Abstract
Streptococcus pyogenes plays an important role in the pathogenesis of tonsillitis. The present study was conducted to evaluate the in vitro antibacterial activities of 18 essential oils chemotypes from aromatic medicinal plants against S. pyogenes. Antibacterial activity of essential oils was investigated using disc diffusion method. Minimum Inhibitory Concentration of essential oils showing an important antibacterial activity was measured using broth dilution method. Out of 18 essential oils tested, 14 showed antibacterial activity against S. pyogenes. Among them Cinnamomum verum, Cymbopogon citratus, Thymus vulgaris CT thymol, Origanum compactum, and Satureja montana essential oils exhibited significant antibacterial activity. The in vitro results reported here suggest that, for patients suffering from bacterial throat infections, if aromatherapy is used, these essential oils, considered as potential antimicrobial agents, should be preferred.
1. Introduction
Since ten years, the optimization of the use of antibiotics concerns national health agencies that try, through many advertising campaigns and famous slogans, to inform and sensitize people. For example, most cases of tonsillitis are viral and do not need antibiotic treatment. For instance, in about 37% of tonsillitis occurring among children the etiology is bacterial, with Streptococcus pyogenes being the most common bacterial etiology [1]. In this particular context, inflamed tonsils have to be treated by antibiotics. Penicillin is the first choice of antibiotic for the treatment of S. pyogenes tonsillitis. No S. pyogenes resistant to penicillin has been reported. Unfortunately, failures of penicillin treatments to eradicate S. pyogenes from tonsillitis/pharyngitis have been reported [2–4].
Among the alternative therapeutic arsenal, the essential oils (EOs) could be an interesting choice against this pathogen; the EOs antiseptic properties have been demonstrated, at least in vitro (more than 2000 publications about antimicrobial activity of EOs referenced in PubMed since 2002). In spite of all the information available on EOs, we wanted to evaluate their place in alternative or complementary treatments of S. pyogenes tonsillitis.
We carried out in vitro experiments to evaluate antibacterial activity of EOs described as active against S. pyogenes. Disc diffusion method was performed to test antibacterial activity of 18 EOs; MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) of the 5 most effective EOs were determined. Similar experiments were carried out with amoxicillin, the benchmark treatment in this pathology.
2. Material and Methods
2.1. Essential Oils
Eighteen essential oils (Cinnamomum verum, Cymbopogon citratus, Origanum compactum, Thymus vulgaris CT thymol, Satureja montana, Eugenia caryophyllus, Cymbopogon martinii var. motia, Cinnamomum camphora CT linalool, Mentha piperita, Thymus vulgaris CT thujanol, Origanum marjorana, Lavandula stoechas, Melaleuca cajuputi, Melaleuca alternifolia, Ocimum basilicum spp. basilicum, Melaleuca quinquenervia CT cineole, Cinnamomum camphora CT cineole, and Rosmarinus officinalis CT cineole) were furnished by Pranarôm Science, France. Major components of these EOs are listed in Table 1.
Table 1.
Relative percentage composition in the major components for each tested EO according to Pranarôm.
(a)
| Major components classified by organic functions | Botanical name—part of the plant (lot number) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cinnamomum verum—bark (CVB12) | Cymbopogon citratus—aerial part (CCH11) | Origanum compactum—flowering top (OCH11) | Thymus vulgaris CT thymol—flowering top (TV6H9) | Satureja montana—flowering top (SMH11) | Eugenia caryophyllus—bud (ECF9) | Cymbopogon martinii var. motia—aerial part (OF0527) | Cinnamomum camphora CT linalool—wood (HOB9) | Mentha piperita—leaves (MPH29) | ||
| Aldehydes | E-cinnamaldehyde | 65.5 | ||||||||
