Abstract
One important challenge for regenerative medicine is to produce a clinically relevant number of cells with consistent tissue-forming potential. Isolation and expansion of cells from skeletal tissues results in a heterogeneous population of cells with variable regenerative potential. A more consistent tissue formation could be achieved by identification and selection of potent progenitors based on cell surface molecules. In this study, we assessed the expression of stage-specific embryonic antigen-4 (SSEA-4), a classic marker of undifferentiated stem cells, and other surface markers in human articular chondrocytes (hACs), osteoblasts, and bone marrow-derived mesenchymal stromal cells (bmMSCs) and characterized their differentiation potential. Further, we sorted SSEA-4-expressing hACs and followed their potential to proliferate and to form cartilage in vitro. Cells isolated from cartilage and bone exhibited remarkably heterogeneous SSEA-4 expression profiles in expansion cultures. SSEA-4 expression levels increased up to ∼5 population doublings, but decreased following further expansion and differentiation cultures; levels were not related to the proliferation state of the cells. Although SSEA-4-sorted chondrocytes showed a slightly better chondrogenic potential than their SSEA-4-negative counterparts, differences were insufficient to establish a link between SSEA-4 expression and chondrogenic potential. SSEA-4 levels in bmMSCs also did not correlate to the cells' chondrogenic and osteogenic potential in vitro. SSEA-4 is clearly expressed by subpopulations of proliferating somatic cells with a MSC-like phenotype. However, the predictive value of SSEA-4 as a specific marker of superior differentiation capacity in progenitor cell populations from adult human tissue and even its usefulness as a stem cell marker appears questionable.
Introduction
Treatment options for musculoskeletal disorders are limited and new therapeutic approaches are being investigated, including the repair of lost or damaged cartilage and bone with tissue engineering and regenerative medicine (TE/RM) techniques. One important challenge in the area of TE/RM is to produce a clinically relevant number of cells with consistent tissue-forming potential. However, isolating and culturing cells from various tissue sources, such as bone marrow (bm), cartilage, and bone, results in a heterogeneous cell population with variable amounts of progenitors,1,2 and therefore, a heterogeneous and often unpredictable tissue formation.3,4
Identification and isolation of specific cells with a regenerative capacity could lead to a more consistent tissue formation.5–7 Cell surface markers can be used, to simultaneously characterize and sort cells using magnetic or fluorescent antibodies, resulting in populations with specific surface characteristics. A wide range of cell surface antigens have been studied on the various progenitor cell types,8 with the most frequently reported negative and positive markers of mesenchymal stromal cells (MSCs) being CD14, CD34, CD45, and CD44, CD90, CD73, CD105, respectively.9 Given that mature stromal cells, including articular chondrocytes, lose their differentiated state during in vitro expansion and adopt a mesenchymal-like phenotype,10–12 it is not surprising that propagated chondrocytes and osteoblasts express a similar range of surface markers to MSCs.13–15 However, there are conflicting reports about the antigenic characteristics of mesenchymal progenitor cells and reliable markers of progenitor populations have yet to be identified.1,16
Stage-specific embryonic antigen-4 (SSEA-4) is one cell surface molecule with a potential use for the identification and sorting of progenitor cells for musculoskeletal regeneration. The SSEA-4 epitope has been identified as a globo-series glycosphingolipid (GSL) with a terminal sialic acid (monosialyl-Gb5).17 It is commonly used to characterize undifferentiated human embryonic stem cells.18 Yet, SSEA-4 is also expressed by human MSCs isolated from a variety of sources, including placenta,19 umbilical cord blood,20 amniotic fluid,21 bm,22 dermis,23 and ligament.24 By using both SSEA-4 expression and plastic adherence, Gang et al. were able to isolate cells with multipotent differentiation potential from human bm aspirates.22 However, bone marrow-derived mesenchymal stromal cells (bmMSCs) started to express SSEA-4 levels at a remarkably wide intensity range after expansion, pointing to increasing heterogeneity in originally SSEA-4-positive cells. This raises the question if changes in the SSEA-4 levels of cells are also indicative of shifts in their differentiation potential. Moreover, SSEA-4 expression by in vitro cultured, dedifferentiated stromal cells important for musculoskeletal regeneration, including human articular chondrocytes (hACs) and osteoblasts (hOB), has not been investigated.
Therefore, we have characterized the expression profiles of SSEA-4 and other cell surface markers on human cell populations relevant for the regeneration of skeletal tissues, at different stages of cultivation. In particular, we have compared the SSEA-4 levels on propagated hACs, hOB, and bmMSCs with the differentiation potential of these cells. Further, we have sorted SSEA-4-expressing hACs and followed their SSEA-4 expression during further expansion and also their potential to form cartilage in vitro. Finally, we have investigated the potential functional relevance of SSEA-4 expression in hACs and bmMSCs.
Materials and Methods
Cell isolation and expansion
Articular cartilage of nonosteoarthritic origin (non-OA; donors: two male+two female, age 54–77 years) was collected with institutional ethics approval from consenting patients undergoing limb amputations. Osteoarthritic (OA) cartilage (donors: two male+four female, age 59–78 years) from visually normal regions (International Cartilage Repair Society anatomical grade 0–125) and trabecular bone samples (three female donors, age 59–82 years) were obtained from consenting patients during joint replacement surgery.
Chondrocytes were isolated either from full-thickness cartilage or the superficial and the middle/deep tissue layers separately, as described previously.26 Chondrocytes were propagated on tissue culture plastic (3000 cells/cm2) in the chondrocyte basal medium (low-d-glucose Dulbecco's modified Eagle's medium with 4 mM l-alanyl-l-glutamine, 1 mM sodium pyruvate, 10 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid), 0.1 mM nonessential amino acids, 50 U/mL penicillin/50 μg/mL streptomycin (Pen/Strep) (all from Invitrogen, Carlsbad, CA), 0.1 mM l-ascorbic acid 2-phosphate, and 0.4 mM l-proline (both Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT).
Human primary osteoblasts were isolated by outgrowth from minced and washed bone specimens,27 cultured in the Minimum Essential Medium Alpha (α-MEM) (Invitrogen) with Pen/Strep and 10% FBS, and subsequently plated at 2000 cells/cm2.
bmMSCs were either purchased from the laboratory of Dr. Darwin Prockop, Texas A&M University (donors #1–3) or isolated from bm aspirates obtained under appropriate ethical approval from the iliac crest of consenting patients undergoing spinal fusion surgery (donors #4–6), (gender and age of bmMSC donors in Table 1). Total bm cells were plated in the α-MEM supplemented with 10% FBS, Pen/Strep, and 1 ng/mL of the fibroblast growth factor 2 (Millipore, Billerica, MA). bmMSCs were initially seeded at 100 cells/cm2, subsequently plated at 1000 cells/cm2, and used between passages 3 and 4.
