Abstract
Interpenetrating network (IPN) hydrogels were recently introduced to the cartilage tissue engineering literature, with the approach of encapsulating cells in thermally gelling agarose that is then soaked in a poly(ethylene glycol) diacrylate (PEGDA) solution, which is then photopolymerized. These IPNs possess significantly enhanced mechanical performance desirable for cartilage regeneration, potentially allowing patients to return to weight-bearing activities quickly after surgical implantation. In an effort to improve cell viability and performance, inspiration was drawn from previous studies that have elicited positive chondrogenic responses to aggrecan, the proteoglycan largely responsible for the compressive stiffness of cartilage. Aggrecan was incorporated into the IPNs in conservative concentrations (40 μg/mL), and its effect was contrasted with the incorporation of chondroitin sulfate (CS), the primary glycosaminoglycan associated with aggrecan. Aggrecan was incorporated by physical entrapment within agarose and methacrylated CS was incorporated by copolymerization with PEGDA. The IPNs incorporating aggrecan or CS exhibited over 50% viability with encapsulated chondrocytes after 6 weeks. Both aggrecan and CS improved cell viability by 15.6% and 20%, respectively, relative to pure IPNs at 6 weeks culture time. In summary, we have introduced the novel approach of including a raw material from cartilage, namely aggrecan, to serve as a bioactive signal to cells encapsulated in IPN hydrogels for cartilage tissue engineering, which led to improved performance of encapsulated chondrocytes.
Introduction
Numerous biomaterials have been explored for cartilage tissue engineering, including various hydrogels.1–4 The hydrogels most common to cartilage tissue engineering are alginate,5–8 agarose,5,9–12 poly(ethylene glycol) (PEG),13–17 and their derivatives. Hern and Hubbell18 established a methodology in 1998 for tethering the peptide sequence RGD to poly(ethylene glycol) diacrylate (PEGDA), a technique that has been shown by their group and others to facilitate cell spreading and alignment18–20 to promote viability21–23 and proliferation,24,25 as well as to improve matrix synthesis.16,24–26 A primary interest of the current study was to provide a signal that may potentially not only serve as an adhesion molecule, but perhaps also to simultaneously serve as a signal favorable to and specific to cartilage tissue engineering, and to then incorporate this strategy in interpenetrating network (IPN) hydrogels. An IPN is a polymer comprising two or more networks that are at least partially interlaced on a polymer scale, but not covalently linked to each other. As the two networks are independent of each other, while being physically interlocked, this type of network is termed an IPN.
IPN hydrogels have properties that typically either retain the characteristics of single networks or are an average of the two independent networks.27,28 Moreover, a recently published study on semi-IPNs of PEGDA and hyaluronic acid intended for a fibroblast application exhibited tensile moduli in the 25–50 kPa range, which is far below the modulus required for engineered cartilage.28 However, the research group of Gong and Osada discovered that for a variety of hydrogel IPNs and semi-IPNs based on synthetic and biopolymers, the mechanical properties of the IPNs were far superior to either of the individual networks.29–37 With regard to biomedical applications, they recognized that the properties they measured with these IPNs compared favorably with tissues such as cartilage. All of these attempts were focused on the importance of mechanical performance of hydrogels in tissue engineering, but they did not encapsulate live cells to study their cell–matrix interaction for tissue engineering. In our previous report, we investigated agarose-PEG-based IPN hydrogels that exhibited cytocompatibility and improved mechanical properties relative to the component networks.38
Although several studies have investigated the effect of biomolecules in improved cell response and promoting ECM synthesis in a single network hydrogel,39–44 there have been no reports investigating the chondrocyte performance in IPN hydrogels with incorporated bioactive molecules. Aggrecan, a key structural component of cartilage, has not been used as a bioactive signal in scaffolds for cartilage tissue engineering, although its demonstrated ability to promote chondrogenesis in monolayer cultures provides a rationale for evaluating aggrecan as a signal in cartilage tissue engineering.45,46 Chondroitin sulfate (CS) represents a major structural component of aggrecan, and has improved chondrogenesis in cartilage tissue engineering applications.47–50 Recently, we have developed a novel synthesis protocol to incorporate methacrylated chondroitin sulfate (MCS) into the agarose-PEGDA IPN for improving cell viability and biosynthesis.51 Therefore, in the present study, we hypothesized that cell performance in IPNs would be improved with the incorporation of aggrecan at a level similar to or exceeding that associated with the inclusion of CS. The goal of the current study was thus to develop a novel synthesis protocol to incorporate aggrecan into the agarose-PEGDA IPN for improving cell viability and biosynthesis. The overall objective of this study was then to combine recent advances in creating high toughness IPNs and the chondrogenic ability of aggrecan to create a significant new class of biomaterials for cartilage regeneration. In this study, physical entrapment of aggrecan was contrasted with covalently linked CS.
Materials and Methods
Materials
2-hydroxyethyl agarose (Type VII) and high purity PEGDA (molecular weight 2000 Da) were obtained from Sunbio, Inc. The photoinitiator, 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl] 2-methyl-1-propanone (Irgacure 2959), was purchased from Ciba Specialty Chemicals Corp. Aggrecan from bovine articular cartilage, CS A sodium salt (Type A 70%, balanced with Type C, from bovine trachea), papain (from papaya latex), N-acetyl cysteine, ethylenediaminetetraacetic acid (EDTA), potassium phosphate buffer, and phosphate-buffered saline (PBS) were purchased from Sigma. Glycidyl methacrylate (for GC, 97.0%) was purchased from Sigma-Aldrich.
Methacrylation of CS
CS A sodium salt was methacrylated using methods adopted from Li et al.39 with slight modifications. First, 5 g of CS was dissolved in 50 mL of PBS. About 5 mL of GMA was added to the CS solution and the reaction was stirred at room temperature for 15 days. Next, MCS was precipitated in acetone (1:20 v/v), then filtered, and redissolved in 100 mL of Milli-Q purified (Millipore Corp.) water followed by chloroform extraction (1:1 v/v). The aqueous polymer solution was separated from the chloroform via a separatory funnel and concentrated back to about 50 mL by rotavap, followed by a second acetone precipitation (1:20 v/v), centrifugation, and acetone removal. Acetone was removed by drying in air at room temperature. The resulting precipitant was collected and dried at room temperature for 48 h. The MCS was redissolved in water and lyophilized (−46°C, 0.0140 mbar). The degree of methacrylation was quantified by the NMR method as described previously.51
Chondrocyte isolation and culture
Articular cartilage samples were dissected aseptically from 15 ankles of 8–9 month old male Duroc hogs, which were obtained from a local butcher. Cells were harvested within 36 h after slaughter following aseptic procedures in our recent reports.52,53 The articular cartilage samples were diced into approximately 1 mm3 pieces using a scalpel in autoclaved PBS. After rinsing with PBS three times, the cartilage samples were digested in a sterile filtered solution of 30 mL of 2 mg/mL type II collagenase (305 U/mg; Worthington Biochemical) for 18 h on an orbital shaker in a humidified 37°C, 5% CO2 incubator. The digested cartilage solution was filtered through a 100 μm filter cell strainer to remove undigested cartilage lumps. The filtrate was then centrifuged at 1500 rpm for 5 min, and then the cell pellet was resuspended in a chondrocyte culture medium in a humidified incubator (37°C, 5% CO2). The culture medium consisted of the Dulbecco's modified Eagle's medium (DMEM) with 4.5 g/L D-glucose supplemented with 10% fetal bovine serum (FBS), 1% nonessential amino acids, 1% sodium pyruvate, 50 μg/mL ascorbic acid, and 0.25 mg/mL penicillin–streptomycin fungicide. The DMEM and supplements were obtained from Invitrogen. The cell number was determined using a hemocytometer. The freshly isolated cells were grown to 90–95% confluence in T75 flasks, and then retrieved using trypsin-EDTA. First passage chondrocytes were used in this study, and the culture medium was changed every 2 to 3 days.
