Abstract
Few studies have examined the trajectory of recovery of executive function (EF) after mild TBI (mTBI). Therefore, consensus has not been reached on the incidence and extent of EF impairment after mTBI. The present study investigated trajectory of change in executive memory over 3 months after mTBI on 59 right-handed participants with mTBI, as defined by Centers for Disease Control criteria, ages 14–30 years, recruited within 96 hours post-injury and tested <1 week (baseline), 1 month, and 3 months after injury. Also included were 58 participants with orthopedic injury (OI) and 27 typically developing (TD) non-injured participants with similar age, socioeconomic status, sex, and ethnicity. MRI data were acquired at baseline and 3 months. Although criteria included a normal CT scan, lesions were detected by MRI in 19 mTBI patients. Participants completed the KeepTrack task, a verbal recall task placing demands on goal maintenance, semantic memory, and memory updating. Scores reflected items recalled and semantic categories maintained. The mTBI group was divided into two groups: high (score ≥12) or low (score <12) symptoms based on the Rivermead Post-Concussion Symptoms Questionnaire (RPQ). Mixed model analyses revealed the trajectory of change in mTBI patients (high and low RPQ), OI patients, and TD subjects were similar over time (although the TD group differed from other groups at baseline), suggesting no recovery from mTBI up to 90 days. For categories maintained, differences in trajectory of recovery were discovered, with the OI comparison group surprisingly performing similar to those in the mTBI group with high RPQ symptoms, and different from low RPQ and the TD groups, bringing up questions about utility of OIs as a comparison group for mTBI. Patients with frontal lesions (on MRI) were also found to perform worse than those without lesions, a pattern that became more pronounced with time.
Key words: cognition; executive function, mild traumatic brain injury; memory
Introduction
Approximately 1.4 million victims in the United States undergo medical treatment for traumatic brain injury (TBI) per year. Of those, 1.1 million receive a dianosis of mild TBI (mTBI).1 Currently, clinical care for patients with mTBI is largely performed by emergency departments, which have shown recent dramatic increases in incidence of mTBI,2 although it should be acknowledged that this may be an artifact because of policy changes resulting in the greatly reduced hospitalization of these patients rather than higher incidence of mTBI. The Centers for Disease Control (CDC) estimated that total lifetime cost of mTBI in 1995 was $16.7 billion, which figure does not include the costs of lost productivity, declining quality of life, and the indirect costs assumed by family members and friends who care for patients with mTBI.3
Definitions of mTBI have varied slightly from study to study, but the CDC defines it as injury to the head from blunt trauma, acceleration, or deceleration forces that results in one or more of the following conditions: confusion, disorientation, impaired consciousness, dysfunction of memory around the time of the injury, loss of consciousness (LOC) of less than 30 minutes in conjunction with post-concussion symptoms, such as headache, dizziness, fatigue, irritability, and poor concentration shortly after injury. This definition was elaborated by a World Health Organization (WHO) task force on mTBI4 to include a Glasgow Coma Scale (GCS) score of 13–15, a period of post-traumatic amnesia (PTA) of less than 24 hours, and exclusion of confounders such as intoxication and effects of other injuries that obscure the symptoms of mTBI.
Patients with mTBI are commonly discharged within hours if CT is not abnormal. Most patients are unaware that persisting behavioral and cognitive symptoms, such as memory dysfunction or difficulties with concentration, may ensue. Yet, a proportion of these may have cognitive and behavioral dysfunction as a consequence of their injury.5–9 There are gaps in the literature regarding the neurocognitive sequlae of mTBI. Likewise, the relation of behavioral symptoms to cognitive function is understudied, particularly given the prevalence of mTBI in youth and young adults.
Studies of mild TBI have generally concluded that the prognosis is positive for children who have sustained mTBI, with most attaining a good recovery by 3 months4 as measured by the Glasgow Outcome Scale (GOS)10 The rate of residual disability is higher in patients with GCS scores of 13–14 than in those with GCS scores of 15,11 which suggests that TBI severity may be a factor. Studies have reported similar disability at 3 months, however, in patients with extracranial injury,12,13 which suggests that factors other than brain injury may be involved.
In a comprehensive literature review, Carroll and associates4 screened 428 studies of mild TBI and eliminated 72% of them for various flaws or inconsistencies in methodology. The remaining 120 studies were considered the best evidence for the outcome of mTBI. These studies provided consistent evidence of few residual cognitive, behavioral, or academic deficits attributable to TBI beyond 3 months. Catale and colleagues,14 however, suggested that results are mixed on studies of executive function (EF) after mTBI. For example, Roncadin and coworkers15 found that children with mTBI performed within the normal range on verbal recognition memory, although it should be noted that this study examined patients with mild, moderate, and severe TBI, with no uninjured or extra-cranial injury comparison group.
