Abstract
Gintonin is a unique lysophosphatidic acid (LPA) receptor ligand found in Panax ginseng. Gintonin induces transient [Ca2+]i through G protein-coupled LPA receptors. Large-conductance Ca2+-activated K+ (BKCa) channels are expressed in blood vessels and neurons and play important roles in blood vessel relaxation and attenuation of neuronal excitability. BKCa channels are activated by transient [Ca2+]i and are regulated by various Ca2+-dependent kinases. We investigated the molecular mechanisms of BKCa channel activation by gintonin. BKCa channels are heterologously expressed in Xenopus oocytes. Gintonin treatment induced BKCa channel activation in oocytes expressing the BKCa channel α subunit in a concentration-dependent manner (EC50 = 0.71 ± 0.08 µg/mL). Gintonin-mediated BKCa channel activation was blocked by a PKC inhibitor, calphostin, and by the calmodulin inhibitor, calmidazolium. Site-directed mutations in BKCa channels targeting CaM kinase II or PKC phosphorylation sites but not PKA phosphorylation sites attenuated gintonin action. Mutations in the Ca2+ bowl and the regulator of K+ conductance (RCK) site also blocked gintonin action. These results indicate that gintonin-mediated BKCa channel activations are achieved through LPA1 receptor-phospholipase C-IP3-Ca2+-PKC-calmodulin-CaM kinase II pathways and calcium binding to the Ca2+ bowl and RCK domain. Gintonin could be a novel contributor against blood vessel constriction and over-excitation of neurons.
1. Introduction
Ginseng, the root of Panax ginseng C. A. Meyer, has been used as a representative tonic or an adaptogen to promote longevity and to enhance bodily functions against hypertension and as a neuroprotectant for several hundred years in Far East countries like Korea, China, and Japan. Currently, ginseng is one of the most famous and precious herbal medicines consumed around the world [1]. Recently, we isolated and characterized a novel glycolipoprotein, designated as gintonin, from ginseng. Gintonin is a lysophosphatidic-acids- (LPAs-) ginseng major latex-like protein (MLP151) and ginseng ribonuclease-like storage protein complex, in which the lysophosphatidic acids (LPAs) bind to ginseng proteins through hydrophobic interactions, and this is the main principle underlying gintonin action [2–6], whereas most of other LPA receptor ligands are derivatives of LPA or LPA analogs [7]. Gintonin induces transient [Ca2+]i through LPA receptor activation via pertussis toxin- (PTX-) sensitive and -insensitive G proteins in animal cells [2–6]. Thus, gintonin-mediated transient [Ca2+]i induction via LPA receptors could be further coupled to the regulation of Ca2+-dependent enzymes and Ca2+-dependent ion channel activities, which play important roles in biological systems.
Large-conductance Ca2+-activated K+ (BKCa) channels are a family of outward K+-selective ion channels activated in response to membrane depolarization. BKCa channels are activated by intracellular Ca2+ elevation and/or Ca2+-dependent kinases [8, 9]. BKCa channels play key roles in neuronal and nonneuronal cell functions. For example, in neuronal cells, BKCa channels regulate the frequency of firing, action potentials following hyperpolarization, and neurotransmitter release. In vascular smooth muscle cells, BKCa channels are one of the main ion channels that are involved in vasorelaxation [10, 11].
BKCa channels are composed of two subunits: the α subunit (also called rSlo), which forms the channel pore [12], and the β subunit [13, 14], which modifies the voltage and calcium sensitivity of the pore-forming α subunit [15, 16]. The α subunit has a large cytoplasmic C terminus and is responsible for the Ca2+-dependent activation of the channel. Furthermore, the cytoplasmic C terminus of the α subunit has two domains that are responsible for the Ca2+-dependent activation of the channel, namely, the Ca2+ bowl and the regulator of K+ conductance (RCK) domain [17–21]. The cytoplasmic C terminus of the α subunit has amino acid residues that can be phosphorylated by a variety of protein kinases such as CaM kinase II, PKA, and PKC [8, 9]. Accumulating evidence shows that BKCa channels play key roles in excitable cells and are regulated by diverse Ca2+ and Ca2+-dependent kinases [10, 11]. Although the signaling pathways of LPA as well as gintonin are well characterized through the biochemical and pharmacological experiments [2–6, 22], relatively little is known about the molecular mechanisms how gintonin-mediated [Ca2+]i transient is linked to BKCa channel regulation.
In the present study, we examined how LPA receptor activation by gintonin may regulate BKCa channel activity in Xenopus oocytes expressing the α subunit of BKCa alone or in Xenopus oocytes coexpressing BKCa channels and other BKCa channel regulators. We found that treatment of gintonin induces BKCa channel activation. Gintonin-mediated BKCa channel activation is achieved through the LPA1 receptor, the phospholipase C-IP3-Ca2+ pathway, and CaM kinase II phosphorylation of the α subunit. We further demonstrated that site-directed mutations of the Ca2+ bowl, RCK domain, and CaM kinase II phosphorylation site of channels greatly attenuated gintonin action. We compared the regulatory modes between gintonin and ginsenoside Rg3 in BKCa channel activation. We further discuss how signal coupling of gintonin to the BKCa channel through the LPA receptor is associated with the beneficial physiological and pharmacological effects of ginseng on blood vessels and the nervous system.
2. Materials and Methods
2.1. Materials
Gintonin was isolated from P. ginseng as described previously [23]. In the present study, we used the crude gintonin fraction, which contains about 9.5% LPAs, the majority being LPAC18:2 [2–6]. Ginsenoside Rg3 was provided by the AMBO Institute (Seoul, Republic of Korea). The stock solution of ginsenoside Rg3 was prepared and used as described previously [24]. M1 muscarinic acetylcholine receptor was purchased from Guthrie Research Institute (Sayre, PA, USA). CaM kinase II gene was kindly provided by OriGene (Rockville, MD, USA). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. In Vitro Synthesis of cRNA
Recombinant plasmids containing cDNA inserts for M1 muscarinic receptor, α subunit (rSlo), and constitutively active CaM kinase II were linearized by digestion with appropriate restriction enzymes. The cRNAs from linearized templates were obtained with an in vitro transcription kit (mMessage mMachine; Ambion, Austin, TX, USA) using a SP6, T3, or T7 RNA polymerase. The RNA was dissolved in RNase-free water at 1 μg/μL, divided into aliquots, and stored at −70°C.
2.3. Preparation of Xenopus Oocytes and Microinjection
Xenopus laevis was purchased from Xenopus I (Ann Arbor, MI, USA). Their care and handling were in accordance with the highest standard of institutional guidelines of Konkuk University. For the isolation of oocytes, frogs were anesthetized with an aerated solution of 3-amino benzoic acid ethyl ester followed by the removal of ovarian follicles. The oocytes were subsequently treated with collagenase and then agitated for 2 h in Ca2+-free OR2 medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin. Stages V-VI oocytes were collected and stored in ND96 medium (in mM: 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES, pH 7.5) supplemented with 50 μg/mL gentamicin. The oocyte-containing solution was maintained at 18°C with continuous gentle shaking and was renewed daily. Electrophysiological experiments were performed within 5-6 days of oocyte isolation, with gintonin or ginsenoside applied to the bath. For BKCa channel experiments, BKCa channel-encoding cRNAs (40 nl) were injected into the animal or vegetal pole of each oocyte one day after isolation, using a 10-μL microdispenser (VWR Scientific, San Francisco, CA, USA) fitted with a tapered glass pipette tip (diameter, 15–20 μm) [25].
