Abstract
Purpose.
To study and correct for the limiting effect of iris mechanics on the amplitude of light-evoked pupil contractions in order to derive a more clinically accurate assessment of afferent input to the visual system.
Methods.
Transient pupil responses were recorded to a series of 1-second red Ganzfeld light stimuli with a stepwise increase in stimulus intensity using a binocular infrared computerized pupillometer. One eye of eight healthy subjects was treated with 0.2% brimonidine tartrate ophthalmic solution to induce pupil size reduction. The amount of pupil contraction as a function of stimulus intensity was compared between the brimonidine-treated, miotic eye and the untreated eye.
Results.
Brimonidine treatment produced significant reduction in pupil size in healthy subjects (mean reduction in pupil size: 1.78 ± 0.35 mm, P < 0.05). For increasing light intensity, the treated pupil started to show reduced pupil contractions compared with the contralateral untreated pupil when the peak of pupil contraction reached an average pupil size of 3.25 ± 0.61 mm (range, 2.38–4.44 mm). When measured by percent pupil contraction (contraction amplitude/baseline pupil diameter), the pupil response as a function of stimulus intensity in the treated, miotic eye did not differ from that in the untreated eye.
Conclusions.
Iris mechanics limits the amount of pupil contraction and can act to reduce the assessed neuronal integration of the pupil light reflex. Pupil response assessed by using percent contraction amplitude is least affected by mechanical effects and provides a more accurate approximation of afferent input.
Keywords: iris mechanics, pupil contraction, brimonidine
Iris mechanics start to limit pupil contraction at an average pupil size of 3.3 mm in diameter. Pupil response assessed by using percent contraction amplitude is least affected by iris mechanics and might be a more reasonable approximation of afferent input.
Introduction
The pupillary light reflex (PLR) has a long history of being employed as a useful clinical test for evaluation of visual function.1–11 In addition, there has been increasing evidence suggesting the role of the PLR as a means to evaluate cognitive function,12–16 autonomic neuropathy,17–20 and behavior of melanopsin retinal ganglion cells.21–25 Although pupil-based testing has advantages of being objective and easy to perform by patients, interpretation of pupil contractions recorded can be confounded by the limiting effects of iris mechanics on how much the pupil can contract. Understanding how iris mechanics affect PLR is thus essential to the interpretation of pupil recording and estimation of afferent input. The mechanical effect becomes increasingly more important when measuring pupil response in patients who have smaller pupils, such as in elderly patients or those using narcotic-derived medications for pain control.26,27
Several studies conducted decades ago28–30 investigated the impact of mechanical properties of the iris to PLR. It was suggested that the movement of the pupil is not linear when pupil size exceeded a certain range, and the mechanical properties of the iris muscles contributed to this nonlinearity. In the present study, we used a novel approach to quantify the mechanical limitations of the iris on pupil contraction, by using a sympatholytic agent, brimonidine, to induce anisocoria.31–33 By producing anisocoria and simultaneously recording both pupil responses to light, the main factor responsible for any reduction in contraction from the more miotic eye is due to iris mechanics, because the neuronal input to each pupil is matched for a given light stimulus.
Methods
Subjects
Eight healthy, young subjects (5 males and 3 females) ranging in age from 29 to 35 years (mean ± SD, 32.0 ± 4.1) were tested. The exclusion criteria included history of ocular disease, eye trauma, or ocular surgery; presence of systemic diseases with known ocular involvement; or current use of ophthalmic solution or systemic medications that can affect the PLR. An eye examination, including visual acuity, slit lamp, and undilated fundus exam, was performed to rule out any ocular abnormalities. The procedures conformed to the tenets of the Declaration of Helsinki. The study was approved by the University of Iowa Institutional Review Board. Written consent form was obtained after the details of the test were explained.
A 0.2% brimonidine tartrate ophthalmic solution was placed in the right eye in three subjects and in the left eye in five subjects. All subjects tolerated brimonidine treatment with few side effects and post-brimonidine pupil recording commenced after waiting for at least 30 minutes after treatment.