On tests of attention, some studies have revealed mild deficits after mTBI even several years later. For example, Catroppa and colleagues16 found that 10 years after injury, children with mTBI were significantly impaired on a test of attentional gating or freedom from distractibility compared with typically developing (TD) children. Other studies have found deficits in tests of cognitive flexibility,14 but few studies examined the trajectory of recovery after mTBI.17 Therefore, consensus has not been reached with regard to the incidence and extent of EF impairment after mild TBI.
We examine patients with mTBI in comparison with patients with orthopedic injuries (OI) and healthy, uninjured controls with regard to performance on a test of executive memory, the KeepTrack Task.18 In addition, we examined the relation of regional brain lesions as revealed by MRI (but with normal CT scans) to performance with the expectation that frontal lesions would show the greatest relation to this EF task. The study is longitudinal and reports findings from the first week after injury to 3 months post-injury. Given the high incidence of mTBI in the adolescent and young adult population, we focused on this age range. Based on general findings on studies of mTBI, we hypothesized that patients with mTBI would show mild deficits on the EF task, which would resolve over time.
Methods
Participants
There were 117 right-handed participants of ages 12–30 years who were recruited and tested at baseline (within 96 hours of injury) and at follow-up sessions at 1 month and 3 months. Recruitment was from consecutive admissions to emergency centers in the Texas Medical Center, Houston, including Ben Taub General Hospital (BTGH), Texas Children's Hospital (TCH), and Memorial Herman Hospital (MHH). In addition, 29 uninjured participants were recruited and tested at the same intervals.
Mild TBI
Fifty-nine patients with mTBI, as defined by criteria from the CDC, had an injury to the head from blunt trauma, acceleration, or deceleration forces with one or more of the following conditions: (1) observed or self-reported confusion, disorientation, or impaired consciousness, dysfunction of memory at the time of the injury, loss of consciousness lasting less than 30 minutes; and, (2) symptoms such as headache, dizziness, fatigue, irritability, and poor concentration (typically referred to as “post-concussion symptoms”) soon after the injury. In addition, as recommended by the WHO task force on mTBI,4 inclusions also included a GCS19 score of 13–15 on examination at an emergency center, no abnormal findings on CT, duration of loss of consciousness for no more than 30 minutes, PTA for less than 24 hours, and an extracranial Abbreviated Injury Score (AIS) ≤3 and an Injury Severity Score (ISS) of <12.
Comparison groups
Fifty-eight participants with OI were recruited less than 96 hours post-injury provided they met the following criteria: right-handed, 12–30 years old, no loss of consciousness, no PTA, and no intracranial injury; AIS <3 for any body region and an ISS ≤12; and a normal CT (if performed). In addition, we tested 27 TD adolescents and young adults who had not sustained any injury, but who were similar to the injury groups in age, sex, and level of education.
Exclusions for all groups included non-English or Spanish speakers, undocumented immigration status, blood alcohol level >200 mg/dL, previous hospitalization for head injury, pregnancy when screened before brain imaging at baseline or 3 months post-injury, pre-existing neurologic disorder associated with cerebral dysfunction and/or cognitive deficit (e.g., cerebral palsy, mental retardation, epilepsy) or diagnosed dyslexia, pre-existing severe psychiatric disorder (bipolar disorder, schizophrenia), and contraindications to undergoing MRI including implant of metal. The OI comparison group was included to control for risk factors20–22 that predispose to injury, including pre-existing behavioral problems, subtle learning disabilities, and family variables.
Further, it has been suggested that persons with OI have suffered a physical trauma and have been through the emergency department experience, making their general trauma context similar to those with mTBI. The uninjured group was included as a means of estimating effects over time not from injury (such as task practice effects) and to compare recovery of injured patients to the general young adult population. Demographic and injury characteristics are shown in Table 1.
Table 1.