Encapsulation of chondrocytes in the agarose gel
Chondrocytes were encapsulated in the bioactive IPN hydrogels using our previously published methods.38 The chondrocytes were detached from T75 flasks using trypsin-EDTA and resuspended in PBS at a high concentration (5–10 million cells/mL). Cells from the PBS suspension were counted using a hemocytometer. The encapsulating agarose solution was prepared by adding 0.3 g agarose powder (cell culture grade) to 10 mL PBS and autoclaving for 30 min. The agarose solution temperature was monitored under an aseptic environment until 39°C was reached. At this point, the chondrocyte suspension was mixed with the liquid agarose at a 1:2 ratio for a final concentration of 25 million cells/mL in 2% agarose. The cell suspension in the agarose solution was pipetted into sterilized cylindrical silicone rubber molds measuring 5 mm in diameter and 2 mm in height. The molds were clamped between glass plates and cooled at 4°C for 9–10 min, and then chondrocyte-encapsulated agarose gel constructs were transferred to non-tissue cultured 24-well plates. Each well was supplied with 1.5 mL of a fresh growth medium, and placed in a 37°C incubator overnight for 12–16 h.
Synthesis of agarose-PEGDA IPNs
IPNs are created by soaking for 2.5 h the agarose-encapsulated porcine chondrocytes constructs (after overnight incubation) in a solution of PEGDA (15% w/v), photoinitiator Irgacure 2959 (0.1% w/v), which is subsequently photopolymerized to form a copolymer second network that interpenetrates the agarose constructs, as shown in our previous work.38 Three sets of IPNs were made: those with no biomolecules, those with aggrecan incorporated, and those with CS incorporated. When cells were incorporated in the agarose as described in the previous section, aseptic conditions were used, but for physical characterizations such precautions were not necessary.
In one case, 40 μg/mL MCS was mixed with the chondrocyte-encapsulated agarose, and then copolymerized with the PEGDA during photopolymerization (Fig. 1) and in the other, 40 μg/mL of aggrecan was mixed with the chondrocyte-encapsulated agarose to physically entrap it within the IPN (Fig. 2). Afterward, the equilibrated gels were placed in rectangular silicon molds between optical glass microscope slides (1 mm thick) and the surrounding space was filled with the excess PEGDA/PBS solution from the soak vials to avoid air bubbles. The gels were exposed to ultraviolet light for 5 min on each side using a 312 nm light, 3.0 mW/cm2 (XL-100; Spectronics Corp.). Pure agarose-PEGDA IPNs without CS or aggrecan were synthesized using the same procedure as described above. Using a 3 mm biopsy punch, gel samples were then cut from the center of the polymerized IPN area of each construct, whether agarose-PEGDA, agarose-PEGDA with aggrecan, or agarose-PEGDA with CS IPN gels. The IPN constructs with encapsulated cells from each group were then cultured for 6 weeks at 37°C with 5% CO2 in 1.5 mL of the chondrocyte growth medium (DMEM with 4.5 g/L D-glucose supplemented with 10% FBS, 1% nonessential amino acids, 1% sodium pyruvate, 50 μg/mL ascorbic acid, 0.25 mg/mL penicillin–streptomycin fungicide, and 100 ng/mL human recombinant insulin-like growth factor [PeproTech, Inc.]), which was replaced every 2–3 days.
FIG. 1.
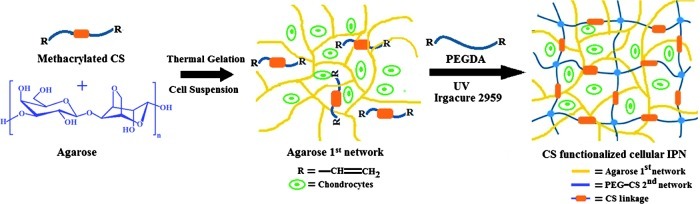
Schematic diagram showing formation of a bioactive interpenetrating network (IPN) gel containing chondroitin sulfate (CS) as a bioactive signal. Color images available online at www.liebertpub.com/tea
FIG. 2.
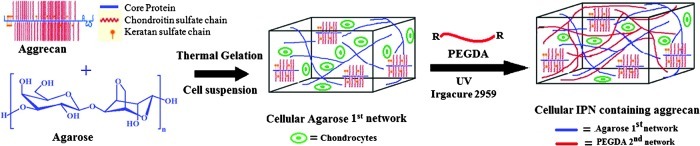
Schematic representation showing formation of agarose-poly(ethylene glycol) diacrylate (PEGDA) double network containing aggrecan as a bioactive signal. Color images available online at www.liebertpub.com/tea
Similarly, acellular agarose-PEGDA IPNs containing CS and aggrecan were synthesized non-aseptically using the above photopolymerization procedure, which was done for the purpose of providing baseline values for glycosaminoglycan (GAG) quantification and Safranin-O staining as a comparison for cellular constructs. Using a 3 mm biopsy punch, gels (3 mm diameter and 2 mm height) were then cut from the IPN area and allowed to equilibrate in PBS and deionized (DI) water for at least 24 h before use.
Swelling degree of IPN gels
IPN gels were placed in excess DI water for at least 24 h to remove extractable materials from the polymer networks. Equilibrated cell-free gel samples were weighed and placed into a desiccation chamber. After at least 48 h, the dried gel samples were removed and weighed again. The swelling degree, Q, was calculated as the ratio of the weight of the equilibrated IPN hydrogel to its dry weight.
Analysis of cell-seeded constructs
We selected two time points (0 and 6 weeks) to evaluate compression properties, and three time points (0, 3, and 6 weeks) to evaluate viability and biosynthesis. Week 0 for cell viability and biosynthesis was defined as 24 h following cell encapsulation in the IPN. Similarly, for the cell-seeded groups evaluated under compression, week 0 was defined as 24 h following cell encapsulation. We emphasize that for all sample sizes provided, these indicate different batches of synthesized IPNs, as opposed to multiple samples being tested from a single batch.
Compression testing
The compressive modulus of the IPN hydrogels was determined at room temperature on an RSA-III dynamic mechanical analyzer (TA Instruments) and tested under unconfined uniaxial compression with a 35 N loading cell (n=5). All measurements were performed on IPN gels swollen to equilibrium in PBS, and the compression plates were lubricated with mineral oil both to minimize any gel plate adhesion and to prevent gel drying during testing. Hydrogels were then compressed in the direction normal to the circular face of the IPN gel at a rate of 0.0005 mm/s (1.7%/min) until mechanical failure occurred. The compressive elastic modulus, defined as the slope of the linear region of the stress–strain curve of a material under compression, was calculated from the initial linear portion of the curve (<20% strain). Fracture points were identified at the peak stress after which a significant (>10%) decrease in stress occurred. Shear modulus values were calculated using the neo-Hookean model for ideal elastomers. The plot of stress versus the strain function, λ−1/λ2, where λ=L/L0 (neo-Hookean Model), yields a straight line for ideal elastomers, where the slope of the line is equal to the gel shear modulus.54 The values were used in the calculations for a set of at least five samples.
Live/dead assay
To compare the viability of cells encapsulated in the hydrogels, a live/dead assay was performed immediately after 0, 3, and 6 weeks of culture (n=3), using a live/dead viability cytotoxicity kit (Molecular Probes). This kit contained 2 mM Calcein-AM to stain the living cells and 4 mM ethidium homodimer-1 to stain the dead cells. Cylindrical hydrogel constructs, 3 mm in diameter, were sectioned horizontally into two equal halves (each of 1 mm thickness) and incubated in live/dead reagents for 30 min before imaging to promote thorough staining. Fluorescence spinning-disk confocal microscopy was used to visualize the green living cells and the red dead cells, using a Yokugawa CSU10 spinning-disk attached to an Olympus IX 81 microscope with 488 nm excitation/515–540 nm emission and 561 nm excitation/585 LP emission filters with a charge-coupled device camera (CoolSnap HQ2; Photometrics) controlled by Slidebook software (version 4.2; Intelligent Imaging Innovations, Inc.). Z-scans were performed to a resolution depth of 350–500 μm in areas representative of the overall IPN gels. Images were acquired in a 2×2 binning mode. The three-dimensional images were deconvoluted using a constrained iterative algorithm (Slidebook). The percentage of total viable cells was calculated using the SlideBook (version 5.0) Mask Statistic module.
