Abstract
Significance: Failure to maintain myoglobin (Mb) in the reduced state causes the formation of metMb, ferryl Mb species, and cross-linked Mb. Dissociation of ferriprotoporphyrin IX from the globin and release of iron atoms can also occur as oxidized Mb accumulates. These modifications may contribute to various oxidative pathologies in muscle and muscle foods. Recent Advances: The mechanism of ferryl Mb-mediated oxidative damage to nearby structures has been partially elucidated. Dissociation of ferriprotoporphyrin IX from metMb occurs more readily at acidic pH values. The dissociated ferriprotoporphyrin IX (also called hemin) readily decomposes preformed lipid hydroperoxides to reactive oxygen species. Heme oxygenase as well as lipophilic free radicals can degrade the protoporphyrin IX moiety, which results in the formation of free iron. Critical Issues: The multiple pathways by which Mb can incur toxicity create difficulties in determining the major cause of oxidative damage in a particular system. Peroxides and low pH activate each of the oxidative Mb forms, ferriprotoporphyrin IX, and released iron. Determining the relative concentration of these species is technically difficult, but essential to a complete understanding of oxidative pathology in muscle tissue. Future Directions: Improved methods to assess the different pathways of Mb toxicity are needed. Although significant advances have been made in the understanding of Mb interactions with other biomolecules, further investigation is needed to understand the physical and chemical nature of these interactions. Antioxid. Redox Signal. 18, 2342–2351.
Introduction
Understanding the redox chemistry of myoglobin (Mb) is challenging due to the multiple forms that can simultaneously be present under oxidative conditions. These include O2(II)Mb, deoxy(II)Mb, met(III)Mb, cross-linked Mb, hemochrome, and hemichrome (see List of Definitions). Ferryl forms of Mb may also be present [Mb(IV)=O and Mb•+(IV)=O]. In addition, hemin (also termed ferriprotoporphyrin IX) can dissociate from the globin at low pH values found in muscle foods and at sites of inflammation and ischemia (68). Ferriprotoporphyrin IX is indicative of the protoporphyrin that contains a ferric iron (Fe3+) atom. Ferriprotoporphyrin IX dissocation from sperm whale metMb at 37°C is 140-fold faster at pH 5.0 compared to pH 7.0 (26). Ferriprotoporphyrin IX dissociation from Asian carp metMb at 4°C occurred readily at pH 5.5, while little dissociation occurred at pH 6.0 (74). Iron atoms can be released upon destruction of the protoporphyrin IX ring by hydrogen peroxide (H2O2) and lipophilic free radicals (LFR) (45). A minireview related to the role of released iron atoms from Mb in renal dysfunction is available (29).
It is also important to differentiate redox reactions of Mb from those of hemoglobin (Hb). The hemin affinity of metHb (and its subunits) is 27-fold to ∼3000-fold lower compared to metMb (27). Thus, Hb appears particularly suited to promote oxidative damage through release of its ferriprotoporphyrin IX moiety, whereas the situation with Mb is less clear. Mb can form a protein-bound heme adduct with oxidase activity (based on oxygen consumption) that greatly exceeds that of Hb (49). Nitrite facilitates the formation of a ferryl protein radical in the case of oxyMb, but not oxyHb (37).
Mb is reactive with various biomolecules, including preformed lipid hydroperoxides (LOOH), polyunsaturated fatty acids (PUFA), ascorbate, phenols, nitric oxide (NO), and copper. This review will focus on the wide range of redox reactions that involve Mb at various valence states as well as reactions involving dissociated hemin and liberated iron atoms.
Practical Considerations
Metal chelators can be used to probe the effect of iron released from Mb (20, 72, 75). However, electron donation from desferrioxamine, a metal chelator, can inhibit oxidative action of Mb by scavenging free radicals (36). Free radical scavenging by desferrioxamine can thus inhibit lipid oxidation by a mechanism independent from chelating iron that is released from protoporphyrin IX (61). This observation indicates that caution is necessary when interpreting effects of certain inhibitors that are errantly considered to be specific.
Ethanol can be used as a solvent for amphiphilic molecules (e.g., unsaturated aldehydes) that affect the redox properties of Mb. The ability of ethanol to reduce Mb(IV)=O to deoxy(II)Mb, producing acetaldehyde, has been reported (25). Thus, the effects of solvents should be considered when assessing oxidation–reduction reactions of Mb.
Mb rarely is obtained fully reduced due to some Mb oxidation during purification and handling. Reduction of metMb can be accomplished with sodium dithionite. However, during desalting, a mixture of dithionite, O2, and deoxy(II)Mb can facilitate rapid H2O2 formation that oxidizes Mb. Maintaining an anaerobic atmosphere during reduction followed by rapid gel filtration can minimize oxidation (15). A chromatographic procedure of resolving ferrous and ferric Mb is available (32).
Formation of superoxide radicals and H2O2 during Mb oxidation can hamper quantification of the rate of autoxidation, kox, a measure of met(III)Mb formation. H2O2 can react with deoxy(II)Mb to form mixtures of Mb(IV)=O and Mb(III). The optical absorbance spectra in the UV and visible regions of Mb(IV)=O and met(III)Mb is similar, which makes it challenging to quantify the concentration of met(III)Mb in the presence of Mb(IV)=O. Superoxide dismutase and catalase (3 mmol/mol of heme) can be added to Mb solutions to scavenge superoxide radicals and H2O2, respectively, so that conversion of deoxy(II)Mb to met(III)Mb can be quantified precisely without interference from Mb(IV)=O (15). Equations have been described to calculate the relative concentration of O2(II)Mb, met(III)Mb, and Mb(IV)=O utilizing optical absorbance values at 490, 560, and 580 nm in the event that no additional Mb forms are present (33). Adjustments of extinction coefficients for different Mb forms may be necessary when pH is varied (11). Hemichromes must also be accounted for when present (14). The presence of hemichrome can be assessed by adding excess dithionite to Mb buffered at pH 8, which will result in hemochrome spectra with a large peak at 560 nm (Table 1) if the nonreduced sample was a hemichrome. Electron paramagnetic resonance is useful in the detection of ferryl radical forms of Mb and hemichromes (53).
Table 1.
UV and Visible Optical Absorbance Spectra of Different Myoglobin Forms, Ferriprotoporphyrin IX (Hemin), and a Hemin Degradation Product
| Form | Soret peak (nm) | Visible range peak(s) (nm) | Visible range peak(s) (nm) | References |
|---|---|---|---|---|
| O2(II)Mb | 418 | 543 | 581 | (5) |
| deoxy(II)Mb | 435 | 560 | (5) | |
| met(III)Mb | 408 | 502 | 630 | (5) |
| Mb(IV)=O | 420 | 549 | 582 (shoulder) | (33, 55) |
| Crosslinked Mb (pH 7.0) | 405–408 | 589 | (50, 59) | |
| Crosslinked Mb (pH 1.9) | 398 | 482, 546 | 580, 720 | (59) |
| Hemichrome | 415 | 535 | 565 (shoulder) | (17) |
| Hemochromea | 529 | 558 | (53) | |
| CO(II)Mb | 424 | 540 | 579 | (5) |
| NO(II)Mb | 421 | 543 | 575 | (5) |
| CN(III)Mb | 423 | 540 | 560 (shoulder) | (5) |
| Sulf(II)Mb | 420 | 617 | (46) | |
| Sulf(III)Mb | 404 | 595 | 715 | (46) |
| Heminb | 384 | 508, 538 | 639 | (12) |
| Biliverdinc | 350 | 674 | (82) |
Reported for hemoglobin (Hb) β chain.
When present in 90% acetone, 8% water, and 2% HCl (12 N).
Biliverdin results from enzymatic reaction of heme oxygenase with hemin.
Mb, myoglobin.
Cross-linked forms of Mb can be detected utilizing high-performance liquid chromatography (HPLC) (59). H64Y/V68F apoMb can be used to measure dissociation of ferriprotoporphyin IX from metMb (26). The Tyr64 substitution causes there to be strong optical density at 600 nm upon binding of ferriprotoporphyrin IX. The Phe68 substitution increases protein stability. Measurement of iron that is released from degraded protoporphyrin IX can be assessed with bathophenanthroline disulfonic acid, assuming that a chelator with a higher affinity for iron is not present (65).