Demographic and Injury Characteristics
| |
TD (n=27) |
OI (n=58) |
RPQ_low (n=28) |
RPQ_high (n=31) |
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | P value | |
| Age at baseline | 20.29 (5.10) | 12.22–0.25 | 20.21 (5.52) | 12.07–0.78 | 18.58 (5.26) | 12.17–9.89 | 17.94 (3.98) | 12.24–6.61 | 0.1380 |
| Interval since injury (days) | |||||||||
| BL | 3.1 (1.1) | 1–7 | 2.3 (14.1) | 1–5 | 3.0 (1.1) | 1–5 | 0.0151a | ||
| 1M | 34.0 ( 5.1) | 23–42 | 32.1 (4.9) | 22–47 | 37.1 (6.8) | 26–51 | 33.1 (7.4) | 24–56 | 0.0116a |
| 3M | 98.2 (12.5) | 63–119 | 94.9 (12.7) | 75–136 | 99.7 (11.8) | 81–123 | 93.7 (9.3) | 70–112 | 0.2791 |
| SCI (z-score) | 0.31 (0.84) | −1.18–2.27 | −0.08 (0.80) | 1.57–2.33 | −0.33 (0.63) | 1.57–1.02 | 0.06 (0.86) | −0.89–1.98 | 0.0224a |
| RPQ | 2.00 (2.83) | 0–11 | 3.33 (3.67) | 0–12 | 3.86 (3.50) | 0–11 | 20.1 (6.21) | 12–37 | |
| GCS | N/A | N/A | N/A | N/A | 14.82 (0.48) | 13–15 | 14.74 (0.51) | 13–15 | 0.4457 |
| ISS * | N/A | N/A | 1.17 (1.1) | 05 | 0.63 (1.8) | 09 | 0.79 (1.26) | 0–4 | 0.17 |
| Frequency | % | Frequency | % | Frequency | % | Frequency | % | ||
|---|---|---|---|---|---|---|---|---|---|
| Sex | M=15 | 51.7 | M=43 | 74.14 | M=20 | 71.43 | M=21 | 67.74 | 0.1977 |
| Pre-ADHD | 1 | 3.45 | 7 | 12.07 | 1 | 3.57 | 5 | 16.13 | 0.2603 |
| Race (Black) | 9 | 31.03 | 24 | 41.38 | 12 | 42.86 | 10 | 32.26 | 0.6614 |
| MOI | |||||||||
| MV | N/A | 6 | 10.34 | 10 | 35.71 | 14 | 45.16 | 0.0094a | |
| Non-MV | 20 | 34.48 | 7 | 25.00 | 6 | 19.35 | |||
| Sports | 25 | 43.10 | 6 | 21.43 | 8 | 25.81 | |||
Significant result.
TD, typically developing; OI, orthopedic injuries ; RPQ, Rivermead Post-Concussive Symptom Questionnaire; SD, standard deviation; BL, baseline; SCI, Socioeconomic Composite Index; GCS, Glasgow Coma Scale; ISS, Injury Severity Scale—non-head injury score; ADHD, attention deficit hyperactivity disorder; MOI, mechanism of injury; MV, motor vehicle.
Measures
KeepTrack task18,23
This novel updating task requires adding and deleting items in working memory according to semantic category, thus involves episodic memory, retrieval inhibition, and semantic processing. The KeepTrack task is considered a test of EF and appears to be related to intelligence.18 On each trial, participants are presented two to four target categories (e.g., animals, colors) from a pool of six possible categories (animals, colors, countries, measures, metals, relatives) and afterward are shown a series of 15 words that are exemplars of the categories and that include at least two exemplars in each target category. The words are presented one word at a time with the instruction to recall the last item presented in each of the target categories.
Table 2 displays an example list with target categories of metals and countries. There are three levels of difficulty, which correspond to the number of target categories that the subject must keep track of for a given list, with the easier level requiring tracking of two categories and the hardest, four categories. The score is the number of correct items updated per trial (Items). Because the subject must keep in mind the target categories for each trial in addition to keeping track of the most recent exemplars of the target category, we also derived a score for the number of correct categories for each trial (Categories).
Table 2.
Example List for KeepTrack Task, with Target Categories of Metals and Countries
| METALS COUNTRIES |
| YELLOW |
| BEAR |
| IRON |
| MOTHER |
| ORANGE |
| GERMANY |
| YARD |
| LION |
| UNCLE |
| CANADA* |
| METER |
| COPPER* |
| OUNCE |
| RED |
Target items (last in list for target category) are denoted by *.
Rivermead Post-Concussive Symptom Questionnaire (RPQ)7
A well-established, abbreviated questionnaire used to measure the presence and severity of post-concussive symptoms after a concussion or other type of traumatic injury to the head. The test may be self-report or administered by an interviewer, and requires patients to rate on a scale of 0 to 4 the severity of 16 symptoms that occur commonly after mTBI relative to pre-injury level, with higher scores associated with worse symptoms.
Socioeconomic Composite Index (SCI)24
The SCI provides a distal measure of the family socioeconomic status (SES) by computing z scores based on three variables: annual family income coded on an eight-point scale; a seven-point scale of maternal education; and a measure of occupational prestige, with higher scores indicating higher SES.
MRI
To address the relationship of EF performance to the presence and site of brain lesions, MRI was acquired for patients (OI and TBI) at the time of the baseline assessment and at 3 months post-injury. The TD group had a single MRI. The MRI protocol included high resolution T1, T2, fluid level adjusted inversion recovery, and gradient echo sequences to detect contusions, hemorrhage, gliosis, and other pathology associated with TBI. Imaging was acquired on a Philips 3T scanner. The MRI data were reviewed by the project neuroradiologist who has extensive experience in TBI research. Presence, location, and pathology for each abnormality were coded on a form designed for TBI research.
