume, 1.3-cm internal diameter, and 0.5-cm height; Department of Chemical Engineering, The University of Melbourne, Melbourne, Australia). The base of the chamber was anchored to the inguinal ligament and surrounding tissues with sutures. After implantation of 200 μL of hydrogels with and without cells, the lid of the chamber was attached, and the wound closed. After 7 days, the rats were anesthetized and chambers were exposed, the vessels examined for patency, and the chamber tissue harvested for histological analysis. Twenty-four rats were used for four groups (six rats for each group, cardiogel with or without ASCs and alginate with or without ASCs). All procedures were performed with the approval of the St. Vincent's Hospital Animal Ethics Committee, under National Health and Medical Research Council guidelines for animal welfare.
Histological analysis
The volume and weight of the generated tissues were measured, and the tissue then fixed in 4% paraformaldehyde for 24 h. Tissue constructs were divided into serial 2-mm-thick transverse slices that were embedded in paraffin, and 5-μm-thick histological sections were made and stained with hematoxylin and eosin for evaluation of morphology. Immunohistochemistry was also performed to detect endothelial cells in the newly formed tissue using immunohistochemistry with mouse anti-endothelial nitric oxide synthase (eNOS) (BD Biosciences) with hematoxylin counterstaining.
Morphometry
Hematoxylin–eosin stained sections were analyzed by videomicroscopy with a computer-generated 6×6-point square grid (CAST system) as previously described.14–18 To determine the proportional volume of tissue components (newly formed connective tissue, remaining hydrogel, arterio-venous (AV) loop or fibrin), random fields were sampled systematically and the number of points falling on each of the tissue categories were counted and divided by the total number of points. At least 330 points were examined for each specimen. For evaluation of vascularization in the newly formed connective tissue, immunohistochemistry with mouse anti-eNOS/NOS type III (BD Biosciences) was used and counted similarly, but with a 2×2-point square grid. eNOS immunoperoxidase staining was by an indirect method (avidin biotin peroxidase; Vector ABC method) with negative controls (normal mouse IgG), as previously described.15 The vascularization within the newly formed tissue was determined by counting the points falling on eNOS-positive blood vessels and dividing by the sum of points on newly formed connective tissue, expressed as a percentage. Morphometric counts were made independently by two blinded observers (K.M. and R.D.).
Tracking cell fate in the chamber
One unstained slide from each sample was cover-slipped and evaluated by fluorescence microscopy with the fluorescence mounting medium for visualization and localization of CM-DiI-labeled human ASCs in the chamber construct.
Statistics
Data are expressed as mean±standard error of the mean (SEM). Statistical significance was calculated using one- or two-way analysis of variance with a Bonferroni post hoc test between treatment groups. A value of p<0.05 was considered significant.
Results
ASCs express angiogenic factors
Quantitative RT-PCR was used to show expression of IL-8, VEGF, bFGF, and SDF-1 mRNA in ASCs and to measure changes in cells cultured for 24 h on tissue culture plastic, cardiogel, or fibronectin. ASCs were found to express all angiogenic factors tested. In particular, IL-8 transcript levels were found to be significantly altered by plating ASCs on cardiac-derived matrix substrate for 24 h (p<0.001, Fig. 1a). IL-8 mRNA levels in ASCs plated on cardiogel were increased up to 6.5-fold compared with ASCs plated on plastic (p<0.01) and were dependent on the cardiogel concentration used to coat the plate (p<0.001). Plating cells on fibronectin had negligible effects on IL-8 expression. In contrast, expression levels of VEGF, bFGF, and SDF-1 remained constant when ASCs were plated on different concentrations of cardiogel or on fibronectin, as compared to plastic (Fig. 1b–d).
FIG. 1.
mRNA expression of candidate angiogenic factors in adipose-derived stem cells (ASCs) on matrix substrates. Transcript levels of (a) interleukin-8 (IL-8) show dose-dependent increases in ASCs plated on increasing concentrations of cardiogel. Expression of (b) vascular endothelial growth factor (VEGF), (c) basic fibroblast growth factor (bFGF), and (d) stromal cell-derived factor-1 (SDF-1) was unchanged by treatment. Results are expressed as relative fold change in the mRNA level compared to plastic (mean±standard error of the mean (SEM)). *represents p<0.05 compared to plastic.