Table 1.
Chondrogenic and Osteogenic Potential of Bone Marrow Mesenchymal Stromal Cells from Different Donors with Varying Stage-Specific Embryonic Antigen-4 Expression Levels
| Donor | Gender, age | SSEA-4+ cells (%, Overton) | COL2A1 (log fold change to d0) | ACAN (log fold change to d0) | GAG per DNA d14 | ALP assay (fold difference to control) | Ca2+ assay (fold difference to control) |
|---|---|---|---|---|---|---|---|
| #1 | male, 22 | 66.3 | 0.9 | −0.6 | 4.1 | ||
| #2 | female, 37 | 17.0 | 1.1 | −1.1 | 4.6 | ||
| #3 | male, 18 | 67.5 | 5.5 | 2.3 | 9.4 | ||
| #4 | male, 22 | 65.5 | 3.3 | −0.1 | 4.3 | 1.6 | 4.6 |
| #5 | female,75 | 33.5 | 3.4 | 0.02 | 3.2 | 72.7 | |
| #6 | female, 32 | 81.9 | N.D. | −0.3 | 1.1 | 29.7 |
Percentages of SSEA-4+ bmMSCs (P3 or 4) were determined by flow cytometry. Chondrogenic markers COL2A1 and ACAN, and the amounts of GAG and DNA in pellets were quantified by real-time reverse transcriptase–polymerase chain reaction and biochemical assays, respectively, after 2 weeks of chondrogenic culture. The mRNA data from day 14 were normalized to expression levels of undifferentiated bmMSCs of day 0 and expressed as logarithms.
ALP activity in media and deposited calcium were also determined after 3 or 4 weeks of osteogenic induction, respectively, and normalized to undifferentiated controls.
SSEA-4, stage-specific embryonic antigen 4; bmMSCs, bone marrow mesenchymal stromal cells; ALP, alkaline phosphatase; GAG, glycosaminoglycans; N.D., not detected.
All cells were maintained at 37°C in a humidified 5% CO2/95% air CO2 incubator with the medium refreshed twice per week. Cells were passaged when subconfluent with 0.25% trypsin with 1 mM ethylenediaminetetraacetic acid (trypsin/EDTA) (Invitrogen) at 37°C for 5 min. Cells were counted by trypan blue (Invitrogen) exclusion in a hemocytometer. Cell population doubling (PD) was calculated using the logarithm (base 2) of the quotient between the final and initial cell numbers.
Flow cytometry analysis and fluorescence-activated cell sorting
Harvested cells were incubated for 30 min in phosphate-buffered saline (PBS) with 3% m/v bovine serum albumin (BSA; Sigma) (3% BSA/PBS) and one of the following antibodies: CD14 (phycoerythrin [PE]-conjugated, 1:20, UCHM-1; Millipore), CD45 (1:20, H5A5; Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA), CD44 (1:20, H4C4; DSHB), CD90 (fluorescein isothiocyanate [FITC]-conjugated, 1:20, F15-42-1; Millipore), CD105 (1:40, P4A4; DSHB), SSEA-1 (1:40, MC-480; DSHB), SSEA-3 (1:40, MC-631; DSHB), SSEA-4 (1:80, MC-813-70; DSHB), or immunoglobulin G (IgG) isotype control (1:50; Invitrogen). Cells were washed twice with cold PBS and samples with unconjugated primary antibodies were incubated for 45 min in 3% BSA/PBS containing either the FITC-labeled goat anti-mouse IgG antibody (1:80; CALTAG, Burlingame, CA) or the DyLight™649-conjugated goat anti-rat IgG antibody (1:40; Jackson ImmunoResearch, West Grove, PA). Finally, samples were washed with cold PBS and resuspended in 3% BSA/PBS.
For cell cycle analysis, harvested cells were fixed with ice-cold 70% v/v ethanol for 15 min and incubated in 3% BSA/PBS with 0.1 mg/mL RNase A solution (Invitrogen), 0.1% Triton X-100, and 0.1 mg/mL propidium iodide (PI) (Invitrogen) for 45 min in the dark.
Approximately 104 cells were analyzed on a FC500 flow cytometer (Beckman Coulter, Fullerton, CA) with appropriate filters for FITC, PE, PI, or DyLight649. For each cell surface antigen, histograms and the percentage of positive cells were determined after the Overton's method28 with CXP software (Beckman Coulter).
Fluorescence-activated cell sorting (FACS) was used to separate SSEA-4-positive from SSEA-4-negative subpopulations of chondrocytes. In brief, 4×106 hACs harvested at passage 2 (four donors; mean PD: 4.4±1.1 standard deviation) were stained for SSEA-4-FITC and sorted on a FACS Aria IIu (BD Biosciences, Franklin Lakes, NJ). One-third of the total sorted cells with lowest or highest fluorescence intensity values were considered SSEA-4-negative (SSEA-4−) or SSEA-4-positive (SSEA-4+), respectively, and used in subsequent proliferation and differentiation experiments.
Immunofluorescence analysis
Immunofluorescence was used to determine localization of SSEA-4 in cartilage and in cell cultures. Formalin-fixed human cartilage specimens were dehydrated in a graded ethanol series, embedded in paraffin, and cut to yield ∼5-μm-thick cross sections. Cells grown on chamber glass slides (BD Biosciences) were washed with PBS and fixed for 20 min in PBS with 4% w/v formaldehyde. Samples were treated with 0.025% w/v hyaluronidase (Sigma) in PBS for 20 min at 37°C and blocked with 1% BSA/PBS for 30 min. Selected samples received an additional treatment with either 0.2% v/v Triton X-100 or ice-cold 100% v/v methanol for 10 min after the antigen retrieval. Samples were subsequently incubated with SSEA-4, SSEA-3, or IgG-matched control antibodies in 1% BSA/PBS for 45 min. Subsequently, Alexa Fluor®488 or Alexa Fluor®633 goat anti-mouse or DyLight649 goat anti-rat antibodies as well as 5 μg/mL 4′,6-diamidino-2-phenylindole (Sigma) and—where required—0.8 U/mL phalloidin rhodamine (Invitrogen) were applied for 1 h in the dark. For double staining with CD14 or CD90, samples were treated with the specified antibodies for 30 min in the dark in a final step. Images were captured using a confocal laser scanning microscope (Leica, Wetzlar, Germany).
Cell proliferation of SSEA-4-sorted cells
To compare the proliferation capacity, SSEA-4+ and SSEA4− hACs were seeded at 2000 cells/cm2 (two donors) and cultured as described under chondrocyte expansion conditions. At day 1, 4, and 7 of culture, cells were harvested for DNA quantification by incubation with 0.5 mg/mL proteinase K (Invitrogen) in a phosphate buffer (20 mM Na2HPO4, 30 mM NaH2PO4·H2O, 5 mM EDTA, pH 7.1) for 15 min and complete digestion in 1.5-mL tubes overnight at 60°C. PD times were calculated by dividing the cultivation period by the PDs, which were determined based on the change in the DNA content, as described above.