Apparatus
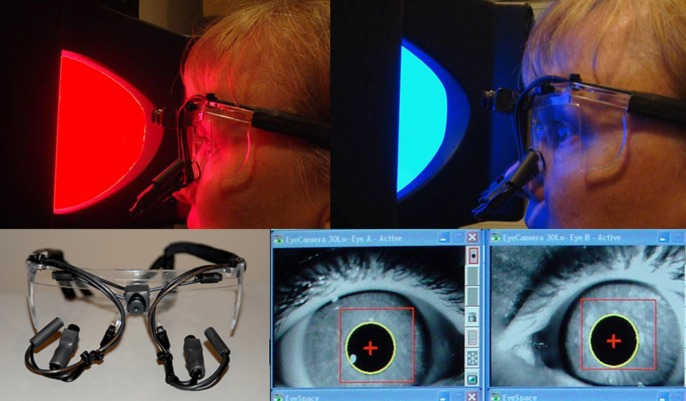
The details of the apparatus set up are described elsewhere.34 Briefly, a dual-channel binocular eye frame pupillometer (Arrington Research, Scottsdale, AZ) was used to record pupil response (Fig. 1). The maximal horizontal pupil diameter was recorded in real time. The pupil of the brimonidine-treated, miotic eye was stimulated; the fellow eye was occluded by an eye patch made of a near infrared passing 780-nm filter. The pupil responses from both eyes were recorded simultaneously. The 780-nm filter blocks the visual stimulus of lesser wavelengths of light to enter retina in the occluded eye; however, it still enables recording of pupil responses by the infrared camera. Pupil recording began 5 seconds before the first stimulus and continued throughout the stimulus paradigm, including 30 seconds of the poststimulus dark period.
Figure 1. .
Ganzfeld bowl stimulus and pupil recording apparatus used in this study. Top left: A red stimulus light is presented to the subject who is seated at a fixed distance from the bowl. Top right: The eye frame with miniature video cameras and infrared light illuminators are shown. Bottom: The infrared video image of pupils being recorded is shown. Reprinted with permission from Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptormediated pupil light reflex. Ophthalmology. 2009;116:1564–1573.34 Copyright Elsevier 2009.
Stimulus
Details of the stimulus set up are described elsewhere.35 Briefly, a diffuse, wide-field stimulus was produced using a light-emitting diode Color Dome Ganzfeld electroretinogram apparatus (Diagnosys, Lowell, MA) (Fig. 1). At a distance of 75 mm from the front of the eye to the opening of the bowl, the horizontal radius of the viewing angle was 45°. Light stimuli with a spectral band of 640 ± 10 nm (red light) were chosen to elicit PLR. Before the pupil recording was started, subjects were dark adapted for 10 minutes. A trial consisted of a series of 1-second duration red stimuli, each followed by a period of darkness. The stimulus intensity ranged from −4.0 to 2.6 log cd/m2 (0.0001–450 cd/m2), with stepwise increment of 0.5 log cd/m2.
Analysis of Pupil Records
Analysis of the pupil recording was performed by a custom-designed software program using IgorPro 6.1 (WaveMetrics, Inc., Lake Oswego, OR) and Excel (Microsoft Corp., Redmond, WA). Baseline pupil diameter for each eye was calculated as the average pupil diameter over a 1-second period prior to stimulus onset. Pupil contraction amplitude (in millimeters) was the difference of pupil size at its peak contraction from baseline. Percent pupil contraction (%) was calculated as the ratio of the pupil contraction amplitude (in millimeters) to baseline pupil diameter:
 |
Tracings showing pupil diameter were displayed against time to visualize the dynamics of the pupil response, and pupil contraction in both millimeters and percentage was plotted as a function of log stimulus intensity.
Statistics
A paired t-test was used to assess for significant pupil size reduction due to brimonidine-induced miosis. Paired t-tests were used to assess for differences in pupil contraction in millimeters and in percentage between the treated and untreated eyes at each stimulus intensity level. P values were Holm adjusted due to the multiple tests of the outcome at 14 different stimulus intensities. Holm-adjusted P values less than 0.05 were considered statistically significant.
Results
Brimonidine treatment resulted in absolute pupil size reduction of 1.47 to 2.60 mm (mean ± SD, 1.77 ± 0.35 mm), and percentage pupil size reduction of 22% to 35% (mean ± SD, 29% ± 6%) across all subjects (P < 0.05).