Structural Aspects of Mb and Nomenclature Considerations
Mammalian Mb is comprised of 153 amino acids and contains the protoporphyrin IX moiety. The central part of protoporphyrin IX contains iron that has six coordination sites. Four of the sites are occupied by nitrogen atoms of the protoporphyrin IX ring and one is attached to the proximal imidazole group of a histidine that is part of the globin (Fig. 1). The sixth site forms a complex with ligands, such as O2. The terms “heme” and “hemin” can be used to describe the protoporphyrin ring containing a ferrous (Fe2+) and ferric (Fe3+) iron atom, respectively. O2(II)Mb and deoxy(II)Mb contain heme and met(III)Mb contains hemin. The preferred nomenclature is ferroprotoporphyrin IX for heme and ferriprotoporphyrin IX for hemin (see List of Definitions). The term “heme degradation” implies that only ferroprotoporphyrin IX can be degraded which is not the case, and thus this nomenclature should be avoided. The term “hemin affinity” is often used when specifically referring to affinity of ferriprotoporphyrin IX for the globin of metMb.
FIG. 1.
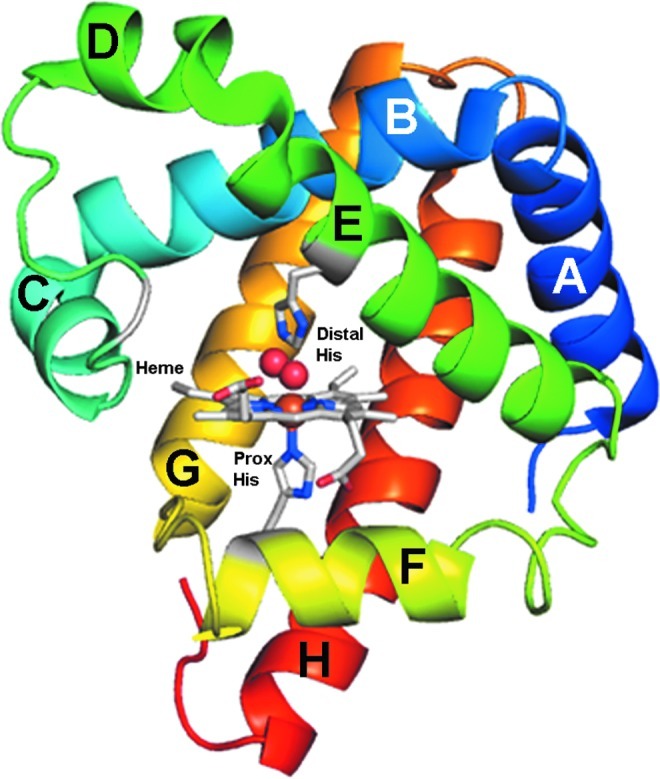
Ribbon representation of O2(II)Mb including the ferroprotoporphyrin IX moiety. Structural aspects of sperm whale O2(II)Mb are shown. The distal histidine, proximal histidine, and ferroprotoporphyrin IX (heme) moiety are shown in stick representation. Each helix is labeled. The iron atom in the center of the heme ring and ligand O2 are shown as spheres. The heme-6-propionate (H6P) group is to the left of the heme-7-propionate. The PDB structure 1MBO (51) was used to prepare the image shown using PyMOL software. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars. Mb, myoglobin; PDB, Protein Data Bank.
The polypeptide chain is organized as eight helices denoted A through H (Fig. 1). The proximal histidine that anchors the globin to the iron atom of protoporphyrin IX is at position F8, the eighth residue along the F helix. In sperm whale Mb, this is residue 93 in the polypeptide chain. The distal histidine which hydrogen bonds with ligands, such as O2, is at site E7, the seventh residue along the E helix. Nomenclature also exists for residues organized as coils between helices. For example, site CD3 represents the third residue between the C and D-helix. Arginine at CD3 in sperm whale Mb forms electrostatic contacts with the heme-6-propionate (H6P) group and His(FG3) forms electrostatic contacts with the heme-7-propionate (Fig. 1). The equilibrium association constants (μM−1) of Mb for O2, CO, and NO are 1, 27, and 2.2×105, respectively (48). Butyl isocyanide was shown to bond to the iron atom of ferriprotoporphyrin IX in Mb (70). This demonstrated that relatively large molecules can fit in the distal pocket of undenatured Mb.
Autooxidation of Mb
“Autooxidation” is the preferred terminology to describe spontaneous conversion of O2(II)Mb or deoxy(II)Mb to met(III)Mb. Shikama (66) describes inner sphere and outer sphere electron transfer mechanisms of Mb autooxidation. Early work describes the iron atom in oxyhemoglobin to be ferric, which may suggest that the O2 bound exists as the superoxide anion radical (80). Formation of met(III)Mb from O2(II)Mb is proton-mediated (15). Protons enter the heme pocket and protonate liganded O2. The positive charge on O2, from the protonation, causes a one-electron removal from the iron atom of ferroprotoporphyrin IX to be energetically favorable. The neutral superoxide radical then dissociates resulting in met(III)Mb formation (Reaction 1):
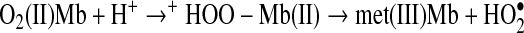 |
(Reaction 1) |
The neutral form of the distal histidine (E7) forms a hydrogen bond with liganded O2, which hinders protonation of bound O2 (Fig. 2). However, protonation of E7 due to lower pH will weaken this hydrogen bond by causing the cationic distal histidine to rotate out into solvent, which facilitates met(III)Mb formation. Thus, decreasing pH from 6 to 4 opened the distal histidine gate of CO(II)Mb (81). Protonation of the proximal histidine (F8) disrupts the heme-globin linkage, which can increase access of protons and water to the heme cleft. Protonation of the H6P will also weaken the heme-globin linkage by decreasing the electrostatic interaction of H6P with site CD3, which is a lysine or arginine residue in most Mbs. It should be noted that the distance between H6P and CD3 is 3.8 Å at pH 8.0 and increases to 9.0 Å at pH 5.7 in perch Hb, which shows extremely high rates of autooxidation under acidic conditions (6).
FIG. 2.
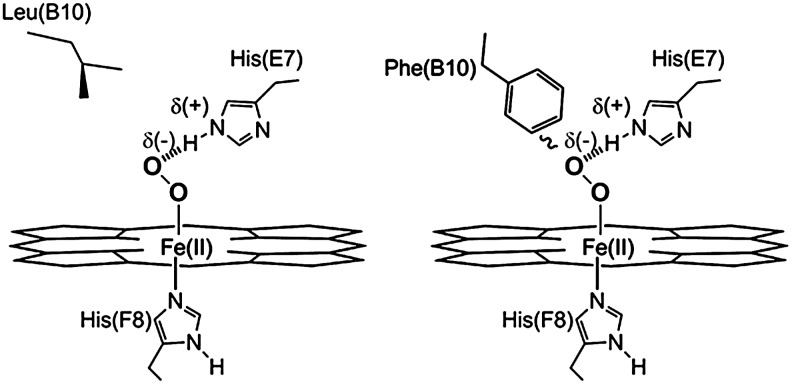
Amino acid substitution of Leu29 with phenylalanine increases O2 affinity. Phenylalanine at site B10 stabilizes O2 that is liganded to the iron atom of ferroprotoporphyrin IX in Mb. L29F is used to describe this Mb mutant because site B10 is the 29th residue in sperm whale Mb. Leucine at B10 in native Mb does not stabilize O2 to the iron atom of ferroprotoporphyrin IX, which results in lower O2 affinity. Image is adapted from ref. (63).
Deoxy(II)Mb reacts with O2 to produce met(III)Mb and superoxide radical by a bimolecular outersphere mechanism (15). Low oxygen partial pressures will facilitate formation of met(III)Mb from deoxy(II)Mb (Reaction 2):
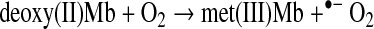 |
(Reaction 2) |
Conversely, increasing oxygen affinity decreases met(III)Mb formation as can be demonstrated by site-directed mutagenesis at site B10, which is residue 29 and the 10th residue along the B helix (Fig. 1). Substitution of leucine at site B10 with phenylalanine (L29F) decreased the equilibrium O2 dissociation constant of Mb 16-fold and decreased met(III)Mb formation 10-fold (15). The partial positive edge of the benzyl side chain of phenylalanine at B10 stabilizes the bond between O2 and the iron atom of the heme (Fig. 2). Phe(B10) may also prevent protonation of liganded O2 by steric hindrance. These effects greatly reduce met(III)Mb formation.