Biochemical analyses
Biochemical assays were performed on the cellular CS-incorporated IPNs, aggrecan-incorporated IPNs, and IPN constructs without bioactive signals to measure the total collagen (by hydroxyproline assay), GAG, and DNA present in the constructs at the end of each time point. At 0, 3, and 6 weeks, four samples were removed aseptically from the culture and placed in microcentrifuge tubes. Samples were mechanically crushed, and then homogenized with a papain mixture (125 mg/mL papain [from papaya latex], 5 mM N-acetyl cysteine, 5 mM EDTA, and 100 mM potassium phosphate buffer) and allowed to digest overnight in a 60°C water bath. The digested constructs were then stored at −20°C. The digested constructs were thawed in a 37°C water bath and centrifuged at 10,000 rpm for 10 min to pellet fragments of polymers before conducting assays. The supernatant was used to determine the GAG, DNA, and hydroxyproline content. The DNA content was quantified using the PicoGreen assay (Molecular Probes) according to the manufacturer's instructions. The total GAG content was determined using the dimethylmethylene blue (Biocolor; Carrickfergus) spectrophotometric assay, with CS as the standard.55 The GAG content associated with the incorporated CS was measured using samples (n=4) collected from acellular IPN constructs containing CS (10.6±2.4 μg/construct, 7.2±3.2 μg/construct, and 6.5±2.5 μg/construct at 0, 3, and 6 weeks, respectively), while those associated with incorporated aggrecan were measured using samples (n=4) collected from acellular IPN constructs containing aggrecan (11.4±5.8 μg/construct, 5.8±0.7 μg/construct, and 2.9±1.5 μg/construct at 0, 3 and 6 weeks, respectively). These values were subtracted from the GAG values of cellular IPN constructs containing CS samples to provide the reported GAG contents for the experimental groups. Similarly, acellular GAG values were subtracted from GAG values obtained from cellular IPN constructs containing aggrecan. As an indicator of the collagen content, a hydroxyproline assay was assessed as described in our previous publication.56 Both GAG and hydroxyproline contents were normalized to the DNA content.
Histological analyses
Histological analysis was performed using Safranin-O and hematoxylin and eosin (H&E) staining on cell-encapsulated constructs at 0, 3, and 6 weeks (n=2). Constructs were fixed in 10% formalin for 2–3 h, and soaked in an optimal cutting temperature medium (Ted Pella, Inc.) overnight at 44°C. Constructs were then placed in a −20°C freezer until further processing. Sections were cut at −20°C using a cryostat (MICROM HM 550) to 8-μm thickness, mounted on a microscope slide and allowed to dry for 1 h at room temperature. Following standard histological procedures, the sections were stained with hematoxylin and Safranin-O, which stain nuclei purple and negatively charged GAGs orange, respectively. Eosin stains cytoplasm, connective tissues, and other extracellular substances red or pink.
Statistical analysis
All data are expressed as mean±standard deviations. Statistical significance was determined by single-factor analysis of variance followed by Tukey's post hoc analysis. Analysis was performed using the SPSS 17.0 statistical software package. Significance was defined as p<0.05 throughout the study.
Results
The aim of the present study was to analyze the effects of CS and aggrecan in agarose-PEGDA IPNs. To study this incorporation effect, three different IPNs were synthesized and examined: (1) agarose-PEGDA IPN without any biomolecules (Pure IPN); (2) agarose-PEGDA IPN with CS (IPN-CS); and (3) agarose-PEGDA IPN with aggrecan (IPN-aggrecan).
Material and structural properties of IPN gels
The IPN gels were synthesized via a two-step network formation: the first step was the formation of a rigid, brittle gel network and the second was the formation of a softer and more ductile network within the first network. The bioactive IPN was synthesized in this study using MCS, which was 6.3% methacrylated as confirmed by the NMR method.51
At room temperature, the equilibrium-swelled IPNs were optically transparent in the absence of cells (Fig. 3A), but are translucent when chondrocytes have been encapsulated (Fig. 3B). The acellular equilibrium IPN-CS gels showed a 2.2-fold increase in swelling degree in DI water when compared with the pure IPN gels (p<0.05, n=3), while the swelling degree of IPN-aggrecan was not statistically significant from both the pure IPN and IPN-CS group at initial time point (Table 1). These acellular IPN gels were soaked in DI water for a 6-week period and characterized again for swelling degrees. It was observed that there was a decrease in swelling degrees from week 0 to 6 in all the three IPN gels. IPN-CS gels showed a statistically significant decrease in Q (p<0.05, n=3), while the decrease in Q of IPN-aggrecan was not significant. After 6 weeks, all three of the IPN gel groups exhibited similar swelling degrees.
FIG. 3.
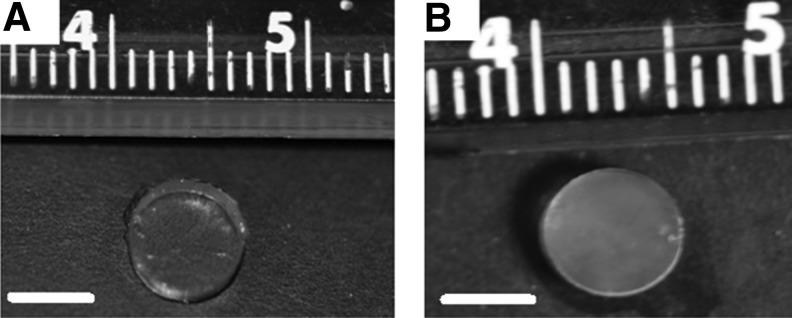
Macroscopic views of IPNs gels: representative images of equilibrium swelled IPNs without (A) and with (B) encapsulated chondrocytes. Note the reduction in transparency with encapsulated chondrocytes. Scale bar=4 mm.
Table 1.
Mechanical and Swelling Properties of Acellular and Cellular IPN Gels
| |
Compressive elastic moduli (E) (kPa)a,b |
Compressive shear moduli (G) (kPa)a,b |
E/G |
Swelling degree Qc |
||||
|---|---|---|---|---|---|---|---|---|
| Gel type | Week 0 | Week 6 | Week 0 | Week 6 | Week 0 | Week 6 | Week 0 | Week 6 |
| Pure IPN | 129±13 | 107±10 | 40.4±10.3 | 35.7±4.4 | 3.2±0.34 | 3.0±0.64 | 11.2±3.2 | 10.2±4.2 |
| IPN-CS | 139±16 | 83±11 | 49.9±9.4 | 25.4±7.3 | 2.8±0.45 | 3.3±0.35 | 25.4±5.2 | 11.5±3.3 |
| IPN-aggrecan | 141±15 | 120±14 | 48.9±8.5 | 33.4±9.2 | 2.9±0.62 | 3.6±0.97 | 16.8±6.3 | 12.7±6.1 |
| Pure IPN+cells | 109±18 | 75±13 | 32.3±6.3 | 20.3±8.2 | 3.4±0.75 | 3.7±0.88 | — | — |
| IPN-CS+cells | 130±20 | 91±10 | 42.1±12.4 | 25.4±8.9 | 3.1±0.23 | 3.6±0.34 | — | — |
| IPN-aggrecan+cells | 101±12 | 74±9 | 31.8±6.9 | 22.5±9.6 | 3.2±0.18 | 3.3±0.57 | — | — |
All values are reported as mean±standard deviation, n=5 for compression testing and 3 for swelling measurements.