2.4. Site-Directed Mutagenesis of the BKCa α and In Vitro Transcription of BKCa Channel cDNAs
Single amino acid substitutions of the BKCa channel (Figure 1(a)) were made using a QuikChange XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA), along with Pfu DNA polymerase and sense and antisense primers encoding the desired mutations. Overlap extension of the target domain by sequential polymerase chain reaction (PCR) was carried out according to the manufacturer's protocol. The final PCR products were transformed into E. coli strain DH5α, screened by PCR, and confirmed by sequencing of the target regions. The mutant DNA constructs were linearized at their 3′ ends by digestion with NotI, and run-off transcripts were prepared using the methylated cap analog, m7G(5′)ppp(5′)G. The cRNAs were prepared using an mMessage mMachine transcription kit (Ambion, Austin, TX, USA) with T7 RNA polymerase. The absence of degraded RNA was confirmed by denaturing agarose gel electrophoresis followed by ethidium bromide staining. Similarly, recombinant plasmids containing rat BKCa channel cDNA inserts were linearized by digestion with the appropriate restriction enzymes, and cRNAs were obtained using the mMessage mMachine in vitro transcription kit (Ambion, Austin, TX, USA) with SP6 RNA polymerase or T7 polymerase. The final cRNA products were resuspended at a concentration of 1 μg/μL in RNase-free water and stored at −80°C [25].
Figure 1.

The primary amino acid sequence of the mutated BKCa channel α subunit and the chemical structure of ginsenoside Rg3. (a) The brief sequence alignment of the BKCa channel and the mutated amino acid residues in the CaM kinase II phosphorylation sites (462, 512), PKA (939), PKC (1061), or Ca2+ binding sites of the Ca2+ bowl (989, 991, 992, 993, 994, 995) and RCK domains (433, 579). (b) The chemical structure of ginsenoside Rg3. Glc: glucose.
2.5. Data Recording
Data recording for BKCa channel currents was performed, as described by Liu et al. [26], to study the detailed downstreams of gintonin-mediated signaling transduction pathways. Therefore, a custom-made Plexiglas net chamber was used for two-electrode voltage-clamp recordings, as previously reported [25]. The oocytes were impaled with two microelectrodes filled with 3 M KCl (0.2–0.7 MΩ), and electrophysiological experiments were carried out at room temperature using an Oocyte Clamp (OC-725C, Warner Instruments, Hamsden, CT, USA). Stimulation and data acquisition were controlled with a pClamp 8 (Axon Instruments, Union City, CA, USA). For most electrophysiological experiments, oocytes were perfused initially with a Cl−, and Ca2+-free solution (in mM: 96 NaOH, 2 KOH, 8 Mg-gluconate, 5 HEPES, and 5 EGTA, pH 7.4 with methanesulfonic acid) in the presence of a Cl− channel blocker (500 μM anthracene-9-carboxylic acid) [26] to inhibit endogenous Cl− channels. The oocytes were then clamped at a holding potential of −80 mV; membrane potential was depolarized to +40 mV for 400 ms at 10-s intervals, and currents were recorded as indicated.
2.6. Data Analysis
To obtain the concentration-response curve of the effect of gintonin or ginsenoside Rg3 on the K+ currents from the BKCa channel, the peak amplitudes at different concentrations of gintonin were plotted. The current activation or enhancement evoked by drug treatment was analyzed after the subtraction of currents elicited by H2O injection. Origin software (Origin, Northampton, MA, USA) was used to plot the Hill equation: y/y max = [A]nH/([A]nH + [EC50]nH), where y represented the peak current at a given concentration of gintonin, y max was the maximal peak current, EC50 was the concentration of gintonin producing a half-maximal effect, [A] was the concentration of gintonin, and nH was the Hill coefficient. All values are presented as mean ± S.E.M. The significance of differences between mean control and treatment values was determined using Student's t-test, where P < 0.05 was considered statistically significant.
3. Results
3.1. Gintonin Induces BKCa Channel Activation in a Concentration-Dependent and Voltage-Dependent Manner in Xenopus Oocytes Expressing BKCa Channels
In the present study, we first examined the effect of gintonin on BKCa channel activity in Xenopus oocytes expressing BKCa channel α (rSlo) subunits. Application of gintonin to oocytes injected with α subunit cRNAs resulted in the activation of the BKCa channel as monitored with a clamp step to +60 from −80 mV holding potential (n = 7; current examined at 20-s intervals). The mean current activation by gintonin (10 μg/mL) was 2855.1 ± 415.0% (Figure 2(a)), whereas gintonin had no effect on the control oocytes that were not injected with cRNA encoding the BKCa α subunit gene (data not shown). Gintonin-induced BKCa channel activation occurred in a concentration-dependent manner (Figure 2(a)). The EC50 was observed to be 0.71 ± 0.08 μg/mL. Charybdotoxin and iberiotoxin, highly specific inhibitors of maxi-K channels [27, 28], greatly attenuated BKCa channel activation induced by gintonin (data not shown), indicating that BKCa channels are functional [29]. BKCa channel activation by gintonin was observed over the entire voltage range examined from 0 mV. Thus, gintonin-induced BKCa channel activation occurs in a voltage-dependent manner since marked activations at more positive potentials were observed, as shown in a current-voltage relationship (Figure 2(b)).
Figure 2.
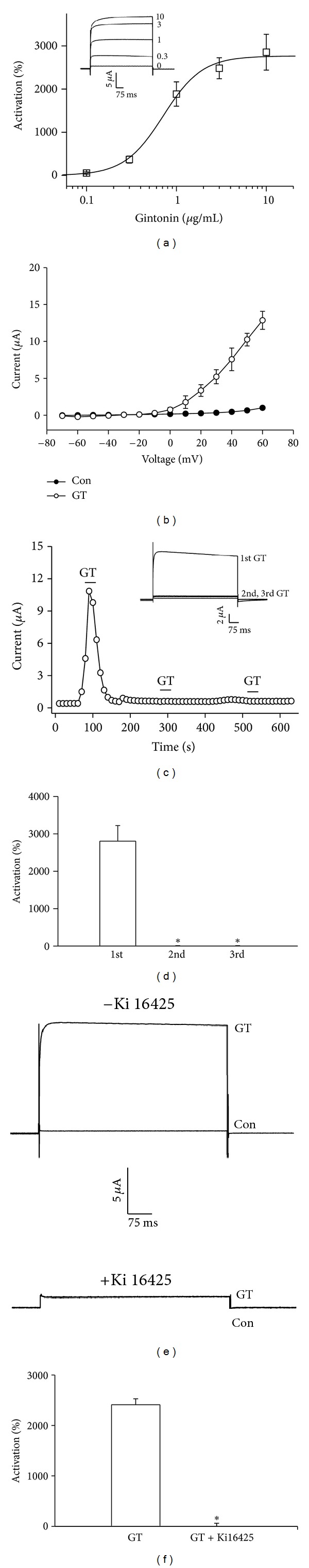
Effects of gintonin on BKCa channel activity. (a) Gintonin concentration-response curve (mean ± S.E.M; n = 13–15 oocytes each). Inset, the representative traces of gintonin-mediated BKCa channel activation at various concentrations. (b) Effects of gintonin (10 μg/mL) on the current-voltage (I-V) relationship of the wild-type BKCa channel (mean ± S.E.M; n = 13–15 oocytes each). (c) Time-current relationship plotted against time before and after repeated applications of gintonin (10 μg/mL) for 30 s in oocytes expressing the BKCa channel. Inset, the representative peak outward current amplitude at +40 mV from a holding potential of −80 mV was measured in the absence or presence of gintonin. (d) Summary histograms show that repeated application of gintonin induces the desensitization of gintonin-mediated BKCa channel activation (*P < 0.001, compared to 1st gintonin treatment). (e) The representative traces on blockage of gintonin-mediated BKCa channel activation by the LPA1/3 receptor antagonist, Ki16425. (f) Summary histograms show that gintonin-mediated activation of the BKCa channel is blocked by the LPA1/3 receptor antagonist, Ki16425 (mean ± S.E.M; n = 12–14 each) (*P < 0.001, compared to gintonin alone).