As O2(II)Mb and deoxy(II)Mb oxidation proceeds, superoxide anion radicals 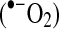 and neutral superoxide radicals
and neutral superoxide radicals
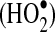 are released, reacting with each other (and protons) to form H2O2 (Reactions 3–5). The pKa for the acid/base pair of superoxide radical is ∼5, and thus pH will dictate whether
are released, reacting with each other (and protons) to form H2O2 (Reactions 3–5). The pKa for the acid/base pair of superoxide radical is ∼5, and thus pH will dictate whether
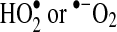 is the dominant by-product of Mb autooxidation. Note the rapid rate of H2O2 formation from
is the dominant by-product of Mb autooxidation. Note the rapid rate of H2O2 formation from
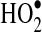 compared to
compared to 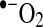 shown in Reactions 3–5 (24). Enzymatic conversion from superoxide radical to H2O2 by superoxide dismutase also occurs. Activated leukocytes can generate H2O2 concentrations up to 200 μM in vitro (1).
shown in Reactions 3–5 (24). Enzymatic conversion from superoxide radical to H2O2 by superoxide dismutase also occurs. Activated leukocytes can generate H2O2 concentrations up to 200 μM in vitro (1).
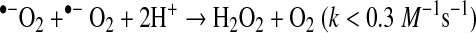 |
(Reaction 3) |
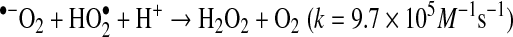 |
(Reaction 4) |
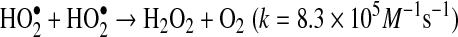 |
(Reaction 5) |
NADH cytochrome b5 reductase converts met(III)Mb to deoxy(II)Mb (23). An active b5 reductase can cause Mb to be a continuous source of H2O2. This is because the reduction of met(III)Mb to deoxy(II)Mb will result in subsequent Mb autooxidation that provides additional superoxide radicals as each equivalent of met(III)Mb formation occurs. One function of NADPH cytochrome P450 reductase is to convert O2 to superoxide anion radical (34). NADH oxidase of activated granulocytes converts O2 to superoxide radical (78). Xanthine oxidase utilizes O2, water, and xanthine (or hypoxanthine) to generate H2O2 (12).
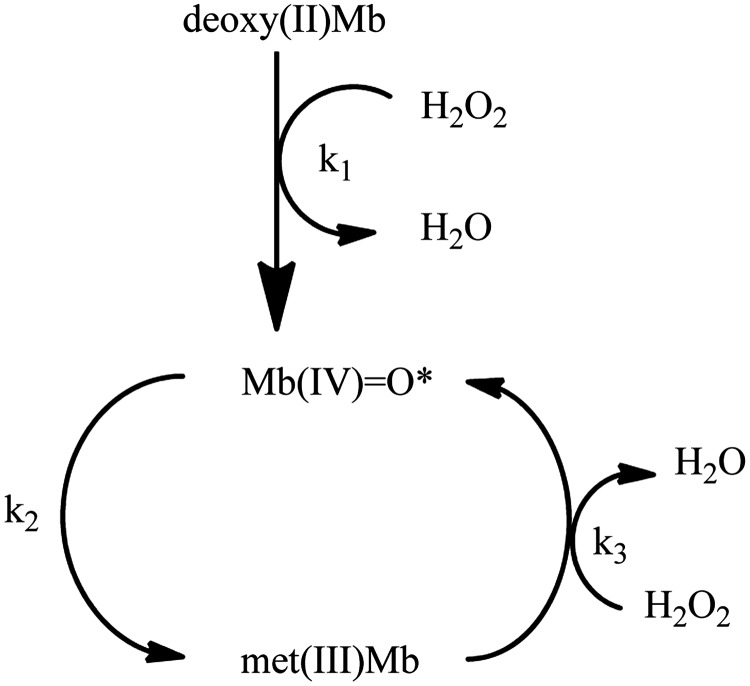
Reaction of Mb with H2O2
Reviews regarding reactions of Mb and Hb with H2O2 are available (56, 58). A mechanism involving three steps was described when 2.5-fold excess of H2O2 was added to O2(II)Mb at pH 7.0 (1). Step 1 is oxidation of deoxy(II)Mb to Mb(IV)=O, followed by autoreduction to met(III)Mb. Met(III)Mb then reacts with an additional H2O2 molecule to regenerate Mb(IV)=O, creating a pseudoperoxidase catalytic cycle (Fig. 3). There can be complexity to the ferryl species formed as described below.
FIG. 3.
Pseudoperoxidase cycle involving Mb and H2O2. Deoxy(II)Mb reacts with hydrogen peroxide (H2O2) resulting in a Mb(IV)=O and water. Mb(IV)=O undergoes auto-reduction to met(III)Mb. Met(III)Mb reacts with an additional H2O2 molecule resulting in regeneration of Mb(IV)=O. *Formation of Mb(IV)=O after reaction of met(III)Mb with H2O2 is often described as coupled with the formation of a porphyrin or protein ferryl Mb radical. Reaction scheme is adapted from ref. (1).
The reaction of met(III)Mb with H2O2 results in formation of a ferryl Mb radical that can initially be cationic (Reaction 6) (34). Reduction of H2O2 to water requires two electrons. One electron is from the ferric iron atom of met(III)Mb and the second electron comes from oxidizing the globin. Oxidation of the globin causes formation of a free radical on the protoporphyrin IX ring or an amino acid of Mb.
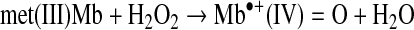 |
(Reaction 6) |
A peroxyl protein radical (denoted as ROO• and not to be confused with a lipid peroxyl radical) can result from a reaction of the neutral ferryl Mb radical with O2. Trp14 in Mb is oriented coplanar with the porphyrin ring and readily forms ROO• (58). Tyrosine residues generally form nonperoxyl Mb radicals.
The reaction of met(III)Mb with H2O2 induce Mb(IV)=O formation, coupled with the formation of Mb•+(IV)=O, in which protonation of Mb(IV)=O generate an Mb(III) radical that oxidizes exogenous substrates or internal amino acid side chains, regenerating met(III)Mb (60). A pK of ∼4.7 was determined for the protonation of Mb(IV)=O (67). The ability of H2O2 to promote formation of cross-linked Mb was described as pH decreased from 7 to 4 (59). One pathway involves protonation of •+Mb(IV)=O. •Mb(IV)-OH then rearranges to form a diradical Mb(III) species that becomes cross linked upon protonation. Cross-linked Mb is a green pigment, which may be due to disruption of the conjugation of one of the pyrrole rings. The distal histidine (His64) has been implicated as a key residue that facilitates formation of cross-linked Mb rather than Tyr103 (57).
Cross-linked Mb was shown to facilitate lipid oxidation in low-density lipoproteins (LDL) and pure phospholipids more readily compared to native Mb at pH 7.4 (76). Conversely, cross-linked Mb resulting from reaction of Mb(III) with H2O2 did not promote lipid oxidation in linoleic acid micelles in the pH range of 5.5–6.5 (9). At pH 7.4, addition of met(III)Mb to linoleate containing H2O2 resulted in competitive formation of Mb•(IV)=O and a Mb hemichrome that was unable to promote lipid oxidation (10). The maximal yield of cross-linked Mb from O2(II)Mb and met(III)Mb was around 29% and 37%, respectively, at a five-fold excess of H2O2 (59). An extinction coefficient of 76 mM−1 cm−1 was reported for cross-linked Mb at 408 nm (76). Thus, a decrease in the Soret peak may be due to conversion of O2(II)Mb (ɛ mM−1 cm−1 ∼157) and met(III)Mb (ɛmM−1 cm−1 ∼188) to cross-linked Mb. However, the potential for ferriprotoporphyrin IX dissociation from the globin and protoporphyrin IX degradation should also be assessed, both of which will decrease the Soret peak.
Reaction of Mb with LOOH
Heterolytic and hemolytic cleavage of LOOH by met(III)Mb is expressed as follows:
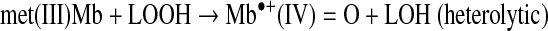 |
(Reaction 7) |
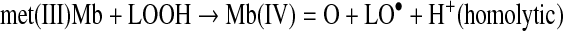 |
(Reaction 8) |
Sperm whale met(III)Mb catalyzed twice as much heterolytic as homolytic scission of a tert-butyl type LOOH at pH 7.4 (2). In the heterolytic mechanism, transfer of electrons from ferriprotoporphyrin IX to the oxygen is facilitated by electron release from the proximal histidine-iron bond and by proton transfer to the departing oxygen from the protonated distal histidine (52). Formation of a peroxyl radical (LOO•) is also possible by the ferryl species that is formed by the heterolytic scission (2). The alkoxyl radical (LO•) readily undergoes intramolecular cyclization to expoxyallylic radical that couples with O2 to form the epoxyperoxyl radical; the steady state concentration of LO• was described to be low relative to the epoxy radical forms (42).
