Abstract
Combined chemotherapeutic regimens in conjunction with oxaliplatin are considered safe and effective treatment options in the clinical management of metastatic colorectal cancer. A 62-year-old male patient with a metastatic rectal carcinoma developed a pulmonary reaction after the first application of the combined standard chemotherapy regimen (5-fluorouracil and sodium folinic acid as a 24 h infusion and oxaliplatin). Following the first dose of chemotherapy, the patient developed acute dyspnoea and fever. A computerised scan of the chest revealed bilateral pulmonary patchy consolidation. Despite high-dose empiric antibiotic and antimycotic treatment, no clinical improvement was seen. The patient's condition deteriorated, and he required invasive mechanical ventilation. Diagnostic thoracoscopic wedge resections were performed for further diagnosis. The histological workup revealed distinct granulomatous inflammation, but no microbial pathogens were to be found. Thereupon, a drug-induced reaction to chemotherapy was suspected and high-dose steroid treatment initiated. Subsequently, the patient's respiratory condition improved and he was extubated. The present case exemplifies the rare course of a bilateral pneumonia-like, drug-induced granulomatous reaction following a single application of oxaliplatin. In addition to the known side effects of oxaliplatin-containing combination chemotherapy, unexpected serious adverse events in the form of pulmonary toxicities should also be taken into account.
1. Introduction
The standard palliative treatment of patients with metastatic colorectal cancer involving the application of 5-fluorouracil-(5-FU-) based chemotherapy combined with irinotecan or oxaliplatin in first- and second-line treatment extended patient survival by up to 22 months [1–3]. In addition, innovative molecular approaches such as antiangiogenesis or inhibition of signal epidermal growth factor receptor transmission have become more important [4, 5]. Currently, the interdisciplinary management of colon cancer patients who initially do not respond to curative therapy is at the focus of efforts to prolong survival and maintain quality of life. In some cases, a secondary metastatic resection of primarily unresectable metastases after downstaging offers a curative option. A necessary proviso, however, is close interdisciplinary cooperation on the part of all those involved [6, 7].
The occurrence of adverse events during chemotherapy makes it necessary to modify or, in rare cases, even to discontinue treatment. Depending on the treatment regimen applied, the most frequent serious side effects that may occur following the use of oxaliplatin (in combination with) 5-FU and folinic acid are haematological (13%–52%), gastrointestinal (10%–33%), and neurological (0%–8%) toxicities. Although most of these reactions can be readily controlled, in rare cases they may necessitate discontinuation of treatment. In comparison with the high level of oxaliplatin usage, pulmonary toxicity is very rare [8–10].
2. Case Presentation
A 62-year-old male patient attended the outpatient department for endoscopic clarification of lower gastrointestinal bleeding haemorrhage. Since the patient had atrial fibrillation, he was on phenprocoumon. Colonoscopy revealed a circular exulcerating carcinoma located in the upper third of the rectum. Histological workup revealed an adenocarcinoma. Ultrasonography of the abdomen showed a number of virtually hypoechoic lesions compatible with liver metastases. Consequently, deep anterior rectal resection plus peritonectomy in the region of the posterior wall of the bladder combined with a descendorectostomy were performed to resolve a high-grade stenosis caused by the rectal carcinoma. Intraoperatively, peritoneal carcinomatosis was also found. The findings were categorized pathohistologically as pT4b, pN2 (5/28), L0, V1, pM1 (PER, HEP), and G2 (UICC Stage IV). The postoperative course was uneventful. Diffuse, nonresectable liver metastases and peritoneal carcinomatosis were diagnosed, and palliative treatment with combination chemotherapy comprising high-dose 5-FU and sodium folinic acid in the form of a 24 h infusion plus oxaliplatin every second week was scheduled [7].