Statistics
To retain the maximum observations for our study, which contains missing data at random, growth curve analysis using a general linear mixed model with random intercept and slope was used to examine the pattern of recovery from TBI at baseline through 3 months.25 The dependent variable is the KeepTrack outcome variable (either Items Correct or Categories Correct). The independent variables include interval (between baseline and follow-up), group and demographic variables (e.g., age, SES as measured by SCI, etc.). The variances of the intercept and slope were tested in a level 1 (unconditional) model, and both were significant. After adjusting for other covariates (i.e., age, SCI), the slope no longer varied randomly, so it was fixed for a level 2 model to see its effect on outcome measurement.
The injury group variable and other time-invariant variables, such as age at baseline (age at injury for TBI and OI patients), sex, SCI, and race, were entered into the level 2 model with the group variable to examine their effects on recovery patterns. All possible three-way interactions of group by growth parameters (linear or quadratic) of the post-injury interval by other variables were tested. To accurately capture the recovery patterns over time and eliminate loss of data from an “out-of-window” testing time, we used the exact interval that had elapsed between time of injury and time of test (in days) for the “interval” variable, instead of the designated time points for baseline, 1 month, and 3 months. Post-injury interval was centered at 30 days, and age was centered at its grand mean of 19 years for meaningful interpretation of the intercept and convenience of the estimation. Effect sizes are not appropriate for this kind of model, so, to facilitate interpretation, estimates are provided (Table 3). Demographic statistics were compared using t test for continuous variables, age at injury, and SCI. The Fisher exact test or chi-square (χ2) was used for categorical variables, such as sex, mechanism of injury, race, and pre-injury attention deficit hyperactivity disorder (ADHD).
Table 3.
Parameter Estimates from the Growth Curve Model Examining Group Differences in Keeptrack Performance
| Parameter | Estimates | t value | p value |
|---|---|---|---|
| Hit percentage | |||
| Intercept | 71.0441 | 12.56 | <0.0001 |
| RPQ_low vs. TD | −8.2255 | −2.05 | 0.0419 |
| RPQ_high vs. TD | −9.9248 | −2.58 | 0.0104 |
| OI vs. TD | −11.1839 | −3.30 | 0.0011 |
| Age at baseline | .5141 | 2.13 | 0.0340 |
| SCI | 2.5447 | 1.63 | 0.1050 |
| Interval (linear slope) | 0.1100 | 5.24 | <0.0001 |
| Interval2 (quadratic) | −.00075 | −1.87 | 0.0627 |
| CC category percentage | |||
| Intercept | 96.2942 | 76.52 | <0.0001 |
| RPQ low vs. TD | −7.1545 | −4.03 | 0.0001 |
| RPQ high vs. TD | −4.9167 | −2.90 | 0.0047 |
| OI vs. TD | −4.6198 | −3.07 | 0.0028 |
| SCI | .9628 | 1.52 | 0.1312 |
| Interval (Slope) | .04876 | 2.76 | 0.0070 |
| Group*slope (RPQ low vs. TD) | .06023 | 2.89 | 0.0048 |
| Group*slope (RPQ high vs. TD) | −.00867 | −.42 | 0.6775 |
| Group*slope (OI vs. TD) | .01630 | .90 | 0.3713 |
| Interval2 (quadratic) | −.00071 | −3.23 | 0.0017 |
RPQ, Rivermead Post-Concussive Symptom Questionnaire; TD, typically developing; OI, orthopedic injuries; SCI, Socioeconomic Composite Index; CC, Categories Correct.
To examine the effect of lesions on performance, t tests were used, although the limited sample size requires that these analyses be considered exploratory.
Results
Preliminary analyses
Initial analyses revealed no differences in performance across the three levels of difficulty of the KeepTrack task, so scores for the levels were collapsed into a mean score, which was used for all analyses. Similarity between the groups was established for age at injury F(3,142)=1.87, p=0.1380; sex, χ2=0.4.6696, p=0.1977; race, χ2=1.5912, p=0.6614; or prevalence of ADHD, Fisher exact test, p=0.2603, which was included to address the possibility that children who sustain injuries may have attentional problems as a risk factor. SES (as measured by the SCI) varied among groups, with the three injury groups similar, but the TD group having a higher SCI score, F(3,142)=3.30, p=0.0.024. As well, the mechanism of injury varied among the injury groups, χ2= 16.9773, p=0.0094, with patients with OI more likely to be injured in sports accidents or non-vehicular accidents, and patients with mTBI more likely to be injured as the result of motor-vehicle crashes. KeepTrack variables (Items and Category) were related to age-at-injury and the SCI, and therefore both variables were included in the model.
