Abstract
Objective. This study intended to systematically evaluate the effectiveness and safety of modified Dachengqi Decoction (MDD) combined with conventional treatment for treating acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Method. An extensive search was performed within 6 English and Chinese electronic databases from inception to April 2012. Methodological quality was assessed according to Cochrane risk of bias assessment. Data were analyzed using Review Manager 5.1. Results. A total of 16 studies (involving 1112 patients) were included. The result showed that MDD and its modification combined with routine treatment were more effective in improving FEV1%pred, enhancing the significant effectiveness, reducing PCO2, and shortening duration of mechanical ventilation. Adverse events were reported in two trials with symptom of diarrhea, while no serious adverse effect was reported. Conclusion. Modified Dachengqi Decoction appears to be effective for treating AECOPD. However, more regular designed RCTs are needed because of insufficient methodological problems.
1. Introduction
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (COPD) [1] for the first time revised the goal of the treatment of COPD into relief symptoms rapidly, and reduces risk of patients' health, such as recurrent episodes or rapid decrease of pulmonary function [2]. Therefore, how to control the symptoms and reduce the frequency of disease onset became the research emphasis in academic field.
COPD belongs to the category of “lung distention” in Chinese medicine. Syndrome of phlegm-heat obstructing in the lung is one of the most common syndromes in the acute stage. The main clinical features include yellow sputum, dyspnea, thirsty, and constipation [3]. “Interior and Exterior Relationship between the Lung and Large Intestine” is one of the most typical viscera correlation theories. It describes the corelationship of viscera, meridian, physiological relation, and pathological changes, which is the foundation for further clinical treatment of AECOPD by using purgative drugs. Many domestic and foreign researches [4, 5] found that COPD patients have digestive tract symptom such as abdominal distension, constipation besides cough, wheezing, phlegm, and dyspnea. So, it is viable to do research on using purgative decoction in the treatment of AECOPD.
Dachengqi decoction, a representative recipe of dredging intestines in Treatise on Febrile Diseases (Shang Han Lun), has been widely used to treat Yangming Fushi Syndrome. It is composed by Rheum, Magnolia officinalis, immature bitter orange, and Mirabilite. In a recent study, it effectively treated critical patients with gastroenteric function disorder, and reduce the incidence and fatality of MODS [6].
This study aimed to determine the effects and safety of purgative decoction on pulmonary function, artery blood gas analysis, ventilator weaning time in patients with AECOPD by systematically evaluating the effectiveness of oral decoctions, or Chinese patent medicine based on Dachengqi Decoction plus conventional treatment compared with western medicine alone in the treatment of AECOPD.
2. Materials and Methods
2.1. Search Strategy
We searched the Chinese literature from CNKI, CBM, VIP, WANFANG, and foreign literature from PubMed and Cochrane library. The searching was from the inception of the databases to April 2012. We utilized the medical subject headings “COPD” or “chronic obstructive pulmonary disease” and “Chinese medicine” in PubMed, Cochrane, while we use “COPD” or “chronic obstructive pulmonary disease” and “Dachengqi Decoction” or “Radix et Rhizoma Rhei” or “Natrii Sulfas” or “Fructus Aurantii Immaturus” or “Cortex Magnoliae officinalis” in Chinese database.
2.2. Inclusion Criteria
Inclusion criteria were the following: (1) RCTs in English or Chinese involving decoctions based on Dachengqi Decotion compared with placebo, no treatment, or conventional treatment without Chinese medicine as controls. (2) Patients must be aged 18 years or over and of any gender or ethnic origin. Patients are diagnosed with COPD in the severe stage with or without respiratory failure. COPD is defined as “The Global Initiative for Chronic Obstructive Lung Disease (GOLD)” which is pulmonary function includes FEV1%pred <80% and FEV1/FVC% <70% after using bronchodilator. (3) Primary outcome measures were pulmonary function (FEV1%pred, FEV1/FVC%), safety, and significant effectiveness based on clinical symptoms relief. The significant effectiveness was defined as the symptoms scores improvement rate ≥70% according to “the guide for clinical trials of new drugs” [7]. Clinical symptoms involved cough, cough-up phlegm, dyspnea, constipation, and wheeze. Secondary outcome measures were artery blood gas analysis (PO2, PCO2) and duration of mechanical ventilation.