One day after receiving the first dose (5-FU 2.000 mg/m2, sodium folinic acid 500 mg/m2 combined with a previous application of oxaliplatin 85 mg/m2) the patient presented at our emergency unit with progressive dyspnoea and a subfebrile temperature (38.4°C). Laboratory markers of inflammation were elevated (leucocytes 12.400/μL (normal range: 4.000–10.000/μL), CRP 41 mg/L (normal range < 5 mg/L)). Empirical antibiotic treatment with ampicillin/sulbactam was initiated. At that time the conventional chest X-ray and the previous computerised tomography of the chest (obtained one month prior to the initiation of chemotherapy) were inconspicuous (Figure 1). Nevertheless, the patient's condition continued to deteriorate. On the patient's third day in hospital, his antibiotic therapy was adjusted to piperacillin/sulbactam and ciprofloxacin. From the fifth day onwards, fluconazole was added. During the course of treatment, both the conventional X-ray and the CT scan of the chest revealed bilateral patchy consolidations of both lungs and low-grade pleural effusions (Figure 2). After seven days, the patient was transferred to the intensive care unit with respiratory failure (O2-saturation 70%). Antibiotic treatment was continued, and noninvasive mechanical ventilation initiated. On the following day, however, the patient had to be intubated because of progressive respiratory distress (arterial blood gases: FiO2 0.55, paO2 36.2 mmHg, pCO2 46.7 mmHg, Horowitz-Index 65.8). Bronchoalveolar lavage was performed. Neither microbiological nor virological examinations revealed the presence of pathogens. Ten days after intubation, the patient's gas exchange parameters showed no signs of clinical improvement (arterial blood gases: FiO2 1.0, paO2 99 mmHg, pCO2 43.0 mmHg, Horowitz-Index 99). The laboratory markers of inflammation continued to increase (maximum values: leucocytes 15.500/μL (normal range: 4.000–10.000/μL), CRP 174 mg/L (normal range < 5 mg/L)). Antibiotic medication was temporarily discontinued, and thoracoscopic wedge resections were performed in all three right pulmonary lobes. Prior to the availability of the microbiological and histological results, antibiotic treatment with ceftazidime and fosfomycin was applied. Neither the Grocott nor the Ziehl-Neelsen or Auramine staining revealed evidence of bacterial or mycotic pathology. Both the mycobacterial PCR and the bacteriological cultures were negative. Repeated microbiological and viral examinations (bronchoalveolar lavage, bloodcultures, galactomannan assay, central venous catheter tip, urine, and legionella-antigens in the urine) all remained negative. No signs of systemic autoimmune disease were to be found. The histological findings revealed extensive granulomatous inflammation with no evidence of malignancy (Figure 3). No eosinophilic infiltration, fibroinflammatory buds, or collagen bundles as evidence of a hypersensitivity reaction or pulmonary fibrosis were seen. Since a drug-induced lung reaction to chemotherapy was suspected, i.v. high-dose steroid treatment (initial application: 1 g prednisolone per day) was started. Within 36 hours, the gas exchange parameters improved, and the patient was extubated after 4 days on steroid treatment and 16 days on invasive mechanical ventilation. During the following days, intermittent noninvasive mechanical ventilation was gradually reduced and finally discontinued on the third postextubation day. The daily prednisolone dose was reduced to 70 mg after eight days, to 50 mg after 12 days, and finally tapered off over 3 weeks.
Figure 1.