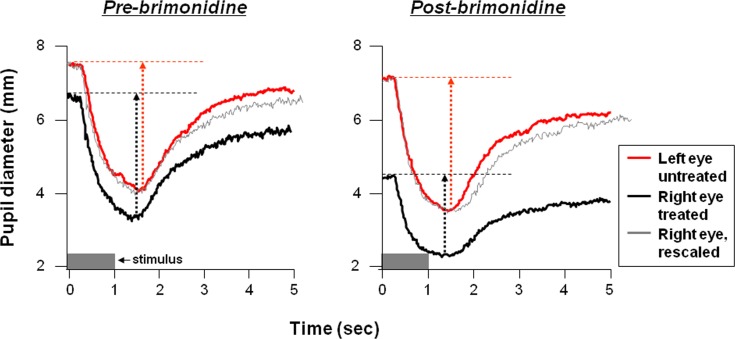
Figure 2 shows an example of a pupil tracing to one stimulus intensity in one subject before and after brimonidine treatment. The right eye was treated and marked black, the left eye was untreated and marked red. Before brimonidine treatment (left, pre-brimonidine), a small anisocoria was present between the two eyes in this subject (separation of the red and black tracings of approximately 0.50 mm along the y-axis). The pupil contraction was similar between the two eyes (length of red and black dotted arrows approximately equal); better demonstrated after the right eye recording (black) is shifted vertically (gray). After brimonidine treatment (right, postbrimonidine), the pupil constricted from 7.31 mm to 3.50 mm (contraction amplitude 3.61 mm) in the untreated left eye (red), whereas the pupil constricted from 4.42 mm to 2.31 mm (contraction amplitude 2.11 mm) in the treated right eye (black); the waveform shape of the pupil light reflex was not significantly altered by brimonidine, as evidenced by rescaling the right pupil tracing to equal the same contraction amplitude as the left pupil (superimposed gray tracing).
Figure 2. .
Examples of pupil recordings (pupil diameter in millimeters against time in seconds) before (left, pre-brimonidine) and after (right, postbrimonidine) treatment in one subject. The stimulus is marked by the gray bar at the lower left of each plot; the stimulus was a flash of red light of 1-second duration with intensity of 2.0 log cd/m2. The contraction amplitude, defined as the maximum amount of pupil contraction from its baseline, is marked by dotted arrows. For purpose of comparing characteristics of the pupil constriction curve (e.g., latencies, time for peak constriction) between the left and right eye, the pupil tracing of the right eye is aligned and superimposed (gray) to the left eye (red). In pre-brimonidine condition (left), the alignment is achieved by vertically shifting the right eye tracing. In the postbrimonidine condition, the alignment is achieved by scaling the right eye trace to equal the contraction amplitude of the right eye to the left eye and aligning the traces horizontally before the start of the constriction to show that the shape of the pupil waveform has not been significantly altered by brimonidine in spite of the reduction in pupil size and contraction amplitude.