| Geranial | 43.4 | |||||||||
| Neral | 31.1 | |||||||||
|
| ||||||||||
| Phenolics derivatives | Carvacrol | 41.8 | 2.7 | 50.0 | ||||||
| Estragole | ||||||||||
| Eugenol | 6.2 | 81.4 | ||||||||
| Thymol | 16.2 | 43.6 | 6.8 | |||||||
|
| ||||||||||
| Terpene alcohols | Borneol | |||||||||
| Geraniol | 4.3 | 82.0 | ||||||||
| Linalool | 3.2 | 4.9 | 3.2 | 98.5 | ||||||
| Menthol | 43.4 | |||||||||
| Myrcenol | ||||||||||
| Neomenthol | 4.3 | |||||||||
| α-terpineol | ||||||||||
| Terpinen-4-ol | ||||||||||
| Cis-thujanol | ||||||||||
| Trans-thujanol | ||||||||||
| Viridiflorol | ||||||||||
|
| ||||||||||
| Ketones | Camphre | |||||||||
| Fenchone | ||||||||||
| Isomenthone | 3.0 | |||||||||
| Menthone | 17.6 | |||||||||
|
| ||||||||||
| Terpenes | Camphene | |||||||||
| β-caryophyllene | 4.9 | 6.1 | ||||||||
| p-cymene | 11.4 | 23.5 | 15.0 | |||||||
| Limonene | 7.1 | 3.3 | ||||||||
| β-myrcene | 2.5 | |||||||||
| β-phellandrene | 2.9 | |||||||||
| α-pinene | ||||||||||
| β-pinene | ||||||||||
| Sabinene | ||||||||||
| α-terpinene | ||||||||||
| γ-terpinene | 16.6 | 8.2 | 4.9 | |||||||
| Terpinolene | ||||||||||
| Bornyl acetate | ||||||||||
| Eugenyl acetate | 9.7 | |||||||||
|
| ||||||||||
| Terpenes | Geranyl acetate | 2.4 | 6.2 | |||||||
| Linalyl acetate | ||||||||||
| Menthyl acetate | 6.2 | |||||||||
| Myrcenyl acetate | ||||||||||
| Myrtenyl acetate | ||||||||||
|
| ||||||||||
| Ethers | 1,8-cineole | 4.7 | ||||||||
(b)
| Major components classified by organic functions | Botanical name—part of the plant (lot number) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RowSpanEmpty | Thymus vulgaris—CT thujanol flowering top (OF0282) | Origanum majorana—flowering top (OMH9) | Lavandula stoechas—flowering top (OF0625) | Melaleuca cajuputi—leave (MCL6) | Melaleuca alternifolia—leave (OF0484) | Ocimum basilicum spp. basilicum—flowering top (OF0761) | Melaleuca quinquenervia CT cineole—leave (BMQ1L23) | Cinnamomum camphora CT cineole—leave (OF0481) |
Rosmarinus officinalis CT cineole—flowering top (RO2H14) |
|
| Aldehydes | E-cinnamaldehyde | |||||||||
| Geranial | ||||||||||
| Neral | ||||||||||
|
| ||||||||||
| Phenolics derivatives | Carvacrol | |||||||||
| Estragole | 70.9 | |||||||||
| Eugenol | ||||||||||
| Thymol | ||||||||||
|
| ||||||||||
| Terpene alcohols | Borneol | 3.1 | ||||||||
| Geraniol | ||||||||||
| Linalool | 3.2 | 20.6 | ||||||||
| Neomenthol | ||||||||||
| Menthol | ||||||||||
| Myrcenol | 9.1 | |||||||||
| α-terpineol | 2.9 | 4.5 | 10.7 | 2.7 | 5.6 | 8.1 | ||||
| Terpinen-4-ol | 13.0 | 29.2 | 39.4 | |||||||
| Cis-thujanol | 5.2 | 11.8 | ||||||||
| Trans-thujanol | 26.0 | 2.8 | ||||||||
| Viridiflorol | 3.4 | |||||||||
|
| ||||||||||
| Ketones | Camphre | 31.8 | 10.2 | |||||||
| Fenchone | 29.8 | |||||||||
| Isomenthone | ||||||||||
| Menthone | ||||||||||
|
| ||||||||||
| Terpenes | Camphene | 4.4 | 4.3 | |||||||
| β-caryophyllene | 3.3 | |||||||||
| p-cymene | 3.0 | |||||||||
| Limonene | 3.0 | 5.3 | 7.8 | 2.2 | ||||||
| β-myrcene | 5.1 | |||||||||
| β-phellandrene | ||||||||||
| α-pinene | 2.9 | 3.6 | 2.6 | 9.3 | 5.0 | 10.3 | ||||
| β-pinene | 2.4 | 3.5 | 8.5 | |||||||
| Sabinene | 2.3 | 6.4 | 14.9 | |||||||
| α-terpinene | 3.9 | 7.2 | 9.2 | |||||||
| γ-terpinene | 6.6 | 12.6 | 20.8 | |||||||
| Terpinolene | 2.8 | 3.3 | ||||||||
| Bornyl acetate | 3.8 | |||||||||
| Eugenyl acetate | 4.0 | |||||||||
| Geranyl acetate | ||||||||||
| Linalyl acetate | 2.2 | |||||||||
| Geranyl acetate | ||||||||||
| Menthyl acetate | ||||||||||
| Myrcenyl acetate | 3.4 | |||||||||
| Myrtenyl acetate | 3.7 | |||||||||
|
| ||||||||||
| Ethers | 1,8-cineole | 60.3 | 2.8 | 58.7 | 53.8 | 44.5 | ||||
2.2. Bacterial Strains and Culture Conditions
Streptococcus pyogenes CIP 104226 strain was used in this study (Collection de l'Institut Pasteur, France). The strain was clinically isolated from pharynx of a child following episode of pharyngitis.