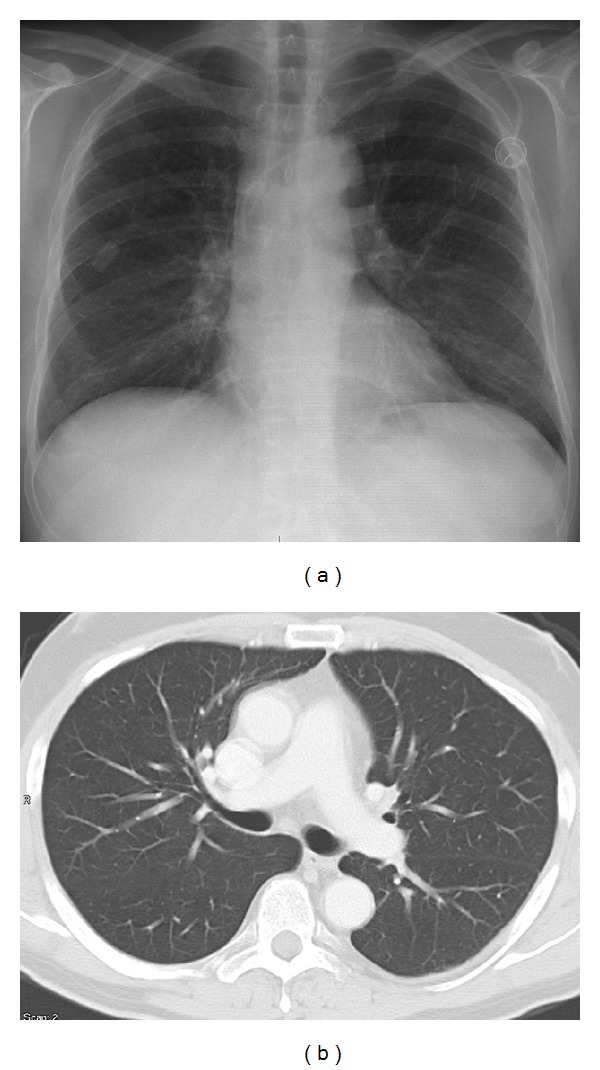
X-ray (one day after inpatient admittance) and CT scan of the chest (one month prior to first chemotherapy administration). Normal finding in both lungs.
Figure 2.
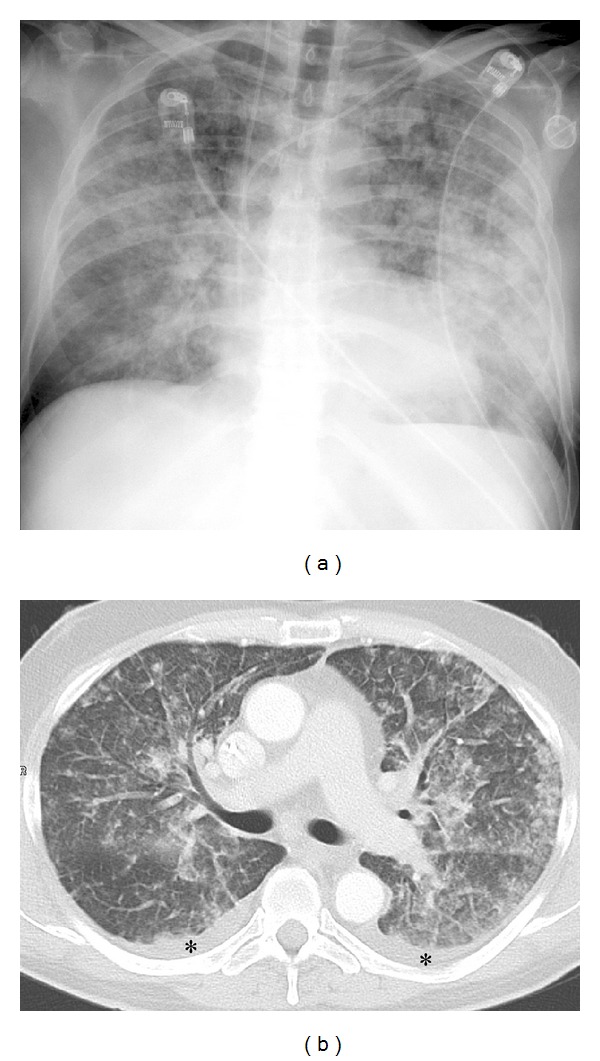
X-ray (seven days after inpatient admittance) and CT scan of the chest (five days after initiation of chemotherapeutic treatment). Patchy airspace consolidation with peribronchial and peripheral distribution. Small pleural effusion bilaterally (black asterisk).
Figure 3.