2.3. Data Extraction
Study characteristics included trial design, sample size, mean and standard deviation of participants' age, and history of COPD; severity of COPD differentiation of syndrome, methodological quality, intervention, outcome measures, treatment duration and follow-up period, and adverse events were extracted to a predefined form and checked by a second reviewer.
2.4. Risk of Bias
The methodological quality of the included studies was independently assessed by 2 authors using the Cochrane risk of bias assessment [8]. Assessment of Cochrane risk of bias consists of seven domains: (1) random sequence generation (2) allocation concealment (3) blinding of participants and personnel (4) blinding of outcome assessment (5) incomplete outcome data (6) selective reporting and (7) other bias. For each domain, evaluation was by denoting “yes”-adequate (low risk of bias); “no”: inadequate (high risk of bias); or “unclear”: unclear or not used (uncertain risk of bias) according to the descriptions of the method in each study. Any disagreement was resolved by discussion with a third reviewer.
2.5. Data Analysis
Meta-analysis was performed using RevMan 5.1. For categorical data, we used risk ratios (RR), while for continuous data, mean differences (MD) were calculated and expressed in effect value and 95% confidence (CI). Heterogeneity was calculated by X 2 and I 2 statistics. When heterogeneity inspection result showed significant heterogeneity (P < 0.05), we used random effects model, otherwise we applied fixed effects model.
3. Results
3.1. Overview of Included Studies
We initially identified 622 citations, after screening for potential relevance, 16 full papers [9–24] were assessed for possible inclusion (Figure 1). All studies were conducted in China. The characteristics of the included studies are summarized in Table 1. The different compositions of Chinese herbal formula MDD are presented in Table 2. The 16 studies involved a total of 1112 acute COPD patients, and 12 studies were included in meta-analysis. All studies reported diagnosis standard. All 12 studies were about Chinese medicine combined with western medicine routine treatment compared with conventional treatment alone (Table 1). Fifteen studies [9–13, 15–18, 20–24] were about oral decoction combined with western medicine routine treatment. Two [14, 19] studies used Chinese patent medicine based on MDD.
Figure 1.
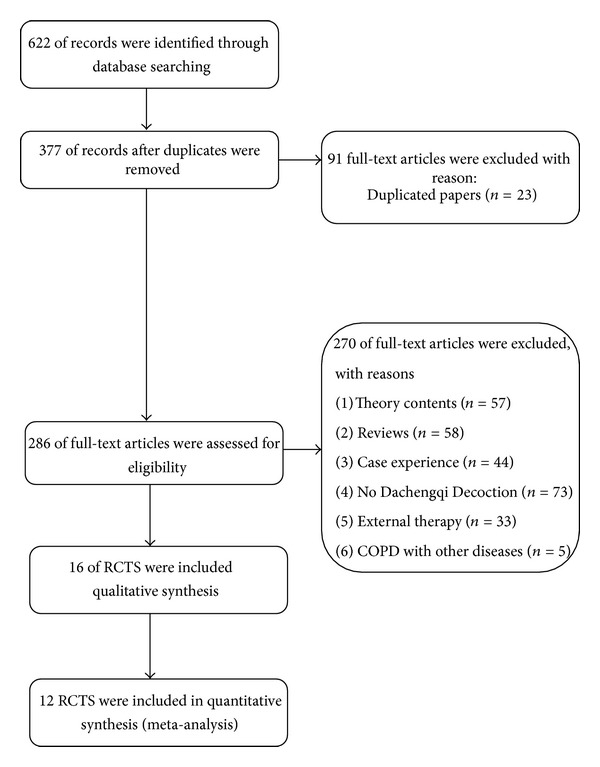
Study selection process.
Table 1.