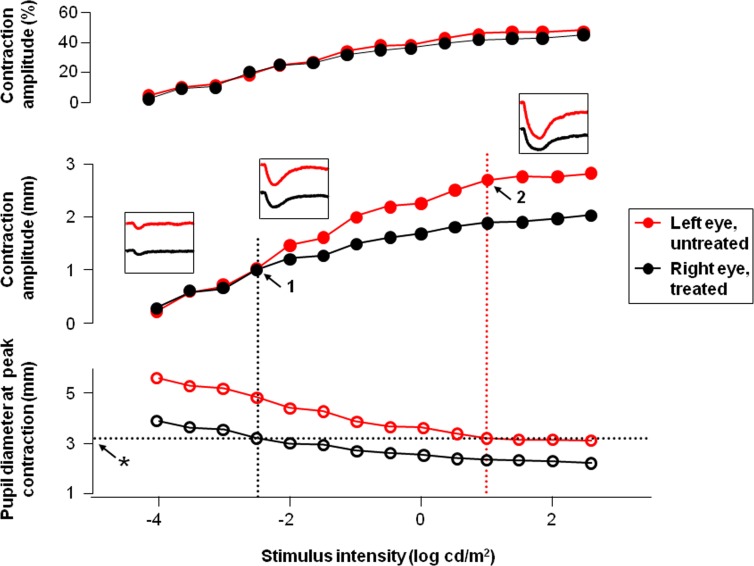
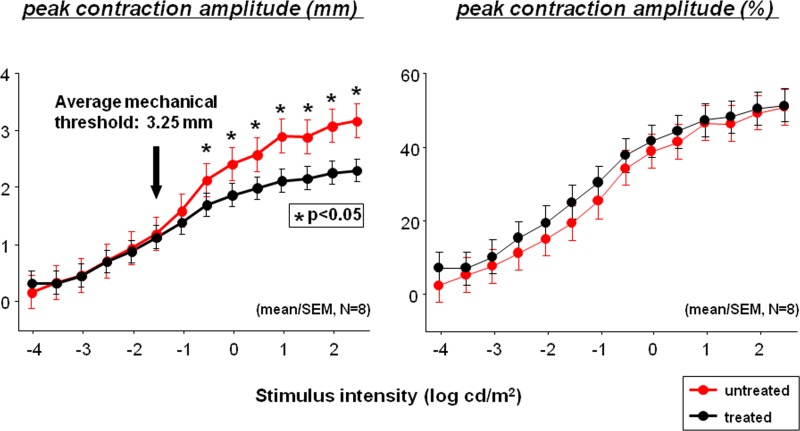
When pupil contraction (in mm) was plotted against stimulus intensity (Fig. 3, middle), the contraction amplitude was similar (left inset) in the treated (black filled circle) and untreated eye (red filled circle) at lower stimulus intensities (−4.0 to −2.5 log cd/m2). At medium stimulus intensities, the pupil contraction amplitude in the treated eye started to become less compared to the untreated eye (the middle inset). The difference in contraction amplitude became most noticeable at high stimulus intensities (right inset). When contraction amplitude is replotted in percentage (top), the pupil response intensity curve is similar for the treated right versus untreated left eye.
Figure 3. .
Plots of contraction amplitude in percentage (top), contraction amplitude in millimeters (middle), and pupil size at the peak of contraction in millimeters (bottom) as a function of stimulus intensity are shown for the treated right eye (black) and untreated left eye (red) for the same subject as in Figure 2. The insets show pupil recordings at low (left), medium (middle), and high (right) stimulus intensities. The vertical dotted black line (1 at arrow) represents the log stimulus intensity where the contraction amplitude of the treated pupil (right eye, black plot line) starts to become less than the untreated pupil (left eye, red plot line). The intersection of the vertical dotted black line with the plot of the pupil diameter at its peak of contraction represents the size of the treated pupil (3.2 mm) where iris mechanics start to limit pupil contraction, represented by the horizontal dotted black line (asterisk at arrow). The vertical dotted red line intersects the untreated pupil diameter where the mechanical limit was reached in the untreated left eye. The contraction amplitude at which this occurred is shown by the arrow at “2.”
The pupil size at which iris mechanics started to limit pupil contraction was derived by identifying the pupil size at the peak of contraction (Fig. 3, bottom,) at which the pupil contraction amplitude in the treated eye started to fall below that of the untreated eye (marked by “1” on the middle plot of Fig. 3; 3.20 mm in this subject as marked by asterisk). One would expect that the mechanical property of the iris remains to be the same between the two eyes for a particular subject. The log intensity at which the same mechanical limit (3.20 mm) was reached in the untreated eye was then identified (intersection of the red vertical, dotted line with the black horizontal dotted line in the lower plot and marked in the middle plot by a “2”). The mechanical limit in the untreated eye was reached at higher stimulus intensity (1 log cd/m2).