3.2. Gintonin-Induced BKCa Channel Activation Is Rapidly Desensitized following Repeated Application of Gintonin and Is Blocked by a LPA1/3 Receptor Antagonist
We next examined the changes in gintonin-induced BKCa channel activation following repeated application of gintonin. As shown in Figures 2(c) and 2(d), an initial treatment of gintonin induced a marked activation of BKCa channels. Oocytes stimulated with gintonin were then washed with recording buffer for 3 min until the basal current was recovered and subsequently restimulated with gintonin. We observed that secondary and tertiary BKCa channel current responses to gintonin retreatment were dramatically diminished. The magnitudes of the BKCa current were 2700 ± 121.6, 55.3 ± 33.1, and 26 ± 10.6%, respectively, of the initial, secondary, or tertiary responses of gintonin treatment (n = 15, oocytes each; from three different batches of donors) (Figure 2(d)). We next examined the effect of an LPA1/3 receptor antagonist, Ki16425, on gintonin-induced BKCa channel activation. In the presence of Ki16425, gintonin-mediated BKCa channel activation was abolished, from 2410 ± 115.56 to 10.64 ± 50.54% (Figures 2(e) and 2(f)). This result indicates that gintonin-mediated BKCa channel activation was achieved through activation of the LPA receptor in Xenopus oocytes, which endogenously express LPA1 receptors [30].
3.3. The Signal Transduction Pathway of Gintonin-Mediated BKCa Channel Activation
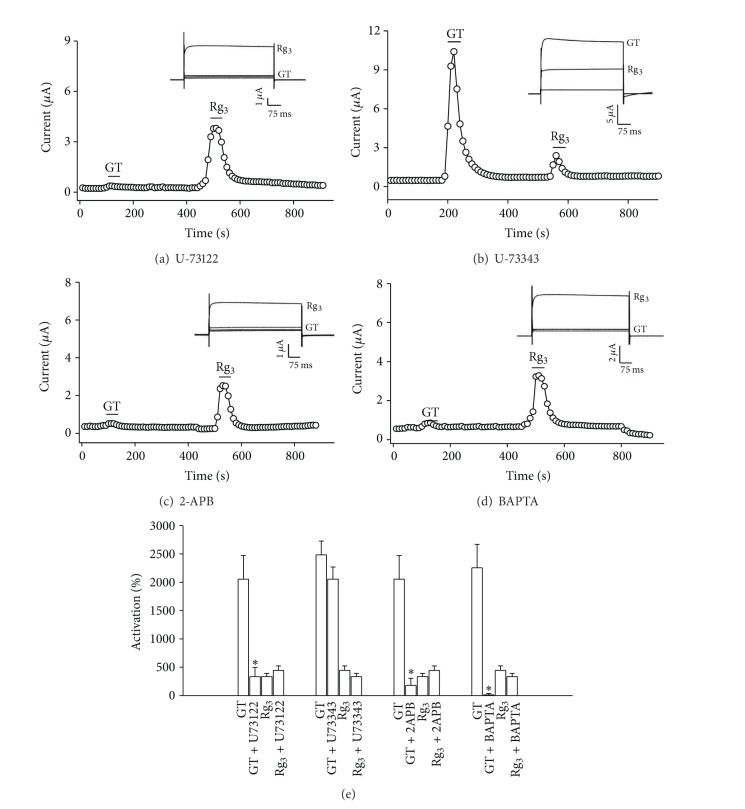
We next examined the signal transduction pathways involved in gintonin-mediated BKCa channel activation. We first examined the involvement of phospholipase C (PLC) in gintonin-mediated BKCa channel activation. To test this possibility, the effects of the active PLC inhibitor U-73122 and its inactive analogue U-73343 were examined on gintonin action [31]. Bath application of U-73122 significantly suppressed gintonin action, whereas the current in the presence of U-73343 was not affected (Figures 3(a) and 3(b)). These results indicate that gintonin-mediated BKCa channel activation requires PLC activation.
Figure 3.
The signal transduction pathways in gintonin-mediated BKCa channel activation. ((a) and (b)) Time-current relationship following application of gintonin (10 μg/mL) or ginsenoside Rg3 (100 μM) for 30 s in the presence of U73122, an active PLC inhibitor, or U73343, an inactive PLC inhibitor, in oocytes expressing BKCa channels. Inset, the representative peak outward current amplitude at +40 mV from a holding potential of −80 mV was measured in the presence of gintonin or ginsenoside Rg3. The active or inactive PLC inhibitor was pretreated for 5 min before gintonin or ginsenoside Rg3 application. ((c) and (d)) Time-current relationship after application of gintonin (10 μg/mL) or ginsenoside Rg3 (100 μM) for 30 s in the presence of 2-APB, an IP3 receptor antagonist, or BAPTA, an intracellular Ca2+ chelator, in oocytes expressing BKCa channels. Inset, the representative peak outward current amplitude at +40 mV from a holding potential of −80 mV was measured in the presence of gintonin or ginsenoside Rg3. The application of 2-APB or BAPTA preceded the gintonin application by 2 h. (e) Summary histograms show the peak outward BKCa channel currents (mean ± S.E.M; n = 13-14 oocytes each) recorded in oocytes expressing the BKCa channel in the absence or presence of the indicated agents. (*P < 0.001, compared to gintonin alone).
