Abstract
Significance: There has been a striking advancement in our understanding of red cell substitutes over the past decade. Although regulatory oversight has influenced many aspects of product development in this period, those who have approached the demonstration of efficacy of red cell substitutes have failed to understand their implication at the level of the microcirculation, where blood interacts closely with tissue. Recent Advances: The understanding of the adverse effects of acellular hemoglobin (Hb)-based oxygen carriers (HBOCs) has fortunately expanded from Hb-induced renal toxicity to a more complete list of biochemical mechanism. In addition, various unexpected adverse reactions were seen in early clinical studies. The effects of the presence of acellular Hb in plasma are relatively unique because of the convergence of mechanical and biochemical natures. Critical Issues: Controlling the variables using genetic engineering and chemical modification to change specific characteristics of the Hb molecule may allow for solving the complex multivariate problems of acellular Hb vasoactivity. HBOCs may never be rendered free of negative effects; however, quantifying the nature and extent of microvascular complications establishes a platform for designing new ameliorative therapies. Future Directions: It is time to leave behind the study of vasoactivity and toxicity based on bench-top measurements of biochemical changes and those based solely on systemic parameters in vivo, and move to a more holistic analysis of the mechanisms creating the problems, complemented with meaningful studies of efficacy. Antioxid. Redox Signal. 18, 2329–2341.
Introduction
In the United States of America, allogeneic red blood cell (RBC) transfusion has long been considered an important treatment option for patients suffering from blood loss (56). However, infectious agent viruses and other pathogens may put the blood supply at risk (71). Currently, the American Red Cross tests donated blood for Hepatitis B and C viruses, human immunodeficiency virus (HIV), human T-cell lymphotropic virus, syphilis, West Nile virus, and the agent of Chagas disease (22, 48, 144, 145). As a result, the safety of the U.S. blood supply in terms of transfusion-transmitted diseases is good. However, screening for more infectious agents will increase the cost of blood. Of more concern is the fact that donated blood may contain yet to be identified infectious agents (48). Currently, in the United States alone, about 14 million units of whole blood are collected annually, and ∼12 million are processed and given for transfusion (1, 2). Transfusion-related adverse events, both short- and long-term, are among the costliest contributors to healthcare expenditures, without including future illness, lost wages, and impact on quality of life (136). Despite the increasing cost of transfusion, its practice remains quite liberal (67). Additionally, there are new concerns regarding the safety and effectiveness of stored blood transfusions, due to RBC-induced changes induced by ex vivo aging, so called RBC storage lesions (89, 90). To further compound the problem, blood availability can be limited in emergency situations such as war scenarios or natural disasters (143). Therefore, it has been a long-term goal of scientists to develop an efficacious and safe RBC substitute (i.e., oxygen bridge) for use in transfusion medicine.
Hemoglobin (Hb)-based oxygen carriers (HBOCs) are currently being developed as RBC substitutes for use in transfusion medicine. Despite significant commercial efforts, recent late-stage clinical results of HBOC studies hamper further development (46, 73, 165). The key issues observed during clinical trials include vasoconstriction, the persistence of long-term systemic hypertension, and oxidative-stress-induced tissue toxicity (69, 138). These studies have highlighted the importance of the clinical contexts in which RBC substitutes are to be used, as these scenarios strongly affect safety and efficacy outcomes that are observed after infusion. For example, although the injury that created the need for transfusion can lead to adverse outcomes by itself, subsequent blood transfusion can trigger a second insult, which is termed ischemia-reperfusion injury. Mechanistically, sublethal ischemia and hypoxia lead to priming of secondary messengers and to the production of reactive oxygen species (ROS) (35). Injured cells are then more susceptible to further production of ROS and to active participation in inflammatory responses. ROS exposure triggers cytotoxic reactions and induces production and release of inflammatory cytokines that result in activation of leukocytes, platelets, and macrophages. Another result is acute endothelial cell dysfunction, which prevents transfusion from achieving its intended objective (111, 155).
The Pathophysiology of Blood Loss
Blood substitutes have been formulated to address the deficit of blood's intrinsic oxygen-carrying capacity resulting from the loss of RBCs. However, the decrease of circulating RBCs (anemia) and blood volume are not the only factors affected during blood loss. Detailed studies of the circulation, and specifically the microcirculation, have shown that normal blood mechanical properties are optimal to maintain a functional central circulation and to insure uniformly perfused tissue capillaries (27, 84, 161). A blood loss lowers blood pressure, and thus the pressure at the capillaries decreases, and most vascular beds initiate reflexes in efforts to maintain pressure via vasoconstriction. This further reduces the pressure at the arteriolar end of the capillaries, which affects the fluid movement across the capillary membrane that occurs as a result of filtration. Low pressure results in a reduction in the region of the capillary where fluid is lost (filter out) from the plasma into the tissues and in an increase in the region of the capillary where fluid returns (filter in) back to the plasma, producing a net gain of fluid to the plasma. This fluid gain, taken over most of the vascular beds, leads to fluid autotransfusion that partially compensates for volume loss during blood loss. Therefore, in addition to reducing the intrinsic blood oxygen-carrying capacity, blood viscosity is reduced. Historically, this has been regarded as a positive outcome, since lower viscosity reduces vascular resistance (93) (Fig. 1). Viscosity is a commonly overlooked parameter in the transfusion medicine. The loss of mechanotransduction on the endothelial cells hampers the production of nitric oxide (NO) and prevents the normal vascular signaling and regulation, thus increasing peripheral vascular resistance (30, 85, 118). Vessel wall shear stress (WSS) is the product of blood viscosity and shear rate determined by the flow velocity profile. WSS modulates peripheral vascular resistance and blood flow, because WSS is the local signal for the endothelium to produce vasorelaxation. Blood losses lower blood viscosity, thus increasing blood flow velocity; however, this effect is not able to preserve WSS due to the two-phase nature of blood (RBCs and plasma). When blood flows, an erythrocyte cell-free layer (CFL) is interposed between the RBC blood column and the vessel wall (97, 140). This hydrodynamic layer is generated by the axial migration, resulting from the flexibility of red cells that causes them to migrate toward the center of the flow stream (117, 134, 135, 163). Blood losses (and hemodilution) increase the CFL width and the concentration of all molecular species dissolved in plasma. Thus, the physiological shortcomings induced by hemodilution tend to lower the NO concentration in the arteriolar wall, preventing vasorelaxation and creating a proinflammatory environment.
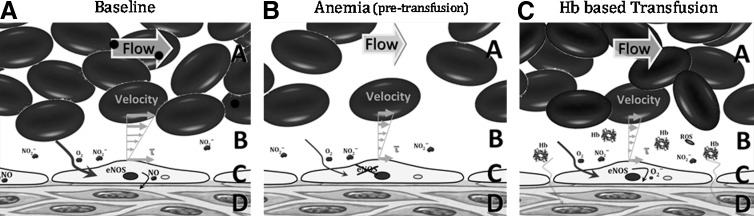
FIG. 1.
Mechanistic analysis of Hb based transfusion associate vascular dysfunction. In physiological condition (panel A), blood flow provides oxygen and mechanical signals (τ, wall shear stress) to the vasculature, which serve to self-regulate local blood flow. After severe blood losses, anemia or any condition that originates a need for blood transfusion (panel B), oxygenation and vascular shear stress are compromised, thus the local vascular regulatory processes become impaired. Consequently, when the blood transfusions are given or a HBOC is infused (panel C), they do not achieve their intended goal; because they only increase oxygen carrying capacity without restoring blood flow. Even following perfusion recovery, vascular regulatory processes are not fully functional, such as endothelial NO synthase, which becomes uncoupled and instead of generating NO produces superoxide, contributing to oxidative stress, and triggering an inflammatory cascade. The additive role of oxidative and nitrosative stress results in increased NO release from inducible NO synthase (iNOS) in perivascular tissue and from macrophages/monocytes. The NO generated by iNOS is metabolized to peroxynitrite, which is cytotoxic to tissues. Within panels A, B and C: The flow arrow shows blood flow direction. A, RBC column; B, cell-free layer (CFL) width; C, endothelial cells; and D, tissue space.
Transfusion of Stored Blood and the Potential for Toxicity
Considerable progress has been made in improving blood product safety protocols during the past 20 years, significantly reducing the risk of infection and the immune response to transfusion (2, 92). Blood transfusion in some patients has been shown to provide no benefit, and in fact is associated with increased morbidity and mortality (90, 122). Several studies have indicated that the transfusion of stored blood for more than 14 days decreased oxygen delivery (95, 148), contributed to tissue hypoxia (64), and increased mortality (53). A recent systematic review and meta-analysis that evaluated the efficacy of stored blood transfusion (including 272, 596 patients) revealed that in 42 of 45 studies, the risk of transfusion outweighed the benefit by increasing the mortality rate by 70% (107). Additionally, several studies have shown that the survival rate decreases with the mean blood storage duration, and that the storage duration is a strong predictor and risk factor of multiple-organ failure (107).
From the moment that blood is withdrawn, erythrocytes begin to exhibit changes on their membrane due to stress during collection, which only increases upon manipulation, storage, and reinfusion, consequently affecting the degree of intravascular hemolysis post-transfusion (91, 96). Stored blood transfusions release small amounts of Hb in the plasma, inducing vasoconstriction, reducing blood flow and oxygen delivery (86, 162). Low levels of Hb in plasma severely disrupt NO bioavailability, via NO dioxygenation (NOD) reactions (87, 124, 156). At the smooth muscle, this process primarily decreases intracellular NO concentration by limiting the activation of the NO receptor, soluble guanylate cyclase (sGC), which results in decreased production of cyclic guanosine monophosphate (cGMP), and ultimately causes vasoconstriction (9, 123).