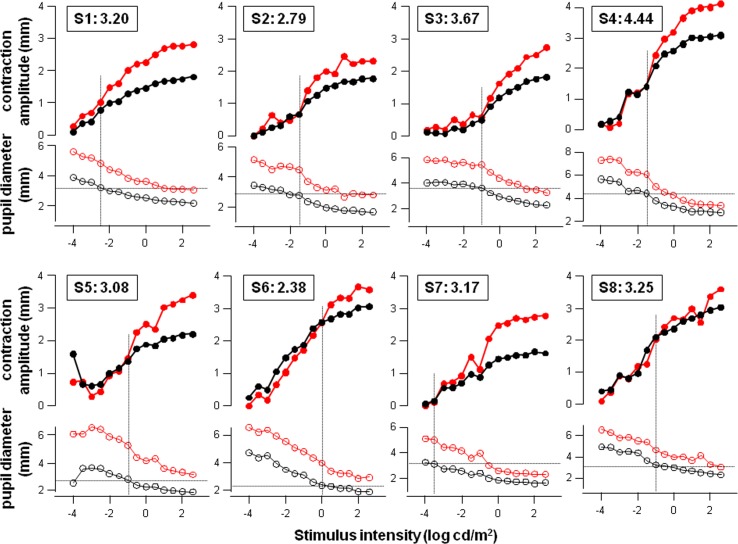
The same plot as shown in Figure 3 was produced for individual subjects (Fig. 4). As described in Figure 3, the point at which the pupil response in the treated eye started to deviate from that in the untreated eye is identified by observation on the top plot for each subject, and marked by a vertical dotted line. The intersection of the vertical dotted line and pupil diameter at peak contraction in the treated eye (black open circles at bottom of each plot) determines the pupil size at which iris mechanical limit is reached (demonstrated inside the box on top of each plot), and ranges from 2.38 to 4.44 mm (mean ± SD, 3.25 ± 0.61 mm) across eight subjects.
Figure 4. .
Plots of contraction amplitude (top red and black curves of each plot) and pupil size at peak contraction (bottom red and black curves of each plot) against stimulus intensity in individual subjects (S1 through S8; n = 8), using the same layout as shown in Figure 3. The box on top of each plot identifies the subject and the pupil size (in millimeters) at which the iris mechanical limit is reached. The treated eye is plotted in black, and untreated eye is plotted in red.
The average pupil contraction across eight subjects is summarized in Figure 5. When measured by percent contraction amplitude (Fig. 5, right), there were no statistically significant differences between the treated (black) and untreated eyes (red) at any of the 14 stimulus intensities among eight subjects (paired t-test, P > 0.05). The absolute pupil contraction in millimeters (Fig. 5, left) was significantly smaller (paired t-test, P < 0.05, asterisk) in the treated (black) than in the untreated (red) eye at stimulus intensities equal to or greater than −0.5 log cd/m2. The average pupil size at which the mechanical limit was reached was approximately 3.25 mm (left, black arrow).
Figure 5. .
Plot of average (mean ± SEM) pupil contraction against log stimulus intensity across eight subjects. The contraction amplitude in millimeters is shown on the left and the percent contraction amplitude (%) is shown to the right.
Discussion
In this study, an average reduction in resting pupil size of 1.78 mm was observed in brimonidine-treated eyes, which is consistent with the results observed in the other studies.32,36,37
The relationship between log stimulus intensity and pupil contraction amplitude will vary depending on the area of retina being illuminated, in addition to stimulus brightness. The Ganzfeld bowl light stimulus used in this study will undoubtedly produce a greater pupil response than a smaller-sized light stimulus, although scatter of bright light across the retina even with a smaller-sized light tends to recruit larger areas of retina at brighter lights. Nonetheless, the mechanical constraints on pupil movement are likely to come into play at the same pupil size in a given eye, no matter what area of retina is stimulated, as long as the summated area of retina at a given brightness causes the pupil size to drop below its mechanical limit.
Limitations on pupil contraction were observed in all brimonidine-treated eyes. The first sign of a limitation of pupil contraction in the miotic eye was at the point where pupil contraction (in millimeters) in the treated eye started to fall below that in the untreated eye. This point is presumed to be where iris mechanics starts to limit pupil contraction, which we term the “mechanical threshold” (Fig. 4). The “mechanical threshold” found in our study (range from 2.38 to 4.44 mm; mean 3.25 ± 0.61 mm) is strikingly similar to what was observed by Loewenfeld and Newsome28 (the lower limit of linear range of pupil contraction ranged from 2.75 to 3.80 mm, mean ± SD 3.41 ± 0.37 mm), when anisocoria was produced in six healthy subjects using cocaine, an indirect sympathomimetic, to produce mydriasis instead of miosis, as was done in the present study. It was not surprising to see that plotting pupil response in percent contraction amplitude reduced the confounding effect of iris mechanics on the assessment of afferent light transduction (Fig. 5, right), because percent contraction represents pupil contraction relative to baseline pupil size. However, even the percent contraction appears to plateau at high stimulus intensities, as repeatedly observed in the study of Park et al.,35 using the same stimulus protocol. A question was raised as to whether the plateau of the pupil response was contributed solely by a limitation imposed by iris mechanics or also by a plateau signifying neuronal nonlinearity of the PLR at high stimulus intensities.