No swelling degree measurements were performed on cellular constructs due to limited sample numbers.
There were no statistically significant differences among the groups at week 0 and 6.
Statistically significant differences between week 0 and 6 for IPN-CS and IPN-CS+cells (p<0.05).
Statistically significant differences among pure IPN and IPN-CS at week 0 (p<0.05).
Pure IPN, agarose-PEGDA interpenetrating network with no bioactive signals; IPN-CS, agarose-PEGDA interpenetrating network with 40 μg/mL chondroitin sulfate; IPN-aggrecan, agarose-PEGDA interpenetrating network with 40 μg/mL aggrecan; PEGDA, poly(ethylene glycol) diacrylate.
The shear modulus (G) of the acellular IPN-CS gel was 23.5% higher than pure IPN, while it was 21% higher than IPN-aggrecan gels at week 0 (n=5, p<0.05) (Table 1). However, the shear moduli in IPN-CS gels decreased by 49% from week 0 to 6 (p<0.05), while the decrease in shear moduli from week 0 to 6 in all other IPN gels was not statistically significant (Table 1). The shear moduli of cellular IPNs gel were determined at week 0 and 6. The presence of encapsulated chondrocytes did not significantly compromise mechanical properties of IPN gels at week 0 (see Table. 1). After the 6-week culture period, all of the cellular IPN gels showed a decrease in shear moduli, although differences were not statistically significant. The shear modulus in cellular IPN-CS gels decreased significantly by 39.6% from week 0 to 6 (p<0.05, n=5). As E ∼3G for incompressible materials such as hydrogels, a similar behavior in the elastic modulus, E, was observed (see Table 1).
Acellular IPN-CS gels displayed a 33% increase in fracture properties (1.2 vs. 0.9 MPa) and a 20% increase in toughness (151 vs. 125 kJ/m3) relative to the pure IPN (p<0.05, n=5), while the IPN-aggrecan gels showed an increase of 11% in fracture properties (1.0 vs. 0.9 MPa) and a 17% increase in toughness (147 vs. 125 kJ/m3) relative to the pure IPN at week 0. These fracture properties decreased significantly from week 0 to 6 in the IPN-CS gel group (p<0.05, n=5) (Table 2).
Table 2.
Compressive Failure Properties of Acellular and Cellular Interpenetrating Network Gels
| |
Fracture strain (%) |
Fracture stress (MPa)a,b |
Toughness (kJ/m3)a,b |
|||
|---|---|---|---|---|---|---|
| Gel type | Week 0 | Week 6 | Week 0 | Week 6 | Week 0 | Week 6 |
| Pure IPN | 78±10 | 70±20 | 0.9±0.3 | 0.7±0.2 | 125±38 | 89±21 |
| IPN-CS | 79±14 | 73±11 | 1.2±0.7 | 0.9±0.3 | 151±47 | 77±35 |
| IPN-aggrecan | 80±12 | 78±18 | 1.0±0.6 | 0.8±0.2 | 147±28 | 99±29 |
| Pure IPN+cells | 77±17 | 74±21 | 0.8±0.3 | 0.6±0.3 | 96±39 | 60±27 |
| IPN-CS+cells | 76±13 | 69±19 | 1.1±0.5 | 0.7±0.4 | 127±82 | 105±30 |
| IPN-aggrecan+cells | 79±16 | 75±20 | 0.7±0.3 | 0.6±0.3 | 108±77 | 76±31 |
All values are reported as mean±standard deviation, n=5.
There were no statistically significant differences among the groups at week 0 and 6.
Statistically significant differences between week 0 and 6 for IPN-CS and IPN-CS+cells (p<0.05).
Chondrocyte viability
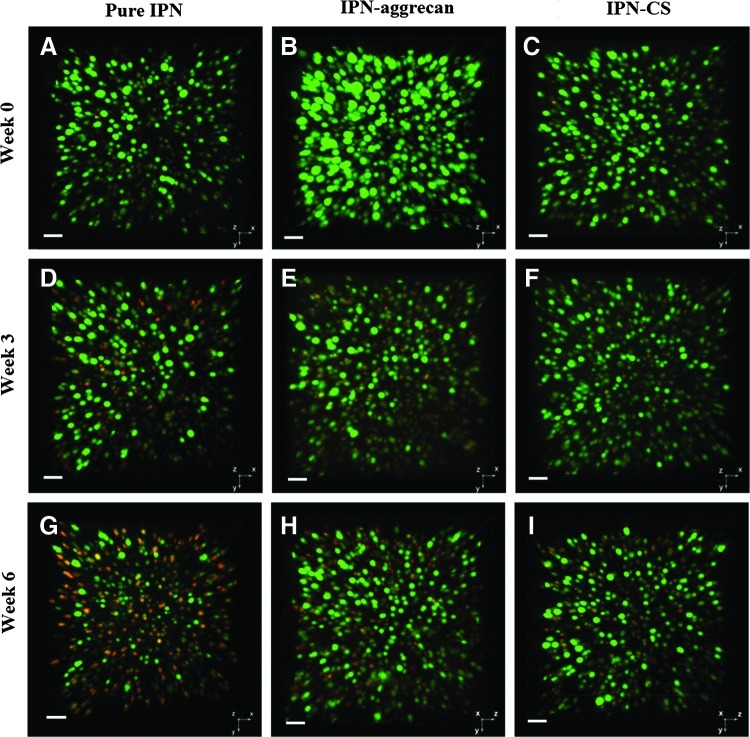
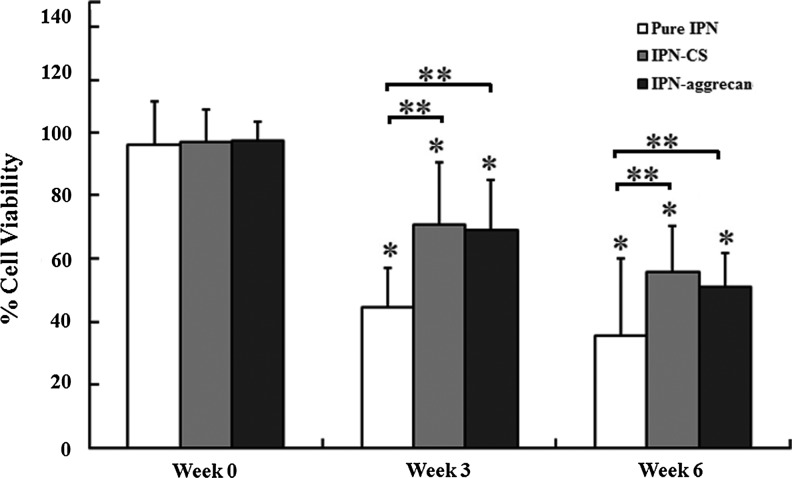
Live/dead (Calcein-ethidium dye) cell staining indicated that the majority of cells encapsulated in IPN-CS and IPN-aggrecan gels remained viable (stained fluorescent green with Calcein) over the culture period of 6 weeks (Fig. 4). Mask statistics analysis showed that in the absence of any bioactive signals (pure IPN gels), viability dropped from 96%±13% at 0 week to 44%±12% and 35%±24% at 3 and 6 weeks postencapsulation, respectively, while by incorporating CS within the IPN gels (IPN-CS gels) it dropped from 97%±10% at week 0 to 70%±20% and 55%±14% at weeks 3 and 6, respectively (Fig. 5). Similarly, viability was 97%±6%, 69%±16%, and 51%±11% at 0, 3, and 6 weeks, respectively, in IPN-aggrecan gels. Values for all three groups at weeks 3 and 6 were significantly less than their respective week 0 values (p<0.05, n=3). At week 6, the IPN-CS and IPN-aggrecan groups had 20% and 15% higher viabilities, respectively, compared to the pure IPN group (p<0.05, n=3).
FIG. 4.