2.3. Disk Diffusion Assay
Antimicrobial activity was investigated by disc diffusion method as already described [5]. The bacterial suspension was adjusted to a density of bacterial cells of 1.0 × 108 UFC/mL (or 0.5 McFarland turbidity units). A sterile swab immersed in this bacterial suspension was used to inoculate the entire surface of a sheep blood agar (Biomerieux). 6 μL of each EO was applied on a sterile paper disc (Biomerieux) aseptically placed on the inoculated plates. Then, plates were incubated for 15 minutes at room temperature. Only one disc was tested per plate. After 24 h of incubation at 37°C in a CO2 incubator, the inhibition zones were measured in millimetres. Amoxicillin (25 μg/disc, Bio-Rad) was used as a positive control for bacterial inhibition. All experiments were done in triplicate. The average of inhibition diameters was calculated to classify the EOs as follows: S. pyogenes is not sensitive (0) for a diameter smaller than 8 mm, moderately sensitive (+) for a 8–14 mm diameter, sensitive (++) for a 14–20 mm diameter, and very sensitive (+++) for a diameter larger than 20 mm [5, 6].
2.4. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
Essential oils with a large inhibition diameter (>20 mm) were examined for their antimicrobial activity against S. pyogenes. The Minimum Inhibitory Concentration (MIC) was estimated by the broth dilution method in Brain-Heart broth (BH, Biomerieux) using the standardized method described by Courvallin et al. [7]. Briefly, each EO was first diluted in DMSO (diméthylsulfoxyde): 40% (v/v) for Cinnamomum verum and 80% (v/v) for the other EOs tested. Serial dilutions of EOs were carried out in distilled water with concentrations ranging from 0.025% to 1% (v/v), depending on the EO tested. One milliliter of a S. pyogenes inoculum (106 UFC/mL) and 0.1 mL of each EO dilution were added to 2.9 mL of Brain-Heart broth. Controls without EO were prepared. After 24 h of incubation at 37°C under agitation, on hermetic tubes, MIC was determined as the lowest concentration of the EO inhibiting visible bacterial growth.
To determine the Minimum Bactericidal Concentration (MBC), 10 μL of bacterial inoculum was taken aseptically from tubes that had not presented visible turbidity and was plated onto sheep blood agar [7]. The MBC was considered as the lower concentration of EOs that allowed less than 0.1% of the original inoculum treated with the EO to grow on the surface of the sheep blood agar. Each MIC and MBC value was obtained from three independent experiments. To determine the nature of antibacterial effect of EOs, the MBC/MIC ratio was used; when the ratio was lower than 4, the EO was considered as a bactericidal EO and when the ratio was higher than 4, it was considered as a bacteriostatic EO [8].
3. Results
3.1. Essential Oils Composition
As depicted in Table 1, essential oils were chosen according to their chemical composition, in particular to their major components. Major compounds of Cinnamomum verum and Cymbopogon citratus were aldehydes. Origanum compactum, Thymus vulgaris CT thymol, Satureja montana, Eugenia caryophyllus, and Ocimum basilicum mainly contained phenolic derivatives. Analysis of Cymbopogon martinii var. motia, Cinnamomum camphora CT linalool, Mentha piperita, Thymus vulgaris CT thujanol, Origanum majorana, and Melaleuca alternifolia indicated terpene alcohols. Ketones were major compounds from Lavandula stoechas. At least, as indicated by their chemotypes, Melaleuca cajuputi, Melaleuca quinquenervia CT cineole, Cinnamomum camphora CT cineole, and Rosmarinus officinalis CT cineole mainly contained cineole, a monoterpene ether.