Medium conditioned by ASCs induces endothelial cell proliferation
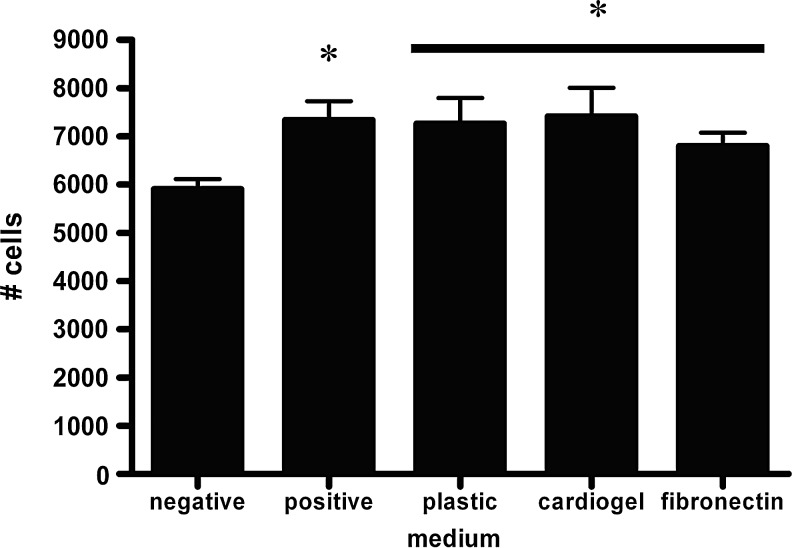
To determine whether expression of angiogenic factors by ASCs correlated with increased endothelial cell proliferation, we tested the effect of ASC-conditioned media on human microvascular endothelial cell cultures. ASC-conditioned media induced more endothelial cell proliferation than did the standard medium (p<0.05, Fig. 2), with no significant difference between media conditioned by ASCs on cardiogel or fibronectin substrates compared to plastic.
FIG. 2.
Endothelial cell proliferation in the medium conditioned by ASCs grown on tissue culture plastic, cardiogel, or fibronectin was greater than in the unconditioned (negative control) medium, and was at least that seen in the high serum (positive control) medium. * represents p<0.05 compared to the negative control.
Influence of ASCs on tissue engineering in vivo
To determine the effects of ASCs on tissue-engineering processes in vivo, we evaluated the formation and vascularization of tissue constructs 7 days after implantation of cells, cardiogel matrix, or a combination of the two (Fig. 3). The macroscopic appearance, volume, and weight of the construct were assessed. After 7-day incubation of vascularized chambers in vivo, all of the chambers contained a single solid piece of vascularized tissue, able to support its own weight and shape ex vivo (Fig. 4). The macrovascular supply (AV loop) was intact in all cases and the constructs were viable (Supplementary Fig. S1). The volume and weight of the constructs were similar for each treatment group (Supplementary Fig. S2).
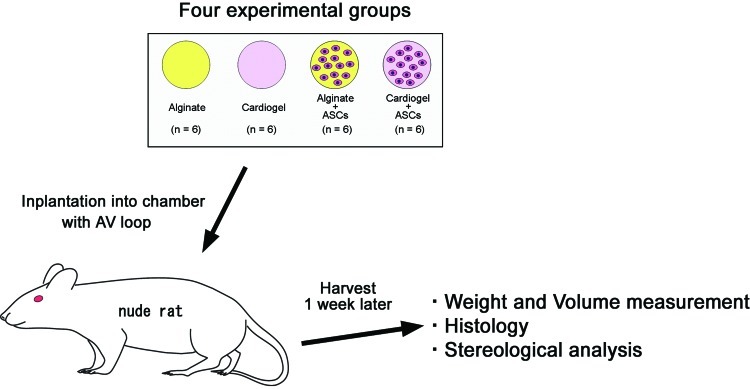
FIG. 3.