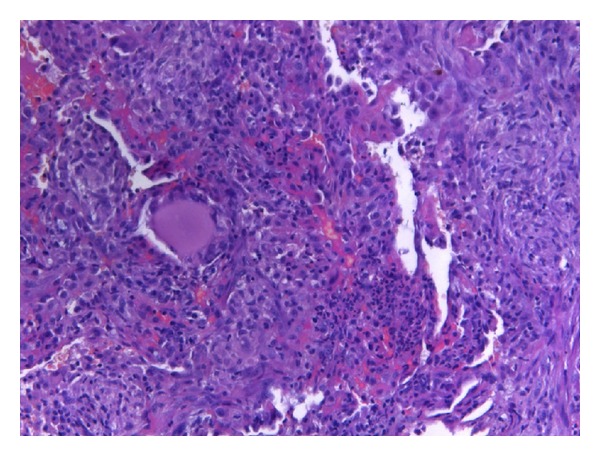
Lung biopsy specimen obtained by video-assisted thoracoscopic wedge resection (HE ×10). Extensive granulomatous inflammation. No evidence of fibrosis or malignancy.
On discharging the patient from the inpatient department, we reevaluated his clinical condition and radiological findings and discussed his treatment options with him in detail. His general state of health (ECOG 1) and alimentary status were both good. A dihydropyrimidine-dehydrogenase deficiency, which is sometimes associated with high-grade 5-FU toxicity, was excluded. A CT scan of the chest 5 months after respiratory failure (Figure 4) showed virtually complete resolution with only slight residual subpleural scarring and pleural calcifications.
Figure 4.
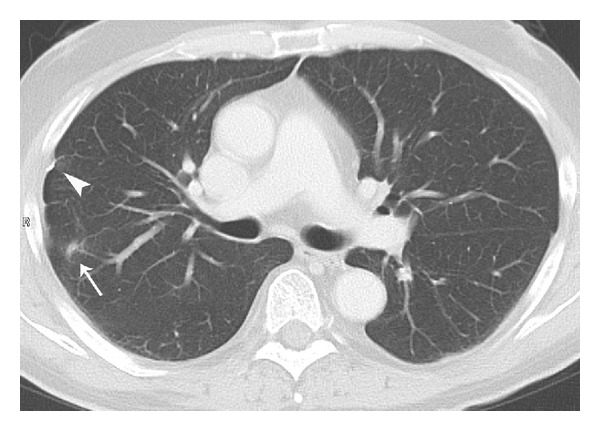
CT scan of the chest five months after the first application of chemotherapy. Small scar in the right upper lobe (white arrow) and small pleural calcification (white arrowhead) as residual lesions of the inflammatory process.
Finally, with the informed consent of the patient, we decided to implement palliative second-line treatment (5-FU and sodium folinic acid in the form of a 24 h infusion plus weekly irinotecan) [11]. All in all, the patient received three cycles of palliative second-line treatment, one cycle of palliative third-line treatment (5-FU and sodium folinic acid in the form of a 24 h infusion combined with weekly irinotecan and cetuximab), one cycle of palliative fourth-line treatment (5-FU and sodium folinic acid in the form of a 24 h infusion combined with irinotecan, and bevacizumab every two weeks), and finally additional internal radiotherapy (SIRT). The additional palliative treatment regimens were well tolerated, and no more serious toxic side effects occurred. Pulmonary symptoms did not reoccur. From initiation of palliative first-line treatment, the patient survived 21 months. He died of tumour-associated cardiocirculatory failure caused by progression of the liver metastases.