Characteristics of included trials.
| Study ID | Sample | CM syndrome | Intervention | Controlled | Couse | Adverse event | Outcome measures |
|---|---|---|---|---|---|---|---|
| Fang and Shi, 2006 [9] | T: 20/18 C: 20/17 |
NS | MDD | Aminophylline 0.25 g qd, Methylprednisolone 80 mg q12h, Cefoperazone and Sulbactam 2 g q12h, Mucosolvan 75 mg q12h | 5D | NS | BGA, DMV |
| Fu, 2010 [10] | T: 30/30 C: 30/30 |
SPHOL | MDD | Conventional treatment | 9D | NS | PF |
| Guo and Liang, 2011 [11] | T: 60/60 C: 60/60 |
SPHOL | MDD | Mucosolvan 60 mg and conventional treatment | 14D | NS | ER |
| Guo and Zhang, 2008 [12] | T: 30/30 C: 30/30 |
NS | MDD | Conventional treatment | 7D | NS | PF, BGA, ER |
| Li, 2009 [13] | T: 29/29 C: 27/27 |
SPHOL | Tongsai granule 6 g tid | Aminophylline 0.5 g, antibacterial | 15D | NS | Inflammation factor |
| Li et al., 2003 [14] | T: 60/60 C: 60/60 |
SPHOL | MDD | Conventional treatment | 12D | NS | ER |
| Li, 2006 [15] | T: 30/30 C: 30/30 |
SPHOL | MDD | Cefaclor capsules 0.25 g tid, Azithromycin tablets 0.5 g, Mucosolvan 30 mg tid. | 14D | YSE | PF, BGA, ER |
| Liang, 2011 [16] | T: 30/30 C: 30/30 |
NS | MDD | Conventional treatment | 15D | NS | PF, ER |
| Liu et al., 2002 [17] | T: 25/25 C: 25/25 |
NS | MDD | Conventional treatment | 12D | NS | Offline success rate |
| Lu, 2010 [18] | T: 21/21 C: 21/21 | SPHOL | MDD | Conventional treatment | 10D | NS | PF, BGA, ER |
| Mao, 2010 [19] | T: 52/52 C: 48/48 |
NS | Tongfupaiqi mixture | Conventional treatment | 14D | YES | PF |
| Meng, 2012 [20] | T: 30/30 C: 30/30 |
SPHOL | MDD | Conventional treatment | 7D | NS | PF, ER |
| Pang, 2009 [21] | T: 42/42 C: 40/40 |
SPHOL | MDD | Conventional treatment | 7D | NS | BGA |
| Peng and Li, 2009 [22] | T: 30/30 C: 30/30 |
SPHOL | MDD | Conventional treatment | 14D | NS | ER |
| Shi et al., 2010 [23] | T: 50/38 C: 30/20 |
NS | MDD | Conventional treatment | 28D | NS | DMV |
| Zhang, 2011 [24] | T: 32/32 C: 30/30 |
SPHOL | MDD | Conventional treatment | 7D | NS | PF, ER |
T: treatment; C: control; NS: not specified; conventional treatment: antibiotics, antispasmodic, expectorant (the drug is unknown); SPHOL: syndrome of phlegm-heat obstructing lung; BGA: blood gas analysis. PF: pulmonary function; ER: effective rate; DMV: duration of mechanical ventilation.
Table 2.
The compositions of MDD.