Chondrogenic differentiation
Chondrogenic potential was evaluated using either the micromass pellet or alginate hydrogel cultures. Pellets (2×105 cells) of both chondrocytes and bmMSCs were formed by centrifugation.26 Chondrocytes were also encapsulated in 2% w/v sodium alginate (Pronova UP LVG; Novamatrix/FMC BioPolymers, Sandvika, Norway) at 107 cells/mL and crosslinked with 102 mM of the CaCl2 solution in wells (20 μL volume) of a custom-made mould. Cells were cultivated in the serum-free high-glucose chondrocyte basal medium supplemented with 1.25 mg/mL BSA, 10−7 M dexamethasone, 1% v/v ITS+1 (all from Sigma), and 10 ng/mL of the transforming growth factor type beta 1 (Invitrogen) and kept under reduced (5%) oxygen tension in a ProOx C-Chamber (Biospherix, Redfield, NY) inside a cell culture incubator. Cultures were maintained for up to 2 weeks with media refreshed twice per week. For flow cytometry analysis, cells were released from alginate constructs by dissolving the hydrogel in 0.15% trypsin/20 mM EDTA for 15 min.
Quantification of glycosaminoglycans and DNA
The glycosaminoglycans (GAG) content of digested cell pellets was quantified using the 1,9-dimethylmethylene blue dye assay, with absorbance at 525 nm measured in a Benchmark Plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA).29
The Quant-iT™ PicoGreen® dsDNA assay (Invitrogen) was used to quantify the DNA content in digested samples, with fluorescence (485 nm excitation, 520 nm emission) measured using a POLARstar OPTIMA fluorescence microplate reader (BMG Labtech, Offenburg, Germany).
Ribonucleic acid extraction and real-time reverse transcriptase–polymerase chain reaction
To measure the absolute expression levels of selected genes of interest, real-time reverse transcriptase–polymerase chain reaction (qRT-PCR) was used. Ribonucleic acid (RNA) was isolated using the PureLink™ RNA Micro Kit (Invitrogen) according to the manufacturer's instructions, which included mechanic disruption of cell pellets with pestles, sample homogenization with a 20-gauge needle, and the removal of genomic DNA by on column treatment with DNase I (Invitrogen).
First-strand complementary DNA (cDNA) synthesis was performed using the SuperScript™ III first-strand synthesis supermix for qRT-PCR (Invitrogen) with no more than 300 ng of total RNA in 20 μL reactions following the manufacturer's protocol.
Primers were either taken from the literature for RPL13A,30 used as published previously (COL1A1, COL2A1, COL10A1, ACAN, and 18S rRNA)26 or designed using Primer-BLAST (NCBI, Bethesda, MD) for MKI67 (5′→3′ F: AATTCAGAC TCCATGTGCCTGAG, R: CATTGTCCTCAGCCTTCTTTGG) and B2M (5′→3′ F: ATGAGTATGCCTGCCGTGTGA, R: GGCATCTTCAAACCTCCATGATG). All PCR reactions were done in duplicates in 10 μL volumes in a 7900HT PCR system (Applied Biosystems, Foster City, CA). Reactions contained 1 × SYBR® Green PCR Master Mix (Applied Biosystems), 200 nM of each forward and reverse primers, and 0.2 μL of undiluted cDNA and started with 2-min denaturation at 95°C followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. Absolute quantification using standard curves and postamplification analysis were carried out as described previously.26 The cDNA copy number for each reaction was then calculated by a direct comparison to the known standards and normalized to the geometric mean of copy numbers of three housekeeping genes (18S rRNA, RPL13A, and B2M). Transcript levels of SSEA-4+ cells of each data point were further normalized to mRNA levels of the respective SSEA-4− samples. Transcript levels in bmMSCs after 14 days of chondrogenic differentiation were normalized to undifferentiated control cells from day 0.
Osteogenic differentiation
Osteogenic potential was evaluated in vitro. bmMSCs (2500 cells/cm2) were cultured in six-well plates in the bmMSC expansion medium for 2 weeks, and for an additional 4 weeks in the bmMSC expansion medium (controls) or the osteoblast expansion medium supplemented with 10−7 M dexamethasone, 50 μg/mL l-ascorbic acid 2-phosphate (Sigma), and 10 mM β-glycerophosphate.
Alkaline phosphatase activity and calcium deposition assays
After 3 weeks of osteogenic induction, the alkaline phosphatase (ALP) activity was measured in the media supernatant using a colorimetric assay. Briefly, media were changed to phenol red and FBS-free α-MEM for 24 h before measurements, and then incubated for 3 h with an equal volume of 1 mg/mL p-nitrophenylphosphate in 0.2 M Tris buffer (Sigma), and absorbance was measured at 405 nm. Calcium deposition within the cell layer was measured using the Wako HRII calcium assay (Wako, Osaka, Japan) after 4 weeks of osteogenic induction, as described previously.27 Data were normalized to the values from undifferentiated controls.
Statistical analysis
Statistical analyses were performed using Minitab 15 (Minitab, State College, PA). Correlations between PD and SSEA-4-positive chondrocytes were analyzed by polynomial regression. To test for differences in the gene expression and pellet sizes, data from SSEA-4+ cells were normalized to SSEA-4-negative controls from the same donor (n=5 donors) and analyzed with a one-sample t-test compared to the value of 1. Statistical significance in the proliferation assay (n=6, two donors) and the comparison of surface markers expressions in zonal chondrocytes (n=3 donors) were tested by analysis of variance using a general linear model, with a donor considered as a random effect. Statistically significant differences were considered to be present at p<0.05.