To see if the IP3 receptor was involved in gintonin action, oocytes were stimulated with gintonin in the presence of 2-APB, an IP3 receptor antagonist. We observed that 2-APB treatment also greatly attenuated the effect of gintonin on BKCa channel activation (Figure 3(c)). These observations suggest that gintonin first induces an activation of the IP3 receptor to mobilize intracellular Ca2+, and then mobilized [Ca2+]i is coupled to BKCa channel activation. We have demonstrated in a previous report that gintonin-mediated activation of Ca2+-activated Cl− channels is dependent on cytosolic Ca2+ [23]; thus, we examined whether the effect of gintonin on BKCa channel activation was also dependent on cytosolic Ca2+. To this end, we first treated oocytes with BAPTA-AM, a membrane permeable Ca2+ chelator, to chelate free cytosolic Ca2+ and examined gintonin effects. We found that BAPTA-AM completely abolished gintonin action on BKCa channel activation (Figure 3(d)), indicating that gintonin-mediated BKCa channel activation is also achieved through mobilization of intracellular Ca2+ from endoplasmic reticulum. However, while ginsenoside Rg3 (100 μM) also enhanced BKCa channel currents, as shown previously [24], gintonin exhibited a greater (3-4-fold) activation of the BKCa channel than ginsenoside Rg3. In addition, the enhancing effects of ginsenoside Rg3 on BKCa channel currents were not sensitive to the PLC inhibitor, the 2-APB antagonist, and BAPTA (Figure 3(e)), indicating that the regulatory mode on BKCa channel activity by gintonin differs from that of ginsenoside Rg3.
3.4. Involvement of PKC but Not PKA in Gintonin-Mediated BKCa Channel Activation
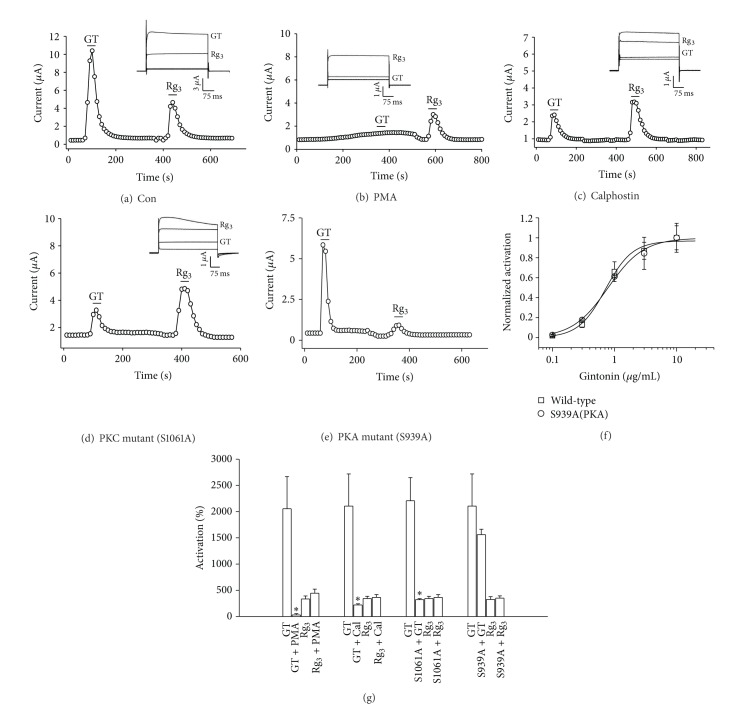
The previous reports have shown that the activation of the PLC pathway also produces lipid-soluble 1,2-diacylglycerol (DAG), an endogenous protein kinase C (PKC) activator. Activation of PKC by treatment with PMA, a DAG analogue, causes receptor phosphorylation and receptor uncoupling from PLC-mediated inositol phospholipid metabolism and results in a loss of Ca2+-activated Cl− channel activation by 5-HT or muscarinic acetylcholine receptor agonist stimulations in Xenopus oocytes [4, 5, 12, 32]. Similarly, in the present study we also first examined the effects of the PKC activator PMA on the gintonin-mediated BKCa channel activation. As shown in Figure 4(b), we found that treatment with PMA induced a loss of gintonin-mediated BKCa channel activation. To confirm PKC involvement in gintonin-mediated BKCa channel activation, we next examined the effect of PKC inhibitor, calphostin, with gintonin action and found that calphostin also prevented gintonin-mediated BKCa channel activation (Figure 4(c)), indicating that gintonin-mediated BKCa channel activation is achieved via PKC activation through LPA receptor. We also tested whether mutation of the PKC phosphorylation site affects gintonin-mediated BKCa channel activation. As shown in Figure 4(d), mutation of Ser1061 to S1061A significantly attenuated gintonin-mediated BKCa channel activation [33]. Similarly, we examined whether mutation of the PKA phosphorylation site affects gintonin-mediated BKCa channel activation. Interestingly, we found that mutation of the PKA phosphorylation site did not affect gintonin-mediated BKCa channel activation (Figures 4(e) and 4(f)). Thus, these results indicate that channel protein phosphorylation by PKC, but not PKA, is involved in gintonin-mediated BKCa channel activation. However, the enhancing effects of ginsenoside Rg3 on BKCa channel currents were not affected by PMA, calphostin, and mutant BKCa channels at the PKC phosphorylation site (Figure 4(g)). These results collectively indicate that the gintonin-mediated but not ginsenoside Rg3-mediated BKCa channel activation involves PKC activation.
Figure 4.
Involvement of PKC but not PKA in gintonin-mediated BKCa channel activation. (a) Gintonin or ginsenoside Rg3 induces activation of BKCa channels in oocytes. ((b)–(d)) Time-current relationships show the effects of gintonin or ginsenoside Rg3 in the pretreatment of PMA (1 μM) for 10 min, calphostin (Cal 1.5 μM) for 10 min, or the mutation of the PKC phosphorylation site (S1061A). Peak outward currents were recorded during bath application of gintonin (10 μg/mL). Insets, the representative gintonin-mediated or ginsenoside Rg3-mediated peak outward current amplitude at +40 mV from a holding potential of −80 mV was measured in the presence of PMA, calphostin, or mutant BKCa channels. (e) Time-current relationships following the application of gintonin (10 μg/mL) or ginsenoside Rg3 (100 μM) for 30 s in oocytes expressing S939A mutant BKCa channels. (f) Concentration dependency of wild-type and S939A mutant BKCa channels on gintonin-mediated BKCa channel activation. (g) Summary histograms show that peak outward BKCa channel currents (mean ± S.E.M; n = 11-12 oocytes each) recorded in oocytes expressing the BKCa channel in the absence or presence of the indicated agents or mutation (*P < 0.001, compared to gintonin alone).
3.5. Involvement of Calmodulin and Calcium-/Calmodulin-Dependent Kinase II (CaM Kinase II) in Gintonin-Mediated Activation of Channels
Since calmodulin and CaM kinase II have been reported to be involved in the regulation of BKCa channel activation [8], we determined whether calmodulin and CaM kinase II are involved in gintonin-mediated BKCa channel activation. To this end, we first examined gintonin-mediated BKCa channel activation following treatment with the calmodulin antagonist, calmidazolium. As shown in Figure 5(b), calmidazolium treatment significantly attenuated gintonin-mediated BKCa channel activation, indicating that calmodulin is involved in gintonin-mediated BKCa channel activation. In contrast, the enhancing effects of ginsenoside Rg3 on BKCa channel currents were not affected by calmidazolium (Figure 5(c)). Since calmodulin is closely related with CaM Kinase II activation, which is known to regulate BKCa channel activity [34, 35], we next examined if gintonin-mediated BKCa channel activation is achieved through CaM kinase II. To this end, we constructed two different kinds of mutant BKCa channels at CaM kinase II phosphorylation sites, T462 and S512, by replacing these residues with alanine (T462A and S512A) [26]. As shown in Figures 5(d) and 5(e), the concentration-response curve shifted rightward, indicating that gintonin-mediated BKCa channel activation was greatly attenuated in mutant channels compared to wild-type channels. Thus, the EC50 was 0.62 ± 0.04, 9.61 ± 0.15, and 1.38 ± 0.25 μg/mL in wild-type and T462A and S521A mutants, respectively. Interestingly, gintonin action on BKCa channel activation was more strongly inhibited in T462A rather than S512A mutants. These results indicate that gintonin induces CaM kinase II activation and links to BKCa channel activation through BKCa channel phosphorylation at T462 and S512.
