Abstract
Background
This study aimed to evaluate the adverse effects of various doses of nicotine and protective effects of different concentrations of γ-tocotrienol (γ-TCT) on in vitro embryonic development and lipid peroxidation in mice.
Material/Methods
A) Effects of various doses of nicotine on in vitro embryonic development: Female mice were treated with 1.0, 3.0, or 5.0 mg/kg/day nicotine for 7 consecutive days. Animals were superovulated, cohabited overnight, and sacrificed. Embryos were cultured in vitro. Plasma was assayed. B) Effects of concomitant treatment of nicotine concurrently with various doses of γ-TCT on in vitro embryonic development: Female mice were treated with nicotine (5.0 mg/kg/day), gavaged γ-TCT of 30, 60, or 90 mg/kg/day or nicotine concurrently with γ-TCT of 3 different doses for 7 consecutive days. Animals were superovulated, cohabited overnight, and sacrificed. Embryos were cultured and plasma was assayed.
Results
A) Effects of various doses of nicotine on in vitro embryonic development: Number of hatched blastocysts decreased in 1.0 and 3.0 mg/kg/day nicotine groups. Nicotine at 5.0 mg/kg/day stopped embryo development at morula. MDA concentrations increased following all nicotine doses. B) Effects of concomitant treatment of nicotine concurrently with various doses of γ-TCT on in vitro embryonic development: Embryo development was completed in all groups. MDA concentration increased only in the group treated with nicotine concurrently with 30 mg/kg/day γ-TCT.
Conclusions
Nicotine impairs in vitro embryo development and increases MDA in plasma. The deleterious impact of nicotine on embryo development is reversed by supplementing γ-TCT concurrently with nicotine.
Keywords: preimplantation embryonic development, nicotine, oxidative stress, γ-tocotrienol, malondialdehyde
Background
The effects of oxidative stress induced by nicotine have been reported to cause damage to the membrane structure even at the chromosomal level [1] and ultrastructural level [2], which subsequently alter multiple physiological processes such as oocyte maturation [3], fertilization and preimplantation embryonic development [4–6], implantation [7], as well as pregnancy and fetal outcome [5]. Oxidative stress occurs as a result of an interrupted balance between reactive oxygen species (ROS) such as O2, H2O2 or OH–, and endogenous antioxidants such as glutathione peroxidase (GP), superoxide dismutase (SOD) or catalase [8]. In health, these 2 systems usually remain in balance. Another type of antioxidant is exogenous antioxidants or non-enzymatic antioxidants such as vitamins A, C and vitamin E derivatives, including tocopherols and tocotrienols, which are only available in the form of dietary supplements.
Our previous study showed that nicotine-induced oxidative stress on pregnant mice is possibly mediated through lipid peroxidation [4]. In female reproduction, lipid peroxidation is associated with impaired mitochondrial functions and inhibition of antioxidant enzymes [9], which subsequently results in impaired oocyte ultrastucture [2], oocyte maturation failure [3], altered tubal embryo transport to the uterus for implantation [7], arrested preimplantation embryonic development [4], and extended length of gestation with altered pregnancy outcome [5].
Recently, γ-TCT [10], a vitamin E derivative, has come to be regarded as a potent lipid antioxidant [11]. The additional 3 double bonds in its side chain allow tocotrienols to be more mobile in cellular membranes, which gives them specific biological and therapeutic properties. In an oocyte ultrastructural study conducted by our group, γ-TCT was found to repair nicotine-induced ultrastructural damage of the preovulatory oocyte by retaining its shape and smooth boundary of zona pellucida with tight perivitelline space [2]. In another study, γ-TCT supplementation was found to increase the number of retrieved 2- and 4-cell stage embryos with improved pregnancy rate and pregnancy outcome in rats [5]. Antioxidative effects of γ-TCT have also been documented in a hypercholesterolemic study [12] and in human diseases [13].
The effective dosage and duration of nicotine treatment in altered in vitro embryo development, implantation of blastocysts and pregnancy outcome [4–6], as well as the optimal beneficial dosage of tocotrienol in reversing the deleterious impact of nicotine on reproductive processes [5,6], are not completely understood. Therefore, our study was carefully designed to optimise the dosage and duration of nicotine treatment in altered embryo development and its reversal by tocotrienol. The selective doses of nicotine used were 1.0, 3.0, or 5.0 mg/kg/day for 7 consecutive days to evaluate in vitro development of embryos and the level of lipid peroxidation. To optimize the beneficial effect, doses of 30, 60, or 90 mg/kg/day of γ-tocotrienol were evaluated.