Dissociation of Ferriprotoporphyrin IX (hemin) from met(III)Mb
Acidic conditions and oxidation of Mb dramatically decrease the ability of Mb to retain the protoporphyrin IX moiety. Dissociation of ferriprotoporphyrin IX from met(III)Mb at 37°C was 140-fold faster at pH 5.0 compared to pH 7.0 (26). Protonation of the proximal histidine (F8) disrupts coordination with the iron atom of ferriprotoporphyrin IX, whereas the stronger covalent bond in deoxy(II)Mb and O2(II)Mb prevents protonation until the pH drops below 3.0. Dissociation of ferroprotoporphyrin IX from deoxy(II)Mb is mediated by global unfolding and solvation of ferroprotoporphyrin IX, which disrupts the ferrous iron-proximal histidine bond (73).
Decreasing hemin affinity of met(III)Mb by site-directed mutagenesis [His(FG3)97Ala] increased lipid oxidation in washed muscle fibers at pH 5.7, whereas increasing hemin affinity [Val(E11)68Thr] decreased lipid oxidation (22). The relatively small and apolar alanine substitution at FG3 negates the electrostatic or hydrogen bonding interaction of His97 with the heme-7-propionate (Fig. 4), decreasing hemin affinity 39-fold (28). The threonine substitution at E11 stabilizes coordinated water in met(III)Mb increasing hemin affinity 25-fold compared to wild-type Mb that contains valine at E11, which cannot hydrogen bond with the water (Fig. 4). The ability of dissociated ferriprotoporphyrin IX to react with LOOH and facilitate formation of LO• and LOO• is as follows:
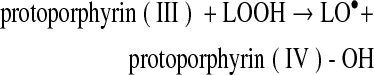 |
(Reaction 9) |
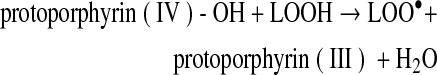 |
(Reaction 10) |
FIG. 4.
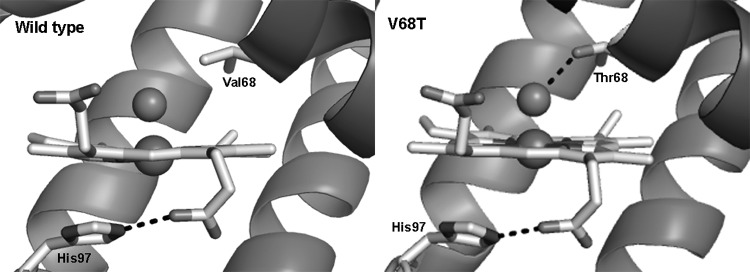
Amino acid substitutions of Val68 and His97 alters hemin affinity in met(III)Mb. Hemin affinity of met(III)Mb is decreased 39-fold by substitution of the native His97(FG3) with alanine. Substitution with Ala97 negates the electrostatic or hydrogen bonding interaction of His97 with the heme-7-propionate, which decreases hemin affinity. Hemin affinity is increased 25-fold by substitution of the native Val68(E11) with threonine. Thr68 hydrogen bonds with liganded water in met(III)Mb, which increases hemin affinity since the native Val68 cannot hydrogen bond with the water. The O atom of the threonine side chain hydrogen bonds to the H atom of the water molecule. The PDB structures, 1MYG (47) and 1MNK (69), were used to prepare the images shown using PyMOL software.
This cycling of ferric and ferryl protoporphyrin IX suggests that the protoporphyrin moiety can act pseudo-catalytically as long as LOOH is available. However, LO• and LOO• (Reactions 9 and 10) degrade the porphyrin moiety, which would negate pseudo-catalytic activity (45). Protoporphyrin IX is an amphiphilic molecule, which favors incorporation into the phospholipid bilayer, where preformed LOOH are located. Acidic pH may be necessary for ferriprotoporphyrin IX that dissociates from metMb to intercalate into phospholipids because apoMb effectively removed hemin from liposomes at pH 8.0 (40, 41). At the same time, met(III)Mb was reported to transfer some ferriprotoporphyrin IX into LDL at pH 7.4 in the presence of H2O2, leading to apoB and LDL oxidation (21). Thus, dissociated ferriprotoporphyrin IX from metMb is a relevant oxidant in various biological systems; however, other reactive forms of Mb and iron derived from the degraded porphyrin of Mb should also be considered. The much lower hemin affinity of Hb compared to Mb (27-fold to ∼3000-fold lower) also merits consideration (27). Thus, Hb represents a larger pool of protoporphyrin IX and iron than Mb.
The ability of ferriprotoporphyrin IX intercalated in endothelial cells to promote lipid oxidation was facilitated by the addition of H2O2 or activated leukocytes (8). A multitude of interactions involving ferriprotoporphyrin IX, ferroprotoporphyrin IX, and H2O2 that can facilitate lipid oxidation have been postulated, including the ability of dissociated ferryl protoporphyrin IX to be an initiator of lipid oxidation (64).
Release of Iron Atoms from Mb
Degradation of protoporphyrin IX will release iron atoms that potentially can participate in redox reactions. In biological systems, iron atoms are complexed to a chelator, which can enhance or decrease reactivity of the metal. Amino acids, peptides, proteins, negatively charged phospholipids, and nucleotides are common iron chelators. A review describing the roles of different buffers and chelators on iron autoxidation, iron valency state, and free radical generation is available (79). The ability of iron released from Mb to facilitate oxidative reactions compared to other oxidative forms of Mb (e.g., ferryl Mb) and dissociated ferriprotoporphyrin IX should be further investigated. Interestingly, a Mb mutant more susceptible to protoporphyrin IX degradation and iron release (H64Q/L29F) promoted lipid oxidation in washed muscle less effectively compared to wild-type Mb at pH 5.7 (22). The exceptional ability of H64Q/L29F to remove H2O2 produced by autoxidation should also inhibit lipid oxidation (1). Light emitted from Hg-Ag Oriel pen lights was used to degrade ferriprotoporphryin IX of metMb and release iron, which negated the ability of metMb to promote lipid oxidation in linoleic acid micelles (43). It is interesting to note that soluble and bound iron in sarcoplasmic reticulum were equally effective at promoting lipid oxidation (31).
In biological systems, the apo form of heme oxygenase will convert ferriprotoporphyyrin IX to carbon monoxide, iron, and biliverdin (Table 1). Biliverdin reductase will convert biliverdin to bilirubin, which has free radical scavenging properties. Transferrin can chelate the released iron atoms, rendering the metal weakly reactive. Characterizing the redox reaction of the many possible low molecular weight iron complexes is beyond the scope of this review. Some relevant reactions of ferrous(II) and ferric(III) iron are as follows (24):
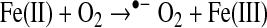 |
(Reaction 11) |
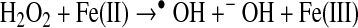 |
(Reaction 12) |
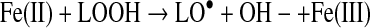 |
(Reaction 13) |
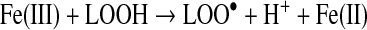 |
(Reaction 14) |
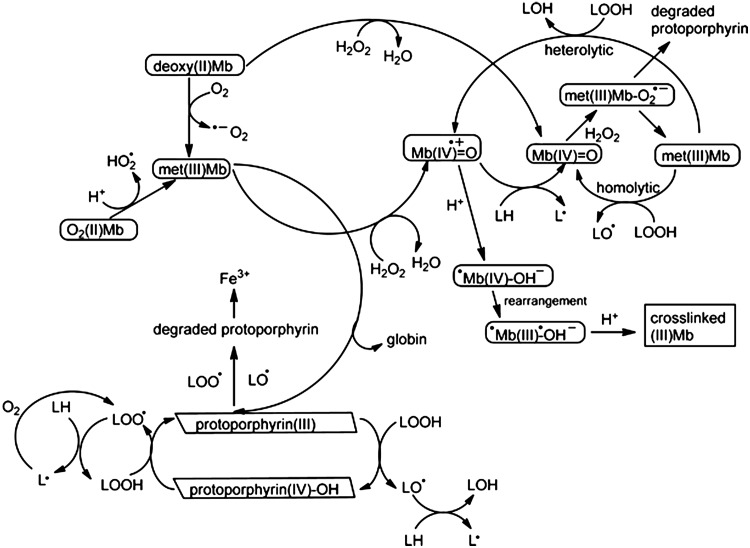
A major challenge is to separate effects from the many reactions that involve LOOH dec omposition. Decomposition of LOOH by Fe(II) was described to occur faster compared to Fe(III) (24). Reaction of Fe(II) with LOOH was 20-fold faster compared to H2O2 (24). It remains unknown if iron atoms derived from Mb facilitate any meaningful degree of oxidation in biological systems compared to other reactants, such as dissociated ferriprotoporphyrin IX or cross-linked Mb. A related challenge that remains is to separate effects from met(III)Mb, ferryl Mb species, cross-linked Mb, dissociated ferriprotoporphyrin IX, and iron derived from destruction of protoporphyrin IX. The fact that ferriprotoporphyrin IX-mediated decomposition of LOOH results in lipid radicals that (i) oxidize lipids, and (ii) degrade the protoporphyrin ring, which liberates iron atoms, makes it difficult to discern between ferriprotoporphyrin IX and iron-mediated lipid oxidation. Decreases in the optical density of the Soret peak can be indicative of cross-linked Mb formation, ferriprotoporphyrin IX dissociation from the globin, and degradation of the protoporphyrin ring. A schematic representation of various redox reactions involving Mb and Mb degradation products is shown in Figure 5.