Symptom groups
We were interested primarily in determining the trajectory of recovery for persons with mild TBI. Reports indicate that some patients with mTBI recover quickly and demonstrate no residual effects, whereas other patients show persistent effects of mTBI. Within the mild injury severity group we examined, GCS scores have a truncated range and lacked sensitivity to gradations of injury. We therefore divided the mTBI patients into two groups depending on post-concussive symptoms, as measured within 5 days after injury by the RPQ,7 which provided a much wider range of symptoms and which we expected to illuminate factors relating to recovery. To do so, we used the median score of 12 to divide mTBI patients into groups with a RPQ score <12 (RPQ_low, n=28) and those with a score ≥12 (RPQ_high, n=31). The two RPQ groups did not differ on mean GCS score, Wilcoxon rank sum test, Z=0.7626, p=0.4457.
Recovery after mTBI
Percentage Items Correct
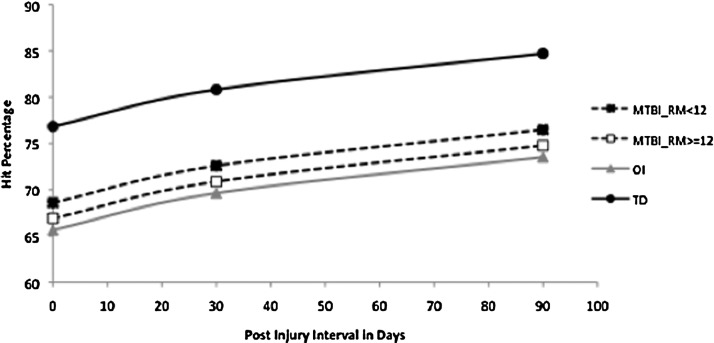
Growth curve analysis revealed a significant linear component of gains over time, F(1,218)=27.44, p<0.0001, and marginally significant quadratic component, F(1,218)=3.50, p=0.0627. This pattern, however, did not differ by groups. The effect of group was significant on the intercept (at baseline), F(3,218)=3.85, p=0.0103, largely because of the TD group performing significantly better than the other three groups. Within the other three groups, the least square means KeepTrack items score was in order, RPQ_low>RPQ_high>OI, but the means were not significantly different from each other. Age was a significant factor in performance across all groups, F(1,218)=4.55, p=0.0340, but SCI was not significantly related to performance, F(1,218)=2.65, p=0.105 (Fig. 1).
FIG. 1.
Items Correct on the KeepTrack task by group and interval after injury.
Percentage Categories Correct
Because the KeepTrack task requires the within-trial maintenance (but not updating) of target categories to successfully complete the task, we decided to examine the ability to maintain semantic category representations (e.g. “metal” or “animals”) separately from updating items (e.g., “iron” or “lion”).
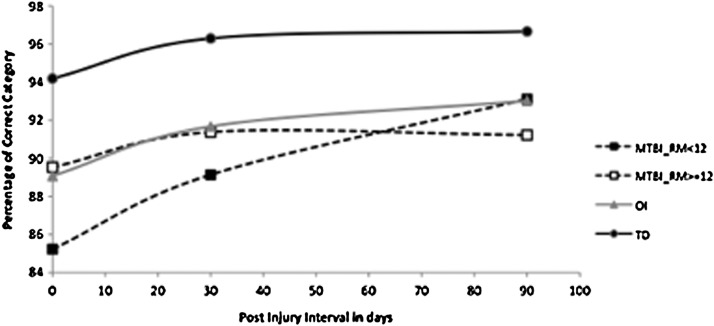
Growth curve analysis revealed that the groups had different recovery patterns (Fig. 2). The groups significantly differed on the baseline intercept, F(3,92)=5.83, p=0.0011, and slopes, F(3, 92)=4.36, p=0.0064), but not on the acceleration rate (quadratic term of interval), although the acceleration rate is significant for all four groups, F(1, 92)=10.41, p=0.0017. Neither age nor SCI significantly affected the performance on the percentage Categories Correct.
FIG. 2.
Categories C5. orrect on the KeepTrack task by group and interval after injury.
Planned comparisons for Categories Correct
To further investigate the interactions of group by interval on categories, we looked at differences in performance on the KeepTrack task among groups at each test interval.
Baseline
The TD group performance differed significantly from all three injury groups: RPQ low versus TD, t(92)=−4.21, p<0.0001; RPQ high versus TD, t(87)=−2.27, p=0.0256; OI versus TD, t(87)=−2.83, p=0.0058. Performance of the OI group was significantly better than the RPQ low, t(92)=2.10, p=0.0385, but not the RPQ high group. Finally, and unexpectedly, performance of the RPQ high was significantly better than RPQ low group, t(92)=2.05, p=0.0428.