| Study ID | Composition of formula |
|---|---|
| Fang and Shi, 2006 [9] | MDD (Radix et Rhizoma Rhei, Fructus Aurantii Immaturus, Cortex Magnoliae officinalis, Trichosanthis, Semen Armeniacae Amarum, Radix Glycytthizae, Semen Raphani.) 100 mL bid |
| Fu, 2010 [10] | MDD (Radix et Rhizoma Rhei, Fructus Aurantii Immaturus, Cortex Magnoliae officinalis) qd |
| Guo and Liang, 2011 [11] | MDD (Radix et Rhizoma Rhei, Fructus Aurantii Immaturus, Cortex Magnoliae officinalis, Rhizoma Pinelliae, Semen Raphani, Radix Astragali) 200 mL bid |
| Guo and Zhang, 2008 [12] | MDD (Gypsum Fibrosum, Radix Scutellariae, Semen Armeniacae Amarum, Fructrs Trichosanthis), 200 mL bid |
| Li, 2009 [13] | Tongsai granule (Radix et Rhizoma Rhei, Herba Ephedrae, Semen Lepidii, Bulbus Fritillariae Cirrhosae) 6 g tid |
| Li et al., 2003 [14] | MDD (Radix et Rhizoma Rhei, Fructus Aurantii Immaturus, Natrii Sulfas, Fructrs Trichosanthis, Semen Raphani, Semen Lepidii) 200 mL bid |
| Li, 2006 [15] | MDD (Radix et Rhizoma Rhei 10 g, Gypsum Fibrosum 30 g, Fructrs Trichosanthis 15 g, Semen Armeniacae Amarum 10 g, Radix Scutellariae 15 g, Semen Lepidii 15 g, Herba Houttuyniae 15 g, Bulbus Fritillariae Thunbergii 15 g, Radix Glycytthizae 15 g) 100 mL tid |
| Liang, 2011 [16] | MDD (Radix et Rhizoma Rhei 7 g, Fructus Aurantii Immaturus 9 g, Cortex Magnoliae officinalis 9 g, Radix Scutellariae 12 g, Rhizoma Pinelliae 14 g, Pericarpium Citri Reticulatae 11 g, Radix Glycytthizae 7 g) 200 mL bid |
| Liu et al., 2002 [17] | MDD (Radix et Rhizoma Rhei, Fructus Aurantii Immaturus, Cortex Magnoliae officinalis, Fructrs Trichosanthis, Semen Armeniacae Amarum, Poria, Radix Glycytthizae) 100 mL bid |
| Lu, 2010 [18] | MDD (Radix et Rhizoma Rhei 3 g, Fructrs Trichosanthis 15 g, Semen Armeniacae Amarum 10 g, Semen Cannabis 10 g, Radix Scutellariae 15 g, Herba Houttuyniae 15 g, Radix Glycytthizae 9 g) 200 mL bid |
| Mao, 2010 [19] | TongFuPaiQi mixture (Radix et Rhizoma Rhei, Semen Raphani, Semen Persicae, Radix Paeoniae Rubra) 25 mL tid |
| Meng, 2012 [20] | MDD (Radix et Rhizoma Rhei, Fructrs Trichosanthis, Semen Armeniacae Amarum Gypsum Fibrosum), 200 mL bid |
| Pang, 2009 [21] | MDD (Radix et Rhizoma Rhei, Cortex Magnoliae officinalis, Fructrs Trichosanthis, Semen Armeniacae Amarum, Rhizoma Pinelliae, Gypsum Fibrosum, Radix Astragali, Radix Glycytthizae) 200 mL bid |
| Peng and Li, 2009 [22] | MDD (Radix et Rhizoma Rhei 6 g, Natrii Sulfas 12 g, Semen Lepidii 15 g, Radix Scutellariae 15 g) qd |
| Shi et al., 2010 [23] | MDD (Radix et Rhizoma Rhei, Fructus Aurantii Immaturus, Fructrs Trichosanthis, Semen Armeniacae Amarum, Semen Raphani, Radix Glycytthizae) 100 mL bid |
| Zhang, 2011 [24] | MDD (Radix et Rhizoma Rhei, Cortex Magnoliae officinalis, Fructrs Trichosanthis, Semen Armeniacae Amarum, Rhizoma Pinelliae, Gypsum Fibrosum, Radix Scutellariae) 200 mL bid |
3.2. Assessment of Risk of Bias
Information of sequence generation was adequate for five studies at low risk of bias [9, 13, 18, 22, 23] and inadequate for seventeen studies with unclear risk of bias [10–12, 14–17, 19–21, 24]. All the five studies reported that they used random number table for sequence generation. Allocation concealment was not reported in all studies (no). Blinding of participants, physicians, and study personnel was not reported in these studies, but all studies were carried without placebo, so they were of high risk of bias. None of the studies reported lost to followup, withdraw and dropoff; thus, the risk of bias of incomplete outcome data over all studies was graded as unclear. As far as selective reporting was concerned, for we could not find any pre-defined outcome measurements in all the icluded studies we classified them as unclear risk of bias. Due to the limited number of included studies, we were not able to implement funnel plot.