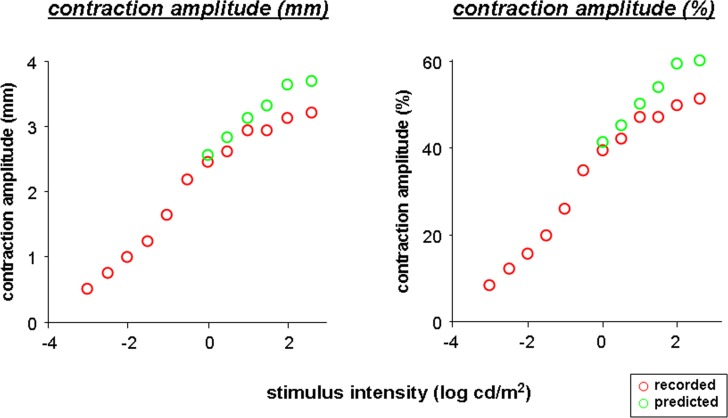
Our results from brimonidine testing suggest that most of the nonlinearity of the pupil response likely comes from a limitation on iris movement and not from nonlinearity of neuronal activity. The relative contribution of the sphincter and dilator muscles to iris mechanics are best demonstrated by the plot of the pupil size at peak contraction in the untreated and treated eyes (Fig. 3, bottom of each plot). When pupil size in the treated, more miotic eye (black opened circles) plateaus, the limitation on pupil contraction should be mainly due to the maximum contraction that can be attained by the iris sphincter muscles; the sympathetic tone is blocked by brimonidine. The pupil of the untreated eye was never able to reach as small a pupil size even at the peak contraction as the treated eye (see Fig. 3, comparing lower red and black curves with open circles), indicating difference in iris muscle tone between untreated versus treated eye at the peak contraction. We modeled what would be the full excursion of the pupil in the untreated eye, should the untreated eye achieve the minimal pupil size at peak contraction that was reached by the treated eye. The pupil contraction amplitude of the untreated eye (in both millimeters and percentage) was recalculated by subtracting the minimum pupil size achieved in the brimonidine-treated eye from the baseline pupil size in the untreated eye for each contraction (predicted pupil contractions, green open circles in Fig. 6). The resulting pupil response curve appears to be more linear. This linear pupil response curve more likely represents parasympathetic mediated pupil contraction from the sphincter muscles when sympathetic tone is blocked by brimonidine.
Figure 6.
Plot of mean pupil contraction amplitude in millimeters (left) and percentage (right) as function of log stimulus intensity, showing predicted pupil response of the untreated eye at high stimulus intensities (open green circles).
These findings seem to suggest that the limitation of peak pupil contraction in the untreated eye compared with the brimonidine-treated eye might be at least partly contributed by the presence of residual dilator muscle tone through sympathetic activity that is still present in the untreated eye at peak of contraction. This finding is somewhat different from the previous hypothesis that the behavior of PLR was attributed to the length-tension characteristics of the sphincter muscle alone.28,29 One has to be aware that this is a simplified model with respect to the receptor mechanism of the iris smooth muscles. The iris is found to be heavily innervated by sympathetic, parasympathetic, and sensory nerve terminals through both postsynaptic and prejunctional receptors in various species.38–40 The innervation of iris sphincter muscle in humans is found to be mainly contributed by cholinergic nerve fibers and substance P,33,41 although other adrenergic receptors have been found on sphincter muscles33,42,43 and may modulate its tone. Brimonidine exerts most of its pharmacologic effect through α2-adrenoreceptor–mediated downregulation of norepinephrine release.44–51 At present, there is no evidence that the α2 agonist activity of brimonidine exerts a modulatory effect on the iris sphincter and its α1 biological activity has been found to be insignificant.47
Conclusions
Iris mechanics start to limit the excursion of the pupil, on average, when pupil size reaches 3.3 mm in diameter among subjects. Pupil response assessed by using percent contraction amplitude is least affected by iris mechanics and might be a more reasonable approximation of afferent input. Pupil constriction to a light stimulus appears to be limited by residual iris dilator muscle tone through sympathetic activity at peak contraction. These results provide further insights into the physiology and pathology of the pupillary system, and help to improve the analysis and interpretation of pupil recordings in clinical practice.