3. Discussion
Acute lung injury after combination chemotherapy including oxaliplatin does occasionally occur. In view of the frequency of such treatment, however, reports of acute respiratory deterioration (sometimes taking a fatal course) associated with the use of oxaliplatin are rare, and only a few publications describe pulmonary fibrosis, hypersensitivity reactions, or organizing pneumonia [12–25]. Drug-induced pneumonitis is usually a diagnosis of exclusion. Symptoms are nonspecific and may include dyspnoea, fever, or respiratory failure. Infections, alveolar haemorrhage, lymphangiosis, and heart failure are the main differential diagnoses in cancer patients on chemotherapy who develop respiratory distress. In our patient, extensive diagnostic workup, which included blood, bronchoalveolar lavage, urine, and histological examinations, failed to produce a specific diagnosis. Retrospectively, no pathogens were ever detected, despite extensive diagnostic measures during the period of hospitalisation, and the response to empirical broad-spectrum antibiotic treatment failed to bring about any consistent clinical improvement. A lung biopsy viathoracoscopic wedge resection in all three right pulmonary lobes was deemed necessary. The histological workup of all three lobes showed the same pattern of a granulomatous infiltration with no signs of pulmonary fibrosis or malignancy. This patient report is the first to describe acute lung injury with a distinct granulomatous reaction associated with this combination of drugs. No other causes of granulomatous lung disease (e.g. sarcoidosis, Wegener disease, or mycobacterial infection) were to be found. The rapid and sustained improvement achieved with high-dose steroid therapy in the absence of any signs of autoimmune disease permits the conclusion to be drawn that the inflammatory changes had been caused by a drug-induced pulmonary reaction.
Since the concomitant medication was unlikely to be responsible for the initial findings, the pathological course must have been caused by the oxaliplatin. After having survived the acute reaction, the patient underwent repeated treatment with palliative combination therapy with 5-FU and sodium folinic acid in addition to other agents in the second-, third-, and fourth line treatments.
Reexposure to 5-FU and sodium folinic acid did not provoke any signs of pulmonary toxicity. We therefore conclude that the observed lung disease was triggered by the oxaliplatin alone or—less likely—in combination with 5-FU and sodium folinic acid.
Various agents have previously been reported to cause such pulmonary side effects as fibrosing alveolitis (e.g. bleomycin, busulfan, or trastuzumab) or granulomatous lung disease (e.g. methotrexate, BCG, TNF-α blocking agents, gefitinib, or everolimus) [26–32]. Usually the disorders develop slowly, and a continued worsening of unspecific symptoms (e.g. cough, dyspnea, or haemoptysis) can be found. These are often reversible after discontinuation of the suspected medication. In some cases, further diagnostic workup and steroid treatment has been necessary. Apart from the presumed involvement of immunological factors in the development of drug-induced pulmonary pathologies, a histological examination of the lung for possible infectious conditions (e.g. caseating granulomas in mycobacterial infections) may help to establish the differential diagnosis of suspected drug-induced lung disease. A drug-induced granulomatous lung reaction after a single administration of oxaliplatin has not so far been reported.
4. Conclusions
Clinical application of combined chemotherapy with oxaliplatin is considered a safe and efficient method of treating advanced colorectal cancer. Apart from the consideration of haematological, gastrointestinal, and neurological side effects, possible pulmonary toxicities should also be taken into account. Lung biopsy should be considered in otherwise unexplained pulmonary disease in patients with chemotherapy.
Authors' Contribution
D. Wildner and F. Boxberger contributed equally to this work.
Acknowledgment
Cedric Morris is acknowledged for language assistance.
Abbreviations
- 5-FU:
5-Fluorouracil
- UICC:
Union Internationale Contre le Cancer
- CRP:
C-reactive protein
- PCR:
Polymerase chain reaction
- ECOG:
Eastern cooperative oncology group
- CT:
Computed tomography
- SIRT:
Selective internal radiotherapy
- BCG:
Bacillus Calmette-Guérin
- TNF-α:
Tumour necrosis factor-alpha.