Material and Methods
A) Effects of various doses of nicotine on in vitro embryonic development
Thirty-two 4–6-week-old (25–30 g) female mice (Mus musculus) were equally divided into groups and injected subcutaneously (s.c.) with either 0.9% saline or nicotine at a dose of 1.0, 3.0, or 5.0 mg/kg/day for 7 consecutive days. Twenty-four hours later, mice were superovulated by injecting (s.c.) PMSG (5 IU) and 48 hours later by intraperitoneal injection of hCG (5 IU). Animals were cohabitated overnight with fertile male mice at a 1: 1 ratio and sacrificed at 48 hours post-coitum.
Sample collection
Blood samples were collected via cardiac puncture, centrifuged (2500 rpm, 4°C for 15 minutes) and plasma samples were frozen at –70°C until analyzed. Fallopian tubes were excised and embryos were flushed under a dissecting microscope (Leica Zoom 2000, Japan), categorized, and counted.
Embryo culture
Normal and abnormal embryos were categorized according to the criteria set by Ertzeid and Storeng [14]. Only good quality embryos were cultured. The embryos were cultured in a 35-mm culture dish filled with 100 μl droplets of Whitten’s medium (Sigma Chemical Co., USA) overlaid with mineral oil (Sigma, USA). Cultures were maintained in a humidified atmosphere containing 5% CO2 and 95% air at 37°C for 5 days. Assessment of embryonic development was made under the reverse phase (Leica IRB, Japan) and inverted microscope (Olympus 1X81 SF-3, Japan).
Determination of MDA in plasma
Plasma samples were processed accordingly for evaluation of MDA levels using the thiobarbituric acid reactive substances (TBARS) method [15]. The absorbance was measured photometrically at 532 nm and the concentrations were expressed as nanomoles MDA per gram protein (nmol/g).
Statistical analysis
Data were analyzed using the SPSS package program (SPSS 17.0, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to test the normality of data distribution. Statistical methods included paired and unpaired 2-tailed Student’s t-test with normal distribution. All continuous variables are expressed as mean ±SEM. A p value of <0.05 or <0.001 was considered statistically significant.
B) Effects of concomitant treatment of nicotine concurrently with various doses of γ-TCT on in vitro embryonic development
Sixty-four 4–6-week-old (25–30 g) female mice were housed in polyurethane cages at 27°C with a 12-hour light-dark cycle. Animals were given food pellets and water ad libitum and divided into 8 groups of 8 animals each. Animals in groups 1 (G1) and 2 (G2) received daily subcutaneous injection of 0.9% saline or nicotine (5.0 mg/kg/day) for 7 consecutive days. Animals in groups 3, 4, and 5 were gavaged with 30 (G3), 60 (G4) or 90 (G5) mg/kg/day γ-TCT (Golden Hope) for 7 consecutive days. Animals in groups 6, 7, and 8 received concomitant treatment of nicotine (5.0 mg/kg/day) and 30 (G6), 60 (G7), or 90 (G8) mg/kg/day γ-TCT for 7 consecutive days. The following day, animals were superovulated using PMSG followed by hCG [Intervet, Holland], mated with fertile males, and sacrificed at 48 hours post-coitum.
Sample collection, embryo culture, determination of MDA and statistical analysis were done as described previously.