One month
The TD group once again showed a significant advantage in performance over each of the injury groups: RPQ low versus TD, t(92)=−4.03, p=0.0001; RPQ high versus TD, t(87)=−2.90, p=0.0047; OI versus TD, t(92)=−3.07, p=0.0028. For Categories Correct at 1 month, the three injury groups did not differ from each other.
Three months
The TD group performed better than the RPQ high group, t(92)=−3.42, p=0.0009, the RPQ low group, t(92)=−2.14, p=0.0349 and also the OI group, t(92)=−2.54, p=0.0126. No other comparisons were significant at 3 months.
Relation of MRI findings to performance on the KeepTrack task
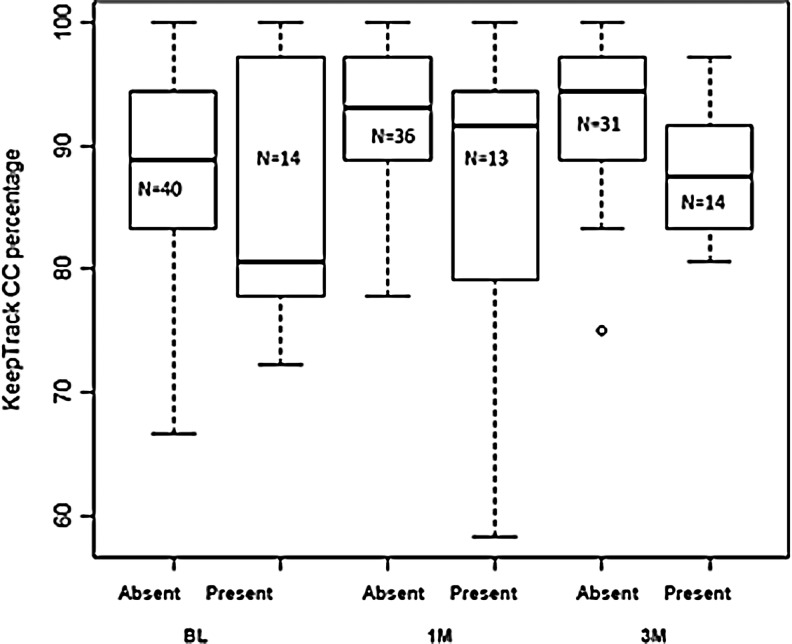
Abnormal findings on MRI were found in 19 (32%) of the patients with mTBI (Table 4). For exploratory purpose, we used t tests to examine the relation of lesions by brain region to performance on the KeepTrack task. We compared updating items correct (Items Correct) and category maintenance (Categories Correct) in all mTBI patients with lesions in specific brain regions (ignoring Rivermead symptom scores) compared with patients without lesions. Within the most common lesion sites (frontal, temporal, parietal), there were no significant associations for lesions in any region and performance on KeepTrack as measured by the number of items correct at baseline, 1 month, or 3 months. For Categories Correct, there were no significant associations for baseline for any region. At 1 month, however, lesions in the frontal lobes were marginally associated with poorer performance: t(12)=1.54, p=0.1011; effect size, d=0.744. At 3 months, the effect of frontal lesions was even greater for Categories Correct: t(12)=2.55, p=0.0144; effect size, d=0.915. The relations between frontal lesions and category maintenance performance by testing occasion is shown in Figure 3.
Table 4.
Lesion Location and Type in the 19 Patients with Mild Traumatic Brain Injury Who Demonstrated Abnormal Findings Either on the Baseline MRI or on the Follow-Up MRI at 1 Month Post-Injury
| |
Right |
Left |
||||
|---|---|---|---|---|---|---|
| ID # | Frontal | Temporal | Parietal | Frontal | Temporal | Parietal |
| 101 | Cerebral hemorrhage | |||||
| 105 | Cerebral hemorrhage | |||||
| 127 | Contusion | |||||
| 129 | Contusion | |||||
| 139 | Contusion | |||||
| 144 | Contusion | Contusion | ||||
| 154 | Cerebral hemorrhage or Gliosis | Cerebral hemorrhage Gliosis | Contusion | Cerebral hemorrhage Gliosis | Cerebral hemorrhage Gliosis | |
| 163 | Axonal | |||||
| 165 | Cerebral hemorrhage | |||||
| 174 | Contusion | |||||
| 184 | Contusion | |||||
| 191 | Cerebral hemorrhage | |||||
| 194 | Paranasal | |||||
| 196 | Axonal contusion | Contusion | ||||
| 209 | Cerebral hemorrhage | |||||
| 214 | Contusion | |||||
| 218 | Gliosis | |||||
| 268 | Contusion | |||||
| 291 | Gliosis | |||||
FIG. 3.
Boxplot of the relation between the presence and absence of frontal lesions as determined by MRI on category maintenance performance of the KeepTrack task.