Spinning disc confocal microscope live/dead images of encapsulated chondrocytes in IPN at week 0 (A–C), week 3 (D–F), and week 6 (G–I) in pure IPN, IPN-aggrecan, and IPN-CS gels. Green (Calcein AM) dye indicates viable (live) cell populations, while red (ethidium homodimer) dye indicates dead cells. Note the improved cell viability emerging at 3 weeks and especially evident at 6 weeks in the CS and aggrecan IPNs relative to the pure IPN. Scale bar=50 μm. Color images available online at www.liebertpub.com/tea
FIG. 5.
Percent cell viability of encapsulated chondrocytes by image analysis mask statistics. Multiple confocal Z-scan series were performed on a representative sample in each group (mean±standard deviation). *Values statistically significant from week 0 (p<0.05 and n=3), while **values indicate significant differences from the control group (pure IPN) at that time point (p<0.05 and n=3).
Biochemical synthesis
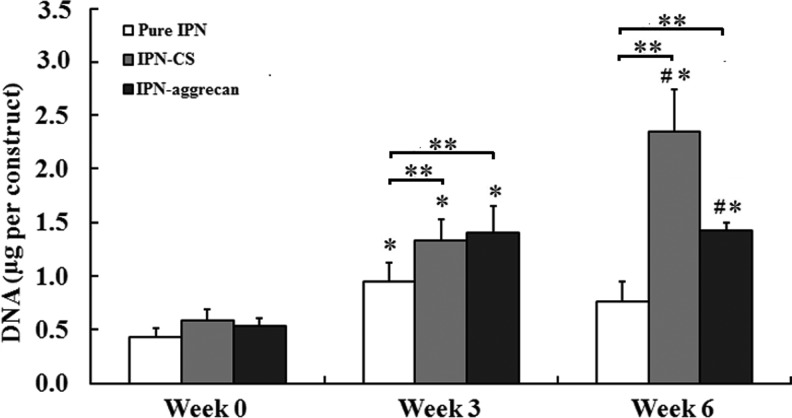
The DNA content data for each type of IPN gel construct are summarized in Figure 6. At week 0, all three of the IPN groups had similar DNA values per construct. At week 6, the DNA content was 3.9 times higher than the week 0 DNA content for the IPN-CS group (p<0.05), while that of the IPN-aggrecan group was 2.6 times higher at week 6 than at week 0 (p<0.05) (Fig. 6). The DNA content in the pure IPN gels increased significantly from week 0 to 3 (p<0.05), but there was a slight decrease from week 3 to 6 (not significant). At week 3, there was no statistically significant difference between the IPN-CS and IPN-aggrecan groups, although the DNA contents in both of these groups were significantly larger than control, by 40% and 47%, respectively (p<0.05). At week 6, the DNA content in the IPN-CS group was 65% higher than in the IPN-aggrecan group (p<0.05), and was three times larger than in the control, while the DNA content in the IPN-aggrecan group was two times larger than in the control (p<0.05).
FIG. 6.
The total DNA content within pure IPN, IPN-CS, and IPN-aggrecan gel constructs at 0, 3, and 6 weeks. Error bars represent mean±standard deviation. *Values statistically significant from week 0 time point, **values indicate statistically significant differences from the control at that time point, and #values indicate statistically significant differences between the groups at that time point (p<0.05, n=4).
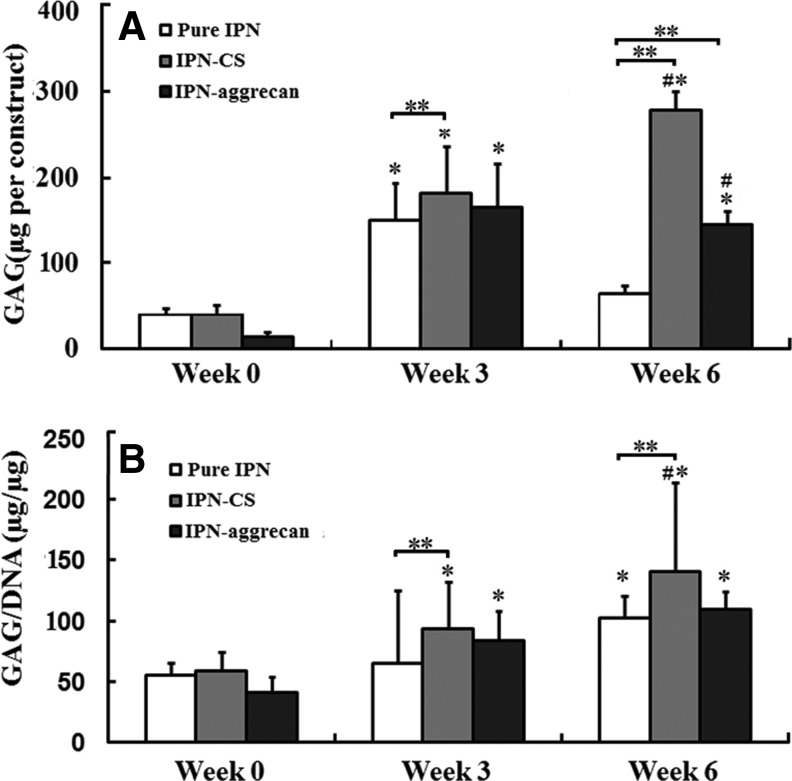
For the reported GAG content values (Fig. 7), the average GAG values of acellular IPN-CS and IPN-aggrecan gels at a given time point were subtracted from the total measured values for the respective cellular group (IPN-CS or IPN-aggrecan) at that time point. The GAG content per construct for all of the groups increased significantly from week 0 to 3 (p<0.05, n=4), while only the IPN-CS gels had a significant increase in the total GAG content from week 3 to 6 (p<0.05) (Fig. 7A). For all groups, the GAG content values normalized to total DNA significantly increased from week 0 to 6 (p<0.05) (Fig. 7B). There were no statistically significant differences in either the total or normalized GAG content among groups at week 0. At week 3, there was no statistically significant difference in the total as well as in the normalized GAG content between IPN-CS and IPN-aggrecan groups, although the total and normalized GAG contents in the IPN-CS group were significantly higher than in the control group, by 20% and 43%, respectively (p<0.05). At week 6, the total and normalized GAG contents in the IPN-CS group were significantly higher than in the IPN-aggrecan group, by 93% and 28%, respectively (p<0.05), and were 4.3 and 1.3 times larger than in the control, respectively (p<0.05). The total GAG content in the IPN-aggrecan group was 2.3 times higher than in the control group (p<0.05).
FIG. 7.
(A) Total sulfated glycosaminoglycan (GAG) accumulated per IPN gel (construct). (B) Total GAG accumulated normalized to the DNA content. Values represent mean±standard deviation. The GAG content associated with incorporated CS and aggrecan was subtracted from the actual GAG value to provide the values reported here. *Values indicate statistically significant increase in GAG production vs. week 0 time point, **values indicate a statistically significant increase in GAG production from the control at that time point and #values indicate statistically significant differences among the groups at that time point (p<0.05, n=4).