Results
A) Effects of various doses of nicotine on in vitro embryonic development
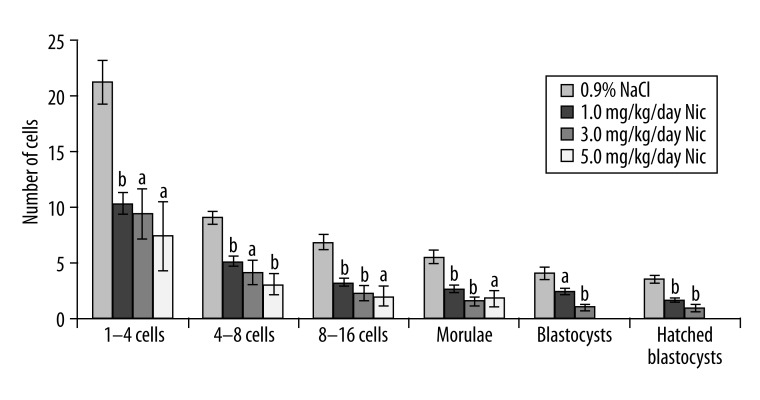
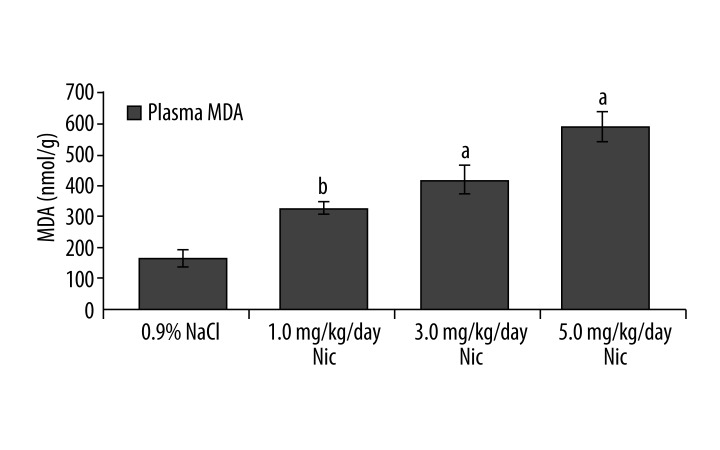
Figure 1 shows the number of preimplantation embryos developed in mice following nicotine treatment at a dose of 1.0, 3.0, or 5.0 mg/kg/day for 7 consecutive days. The number of hatched blastocysts were decreased in the 1.0 mg/kg/day (1.63±0.18, p<0.001) and 3.0 mg/kg/day nicotine-treated groups (0.88±0.3, p<0.001), respectively, compared to their respective controls (3.5±0.34), while embryos in the 5.0 mg/kg/day nicotine-treated group stopped developing at the morula stage (1.75±0.77, p<0.05). Nicotine at 5.0 mg/kg/day for 7 consecutive days was therefore considered to be the most effective dose to attenuate development of the preimplantation embryos.
Figure 1.
Number of preimplantation embryos developed following different doses of nicotine in mice. a – p<0.05, b – p<0.001.
B) Effects of concomitant treatment of nicotine concurrently with various doses of γ-TCT on in vitro embryonic development
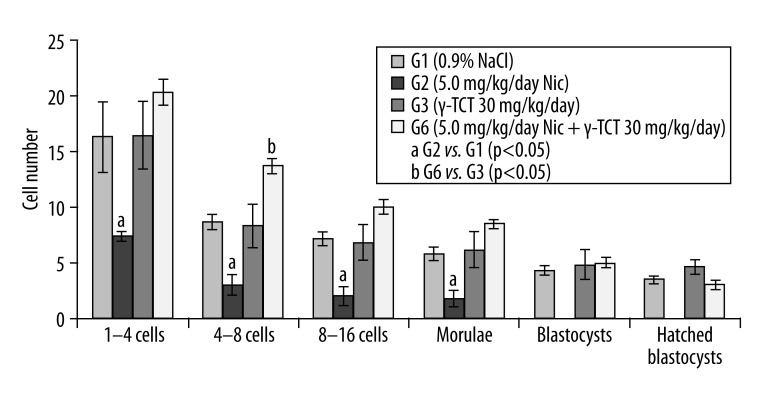
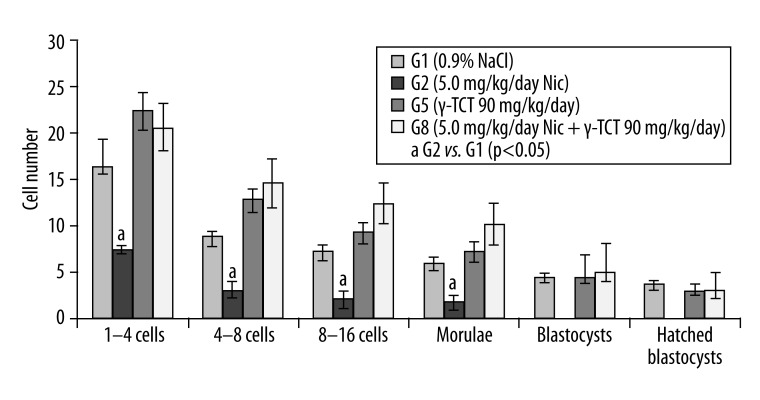
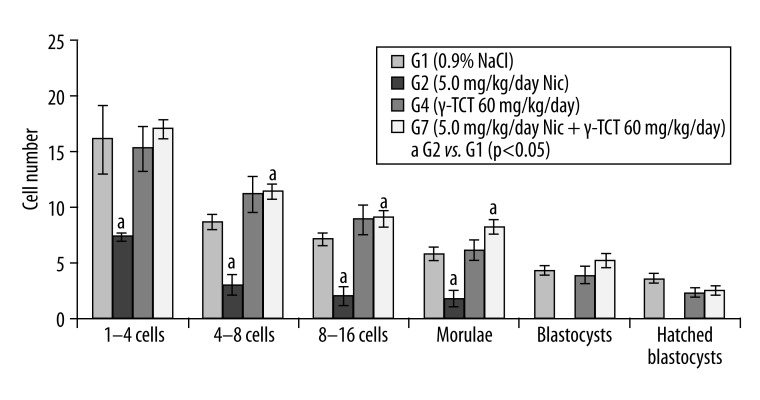
Figures 2–4 show the number of embryos developed in animals treated with nicotine (5.0 mg/kg/day) and a concurrent supplementation of γ-TCT at 30 (Figure 2), 60 (Figure 3), and 90 (Figure 4) mg/kg/day for 7 consecutive days. In all the 3 combined treatment groups, development of the preimplantation embryos was completed at hatched blastocysts, unlike the only nicotine-treated embryos where the embryo development was found to be completely ceased at the morula stage. These results indicate that γ-TCT at all 3 trial doses could effectively reverse the deleterious impact of nicotine on in vitro development of embryos.