Schematic of the in vivo study design. Four experimental groups were made: alginate only, cardiogel only, alginate with ASCs, and cardiogel with ASCs (n=6 each, N=24 total). Tissues were taken 7 days later for histological and morphometric assessment. Color images available online at www.liebertpub.com/tea
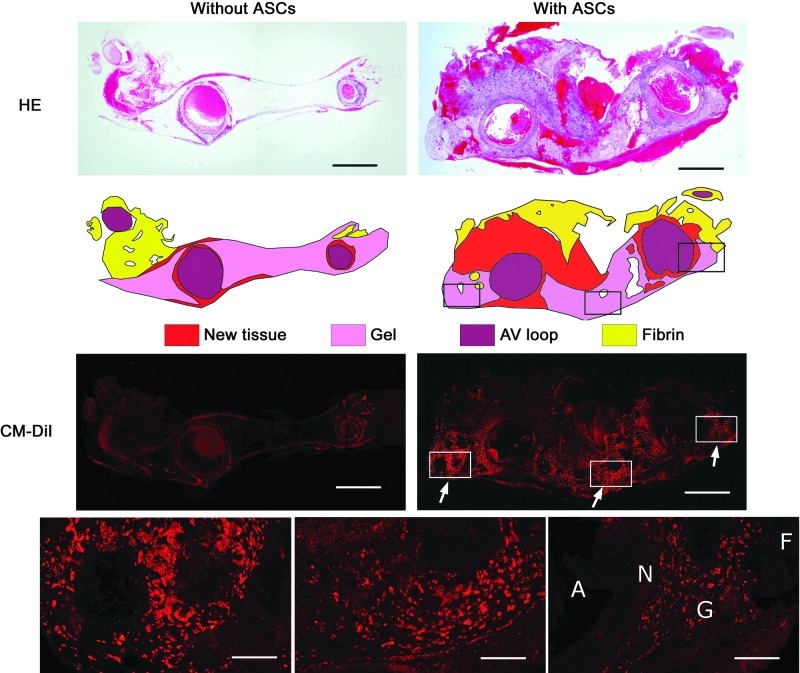
FIG. 4.
Photomicrographs of hematoxylin and eosin (HE) stain (upper panels) and CM-DiI fluorescence (lower panels) of the chamber without (left) and with (right) ASCs (arrows). Tissue was categorized into new tissue, gel, arterio-venous (AV) loop, and fibrin (middle panels) (scale bar=1 mm). Higher power micrographs (bottom panels) show the DiI fluorescence predominantly localized to the gel space (G) (F, fibrin; N, new tissue; A, AV loop; scale bar=175 μm). Color images available online at www.liebertpub.com/tea
Tissue composition analysis by histomorphometry
Histological analysis of the constructs revealed chamber tissue composed predominantly of the original AV loop constituents, a variable amount of newly formed granulation tissue around the loop vessels as well as the residual hydrogel scaffold and fibrin network throughout the remaining chamber space. Morphometric analysis of chamber contents showed that significantly more new tissue was formed in the chambers when ASCs were implanted (Fig. 5). The presence or absence of cardiogel had no effect on tissue formation. Likewise, vascularization in the new tissue was significantly increased with ASCs present, regardless of the presence of cardiogel (Fig. 6).
FIG. 5.
Stereological analysis showing percentage volume of the constructs. In the groups with ASCs, percentage volume of the new tissue was significantly increased [p<0.05, analysis of variance (ANOVA)].
FIG. 6.
(a) Photomicrographs of newly formed tissue with endothelial nitric oxide synthase immunohistochemistry without (left) and with (right) ASCs (scale bar=50 μm). (b) Stereological analysis showing vascularization in the newly formed tissue (mean±SEM). In the groups with ASCs, percentage vascularization in newly formed tissue were significantly higher than without ASCs (p<0.05, ANOVA). Color images available online at www.liebertpub.com/tea
Cell tracking in the chamber
Residual CM-DiI fluorescence was observed in histological sections of tissues implanted with ASCs (Fig. 4). Labeled cells were clearly visible in cardiogel/ASC and alginate/ASC constructs and could be easily discriminated from background fluorescence as brightly stained clumps of cells within lacunae in the hydrogel scaffold. Some smaller, high-intensity fluorescent regions were also observed in new tissue adjacent to the gel. No stained cells were seen incorporated into the walls of the AV loop or in distal regions of the construct.