Figure 5.
Involvement of calmodulin and CaM kinase II in gintonin-mediated BKCa channel activation. ((a) and (b)) Oocytes expressing the BKCa channel were incubated in the absence (a) or presence (b) of calmidazolium (1.5 μM) for 10 min. Insets, the representative gintonin-mediated or ginsenoside Rg3-mediated peak outward current amplitude at +40 mV from a holding potential of −80 mV was measured in the absence or presence of calmidazolium. (c) Summary histograms show peak outward BKCa channel currents recorded in oocytes expressing the BKCa channel in the absence or presence of the calmidazolium (CMZ) (mean ± S.E.M; n = 13-14 oocytes each; *P < 0.001, compared to gintonin alone). (d) The oocytes expressing mutant BKCa channel at the CaM kinase II phosphorylation site (T462A or S521A) were treated with gintonin by bathing application for 60 s. Mutation of CaM kinase II phosphorylation sites resulted in a rightward shift of the gintonin concentration-response curve (mean ± S.E.M; n = 10–12 oocytes each). (e) Summary histograms show that the gintonin-mediated peak outward BKCa channel currents recorded in oocytes expressing mutant BKCa channel at the CaM kinase II phosphorylation site (T462A or S521A) were significantly attenuated (mean ± S.E.M; n = 10–12 oocytes each; *P < 0.001, compared to wild-type).
3.6. Involvement of the Ca2+-Binding Domain (Ca2+ Bowl) and RCK Domain in Gintonin-Mediated BKCa Channel Activation
BKCa channels have unique structures called the Ca2+ bowl and the RCK domain. These two domains play important roles in Ca2+-dependent regulation of BKCa channels [36, 37]. To confirm the involvement of the Ca2+ bowl and RCK domains in gintonin-mediated BKCa channel activation, we mutated residues at these domains since C-terminus mutations have been shown to affect Ca2+-mediated regulation of BKCa channel activity [17, 38]. To this end, we constructed six different mutant BKCa channels in Ca2+-bowl residues, D989, D991, D992, D993, D994, and D995, by replacing these residues with alanine (D989A, D991A, D992A, D993A, D994A, and D995A) [39]. Moreover, we constructed two different kinds of mutant BKCa channels in RCK domain residues such as D433 and M579 by replacing these residues with alanine and isoleucine (D433A and M579I) [21].
We then examined the effects of gintonin on the activity of these mutant channels. In concentration-response curves, the stimulatory effects of gintonin on BKCa channel activity were observed to be greatly attenuated in oocytes expressing the mutants compared to wild-type channels in the order of D994A > D989A > D922A > D991 > D993A (Figure 6(a)). The EC50 values were 0.64 ± 0.08, 1.00 ± 0.01, 2.70 ± 0.06, 4.01 ± 0.06, 1.38 ± 0.03, 11.31 ± 4.62, and 1.35 ± 0.22 μg/mL in wild-type, D989A, D991A, D992A, D993A, D994A, and D995A, respectively. Interestingly, gintonin action on BKCa channel activation was more strongly inhibited in D994A rather than in other Ca2+ bowl mutants. We also examined the effects of gintonin on RCK domain mutant channels. As shown in Figure 6(b), gintonin-mediated BKCa channel activation was greatly attenuated in oocytes expressing the mutant channels D433A and M579I compared to wild-type channels. The representative concentration-response curves are also shown in Figure 6(b). The EC50 values were 0.51 ± 0.07, 10.71 ± 0.60, and 2.26 ± 0.06 μg/mL in wild-type, D433A, and M579I mutants, respectively. Interestingly, gintonin action on BKCa channel activation was more strongly inhibited in D433A rather than M579I RCK domain mutants. As a positive control, we injected cRNA encoding M1 muscarinic acetylcholine receptor (mAChR) into the oocytes, which are reported to induce transient [Ca2+]i via the Gα q/11-PLC-IP3 pathway [40]. As shown in Figure 6(c), in Ca2+ bowl mutants such as D991A, D992A, and D994A mutants, treatment of acetylcholine caused a right shift in the concentration-response curves. In RCK domain mutants, such as D433S and M579I mutants, treatment of acetylcholine also caused a right shift of the concentration-response curves (Figure 6(d)). These results indicate that the released Ca2+ induced by gintonin or acetylcholine treatment binds to the Ca2+ bowl and RCK domain and induces BKCa channel activation.
Figure 6.
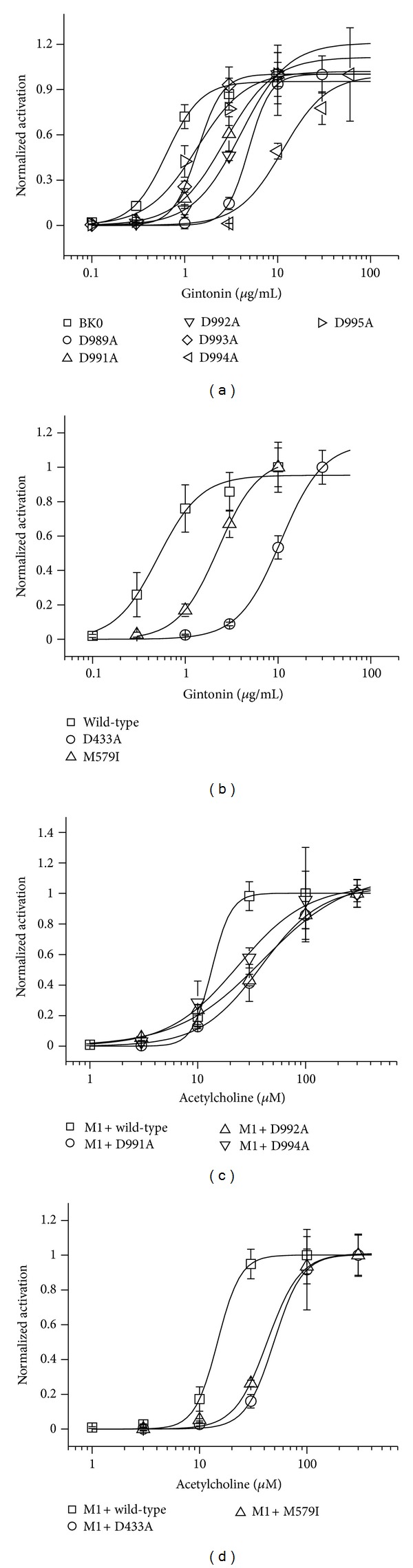
Involvement of the Ca2+ bowl and RCK domain in gintonin-mediated BKCa channel activation. (a) The oocytes expressing wild-type or various mutant BKCa channels at the Ca2+ bowl were treated by bath application of gintonin (10 μg/mL) for 60 s, and peak outward currents were recorded. Mutations of the Ca2+ bowl caused a rightward shift of the gintonin concentration-response curve. (b) The oocytes expressing wild-type or RCK domain mutant BKCa channels, D433A or M579I, were treated with gintonin by bathing application for 60 s. Mutation of the RCK domain also caused a rightward shift of the gintonin concentration-response curve. (c) The oocytes coexpressing M1 muscarinic receptor or various mutant BKCa channels with M1 muscarinic receptor were treated by bath application of acetylcholine (100 μM), and peak outward currents were recorded. Mutations of the calcium bowl caused a rightward shift of the acetylcholine concentration-response curve. (d) Oocytes coexpressing wild-type with M1 muscarinic receptor or mutant BKCa channels at the RCK domain such as D433A or M579I with M1 muscarinic receptor were treated with gintonin by bathing application for 60 s. Mutations in the RCK domain caused a rightward shift of the gintonin concentration-response curve (mean ± S.E.M; n = 13-14 oocytes each).