Acknowledgments
The authors thank Susan Anderson for technical assistance and Scott Hetzel for statistical assistance.
Supported in part by the University of Wisconsin Institute for Clinical and Translational Research, funded through a National Institutes of Health Clinical and Translational Science Award (Grant number 1 UL 1 RR025011), as well as by an unrestricted research grant to the Department of Ophthalmology and Visual Sciences at the University of Wisconsin from Research to Prevent Blindness, Inc., and a Center of Excellence grant for the Iowa City Center for the Prevention and Treatment of Visual Loss from the Department of Veterans Affairs.
Disclosure: Y. Chen, None; R.H. Kardon, None
References
- 1. Cox TA, Thompson HS, Hayreh SS, Snyder JE. Visual evoked potential and pupillary signs. A comparison in optic nerve disease. Arch Ophthalmol. 1982; 100: 1603–1607 [DOI] [PubMed] [Google Scholar]
- 2. Fankhauser F, Flammer J. Puptrak 1.0—a new semiautomated system for pupillometry with the Octopus perimeter: a preliminary report. Doc Ophthalmol. 1989; 73: 235–248 [DOI] [PubMed] [Google Scholar]
- 3. Kankipati L, Girkin CA, Gamlin PD. Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci. 2010; 51: 2764–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kardon RH. Pupil perimetry. Curr Opin Ophthalmol. 1992; 3: 565–570 [DOI] [PubMed] [Google Scholar]
- 5. Kardon RH, Kirkali PA, Thompson HS. Automated pupil perimetry. Pupil field mapping in patients and normal subjects. Ophthalmology. 1991; 98: 485–495 [DOI] [PubMed] [Google Scholar]
- 6. Kwon YH, Pereira ML, Anderson SC, Kim YI, Kardon RH. Quantitative correlation of elevated intraocular pressure with relative afferent pupillary defect change in unilateral glaucoma. Acta Ophthalmol Scand. 2005; 83: 127–129 [DOI] [PubMed] [Google Scholar]
- 7. Lagreze WD, Kardon RH. Correlation of relative afferent pupillary defect and estimated retinal ganglion cell loss. Graefes Arch Clin Exp Ophthalmol. 1998; 236: 401–404 [DOI] [PubMed] [Google Scholar]
- 8. Lowenstein O, Murphy SB, Loewenfeld IE. Functional evaluation of the pupillary light reflex pathways; experimental pupillographic studies in cats. AMA Arch Ophthalmol. 1953; 49: 656–657 [DOI] [PubMed] [Google Scholar]
- 9. Ukai K. Spatial pattern as a stimulus to the pupillary system. J Opt Soc Am A. 1985; 2: 1094–1100 [DOI] [PubMed] [Google Scholar]
- 10. Wilhelm H, Neitzel J, Wilhelm B, et al. Pupil perimetry using M-sequence stimulation technique. Invest Ophthalmol Vis Sci. 2000; 41: 1229–1238 [PubMed] [Google Scholar]
- 11. Yoshitomi T, Matsui T, Tanakadate A, Ishikawa S. Comparison of threshold visual perimetry and objective pupil perimetry in clinical patients. J Neuroophthalmol. 1999; 19: 89–99 [PubMed] [Google Scholar]
- 12. Goldwater BC. Psychological significance of pupillary movements. Psychol Bull. 1972; 77: 340–355 [DOI] [PubMed] [Google Scholar]
- 13. Hayashi N, Someya N, Fukuba Y. Effect of intensity of dynamic exercise on pupil diameter in humans. J Physiol Anthropol. 2010; 29: 119–122 [DOI] [PubMed] [Google Scholar]
- 14. Siegle GJ, Ichikawa N, Steinhauer S. Blink before and after you think: blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology. 2008; 45: 679–687 [DOI] [PubMed] [Google Scholar]