FIG. 5.
Reaction pathways for O2(II)Mb, deoxy (II)Mb, met (III)Mb, ferryl Mb species, and protoporphyrin IX. Various pathways lead to hypervalent states of Mb with the concomitant generation of hydrophilic and hydrophobic oxidants. Oxidant challenge results in formation of met(III)Mb, cross-linked Mb, dissociation of ferriprotoporphyrin IX, and degradation of the protoporphyrin ring, which releases iron atoms. Phagocytes can facilitate bursts of H2O2 that degrade the protoporphyrin. LH represents a polyunsaturated fatty acid. LOOH represents a lipid hydroperoxide. Cross linking of Mb, dissociation of ferriprotoporphyrin IX, and ferryl Mb-mediated lipid oxidation occur more readily at reduced pH values. Image is adapted from ref. (62).
Reactions of Mb with Other Biomolecules
Mb•+(IV)=O and Mb•(IV)=O can abstract a hydrogen atom from polyunsaturated fatty acids (LH), which is an initiating step of lipid oxidation (35):
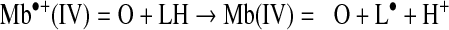 |
(Reaction 15) |
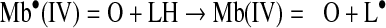 |
(Reaction 16) |
The protonated form of Mb(IV)=O can be denoted as Mb(IV)-OH− and is considered much more reactive with LH than the unprotonated form (58).
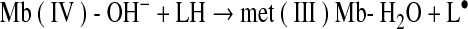 |
(Reaction 17) |
Lipid radicals (LO• and LOO•) that result from met(III)Mb and hemin-mediated decomposition of LOOH (Fig. 5) also are capable of hydrogen abstraction from LH (16). The electrostatic charge of the lipid phase (or domains in the lipid phase) may be an important factor that affects the ability of Mb to promote lipid oxidation. Cytochrome c-promoted lipid oxidation occurs more effectively in negatively charged liposomes than in neutral and positively charged liposomes at pH 7.4 (54). Mbs have a net positive charge at acidic to neutral pH values.
NO reacts very rapidly with O2(II)Mb to generate met(III)Mb and nitrate (19). NO(II)Mb reacts very slowly with O2 causing formation of met(III)Mb and nitrate. This autooxidation of NO(II)Mb is the result of slow dissociation, O2 binding, and subsequent dioxygenation of the released NO, producing a low-spin ferric Mb dihistidyl hemichrome (7). NO was shown to reduce activated Mb(IV)=O to met(III)Mb with dependence on NO and H2O2 concentration (18).
Ascorbate can reduce Mb(IV)=O to met(III)Mb with formation of the dehydroascorbyl radical (39). Met(III)Mb can be reduced by ascorbate in the presence of O2 to O2(II)Mb and dehydroscorbate (4):
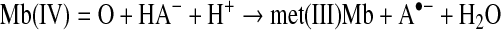 |
(Reaction 18) |
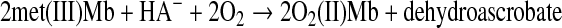 |
(Reaction 19) |
In the curing reaction of meat, added sodium nitrite (NaNO2) goes through a series of reactions that result in the formation of NO and met(III)Mb. Added sodium ascorbate as well as endogenous reductants in the muscle reduce met(III)Mb to deoxy(II)Mb. The simultaneously generated NO from nitrite binds to deoxy(II)Mb or can reductively nitrosylate met(III)Mb under anaerobic conditions, resulting in the formation of NO(II)Mb. NO(II)Mb is denatured during thermal processing, which produces a stable denatured hemochrome that along with Mb(II)CO and Mb(II)NO contributes to the characteristic pink color of cured meats. Residual Hb in the meat provides additional pigments for the curing reactions. Addition of ascorbate to Mb(III) containing LOOH decreased the proportion of homolytic scission products presumably by converting the alkoxyl radical intermediate (Fig. 5) to an alkoxyl anion (2).
The ability of phenols to convert O2(II)Mb to met(III)Mb is plausible considering that Hb oxidation by phenols was demonstrated previously at elevated pH with direct dependence on the concentration of O2(II)Hb (77). Apparently, the phenol can donate an electron to bound O2 within Hb (Reaction 20). This unstable intermediate is then converted to metHb and H2O2 (Reaction 21).
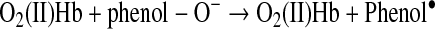 |
(Reaction 20) |
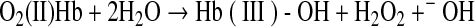 |
(Reaction 21) |
The ability of thymoquinone in the presence of GSH, NADH, and NADPH to reduce Mb(IV)=O and met(III)Mb to deoxy(II)Mb with subsequent oxygenation to O2(II)Mb has been described (38). Epigallocatechin gallate reduced Mb(IV)=O to met(III)Mb by an outer-sphere electron transfer mechanism based on enthalpy and entropy determinations (30).
Acid-catalyzed met(III)Mb formation due to NaCl was ascribed to the nature of the anion rather than the cation (3). Mammalian O2(II)Mb can be oxidized by copper ions with a dependence on binding of the metal to histidine residues in a species (pig, sperm whale, and horse)-specific manner (44). The lipid oxidation product, 4-hydroxy-2-nonenal (HNE), binds to His 24, 36, and 119 in pig Mb and His 24, 36, 81, 88, 93, 119, and 152 in bovine Mb (71). HNE binding to Mb has been shown to accelerate Mb oxidation.
Summary
The multiple pathways by which Mb can incur toxicity (Fig. 5) create a major challenge in determining which mechanism incurs the majority of oxidative damage in a particular system. The most oxidative form of Mb can be hiding in the weeds among all the other quantifiable species that include met(III)Mb, hemichromes, ferryl Mb forms, cross-linked Mb, dissociated ferriprotoporphyrin IX, and released iron. The fact that peroxides and acidic pH values activate nearly all of these species hampers differentiation. Rhabdomyolysis-induced renal failure is attenuated with acetaminophen, apparently by reducing ferryl Mb to metMb, yet other mechanisms may be involved (13). Iron atoms are often released due to the ability of dissociated ferriprotoporphyrin IX to decompose LOOH to LFR that degrade the protoporphyrin ring and initiate lipid oxidation. This degradation can cause an errant conclusion that iron atoms facilitated the oxidative damage observed. The ability of met(III)Mb to decompose preformed LOOH to free radicals that cause oxidative damage has received relatively little attention. This may be due to challenges of assessing this met(III)Mb pathway. Solubility issues can arise when working with LOOH as a reactant.
Future research should include developing improved methodology to assess specific mechanisms of Mb toxicity. Iron chelators can also function as free radical scavengers, and thus are not specific inhibitors. Apohemopexin can be used as a specific inhibitor of oxidative damage due to removal of free ferriprotoporphyrin IX (at appropriate pH and in the presence of sodium and chloride ions). HPLC can be used to measure cross-linked Mb, but this methodology is fairly tedious. Ferryl Mb species are transitory especially in the presence of reductants, which creates difficulties in quantitative measurements. The ability of Hb toxicity to compete with Mb toxicity must also be considered in studies when both heme proteins are present. Fish Hbs have very low hemin affinity at pH ∼6 compared to mammalian Hbs (6). The ability of dissociated protoporphyrin IX to incur toxicity can be examined using fish Hbs in model systems. Additional research is also needed to differentiate toxicity due to LOOH compared to H2O2.