3.7. Dual Mutations of the Ca2+ Bowl or RCK Domain with BKCa Channel Phosphorylation Sites Further Attenuate Gintonin-Mediated BKCa Channel Activation
We further examined whether dual mutations of amino acid residues in the Ca2+ bowl or the RCK domain and in CaM kinase II phosphorylation sites in the BKCa channel further affect gintonin-mediated BKCa channel activation. As shown in Figure 7(a), double mutations of CaM kinase II and the Ca2+ bowl (T462/F994A) further attenuated the gintonin-mediated BKCa channel activation with a concomitant right shift of the concentration-response curves (Figure 7(c)). The EC50 was 31.23 ± 1.20 μg/mL. Additionally, double mutations of CaM kinase II and the RCK domain further attenuated the gintonin-mediated BKCa channel activation (Figure 7(d)). The EC50 was 22.5 ± 0.80 μg/mL, again confirming that gintonin-mediated BKCa channel activation includes Ca2+-mediated CaM kinase II activation and Ca2+ binding to the Ca2+ bowl and RCK region. However, the enhancing effects of ginsenoside Rg3 on BKCa channel currents were not affected by the double mutations of CaM kinase II and the Ca2+ bowl or by mutations in CaM kinase II and the RCK domain (Figures 7(a) and 7(b)).
Figure 7.
Double mutations of CaM kinase II and Ca2+ bowl or RCK domain further attenuate gintonin-mediated BKCa channel activation. (a) Time-current relationship after application of gintonin (10 μg/mL) or ginsenoside Rg3 (100 μM) for 60 s in oocytes coexpressing BKCa channels and CaM kinase II + Ca2+ bowl mutants. Insets, the representative peak outward current amplitude at +40 mV from a holding potential of −80 mV was measured in the presence of gintonin or ginsenoside Rg3. (b) Time-current relationship after application of gintonin (10 μg/mL) or ginsenoside Rg3 (100 μM) for 60 s in oocytes coexpressing BKCa channels and CaM kinase II + RCK domain mutants. Inset, the representative peak outward current amplitude at +40 mV from a holding potential of −80 mV was measured in the presence of gintonin or ginsenoside Rg3. (c) Coexpression of BKCa channels with CaM kinase II + Ca2+ bowl mutants caused a further rightward shift of the gintonin concentration-response curve. (d) Coexpression of BKCa channels with CaM kinase II + RCK domain mutants caused a further rightward shift of the gintonin concentration-response curve (mean ± S.E.M; n = 13 oocytes each).
4. Discussion
BKCa channels exist in excitable cells such as neurons and vascular smooth muscle cells. Their main roles are to induce repolarization following depolarization or to restore the resting membrane potential of neurons and vascular smooth muscles. Thus, the physiological functions of BKCa channels are to regulate synaptic transmission in the nervous system and to relax the blood vessels. The activation of BKCa channels is closely linked to transient [Ca2+]i induction by voltage-gated Ca2+ channel activation after depolarization since BKCa channels colocalize with Ca2+ channels [41, 42]. The cytoplasmic C terminus of the BKCa channel α subunit contains two main Ca2+ binding sites, that is, the Ca2+ bowl and a high Ca2+ affinity RCK domain [37]. In addition, various kinases also regulate BKCa channel activities through the phosphorylation of BKCa channel proteins [43, 44].
The present study was performed to elucidate the molecular mechanisms coupling gintonin to BKCa channel activation by using a Xenopus oocyte gene expression system. Our results revealed four major findings. Firstly, we observed that gintonin treatment induced BKCa channel activation in a concentration- and voltage-dependent manner via LPA receptor activation but the repeated treatment of gintonin induced a rapid desensitization. Secondly, the presence of a PLC inhibitor, an IP3 receptor, antagonist, an intracellular Ca2+ chelator, or a PKC inhibitor greatly attenuated the action, of gintonin. Thirdly, treatment with a calmodulin inhibitor attenuated gintonin action and mutations of PKC and CaM kinase II phosphorylation sites, but not by PKA phosphorylation sites, on the BKCa channel greatly attenuated gintonin action. Fourthly, mutations of amino acid residues in the Ca2+ bowl and RCK domains greatly attenuated gintonin-mediated enhancement of BKCa channel currents. Thus, since BKCa channels play an important role in presynaptic nerve terminals and blood vessel smooth muscle cells, the findings in the present study show the possibility that gintonin may be a novel BKCa channel regulator in the nervous and vascular systems via the PLC-IP3-Ca2+ and Ca2+-PKC-CaM kinase II signal transduction pathways.
Interestingly, although gintonin- and acetylcholine-mediated BKCa channel activations are attenuated by site-directed mutations of amino acid residues of Ca2+ bowl and RCK domain, it appears in D994A and D433A mutant channels that the degree of gintonin-mediated BKCa channel activation was more strongly attenuated than that of acetylcholine-mediated BKCa channel activation. These results imply that although both agents use the same signaling pathway for BKCa channel activation, D994 residue in Ca2+ bowl and D433 residue in RCK domain might play more important role in gintonin- rather than acetylcholine-mediated BKCa channel activation.