Discussion
Successful translation of experimental tissue-engineering methods from in vitro to in vivo promise a real advance for regenerative therapies. The implementation of these approaches will rely on rapid development of a vascular supply to support cell survival and tissue assembly into sufficient volume of an engineered tissue.18 In this study, we demonstrate that incorporation of ASCs into a vascularized chamber promotes vascularization and tissue formation within an alginate scaffold.
The identity of factors regulating the angiogenic activity from bone marrow-derived MSCs has been the subject of a number of studies.4,6,7,11 Our in vitro experiments here confirm the proliferative activity of ASC-conditioned media on human endothelial cells and basal expression of angiogenic factor mRNA. We go further to show a significant dose-dependent increase in IL-8 expression with the cardiac-derived biomaterial cardiogel. The constituents of cardiogel, which might regulate gene expression are not known, but the finding does confirm that cell–ECM interactions have potential to regulate ASC cytokine expression. Further study is required to determine whether a similar effect is evident upon engraftment of ASCs in a cardiac environment. The specific molecules responsible for the angiogenic effect of ASCs remain unknown. It is unlikely to be predominantly IL-8, but rather a combination of various factors as the increased IL-8 expression by ASCs on matrix substrates did not correlate with increased angiogenic readouts. We recently showed that a significant proportion of the ASC paracrine angiogenic activity is produced by VEGF A and VEGF D in the conditioned medium.6 Further studies are required to identify roles of other angiogenic growth factors. Exposure of ASCs to cardiogel in the rat vascularized chamber did not appear to influence tissue formation beyond the basal effect of ASCs. The ASC secretome is complex,19 and so, other factors secreted by the ASCs may regulate tissue formation and have a dominating influence in this chamber environment, or the effect of cardiogel might be modified in the more complex in vivo environment.
Incorporation of ASCs into the tissue-engineering chamber resulted in the generation of a greater volume of new tissue compared to chambers lacking ASCs. In addition, this newly formed tissue had a higher vascularization, suggesting that at least part of the ASC effect was driven by enhanced angiogenesis. Although the actual proportion of ASCs surviving implantation remains unknown, the fact that ASCs predominantly remain in the gel space and are not incorporated into distant regions of the tissue constructs strongly suggests a paracrine effect. There are many reports that introduction of MSCs has beneficial effects in vivo, for example, more rapid skin wound healing,20 improvement of functional recovery in cerebral infarction models,21,22 or myocardial infarction models.23–25 In these models, implanted MSCs are thought to do more than just differentiate and proliferate. The MSCs clearly act additionally as a source of bioactive factors that inhibit scar formation, inhibit apoptosis, increase angiogenesis, and stimulate intrinsic progenitor cells.26 After cerebral infarction in rats, delivery of MSCs, which are not known to differentiate into neurons or neuronal support cells, produces a significant functional improvement.21,27 It is difficult to assess what portion of these positive effects can be ascribed to MSC-derived cells per se and what contribution is attributable to those other factors,26 but the latter are supposed to be dominant at least in the days following their introduction.26,28 These hypotheses are compatible with our in vivo results for CM-DiI-labeled ASCs concentrated in the gel space-promoted growth of vascular-rich tissue in the chamber without any incorporation into the walls of the AV loop or in distal regions of the construct, suggesting that the angiogenic effect of ASCs occurs through paracrine pathways.
For successful generation and maintenance of the tissue constructs, angiogenic growth factors, such as VEGF and FGF, are thought to play important roles.10,11,17 Because of their short biological half life,29,30 long-lasting and slow-release delivery systems would be preferred. There are numerous reports about long-lasting delivery systems for growth factors, using alginate,31,32 gelatin,33,34 poly lactic co-glycolic acid,35,36 chitosan-albumin,37 and fibrin.38,39 In addition, application of genes40–42 or genetically modified cells11,43 encoding angiogenic growth factors is another approach for the long-lasting growth factor delivery system. Our study shows that the delivery of ASCs in a slowly resorbing alginate scaffold may be beneficial, although the short-term nature of the current study limits our conclusions and indicates further study is required. Finally, vascular progenitor cells have been previously transplanted into the AV loop separation chamber, demonstrating that the cells can migrate and become distributed within the construct.44 We also have shown that ASCs implanted into a chamber over a long period may integrate into the tissue and contribute to new vessels.9 Similarly, other stem cell populations may prove useful with this method.6 Using autologous stem cells from an easily harvested and safe source provide a unique approach that may integrate into the developing tissue in patients and continue to have beneficial effects over an extended period.