Transfusion of HBOCs and the Potential for Toxicity
Acellular Hb is present in the circulation in minute quantities due to the natural breakdown of RBCs that are readily disposed of without consequence in the healthy organism (113, 130, 147). However, elevated levels of Hb in the plasma can be observed in the blood and/or urine. Small amounts of plasma Hb are removed from the circulating plasma by haptoglobin (Hp). Other plasma proteins such as hemopexin, α1-microglobulin, and albumin have a role to detoxify free heme (11). Plasma circulating Hp transports the Hb to be removed by the reticuloendothelial system in the liver and spleen (11). The Hp-Hb complex is also removed from the plasma by deposition in various body tissues, but chiefly in the liver and renal cortex (18). Further accumulation of Hb in the plasma, beyond the combining power of Hp, is filtered through the glomeruli of the kidneys and reabsorbed by tubular cells, where it may lead to precipitate formation, termed hemosiderin, during conditions with Hb overload, causing oxidative damage and glomerular and tubular necrosis (18). Exceeding this threshold has toxic consequence during trauma, acute hemolytic anemia, breakdown of stored blood in transfusion, or the deliberate introduction of Hb as an HBOC (78, 128, 147).
Hb is also the precursor for synthesis and formulation of HBOCs to be used as blood substitutes (55, 57, 75). The first intent to develop an HBOC was stroma-free Hb (8, 51). However, transfusion of stroma-free (i.e., cell-free) Hb led to several major side effects (6, 19, 36, 45, 176). In the circulation, extracellular tetrameric Hb (α2β2) easily dissociates into two pairs of αβ dimers (19, 36), which are extremely prone to oxidation (176) and increased renal excretion (19–21). The process of Hb oxidation to methemoglobin (metHb) promotes unfolding of the globin chains and releases cytotoxic heme into the circulation, leading to kidney tubule damage and eventual renal failure (19, 36). metHb generates harmful ROS (6), which damage cell membranes, oxidize nucleic acids, and proteins (45). The extent of tissue toxicity by plasma Hb is further magnified by the extravasation of Hb through fenestrated capillaries and endothelial cell junctions (Fig. 2).
FIG. 2.
Hb structures, auto-oxidation products, and toxicity. Hb is safely contained within the RBCs, although it appears in the plasma, when the RBCs rupture (hemolysis), or when HBOCs are infused. All Hb chains contain an Fe-containing protoporphyrin IX group, which binds molecular oxygen. Cell-free Hb auto-oxidizes, leading to formation of reactive oxygen species (ROS) and degradation products as heme and methemoglobin (metHb). All of these molecules are highly reactive and have the ability to damage lipids, protein, and DNA, and are therefore proinflammatory, pro-oxidant, and toxic. Furthermore, Hb also binds and reacts with NO, thereby affecting the vasomotor tone. Thus, hemolysis and elevated extracellular Hb concentrations are associated with vasoconstriction and hypertension. Finally, the metabolites formed by the Hb degradation are hemolytic, and thus oxidative stress accelerates the hemolysis, in a positive feedback loop.
The two major theories to explain the occurrence of acellular Hb-induced vasoconstriction are that plasma Hb scavenges NO produced by the endothelium and oversupplies oxygen to vasculature via Hb-facilitated diffusion of oxygen and oxygenated Hb (170). Limiting NO prevents the relaxation of the smooth muscle (77, 149), and hyperoxygenation triggers regulatory response to limit blood flow and to reduce the surface area for oxygen transport (3, 4). HBOC-induced vasoconstriction has been clinically observed as hypertension. Scavenging of NO can occur either through binding to deoxyhemes, resulting in the highly stable ferrous nitrosyl Hb derivative, or through the NOD reactions between NO and oxyHb to produce metHb and nitrate. NO binding to deoxygenated Hb and NO reaction rate with oxyHb are virtually identical for most HBOC formulations (129); however, the vasoconstrictive effects vary between HBOC formulations (32, 129). Recombinant Hbs (rHbs) in which NO accesses to the oxyheme is sterically hindered showed correlation between NO reaction rates and the level of hypertension (42), although in vivo vasoconstriction was not studied. The rHbs reduced Hb NOD reaction rates by a maximum of one order of magnitude (42, 44, 124). However, mathematical models suggest that reaction rates need to be reduced by several orders of magnitude to obtain an NO concentration of 100–150 nM at the abluminal side of a blood vessel (82, 139), which is required to activate 50% of sGC (39, 142).
NO scavenging does not yield a unifying explanation to the vasoconstriction for all HBOCs. Polyethylene glycol (PEG)-conjugated Hbs (PEG-Hbs) show minimal levels of vasoconstriction, but they lower perivascular NO concentration, to a similar extent, as do vasoconstrictive HBOCs (149). Insight into the mechanisms behind these effects was obtained by direct measurements of microvascular pressure and resistance (27, 32). After hemodilution with PEG-Hb, capillary pressure was maintained; blood flow increased; and vascular resistance was lowered, when compared to other HBOCs (27, 32). The increase in blood flow with PEG-Hbs compared to other HBOCs has been consistently documented in other studies of hemodilution and hemorrhagic shock resuscitation (152, 167, 169, 172). PEG-Hb is a good plasma expander because of its high colloid osmotic pressure (COP) (170); when PEG-Hb is infused, it affects capillary filtration and promotes reabsorption of water, increasing plasma volume and reducing blood protein concentration, although lowering perivascular NO (149).
In the second theory, it is hypothesized that HBOCs oversupply oxygen to the surrounding vasculature via Hb-facilitated diffusion of oxygen and oxygenated Hb (168). Therefore, an autoregulatory response induces vasoconstriction to limit blood flow and the available surface area for oxygen transport through the blood vessel wall (3, 4). The oxygen-facilitated diffusion hypothesis is not entirely satisfactory, because while it is operational in the vascular compartment, it ceases to work across the vessel wall, as Hbs cannot diffuse across the vessel wall, and the oxygen flux across the vessel wall is determined by the oxygen tension gradient. Thus, facilitated diffusion only increases the amount of oxygen transported by the blood to the tissue interface. Studies using an artificial capillary system compared various acellular Hb and HBOCs and showed that unmodified Hb and small HBOCs increased oxygen release, relative to larger chemically modified HBOCs regardless of their oxygen affinity (109). Experiments demonstrating increased fluxes due to facilitated diffusion have used infinite sinks at the system boundary through which the oxygen exits (109), and such boundary conditions are not present in blood vessels.
Acellular Hb Oxidative Damage
Hb-based transfusion strategies inevitably restore oxygenation to oxygen-deficient tissues. This often results in ischemia–reperfusion injury, which exacerbates underlying conditions and worsens clinical outcomes. Mitigating the problems associated with ischemia–reperfusion injury has proven itself to be difficult, and there have been a number of studies of complementary mechanisms aimed at increasing protection (116, 127, 177). Studies suggest that pathogenesis of ischemia-reperfusion-reoxygenation injury is strongly linked to oxidative stress and the formation of ROS. In vascular endothelial cells, active consumption of NO by ROS contributes to vascular inflammation and endothelial dysfunction (ED) (60). Restoration of vascular levels of NO has been shown to sustain mitochondrial and cellular function, thus reducing oxidative damage (112, 127). The antioxidant defenses of plasma revolve around urate and ascorbate. In the case of HBOCs, the maintenance of the ferrous form of Hb is necessary for effective oxygen delivery, and oxidation to metHb (nonoxygen carrying) leaves Hb void of efficacy and prone to degradation.
Status of Commercial Developments
Vasoconstriction, heme-derived ROS, and free iron can account for the myocardial infarction observed in clinical trials of small-sized commercial polymerized Hbs (PolyHbs) such as Hemopure® (OPK Biotech., Boston, MA) and PolyHeme® (Northfield Laboratories, Inc., Evanston, IL) (120). Hemopure is developed and manufactured as a chemically stabilized, cross-linked bovine Hb suspended in an electrolyte solution for human use, with a P50 (i.e., O2 affinity) of 38 mm Hg, and an average molecular weight of 250 kDa with <2% α2β2 (49, 75, 99, 108). Hemopure is also known as Hb Glutamer-250 or HBOC 201. PolyHeme is the only HBOC to reach a phase III trial, polymerized in a multistep polymerization and purification process. The final product is a pyridoxylated polymerized human Hb in an electrolyte solution with a P50 ranging from 28 to 30 mm Hg, an average MW of 150 kDa with <1% α2β2 (55, 76, 115). Glutaraldehyde has been widely employed to nonspecifically crosslink/polymerize Hb (4, 7, 58, 59). Despite commercial development of PolyHbs, hypertension and other important safety concerns remain critical impediments to their clinical use (65, 141).
Polymerized bovine Hb for veterinary use (oxyglobin; OPK Biotech, formerly Biopure) was evaluated in hemorrhagic shock resuscitation and hemodilution as a function of Hb concentration administered. Although oxyglobin was vasoconstrictive, it improved survival when used in small dosage by comparison to nonoxygen-carrying colloidal fluids (29, 31). However, when used at higher dosages, tissue oxygenation was significantly impaired, and toxic effects on tissue viability were observed within 8 h (28). In a similar model, MP4OX, a PEG-Hb developed by Sangart, Inc. (San Diego, CA), was found to be a plasma expander rather than an oxygen carrier, as its high COP precludes PEG-Hb from being used at concentrations required for increasing oxygen transport (152, 169). If the benefits of PEG-Hb are plasma expansion, the Hb can be replaced with a less-toxic protein like albumin, especially when PEG-conjugated albumin appears to show no difference in terms of perfusion compared to PEG-Hb (33). The gain in oxygen-carrying capacity with MP4OX is not physiologically relevant in clinical situations.
Basic Strategies to Mitigate Acellular Hb Toxicity
Acellular Hb can arise from hemolysis, transfusion of stored blood, RBC parasitic diseases, and the infusion of HBOCs (52, 72, 126, 171). Acellular Hb toxicity is exacerbated by pre-existing vascular dysfunction found in large segments of the population who suffer from diabetes, hypertension, and obesity (68). Several approaches have been implemented to control HBOC toxicity using either chemical or genetic modifications of the Hb, and then developing strategies for mitigating pathological effects with targeted biochemical and rheological therapies.