In a previous study, we demonstrated that ginsenoside Rg3 enhances BKCa channel currents following depolarization [24]. By comparing the regulatory mode of gintonin action with ginsenoside Rg3 action for BKCa channel activation, we determined that gintonin differs from ginsenoside Rg3. Ginsenoside Rg3-induced enhancement of BKCa channel currents was not achieved through receptor-mediated transient [Ca2+]i (Figures 2 and 3). Thus, ginsenoside Rg3-induced BKCa channel current enhancement did not include membrane receptor signaling transduction pathways. Instead, as a kind of dammarane glycosides (Figure 1(b)), ginsenoside Rg3-induced enhancement of BKCa channel currents was abolished by substitution of a Tyr360 residue, located at the channel pore entrance, and the enhancement of BKCa channel currents by Rg3 did not show desensitization after repeated treatment [24]. Thus, ginsenoside Rg3 regulates BKCa channel activity through direct interaction with channel proteins at the channel pore entrance. In contrast, as a G protein-coupled LPA receptor ligand, gintonin amplifies BKCa channel activation via a series of signal transductions through membrane bound G protein-coupled LPA receptor activation (Figure 8). Supporting this notion, gintonin, even at much lower concentrations than ginsenoside Rg3, induces greater amplitudes of outward BKCa channel currents (by 4-5-fold) than ginsenoside Rg3 (Figure 3). The EC50 of gintonin is about 35 nM (under the assumption that the molecular weight of gintonin is 20 kDa), whereas that of ginsenoside Rg3 was about 15 μM for BKCa channel activation [24]. In addition, interruptions of the receptor signaling pathway by inhibitors or mutations abolished or attenuated gintonin-mediated but not ginsenoside Rg3-mediated BKCa channel activation. These results indicate that although ginseng contains two agents with two different action modes for the regulation of BKCa channel activity, gintonin is more efficient for BKCa channel activation than ginsenoside Rg3 (Figure 8).
Figure 8.
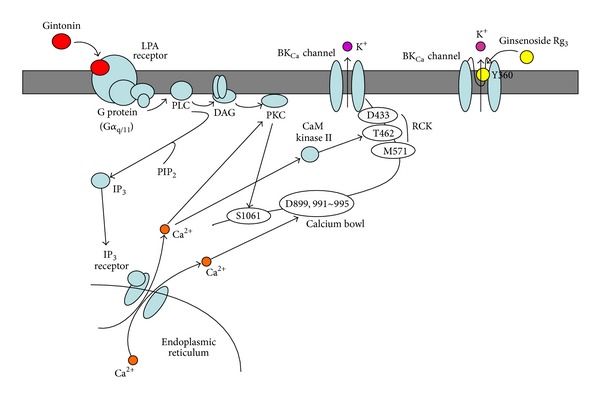
A comparative drawing of the action modes between gintonin and ginsenoside Rg3 in BKCa channel activation. Gintonin activates BKCa channels via G protein-coupled LPA1 receptors. Gintonin-mediated BKCa channel activations are mediated by Ca2+ binding to the Ca2+ bowl, RCK, or via activations of Ca2+-dependent kinases, whereas ginsenoside Rg3 activates BKCa channels through direct interaction with a specific amino acid located at the pore entryway of channel proteins following depolarization but not receptor activation [24].
BKCa channels are widely distributed in nervous and vascular systems [10, 45, 46]. In vitro gintonin-mediated BKCa channel activation might be associated with the in vivo pharmacological effects of ginseng. In previous studies, ginseng has exhibited neuroprotective effects against a variety of excitatory neurotransmitters, toxins, or ischemic stroke [1]. In addition, ginseng is also reported to induce relaxation of blood vessels constricted by adrenergic receptor stimulations [47, 48]. Thus, gintonin might be utilized for the reduction of overexcitability of the nervous system or to downregulate hyperactivity of blood vessels. Thus, the present studies show the possibility using a Xenopus oocyte gene expression model system that gintonin might participate in the regulation of synaptic transmission in nerve terminals and vascular muscle tone. However, more investigations are needed to extend from Xenopus oocytes to neuron or muscle cells.
In summary, we found that gintonin induces BKCa channel activation via membrane G protein-coupled LPA receptor signaling pathways. Using site-directed mutagenesis, we further confirmed the molecular mechanisms between the Ca2+ bowl, RCK domain, and CaM kinase II, which are involved in gintonin-mediated BKCa channel regulation. These novel findings provide insight into the molecular basis of the pharmacological effects of ginseng in the nervous and vascular systems.
Author's Contribution
S. H. Choi and B. H. Lee equally contributed to this paper.
Acknowledgment
This study was supported by the SMART Research Professor Program of Konkuk University.
References
- 1.Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Reviews. 2007;13(4):381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang SH, Shin TJ, Choi SH, et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Molecules and Cells. 2012;33(2):151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orellana S, Solski PA, Brown JH. Guanosine 5′-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. Journal of Biological Chemistry. 1987;262(4):1638–1643. [PubMed] [Google Scholar]
- 4.Moran O, Dascal N. Protein kinase C modulates neurotransmitter responses in Xenopus oocytes injected with rat brain RNA. Brain Research. 1989;5(3):193–202. doi: 10.1016/0169-328x(89)90035-1. [DOI] [PubMed] [Google Scholar]
- 5.Lupu-Meiri M, Shapira H, Oron Y. Dual regulation by protein kinase C of the muscarinic response in Xenopus oocytes. The European Journal of Physiology. 1989;413(5):498–504. doi: 10.1007/BF00594180. [DOI] [PubMed] [Google Scholar]
- 6.Singer D, Boton R, Moran O, Dascal N. Short- and long-term desensitization of serotonergic response in Xenopus oocytes injected with brain RNA: roles for inositol 1, 4, 5-trisphosphate and protein kinase C. The European Journal of Physiology. 1990;416(1-2):7–16. doi: 10.1007/BF00370215. [DOI] [PubMed] [Google Scholar]
- 7.Im DS. Pharmacological tools for lysophospholipid GPCRs: development of agonists and antagonists for LPA and S1P receptors. Acta Pharmacologica Sinica. 2010;31(9):1213–1222. doi: 10.1038/aps.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toro L, Wallner M, Meera P, Tanaka Y. Maxi-K(Ca), a unique member of the voltage-gated K channel superfamily. News in Physiological Sciences. 1998;13(3):112–117. doi: 10.1152/physiologyonline.1998.13.3.112. [DOI] [PubMed] [Google Scholar]
- 9.Weiger TM, Hermann A, Levitan IB. Modulation of calcium-activated potassium channels. Journal of Comparative Physiology A. 2002;188(2):79–87. doi: 10.1007/s00359-002-0281-2. [DOI] [PubMed] [Google Scholar]
- 10.Ghatta S, Nimmagadda D, Xu X, O’Rourke ST. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacology and Therapeutics. 2006;110(1):103–116. doi: 10.1016/j.pharmthera.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Koike K, Toro L. MaxiK channel roles in blood vessel relaxations induced by endothelium-derived relaxing factors and their molecular mechanisms. Journal of Smooth Muscle Research. 2004;40(4-5):125–153. doi: 10.1540/jsmr.40.125. [DOI] [PubMed] [Google Scholar]
- 12.Orellana S, Solski PA, Brown JH. Guanosine 5′-0-(thiotriphosphate)-dependent inositol triphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. Journal of Biological Chemistry. 1987;262(4):1638–1643. [PubMed] [Google Scholar]