Figure 2.
Preimplantation embryo development in G1, G2, G3 and G6. a, b – p<0.05.
Figure 4.
Preimplantation embryo development in G1, G2, G5 and G8. a – p<0.05.
Figure 3.
Preimplantation embryo development in G1, G2, G4 and G7. a – p<0.05.
Figure 2 shows embryo development between G1 and G2. In G1 (control), the number of 1–4 cells retrieved embryos was 16.33±0.4, whereas in G2 (nicotine of 5.0 mg/kg/day) the number of 1–4 cells embryos was significantly lower (7.38±3.1, p<0.05). This decreasing trend in the number of embryos induced by nicotine as compared to its control also applied to 4–8 cells (3.0±0.7 [treated] vs. 8.67±0.9 [control] p<0.05), 8–16 cells (2.0±0.6 [treated] vs. 7.17±0.9 [control] p<0.05), and at the morula stage (1.75±0.6 [treated] vs. 5.83±0.8 [control] p<0.05). No blastocysts were found in G2 on day 5 of in vitro culture. Conversely, supplementation of γ-TCT (30 mg/kg/day) along with nicotine, allowed morulae to turn into blastocysts (5.0±0.4 [treated] vs. 4.83±1.4 [control]), and the number of hatched blastocysts was found to be 3.0±0.4 compared to controls (4.67±0.7). A significant difference was also found in the number of 4–8 cell embryos in this γ-TCT supplemented group (13.67±0.7 [treated] vs. 8.33±1.9 [control]).
Figure 3 shows results of G2 and G7. In G2 (nicotine of 5.0 mg/kg/day), the number of 1–4 cell retrieved embryos, 4–8 cells, 8–16 cells, and morulae count were significantly lower than their respective controls. Moreover, preimplantation embryo development was ceased at the morula stage. No blastocysts were found in G2. On the other hand, in G7 (nicotine + 60 mg/kg/day γ-TCT), the number of morulae that turned into blastocysts was 5.25±0.6 (3.83±0.8 control) and the number of hatched blastocysts was 2.5±0.3 (2.33±0.4 control), respectively.