Discussion
These data support previous findings of variation in outcome in mTBI, which, in this case, was more sensitively captured with the RPQ than by injury severity as measured by GCS scores. Although age was a factor for the Items Correct measure, there was no interaction with group, indicating similar effects across all of the groups. SES (as measured by the SCI) was not a significant factor in recovery over time.
We examined two measures on the KeepTrack task. The first, Items Correct, requires the maintenance of target categories and updating of individual items within a category. The second measure, Categories Correct, focuses on the ability to maintain target semantic representations during a memory task.
On Items Correct, each of the four groups showed similar gains in KeepTrack performance over time, including the uninjured TD group, which suggests that at least part of the observed changes over time are from practice effects, generalized familiarity effects, development, or other causes unrelated to injury.
In contrast, maintaining a semantic representation as indexed by the Categories Correct measure is better preserved than Items Correct in all groups, with performance near ceiling for the TD group. Among the injury groups, only the RPQ low group shows dramatic change over the time between baseline and 3 months, largely because of significantly poorer performance at the baseline measure. In contrast, both the RPQ high and the OI group show increases in the first month after injury, but beyond a month, postinjury advances are much reduced or non-existent.
The poor showing of the RPQ low group at baseline on Categories Correct relative to the RPQ high group is a puzzle. Ancillary analyses indicated that the discrepancy cannot be explained by age, sex, SES, race, mechanism of injury, presence of premorbid ADHD, acute pain scale, GCS score or severity of injuries other than the head (ISS score).
Interestingly, performance of the KeepTrack task in the OI group is most similar to the RPQ high group, despite the OI group demonstrating RPQ scores quite similar to the RPQ low group (Table 1). Because the inclusion of an OI group has been considered advisable to account for non-brain injury variables such as general trauma and predisposing risk factors, it might be expected that the performance of the OI group would be similar to that of the mTBI group with the mildest injuries or symptoms. The finding that they instead more closely resemble performance of the RPQ high group allows for a discussion of the use of OI injury groups as a comparison group for TBI in children.
Presence and site of lesions on MRI were analyzed in relation to KeepTrack performance for exploratory purposes. Despite the eligibility criterion of a normal CT scan at the time of clinical assessment in the emergency center within 24 hours after injury, about one-third of the mTBI patients had focal lesions on MRI acquired at baseline and/or 3 months. We found a marginal association of frontal lesions with number of categories correctly recalled at 1 month and a significant association at 3 months. The associations of performance with frontal lesions had large effect sizes in both the 1 month and 3 month data. We acknowledge that these findings should be interpreted with caution, given the small number of patients in the lesion analyses.
Limitations of the study
Given reports of rapid recovery after mild TBI, the window of 96 hours allowed between time of injury and recruitment may have contributed to an underestimate of impairment at the baseline assessment, thus potentially affecting the trajectory of change. Although a design with three time points allows detection of some non-linear patterns of change, it does not allow elucidation of more complex patterns that may occur quickly over time. Mechanism of injury may potentially contribute to differences in recovery. Therefore, because there were proportionally more high speed (motor-vehicle accident) injuries in the mTBI groups relative to the OI group and more low speed (sports) injuries in the OI group, there was a potential for confounding, although analyses indicted that this variable did not significantly impact recovery in this study. Finally, because our study was intended to examine the effect of diffuse injury of the brain, potential participants who showed focal lesions on CT were excluded, which, although enhancing the homogeneity of the sample, limited generalizability to the population of patients with mTBI.
Conclusion
Our data indicated that the trajectory of change differed by outcome measure and interacted with injury group. We also conclude that the inclusion of a TD group with the injury groups is critical in determining whether change over time is related to recovery or to non-injury–related variables, as was observed in this study on at least one outcome measure. We conclude that recovery from mTBI may be more complex than previously appreciated.
Author Disclosure Statement
No competing financial interests exist.
Acknowledgments
The authors gratefully acknowledge support from the following grants: NINDS grant 5 P01 NS056202 and DoD grant W81XWH-08-2-0133 PT074693P2.