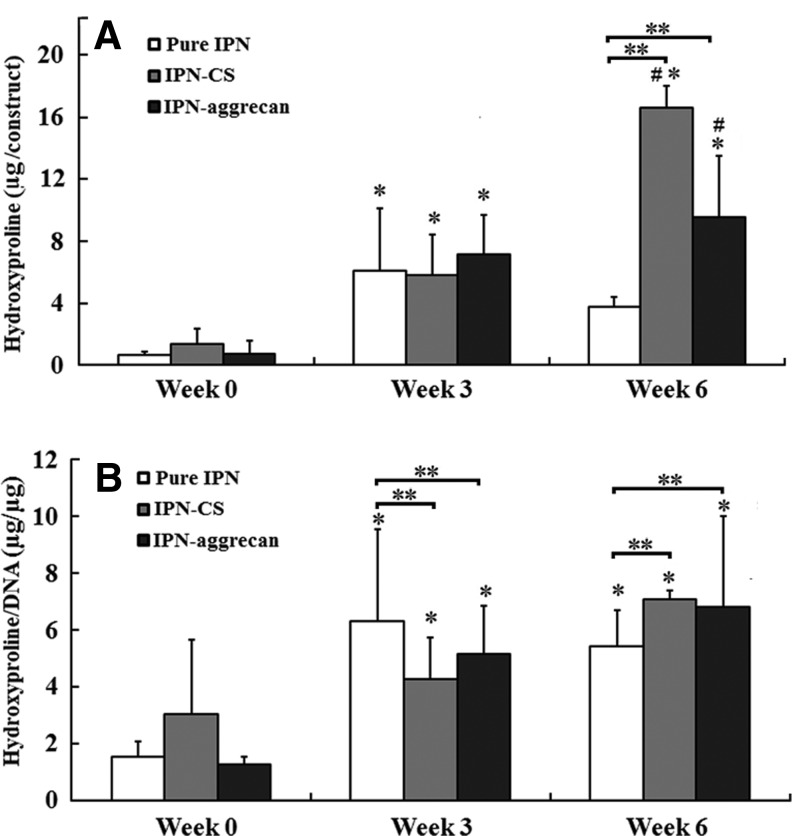
Similar to GAG content, hydroxyproline content per construct increased significantly in all three of the groups from week 0 to 3 (p<0.05, n=4), whereas only the IPN-CS group had a significant increase in the total hydroxyproline content from week 3 to 6 (p<0.05) (Fig. 8A). At week 6, the normalized hydroxyproline content in the IPN-CS and IPN-aggrecan groups increased by 2.3- and 5.6-fold relative to week 0, respectively (p<0.05). There were no statistically significant differences in either the total or normalized hydroxyproline content among groups at week 0. At week 3, there were no statistically significant differences in the hydroxyproline content among groups, although the normalized hydroxyproline was 50% and 20% higher in the control group than in the IPN-CS and IPN-aggrecan groups, respectively (p<0.05) (Fig. 8B). At week 6, the hydroxyproline content in the IPN-CS group was 77% higher than in the IPN-aggrecan group (p<0.05), and 4.2 times larger than in the control (p<0.05). However, the normalized hydroxyproline contents were not significantly different between the IPN-CS and IPN-aggrecan groups at week 6, although they were both significantly larger than the control, by 40% and 36%, respectively (p<0.05) (Fig. 8B).
FIG. 8.
(A) Total hydroxyproline accumulated per IPN (construct) at 0, 3, and 6 weeks. (B) The total accumulated hydroxyproline content normalized to the DNA content. Values represent mean±deviation. *Values indicate a statistically significant increase in hydroxyproline production vs. week 0 time point, **values indicate statistically significant differences from the control at that time point and #values indicate statistically significant differences among the groups at that time point (p<0.05, n=4).
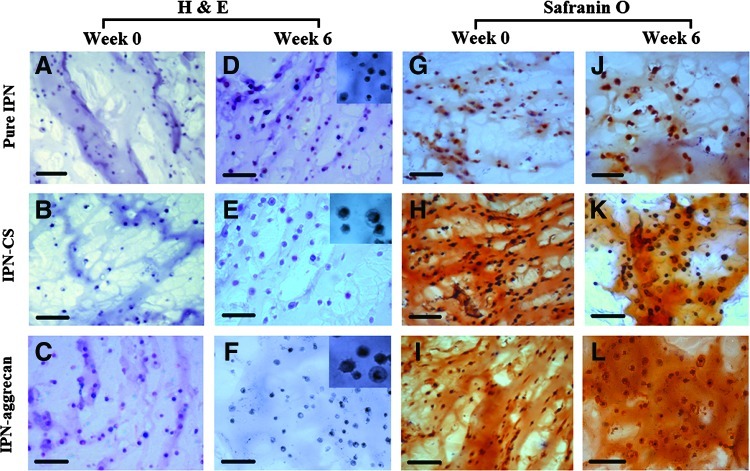
Histological evaluations
Histological evaluation of chondrocytes encapsulated in IPN gels after the 6-week culture period indicated a slight increase in the staining intensity (Fig. 9). H&E staining demonstrated uniform cell distribution at week 0 in all of the groups, namely, pure IPN, IPN-CS, and IPN-aggrecan (Fig. 9A–C), and pericellular matrix accumulation was evident at week 6 for each group (Fig. 9D–F). In addition, the diameters of the pericellular matrices visualized with H&E staining (inset figures, Fig. 9) was generally larger in the IPN-CS (Fig. 9E) and IPN-aggrecan sections (Fig. 9F) compared to the pure IPN sections (Fig. 9D) at week 6.
FIG. 9.
Histology of representative sections of IPN gels encapsulated with chondrocytes at week 0 and 6: Hematoxylin and eosin (H&E) staining (A–F) and Safranin-O staining (G–L). Inset pictures showing the pericellular area. Both IPN-CS and IPN-aggrecan stained positive for incorporated CS. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Safranin-O staining for GAGs was observed in a pericellular distribution initially at week 0 (Figs. 9G–I), with the staining intensity slightly increasing throughout the constructs from week 0 to 6 (Figs. 9J–L). Chondrocytes in the IPN-CS gel (Fig. 9K) as well as IPN-aggrecan gel (Fig. 9L) constructs produced a slightly stronger Safranin-O staining (pericellular as well as in the interterritorial region) than those in the pure IPN (Fig. 9J). Pericellular accumulation was not seen in pure IPN sections.
Discussion
In our previous study, we introduced a rationally designed agarose/PEGDA IPN hydrogel that exhibited dramatically improved compressive moduli and shear moduli relative to its component networks, while maintaining an unprecedented cell viability for such a network.38 However, there was still opportunity for improvement in cell performance and viability, which we hypothesized could be achieved with the inclusion of bioactive raw materials from cartilage and evaluated in a full 6-week study. Therefore, in the current study, we developed a novel bioactive IPN based on agarose-PEGDA hydrogels and evaluated the ability of this IPN material to support a 3D culture of encapsulated chondrocytes by incorporation of CS and aggrecan as bioactive signals. The highly negatively charged GAGs associated with aggrecan57 are critical determinants of the unique biomechanical properties of cartilage, in particular, its compressive58 and shear59 stiffness.
Aggrecan was physically entrapped, while CS was chemically attached to the PEGDA network within the IPN gels. Since CS is a highly charged molecule, incorporation of CS or aggrecan altered the swelling properties of IPN gels. The osmotic pressure of the counter ions of negative charged groups causes CS and aggrecan to increase swelling. Therefore, IPN-CS and IPN-aggrecan gels had higher swelling degrees than pure IPN gels. An IPN system containing one stiff and brittle first network along with one soft and ductile second network has been proposed by Gong and Osada to yield gels with excellent overall mechanical properties in both strength and toughness.29–37 The decrease in shear moduli relative to acellular IPN gels may have originated from a lower conversion of PEGDA and PEGDA CS copolymer within the IPN due to the significant volume fraction of cells that interfere with the network blocking to some extent of UV radiation. The moduli of both cellular and acellular IPN-CS and IPN-aggrecan gels declined after 6 weeks. The significant decrease in the shear modulus and toughness of IPN-CS gels both in the cellular and acellular gels from week 0 to 6 could be due to the hydrolytic degradation of CS linkages. The decrease in the shear modulus of IPN-aggrecan was associated with the leaching of physically entrapped aggrecan molecules in the culture media over the culture time of 6 weeks. The decrease in swelling degrees and GAG content values after week 6 also supported this leaching of biomolecules from the IPN system.