Molecular size
Larger-sized HBOCs, unlike smaller tetrameric HBOCs, can (i) provide a greater degree of physical separation from the blood vessel wall, thus reducing NO scavenging by increasing the diffusion barrier; (ii) have a higher viscosity, and thus increase vessel WSS exerted by the blood, leading to an increased NO synthesis; (iii) reduce extravasation through endothelial cell–cell junctions; and (iv) decrease the Hb diffusion coefficient, and thus reduce the oxyHb flux to the blood vessel wall, thereby decreasing both the rate of NO scavenging and oxygen oversupply (26, 124, 149, 157) (Fig. 3). Attempts to develop large polymerized bovine Hb by increasing the cross-link density (50:1, i.e., molar ratio of glutaraldehyde to Hb) have yielded no vasoconstriction compared to smaller polymeric Hbs (26), and other toxicities have not yet been explored. Natural acellular Hbs of terrestrial (47) and marine (150) worms are acellular and have evolved in this manner. Hb from the marine worm Arenicola marina is being developed as an HBOC (150), and with similar properties, a zero-linked polymeric Hb (63) has been developed by OXYVITA, Inc., New Windsor, NY.
FIG. 3.
Molecular weight and hydrodynamic radius of HBOCs. Schematic representation of Hb, polyethylene glycol-conjugated Hbs (PEG-Hb), encapsulated Hb vesicles (HbV), and polymerized Hb (PolyHb, large, and small) relative to RBCs. The size/molecular mass of the HBOC molecules have a direct impact on their proximity to the vascular endothelium and their molecular diffusivity, which determine NO scavenging and vessel wall oxygen oversupply. In the absence of plasma Hb in the circulation, the plasma layer adjacent to the vessel wall limits the NO scavenging by Hb in the RBCs; however, after infusion of HBOC, the plasma Hb becomes a sink for NO. Large HBOCs have a high solution viscosity relative to smaller HBOCs; thus, they increased plasma viscosities when infused, leading to a hyperviscous oxygen carrier. An increase in plasma viscosity directly increases vessel WSS and endothelial cell mechanotransduction.
Other strategies to increase the molecular size of HBOCs include conjugation of PEG to the surface of Hb (157, 159) and encapsulation of Hb inside PEG-conjugated vesicles (132). The hydrodynamic radius of Hb is about 3 nm, while that of PEG-Hb is 9 nm. Thus, the flux of oxygen due to facilitated diffusion in the plasma in the presence of Hb is threefold than that of PEG-Hb. However, PEG-Hb shows a nonlinear dependence of COP with respect to concentration, reflecting colligative properties, the Donnan effect (121), and effects arising from its molecular-excluded volume (146), increasing capillary reabsorption and diluting plasma PEG-Hb concentration. A different approach to increase the size is to encapsulate Hb vesicles (HbV). This technology was initially developed by E. Tsuchida and consists of encapsulating Hb in 250-nm-diameter phospholipid vesicles coated with PEG (154). HbV must be delivered in solution in a plasma expander, thus allowing the design of its rheological properties. The rheology of HbV suspended in albumin was nearly Newtonian, whereas starch, dextran, and gelatin caused the suspensions to be non-Newtonian with shear-thinning behavior, as solution viscosity decreases as the applied shearing stress increased (131).
Rational Hb mutagenesis
The approach taken by J.S. Olson's group at the Rice University is to alleviate hypertensive effects through rational mutagenesis of the distal pocket of rHb to mitigate NO scavenging (42). Considering the ultrastability of the αβ interface (110), the first site-directed mutagenesis strategy was focused on genetically engineering an α–α-fusion Hb tetramer that was physically unable to dissociate into αβ dimers (98). The first rHb to be developed via this strategy consisted of rHb1.1 (Baxter Hemoglobin Therapeutics, Boulder, CO). Unfortunately, although rHb1.1 exhibited no renal toxicity, it elicited adverse side effects such as decreased heart rate, fever, and chills (160). This approach has been shown to reduce vasoconstriction and hypertension (44, 124, 125); however, obtaining sufficient quantities of recombinant material remains prohibitively expensive.
Enhancing Hb physiological clearance
Pharmacological activation of the glucocorticoid pathway induces expression of the Hb-scavenging Hp in dogs and guinea pigs (15). Hp sequesters plasma Hb and neutralizes much of its vasculotoxic oxidative stress, protecting against systemic hypertension and other vasculopathic outcomes (15). The depletion of NO is compounded by the oxidative stress induced by decomposition of Hb into heme and elemental iron. The canine model of intravascular hemolysis demonstrates the induction of systemic and pulmonary hypertension, as well as impaired creatine clearance, presumably due to decreased renal blood flow (15). The acute experimental canine vasculopathy is partially reversed by administration of NO or by glucocorticoid-induced production of endogenous Hp (15).
Hp is produced mostly by the liver, but also by the skin, lung, and kidney (41). Hp, in its simplest form, consists of two α- and two β-chains, connected by disulfide bridges. Hp exists in two allelic forms in human populations, Hp1 and Hp2, the latter one having arisen due to the partial duplication of the Hp1 gene (102). Three genotypes of Hp found in humans: Hp1-1, Hp2-1, and Hp2-2. Hp of different genotypes binds Hb with different affinities, with Hp2-2 being the weakest binder (94). Hp escorts Hb to the CD163 protein, in which the Hp-Hb complex is avidly bound and cleared from the plasma, depleting plasma Hp in the process (12) (Fig. 4). Hp binding to Hb prevents the generation of oxidant species, and mitigates the hypertension and other adverse vascular outcomes (80). The Hp-Hb complex has high oxygen affinity, which could increase nitrite reductase activity, potentially producing the endogenous antioxidant NO from nitrite (14). Infusion of Hp may have potential benefits during acute sickle-cell vaso-occlusion (12, 80). Hp effects might also explain some of the life-saving effects of glucocorticoids and aggressive plasma exchange transfusion therapy in thrombotic thrombocytopenic purpura (79).
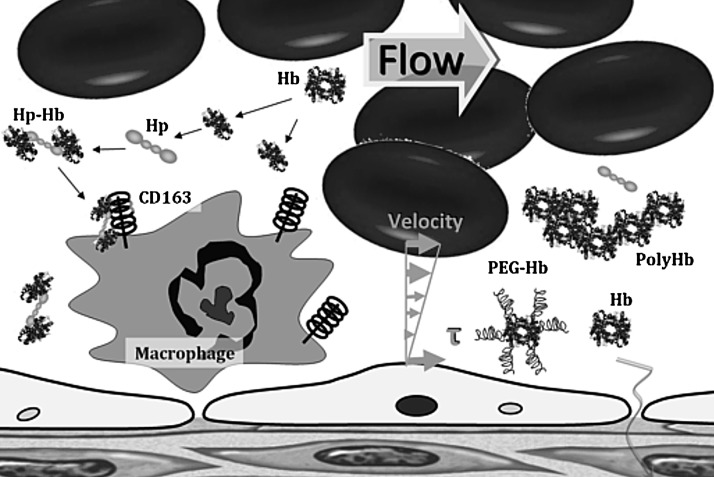
FIG. 4.
Schematic representation of haptoglobin (Hp)-Hb complex formation and endocytosis mediated by CD163 receptor. Hp is synthesized predominantly in the liver. If plasma Hb is released into the circulation, it binds immediately to Hp to form an Hp-Hb complex, which subsequently is removed by the CD163 receptor expressed mainly on monocytes, macrophages, and Kupffer cells in the liver. The binding between Hp and Hb is among the strongest noncovalent interactions known, with very slow dissociation rates. When hemolysis depletes Hp, Hb accumulates in the kidney and is secreted in the urine. Hp can offer an effective protection against kidney damage. The Hb-Hp complex is not filtrated by the kidney, and instead directs Hb to CD163 on macrophages for a process of endocytosis and final degradation. Within macrophages, the heme oxygenase enzyme breaks down the heme group of Hb into bilirubin and CO, which both have been shown to be an antioxidant and vasodilator.
NO-scavenging inhibition
Angeli's salt (sodium α-oxyhyponitrite, Na2N2O3) has been used to compensate for NO scavenging by plasma Hb, by selectively converting plasma Hb to ferric, and ideally iron-nitrosylated heme species that do not actively scavenge NO (66). Nitroxyl oxidizes oxy-Hb to metHb, and it can also convert metHb to a more stable, less-toxic species, iron-nitrosyl Hb (66). This concept can treat intravascular hemolysis, but would not apply to HBOCs, since it will prevent oxygen transport.
NO supplementation
Various compounds have been used to compensate for the NO consumed by HBOCs and the reduction the vasoconstriction. The effects of administering nitroglycerin (NTG) during HBOC fluid resuscitation from hemorrhagic shock were to attenuate the vasoconstrictive complications of HBOC (81). NTG is a weak NO donor (an organic nitrate that requires a three-electron reduction to release NO) that causes vasodilatation (164). NTG did not eliminate the vasoconstriction and caused elevations in metHb levels, thus reducing oxygen delivery to tissues. Similarly, sodium nitrite has been used in a similar fluid resuscitation from hemorrhagic shock with HBOCs, and vasoconstriction was attenuated at high doses of nitrite (10). In addition to increased metHb levels, nitrite supplementations reduced platelet function and increased the risk of pulmonary complications (edema and congestion) (10, 114). Pure NO inhalation has been used to treat NO depletion (174, 175). Inhaled NO prevented pulmonary and systemic hypertension induced by HBOCs (175). NO inhalation restores the NO that the NOD reactions consume within the lungs, and increases metHb levels, without restoring the NO bioavailability to peripheral tissues. Of late, NO-releasing nanoparticles (NOnps) have been used to restore NO bioavailability after infusion of HBOC (24). The slow release of NO from the NOnps, as they circulate, allows small amounts of NO to diffuse abluminally, thus reducing the effects of NO scavenging and limiting metHb formation (24, 25, 118). Additionally, a minor increase in intraluminal NO affects the diffusion gradients form endothelial-derived NO, allowing endothelial-derived NO to diffuse further into tissues, restoring signaling at smooth muscle (Fig. 5).
FIG. 5.
Intravascular NO delivery based on nanoparticles (NO-releasing nanoparticles [NOnps]). NOnps are a hydrogel precursor in conjunction with a glass-forming (glass in the context of a solid comprised of an amorphous network of hydrogen-bonded elements) combination of chitosan and polyethylene glycol to form a fine powder (25). This material retains NO in a stable form when dry, and release NO upon exposure to moisture. NOnps have shown a high efficacy in relaxing blood vessel in vivo compared to NONOates (25). About 30 times less NO is required from NOnps compared to NONOates to produce similar dilation when applied intravascularly. In addition, the effects of NOnps were sustained longer compared to NONOates that only lasted minutes. The higher doses of NO required by NONOate significantly increase metHb levels and decrease the oxygen-carrying capacity (25).