Results
Transient and heterogeneous expression of SSEA-4 by hACs in vitro
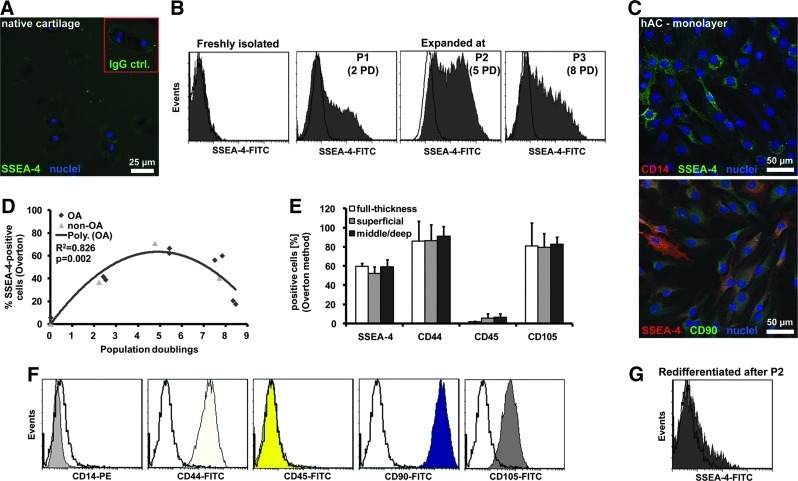
Chondrocytes in human articular cartilage stained at background levels for SSEA-4 (Fig. 1A). Accordingly, SSEA-4 expression was low or undetectable with flow cytometry on primary, freshly isolated hACs (Fig. 1B). However, SSEA-4-expressing cells were detectable by the first passage, levels peaked at ∼5 PD, and decreased again beyond passage 2 (Fig. 1B, D). Chondrocytes from OA and non-OA origin shared similar SSEA-4 expression profiles during monolayer expansion (Fig. 1D). We found no differences in surface marker expressions between expanded cells isolated from different zones of cartilage (Fig. 1E). There were at least two distinct subpopulations (high or low) of SSEA-4-expressing cells (Fig. 1B–D). In contrast, monolayer-expanded chondrocytes homogeneously expressed high levels of the mesenchymal cell markers CD44, CD90, and CD105 and were negative for the hematopoietic cell markers CD14 and CD45, when tested with flow cytometry (Fig. 1F). Expression of the various surface markers was confirmed with confocal microscopy. Propagated hACs exhibited varying staining intensities for SSEA-4, while showing no or homogeneous staining for CD14 or CD90, respectively (Fig. 1C). There were no obvious phenotypic differences between SSEA-4-positive and SSEA-4-negative cells. Interestingly, SSEA-4 expression of chondrocytes was essentially lost following 2 weeks of redifferentiation in alginate hydrogels (Fig. 1G), whereas CD44 was still presented by the majority of cells (data not shown).
FIG. 1.
Expression levels of stage-specific embryonic antigen 4 (SSEA-4) in human articular chondrocytes (hACs) at different stages of cultivation. (A) Human articular cartilage sections (non-osteoarthritic [OA] source) were probed either with SSEA-4-specific or isotype control (immunoglobulin G [IgG]ctrl.) antibodies (red-framed inset) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI). (B) Representative flow cytometry histograms of SSEA-4 plotted with an IgG control (no color) are shown for freshly isolated hACs and for monolayer-propagated chondrocytes after different passages (P1–3) and population doubling (PD) (C) hACs on glass chamber slides were probed with antibodies specific for SSEA-4 and either CD14 or CD90, and counterstained with DAPI. (D) Levels of SSEA-4-positive, monolayer-cultured hACs isolated from OA (rhombus, n=2 donors) and non-OA (triangle, n=1 donor) cartilage. (E) Surface marker levels in OA chondrocytes isolated from different harvest sites of articular cartilage after passage 2 (mean±standard deviation [SD], n=3 donors). (F) Flow cytometry histograms of representative expression patterns of CD14, CD44, CD45, CD90, and CD105 plotted with controls (no color). (G) Representative flow cytometry histogram of SSEA-4 plotted with an IgG control (no color) for P2 chondrocytes redifferentiated 2 weeks in alginate cultures. Color images available online at www.liebertpub.com/tea
Proliferation and chondrogenic potential of hACs sorted for SSEA-4
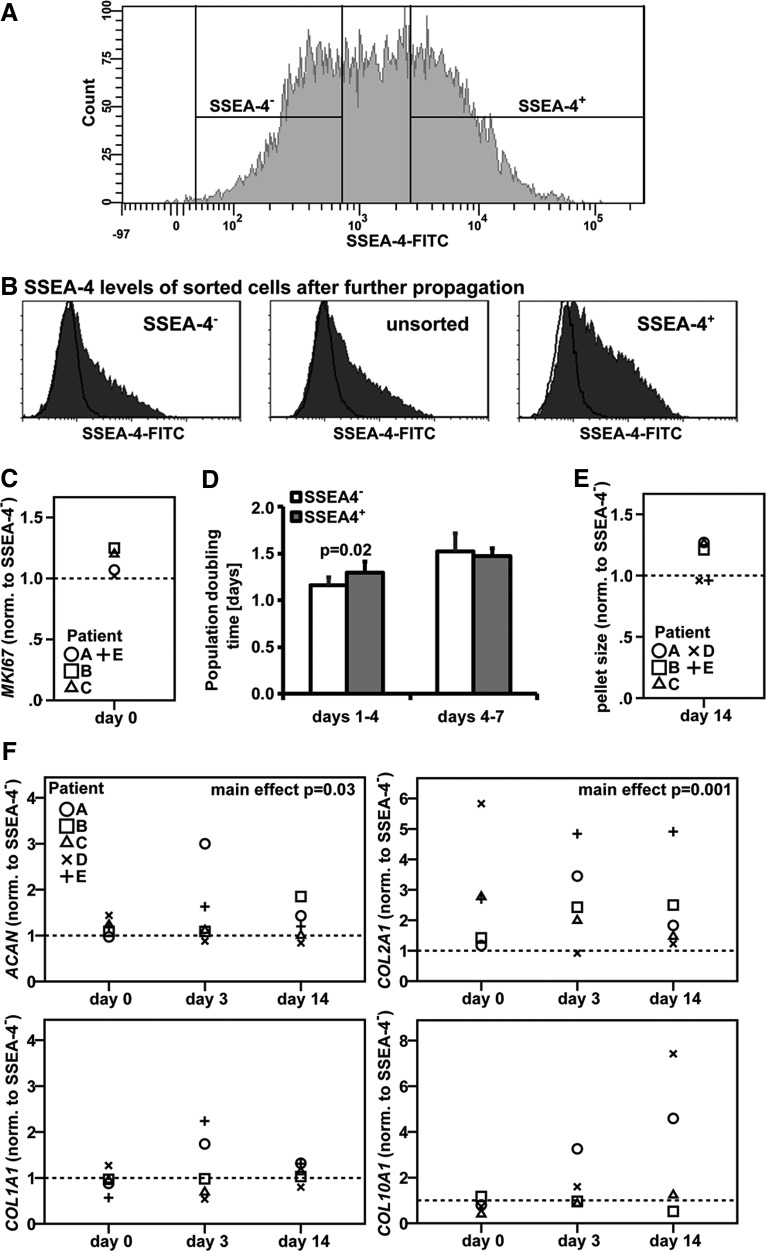
Passage 2 chondrocytes were separated by FACS based on their SSEA-4 expression levels (Fig. 2A) and either further propagated in a monolayer or redifferentiated in micromass pellets. Following an additional passage (∼3 PDs), a high number of SSEA-4+ cells had lost SSEA-4 expression, while a subpopulation of initially SSEA-4− cells gained expression of this marker (Fig. 2B). Gene expression levels of the proliferation marker MKI67, which is detectable during all active phases of proliferation (G1, S, G2, and mitosis),31 were comparable in SSEA-4+ and SSEA-4− cells after sorting (p=0.09) (Fig. 2C). SSEA-4− hACs appeared to proliferate faster initially between day 1 and 4 of culture on tissue culture plastic compared to the SSEA-4+ cells (p=0.02) (Fig. 2D). However, PD times were equal between the two groups between day 4 and 7 of propagation (p=0.7). To test if cells at different stages of the cell cycle account for the differences in SSEA-4 expression levels, we performed a cell cycle analysis on unsorted passage 2 hACs using PI staining; 69.9% of cells were SSEA-4-positive, 83.8% of cells in the G0/1 phase, and 7.9% or 7.3% in the S or G2/M phases, respectively, suggesting that SSEA-4 expression is not simply related to the cell cycle.