References
- 1.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. Journal of Clinical Oncology. 2004;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. Journal of Clinical Oncology. 2005;23(36):9441–9442. doi: 10.1200/JCO.2005.04.4792. [DOI] [PubMed] [Google Scholar]
- 3.Link K, Happich K, Schirner I, et al. Palliative second-line treatment with weekly high-dose 5-fluorouracil as 24-hour infusion and folinic acid (AIO) plus oxaliplatin after pre-treatment with the AIO-regimen in colorectal cancer (CRC) Anticancer Research. 2004;24(1):385–391. [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan- refractory metastatic colorectal cancer. New England Journal of Medicine. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 6.Wein A, Riedel C, Köckerling F, et al. Impact of surgery on survival in palliative patients with metastatic colorectal cancer after first line treatment with weekly 24-hour infusion of high-dose 5-fluorouracil and folinic acid. Annals of Oncology. 2001;12(12):1721–1727. doi: 10.1023/a:1013521430755. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht H, Boxberger F, Wolff K, et al. Palliative first-line treatment with weekly high-dose 5-fluorouracil and sodium folinic acid (s-FA) (AIO-schedule) as 24h-infusion plus biweekly oxaliplatin (ox) in patients with definitively non-resectable metastatic colorectal cancer (CRC) following secondary (sec.) metastatic resection: an interdisciplinary phase II-trial. Journal of Clinical Oncology. 2009;27(supplement, abstract e15073) [Google Scholar]
- 8.Ramanathan RK, Clark JW, Kemeny NE, et al. Safety and toxicity analysis of oxaliplatin combined with fluorouracil or as a single agent in patients with previously treated advanced colorectal cancer. Journal of Clinical Oncology. 2003;21(15):2904–2911. doi: 10.1200/JCO.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. Journal of Clinical Oncology. 2004;22(1):23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy J, Misset JL. Oxaliplatin-related side effects: characteristics and management. Seminars in Oncology. 2002;29(5, supplement 15):11–20. doi: 10.1053/sonc.2002.35524. [DOI] [PubMed] [Google Scholar]
- 11.Stickel F, Jüngert B, Brueckl V, et al. Weekly high-dose 5-fluorouracil as 24-h infusion and folinic acid (AIO) plus irinotecan as second- and third-line treatment in patients with colorectal cancer pre-treated with AIO plus oxaliplatin. Anti-Cancer Drugs. 2003;14(9):745–749. doi: 10.1097/00001813-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Trisolini R, Lazzari Agli L, Tassinari D, et al. Acute lung injury associated with 5-fluorouracil and oxaliplatinum combined chemotherapy. European Respiratory Journal. 2001;18(1):243–245. [PubMed] [Google Scholar]
- 13.Gagnadoux F, Roiron C, Carrie E, Monnier-Cholley L, Lebeau B. Eosinophilic lung disease under chemotherapy with oxaliplatin for colorectal cancer. American Journal of Clinical Oncology. 2002;25(4):388–390. doi: 10.1097/00000421-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Yagüe XH, Soy E, Merino BQ, Puig J, Fabregat MB, Colomer R. Interstitial pneumonitis after oxaliplatin treatment in colorectal cancer. Clinical & Translational Oncology. 2005;7(11):515–517. doi: 10.1007/BF02717006. [DOI] [PubMed] [Google Scholar]
- 15.Pasetto LM, Monfardini S. Is acute dyspnea related to oxaliplatin administration? World Journal of Gastroenterology. 2006;12(36):5907–5908. doi: 10.3748/wjg.v12.i36.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung KH, Kil SY, Choi IK, et al. Interstitial lung diseases in patients treated with oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX) International Journal of Tuberculosis and Lung Disease. 2006;10(10):1181–1182. [PubMed] [Google Scholar]