- 13.Dworetzky SI, Boissard CG, Lum-Ragan JT, et al. Phenotypic alteration of a human BK (hSlo) channel by hSloβ subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. Journal of Neuroscience. 1996;16(15):4543–4550. doi: 10.1523/JNEUROSCI.16-15-04543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McManus OB, Helms LMH, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14(3):645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 15.Chang CP, Dworetzky SI, Wang J, Goldstein ME. Differential expression of the α and β subunits of the large-conductance calcium-activated potassium channel: implication for channel diversity. Molecular Brain Research. 1997;45(1):33–40. doi: 10.1016/s0169-328x(96)00230-6. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi K(Ca) channels in human coronary smooth muscle: predominant α + β subunit complexes. Journal of Physiology. 1997;502(3):545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao L, Kaldany C, Holmstrand EC, Cox DH. Mapping the BKCa channel’s “Ca2+ Bowl”: side-chains essential for Ca2+ Sensing. Journal of General Physiology. 2004;123(5):475–489. doi: 10.1085/jgp.200409052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piskorowski R, Aldrich RW. Calcium activation of BKCa potassium channels lacking the calcium bowl and RCK domains. Nature. 2002;420(6915):499–502. doi: 10.1038/nature01199. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophysical Journal. 1997;73(3):1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber M, Yuan A, Salkoff L. Transplantable sites confer calcium sensitivity to BK channels. Nature Neuroscience. 1999;2(5):416–421. doi: 10.1038/8077. [DOI] [PubMed] [Google Scholar]
- 21.Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418(6900):880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 22.Mutoh T, Rivera R, Chun J, et al. Insights into the pharmacological relevance of lysophospholipid receptors. British Journal of Pharmacology. 2012;165(4):829–844. doi: 10.1111/j.1476-5381.2011.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyo MK, Choi SH, Hwang SH, et al. Novel glycolipoproteins from ginseng. Journal of Ginseng Research. 2011;35(1):92–103. [Google Scholar]
- 24.Choi SH, Shin TJ, Lee BH, et al. Ginsenoside Rg3 enhances large conductance Ca2+-activated potassium channel currents: a role of Tyr360 residue. Molecules and Cells. 2011;31(2):133–140. doi: 10.1007/s10059-011-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Lee BH, Choi SH, et al. Ginsenoside Rg3 inhibits human Kv1.4 channel currents by interacting with the Lys531 residue. Molecular Pharmacology. 2008;73(3):619–626. doi: 10.1124/mol.107.040360. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Chen B, Ge Q, Wang ZW. Presynaptic Ca2+/calmodulin-dependent protein kinase II modulates neurotransmitter release by activating BK channels at Caenorhabditis elegans neuromuscular junction. Journal of Neuroscience. 2007;27(39):10404–10413. doi: 10.1523/JNEUROSCI.5634-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophysical Journal. 1992;63(2):583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao YD, Garcia ML. Interaction of agitoxin2, charybdotoxin, and iberiotoxin with potassium channels: selectivity between voltage-gated and Maxi-K channels. Proteins. 2003;52(2):146–154. doi: 10.1002/prot.10341. [DOI] [PubMed] [Google Scholar]
- 29.Kaczorowski GJ, Knaus HG, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. Journal of Bioenergetics and Biomembranes. 1996;28(3):255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- 30.Kimura Y, Schmitt A, Fukushima N, et al. Two vovel Xenopus homologs of mammalian LPA1/EDG-2 function as lysophosphatidic acid receptors in Xenopus oocytes and mammalian cells. Journal of Biological Chemistry. 2001;276(18):15208–15215. doi: 10.1074/jbc.M011588200. [DOI] [PubMed] [Google Scholar]
- 31.Thompson AK, Mostafapour SP, Denlinger LC, Bleasdale JE, Fisher SK. The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. A role for G(p) in receptor compartmentation. Journal of Biological Chemistry. 1991;266(35):23856–23862. [PubMed] [Google Scholar]
- 32.Singer D, Boton R, Moran O, Dascal N. Short- and long-term desensitization of serotonergic response in Xenopus oocytes injected with brain RNA: roles for inositol 1,4,5-trisphosphate and protein kinase C. The European Journal of Physiology. 1990;416(1-2):7–16. doi: 10.1007/BF00370215. [DOI] [PubMed] [Google Scholar]
- 33.Zhu S, Browning DD, White RE, Fulton D, Barman SA. Mutation of protein kinase C phosphorylation site S1076 on α-subunits affects BKCa channel activity in HEK-293 cells. The American Journal of Physiology. 2009;297(4):L758–L766. doi: 10.1152/ajplung.90518.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sansom SC, Ma R, Carmines PK, Hall DA. Regulation of Ca2+-activated K+ channels by multifunctional Ca2+/calmodulin-dependent protein kinase. The American Journal of Physiology. 2000;279(2):F283–F288. doi: 10.1152/ajprenal.2000.279.2.F283. [DOI] [PubMed] [Google Scholar]
- 35.Wang ZW. Regulation of synaptic transmission by presynaptic CAMKII and BK channels. Molecular Neurobiology. 2008;38(2):153–166. doi: 10.1007/s12035-008-8039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417(6888):515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 37.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nature Reviews Neuroscience. 2006;7(12):921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 38.Zeng XH, Xia XM, Lingle CJ. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. Journal of General Physiology. 2005;125(3):273–286. doi: 10.1085/jgp.200409239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao L, Rapin AM, Holmstrand EC, Cox DH. Elimination of the BKCa channel’s high-affinity Ca2+ sensitivity. Journal of General Physiology. 2002;120(2):173–189. doi: 10.1085/jgp.20028627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishii M, Kurachi Y. Muscarinic acetylcholine receptors. Current Pharmaceutical Design. 2006;12(28):3573–3581. doi: 10.2174/138161206778522056. [DOI] [PubMed] [Google Scholar]
- 41.Berkefeld H, Fakler B. Repolarizing responses of BKCa-cav complexes are distinctly shaped by their cav subunits. Journal of Neuroscience. 2008;28(33):8238–8245. doi: 10.1523/JNEUROSCI.2274-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latorre R, Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biological Research. 2006;39(3):385–401. doi: 10.4067/s0716-97602006000300003. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Zhou Y, Wen H, Levitan IB. Simultaneous binding of two protein kinases to a calcium-dependent potassium channel. The Journal of Neuroscience. 1999;19(10, article RC4) doi: 10.1523/JNEUROSCI.19-10-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Wilson GF, Griffith LC. Calcium/calmodulin-dependent protein kinase II phosphorylates and regulates the Drosophila eag potassium channel. Journal of Biological Chemistry. 2002;277(27):24022–24029. doi: 10.1074/jbc.M201949200. [DOI] [PubMed] [Google Scholar]
- 45.Nelson MT, Cheng H, Rubart M, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 46.Yazejian B, Digregorio DA, Vergara JL, Poage RE, Meriney SD, Grinnell AD. Direct measurements of presynaptic calcium and calcium-activated potassium currents regulating neurotransmitter release at cultured Xenopus nerve-muscle synapses. Journal of Neuroscience. 1997;17(9):2990–3001. doi: 10.1523/JNEUROSCI.17-09-02990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim ND, Kang SY, Park JH, Schini-Kerth VB. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. European Journal of Pharmacology. 1999;367(1):41–49. doi: 10.1016/s0014-2999(98)00898-x. [DOI] [PubMed] [Google Scholar]
- 48.Kang SY, Schini-Kerth VB, Kim ND. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta. Life Sciences. 1995;56(19):1577–1586. doi: 10.1016/0024-3205(95)00124-o. [DOI] [PubMed] [Google Scholar]