3.2. Screening of Antibacterial Activity
Results obtained with disk diffusion assay regarding the growth inhibition zones of the tested S. pyogenes strain are presented in Figure 1. Our results showed that EOs from Cinnamomum verum, Cymbopogon citratus, Thymus vulgaris CT thymol, Origanum compactum, and Satureja montana are the most active oils tested against S. pyogenes, with inhibition zones average ranging from 48.0 mm to 35.0 mm (+++). S. pyogenes is sensitive (++) to Eugenia caryophyllus and Cymbopogon martinii var. motia (means inhibition diameters, resp., 18.3 and 15.3 mm). Most of EOs tested showed a moderate inhibitory activity (+) against S. pyogenes (means inhibition diameters ranging from 13.0 to 9.0 mm): Cinnamomum camphora CT linalool, Mentha piperita, Thymus vulgaris CT thujanol, Origanum majorana, Lavandula stoechas, Melaleuca cajuputi, Melaleuca alternifolia. Four EOs showed no significant activity (0) against the tested strain (inhibition zone diameter ranging from 6.3 to 0.0 mm): Ocimum basilicum spp. basilicum, and Melaleuca quinquenervia CT cineole, Cinnamomum camphora CT cineole, and Rosmarinus officinalis CT cineole. Inhibition zones of almost all the essential oils were significantly lower than the positive control amoxicillin (47.3 ± 2.5 mm).
Figure 1.
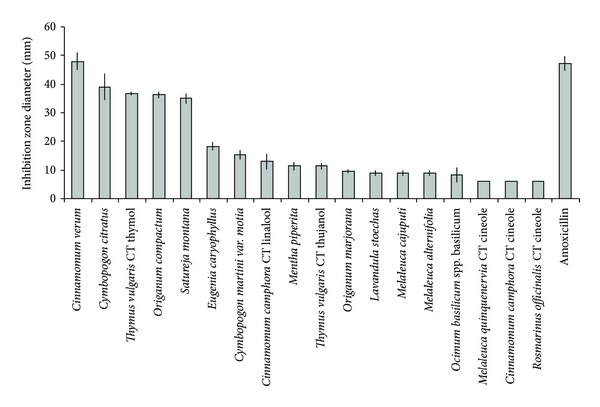
Inhibition zone diameters obtained with the various essential oils against S. pyogenes (means ± SD).
3.3. MIC and MBC Values Determination
Referring to the large inhibition zones observed with disk diffusion method for five essential oils (Cinnamomum verum, Cymbopogon citratus, Thymus vulgaris CT thymol, Origanum compactum, and Satureja montana), the MIC values were determined with broth dilution assays (Figure 2). Cinnamomum verum EO mainly composed of aromatic aldehyde was the most efficient against S. pyogenes (0.19% (v/v)). MIC of Cymbopogon citratus containing mainly terpenic aldehyde was 0.93% (v/v). As far as the EOs rich in aromatic phenols were concerned, MICs ranged from 0.57 to 0.90% (v/v).
Figure 2.
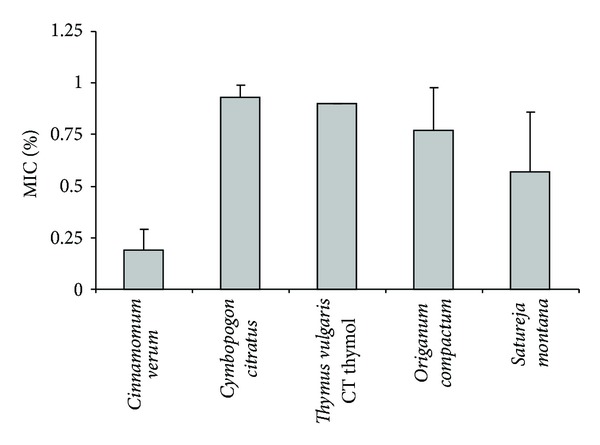
Minimum Inhibitory Concentrations of five selected essential oils against S. pyogenes (means ± SD).
Concerning MBC, in most cases, it was close to the MIC, indicating a good bactericidal activity against S. pyogenes (Table 2), with a ratio of MBC to MIC ranging from 1.02 to 1.53.
Table 2.