In our previous report, we demonstrated that the majority of IPN-encapsulated cells remained viable after the first week of culture.38 The pure IPN gels, due to their bioinert nature, lacked the bioactive signals to further promote cell growth and long-term survivability. Accordingly, in the current study, pure IPN gels experienced a significant loss in cell viability by week 6. For cells to produce cartilaginous tissue, long-term survivability is essential. In the current study, the live/dead data suggested that more cells stayed alive when encapsulated in the presence of CS and aggrecan in IPN gels compared to the pure IPN gels at week 6 postencapsulation. The CS moieties may have various roles such as interaction with growth factors and direct interaction with cells. For example, CS and aggrecan may provide host tissue-mimetic microenvironments in which cells grow and proliferate.
Interestingly, there was a general trend for decreasing percent viability over time juxtaposed with a general trend for increasing DNA content (although trends varied in magnitude among groups). Although these opposing trends may initially appear as a paradoxical result, they may be best explained by considering that the living cells continue to proliferate over time (DNA content increases), and that some of these cells die (resulting in a larger number of dead cells detected by the live/dead assay, but still leaving DNA that can be picked up by the DNA content assay), while others live and again continue to proliferate.
IPN-CS and IPN-aggrecan gels achieved higher levels of matrix production compared to pure IPN gels. IPN-CS and IPN-aggrecan accumulated significantly more normalized GAG compared to the pure IPN at the culture intervals of week 3 and 6. These results suggest that production of the ECM was largely dependent on the surrounding tissue-specific microenvironment provided by CS and aggrecan molecules. Incorporated CS and aggrecan may have also served roles as adhesion molecules and therefore provided an overall positive impact on GAG and collagen (or hydroxyproline) production.
Cells secreted GAGs in both the pericellular and the intercellular space throughout the entire IPN-CS scaffold, confirming the ability of IPN-CS and IPN-aggrecan constructs to produce large amounts of the ECM, which is essential for successful regeneration of cartilage-like tissue.
The current work outlined a technique for enhancing the bioactivity of synthetic IPNs based on agarose and PEGDA to promote the long-term cell viability and biosynthesis needed for cartilage tissue regeneration. The current study has yielded an IPN gel with a notable combination of high mechanical strength, cytocompatibility, and ability to provide a suitable microenvironment for encapsulated cells to grow, proliferate, and produce cartilaginous tissue. We demonstrated here that incorporating raw material biomolecules such as CS and aggrecan within the IPN produced significantly more GAG-rich and collagen-rich tissues. This feature can potentially be a powerful tool for developing a biologically engineered tissue in which the mechanical integrity, long-term cell survivability, and improved biosynthesis are the key properties for regenerating cartilage-like tissue. We are in the process of studying how these biologically engineered IPNs respond to cartilage regeneration by implanting them in rabbit knees. We are also interested in further elucidating the chondroinductive nature of CS and aggrecan in the IPNs by examining both gene expression and collagen II production by both chondrocytes and mesenchymal stem cells.
Conclusion
In this study, we successfully designed and synthesized a promising new class of aggrecan-incorporated and CS functionalized engineered agarose-PEGDA IPN hydrogel scaffold with a unique combination of high mechanical strength and cyto-compatibility. Incorporation of aggrecan and CS molecules within agarose-PEGDA networks created an IPN with a greater compressive modulus (relative to the IPN with no bioactive signals), higher swelling ratio, higher long-term cell viability, and improved biosynthesis. The current study has conclusively demonstrated that the cellular performance is significantly improved in vitro with the addition of CS and aggrecan to the IPNs. However, there was no indication that the more novel approach of aggrecan was more beneficial that the more straightforward, cheaper, and less novel approach of CS incorporation.
Advancing to in vivo investigation would be of great interest, as the aggrecan may be more beneficial in vivo as a more complete raw material for the regenerating cartilage, and its protein core may provide another level of growth factor binding and/or bioactive signaling to autologous cells (e.g., mesenchymal stem cells) that could not possibly be detected in vitro. However, any improvement with aggrecan in vivo relative to CS would need to be significant and substantial to justify the additional regulatory scrutiny and cost. In any case, as we look to the future with the new class of IPN hydrogels in tissue engineering, we have confidence that the inclusion of bioactive raw materials such as CS and aggrecan in cartilage tissue engineering will provide significant benefits in cell performance.
Acknowledgments
The authors acknowledge the financial support from the National Institutes of Health (R21 EB008783). We are grateful to David Moore and Heather Shinogle for their assistance during LIVE/DEAD imaging with spinning disc confocal microscopy.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chung C. Burdick J.A. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elisseeff J. Puleo C. Yang F. Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8:150. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 3.Ifkovits J.L. Burdick J.A. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 4.Vinatier C. Guicheux J. Daculsi G. Layrolle P. Weiss P. Cartilage and bone tissue engineering using hydrogels. Biomed Mater Eng. 2006;16:S107. [PubMed] [Google Scholar]
- 5.Awad H.A. Wickham M.Q. Leddy H.A. Gimble J.M. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 6.Heywood H.K. Bader D.L. Lee D.A. Glucose concentration and medium volume influence cell viability and glycosaminoglycan synthesis in chondrocyte-seeded alginate constructs. Tissue Eng. 2006;12:3487. doi: 10.1089/ten.2006.12.3487. [DOI] [PubMed] [Google Scholar]
- 7.Kuo S.M. Wang Y.J. Weng C.L. Lu H.E. Chang S.J. Influence of alginate on type II collagen fibrillogenesis. J Mater Sci Mater Med. 2005;16:525. doi: 10.1007/s10856-005-0528-x. [DOI] [PubMed] [Google Scholar]
- 8.Lee H.J. Choi B.H. Min B.H. Son Y.S. Park S.R. Low-intensity ultrasound stimulation enhances chondrogenic differentiation in alginate culture of mesenchymal stem cells. Artif Organs. 2006;30:707. doi: 10.1111/j.1525-1594.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 9.Mauck R.L. Seyhan S.L. Ateshian G.A. Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 10.Mauck R.L. Soltz M.A. Wang C.C.B. Wong D.D. Chao P.H.G. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng T Asme. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 11.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Saris D.B. Mukherjee N. Berglund L.J. Schultz F.M. An K.N. O'Driscoll S.W. Dynamic pressure transmission through agarose gels. Tissue Eng. 2000;6:531. doi: 10.1089/107632700750022170. [DOI] [PubMed] [Google Scholar]
- 13.Bryant S.J. Arthur J.A. Anseth K.S. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater. 2005;1:243. doi: 10.1016/j.actbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Hwang N.S. Kim M.S. Sampattavanich S. Baek J.H. Zhang Z. Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006;24:284. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 15.Hwang N.S. Varghese S. Theprungsirikul P. Canver A. Elisseeff J. Enhanced chondrogenic differentiation of murine embryonic stem cells in hydrogels with glucosamine. Biomaterials. 2006;27:6015. doi: 10.1016/j.biomaterials.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Hwang N.S. Varghese S. Zhang Z. Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2695. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 17.Wang D.A. Williams C.G. Yang F. Cher N. Lee H. Elisseeff J.H. Bioresponsive phosphoester hydrogels for bone tissue engineering. Tissue Eng. 2005;11:201. doi: 10.1089/ten.2005.11.201. [DOI] [PubMed] [Google Scholar]