Other alternatives for intravascular NO therapy include compounds containing either NO or an NO precursor in a stable form, typically lack the capacity for controlled and sustained delivery (50, 70). The largest family of NO donors currently in use is NONOates, also known as diazeniumdiolates. The NONOate decomposition rate depends on pH and temperature (105, 106). NONOates have been used to mitigate the vascular inflammation-induced plasma Hb during malaria infection (34). They have limited application to HBOCs, due to short half-life and increased formation of metHb. Other alternatives to prevent vasoconstriction induced by HBOC include coadjunct therapy with Sildenafil, a phosphodiesterase (PDE) type 5 inhibitor (PDE5), by limiting cGMP breakdown (54). PDE5 inhibitor therapy partially prevented some hemodynamic changes (54). Therefore, a combination of PDE5 inhibitor with NOnps may increase the efficacy of a given dose of NOnps and decrease the amount of NOnps needed to counteract HBOC vasoconstriction.
Enhanced Hb nitrite reductase
It has been recently reported that PEG-Hb can convert nitrite to NO at a faster rate than does the native protein, which in part can compensate for the scavenging of NO (100). PEGylation of Hb stabilizes Hb into a conformation and hydration state that facilitates nitrite reductase to NO (103). The heme redox potential becomes more negative, making the iron a more efficient electron donor, thus increasing the rate of nitrite reductase by PEG-Hb (101). The reduction of nitrite to NO results in metHb formation, which clearly causes a negative outcome in terms of oxygen-carrying capacity (87).
Experimental Approaches to Predict Toxicity
The potential efficacy of several physiologically relevant reducing agents (glutathione, NADH, and ascorbic acid) in the redox stabilization of several HBOCs indicated that the rate of reduction by endogenous reducing agents is specific for each HBOC, and that most HBOC reduction by NADH requires the presence of another electron carrier (62). One of the main goals of preclinical toxicological evaluations for HBOCs was to select animal models that closely reflect human physiology. Knockout strains of mice have been generated that lack l-gulonolactone oxidase (LGO), which is the final enzyme in the synthesis of ascorbate. However, as with all transgenic and knockout animals, nonequivalent compensatory mechanisms may limit the predictive power of studies with these animals. Guinea pigs are a suitable species to evaluate human-relevant preclinical toxicokinetics as related to oxidative processes after Hb and HBOC exposure (17, 23). Guinea pigs, like humans and other primates, lack LGO, and hence are incapable of endogenous synthesis of ascorbate (37). They distribute antioxidant capabilities within the circulation and tissue parenchyma, presumably to counteract the reduced plasma ascorbate (119). Cooper et al. demonstrated the role for urate and ascorbate in the reduction of oxidized species of Hb and utilized the unique similarity of the guinea pig to the human to evaluate the role of ascorbate and urate after HBOC administration (40). Infusion of acellular Hb as oxygen therapeutics in animals and humans almost always induces an immediate vasoactive response, leading to elevation of both systematic and pulmonary blood pressure due to the removal of NO (40). In the presence of peroxide, Hb functions as a true enzymatic ascorbate peroxidase.
Buehler and coworkers have evaluated polymerized bovine Hb in guinea pigs to examine homeostatic mechanisms in a model relevant to human physiology (13). In their model, evaluation of blood pressure in response to PolyHb formulation suggested that molecular size was a critical attribute. Uptake by peripheral tissue macrophages was increased in lower-molecular-sized PolyHbs, and iron deposition was highest in the spleen and liver and was partially excreted via the kidneys, whereas the larger-molecular-sized PolyHbs were excreted exclusively by the spleen and liver (13). Future studies should include direct measurements of oxidative stress and lipid peroxidation (4-hydroxynonenal lysine adducts) as reliable indices of in vivo oxidative stress. Undiagnosed ED caused by genetic factors, smoking, hypertension, diabetes, and hypercholesterolemia may have played an important role in unforeseen clinical adverse events (transient ischemic attacks, strokes, and myocardial infarction) observed with HBOCs (137). These events cannot be predicted by toxicology studies conducted using rodent models (16). Therefore, evaluation of HBOCs in species or in transgenic animals with antioxidant states and induced ED similar to those of humans in health and in disease would increase our understanding of oxidative stress safety and efficacy of HBOC infusion.
Microvascular Assessment of Manifestation of Toxicity
Intravital microscopy is a powerful tool for the in vivo analysis of microcirculatory responses at the tissue level, especially to alterations of blood composition. The microcirculation usually is defined as that part of the vascular tree comprising blood vessels smaller than 100 μm, including arterioles, capillaries, and venules. The microcirculation plays a central role in tissue oxygenation, because it is across the walls of the microvessels that oxygen arrives to all tissues. Assessment of tissue oxygenation represents a key factor in evaluating the need for blood transfusion, as most of the clinical tools only provide information subrogate markers of global oxygenation (oxygen saturation, blood lactate, acid–base balance, and gastric pH), and many of them imply invasive monitoring. The current animal models to study the microcirculation all have their drawbacks, but as long as we lack clinical techniques to measure local perfusion and tissue oxygenation, they remain the only way to increase knowledge about the effect of transfusion tissue health and function.
In vivo measurement and characterization of the microcirculation with well-established animal models to visualize tissues in intact conditions, in parallel with systemic circulatory analysis, allow for the study of hemodynamics and vascular WSS, NO levels, oxygen transport, and the effects of strategies to control toxicity of acellular Hb. The in vivo effects of acellular Hb that are first manifested in microvessels can early detect the changes between normal vascular tissue and tissue under acute inflammatory conditions, and ED within short time scales. Vasoconstriction is observed early in the microcirculation, before hypertension and vascular resistance are systemically detected (5, 104). The worst implications of vasoconstriction are at the microcirculation, since constriction lowers capillary pressure and decreases functional capillary density (FCD), producing uneven tissue oxygenation and accumulation of metabolic by-products, causing microvascular dysfunction (83).
A major barrier to progress in the HBOC field has been the lack of a systematic analysis of the toxicity associated with acellular Hb. There is currently no universal gauge that allows comparisons of levels of toxicity between various Hb-containing solutions and whole blood (38, 74, 173, 175). The deliberate introduction of acellular Hb into the bloodstream arose with the development of HBOCs. It has been assumed that the gold standard of transfusion is blood; however, it is increasingly evident that transfused blood may itself be a source of toxicity if it has aged (i.e., >25–30 days) (130). Hb is a colloid, and it could be argued that comparable nontoxic standards should be nonheme-based colloids; however, even if such a comparison were practical, the lack of a measuring tool for toxicity prevents rational evaluation. FCD is a sensitive measurement of toxicity in the microcirculation and a marker for microvascular dysfunction (28, 61, 88, 133, 152, 166, 167); however, FCD can only be measured in experimental studies.
An optimal oxygen carrier has not been devised yet, and blood-banked blood is probably not optimal in this context. Moreover, the design of a HBOC that has the properties of native blood in the circulation may be unattainable, particularly because it is not possible to reproduce the biomechanical characteristics of native blood, and allows the microcirculation to remain functional. Nevertheless, this can be achieved by recognizing that a critical property of a blood substitute is that of maintaining the functionality of the microcirculation, which is significantly dependent on adequate levels of oxygen, NO, and hemodynamic forces on the vascular endothelium.
Abbreviations Used
- CFL
cell-free layer
- ED
endothelial dysfunction
- FCD
functional capillary density
- GC
guanylate cyclase
- GMP
guanosine monophosphate
- Hb
hemoglobin
- HBOC
hemoglobin-based oxygen carriers
- HbV
Hb vesicles
- HIV
human immunodeficiency virus
- Hp
haptoglobin
- iNOS
inducible NO synthase
- LGO
l-gulonolactone oxidase
- methb
methemoglobin
- NO
nitric oxide
- NOD
NO dioxygenation
- NOnps
NO-releasing nanoparticles
- NTG
nitroglycerin
- PDE
phosphodiesterase
- PEG
polyethylene glycol
- PEG-Hb
polyethylene glycol-conjugated Hbs
- Polyhb
polymerized Hb
- RBC
red blood cell
- Rhb
recombinant Hbs
- ROS
reactive oxygen species
- WSS
wall shear stress
Acknowledgments
This work was partially supported by the National Institutes of Health (NIH) under the Program project P01-HL071064, and grants R01-HL52684, R01-HL62354, and R01-HL62318.