3.3. Outcome Measure
3.3.1. Pulmonary Function
FEV1%pred was reported in 5 articles [10, 13, 16, 18, 19] (Figure 2 ) and FEV1/FVC% was reported in 5 studies, respectively [10, 13, 16, 18, 24] (Figure 3). Significant differences showed in FEV1%pred. Decoction group concludes five trials (MD 5.3, 95%CI 1.48 to 9.12). Similar changes were shown in FEV1/FVC% (MD 1.55, 95%CI 0.23 to 2.87).
Figure 2.
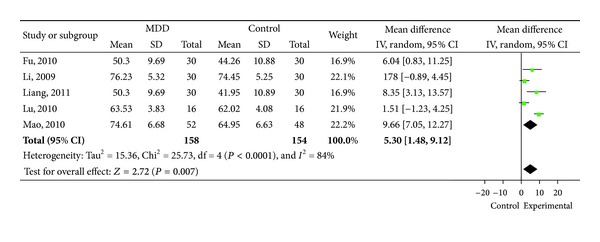
Forest plot of comparison: FEV1%pred (%).
Figure 3.
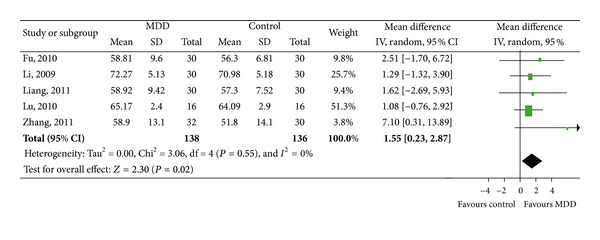
Forest plot of comparison: FEV1/FVC% (%).
3.3.2. Significant Effectiveness
MDD group showed higher percentage of effectiveness when compared with non-MDD formula group (RR 1.62, 95%CI 1.3 to 2.02) [10, 11, 13, 18, 22] (Figure 4).
Figure 4.
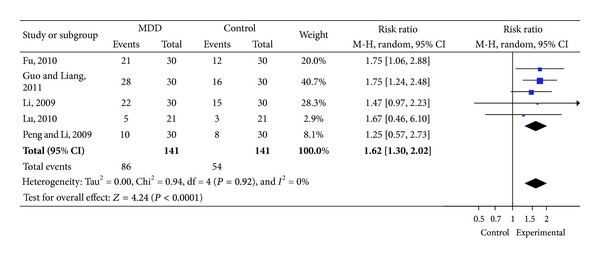
Forest plot of comparison: therapeutic effect of syndrome.
3.3.3. Blood Gas Analysis
PO2 was reported in 6 studies [9, 11, 13, 18, 20, 21] (Table 3) and PCO2 was predicted in 5 studies [9, 11, 18, 20, 21] (Table 4). There was an improvement in PO2 and a reduction in PCO2 when comparing modified Dachengqi Decoction plus conventional treatment to conventional treatment alone.
Table 3.
Outcome measures for PO2 (mmHg).
| Study | Treatment (m ± s) | Control (m ± s) | Mean difference (95% CI) | P value |
|---|---|---|---|---|
| Fang and Shi, 2006 [9] | 90 ± 21 | 68 ± 9 | 22.00 (11.40, 32.60) | P = 0.0004 |
| Guo and Liang, 2011 [11] | 82.3 ± 7.32 | 75.02 ± 8.34 | 7.28 (3.31, 11.25) | P = 0.0007 |
| Li, 2009 [13] | 80.25 ± 5.18 | 79.25 ± 7.36 | 1.00 (−2.22, 4.22) | P = 0.5452 |
| Lu, 2010 [18] | 92.95 ± 4.67 | 91.29 ± 5.02 | 1.66 (−1.70, 5.02) | P = 0.34 |
| Meng, 2012 [20] | 81.2 ± 4.9 | 75.2 ± 5.1 | 6.00 (3.47, 8.53) | P < 0.0001 |
| Pang, 2009 [21] | 69.87 ± 3.96 | 62.43 ± 4.14 | 7.44 (5.68, 9.20) | P < 0.0001 |
| Meta | 5.76 (4.60, 6.93) | P < 0.00001 |
Table 4.