Abbreviations Used
- Hb
hemoglobin
- HNE
4-hydroxy-2-nonenal
- H2O2
hydrogen peroxide
- H6P
heme-6-propionate
- HPLC
high-performance liquid chromatography
- LDL
low-density lipoproteins
- LFR
lipophilic free radicals
- LH
polyunsaturated fatty acid
- LOOH
lipid hydroperoxide
- Mb
myoglobin
- NO
nitric oxide
- PDB
Protein Data Bank
- PUFA
polyunsaturated fatty acid
Acknowledgments
This project was supported, in part, by the National Research Initiative Grant no. 2005-35503-16134 and 2007-35503-18482 from the USDA Cooperative State Research, Education, and Extension Service Improving Food Quality and Value program, and by the College of Agricultural and Life Sciences, University of Wisconsin-Madison, HATCH project 142PRJ28VZ.
Author Disclosure Statement
No competing financial interests exist.
List of Definitions
(II): The +2 valence state of the ferrous iron atom in the protoporphyrin of Mb.
(III): The +3 valence state of the ferric iron atom in the protoporphyrin of Mb.
(IV): The +4 valence state of the ferryl iron atom in the protoporphyrin of Mb.
O2(II)Mb: When O2 is liganded to the ferrous iron atom of the protoporphyrin in Mb. Another term is oxyMb.
Biliverdin: Green pigment produced from the reaction of apoheme oxygenase with ferriprotoporphyrin IX.
Choleglobin: Degradation product of Mb and Hb with green color that can result from gamma radiation. Ascorbic acid and H2O2 can facilitate choleglobin formation from Hb.
Cross-linked Mb: Protonation of the ferryl Mb cation radical results in formation of a ferryl radical anion that rearranges to form crosslinked Mb in which a carbon atom of the ferriprotoporphoryin IX is covalently bound to an amino acid side chain of the polypeptide.
Deoxy(II)Mb: When there is no ligand to the ferrous iron atom of the protoporphyrin in Mb; often a water molecule is coordinated in the distal heme pocket of deoxy(II)Mb.
Ferroprotoporphyrin IX: The ring structure in Mb containing ferrous (2+) iron atom. This can also be called heme. Ferroprotoporphyrin IX oxidizes nearly instantaneously upon dissociation from the globin to ferriprotoporphyrin IX.
Ferriprotoporphyrin IX: The ring structure in metMb containing a ferric (3+) iron atom. This can also be called hemin.
Heme: Term that is analogous to ferroprotoporphyrin IX.
Hemin: Term that is analogous to ferriprotoporphyrin IX. The term ‘hemin’ is specifically used when referring to the affinity of the globin for ferriprotoporphyrin IX (e.g., hemin affinity).
Hemichrome: when a nitrogen base forms a covalent bond with the ferric iron (Fe3+) atom of the hemin moiety within the globin.
Hemochrome: when a nitrogen base (often the distal histidine) forms a covalent bond with the ferrous iron (Fe2+) atom of the heme moiety with the globin.
Mb(IV)=O: Designation for ferryl Mb in which the iron atom of the protoporphyrin is in the ferryl (+4) oxidation state. Ferryl Mb can also exist as a cationic radical, a neutral radical, and as an anionic radical.
Met(III)Mb: The form of Mb in which ferric iron is present in the protoporphyrin; water is liganded at acidic and neutral pH values (aquomet), while hydroxide (hydroxymet) will be liganded to the ferric iron atom of met(III)Mb at elevated pH.
Sulf(II)Mb: Green–purple colored compound produced by incubation of ferrous Mb with hydrogen sulfide.
Sulf(III)Mb: Red colored compound produced by reaction of met(III)Mb with hydrogen sulfide.
References
- 1.Alayash AI. Ryan BA. Eich RF. Olson JS. Cashon RE. Reactions of sperm whale myoglobin with hydrogen peroxide. Effects of distal pocket mutations on the formation and stability of the ferryl intermediate. J Biol Chem. 1999;274:2029–2037. doi: 10.1074/jbc.274.4.2029. [DOI] [PubMed] [Google Scholar]
- 2.Allentoff AJ. Bolton JL. Wilks A. Thompson JA. Ortiz de Montellano PR. Heterolytic versus homolytic peroxide bond cleavage by sperm whale myoglobin and myoglobin mutants. J Am Chem Soc. 1992;114:9744–9749. [Google Scholar]
- 3.Andersen HJ. Bertelsen G. Skibsted LH. Salt effect on acid-catalyzed autoxidation of oxymyoglobin. Acta Chemica Scand A. 1988;42:226–236. [Google Scholar]
- 4.Andersen HJ. Skibsted LH. Kinetics and mechanism of thermal oxidation and photooxidation of nitrosylmyoglobin in aqueous solution. J Agric Food Chem. 1992;40:1741–1750. [Google Scholar]
- 5.Antoni E. Brunoni M. Hemoglobin and Myoglobin in Their Reactions with Ligands. Amsterdam, The Netherlands: North-Holland Publ Co; 1971. [Google Scholar]
- 6.Aranda R., IV He C. Worley CE. Levin E. Li R. Olson JS. Phillips GN., Jr. Richards MP. Structural analysis of fish versus mammalian hemoglobins: effects of the heme pocket environment on autooxidation and hemin loss rates. Proteins. 2009;75:217–230. doi: 10.1002/prot.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold EV. Bohle DS. Jordan PA. Reversible and irreversible hemichrome generation by the oxygenation of nitrosylmyoglobin. Biochemistry. 1999;38:4750–4756. doi: 10.1021/bi982729e. [DOI] [PubMed] [Google Scholar]
- 8.Balla G. Vercellotti GM. Muller-Eberhard U. Eaton J. Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64:648–655. [PubMed] [Google Scholar]
- 9.Baron CP. Skibsted LH. Andersen HJ. Prooxidative activity of myoglobin species in linoleic acid emulsions. J Agric Food Chem. 1997;45:1704–1710. [Google Scholar]
- 10.Baron CP. Skibsted LH. Andersen HJ. Peroxidation of linoleate at physiological pH: hemichrome formation by substrate binding protects against metmyoglobin activation by hydrogen peroxide. Free Radic Biol Med. 2000;28:549–558. doi: 10.1016/s0891-5849(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 11.Benesch RE. Benesch R. Yung S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal Biochem. 1973;55:245–248. doi: 10.1016/0003-2697(73)90309-6. [DOI] [PubMed] [Google Scholar]
- 12.Bir SC. Kolluru GK. Fang K. Kevil CG. Redox balance dynamically regulates vascular growth and remodeling. Semin Cell Dev Biol. 2012;23:745–757. doi: 10.1016/j.semcdb.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutaud O. Moore KP. Reeder BJ. Harry D. Howie AJ. Wang S. Carney CK. Masterson TS. Amin T. Wright DW. Wilson MT. Oates JA. Roberts LJ., 2nd Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci U S A. 2010;107:2699–2704. doi: 10.1073/pnas.0910174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle RC. Tappel AL. Tappel AA. Chen H. Andersen HJ. Quantitation of heme proteins from spectra of mixtures. J Agric Food Chem. 1994:100–104. [Google Scholar]
- 15.Brantley RE. Smerdon SJ. Wilkinson AJ. Singleton EW. Olson JS. The mechanism of autooxidation of myoglobin. J Biol Chem. 1993;268:6995–7010. [PubMed] [Google Scholar]
- 16.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 17.Culbertson DS. Olson JS. Role of heme in the unfolding and assembly of myoglobin. Biochemistry. 2010;49:6052–6063. doi: 10.1021/bi1006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dee G. Rice-Evans C. Obeyesekera S. Meraji S. Jacobs M. Bruckdorfer KR. The modulation of ferryl myoglobin formation and its oxidative effects on low density lipoproteins by nitric oxide. FEBS Lett. 1991;294:38–42. doi: 10.1016/0014-5793(91)81338-9. [DOI] [PubMed] [Google Scholar]