References
- 1.Medical expenditures attributable to injuries—United States, 2000. MMWR Morb Mortal Wkly Rep. 2004;53:1–4. [PubMed] [Google Scholar]
- 2.Global Database on Blood Safety. Geneva: World Health Organization; 2008. Report 2004–2005. [Google Scholar]
- 3.Alayash AI. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants? Nat Biotechnol. 1999;17:545–549. doi: 10.1038/9849. [DOI] [PubMed] [Google Scholar]
- 4.Alayash AI. Hemoglobin-based blood substitutes and the hazards of blood radicals. Free Radic Res. 2000;33:341–348. doi: 10.1080/10715760000300881. [DOI] [PubMed] [Google Scholar]
- 5.Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Disc. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 6.Alayash AI. Patel RP. Cashon RE. Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid Redox Signal. 2001;3:313–327. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- 7.Alayash AI. Summers AG. Wood F. Jia Y. Effects of glutaraldehyde polymerization on oxygen transport and redox properties of bovine hemoglobin. Arch Biochem Biophys. 2001;391:225–234. doi: 10.1006/abbi.2001.2426. [DOI] [PubMed] [Google Scholar]
- 8.Amberson WR. Jennings JJ. Rhode CM. Clinical experience with hemoglobin-saline solutions. J Appl Physiol. 1949;1:469–489. doi: 10.1152/jappl.1949.1.7.469. [DOI] [PubMed] [Google Scholar]
- 9.Arnal JF. Warin L. Michel JB. Determinants of aortic cyclic guanosine monophosphate in hypertension induced by chronic inhibition of nitric oxide synthase. J Clin Invest. 1992;90:647–652. doi: 10.1172/JCI115906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnaud F. Scultetus AH. Kim B. Haque A. Saha B. Nigam S. Moon-Massat P. Auker C. McCarron R. Freilich D. Dose response of sodium nitrite on vasoactivity associated with HBOC-201 in a swine model of controlled hemorrhage. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:195–205. doi: 10.3109/10731199.2010.533126. [DOI] [PubMed] [Google Scholar]
- 11.Ascenzi P. Bocedi A. Visca P. Altruda F. Tolosano E. Beringhelli T. Fasano M. Hemoglobin and heme scavenging. IUBMB Life. 2005;57:749–759. doi: 10.1080/15216540500380871. [DOI] [PubMed] [Google Scholar]
- 12.Baek JH. D'Agnillo F. Vallelian F. Pereira CP. Williams MC. Jia Y. Schaer DJ. Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek JH. Zhou Y. Harris DR. Schaer DJ. Palmer AF. Buehler PW. Down selection of polymerized bovine hemoglobins for use as oxygen releasing therapeutics in a guinea pig model. Toxicol Sci. 2012;127:567–581. doi: 10.1093/toxsci/kfs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benesch RE. Ikeda S. Benesch R. Reaction of haptoglobin with hemoglobin covalently cross-linked between the alpha beta dimers. J Biol Chem. 1976;251:465–470. [PubMed] [Google Scholar]
- 15.Boretti FS. Buehler PW. D'Agnillo F. Kluge K. Glaus T. Butt OI. Jia Y. Goede J. Pereira CP. Maggiorini M. Schoedon G. Alayash AI. Schaer DJ. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buehler PW. Alayash AI. Toxicities of hemoglobin solutions: in search of in-vitro and in-vivo model systems. Transfusion. 2004;44:1516–1530. doi: 10.1111/j.1537-2995.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 17.Buehler PW. D'Agnillo F. Hoffman V. Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther. 2007;323:49–60. doi: 10.1124/jpet.107.126409. [DOI] [PubMed] [Google Scholar]
- 18.Bunn HF. Erythocyte destruction and hemoglobin catabolism. Semin Hematol. 1972;9:3–17. [PubMed] [Google Scholar]
- 19.Bunn HF. Esham WT. Bull RW. The renal handling of hemoglobin. I. Glomerular filtration. J Exp Med. 1969;129:909–923. doi: 10.1084/jem.129.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunn HF. Jandl JH. The renal handling of hemoglobin. Trans Assoc Am Physicians. 1968;81:147–152. [PubMed] [Google Scholar]
- 21.Bunn HF. Jandl JH. The renal handling of hemoglobin. II. Catabolism. J Exp Med. 1969;129:925–934. doi: 10.1084/jem.129.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busch MP. Kleinman SH. Nemo GJ. Current and emerging infectious risks of blood transfusions. JAMA. 2003;289:959–962. doi: 10.1001/jama.289.8.959. [DOI] [PubMed] [Google Scholar]
- 23.Butt OI. Buehler PW. D'Agnillo F. Differential induction of renal heme oxygenase and ferritin in ascorbate and nonascorbate producing species transfused with modified cell-free hemoglobin. Antioxid Redox Signal. 2010;12:199–208. doi: 10.1089/ars.2009.2798. [DOI] [PubMed] [Google Scholar]
- 24.Cabrales P. Han G. Nacharaju P. Friedman AJ. Friedman JM. Reversal of hemoglobin-induced vasoconstriction with sustained release of nitric oxide. Am J Physiol Heart Circ Physiol. 2011;300:H49–H56. doi: 10.1152/ajpheart.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabrales P. Han G. Roche C. Nacharaju P. Friedman AJ. Friedman JM. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010;49:530–538. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabrales P. Sun G. Zhou Y. Harris DR. Tsai AG. Intaglietta M. Palmer AF. Effects of the molecular mass of tense-state polymerized bovine hemoglobin on blood pressure and vasoconstriction. J Appl Physiol. 2009;107:1548–1558. doi: 10.1152/japplphysiol.00622.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrales P. Tsai AG. Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low and high plasma viscosity expanders. Am J Physiol. 2004;287:H363–H373. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 28.Cabrales P. Tsai AG. Intaglietta M. Deferoxamine lowers tissue damage after 80% exchange transfusion with polymerized hemoglobin. Antioxid Redox Signal. 2007;9:375–384. doi: 10.1089/ars.2006.1379. [DOI] [PubMed] [Google Scholar]
- 29.Cabrales P. Tsai AG. Intaglietta M. Isovolemic exchange transfusion with increasing concentrations of low oxygen affinity hemoglobin solution limits oxygen delivery due to vasoconstriction. Am J Physiol Heart Circ Physiol. 2008;295:H2212–H2218. doi: 10.1152/ajpheart.00751.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrales P. Tsai AG. Intaglietta M. Exogenous nitric oxide induces protection during hemorrhagic shock. Resuscitation. 2009;80:707–712. doi: 10.1016/j.resuscitation.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabrales P. Tsai AG. Intaglietta M. Polymerized bovine hemoglobin can improve small-volume resuscitation from hemorrhagic shock in hamsters. Shock. 2009;31:300–307. doi: 10.1097/SHK.0b013e318180ff63. [DOI] [PubMed] [Google Scholar]
- 32.Cabrales P. Tsai AG. Winslow RM. Intaglietta M. Effects of extreme hemodilution with hemoglobin based O2 carriers on microvascular pressure. Am J Physiol. 2005;288:H2146–H2153. doi: 10.1152/ajpheart.00749.2004. [DOI] [PubMed] [Google Scholar]
- 33.Cabrales P. Tsai AG. Winslow RM. Intaglietta M. Extreme hemodilution with PEG-hemoglobin vs. PEG-albumin. Am J Physiol. 2005;289:H2392–H2400. doi: 10.1152/ajpheart.00225.2005. [DOI] [PubMed] [Google Scholar]
- 34.Cabrales P. Zanini GM. Meays D. Frangos JA. Carvalho LJ. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J Infect Dis. 2011;203:1454–1463. doi: 10.1093/infdis/jir058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadenas S. Aragones J. Landazuri MO. Mitochondrial reprogramming through cardiac oxygen sensors in ischaemic heart disease. Cardiovasc Res. 2010;88:219–228. doi: 10.1093/cvr/cvq256. [DOI] [PubMed] [Google Scholar]
- 36.Chan WL. Tang NLS. Yim CCW. Lai FM. Tam MSC. New features of renal lesion induced by stroma free hemoglobin. Toxicol Pathol. 2000;28:635–642. doi: 10.1177/019262330002800501. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee IB. Evolution and the biosynthesis of ascorbic acid. Science. 1973;182:1271–1272. doi: 10.1126/science.182.4118.1271. [DOI] [PubMed] [Google Scholar]
- 38.Chen JY. Scerbo M. Kramer G. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics (Sao Paulo) 2009;64:803–813. doi: 10.1590/S1807-59322009000800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condorelli P. George SC. In vivo control of soluble guanylate cyclase activation by nitric oxide: a kinetic analysis. Biophys J. 2001;80:2110–2119. doi: 10.1016/S0006-3495(01)76184-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper CE. Silaghi-Dumitrescu R. Rukengwa M. Alayash AI. Buehler PW. Peroxidase activity of hemoglobin towards ascorbate and urate: a synergistic protective strategy against toxicity of hemoglobin-based oxygen carriers (HBOC) Biochim Biophys Acta. 2008;1784:1415–1420. doi: 10.1016/j.bbapap.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 41.D'Armiento J. Dalal SS. Chada K. Tissue, temporal and inducible expression pattern of haptoglobin in mice. Gene. 1997;195:19–27. doi: 10.1016/s0378-1119(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 42.Doherty DH. Doyle MP. Curry SR. Vali RJ. Fattor TJ. Olson JS. Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 43. This reference has been deleted.