Figure 4 shows results of G2 and G8. In G2, the number of 1–4 cell retrieved embryos, 4–8 cells, 8–16 cells, and morulae were significantly lower than their respective controls. Preimplantation embryo development ceased at the morula stage. However, in G8 (nicotine + 90 mg/kg/day g-TCT), the number of morulae that turned into blastocysts was 6.1±2.1 (4.83±0.9 control) and the number of hatched blastocysts was 3.5±1.5 (3.0±0.7 control).Various doses of nicotine and plasma levels of MDA in mice.
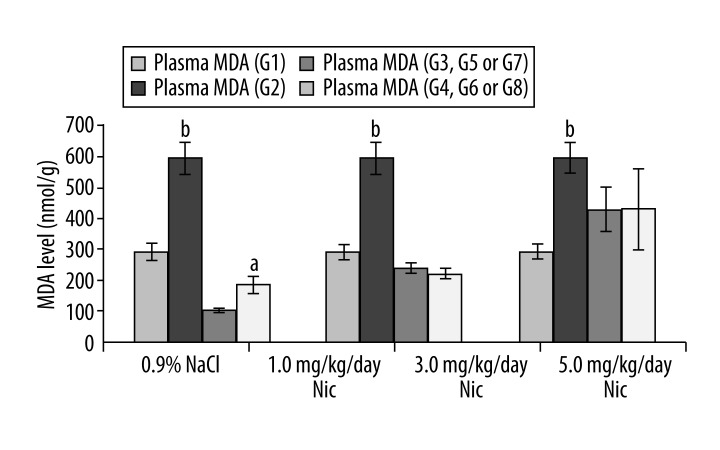
Figure 5 shows plasma levels of MDA following different doses of nicotine treatment. Plasma MDA concentrations were found to be significantly higher in all 3 nicotine-treated groups: 1.0 mg/kg/day (326.4±20.7 nmol/g, p<0.001), 3.0 mg/kg/day (416.9±46.0 nmol/g, p<0.05), and 5.0 mg/kg/day (589.5±49.9 nmol/g, p<0.05), respectively, compared to control (163.5±28.8 nmol/g). Plasma levels of MDA in the nicotine plus γ-TCT supplemented mice.Plasma MDA concentration was significantly higher in animals of G2 (589.5±49.9 nmol/g) compared to control animals of G1 (294.3±23.1 nmol/g, p<0.001). A significant increase in plasma MDA was also recorded in animals of G4 (188.3±27.9 nmol/g) compared to its control G3 (106.1±6.9 nmol/g, p<0.05), suggesting that concomitant administration of γ-TCT at a dose of 30 mg/kg/day concurrently with nicotine (5.0 mg/kg/day) was not enough to neutralize levels of prooxidants in plasma induced by nicotine. However, reduction of plasma MDA concentration almost back to its control level (G5) by γ-TCT of 60 mg/kg/day (G6) suggests that γ-TCT of 60 mg/kg/day could be an appropriate dosage in neutralizing nicotine-induced lipid peroxidation (MDA level). An increased dose of γ-TCT at 90 mg/kg/day (G8) concurrently with nicotine showed almost identical results as recorded between G6 and G5.
Figure 5.
Plasma MDA levels following different doses of nicotine. a – p<0.05, b – p<0.001.
Discussion
Nicotine has been selected as the toxicant for evaluation as it is known from numerous epidemiological studies that nicotine from tobacco smoking may exert a variety of adverse impact on reproductive outcomes [16,17]. It seems that no systematic study has yet assessed the dose-related beneficial effects of γ-TCT over the nicotine-induced cessation of preimplantation embryonic development in mice.
In the present study, 1410 embryos were retrieved, of which 69.1% were found to be normal according to the criteria set by Ertzeid & Storeng [14]. Following nicotine of 5.0 mg/kg/day for 7 days, the percentage of retrieved normal embryo was significantly reduced to 39.5% compared to control (70.6%). However, compared to nicotine-treated retrieved normal embryos (39.5%), a significantly higher percentage (73.8%) of retrieved normal embryos was evident following concomitant supplementation of γ-TCT concurrently with nicotine. This higher percentage of normal retrieved embryos as a result of concurrent supplementation of γ-TCT with nicotine agrees to some extent with other studies [5,18].