References
- 1.Langlois J.A. Rutland-Brown W. Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Thurman D. Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Injury Prevention and Control. 2006. www.cdc.gov/ncipc/pub-res/mtbi/mtbireport.pdf. [Mar 27;2013 ]. www.cdc.gov/ncipc/pub-res/mtbi/mtbireport.pdf
- 4.Carroll L.J. Cassidy J.D. Holm L. Kraus J. Coronado V.G. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004;43(Suppl):113–125. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- 5.Alsop D.C. Murai H. Detre J.A. McIntosh T.K. Smith D.H. Detection of acute pathologic changes following experimental traumatic brain injury using diffusion-weighted magnetic resonance imaging. J Neurotrauma. 1996;13:515–521. doi: 10.1089/neu.1996.13.515. [DOI] [PubMed] [Google Scholar]
- 6.Edna T.H. Cappelen J. Late post-concussional symptoms in traumatic head injury. An analysis of frequency and risk factors. Acta Neurochir. (Wien) 1987;86:12–17. doi: 10.1007/BF01419498. [DOI] [PubMed] [Google Scholar]
- 7.Ingebrigtsen T. Waterloo K. Marup-Jensen S. Attner E. Romner B. Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J. Neurol. 1998;245:609–612. doi: 10.1007/s004150050254. [DOI] [PubMed] [Google Scholar]
- 8.Rimel R.W. Giordiani B. Barth J.T. Boll T.J. Jane J.A. Disability caused by minor head injury. Neurosurgery. 1981;9:221–228. [PubMed] [Google Scholar]
- 9.Rutherford W.H. Merrett J.D. McDonald J.R. Symptoms at one year following concussion from minor head injuries. Injury. 1979;10:225–230. doi: 10.1016/0020-1383(79)90015-9. [DOI] [PubMed] [Google Scholar]
- 10.Jennett B. Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 11.Hsiang J.N. Yeung T. Yu A.L. Poon W.S. High-risk mild head injury. J. Neurosurg. 1997;87:234–238. doi: 10.3171/jns.1997.87.2.0234. [DOI] [PubMed] [Google Scholar]
- 12.Boake C. McCauley S.R. Pedroza C. Levin H.S. Brown S.A. Brundage S.I. Lost productive work time after mild to moderate traumatic brain injury with and without hospitalization. Neurosurgery. 2005;56:994–1003. [PubMed] [Google Scholar]
- 13.Levin H.S. Brown S.A. Song J.X. McCauley S.R. Boake C. Contant C.F. Goodman H. Kotrla K.J. Depression and posttraumatic stress disorder at three months after mild to moderate traumatic brain injury. J. Clin. Exp. Neuropsychol. 2001;23:754–769. doi: 10.1076/jcen.23.6.754.1021. [DOI] [PubMed] [Google Scholar]
- 14.Catale C. Marique P. Closset A. Meulemans T. Attentional and executive functioning following mild traumatic brain injury in children using the Test for Attentional Performance (TAP) battery. J. Clin. Exp. Neuropsychol. 2009;31:331–338. doi: 10.1080/13803390802134616. [DOI] [PubMed] [Google Scholar]
- 15.Roncadin C. Guger S. Archibald J. Barnes M. Dennis M. Working memory after mild, moderate, or severe childhood closed head injury. Dev. Neuropsychol. 2004;25:21–36. doi: 10.1080/87565641.2004.9651920. [DOI] [PubMed] [Google Scholar]
- 16.Catroppa C. Anderson V. Godfrey C. Rosenfeld J.V. Attentional skills 10 years post-paediatric traumatic brain injury (TBI) Brain Inj. 2011;25:858–869. doi: 10.3109/02699052.2011.589794. [DOI] [PubMed] [Google Scholar]
- 17.Stuss D.T. Stethen L.L. Hugenholtz H. Picton T. Pivik J. Richard M.T. Reaction time after head injury: fatigue, divided and focused attention, and consistency of performance. J. Neurol. Neurosurg. Psychiatry. 1989;52:742–748. doi: 10.1136/jnnp.52.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman N.P. Miyake A. Corley R.P. Young S.E. DeFries J.C. Hewitt J.K. Not all executive functions are related to intelligence. Psychol. Sci. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 19.Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 20.Bijur P. Haslum M. Cognitive, behavioral, and motoric sequelae of mild head injury in a national birth cohort, in Traumatic Head Injury in Children. In: Broman S.H., editor; Michel M.E., editor. Oxford University Press; New York: 1995. pp. 147–164. [Google Scholar]
- 21.Stancin T. Kaugars A.S. Thompson G.H. Taylor H.G. Yeates K.O. Wade S. Drotar D. Child and family functioning 6 and 12 months after a serious pediatric fracture. J. Trauma. 2001;51:69–76. doi: 10.1097/00005373-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Stancin T. Taylor H.G. Thompson G.H. Wade S. Drotar D. Yeates K.O. Acute psychosocial impact of pediatric orthopedic trauma with and without accompanying brain injuries. J. Trauma. 1998;45:1031–1038. doi: 10.1097/00005373-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Yntema D.B. Schulman G.M. Response selection in keeping track of several things at once. Acta Psychol. (Amst) 1969;27:325–332. doi: 10.1016/0001-6918(67)90075-3. [DOI] [PubMed] [Google Scholar]
- 24.Yeates K.O. Taylor H.G. Drotar D. Wade S.L. Klein S. Stancin T. Schatschneider C. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J. Int. Neuropsychol. Soc. 1997;3:617–630. [PubMed] [Google Scholar]
- 25.Singer J. Willett J. Oxford University Press; New York: 2003. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. [Google Scholar]