Outcome measures for PCO2 (mmHg).
| Study | Treatment (m ± s) | Control (m ± s) | Mean difference (95% CI) | P value |
|---|---|---|---|---|
| Fang and Shi, 2006 [9] | 67 ± 11 | 74 ± 13 | −7.00 (−14.78, 0.78) | P = 0.0852 |
| Guo and Liang, 2011 [11] | 41.3 ± 5.13 | 48.26 ± 5.34 | −6.96 (−9.61, − 4.31) | P < 0.0001 |
| Lu, 2010 [18] | 41.76 ± 2.1 | 42.76 ± 2.39 | −1.00 (−2.56, 0.56) | P = 0.2184 |
| Meng, 2012 [20] | 65.7 ± 6.3 | 81.7 ± 8.1 | −16.00 (−19.67, − 12.33) | P < 0.0001 |
| Pang, 2009 [21] | 57.54 ± 6.9 | 62.36 ± 5.76 | −4.82 (−7.57, − 2.07) | P = 0.001 |
| Meta | −4.30 (−5.44, − 3.17) | P < 0.00001 |
3.3.4. Duration of Mechanical Ventilation
Duration of mechanical ventilation (days) was reported in 2 studies [9, 23]. Recovery time of MDD group was shorter than that of the conventional treatment group (MD −3.16d, 95%CI −3.9d to −2.43d).
3.3.5. Safety
Two studies [15, 19] reported adverse events. Both trials reported adverse reaction as diarrhea. Other trials did not report it.
4. Discussion
This study focuses on evaluating the effectiveness and safety of modified Dachengqi Decoction for AECOPD based on pulmonary function, blood gas analysis, and effective rates when compared with conventional treatment group. Based on the study of the sixteen studies, DMM may have positive effect on improving patients pulmonary function, improving the symptoms, enhancing the partial pressure of oxygen, decreasing the partial pressure of carbon dioxide, and shortening the duration of mechanical ventilation. As with any meta-analysis, heterogeneity must be considered. We found significant heterogeneity in the outcome measure for FEV1%pred, but the heterogeneity in the outcome measure for FEV1/FVC and clinical symptom relief was very low.
Only two studies [15, 19] reported a total of three adverse events of diarrhea,which suggests that the MDD for COPD is well tolerated. However due to the incomplete evaluation, safety of DMM should be accepted more cautiously. The results need to be monitored rigorously in the future.
Some systematic review [25, 26] has indicated a benefit of using Chinese herb such as oral ginseng formulae for the management of stable COPD which belongs to deficiency syndrome according to TCM (traditional Chinese medicine) theory. Also, there were many studies showing that Chinese medicine had become more and more important in treating COPD/AECOPD [27]. There have been many randomized controlled trials indicating that herbs can release clinical symptoms and improve quality of life.
MDD is not available for it is not widely used in treating COPD/AECOPD, but some studies [5] found that COPD patients have digestive tract symptom such as abdominal distension, constipation besides cough, phlegm, and dyspnea. Thus, it is practical to use MDD in treating COPD which belongs to excess syndrome especially with constipation symptoms in TCM. The theory of “Interior and Exterior Relationship between the Lung and Large Intestine” is one of the most important theories in traditional Chinese Medicine which is of great value in the clinical practice. Also some studies found that “catharsis large intestine” can decrease T cells and enhance the number of serum T cells and affect the balance of CD4+ and CD8+ and can have effects on airway remodeling of lung in rats with COPD [28].
Also, there are several methodological limitations. First, all trials involved were of low quality. No study applied placebo as control; thus, the patients and physicians were not blinded. Although all the trials reported randomization, only 5 studies reported sequence generation and no study addressed the issue of allocation concealment. The quality of studies published in Chinese is majorly poor, and some scholars indicated that China generated virtually no negative studies at all [29]. Therefore, the findings of the meta-analyses should be interpreted with caution. Due to the time limitation, we did not contact the original authors, and further information for better evaluation of risk of bias was inadequate.
Second, all the included studies were published in Chinese journals, and all the results were positive. What is more, study number was not enough to implement funnel plot, so there might be a potential publication bias. We could not rule out the systematic error because the sample size of all studies was limited. So the clinical effect might be exaggerated. Larger sample RCTs are needed in the future for accurate results.