Minimum Bactericidal Concentrations and MBC/MIC ratio of five selected essential oils against S. pyogenes.
| MBC* | MBC/MIC | |
|---|---|---|
| Cinnamomum verum | 0.25 ± 0 | 1.32 |
| Cymbopogon citratus | 0.95 ± 0.07 | 1.02 |
| Thymus vulgaris CT thymol | 0.87 ± 0.15 | 1.13 |
| Origanum compactum | 0.97 ± 0.06 | 1.08 |
| Satureja montana | 0.87 ± 0.15 | 1.53 |
*Means of MBCs ± SD.
4. Discussion
Even if aromatic and medicinal plants have been used from ancient times as natural therapies and are considered as alternatives to synthetic drugs, scientific investigations to evaluate antimicrobial activity of essential oils are needed.
The aim of this work was to focalize in EOs usable against S. pyogenes and to compare their antimicrobial activity specifically against S. pyogenes, a bacteria responsible for human tonsillitis. Eighteen EOs have been selected for their composition. Indeed, in the literature, it has been reported that EOs containing mainly aromatic phenols or aldehydes presented a major antimicrobial activity against respiratory tract pathogens, followed by EOs containing terpene alcohols. EOs containing terpene ether, ketone, or oxide had weaker activity [9, 10]. Then, for example, thyme, cinnamon, lemongrass, tea tree, lavender, oregano, clove, palmarosa, or cajeput EOs are known to be active against S. pyogenes [9–12] while oregano, basil, mint, rosemary, and lavender EOs are known to inhibit another Gram-positive bacteria, Staphylococcus aureus [13].
In this study, we used the standardized disk assay method to select 5 essential oils showing the higher inhibitory activity against S. pyogenes among 18 essential oils tested. The obtained results, in accordance with the literary works, showed that EOs mainly composed of aldehydes or phenols were the most effective against S. pyogenes.
We showed that cinnamon presented the higher activity against S. pyogenes compared to the other EOs tested. These results were consistent with previous works [10, 11]. Therefore, Cinnamomum verum EO containing cinnamaldehyde (an aromatic aldehyde) showed the highest activity. Moreover, EOs containing the aromatic phenols, carvacrol and thymol, were very efficient (+++) against the S. pyogenes strain tested. The other oils containing a phenolic derivative (clove containing eugenol and basil containing estragole) were less active (++). As depicted in Figure 3, these results could be directly linked with the structures of major aromatic phenolic derivatives from EOs. Particularly, the presence of free phenol seems to increase antibacterial activity against S. pyogenes. Basil EO contains mainly estragol without any free phenol. It can be surprising that clove was not selected among the essential oils showing the stronger antibacterial activity. Indeed, previous studies had shown that antibacterial activity of clove EO against S. pyogenes was nearly the same as thyme EO [11]. The differences between our results and previous studies can be due to the fact that the composition of EOs is not strictly defined but is a complex mixture of organic substances, varying in quality and quantity [14].
Figure 3.
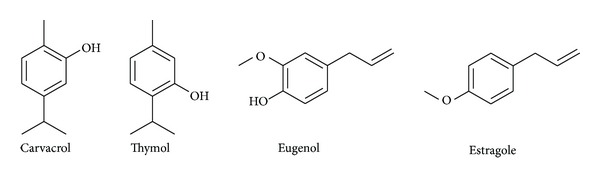
Structure of phenolic derivatives from tested essential oils.
Antibacterial activity of the 5 oils selected was studied by determining the MIC and the MBC. In this study, results of MICs were reliable with diameters of inhibition zones observed with disk diffusion method, with Cinnamomum verum being the more effective EO followed by the other four EOs tested. All tested EOs showed bactericidal activity in vitro (MBCs nearly equal to MICs) but investigations such as pharmacokinetic and pharmacodynamic studies are needed to characterize the antibacterial activity in vivo and their clinical efficacy [15].
The comparison of antibacterial activity and cytotoxicity of EOs and antibiotics must be approached with caution. Indeed, due to the complex and variable chemical composition of EOs, affected by many factors like chemotypes or cultivation conditions, it is still difficult to understand antibacterial activity mechanisms of EOs and to control their cytotoxicity. As discussed later, at least one part of the antibacterial and cytotoxic activities of essential oils is nonspecific but linked with lipophilic compounds targeting membranes.