- 18.Hern D.L. Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Buxton A.N. Zhu J. Marchant R. West J.L. Yoo J.U. Johnstone B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13:2549. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 20.DeLong S.A. Gobin A.S. West J.L. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J Control Release. 2005;109:139. doi: 10.1016/j.jconrel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Benoit D.S. Tripodi M.C. Blanchette J.O. Langer S.J. Leinwand L.A. Anseth K.S. Integrin-linked kinase production prevents anoikis in human mesenchymal stem cells. J Biomed Mater Res A. 2007;81:259. doi: 10.1002/jbm.a.31292. [DOI] [PubMed] [Google Scholar]
- 22.Nuttelman C.R. Tripodi M.C. Anseth K.S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24:208. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Weber L.M. Hayda K.N. Haskins K. Anseth K.S. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Benoit D.S. Anseth K.S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26:5209. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 25.Shu X.Z. Ghosh K. Liu Y. Palumbo F.S. Luo Y. Clark R.A. Prestwich G.D. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J Biomed Mater Res A. 2004;68:365. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- 26.Yang F. Williams C.G. Wang D.A. Lee H. Manson P.N. Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Elisseeff J. McIntosh W. Anseth K. Riley S. Ragan P. Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Kutty J.K. Cho E. Soo Lee J. Vyavahare N.R. Webb K. The effect of hyaluronic acid incorporation on fibroblast spreading and proliferation within PEG-diacrylate based semi-interpenetrating networks. Biomaterials. 2007;28:4928. doi: 10.1016/j.biomaterials.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Gong J.P. Katsuyama Y. Kurokawa T. Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv Mater. 2003;15:1155. [Google Scholar]
- 30.Huang M. Furukawa H. Tanaka Y. Nakajima T. Osada Y. Gong J.P. Importance of entanglement between first and second components in high-strength double network gels. Macromolecules. 2007;40:6658. [Google Scholar]
- 31.Kaneko D. Tada T. Kurokawa T. Gong J.P. Osada Y. Mechanically strong hydrogels with ultra-low frictional coefficients. Adv Mater. 2005;17:535. [Google Scholar]
- 32.Kwon H.J. Osada Y. Gong J.P. Polyelectrolyte gels-fundamentals and applications. Polym J. 2006;38:1211. [Google Scholar]
- 33.Na Y.H. Kurokawa T. Katsuyama Y. Tsukeshiba H. Gong J.P. Osada Y. Okabe S. Karino T. Shibayama M. Structural characteristics of double network gels with extremely high mechanical strength. Macromolecules. 2004;37:5370. [Google Scholar]
- 34.Nakayama A. Kakugo A. Gong J.P. Osada Y. Takai M. Erata T. Kawano S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv Funct Mater. 2004;14:1124. [Google Scholar]
- 35.Tanaka Y. Kuwabara R. Na Y.H. Kurokawa T. Gong J.P. Osada Y. Determination of fracture energy of high strength double network hydrogels. J Phys Chem B. 2005;109:11559. doi: 10.1021/jp0500790. [DOI] [PubMed] [Google Scholar]
- 36.Tsukeshiba H. Huang M. Na Y.H. Kurokawa T. Kuwabara R. Tanaka Y. Furukawa H. Osada Y. Gong J.P. Effect of polymer entanglement on the toughening of double network hydrogels. J Phys Chem B. 2005;109:16304. doi: 10.1021/jp052419n. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda K. Ping Gong J. Katsuyama Y. Nakayama A. Tanabe Y. Kondo E. Ueno M. Osada Y. Biomechanical properties of high-toughness double network hydrogels. Biomaterials. 2005;26:4468. doi: 10.1016/j.biomaterials.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 38.DeKosky B.J. Dormer N.H. Ingavle G.C. Roatch C.H. Lomakin J. Detamore M.S. Gehrke S.H. Hierarchically designed agarose and poly(ethylene glycol) interpenetrating network hydrogels for cartilage tissue engineering. Tissue Eng Part C Methods. 2010;16:1533. doi: 10.1089/ten.tec.2009.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q. Williams C.G. Sun D.D. Wang J. Leong K. Elisseeff J.H. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A. 2004;68:28. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 40.Strehin I. Nahas Z. Arora K. Nguyen T. Elisseeff J. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials. 2010;31:2788. doi: 10.1016/j.biomaterials.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang N.S. Varghese S. Lee H.J. Theprungsirikul P. Canver A. Sharma B. Elisseeff J. Response of zonal chondrocytes to extracellular matrix-hydrogels. FEBS Lett. 2007;581:4172. doi: 10.1016/j.febslet.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H.J. Yu C. Chansakul T. Hwang N.S. Varghese S. Yu S.M. Elisseeff J.H. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue Eng Part A. 2008;14:1843. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- 43.Brigham M.D. Bick A. Lo E. Bendali A. Burdick J.A. Khademhosseini A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng Part A. 2009;15:1645. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice M.A. Anseth K.S. Encapsulating chondrocytes in copolymer gels: bimodal degradation kinetics influence cell phenotype and extracellular matrix development. J Biomed Mater Res A. 2004;70:560. doi: 10.1002/jbm.a.30106. [DOI] [PubMed] [Google Scholar]
- 45.Deng Y. Hu J.C. Athanasiou K.A. Isolation and chondroinduction of a dermis-isolated, aggrecan-sensitive subpopulation with high chondrogenic potential. Arthritis Rheum. 2007;56:168. doi: 10.1002/art.22300. [DOI] [PubMed] [Google Scholar]
- 46.French M.M. Rose S. Canseco J. Athanasiou K.A. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng. 2004;32:50. doi: 10.1023/b:abme.0000007790.65773.e0. [DOI] [PubMed] [Google Scholar]
- 47.Bannwarth B. Acetaminophen or NSAIDs for the treatment of osteoarthritis. Best Pract Res Clin Rheumatol. 2006;20:117. doi: 10.1016/j.berh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Sechriest V.F. Miao Y.J. Niyibizi C. Westerhausen-Larson A. Matthew H.W. Evans C.H. Fu F.H. Suh J.K. GAG-augmented polysaccharide hydrogel: a novel biocompatible and biodegradable material to support chondrogenesis. J Biomed Mater Res. 2000;49:534. doi: 10.1002/(sici)1097-4636(20000315)49:4<534::aid-jbm12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Yang S.H. Chen P.Q. Chen Y.F. Lin F.H. Gelatin/chondroitin-6-sulfate copolymer scaffold for culturing human nucleus pulposus cells in vitro with production of extracellular matrix. J Biomed Mater Res B Appl Biomater. 2005;74:488. doi: 10.1002/jbm.b.30221. [DOI] [PubMed] [Google Scholar]
- 50.Yang S.H. Chen P.Q. Chen Y.F. Lin F.H. An in-vitro study on regeneration of human nucleus pulposus by using gelatin/chondroitin-6-sulfate/hyaluronan tri-copolymer scaffold. Artif Organs. 2005;29:806. doi: 10.1111/j.1525-1594.2005.00133.x. [DOI] [PubMed] [Google Scholar]
- 51.Ingavle G.C. Dormer N.H. Gehrke S.H. Detamore M.S. Using chondroitin sulfate to improve the viability and biosynthesis of chondrocytes encapsulated in interpenetrating network (IPN) hydrogels of agarose and poly(ethylene glycol) diacrylate. J Mater Sci Mater Med. 2012;23:157. doi: 10.1007/s10856-011-4499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L. Detamore M.S. Effects of growth factors and glucosamine on porcine mandibular condylar cartilage cells and hyaline cartilage cells for tissue engineering applications. Arch Oral Biol. 2009;54:1. doi: 10.1016/j.archoralbio.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Wang L. Lazebnik M. Detamore M.S. Hyaline cartilage cells outperform mandibular condylar cartilage cells in a TMJ fibrocartilage tissue engineering application. Osteoarthritis Cartilage. 2009;17:346. doi: 10.1016/j.joca.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Treloar L.R.G. The Physics of Rubber Elasticity. Oxford, United Kingdom: Oxford University Press; 2005. [Google Scholar]
- 55.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 56.Wang L. Tran I. Seshareddy K. Weiss M.L. Detamore M.S. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:2259. doi: 10.1089/ten.tea.2008.0393. [DOI] [PubMed] [Google Scholar]
- 57.Hardingham T.E. Fosang A.J. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861. [PubMed] [Google Scholar]
- 58.Williamson A.K. Chen A.C. Sah R.L. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 59.Jin M.S. Grodzinsky A.J. Effect of electrostatic interactions between glycosaminoglycans on the shear stiffness of cartilage: a molecular model and experiments. Macromolecules. 2001;34:8330. [Google Scholar]