It is arguable that combinations of growth factors might be advantageous. Several studies show synergistic interactions between VEGF and bFGF,39,45–47 VEGF and platelet-derived growth factor-B (PDGF-B),48 and bFGF and PDGF.49 Combinations of such growth factors delivered locally by stem cells could reduce the growth factor concentration required in total and would minimize undesirable systemic side effects such as accelerated atherosclerotic plaque growth,50,51 vasodilatation and hypotension,52 lower-extremity edema,53 and the possibility of triggering neoplasms.54 Since implanted stem cells synthesize and secrete a broad spectrum of cytokines and growth factors26 that might have synergistic effects, they are likely to act as a long-lasting growth factor cocktail delivery system.
The results from this tissue-engineering chamber model show the potential use of ASCs as a long-lasting source of multiple growth factors in combination with cell or tissue implantation and transplantation to improve their survival. Whether particular ECM scaffolds might augment these effects in vivo remains to be explored further.
Supplementary Material
Acknowledgments
These studies were supported by grants from the National Health and Medical Research Council of Australia (509271), the Cass Foundation, and the L.E.W. Carty Trust. The O'Brien Institute acknowledges the Victorian State Government's Department of Innovation, Industry and Regional Development's Operational Infrastructure Support Program. The authors wish to acknowledge the technical advice of Mr. J. Palmer, Ms. X. Han, and staff of the Experimental Medicine and Surgery Unit at St. Vincent's Hospital.
Disclosure Statement
Professor Morrison is an inventor on the Vascularized Tissue Graft patent and entitled to proceeds derived from commercialization of the patent, and is a board member and employee of the O'Brien Institute, which has an interest in the company charged with the commercialization of the Vascularized Tissue Graft patent. The remaining authors report no competing financial interests.
References
- 1.Murry C.E. Reinecke H. Pabon L.M. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs S. Baffour R. Zhou Y.F. Shou M. Pierre A. Tio F.O., et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 3.Kamihata H. Matsubara H. Nishiue T. Fujiyama S. Tsutsumi Y. Ozono R., et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 4.Kinnaird T. Stabile E. Burnett M.S. Shou M. Lee C.W. Barr S., et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 5.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove C.J. Bovenkerk J.E., et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao S.T. Asgari A. Lokmic Z. Sinclair R. Dusting G.J. Lim S.Y. Dilley R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21:2189. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.See F. Seki T. Psaltis P.J. Sondermeijer H.P. Gronthos S. Zannettino A.C. Govaert K.M. Schuster M.D. Kurlansky P.A. Kelly D.J. Krum H. Itescu S. Therapeutic effects of human STRO-3-selected mesenchymal precursor cells and their soluble factors in experimental myocardial ischemia. J Cell Mol Med. 2011;15:2117. doi: 10.1111/j.1582-4934.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amos P.J. Shang H. Bailey A.M. Taylor A. Katz A.J. Peirce S.M. IFATS collection: the role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008;26:2682. doi: 10.1634/stemcells.2008-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi Y.S. Matsuda K. Dusting G.J. Morrison W.A. Dilley R.J. Engineering cardiac tissue in vivo from human adipose-derived stem cells. Biomaterials. 2010;31:2236. doi: 10.1016/j.biomaterials.2009.11.097. [DOI] [PubMed] [Google Scholar]
- 10.Amoh Y. Li L. Yang M. Moossa A.R. Katsuoka K. Penman S. Hoffman R.M. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci U S A. 2004;101:13291. doi: 10.1073/pnas.0405250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnecchi M. Zhang Z. Ni A. Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levenberg S. Rouwkema J. Macdonald M. Garfein E.S. Kohane D.S. Darland D.C. Marini R. van Blitterswijk C.A. Mulligan R.C. D'Amore P.A. Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 13.Mian R. Morrison W.A. Hurley J.V. Penington A.J. Romeo R. Tanaka Y., et al. Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng. 2000;6:595. doi: 10.1089/10763270050199541. [DOI] [PubMed] [Google Scholar]