- 17.Garrido M, O’Brien A, González S, Clavero JM, Orellana E. Cryptogenic organizing pneumonitis during oxaliplatin chemotherapy for colorectal cancer: case report. Chest. 2007;132(6):1997–1999. doi: 10.1378/chest.07-0536. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Casado A, García MD, Racionero MA. Pulmonary toxicity of 5-fluoracil and oxaliplatin. Clinical and Translational Oncology. 2006;8(8):p. 624. doi: 10.1007/s12094-006-0072-2. [DOI] [PubMed] [Google Scholar]
- 19.Mundt P, Mochmann HC, Ebhardt H, Zeitz M, Duchmann R, Pauschinger M. Pulmonary fibrosis after chemotherapy with oxaliplatin and 5-fluorouracil for colorectal cancer. Oncology. 2008;73(3-4):270–272. doi: 10.1159/000127425. [DOI] [PubMed] [Google Scholar]
- 20.Lobera SA, Mariñelarena NS, Echeberría IE, et al. Fatal pneumonitis induced by oxaliplatin. Clinical and Translational Oncology. 2008;10(11):764–767. doi: 10.1007/s12094-008-0285-7. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox BE, Ryu JH, Kalra S. Exacerbation of pre-existing interstitial lung disease after oxaliplatin therapy: a report of three cases. Respiratory Medicine. 2008;102(2):273–279. doi: 10.1016/j.rmed.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Muneoka K, Shirai Y, Sasaki M, Wakai T, Sakata J, Hatakeyama K. Interstitial pneumonia arising in a patient treated with oxaliplatin, 5-fluorouracil, and, leucovorin (FOLFOX) International Journal of Clinical Oncology. 2009;14(5):457–459. doi: 10.1007/s10147-008-0863-2. [DOI] [PubMed] [Google Scholar]
- 23.Ohori H, Takahashi M, Ogasawara N, Suzuki M, Miyate Y, Kato S. Two cases of interstitial lung diseases in patients treated with oxaliplatin, 5-fluorouracil and Leucovorin (FOLFOX) Gan To Kagaku Ryoho. 2009;36(2):295–298. [PubMed] [Google Scholar]
- 24.Ryu C-G, Jung E-J, Kim G, Kim SR, Hwang D-Y. Oxaliplatin-induced pulmonary fibrosis: two case reports. Journal of the Korean Society of Coloproctology. 2011;27(5):266–269. doi: 10.3393/jksc.2011.27.5.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan AK, Choo BA, Glaholm J. Pulmonary toxicity with oxaliplatin and capecitabine/5-fluorouracil chemotherapy: a case report and review of the literature. Onkologie. 2011;34(8-9):443–446. doi: 10.1159/000331133. [DOI] [PubMed] [Google Scholar]
- 26.Cordier JF. Cryptogenic organising pneumonia. European Respiratory Journal. 2006;28(2):422–446. doi: 10.1183/09031936.06.00013505. [DOI] [PubMed] [Google Scholar]
- 27.Daïen CI, Monnier A, Claudepierre P, et al. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology. 2009;48(8):883–886. doi: 10.1093/rheumatology/kep046. [DOI] [PubMed] [Google Scholar]
- 28.Toussirot E, Berthelot JM, Pertuiset E, et al. Pulmonary nodulosis and aseptic granulomatous lung disease occurring in patients with rheumatoid arthritis receiving tumor necrosis factor-α- blocking agent: a case series. Journal of Rheumatology. 2009;36(11):2421–2427. doi: 10.3899/jrheum.090030. [DOI] [PubMed] [Google Scholar]
- 29.Zisman DA, McCune WJ, Tino G, Lynch JP. Drug-induced pneumonitis: the role of methotrexate. Sarcoidosis Vasculitis and Diffuse Lung Diseases. 2001;18(3):243–252. [PubMed] [Google Scholar]
- 30.Foster RA, Zander DS, Mergo PJ, Valentine JF. Mesalamine-related lung disease: clinical, radiographic, and pathologic manifestations. Inflammatory Bowel Diseases. 2003;9(5):308–315. doi: 10.1097/00054725-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 31.De Diego A, Rogado MC, Prieto M, Nauffal D, Perpina M. Disseminated pulmonary granulomas after intravesical bacillus Calmette-Guerin immunotherapy. Respiration. 1997;64(4):304–306. doi: 10.1159/000196693. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Gemma A. Current status of DILD in molecular targeted therapies. International Journal of Clinical Oncology. 2012;17(6):534–541. doi: 10.1007/s10147-012-0494-5. [DOI] [PubMed] [Google Scholar]