FIG. 2.
Proliferation capacity and chondrogenic potential of hACs expressing different levels of SSEA-4. (A) hACs (P2) were separated into SSEA-4+ and SSEA-4− subpopulations by fluorescence-activated cell sorting. (B) SSEA-4 expression levels in sorted and unsorted cells after propagation for an additional passage cycle. Proliferation potential was compared by measuring (C) mRNA levels of MKI67 immediately after sorting (n=4 donors) and (D) determining PD times of sorted cells in monolayer cultures (mean±SD; n=6, two donors). Chondrogenic potential of SSEA-4+ and SSEA-4− was assessed in micromass pellet cultures by comparing (E) pellet sizes after 14 days of redifferentiation and (F) gene expression levels of collagen I (COL1A1), II (COL2A1), X (COL10A1), and aggrecan (ACAN) during pellet culture (donors A–C: non-OA, two male, one female, 54–65 years; D+E: OA, one male, one female, 76+78 years).
In redifferentiation pellet culture, SSEA-4+ cells from three out of five donors tested formed pellets with larger diameters than SSEA-4− cells (p=0.1) (Fig. 2E). Moreover, although SSEA-4+ cells exhibited overall higher levels of the chondrogenic marker genes COL2A1 (p=0.001) and aggrecan (p=0.03) compared to SSEA-4− cells, differences in expression levels of these genes between the two groups did not reach significance at any of the individual time points tested (Fig. 2F). Gene expression levels for COL1A1, a marker for fibroblastic, dedifferentiated chondrocytes, (p=0.4) and for the hypertrophy marker COL10A1 (p=0.1) were comparable in the two subpopulations.
SSEA-4 expression in hOB during in vitro expansion
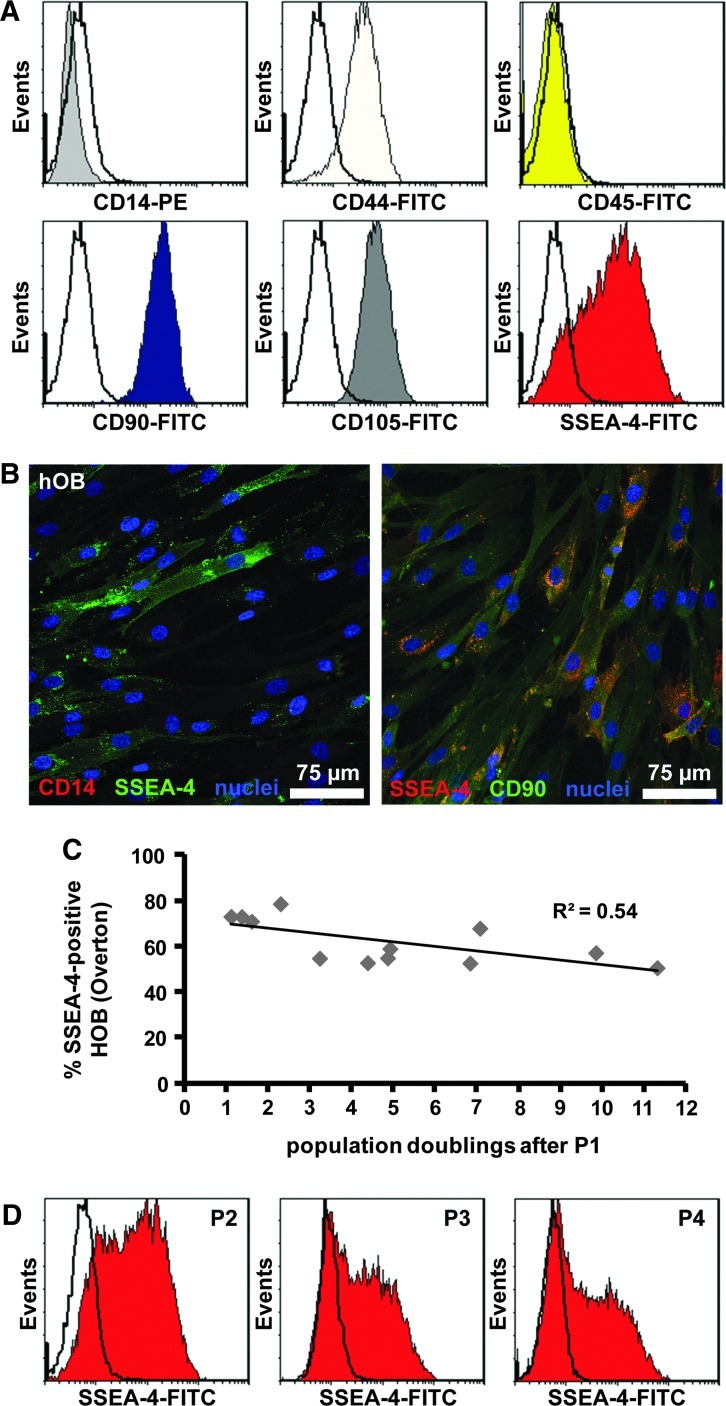
hOB also exhibited at least two distinct subpopulations of SSEA-4-expressing cells during monolayer propagation, while being negative for CD14 and CD45 and showing high, homogeneous expression for CD44, CD90, and CD105 (Fig. 3A, B). Similar to hACs, the SSEA-4+ osteoblast population decreased with continuous cell expansion (Fig. 3C, D).
FIG. 3.