- 14.Lokmic Z. Stillaert F. Morrison W.A. Thompson E.W. Mitchell G.M. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21:511. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- 15.Morritt A.N. Bortolotto S.K. Dilley R.J. Han X. Kompa A.R. McCombe D., et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 16.Tee R. Morrison W.A. Dusting G.J. Liu G. Choi Y.S. Hsiao S.T. Dilley R.J. Transplantation of engineered cardiac muscle flaps in syngeneic rats. Tissue Eng A. doi: 10.1089/ten.tea.2012.0151. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi Y.S. Dusting G.J. Stubbs S. Arunothayaraj S. Han X.L. Collas P. Morrison W.A. Dilley R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J Cell Mol Med. 2010;14:878. doi: 10.1111/j.1582-4934.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lokmic Z. Mitchell G.M. Engineering the microcirculation. Tissue Eng Part B Rev. 2008;14:87. doi: 10.1089/teb.2007.0299. [DOI] [PubMed] [Google Scholar]
- 19.Kilroy G.E. Foster S.J. Wu X. Ruiz J. Sherwood S. Heifetz A. Ludlow J.W. Stricker D.M. Potiny S. Green P. Halvorsen Y.D. Cheatham B. Storms R.W. Gimble J.M. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 20.Altman A.M. Yan Y. Matthias N. Bai X. Rios C. Mathur A.B., et al. IFATS collection: human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27:250. doi: 10.1634/stemcells.2008-0178. [DOI] [PubMed] [Google Scholar]
- 21.Chen J. Li Y. Katakowski M. Chen X. Wang L. Lu D., et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 22.Li Y. McIntosh K. Chen J. Zhang C. Gao Q. Borneman J., et al. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y.L. Zhao Q. Qin X. Shen L. Cheng L. Ge J., et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 24.Min J.Y. Sullivan M.F. Yang Y. Zhang J.P. Converso K.L. Morgan J.P., et al. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg. 2002;74:1568. doi: 10.1016/s0003-4975(02)03952-8. [DOI] [PubMed] [Google Scholar]
- 25.Kawada H. Fujita J. Kinjo K. Matsuzaki Y. Tsuma M. Miyatake H., et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 26.Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 27.Li Y. Chen J. Zhang C.L. Wang L. Lu D. Katakowski M., et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 28.Boomsma R.A. Swaminathan P.D. Geenen D.L. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol. 2007;122:17. doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y. Shansky J. Del Tatto M. Ferland P. Wang X. Vandenburgh H. Recombinant vascular endothelial growth factor secreted from tissue-engineered bioartificial muscles promotes localized angiogenesis. Circulation. 2001;104:594. doi: 10.1161/hc3101.092215. [DOI] [PubMed] [Google Scholar]
- 30.Sprugel K.H. McPherson J.M. Clowes A.W. Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol. 1987;129:601. [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K.Y. Peters M.C. Mooney D.J. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Control Release. 2003;87:49. doi: 10.1016/s0168-3659(02)00349-8. [DOI] [PubMed] [Google Scholar]
- 32.Peters M.C. Isenberg B.C. Rowley J.A. Mooney D.J. Release from alginate enhances the biological activity of vascular endothelial growth factor. J Biomater Sci Polym Ed. 1998;9:1267. doi: 10.1163/156856298x00389. [DOI] [PubMed] [Google Scholar]
- 33.Ikada Y. Tabata Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31:287. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 34.Tabata Y. Nagano A. Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng. 1999;5:127. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 35.Murphy W.L. Peters M.C. Kohn D.H. Mooney D.J. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 36.Eiselt P. Kim B.S. Chacko B. Isenberg B. Peters M.C. Greene K.G., et al. Development of technologies aiding large-tissue engineering. Biotechnol Prog. 1998;14:134. doi: 10.1021/bp970135h. [DOI] [PubMed] [Google Scholar]