- 19.Eich RF. Li T. Lemon DD. Doherty DH. Curry SR. Aitken JF. Mathews AJ. Johnson KA. Smith RD. Phillips GN., Jr. Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 20.Engelmann MD. Bobier RT. Hiatt T. Cheng IF. Variability of the Fenton reaction characteristics of the EDTA, DTPA, and citrate complexes of iron. Biometals. 2003;16:519–527. doi: 10.1023/a:1023480617038. [DOI] [PubMed] [Google Scholar]
- 21.Grinshtein N. Bamm VV. Tsemakhovich VA. Shaklai N. Mechanism of low-density lipoprotein oxidation by hemoglobin-derived iron. Biochemistry. 2003;42:6977–6985. doi: 10.1021/bi020647r. [DOI] [PubMed] [Google Scholar]
- 22.Grunwald EW. Richards MP. Studies with myoglobin variants indicate that released hemin is the primary promoter of lipid oxidation in washed fish muscle. J Agric Food Chem. 2006;54:4452–4460. doi: 10.1021/jf0603228. [DOI] [PubMed] [Google Scholar]
- 23.Hagler L. Coppes RI., Jr. Herman RH. Metmyoglobin reductase. Identification and purification of a reduced nicotinamide adenine dinucleotide-dependent enzyme from bovine heart which reduces metmyoglobin. J Biol Chem. 1979;254:6505–6514. [PubMed] [Google Scholar]
- 24.Halliwell B. Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford University Press; 1999. p. 62. [Google Scholar]
- 25.Harada K. Tamura M. Yamazaki I. The 2-electron reduction of sperm whale ferryl myoglobin by ethanol. J Biochem. 1986;100:499–504. doi: 10.1093/oxfordjournals.jbchem.a121739. [DOI] [PubMed] [Google Scholar]
- 26.Hargrove MS. Singleton EW. Quillin ML. Ortiz LA. Phillips GN., Jr. Olson JS. Mathews AJ. His64(E7)→Tyr apomyoglobin as a reagent for measuring rates of hemin dissociation. J Biol Chem. 1994;269:4207–4214. doi: 10.2210/pdb1mgn/pdb. [DOI] [PubMed] [Google Scholar]
- 27.Hargrove MS. Whitaker T. Olson JS. Vali RJ. Mathews AJ. Quaternary structure regulates hemin dissociation from human hemoglobin. J Biol Chem. 1997;272:17385–17389. doi: 10.1074/jbc.272.28.17385. [DOI] [PubMed] [Google Scholar]
- 28.Hargrove MS. Wilkinson AJ. Olson JS. Structural factors governing hemin dissociation from metmyoglobin. Biochemistry. 1996;35:11300–11309. doi: 10.1021/bi960372d. [DOI] [PubMed] [Google Scholar]
- 29.Holt S. Moore K. Pathogenesis of renal failure in rhabdomyolysis: the role of myoglobin. Exp Nephrol. 2000;8:72–76. doi: 10.1159/000020651. [DOI] [PubMed] [Google Scholar]
- 30.Hu M. Skibsted LH. Kinetics of reduction of ferrylmyoglobin by (-)-epigallocatechin gallate and green tea extract. J Agric Food Chem. 2002;50:2998–3003. doi: 10.1021/jf011535u. [DOI] [PubMed] [Google Scholar]
- 31.Huang CH. Hultin HO. Soluble and bound iron equally effect lipid oxidation of sarcoplasmic reticulum. J Food Biochem. 1992;16:1–13. [Google Scholar]
- 32.Huisman TH. Studies on the heterogeneity of hemoglobin. XI. Chromatographic studies of intermediate forms of oxy- and ferrihemoglobin. Arch Biochem Biophys. 1966;113:427–434. doi: 10.1016/0003-9861(66)90209-8. [DOI] [PubMed] [Google Scholar]
- 33.Jourd'heuil D. Mills L. Miles AM. Grisham MB. Effect of nitric oxide on hemoprotein-catalyzed oxidative reactions. Nitric Oxide. 1998;2:37–44. doi: 10.1006/niox.1998.0167. [DOI] [PubMed] [Google Scholar]
- 34.Kanner J. German JB. Kinsella JE. Initiation of lipid peroxidation in biological systems. Crit Rev Food Sci Nutr. 1987;25:317–364. doi: 10.1080/10408398709527457. [DOI] [PubMed] [Google Scholar]
- 35.Kanner J. Harel S. Initiation of membranal lipid peroxidation by activated metmyoglobin and methemoglobin. Arch Biochem Biophys. 1985;237:314–321. doi: 10.1016/0003-9861(85)90282-6. [DOI] [PubMed] [Google Scholar]
- 36.Kanner J. Harel S. Desferrioxamine as an electron donor. Inhibition of membranal lipid peroxidation initiated by H2O2-activated metmyoglobin and other peroxidizing systems. Free Radic Res Commun. 1987;3:1–5. doi: 10.3109/10715768709069798. [DOI] [PubMed] [Google Scholar]
- 37.Keszler A. Mason RP. Hogg N. Immuno-spin trapping of hemoglobin and myoglobin radicals derived from nitrite-mediated oxidation. Free Radic Biol Med. 2006;40:507–515. doi: 10.1016/j.freeradbiomed.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Khalife KH. Lupidi G. Reduction of hypervalent states of myoglobin and hemoglobin to their ferrous forms by thymoquinone: the role of GSH, NADH and NADPH. Biochim Biophys Acta. 2008;1780:627–637. doi: 10.1016/j.bbagen.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Kroger-Ohlsen M. Skibsted LH. Kinetics and mechanism of reduction of ferrylmyoglobin by ascorbate and D-isoascorbate. J Agric Food Chem. 1997;45:668–676. [Google Scholar]
- 40.Light WR. Olson JS., 3rd The effects of lipid composition on the rate and extent of heme binding to membranes. J Biol Chem. 1990;265:15632–15637. [PubMed] [Google Scholar]
- 41.Light WR. Olson JS., 3rd Transmembrane movement of heme. J Biol Chem. 1990;265:15623–15631. [PubMed] [Google Scholar]
- 42.Marnett LJ. Wilcox AL. The chemistry of lipid alkoxyl radicals and their role in metal-amplified lipid peroxidation. Biochem Soc Symp. 1995;61:65–72. doi: 10.1042/bss0610065. [DOI] [PubMed] [Google Scholar]
- 43.Mikkelsen A. Sosniecki L. Skibsted LH. Myoglobin catalysis in lipid oxidation. Z Lebensm Unters Forsch. 1992;195:228–234. [Google Scholar]
- 44.Moiseeva SA. Postnikova GB. Mechanism of oxidation of oxymyoglobin by copper ions: comparison of sperm whale, horse, and pig myoglobins. Biochemistry (Mosc) 2001;66:780–787. doi: 10.1023/a:1010268813926. [DOI] [PubMed] [Google Scholar]
- 45.Nagababu E. Rifkind JM. Heme degradation by reactive oxygen species. Antioxid Redox Signal. 2004;6:967–978. doi: 10.1089/ars.2004.6.967. [DOI] [PubMed] [Google Scholar]
- 46.Nicholls P. The formation and properties of sulphmyoglobin and sulphcatalase. Biochem J. 1961;81:374–383. doi: 10.1042/bj0810374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oldfield TJ. Smerdon SJ. Dauter Z. Petratos K. Wilson KS. Wilkinson AJ. High-resolution X-ray structures of pig metmyoglobin and two CD3 mutants: Mb(Lys45—Arg) and Mb(Lys45—Ser) Biochemistry. 1992;31:8732–8739. doi: 10.1021/bi00152a008. [DOI] [PubMed] [Google Scholar]
- 48.Olson JS. Phillips GN., Jr. Myoglobin discriminates between O2, NO, and CO by electrostatic interactions with the bound ligand. JBIC. 1997;2:544–552. [Google Scholar]
- 49.Osawa Y. Darbyshire JF. Meyer CA. Alayash AI. Differential susceptibilities of the prosthetic heme of hemoglobin-based red cell substitutes. Implications in the design of safer agents. Biochem Pharmacol. 1993;46:2299–2305. doi: 10.1016/0006-2952(93)90621-3. [DOI] [PubMed] [Google Scholar]
- 50.Osawa Y. Williams MS. Covalent crosslinking of the heme prosthetic group to myoglobin by H2O2: toxicological implications. Free Radic Biol Med. 1996;21:35–41. doi: 10.1016/0891-5849(95)02215-5. [DOI] [PubMed] [Google Scholar]