Expression of surface markers on monolayer-expanded human osteoblasts (hOB). (A) Flow cytometry histograms of representative expression patterns for one donor of CD14, CD44, CD45, CD90, CD105, and SSEA-4, plotted with matched IgG controls (no color). (B) Osteoblasts grown on glass chamber slides were probed with antibodies specific for SSEA-4 as well as either CD14 or CD90 and counterstained with DAPI. (C) Levels of SSEA-4-positive hOB isolated from bone explant cultures (P1) and propagated for various PD. (D) Representative histograms for SSEA-4 levels in osteoblasts after different passages (P2–4). Color images available online at www.liebertpub.com/tea
Chondrogenic and osteogenic potential of bmMSCs with varying levels of SSEA-4 expression
To test if SSEA-4 expression levels on bmMSCs can be predictive of their differentiation potential, we determined the surface marker expression profile in bmMSCs expanded over 3–4 passages and cultured them under conditions to induce either chondrogenic or osteogenic differentiation.
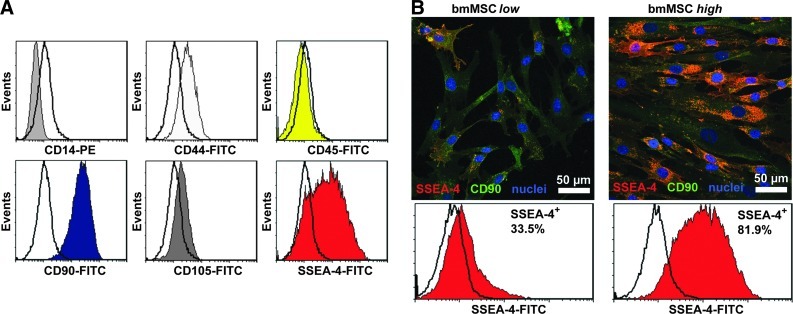
bmMSC preparations were uniformly negative for CD14 and CD45 and positive for CD44, CD90, and CD105 (Fig. 4A). However, flow cytometry and confocal microscopy revealed a remarkable donor variation in SSEA-4 levels (Fig. 4B and Table 1). In general, bmMSC expression of SSEA-4 was heterogeneous compared to CD44, CD90, and CD105. When differentiated in micromass pellets over 2 weeks, chondrogenic markers COL2A1 and aggrecan were highly upregulated in cells from three and one out of six patients, respectively (Table 1). However, there was no correlation between mRNA levels for these genes, or GAG amounts per DNA in pellets, and the percentage of SSEA-4+ cells. Similarly, the levels of SSEA-4 expression did not correlate with ALP activities nor the amount of calcium deposited in bmMSCs cultured under osteogenic conditions (Table 1).
FIG. 4.
Expression of surface markers on bone marrow mesenchymal stromal cells (bmMSCs). (A) Flow cytometry histograms of representative expression patterns of CD14, CD44, CD45, CD90, CD105, and SSEA-4 for one donor, plotted with matched IgG controls (no color). (B) bmMSCs with either low or high expression of SSEA-4 (two representative donors) as shown by immunofluorescence (SSEA-4, CD90, and DAPI) and by flow cytometry with percentage of SSEA-4+ cells. Color images available online at www.liebertpub.com/tea
Characterization of the SSEA-4 antigen
To further characterize the carrier of the SSEA-4 antigen in our cell models, we tested for the presence of SSEA-3, which is found in the very same globo-series GSL containing the SSEA-4 epitope.17 SSEA-3 expression levels were relatively low in both hACs and bmMSCs compared to SSEA-4, as measured with flow cytometry (Fig. 5A). bmMSCs high in SSEA-4 also expressed slightly higher levels of SSEA-3. Notably, the typical staining profile with subpopulations of varying SSEA-4 levels was not detectable for SSEA-3. However, the staining patterns of SSEA-4 and SSEA-3 appeared similar on hACs grown on glass slides (Fig. 5B). Neither bmMSCs nor hACs stained positive for the SSEA-1 antigen (data not shown).
FIG. 5.
Expression of SSEA-3 on hACs and bmMSCs in monolayer cultures. (A) Representative flow cytometry histograms of SSEA-3 expression profiles on hACs, and bmMSCs with high or low in SSEA-4 expression (controls: no color). (B) SSEA-3 immunofluorescence (with DAPI counterstain) on chondrocytes (P2) and bmMSCs (high SSEA-4 levels). Color images available online at www.liebertpub.com/tea
Treating chondrocytes as well as bmMSCs with 0.2% Triton X-100 reduced their immunoreactivity against SSEA-4 and left some residual, punctuate, perinuclear staining (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). After washing with ice-cold 100% methanol, SSEA-4 staining was completely lost in both cell types (data not shown).
Discussion
The aim of this study was to characterize the expression of the stem cell surface marker SSEA-4 in chondro- and osteoprogenitor cells and to test its potential to identify subsets of cells with superior differentiation or proliferation potential. We have shown that propagated hACs exhibit remarkably heterogeneous expression profiles for SSEA-4, which indicates the presence of at least two distinct cell subpopulations with either low or high levels of this surface marker. None of the other surface markers tested showed similar heterogeneity. The existence of chondrocyte subsets with distinct SSEA-4 expression is not explained by the phenotypic variation of cells from different cartilage zones, as chondrocytes isolated from superficial and middle/deep zone of cartilage shared similar levels of SSEA-4. We also found that SSEA-4 was not detectable on cells of native articular cartilage and that it was no longer expressed in chondrocytes redifferentiated in alginate constructs. This is in agreement with studies that observed the loss of SSEA-4 in differentiated embryonic stem cells32 and MSCs.33 The increase in SSEA-4 levels during propagation could be explained by the presence and amplification of a highly proliferative mesenchymal progenitor cell population that is thought to reside in cartilage.34,35 It seems more likely, however, that SSEA-4 is upregulated together with an overall change from a chondrocytic to a more immature, MSC-like phenotype during dedifferentiation in vitro.11 Interestingly, propagated hOB display a similar SSEA-4 expression profile to chondrocytes, suggesting that heterogenic SSEA-4 expression may be a common feature of in vitro expanded cells from skeletal tissues.
SSEA-4 was only transiently expressed in expansion cultures of chondrocytes and osteoblasts with levels declining after a prolonged monolayer cultivation. This was even more evident in chondrocytes sorted for SSEA-4, where a high number of SSEA-4+ cells lost their expression after subsequent passage. Interestingly, some SSEA-4− cells still gained expression of this marker. The loss of SSEA-4 could be caused by increased replicative senescence36 or be indicative of a rapid decrease in the redifferentiation capacity of ex vivo expanded chondrocytes.37,38 This raises the question if it can be used as general marker for chondroprogenitor cells with superior differentiation potential. To explore this, we separated subpopulations of propagated chondrocytes with low and high levels of this surface marker by FACS and determined their differentiation and proliferation capacity. SSEA-4+ chondrocytes expressed higher levels of chondrogenic markers than SSEA-4− populations when redifferentiated in pellet cultures. However, it should be noted that cells devoid of this antigen still exhibited some degree of redifferentiation potential. There were also no clear differences in terms of proliferation capacity between sorted cells. Although SSEA-4− chondrocytes seemed to proliferate at a slightly faster rate in the first 4 days of culture, both groups expressed comparable amounts of the proliferation marker MKI67.31 A cell cycle analyses did not reveal a link to the variation in SSEA-4 levels, either. Taken together, our data speak against a direct involvement or a dominant role of SSEA-4 in cellular processes such as proliferation, cell cycle progression, or differentiation.