- 44.Draghi F. Miele AE. Travaglini-Allocatelli C. Vallone B. Brunori M. Gibson QH. Olson JS. Controlling ligand binding in myoglobin by mutagenesis. J Biol Chem. 2002;277:7509–7519. doi: 10.1074/jbc.M109206200. [DOI] [PubMed] [Google Scholar]
- 45.Dunne J. Caron A. Menu P. Alayash AI. Buehler PW. Wilson MT. Silaghi-Dumitrescu R. Faivre B. Cooper CE. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem J. 2006;399:513–524. doi: 10.1042/BJ20060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmer J. Alam HB. Wilcox SR. Hemoglobin-based oxygen carriers for hemorrhagic shock. Resuscitation. 2012;83:285–292. doi: 10.1016/j.resuscitation.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Elmer J. Zorc K. Rameez S. Zhou Y. Cabrales P. Palmer AF. Hypervolemic infusion of Lumbricus terrestris erythrocruorin purified by tangential-flow filtration. Transfusion. 2012;52:1729–1740. doi: 10.1111/j.1537-2995.2011.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finucane ML. Slovic P. Mertz CK. Public perception of the risk of blood transfusion. Transfusion. 2000;40:1017–1022. doi: 10.1046/j.1537-2995.2000.40081017.x. [DOI] [PubMed] [Google Scholar]
- 49.Freilich D. Pearce LB. Pitman A. Greenburg G. Berzins M. Bebris L. Ahlers S. McCarron R. HBOC-201 Vasoactivity in a phase III clinical trial in orthopedic surgery subjects-extrapolation of potential risk for acute trauma trials. J Trauma. 2009;66:365–376. doi: 10.1097/TA.0b013e3181820d5c. [DOI] [PubMed] [Google Scholar]
- 50.Friedman A. Friedman J. New biomaterials for the sustained release of nitric oxide: past, present and future. Expert Opin Drug Deliv. 2009;6:1113–1122. doi: 10.1517/17425240903196743. [DOI] [PubMed] [Google Scholar]
- 51.Gilligan DR. Altschule MD. Katersky EM. Studies of hemoglobinemia and hemoglobinuria produced in man by intravenous injection of hemoglobin solutions. J Clin Invest. 1941;20:177–187. doi: 10.1172/JCI101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gladwin MT. Role of the red blood cell in nitric oxide homeostasis and hypoxic vasodilation. Adv Exp Med Biol. 2006;588:189–205. doi: 10.1007/978-0-387-34817-9_17. [DOI] [PubMed] [Google Scholar]
- 53.Gong MN. Thompson BT. Williams P. Pothier L. Boyce PD. Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 54.Gotshall RW. Hamilton KL. Foreman B. van Patot MC. Irwin DC. Glutaraldehyde-polymerized bovine hemoglobin and phosphodiesterase-5 inhibition. Crit Care Med. 2009;37:1988–1993. doi: 10.1097/CCM.0b013e3181a00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gould SA. Moore EE. Hoyt DB. Burch JM. Haenel JB. Garcia J. DeWoskin R. Moss GS. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187:113–120. doi: 10.1016/s1072-7515(98)00095-7. discussion 120–122. [DOI] [PubMed] [Google Scholar]
- 56.Greenburg AG. Benefits and risks of blood transfusion in surgical patients. World J Surg. 1996;20:1189–1193. doi: 10.1007/s002689900181. [DOI] [PubMed] [Google Scholar]
- 57.Greenburg AG. Kim HW. Use of an oxygen therapeutic as an adjunct to intraoperative autologous donation to reduce transfusion requirements in patients undergoing coronary artery bypass graft surgery. J Am Coll Surg. 2004;198:373–383. doi: 10.1016/j.jamcollsurg.2003.11.020. discussion 384–385. [DOI] [PubMed] [Google Scholar]
- 58.Guillochon D. Esclade L. Remy MH. Thomas D. Studies on haemoglobin immobilized by cross-linking with glutaraldehyde. Cross-linked soluble polymers and artificial membranes. Biochim Biophys Acta. 1981;670:332–340. doi: 10.1016/0005-2795(81)90105-7. [DOI] [PubMed] [Google Scholar]
- 59.Guillochon D. Esclade L. Thomas D. Effect of glutaraldehyde on haemoglobin: oxidation-reduction potentials and stability. Biochem Pharmacol. 1986;35:317–323. doi: 10.1016/0006-2952(86)90532-0. [DOI] [PubMed] [Google Scholar]
- 60.Haidara MA. Yassin HZ. Rateb M. Ammar H. Zorkani MA. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmacol. 2006;4:215–227. doi: 10.2174/157016106777698469. [DOI] [PubMed] [Google Scholar]
- 61.Hangai-Hoger N. Nacharaju P. Manjula BN. Cabrales P. Tsai AG. Acharya SA. Intaglietta M. Microvascular effects following treatment with polyethylene glycol-albumin in lipopolysaccharide-induced endotoxemia. Crit Care Med. 2006;34:108–117. doi: 10.1097/01.ccm.0000190623.97200.82. [DOI] [PubMed] [Google Scholar]
- 62.Harrington JP. Gonzalez Y. Hirsch RE. Redox concerns in the use of acellular hemoglobin-based therapeutic oxygen carriers: the role of plasma components. Artif Cells Blood Substit Immobil Biotechnol. 2000;28:477–492. [PubMed] [Google Scholar]
- 63.Harrington JP. Orlik K. Zito SL. Wollocko J. Wollocko H. Structural and redox behavior of OxyVita, a zero-linked polymeric hemoglobin: comparison with natural acellular polymeric hemoglobins. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:64–68. doi: 10.3109/10731191003634562. [DOI] [PubMed] [Google Scholar]
- 64.Hayes MA. Timmins AC. Yau EH. Palazzo M. Hinds CJ. Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 65.Hayward R. Lefer AM. Administration of polymerized bovine hemoglobin improves survival in a rat model of traumatic shock. Methods Find Exp Clin Pharmacol. 1999;21:427–433. doi: 10.1358/mf.1999.21.6.541924. [DOI] [PubMed] [Google Scholar]
- 66.He X. Azarov I. Jeffers A. Presley T. Richardson J. King SB. Gladwin MT. Kim-Shapiro DB. The potential of Angeli's salt to decrease nitric oxide scavenging by plasma hemoglobin. Free Radic Biol Med. 2008;44:1420–1432. doi: 10.1016/j.freeradbiomed.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hebert PC. Wells G. Blajchman MA. Marshall J. Martin C. Pagliarello G. Tweeddale M. Schweitzer I. Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 68.Heidenreich PA. Trogdon JG. Khavjou OA. Butler J. Dracup K. Ezekowitz MD. Finkelstein EA. Hong Y. Johnston SC. Khera A. Lloyd-Jones DM. Nelson SA. Nichol G. Orenstein D. Wilson PW. Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 69.Hess JR. Blood substitutes for surgery and trauma: efficacy and toxicity issues. BioDrugs. 1999;12:81–90. doi: 10.2165/00063030-199912020-00001. [DOI] [PubMed] [Google Scholar]
- 70.Homer K. Wanstall J. In vitro comparison of two NONOates (novel nitric oxide donors) on rat pulmonary arteries. Eur J Pharmacol. 1998;356:49–57. doi: 10.1016/s0014-2999(98)00511-1. [DOI] [PubMed] [Google Scholar]
- 71.Hourfar MK. Themann A. Eickmann M. Puthavathana P. Laue T. Seifried E. Schmidt M. Blood screening for influenza. Emerg Infect Dis. 2007;13:1081–1083. doi: 10.3201/eid1307.060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu LL. Champion HC. Campbell-Lee SA. Bivalacqua TJ. Manci EA. Diwan BA. Schimel DM. Cochard AE. Wang X. Schechter AN. Noguchi CT. Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jahr JS. Akha AS. Holtby RJ. Crosslinked, polymerized, and PEG-conjugated hemoglobin-based oxygen carriers: clinical safety and efficacy of recent and current products. Curr Drug Discov Technol. 2012;9:158–165. doi: 10.2174/157016312802650742. [DOI] [PubMed] [Google Scholar]
- 74.Jahr JS. Mackenzie C. Pearce LB. Pitman A. Greenburg AG. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J Trauma. 2008;64:1484–1497. doi: 10.1097/TA.0b013e318173a93f. [DOI] [PubMed] [Google Scholar]
- 75.Jahr JS. Moallempour M. Lim JC. HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure (R) (Biopure Corporation) Expert Opin Biol Ther. 2008;8:1425–1433. doi: 10.1517/14712598.8.9.1425. [DOI] [PubMed] [Google Scholar]
- 76.Jahr JS. Varma N. PolyHeme. Northfield Laboratories. IDrugs. 2004;7:478–482. [PubMed] [Google Scholar]
- 77.Jeffers A. Gladwin MT. Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–1565. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Josa M. Castella M. Pare C. Bedini JL. Cartana R. Mestres CA. Pomar JL. Mulet J. Hemolysis in mechanical bileaflet prostheses: experience with the Bicarbon valve. Ann Thorac Surg. 2006;81:1291–1296. doi: 10.1016/j.athoracsur.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 79.Kaiser C. Gembruch U. Janzen V. Gast AS. Thrombotic thrombocytopenic purpura. J Matern Fetal Neonatal Med. 2012;25:2138–2140. doi: 10.3109/14767058.2012.666586. [DOI] [PubMed] [Google Scholar]
- 80.Kato GJ. Haptoglobin halts hemoglobin's havoc. J Clin Invest. 2009;119:2140–2142. doi: 10.1172/JCI40258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katz LM. Manning JE. McCurdy S. Sproule C. McGwin G., Jr. Moon-Massat P. Cairns CB. Freilich D. Nitroglycerin attenuates vasoconstriction of HBOC-201 during hemorrhagic shock resuscitation. Resuscitation. 2010;81:481–487. doi: 10.1016/j.resuscitation.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 82.Kavdia M. Tsoukias NM. Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin-based blood substitutes. Am J Physiol Heart Circ Physiol. 2002;282:H2245–H2253. doi: 10.1152/ajpheart.00972.2001. [DOI] [PubMed] [Google Scholar]
- 83.Kerger H. Saltzman DJ. Menger MD. Messmer K. Intaglietta M. Systemic and subcutaneous microvascular Po2 dissociation during 4-h hemorrhagic shock in conscious hamsters. Am J Physiol. 1996;270:H827–H836. doi: 10.1152/ajpheart.1996.270.3.H827. [DOI] [PubMed] [Google Scholar]
- 84.Kerger H. Tsai AG. Saltzman DJ. Winslow RM. Intaglietta M. Fluid resuscitation with O2 vs. non-O2 carriers after 2 h of hemorrhagic shock in conscious hamsters. Am J Physiol. 1997;272:H525–H537. doi: 10.1152/ajpheart.1997.272.1.H525. [DOI] [PubMed] [Google Scholar]