Lockman et al. [19] detected almost identical levels of plasma nicotine between heavy smokers and rats treated with nicotine at a dose of 4.5 mg/kg/day. Moreover, nicotine at 5.0 mg/kg/day for 4 consecutive days reduced the number of blastocysts in rats [20]. Effects of various doses of nicotine over a period of 7 days show that the number of hatched blastocysts decreased in both 1.0 mg/kg/day (1.63±0.18, p<0.001) and 3.0 mg/kg/day nicotine-treated groups (0.88±0.3, p<0.001) compared to their respective control (3.5±0.34). But in the 5.0 mg/kg/day nicotine-treated animals, embryos stopped developing at the morula stage (1.75±0.77 vs. 3.5±0.34, p<0.05). Significant differences in the developmental stages of 1–4, 4–8, 8–16 cells, morulae, blastocysts, and hatched blastocysts were also evident in the nicotine-treated embryos (Figure 1). Our results of delayed embryonic cleavage confirmed the findings of other researchers [4–6]. A previous study reported that embryos in rats developed only up to the 4-cell stage when exposed to nicotine for 30 consecutive days [5]. Nicotine exposure for a similar period in mice is found to prevent blastocyst formation on day 4 in in vitro culture [6]. Delayed embryo cleavage as a result of nicotine exposure is considered in inducing complications in pregnancy because it may lead to a delayed blastocyst implantation as a result of delayed loss of zona pellucida, the growth of the inner cell mass [21], or prolonged gestation [5]. Nicotine has been found to be carried within the embryo during preimplantation embryonic development [22] and possibly results in delayed blastocyst implantation and prolonged gestation.
Apart from cessation of in vitro embryo development, nicotine at 1.0, 3.0, or 5.0 mg/kg/day was also found to elevate plasma MDA, an indicator of oxidative stress (OS), confirmed by others [5,6,22]. Therefore, nicotine-induced impaired in vitro embryo development could possibly be the result of free radical-induced OS. Embryos grew to blastocysts and hatched following concomitant supplementation of γ-TCT, a proven antioxidant [5,23,24], concurrently with nicotine.
Moreover, we are possibly the first to report that 60 mg/kg/day γ-TCT,(the optimum concentration) can effectively reverse nicotine-induced altered in vitro embryo development by suppressing the level of OS.
Conclusions
A dose of 5.0 mg/kg/day nicotine for 7 consecutive days stops in vitro preimplantation embryo development. However, concomitant treatment of γ-TCT at an optimal dose of 60 mg/kg/day concurrently with nicotine consistently maintains in vitro embryo development, as evident in controls.
Figure 6.
Plasma MDA levels following concomitant administration of nicotine concurrently with various doses of γ-TCT. a – p<0.05, b – p<0.001.
Footnotes
Source of support: Funding for this study was made possible by the Fundamental Research Grant Scheme (FRGS) No. 600-IRDC/ST/FRGS.5/3/1343 to Professor Dr Mohd Hamim Bin Rajikin
References
- 1.Wang X, Falcone T, Attaran M, et al. Vitamin C and Vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocysts development rate. Fert Steril. 2002;78:1271–77. doi: 10.1016/s0015-0282(02)04236-x. [DOI] [PubMed] [Google Scholar]
- 2.Rajikin MH, Latif ES, Mar MR, et al. Deleterious effects of nicotine on the ultrastructure of oocytes: role of gamma tocotrienol. Med Sci Monit. 2009;15(12):BR378–83. [PubMed] [Google Scholar]
- 3.Tarin JJ. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum R Prod. 1996;2:717–24. doi: 10.1093/molehr/2.10.717. [DOI] [PubMed] [Google Scholar]
- 4.Kamsani YS, Rajikin MH, Chatterjee A, et al. Impairment of in vitro embryonic development with a corresponding elevation of oxidative stress following nicotine treatment in mice: effect of variation in treatment duration. Biomed Res. 2010;21(4):359–64. [Google Scholar]