A functional role for SSEA-4 in embryonic stem cells has not been identified yet. A reduction of SSEA-4 levels by inhibition of the GSL pathway does not affect the stem cell phenotype, including pluripotency, but points to a role during embryonic stem cell differentiation, instead.39,40 In this respect, it is surprising that SSEA-4 has also been shown to be expressed on the more specialized, committed MSCs isolated from a number of adult human tissues.22–24 Gang et al. have even been able to select multipotent cells from bm by employing SSEA-4 and proposed its expression identifies a homogeneous MSC population with superior biological properties.22 In contrast, Guillot et al. failed to detect SSEA-4 or other stem cell markers in adult bmMSCs.41 We found that the expression of SSEA-4 varies considerably between bmMSC preparations from different donors. This could clearly be reflective of a generally diverse repertoire of different MSC subpopulations in vivo or may be due to unintended, minor variations in the in vitro culture conditions that favored different subpopulations with varying differentiation potential.1 However, the level of SSEA-4 expression did not correlate with the outcome of our in vitro differentiation assays. This suggests that the predictive value of SSEA-4 levels in bmMSCs may be limited in respect to their chondrogenic and osteogenic potentials.
Apart from variations in the handling and the origin of MSC preparations, the nature of the SSEA-4 antigen could be a source of inconsistency and confusion in the literature. Brimble et al. reported that the SSEA-4 antigen was still present despite successful depletion of GSLs and SSEA-3 on their stem cell model.39 SSEA-3, another commonly used stem cell surface marker, recognizes a glycan moiety (Gb5) in the very same globo-series GSL containing the SSEA-4 epitope.17 We found only relatively low levels of SSEA-3 in bmMSCs and chondrocytes, with the latter also lacking the heterogeneous expression profile of SSEA-4. Therefore, the majority SSEA-4 antigen in MSCs or other cell types may not be monosialyl-Gb5. It should be noted that the SSEA-4 antibody, clone MC-813-70, shows some weak cross reactivity with other GSL types as well as glycoproteins, all carrying the NeuAcα2-3Galβ1-3GalNAc epitope.17 Indeed, the SSEA-4 epitope has been identified on the 34/67 laminin receptor of a murine embryonal carcinoma cell line.42 Our observation that Triton X-100 and ice-cold methanol abolished SSEA-4 immunoreactivity cannot answer the question about the carrier of the epitope, since glycolipids, as well as certain glycoproteins could be harbored in detergent-soluble areas on the cell surface. Further investigations into the true identity of the SSEA-4 carrier(s) are necessary to explain the heterogeneity of SSEA-4 on chondrocytes, osteoblasts, and bmMSCs expansion cultures, which may ultimately shed some light on the functional importance of SSEA-4 variations in these cells.
Conclusions
Cells isolated from cartilage and bone adopt the expression of the stem cell surface marker SSEA-4 in in vitro expansion cultures suggesting that it is expressed by subpopulations of cells with an immature, mesenchymal stem cell-like phenotype. Presentation of this surface antigen is remarkably heterogeneous and appears to be of a transient nature during the cultivation period. Although SSEA-4-positive chondrocytes show a slightly better chondrogenic potential compared to chondrocytes that do not present this surface molecule, the differences in the differentiation capacity of the two groups are not pronounced enough to link SSEA-4 expression to chondrogenic progenitors with superior differentiation potency. In addition, SSEA-4 levels in bmMSCs seem to be unrelated to the cells' chondrogenic and osteogenic potential in vitro. These data suggest that SSEA-4 is not a reliable marker of multipotency in bmMSCs and is not specific to dedifferentiated mature cell populations with superior differentiation capacity. The fact that SSEA-4 is also expressed by more committed cell types also raises doubts about its usefulness as a stem cell marker in general.
Supplementary Material
Acknowledgments
The authors thank the Australian Research Council and the New Zealand Lotteries Health Research Grants scheme for funding. We also thank Prof. Ross Crawford and Prof. Michael Schütz for supplying tissue samples from surgeries they performed at the Prince Charles Hospital and the Princess Alexandra Hospital, respectively. The antibodies against SSEA-4, SSEA-3, SSEA-1, CD44, CD45, and CD105, developed by D. Solter, B.B. Knowles, T.F Linsenmayer, J.T. August, J.E.K. Hildreth, E.A. Wayner, respectively, were obtained from the DSHB developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Disclosure Statement
The authors have no conflict of interests.