Third, all the decoctions included in the research were based on Dachengqi Decoction, but the herbs and the dosages were different in each study. This might be the main reason leading to the significant heterogeneity. We can see in the research that all the studies except six pointed out that the syndrome of phlegm-heat obstructing lung is appropriate for MDD. Determination of treatment based on pathogenesis obtained through differentiation of symptoms is one of the most important characteristics in Chinese Medicine, so modification according to symptoms is needed during the treatment. Herbs of cold nature such as Gypsum Fibrosum or Radix Scutellariae are used to clear heat while diminishing sputum herbs such as Fructrs Trichosanthis, Semen Lepidii, and Herba Houttuyniae are commonly used in patients with abundance phlegm on the basic of Dachengqi Decoction.
Despite of the methodological weakness and potential risk of bias, the data from the 16 included studies illustrated that MDD combined with conventional treatment may have better effectiveness than conventional treatment alone, especially in improving FEV1%pred, enhancing significant effectiveness, reducing PCO2, and shortening duration of mechanical ventilation. The study suggests that MDD could improve airway obstruction and relieve respiratory failure so as to improve prognosis.
5. Conclusion
Modified Dachengqi Decoction appears to be effective for treating AECOPD. However, more well-designed and large sample RCTs are needed in the future due to the insufficient methodological problems of existing studies.
Conflict of Interests
The authors declare that there is no conflict of interests.
Authors' Contribution
R. Wu and Y. Li equally contributed to this paper.
Acknowledgments
The authors particularly thank Professor J. P. Liu and Associated Professor Y. T. Fei from the Centre for Evidence-based Chinese Medicine, Beijing University of Chinese Medicine, for their great supports in this study. This study was funded by the China National Key Basic Research Plan (no. 2009CB522704).
References
- 1.GOLD Executive Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/
- 2.Rizkallah J, Man SFP, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD. Chest. 2009;135(3):786–793. doi: 10.1378/chest.08-1516. [DOI] [PubMed] [Google Scholar]
- 3.Professional Committee of Pulmonary Disease of Internal Medicine Branch, China Association of Chinese Medicine. Syndrome diagnostic criteria of traditional Chinese medicine for chronic obstructive pulmonary disease. Zhong Yi Za Zhi. 2012;53(2):177–178. [Google Scholar]
- 4.Zheng FJ, Li YH. Discussion on pathogenesis of COPD and treatment based on intestine. China Journal of Traditional Chinese Medicine and Pharmacy. 2010;(12):1934–1937. [Google Scholar]
- 5.Ludvigsson JF, Inghammar M, Ekberg M, Egesten A. A nationwide cohort study of the risk of chronic obstructive pulmonary disease in coeliac disease. Journal of Internal Medicine. 2012;271(5):481–489. doi: 10.1111/j.1365-2796.2011.02448.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang SL, Li DB. Clinical study on therapy of clearing hallow viscera in treating critical patients with gastro-enteric function disorder. Chinese Journal of Integrative Medicine. 2006;12(2):122–125. doi: 10.1007/BF02857358. [DOI] [PubMed] [Google Scholar]
- 7.Zheng· XY. Guidelines of Clinical Research of New Drugs of Traditional Chinese Medicine. 1st edition. Beijing, China: Medical Press Science of Technology of China; 2002. [Google Scholar]
- 8.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.1) 2008, http://handbook.cochrane.org/
- 9.Fang K, Shi ZL. Observation on clinical effects of Tongfu Pingchuan decoction combined with mechanical ventilation on patients with respiratory failure induced by chronic obstructive pulmonary disease. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 2006;13(5):291–293. [Google Scholar]
- 10.Fu JY. The Study of the Application of the Method of Purging to Relax the Bowels in the Treatment of Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Guangzhou University of Chinese Medicine; 2010. [Google Scholar]
- 11.Guo JH, Liang D. Clinical research of Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD) treated by tonifying Qi and unblocking Fu. China Journal of Chinese Medicine. 2011;26(12):1421–1422. [Google Scholar]
- 12.Guo WX, Zhang FY. Clinical observation of xuanbaichengqitang with mucosolvan in the treatment of Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Hebei Journal of Traditional Chinese Medicine. 2008;30(5):502–503. [Google Scholar]
- 13.Li DL. The clinical study of Xuanbaichengqitang in the treatment of Chronic Obstructive Pulmonary Disease. Liaoning University of Chinese Medicine, 2009.