- 51.Phillips SE. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980;142:531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- 52.Poulos TL. Kraut J. The stereochemistry of peroxidase catalysis. J Biol Chem. 1980;255:8199–8205. [PubMed] [Google Scholar]
- 53.Rachmilewitz EA. Peisach J. Blumberg WE. Studies on the stability of oxyhemoglobin A and its constituent chains and their derivatives. J Biol Chem. 1971;246:3356–3366. [PubMed] [Google Scholar]
- 54.Radi R. Turrens JF. Freeman BA. Cytochrome c-catalyzed membrane lipid peroxidation by hydrogen peroxide. Arch Biochem Biophys. 1991;288:118–125. doi: 10.1016/0003-9861(91)90172-f. [DOI] [PubMed] [Google Scholar]
- 55.Rao SI. Wilks A. Hamberg M. Ortiz de Montellano PR. The lipoxygenase activity of myoglobin. J Biol Chem. 1994;269:7210–7216. [PubMed] [Google Scholar]
- 56.Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal. 2010;13:1087–1123. doi: 10.1089/ars.2009.2974. [DOI] [PubMed] [Google Scholar]
- 57.Reeder BJ. Cutruzzola F. Bigotti MG. Watmough NJ. Wilson MT. Histidine and not tyrosine is required for the peroxide-induced formation of haem to protein cross-linked myoglobin. IUBMB Life. 2007;59:477–489. doi: 10.1080/15216540601178083. [DOI] [PubMed] [Google Scholar]
- 58.Reeder BJ. Svistunenko DA. Cooper CE. Wilson MT. The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology. Antioxid Redox Signal. 2004;6:954–966. doi: 10.1089/ars.2004.6.954. [DOI] [PubMed] [Google Scholar]
- 59.Reeder BJ. Svistunenko DA. Sharpe MS. Wilson MT. Characteristics and mechanism of formation of peroxide-induced heme to protein cross-linking in myoglobin. Biochemistry. 2002;41:367–375. doi: 10.1021/bi011335b. [DOI] [PubMed] [Google Scholar]
- 60.Reeder BJ. Wilson MT. The effects of pH on the mechanism of hydrogen peroxide and lipid hydroperoxide consumption by myoglobin: a role for the protonated ferryl species. Free Radic Biol Med. 2001;30:1311–1318. doi: 10.1016/s0891-5849(01)00534-2. [DOI] [PubMed] [Google Scholar]
- 61.Reeder BJ. Wilson MT. Desferrioxamine inhibits production of cytotoxic heme to protein cross-linked myoglobin: a mechanism to protect against oxidative stress without iron chelation. Chem Res Toxicol. 2005;18:1004–1011. doi: 10.1021/tx049660y. [DOI] [PubMed] [Google Scholar]
- 62.Richards MP. Heme proteins and oxidation in fresh and processed meats. In: Decker E, editor; Elias R, editor. Oxidation in Foods and Beverages and Antioxidant Applications. Cambridge: Woodhead Publishing; 2010. pp. 76–104. [Google Scholar]
- 63.Richards MP. Cai H. Grunwald EW. Phenylalanine substitution at site B10 (L29F) inhibits metmyoglobin formation and myoglobin-mediated lipid oxidation in washed fish muscle: mechanistic implications. J Agric Food Chem. 2009;57:7997–8002. doi: 10.1021/jf901147a. [DOI] [PubMed] [Google Scholar]
- 64.Robinson SR. Dang TN. Dringen R. Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Rep. 2009;14:228–235. doi: 10.1179/135100009X12525712409931. [DOI] [PubMed] [Google Scholar]
- 65.Schricker BR. Miller DD. Stouffer JR. Measurement and content of nonheme and total iron in muscle. J Food Sci. 1982;47:740–743. [Google Scholar]
- 66.Shikama K. The molecular mechanism of autoxidation for myoglobin and hemoglobin: a venerable puzzle. Chem Rev. 1998;98:1357–1373. doi: 10.1021/cr970042e. [DOI] [PubMed] [Google Scholar]
- 67.Silaghi-Dumitrescu R. Reeder BJ. Nicholls P. Cooper CE. Wilson MT. Ferryl haem protonation gates peroxidatic reactivity in globins. Biochem J. 2007;403:391–395. doi: 10.1042/BJ20061421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silver IA. Murrills RJ. Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 69.Smerdon SJ. Krzywda S. Brzozowski AM. Davies GJ. Wilkinson AJ. Brancaccio A. Cutruzzola F. Allocatelli CT. Brunori M. Li T. Interactions among residues CD3, E7, E10, and E11 in myoglobins: attempts to simulate the ligand-binding properties of Aplysia myoglobin. Biochemistry. 1995;34:8715–8725. doi: 10.1021/bi00027a022. [DOI] [PubMed] [Google Scholar]
- 70.Smith RD. Blouin GC. Johnson KA. Phillips GN., Jr. Olson JS. Straight-chain alkyl isocyanides open the distal histidine gate in crystal structures of myoglobin. Biochemistry. 2010;49:4977–4986. doi: 10.1021/bi1001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suman SP. Faustman C. Stamer SL. Liebler DC. Proteomics of lipid-oxidation induced oxidation of porcine and bovine oxymyoglobins. Proteomics. 2006;7:628–640. doi: 10.1002/pmic.200600313. [DOI] [PubMed] [Google Scholar]
- 72.Tampo Y. Onodera S. Yonaha M. Mechanism of the biphasic effect of ethylenediaminetetraacetate on lipid peroxidation in iron-supported and reconstituted enzymatic system. Free Radic Biol Med. 1994;17:27–34. doi: 10.1016/0891-5849(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 73.Tang Q. Kalsbeck WA. Olson JS. Bocian DF. Disruption of the heme iron-proximal histidine bond requires unfolding of deoxymyoglobin. Biochemistry. 1998;37:7047–7056. doi: 10.1021/bi9729413. [DOI] [PubMed] [Google Scholar]
- 74.Thiansilakul Y. Benjakul S. Park SY. Richards MP. Characteristics of myoglobin and hemoglobin-mediated lipid oxidation in washed mince from bighead carp (Hypothalimichthys nobilis) Food Chem. 2012;132:892–900. doi: 10.1016/j.foodchem.2012.02.182. [DOI] [PubMed] [Google Scholar]
- 75.Tien M. Morehouse LA. Bucher JR. Aust SD. The multiple effects of ethylenediaminetetraacetate in several model lipid peroxidation systems. Arch Biochem Biophys. 1982;218:450–458. doi: 10.1016/0003-9861(82)90367-8. [DOI] [PubMed] [Google Scholar]
- 76.Vuletich JL. Osawa Y. Aviram M. Enhanced lipid oxidation by oxidatively modified myoglobin: role of protein-bound heme. Biochem Biophys Res Commun. 2000;269:647–651. doi: 10.1006/bbrc.2000.2349. [DOI] [PubMed] [Google Scholar]
- 77.Wallace WJ. Caughey WS. Mechanism for the autoxidation of hemoglobin by phenols, nitrite, and “oxidant” drugs. Peroxide formation by one electron donation to bound dioxygen. Biochem Biophys Res Commun. 1975;62:561–567. doi: 10.1016/0006-291x(75)90435-0. [DOI] [PubMed] [Google Scholar]
- 78.Weaver H. Shukla N. Ellinsworth D. Jeremy JY. Oxidative stress and vein graft failure: a focus on NADH oxidase, nitric oxide and eicosanoids. Curr Opin Pharmacol. 2012;12:160–165. doi: 10.1016/j.coph.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Welch KD. Davis TZ. Aust SD. Iron autoxidation and free radical generation: effects of buffers, ligands, and chelators. Arch Biochem Biophys. 2002;397:360–369. doi: 10.1006/abbi.2001.2694. [DOI] [PubMed] [Google Scholar]
- 80.Wever R. Oudega B. van Gelder BF. Generation of superoxide radicals during the autoxidation of mammalian oxyhemoglobin. Biochim Biophys Acta. 1973;302:475–478. [Google Scholar]
- 81.Yang F. Phillips GN., Jr. Crystal structures of CO-, deoxy- and met-myoglobins at various pH values. J Mol Biol. 1996;256:762–774. doi: 10.1006/jmbi.1996.0123. [DOI] [PubMed] [Google Scholar]
- 82.Yoshida T. Kikuchi G. Sequence of the reaction of heme catabolism catalyzed by the microsomal heme oxygenase system. FEBS Lett. 1974;48:256–261. doi: 10.1016/0014-5793(74)80481-3. [DOI] [PubMed] [Google Scholar]