References
- 1.Pevsner-Fischer M. Levin S. Zipori D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev. 2011;7:560. doi: 10.1007/s12015-011-9229-7. [DOI] [PubMed] [Google Scholar]
- 2.Barbero A. Ploegert S. Heberer M. Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 3.Kuroda Y. Kitada M. Wakao S. Dezawa M. Bone marrow mesenchymal cells: how do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp (Warsz) 2011;59:369. doi: 10.1007/s00005-011-0139-9. [DOI] [PubMed] [Google Scholar]
- 4.Roberts S. McCall IW. Darby A.J. Menage J. Evans H. Harrison P.E., et al. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5:R60. doi: 10.1186/ar613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saris D.B. Vanlauwe J. Victor J. Haspl M. Bohnsack M. Fortems Y., et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 6.Grogan S.P. Barbero A. Diaz-Romero J. Cleton-Jansen A.M. Soeder S. Whiteside R., et al. Identification of markers to characterize and sort human articular chondrocytes with enhanced in vitro chondrogenic capacity. Arthritis Rheum. 2007;56:586. doi: 10.1002/art.22408. [DOI] [PubMed] [Google Scholar]
- 7.Niemeyer P. Pestka J.M. Salzmann G.M. Sudkamp N.P. Schmal H. Influence of cell quality on clinical outcome after autologous chondrocyte implantation. Am J Sports Med. 2012;40:556. doi: 10.1177/0363546511428879. [DOI] [PubMed] [Google Scholar]
- 8.Aicher W.K. Buhring H.J. Hart M. Rolauffs B. Badke A. Klein G. Regeneration of cartilage and bone by defined subsets of mesenchymal stromal cells—potential and pitfalls. Adv Drug Deliv Rev. 2011;63:342. doi: 10.1016/j.addr.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Mafi P. Hindocha S. Mafi R. Griffin M. Khan W.S. Adult mesenchymal stem cells and cell surface characterization - a systematic review of the literature. Open Orthop J. 2011;5:253. doi: 10.2174/1874325001105010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russ H.A. Ravassard P. Kerr-Conte J. Pattou F. Efrat S. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS One. 2009;4:e6417. doi: 10.1371/journal.pone.0006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Romero J. Nesic D. Grogan S.P. Heini P. Mainil-Varlet P. Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J Cell Physiol. 2008;214:75. doi: 10.1002/jcp.21161. [DOI] [PubMed] [Google Scholar]
- 12.Poloni A. Maurizi G. Leoni P. Serrani F. Mancini S. Frontini A., et al. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cells. 2012;30:965. doi: 10.1002/stem.1067. [DOI] [PubMed] [Google Scholar]
- 13.Chen X.D. Qian H.Y. Neff L. Satomura K. Horowitz M.C. Thy-1 antigen expression by cells in the osteoblast lineage. J Bone Miner Res. 1999;14:362. doi: 10.1359/jbmr.1999.14.3.362. [DOI] [PubMed] [Google Scholar]
- 14.Fujii Y. Fujii K. Nakano K. Tanaka Y. Crosslinking of CD44 on human osteoblastic cells upregulates ICAM-1 and VCAM-1. FEBS Lett. 2003;539:45. doi: 10.1016/s0014-5793(03)00182-0. [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Romero J. Gaillard J.P. Grogan S.P. Nesic D. Trub T. Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202:731. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]
- 16.Pelttari K. Wixmerten A. Martin I. Do we really need cartilage tissue engineering? Swiss Med Wkly. 2009;139:602. doi: 10.4414/smw.2009.12742. [DOI] [PubMed] [Google Scholar]
- 17.Kannagi R. Cochran N.A. Ishigami F. Hakomori S. Andrews P.W. Knowles B.B., et al. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanctot P.M. Gage F.H. Varki A.P. The glycans of stem cells. Curr Opin Chem Biol. 2007;11:373. doi: 10.1016/j.cbpa.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battula V.L. Bareiss P.M. Treml S. Conrad S. Albert I. Hojak S., et al. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007;75:279. doi: 10.1111/j.1432-0436.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 20.Suila H. Pitkanen V. Hirvonen T. Heiskanen A. Anderson H. Laitinen A., et al. Are globoseries glycosphingolipids SSEA-3 and -4 markers for stem cells derived from human umbilical cord blood? J Mol Cell Biol. 2011;3:99. doi: 10.1093/jmcb/mjq041. [DOI] [PubMed] [Google Scholar]
- 21.De Coppi P. Bartsch G., Jr. Siddiqui M.M. Xu T. Santos C.C. Perin L., et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 22.Gang E.J. Bosnakovski D. Figueiredo C.A. Visser J.W. Perlingeiro R.C. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 23.Vaculik C. Schuster C. Bauer W. Iram N. Pfisterer K. Kramer G., et al. Human dermis harbors distinct mesenchymal stromal cell subsets. J Invest Dermatol. 2012;132:563. doi: 10.1038/jid.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J. Pan T. Im H.J. Fu F.H. Wang J.H. Differential properties of human ACL and MCL stem cells may be responsible for their differential healing capacity. BMC Med. 2011;9:68. doi: 10.1186/1741-7015-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aroen A. Loken S. Heir S. Alvik E. Ekeland A. Granlund O.G., et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]
- 26.Schrobback K. Malda J. Crawford R.W. Upton Z. Leavesley D.I. Klein T.J. Effects of oxygen on zonal marker expression in human articular chondrocytes. Tissue Eng Part A. 2012;18:920. doi: 10.1089/ten.TEA.2011.0088. [DOI] [PubMed] [Google Scholar]
- 27.Reichert J.C. Quent V.M. Burke L.J. Stansfield S.H. Clements J.A. Hutmacher D.W. Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials. 2010;31:7928. doi: 10.1016/j.biomaterials.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Overton W.R. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry. 1988;9:619. doi: 10.1002/cyto.990090617. [DOI] [PubMed] [Google Scholar]
- 29.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 30.Curtis K.M. Gomez L.A. Rios C. Garbayo E. Raval A.P. Perez-Pinzon M.A., et al. EF1alpha and RPL13a represent normalization genes suitable for RT-qPCR analysis of bone marrow derived mesenchymal stem cells. BMC Mol Biol. 2010;11:61. doi: 10.1186/1471-2199-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholzen T. Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Draper J.S. Pigott C. Thomson J.A. Andrews P.W. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J Anat. 2002;200:249. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heiskanen A. Hirvonen T. Salo H. Impola U. Olonen A. Laitinen A., et al. Glycomics of bone marrow-derived mesenchymal stem cells can be used to evaluate their cellular differentiation stage. Glycoconj J. 2009;26:367. doi: 10.1007/s10719-008-9217-6. [DOI] [PubMed] [Google Scholar]
- 34.Alsalameh S. Amin R. Gemba T. Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 35.Fickert S. Fiedler J. Brenner R.E. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6:R422. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsch D. Brummendorf T.H. Richter W. Fellenberg J. Replicative aging of human articular chondrocytes during ex vivo expansion. Arthritis Rheum. 2002;46:2911. doi: 10.1002/art.10626. [DOI] [PubMed] [Google Scholar]
- 37.Dell'Accio F. De Bari C. Luyten F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44:1608. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 38.Giovannini S. Diaz-Romero J. Aigner T. Mainil-Varlet P. Nesic D. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. J Cell Physiol. 2009;222:411. doi: 10.1002/jcp.21965. [DOI] [PubMed] [Google Scholar]
- 39.Brimble S.N. Sherrer E.S. Uhl E.W. Wang E. Kelly S. Merrill A.H., Jr., et al. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells. 2007;25:54. doi: 10.1634/stemcells.2006-0232. [DOI] [PubMed] [Google Scholar]
- 40.Fenderson B.A. Radin N. Andrews P.W. Differentiation antigens of human germ cell tumours: distribution of carbohydrate epitopes on glycolipids and glycoproteins analyzed using PDMP, an inhibitor of glycolipid synthesis. Eur Urol. 1993;23:30. doi: 10.1159/000474567. discussion 6. [DOI] [PubMed] [Google Scholar]
- 41.Guillot P.V. Gotherstrom C. Chan J. Kurata H. Fisk N.M. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 42.Katagiri Y.U. Kiyokawa N. Nakamura K. Takenouchi H. Taguchi T. Okita H., et al. Laminin binding protein, 34/67 laminin receptor, carries stage-specific embryonic antigen-4 epitope defined by monoclonal antibody Raft.2. Biochem Biophys Res Commun. 2005;332:1004. doi: 10.1016/j.bbrc.2005.05.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.