Screening the EOs presenting antibacterial effects, Fabio et al. have reported that MICs of the EOs showing antibacterial activity were higher than the nontoxic concentrations on Vero cells [11]. Investigations on cytotoxicity of EOs have to be conducted, particularly in terms of possibility of overdoses and in terms of interactions with drugs. Moreover, the lack of clinical studies (toxicity, pharmacokinetic, etc.) has to be underlined.
Therapeutic doses of EOs containing phenols or aldehydes are usually only a few drops per day (2 drops 3 times a day) per os. The inhalation of such irritating EOs should be avoided. It should be noticed that pharmacokinetic predictions about mixtures such as EOs are difficult [14]. Nevertheless, as essential oils are lipophilic and volatile compounds, they can rapidly reach the systemic compartment and be partially eliminated through respiratory way, that is, on the infectious site in tonsillitis context [16].
Antibacterial treatment of S. pyogenes tonsillitis has several objectives; among them is the reduction of the transmission to family members. Indeed, it seems that there is an increased risk concerning the invasive infections (e.g., bacteraemia and pneumonia) for household contacts of index patients, compared to the annual incidence rate of sporadic invasive infections caused by this bacteria [17]. However, about 20–30% of antibiotic treatments with penicillin fail to eradicate the pathogen [3, 4]. This failure in therapy is not due to a resistant phenotype to penicillin [18] but can be related to various hypothesis such as microbiologic interactions between commensal pharyngotonsillar flora and S. pyogenes, poor penetration and diffusion of penicillin into tonsils, or reacquisition of S. pyogenes from a contact [2]. In this context, synergy of action of EOs with antibiotics could be investigated. With a nonspecific mode of action, EOs could help to control beta-lactamase-producing bacteria causing failure of penicillin treatment to eradicate S. pyogenes (for a review see [19]).
An illustration of this promising strategy of combination is the study performed by Fadli et al. Among 80 EOs/antibiotics combinations tested, 71% showed synergism. For example, it has been noted that carvacrol showed a synergistic effect when combined with ciprofloxacin [20]. Moreover, this alternative strategy could be interesting because it can ideally lead to a reduction of doses of the antibiotics, thus reducing the adverse effects of the therapy. However, the synergistic effects have to be evaluated in vitro and in vivo as effects are variable, depending on many factors like EO composition, exposure time, or mode of action of the active components of the EO. Investigations on EOs mechanisms of action have not been fully established yet. Studies on Cinnamomum sp. and on its major components showed morphological changes (loss of membrane integrity) on Gram-positive bacteria (Staphylococcus aureus or Bacillus cereus) and a decrease in the metabolic activity and in the bacterial replication capacity [21, 22]. Carvacrol exposure causes morphological modifications, as changes in cell surface structure [23, 24]. However, as EOs components are lipophilic, membranes of various organisms are likely targeted but protein targets seem nonspecific; particularly, phenolic alcohols or aldehydes interfere with membrane-integrated or -associated enzyme proteins, stopping their activity. Components of EOs can also interfere with electron transport chain from bacterial or mammalian mitochondria and alter energy production [25].
5. Conclusion
In conclusion, we show an interesting antibacterial activity of some essential oils against S. pyogenes, particularly Cinnamomum verum EO but we need further investigations to evaluate bactericidal properties in practical applications on clinical strains and to assess the potential for therapeutic application. Fronting the fact that there is no evidence of a potential clinical use of these EOs, further researches are needed in order to determine if they could substitute efficiently antibiotics or, perhaps, be used in combination. Additional in vivo studies and clinical trials would help to justify and evaluate the potential of these oils in tonsillitis context.