- 85.Khazaei M. Barmaki B. Role of exogenous nitric oxide donor in treatment of decompensated hemorrhagic shock in normotensive and hypertensive rats. J Biomed Biotechnol. 2012;2012:365195. doi: 10.1155/2012/365195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim-Shapiro DB. Lee J. Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–851. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim-Shapiro DB. Schechter AN. Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 88.Kim HW. Greenburg AG. Mechanisms for vasoconstriction and decreased blood flow following intravenous administration of cell-free native hemoglobin solutions. Adv Exp Med Biol. 2005;566:397–401. doi: 10.1007/0-387-26206-7_52. [DOI] [PubMed] [Google Scholar]
- 89.Koch C. Li L. Figueroa P. Mihaljevic T. Svensson L. Blackstone EH. Transfusion and pulmonary morbidity after cardiac surgery. Ann Thorac Surg. 2009;88:1410–1418. doi: 10.1016/j.athoracsur.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 90.Koch CG. Li L. Sessler DI. Figueroa P. Hoeltge GA. Mihaljevic T. Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 91.Kokk T. Talvik R. Zilmer M. Kutt E. Talvik T. Markers of oxidative stress before and after exchange transfusion for neonatal sepsis. Acta Paediatr. 1996;85:1244–1246. doi: 10.1111/j.1651-2227.1996.tb18239.x. [DOI] [PubMed] [Google Scholar]
- 92.Kuehnert MJ. Roth VR. Haley NR. Gregory KR. Elder KV. Schreiber GB. Arduino MJ. Holt SC. Carson LA. Banerjee SN. Jarvis WR. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion. 2001;41:1493–1499. doi: 10.1046/j.1537-2995.2001.41121493.x. [DOI] [PubMed] [Google Scholar]
- 93.Kuriyama S. Interpreting the history of bloodletting. J Hist Med Allied Sci. 1995;50:11–46. doi: 10.1093/jhmas/50.1.11. [DOI] [PubMed] [Google Scholar]
- 94.Langlois MR. Martin ME. Boelaert JR. Beaumont C. Taes YE. De Buyzere ML. Bernard DR. Neels HM. Delanghe JR. The haptoglobin 2-2 phenotype affects serum markers of iron status in healthy males. Clin Chem. 2000;46:1619–1625. [PubMed] [Google Scholar]
- 95.Leal-Noval SR. Munoz-Gomez M. Arellano-Orden V. Marin-Caballos A. Amaya-Villar R. Marin A. Puppo-Moreno A. Ferrandiz-Millon C. Flores-Cordero JM. Murillo-Cabezas F. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36:1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- 96.Lippa S. Forni F. Candido A. Aureli V. Mango G. Oxidative stress of red blood cells stored for transfusion use. Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:105–110. [PubMed] [Google Scholar]
- 97.Liu X. Miller MJ. Joshi MS. Sadowska-Krowicka H. Clark DA. Lancaster JR., Jr. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 98.Looker D. Abbott-Brown D. Cozart P. Durfee S. Hoffman S. Mathews AJ. Miller-Roehrich J. Shoemaker S. Trimble S. Fermi G, et al. A human recombinant haemoglobin designed for use as a blood substitute. Nature. 1992;356:258–260. doi: 10.1038/356258a0. [DOI] [PubMed] [Google Scholar]
- 99.Lowe KC. Blood substitutes: from chemistry to clinic. J Mater Chem. 2006;16:4189–4196. [Google Scholar]
- 100.Lui FE. Dong P. Kluger R. Polyethylene glycol conjugation enhances the nitrite reductase activity of native and cross-linked hemoglobin. Biochemistry. 2008;47:10773–10780. doi: 10.1021/bi801116k. [DOI] [PubMed] [Google Scholar]
- 101.Lui FE. Kluger R. Enhancing nitrite reductase activity of modified hemoglobin: bis-tetramers and their PEGylated derivatives. Biochemistry. 2009;48:11912–11919. doi: 10.1021/bi9014105. [DOI] [PubMed] [Google Scholar]
- 102.Maeda N. DNA polymorphisms in the controlling region of the human haptoglobin genes: a molecular explanation for the haptoglobin 2-1 modified phenotype. Am J Hum Genet. 1991;49:158–166. [PMC free article] [PubMed] [Google Scholar]
- 103.Malavalli A. Manjula BN. Friedman JM. Acharya AS. Perturbation of the intermolecular contact regions (molecular surface) of hemoglobin S by intramolecular low-O2-affinity-inducing central cavity cross-bridges. J Protein Chem. 2000;19:255–267. doi: 10.1023/a:1007039111556. [DOI] [PubMed] [Google Scholar]
- 104.Manjula BN. Tsai A. Upadhya R. Perumalsamy K. Smith PK. Malavalli A. Vandegriff K. Winslow RM. Intaglietta M. Prabhakaran M. Friedman JM. Acharya AS. Site-specific PEGylation of hemoglobin at Cys-93(beta): correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjug Chem. 2003;14:464–472. doi: 10.1021/bc0200733. [DOI] [PubMed] [Google Scholar]
- 105.Maragos CM. Morley D. Wink DA. Dunams TM. Saavedra JE. Hoffman A. Bove AA. Isaac L. Hrabie JA. Keefer LK. Complexes of. NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 106.Maragos CM. Wang JM. Hrabie JA. Oppenheim JJ. Keefer LK. Nitric oxide/nucleophile complexes inhibit the in vitro proliferation of A375 melanoma cells via nitric oxide release. Cancer Res. 1993;53:564–568. [PubMed] [Google Scholar]
- 107.Marik PE. Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 108.Marinaro J. Smith J. Tawil I. Billstrand M. Crookston KP. HBOC-201 use in traumatic brain injury: case report and review of literature. Transfusion. 2009;49:2054–2059. doi: 10.1111/j.1537-2995.2009.02235.x. [DOI] [PubMed] [Google Scholar]
- 109.McCarthy MR. Vandegriff KD. Winslow RM. The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem. 2001;92:103–117. doi: 10.1016/s0301-4622(01)00194-6. [DOI] [PubMed] [Google Scholar]
- 110.McDonald MJ. Turci SM. Mrabet NT. Himelstein BP. Bunn HF. The kinetics of assembly of normal and variant human oxyhemoglobins. J Biol Chem. 1987;262:5951–5956. [PubMed] [Google Scholar]
- 111.McQuaid KE. Keenan AK. Endothelial barrier dysfunction and oxidative stress: roles for nitric oxide? Exp Physiol. 1997;82:369–376. doi: 10.1113/expphysiol.1997.sp004032. [DOI] [PubMed] [Google Scholar]
- 112.Milsom AB. Patel NS. Mazzon E. Tripatara P. Storey A. Mota-Filipe H. Sepodes B. Webb AJ. Cuzzocrea S. Hobbs AJ. Thiemermann C. Ahluwalia A. Role for endothelial nitric oxide synthase in nitrite-induced protection against renal ischemia-reperfusion injury in mice. Nitric Oxide. 2010;22:141–148. doi: 10.1016/j.niox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 113.Minneci PC. Deans KJ. Zhi H. Yuen PS. Star RA. Banks SM. Schechter AN. Natanson C. Gladwin MT. Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moon-Massat P. Scultetus A. Arnaud F. Brown A. Haque A. Saha B. Kim B. Sagini E. McGwin G., Jr. Auker C. McCarron R. Freilich D. The effect HBOC-201 and sodium nitrite resuscitation after uncontrolled haemorrhagic shock in swine. Injury. 2012;43:638–647. doi: 10.1016/j.injury.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 115.Moore EE. Moore FA. Fabian TC. Bernard AC. Fulda GJ. Hoyt DB. Duane TM. Weireter LJ., Jr. Gomez GA. Cipolle MD. Rodman GH., Jr. Malangoni MA. Hides GA. Omert LA. Gould SA. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 116.Mourouzis I. Dimopoulos A. Saranteas T. Tsinarakis N. Livadarou E. Spanou D. Kokkinos AD. Xinaris C. Pantos C. Cokkinos DV. Ischemic preconditioning fails to confer additional protection against ischemia-reperfusion injury in the hypothyroid rat heart. Physiol Res. 2009;58:29–38. doi: 10.33549/physiolres.931387. [DOI] [PubMed] [Google Scholar]
- 117.Munn LL. Dupin MM. Blood cell interactions and segregation in flow. Ann Biomed Eng. 2008;36:534–544. doi: 10.1007/s10439-007-9429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nachuraju P. Friedman AJ. Friedman JM. Cabrales P. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation. 2011;82:607–613. doi: 10.1016/j.resuscitation.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nandi A. Mukhopadhyay CK. Ghosh MK. Chattopadhyay DJ. Chatterjee IB. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radic Biol Med. 1997;22:1047–1054. doi: 10.1016/s0891-5849(96)00491-1. [DOI] [PubMed] [Google Scholar]
- 120.Natanson C. Kern SJ. Lurie P. Banks SM. Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nguyen MK. Kurtz I. Determinants of plasma water sodium concentration as reflected in the Edelman equation: role of osmotic and Gibbs-Donnan equilibrium. Am J Physiol Renal Physiol. 2004;286:F828–F837. doi: 10.1152/ajprenal.00393.2003. [DOI] [PubMed] [Google Scholar]
- 122.Nordmann AJ. Bucher H. Hengstler P. Harr T. Young J. Primary stenting versus primary balloon angioplasty for treating acute myocardial infarction. Cochrane Database Syst Rev. 2005:CD005313. doi: 10.1002/14651858.CD005313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ohigashi T. Brookins J. Fisher JW. Interaction of nitric oxide and cyclic guanosine 3′,5′-monophosphate in erythropoietin production. J Clin Invest. 1993;92:1587–1591. doi: 10.1172/JCI116740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olson JS. Foley EW. Rogge C. Tsai AL. Doyle MP. Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 125.Olson JS. Mathews AJ. Rohlfs RJ. Springer BA. Egeberg KD. Sligar SG. Tame J. Renaud JP. Nagai K. The role of the distal histidine in myoglobin and haemoglobin. Nature. 1988;336:265–266. doi: 10.1038/336265a0. [DOI] [PubMed] [Google Scholar]
- 126.Pelletier MM. Kleinbongard P. Ringwood L. Hito R. Hunter CJ. Schechter AN. Gladwin MT. Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 127.Phillips L. Toledo AH. Lopez-Neblina F. Anaya-Prado R. Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22:46–55. doi: 10.1080/08941930802709470. [DOI] [PubMed] [Google Scholar]
- 128.Robinson Y. Cristancho E. Boning D. Intravascular hemolysis and mean red blood cell age in athletes. Med Sci Sports Exerc. 2006;38:480–483. doi: 10.1249/01.mss.0000188448.40218.4c. [DOI] [PubMed] [Google Scholar]
- 129.Rohlfs RJ. Brunner E. Chiu A. Gonzales A. Gonzales ML. Magde D. Magde MD., Jr. Vandegriff KD. Winslow RM. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998;273:12128–12134. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 130.Rother RP. Bell L. Hillmen P. Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 131.Sakai H. Sato A. Takeoka S. Tsuchida E. Rheological properties of hemoglobin vesicles (artificial oxygen carriers) suspended in a series of plasma-substitute solutions. Langmuir. 2007;23:8121–8128. doi: 10.1021/la7004503. [DOI] [PubMed] [Google Scholar]