- 5.Mokhtar N, Rajikin MH, Zakaria Z. Role of tocotrienol-rich palm vitamin E on pregnancy and preimplantation embryos in nicotine treated rats. Biomed Res. 2008;19:181–84. [Google Scholar]
- 6.Rozzana MS, Zaiton Z, Rajikin MH, et al. Supplementation with alpha lipoic acid improves the in vitro development of embryos in nicotine-treated mice. Biomed Res. 2005;16:28–32. [Google Scholar]
- 7.Talbot P, Riveles K. Smoking and reproduction: the oviduct as a target of cigarette smoke. Reprod Biol Endocrinol. 2005;3:1–17. doi: 10.1186/1477-7827-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocr. 2005;3:1–21. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashakumary L, Vijayammal PL. Effect of nicotine on antioxidant defense mechanisms in rats fed with a high-fat diet. Pharmacol. 1996;52:153–58. doi: 10.1159/000139379. [DOI] [PubMed] [Google Scholar]
- 10.Raederstorff D, Elste V, Aebischer C, et al. Effect of either gamma-tocotrienol or a tocotrienol mixture on the plasma lipid profile in hamsters. Ann Nutr Metab. 2002;46:17–23. doi: 10.1159/000046748. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Sundaram C, Prasad S, et al. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem Pharma. 2010;80:1613–31. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qureshi AA, Bradlow BA, Brace L, et al. Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids. 1995;30:1171–77. doi: 10.1007/BF02536620. [DOI] [PubMed] [Google Scholar]
- 13.Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ertzeid G, Storeng R. Adverse effects of gonadotrophin treatment on pre- and postimplantation development in mice. J Reprod Fert. 1992;96:649–55. doi: 10.1530/jrf.0.0960649. [DOI] [PubMed] [Google Scholar]
- 15.Lefevre G, Leymarie B, Beyerle M, et al. Evaluation of lipid peroxidation by measuring thiobarbituric acid reactive substances. Ann Biol Clin. 1998;56:305–19. [PubMed] [Google Scholar]
- 16.Freour T, Masson D, Mirallie S, et al. Active smoking compromises IVF outcome and affects ovarian reserve. Reprod Biomed. 2008;16:96–102. doi: 10.1016/s1472-6483(10)60561-5. [DOI] [PubMed] [Google Scholar]
- 17.Berthiller J, Sasco AJ. Smoking (active or passive) in relation to fertility. J Gynecol Obstet Biol Reprod. 2005;34(1):47–54. [PubMed] [Google Scholar]
- 18.Joesbury KA, Edirisinghe WR, Phillips MR, et al. Evidence that male smoking affects the likelihood of a pregnancy following IVF treatment: application of the modified cumulative embryo score. Hum Reprod. 1998;13:1506–13. doi: 10.1093/humrep/13.6.1506. [DOI] [PubMed] [Google Scholar]
- 19.Lockman PR, Van der Schyf CJ, Abbruscato TJ, et al. Chronic nicotine exposure alters blood-brain barrier permeability and diminishes brain uptake of methyllycaconitine. J Neurochem. 2005;94:37–44. doi: 10.1111/j.1471-4159.2005.03162.x. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JA, Hammer RE. Effects of nicotine on oviductal blood flow and embryo development in the rat. J Reprod Fertil. 1985;74:71–76. doi: 10.1530/jrf.0.0740071. [DOI] [PubMed] [Google Scholar]
- 21.Card JP, Mitchell JA. The effects of nicotine on implantation in the rat. Biol Reprod. 1979;20:532–39. doi: 10.1095/biolreprod20.3.532. [DOI] [PubMed] [Google Scholar]
- 22.Sieber SM, Fabro S. Identification of drugs in the preimplantation blastocyst and in the plasma, uterine secretion and and urine of the pregnant rabbit. J Pharmacol Exp Ther. 1971;176(1):65–75. [PubMed] [Google Scholar]
- 23.Raederstorff D, Elste V, Aebischer C, et al. Effect of either gamma-tocotrienol or a tocotrienol mixture on the plasma lipid profile in hamsters. Ann Nutr Metab. 2002;46:17–23. doi: 10.1159/000046748. [DOI] [PubMed] [Google Scholar]
- 24.Hermizi H, Faizah O, Nirwana SI, et al. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in Sprague-Dawley male rats after nicotine cessation. Calcif Tissue Intl. 2009;84:65–74. doi: 10.1007/s00223-008-9190-x. [DOI] [PubMed] [Google Scholar]