- 14.Li SY, Cheng XK, Li JS, Ma LJ, Li CH. Effect of tongse grain of TCM on cytokine in patients with chronic Obstructive Pulmonary Disease. Liaoning Journal of Traditional Chinese Medicine. 2003;30(8):624–625. [Google Scholar]
- 15.Li XY. Through experiments of tong drop turbidity method in the treatment of chronic obstructive pulmonary disease curative effect observation of respiratory failure. Journal of Traditional Chinese Medicine. 2006;28(7):503–504. [Google Scholar]
- 16.Liang WX. The application of purgative method in treating the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Guide of China Medicine. 2011;9(19):126–127. [Google Scholar]
- 17.Liu Y, Jin XY, Huo B, et al. Clinical study on Yiqi Tongfu decoction for difficult to weaning patients with COPD from mechanical ventilation. Academic Journal of Kaifeng Medical College. 2002;21(2):29–31. [Google Scholar]
- 18.Lu JY. The Clinical Research on the Effect of Qingre Xiefu Chinese Traditional Medicine in the Treatment of Acute Exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD) Hebei Medical University; 2010. [Google Scholar]
- 19.Mao LN. The treatment of TongFuPaiQi for AECOPD patients. Yunnan Journal of Traditional Chinese Medicine and Materia Medica. 2010;31(6):p. 33. [Google Scholar]
- 20.Meng FS. Clinical study on xuanbaichengqi decoction for acute exacerbation of chronic obstructive pulmonary disease. Journal of New Chinese Medicine. 2012;44(4):23–24. [Google Scholar]
- 21.Pang K. Treatment Combine traditional Chinese and western medicine for 42 COPD patients. Traditional Chinese Medicinal Research. 2009;22(8):23–25. [Google Scholar]
- 22.Peng WB, Li SF. Clinical observation of lung-purging, blood-activating and fu-unblocking herbs with western medicine in treating acute exacerbation of chronic obstructive pulmonary disease. Shanghai Journal of Traditional Chinese Medicine. 2009;43(7):28–30. [Google Scholar]
- 23.Shi Z-L, Fang K, Li G-H, Mao M-J, Li Z-H. Effect of catharsis method on breathing mechanics and airway inflammatory factors of patients with respiratory failure induced by chronic obstructive pulmonary disease. China Journal of Traditional Chinese Medicine and Pharmacy. 2010;25(10):1710–1713. [Google Scholar]
- 24.Zhang XW. Clinical analysis of 62 cases COPD treated with traditional Chinese and western medicine. Hebei Medicine. 2011;17(10):1362–1363. [Google Scholar]
- 25.Zhou W, Zhong YQ, Yang HM, et al. Traditional chinese medicine in the treatment of chronic obstructive pulmonary disease in stable stage: a systematic review of randomized controlled trials. Chinese Journal of Evidence-Based Medicine. 2009;9(3):311–318. [Google Scholar]
- 26.An XD, Zhang AL, Yang AW, et al. Oral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic review. Respiratory Medicine. 2011;105:165–176. doi: 10.1016/j.rmed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Mao B, Wang G, et al. Effect of Tanreqing injection on treatment of acute exacerbation of chronic obstructive pulmonary disease with Chinese medicine syndrome of retention of phlegm and heat in Fei. Chinese Journal of Integrative Medicine. 2010;16(2):131–137. doi: 10.1007/s11655-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 28.Quan ZX, Zhong XG, Peng G, et al. Effects of catharsis large intestine on T lymphocyte subsets in peripheral blood from rats with COPD. China Journal of Traditional Chinese Medicine and Pharmacy. 2012 ;27(4):992–994. [Google Scholar]
- 29.Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials. 1998;19(2):159–166. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]