- 132.Sakai H. Sou K. Tsuchida E. Hemoglobin-vesicles as an artificial oxygen carrier. Methods Enzymol. 2009;465:363–384. doi: 10.1016/S0076-6879(09)65019-9. [DOI] [PubMed] [Google Scholar]
- 133.Sakai H. Tsai AG. Kerger H. Park SI. Takeoka S. Nishide H. Tsuchida E. Intaglietta M. Subcutaneous microvascular responses to hemodilution with a red cell substitute consisting of polyethyleneglycol-modified vesicles encapsulating hemoglobin. J Biomed Mater Res. 1998;40:66–78. doi: 10.1002/(sici)1097-4636(199804)40:1<66::aid-jbm8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 134.Secomb TW. Hsu R. Pries AR. A model for red blood cell motion in glycocalyx-lined capillaries. Am J Physiol. 1998;274:H1016–H1022. doi: 10.1152/ajpheart.1998.274.3.H1016. [DOI] [PubMed] [Google Scholar]
- 135.Segre G. Silberberg A. Behaviour of macroscopic rigid spheres in Poiseuille flow. J Fluid Mech. 1962;14:115–135. [Google Scholar]
- 136.Shander A. Hofmann A. Ozawa S. Theusinger OM. Gombotz H. Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–765. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 137.Silverman TA. Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Anesthesiology. 2009;111:946–963. doi: 10.1097/ALN.0b013e3181ba3c2c. [DOI] [PubMed] [Google Scholar]
- 138.Sloan EP. Koenigsberg M. Gens D. Cipolle M. Runge J. Mallory MN. Rodman G., Jr. Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock: a randomized controlled efficacy trial. JAMA. 1999;282:1857–1864. doi: 10.1001/jama.282.19.1857. [DOI] [PubMed] [Google Scholar]
- 139.Smagghe BJ. Trent JT., 3rd Hargrove MS. NO dioxygenase activity in hemoglobins is ubiquitous in vitro, but limited by reduction in vivo. PLoS One. 2008;3:e2039. doi: 10.1371/journal.pone.0002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sriram K. Vazquez BY. Yalcin O. Johnson PC. Intaglietta M. Tartakovsky DM. The effect of small changes in hematocrit on nitric oxide transport in arterioles. Antioxid Redox Signal. 2011;14:175–185. doi: 10.1089/ars.2010.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Standl T. Wilhelm S. Horn EP. Burmeister M. Gundlach M. Schulte am Esch J. [Preoperative hemodilution with bovine hemoglobin. Acute hemodynamic effects in liver surgery patients] Anaesthesist. 1997;46:763–770. doi: 10.1007/s001010050466. [DOI] [PubMed] [Google Scholar]
- 142.Stone JR. Marletta MA. Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. Biochemistry. 1996;35:1093–1099. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- 143.Stowell CP. Levin J. Spiess BD. Winslow RM. Progress in the development of RBC substitutes. Transfusion. 2001;41:287–299. doi: 10.1046/j.1537-2995.2001.41020287.x. [DOI] [PubMed] [Google Scholar]
- 144.Stramer SL. Current risks of transfusion-transmitted agents—a review. Arch Pathol Lab Med. 2007;131:702–707. doi: 10.5858/2007-131-702-CROTAA. [DOI] [PubMed] [Google Scholar]
- 145.Stramer SL. Hollinger FB. Katz LM. Kleinman S. Metzel PS. Gregory KR. Dodd RY. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49:1S–235S. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 146.Svergun DI. Ekstrom F. Vandegriff KD. Malavalli A. Baker DA. Nilsson C. Winslow RM. Solution structure of poly(ethylene) glycol-conjugated hemoglobin revealed by small-angle X-ray scattering: implications for a new oxygen therapeutic. Biophys J. 2008;94:173–181. doi: 10.1529/biophysj.107.114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taylor JGt. Nolan VG. Mendelsohn L. Kato GJ. Gladwin MT. Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS One. 2008;3:e2095. doi: 10.1371/journal.pone.0002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsai AG. Cabrales P. Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626–1634. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 149.Tsai AG. Cabrales P. Manjula BN. Acharya SA. Winslow RM. Intaglietta M. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108:3603–3610. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tsai AG. Intaglietta M. Sakai H. Delpy E. Drieu LARC. Rousselot M. Zal F. Microcirculation and NO-CO studies of a natural extracellular hemoglobin developed for an oxygen therapeutic carrier. Curr Drug Discov Technol. 2012;9:166–172. doi: 10.2174/157016312802650814. [DOI] [PubMed] [Google Scholar]
- 151. This reference has been deleted.
- 152.Tsai AG. Vandegriff KD. Intaglietta M. Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol. 2003;285:H1411–H1419. doi: 10.1152/ajpheart.00307.2003. [DOI] [PubMed] [Google Scholar]
- 153. This reference has been deleted.
- 154.Tsuchida E. Sou K. Nakagawa A. Sakai H. Komatsu T. Kobayashi K. Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugate chemistry. Bioconjug Chem. 2009;20:1419–1440. doi: 10.1021/bc800431d. [DOI] [PubMed] [Google Scholar]
- 155.Tsukahara T. Haniu H. Cellular cytotoxic response induced by highly purified multi-wall carbon nanotube in human lung cells. Mol Cell Biochem. 2011;352:57–63. doi: 10.1007/s11010-011-0739-z. [DOI] [PubMed] [Google Scholar]
- 156.Uyuklu M. Meiselman HJ. Baskurt OK. Role of hemoglobin oxygenation in the modulation of red blood cell mechanical properties by nitric oxide. Nitric Oxide. 2009;21:20–26. doi: 10.1016/j.niox.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vandegriff KD. Malavalli A. Woodridge J. Lohman J. Winslow RM. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43:509–516. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 158. This reference has been deleted.
- 159.Vandegriff KD. Winslow RM. Hemospan: design principles for a new class of oxygen therapeutic. Artif Organs. 2009;33:133–138. doi: 10.1111/j.1525-1594.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 160.Viele MK. Weiskopf RB. Fisher D. Recombinant human hemoglobin does not affect renal function in humans: analysis of safety and pharmacokinetics. Anesthesiology. 1997;86:848–858. doi: 10.1097/00000542-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 161.Villela NR. Salazar Vazquez BY. Intaglietta M. Microcirculatory effects of intravenous fluids in critical illness: plasma expansion beyond crystalloids and colloids. Curr Opin Anaesthesiol. 2009;22:163–167. doi: 10.1097/ACO.0b013e328328d304. [DOI] [PubMed] [Google Scholar]
- 162.Wallis JP. Nitric oxide and blood: a review. Transfus Med. 2005;15:1–11. doi: 10.1111/j.1365-3148.2005.00542.x. [DOI] [PubMed] [Google Scholar]
- 163.Wang SK. Hwang NH. On transport of suspended particulates in tube flow. Biorheology. 1992;29:353–377. doi: 10.3233/bir-1992-292-313. [DOI] [PubMed] [Google Scholar]
- 164.Wegria R. Nickerson JL. Case RB. Holland JF. Effect of nitroglycerine on the cardiovascular system of normal persons. Am J Med. 1951;10:414–418. doi: 10.1016/0002-9343(51)90286-0. [DOI] [PubMed] [Google Scholar]
- 165.Weiskopf RB. Hemoglobin-based oxygen carriers: compassionate use and compassionate clinical trials. Anesth Analg. 2010;110:659–662. doi: 10.1213/ANE.0b013e3181c85255. [DOI] [PubMed] [Google Scholar]
- 166.Wettstein R. Tsai AG. Erni D. Lukyanov AN. Torchilin VP. Intaglietta M. Improving microcirculation is more effective than substitution of red blood cells to correct metabolic disorder in experimental hemorrhagic shock. Shock. 2004;21:235–240. doi: 10.1097/01.shk.0000114301.36496.ea. [DOI] [PubMed] [Google Scholar]
- 167.Wettstein R. Tsai AG. Erni D. Winslow RM. Intaglietta M. Resuscitation with polyethylene glycol-modified human hemoglobin improves microcirculatory blood flow and tissue oxygenation after hemorrhagic shock in awake hamsters. Crit Care Med. 2003;31:1824–1830. doi: 10.1097/01.CCM.0000069340.16319.F2. [DOI] [PubMed] [Google Scholar]
- 168.Winslow RM. Vasoconstriction and the efficacy of hemoglobin-based blood substitutes. Transfus Clin Biol. 1994;1:9–14. doi: 10.1016/s1246-7820(05)80050-3. [DOI] [PubMed] [Google Scholar]
- 169.Winslow RM. MP4, a new nonvasoactive polyethylene glycol-hemoglobin conjugate. Artif Organs. 2004;28:800–806. doi: 10.1111/j.1525-1594.2004.07392.x. [DOI] [PubMed] [Google Scholar]
- 170.Winslow RM. Targeted O2 delivery by low-p50 hemoglobin: a new basis for hemoglobin-based oxygen carriers. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:1–12. doi: 10.1081/bio-200046634. [DOI] [PubMed] [Google Scholar]
- 171.Wood KC. Hsu LL. Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 172.Young MA. Riddez L. Kjellstrom BT. Bursell J. Winslow F. Lohman J. Winslow RM. MalPEG-hemoglobin (MP4) improves hemodynamics, acid-base status, and survival after uncontrolled hemorrhage in anesthetized swine. Crit Care Med. 2005;33:1794–1804. doi: 10.1097/01.ccm.0000172648.55309.13. [DOI] [PubMed] [Google Scholar]
- 173.Yu B. Bloch KD. Zapol WM. Hemoglobin-based red blood cell substitutes and nitric oxide. Trends Cardiovasc Med. 2009;19:103–107. doi: 10.1016/j.tcm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu B. Shahid M. Egorina EM. Sovershaev MA. Raher MJ. Lei C. Wu MX. Bloch KD. Zapol WM. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. 2010;112:586–594. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu B. Volpato GP. Chang K. Bloch KD. Zapol WM. Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide. Anesthesiology. 2009;110:113–122. doi: 10.1097/ALN.0b013e318190bc4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang L. Levy A. Rifkind JM. Autoxidation of Hemoglobin Enhanced by Dissociation into Dimers. J Biol Chem. 1991;266:24698–24701. [PubMed] [Google Scholar]
- 177.Zhou JL. Li G. Hai Y. Guan L. Huang XL. Sun P. Protection of carbon monoxide-releasing molecule against lung injury induced by limb ischemia-reperfusion. Chin J Traumatol. 2009;12:71–76. [PubMed] [Google Scholar]