- 37.Elcin Y.M. Dixit V. Gitnick G. Controlled release of endothelial cell growth factor from chitosan-albumin microspheres for localized angiogenesis: in vitro and in vivo studies. Artif Cells Blood Substit Immobil Biotechnol. 1996;24:257. doi: 10.3109/10731199609117438. [DOI] [PubMed] [Google Scholar]
- 38.Wilcke I. Lohmeyer J.A. Liu S. Condurache A. Krüger S. Mailänder P., et al. VEGF(165) and bFGF protein-based therapy in a slow release system to improve angiogenesis in a bioartificial dermal substitute in vitro and in vivo. Langenbecks Arch Surg. 2007;392:305. doi: 10.1007/s00423-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 39.Arkudas A. Pryymachuk G. Hoereth T. Beier J.P. Polykandriotis E. Bleiziffer O. Horch R.E. Kneser U. Dose-finding study of fibrin gel-immobilized vascular endothelial growth factor 165 and basic fibroblast growth factor in the arteriovenous loop rat model. Tissue Eng Part A. 2009;15:2501. doi: 10.1089/ten.tea.2008.0477. [DOI] [PubMed] [Google Scholar]
- 40.Freedman S.B. Clinical trials of gene therapy for atherosclerotic cardiovascular disease. Curr Opin Lipidol. 2002;13:653. doi: 10.1097/00041433-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Morishita R. Perspective in progress of cardiovascular gene therapy. J Pharmacol Sci. 2004;95:1. doi: 10.1254/jphs.95.1. [DOI] [PubMed] [Google Scholar]
- 42.Shah P.B. Losordo D.W. Non-viral vectors for gene therapy: clinical trials in cardiovascular disease. Adv Genet. 2005;54:339. doi: 10.1016/S0065-2660(05)54014-8. [DOI] [PubMed] [Google Scholar]
- 43.Ozawa C.R. Banfi A. Glazer N.L. Thurston G. Springer M.L. Kraft P.E., et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bleiziffer O. Hammon M. Naschberger E. Lipnik K. Arkudas A. Rath S. Pryymachuk G. Beier J.P. Stürzl M. Horch R.E. Kneser U. Endothelial progenitor cells are integrated in newly formed capillaries and alter adjacent fibrovascular tissue after subcutaneous implantation in a fibrin matrix. J Cell Mol Med. 2011;15:2452. doi: 10.1111/j.1582-4934.2010.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ley C.D. Olsen M.W. Lund E.L. Kristjansen P.E. Angiogenic synergy of bFGF and VEGF is antagonized by angiopoietin-2 in a modified in vivo Matrigel assay. Microvasc Res. 2004;68:161. doi: 10.1016/j.mvr.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Rophael J.A. Craft R.O. Palmer J.A. Hussey A.J. Thomas G.P. Morrison W.A., et al. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. Am J Pathol. 2007;171:2048. doi: 10.2353/ajpath.2007.070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asahara T. Bauters C. Zheng L.P. Takeshita S. Bunting S. Ferrara N., et al. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:II365. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- 48.Richardson T.P. Peters M.C. Ennett A.B. Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 49.Cao R. Brakenhielm E. Pawliuk R. Wariaro D. Post M.J. Wahlberg E., et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 50.Moreno P.R. Purushothaman K.R. Sirol M. Levy A.P. Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 51.Khurana R. Simons M. Martin J.F. Zachary I.C. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 52.Horowitz J.R. Rivard A. van der Zee R. Hariawala M. Sheriff D.D. Esakof D.D., et al. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler Thromb Vasc Biol. 1997;17:2793. doi: 10.1161/01.atv.17.11.2793. [DOI] [PubMed] [Google Scholar]
- 53.Baumgartner I. Rauh G. Pieczek A. Wuensch D. Magner M. Kearney M., et al. Lower-extremity edema associated with gene transfer of naked DNA encoding vascular endothelial growth factor. Ann Intern Med. 2000;132:880. doi: 10.7326/0003-4819-132-11-200006060-00005. [DOI] [PubMed] [Google Scholar]
- 54.Epstein S.E. Kornowski R. Fuchs S. Dvorak H.F. Angiogenesis therapy: amidst the hype, the neglected potential for serious side effects. Circulation. 2001;104:115. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.