References
- 1.Chiappini E, Regoli M, Bonsignori F, et al. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clinical Therapeutics. 2011;33(1):48–58. doi: 10.1016/j.clinthera.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Brook I. Overcoming penicillin failures in the treatment of Group A streptococcal pharyngo-tonsillitis. International Journal of Pediatric Otorhinolaryngology. 2007;71(10):1501–1508. doi: 10.1016/j.ijporl.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn SM, Preiksaitis J, Tyrrell GJ, Jadavji T, Church D, Davies HD. Evaluation of potential factors contributing to microbiological treatment failure in Streptococcus pyogenes pharyngitis. Canadian Journal of Infectious Diseases. 2001;12(1):33–39. doi: 10.1155/2001/297304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook I, Gober AE. Failure to eradicate streptococci and beta-lactamase producing bacteria. Acta Paediatrica. 2008;97(2):193–195. doi: 10.1111/j.1651-2227.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 5.Durrafourd C, Lapraz JC, Reynier J. Traité de phytothérapie clinique: endobiogénie et médecine. Paris, France: Masson; 2002. [Google Scholar]
- 6.Franchomme P, Jollois R, Pénoël D. L'aromathérapie exactement. Limoges, France: Roger Jollois; 2001. [Google Scholar]
- 7.Courvallin P, Leclerq R, Bingen E. Antibiogramme. Paris, France: ESKA; 2006. [Google Scholar]
- 8.Levison ME. Pharmacodynamics of antimicrobial drugs. Infectious Disease Clinics of North America. 2004;18(3):451–465. doi: 10.1016/j.idc.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Inouye S, Takizawa T, Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. Journal of Antimicrobial Chemotherapy. 2001;47(5):565–573. doi: 10.1093/jac/47.5.565. [DOI] [PubMed] [Google Scholar]
- 10.Inouye S, Yamaguchi H, Takizawa T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. Journal of Infection and Chemotherapy. 2001;7(4):251–254. doi: 10.1007/s101560170022. [DOI] [PubMed] [Google Scholar]
- 11.Fabio A, Cermelli C, Fabio G, Nicoletti P, Quaglio P. Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. Phytotherapy Research. 2007;21(4):374–377. doi: 10.1002/ptr.1968. [DOI] [PubMed] [Google Scholar]
- 12.Mayaud L, Carricajo A, Zhiri A, Aubert G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Letters in Applied Microbiology. 2008;47(3):167–173. doi: 10.1111/j.1472-765X.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulos A, Kimbaris AC, Plessas S, et al. Antibacterial activities of essential oils from eight Greek aromatic plants against clinical isolates of Staphylococcus aureus . Anaerobe. 2011;17(6):399–402. doi: 10.1016/j.anaerobe.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Bruneton J. Pharmacognosie—Phytochimie—Plantes médicinales. Paris, France: Tec & Doc; 2009. [Google Scholar]
- 15.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clinical Infectious Diseases. 2004;38(6):864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 16.Kohlert C, Schindler G, März RW, et al. Systemic availability and pharmacokinetics of thymol in humans. Journal of Clinical Pharmacology. 2002;42(7):731–737. doi: 10.1177/009127002401102678. [DOI] [PubMed] [Google Scholar]
- 17.Robinson KA, Rothrock G, Phan Q, et al. Risk for severe group A streptococcal disease among patients’ household contacts. Emerging Infectious Diseases. 2003;9(4):443–447. doi: 10.3201/eid0904.020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bidet P, Plainvert C, Doit C, et al. Streptococcus pyogenes or group A streptococcal infections in child: French national reference center data. Archives de Pediatrie. 2010;17(2):201–208. doi: 10.1016/j.arcped.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Brook I. The role of beta-lactamase-producing bacteria in the persistence of streptococcal tonsillar infection. Reviews of Infectious Diseases. 1984;6(5):601–607. doi: 10.1093/clinids/6.5.601. [DOI] [PubMed] [Google Scholar]
- 20.Fadli M, Saad A, Sayadi S, et al. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection—bacteria and their synergistic potential with antibiotics. Phytomedicine. 2012;19(5):464–471. doi: 10.1016/j.phymed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Shan B, Cai YZ, Brooks JD, Corke H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): activity against foodborne pathogenic bacteria. Journal of Agricultural and Food Chemistry. 2007;55(14):5484–5490. doi: 10.1021/jf070424d. [DOI] [PubMed] [Google Scholar]
- 22.Bouhdid S, Abrini J, Amensour M, Zhiri A, Espuny MJ, Manresa A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. Journal of Applied Microbiology. 2010;109(4):1139–1149. doi: 10.1111/j.1365-2672.2010.04740.x. [DOI] [PubMed] [Google Scholar]
- 23.Cristani M, D’Arrigo M, Mandalari G, et al. Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. Journal of Agricultural and Food Chemistry. 2007;55(15):6300–6308. doi: 10.1021/jf070094x. [DOI] [PubMed] [Google Scholar]
- 24.La Storia A, Ercolini D, Marinello F, Di Pasqua R, Villani F, Mauriello G. Atomic force microscopy analysis shows surface structure changes in carvacrol-treated bacterial cells. Research in Microbiology. 2011;162(2):164–172. doi: 10.1016/j.resmic.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Current Medicinal Chemistry. 2003;10(10):813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]