- 15. Silk JS, Dahl RE, Ryan ND, et al. Pupillary reactivity to emotional information in child and adolescent depression: links to clinical and ecological measures. Am J Psychiatry. 2007; 164: 1873–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. Int J Psychophysiol. 2004; 52: 77–86 [DOI] [PubMed] [Google Scholar]
- 17. Alessandri M, Marabini S, Sicuteri R, Boccuni M, Fanciullacci M. Early dysfunction of iris sensory fibres in diabetic patients. Panminerva Med. 1998; 40: 299–303 [PubMed] [Google Scholar]
- 18. Ferrari GL, Marques JL, Gandhi RA, et al. An approach to the assessment of diabetic neuropathy based on dynamic pupillometry. Conf Proc IEEE Eng Med Biol Soc. 2007; 2007: 557–560 [DOI] [PubMed] [Google Scholar]
- 19. Kwon HJ, Kim HY. A pharmacologic pupillary test in the diagnosis of diabetic autonomic neuropathy. Korean J Ophthalmol. 2009; 23: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Micieli G, Manni R, Tassorelli C, Osipova V, Tartara A, Nappi G. Sleep-apnoea and autonomic dysfunction: a cardiopressor and pupillometric study. Acta Neurol Scand. 1995; 91: 382–388 [DOI] [PubMed] [Google Scholar]
- 21. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002; 295: 1070–1073 [DOI] [PubMed] [Google Scholar]
- 22. Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003; 23: 7093–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002; 295: 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003; 424: 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003; 299: 245–247 [DOI] [PubMed] [Google Scholar]
- 26. Taylor WR, Chen JW, Meltzer H, et al. Quantitative pupillometry, a new technology: normative data and preliminary observations in patients with acute head injury. Technical note. J Neurosurg. 2003; 98: 205–213 [DOI] [PubMed] [Google Scholar]
- 27. Winn B, Whitaker D, Elliott DB, Phillips NJ. Factors affecting light-adapted pupil size in normal human subjects. Invest Ophthalmol Vis Sci. 1994; 35: 1132–1137 [PubMed] [Google Scholar]
- 28. Loewenfeld IE, Newsome DA. Iris mechanics. I. Influence of pupil size on dynamics of pupillary movements. Am J Ophthalmol. 1971; 71: 347–362 [DOI] [PubMed] [Google Scholar]
- 29. Semmlow J, Hansmann D, Stark L. Variation in pupillomotor responsiveness with mean pupil size. Vision Res. 1975; 15: 85–90 [DOI] [PubMed] [Google Scholar]
- 30. Sun F, Tauchi P, Stark L. Dynamic pupillary response controlled by the pupil size effect. Exp Neurol. 1983; 82: 313–324 [DOI] [PubMed] [Google Scholar]
- 31. McDonald JE 2nd. El-Moatassem Kotb AM, Decker BB. Effect of brimonidine tartrate ophthalmic solution 0.2% on pupil size in normal eyes under different luminance conditions. J Cataract Refract Surg. 2001; 27: 560–564 [DOI] [PubMed] [Google Scholar]
- 32. Shemesh G, Moisseiev E, Lazar M, Kesler A. Effect of brimonidine tartrate 0.10% ophthalmic solution on pupil diameter. J Cataract Refract Surg. 2011; 37: 486–489 [DOI] [PubMed] [Google Scholar]
- 33. Yoshitomi T, Ito Y, Inomata H. Functional innervation and contractile properties of the human iris sphincter muscle. Exp Eye Res. 1988; 46: 979–986 [DOI] [PubMed] [Google Scholar]
- 34. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009; 116: 1564–1573 [DOI] [PubMed] [Google Scholar]
- 35. Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci. 2011; 52: 6624–6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Besada E, Reed K, Najman P, Shechtman D, Hardigan P. Pupillometry and diagnostic ultrasound of anterior segment effects of brimonidine tartrate 0.2% and apraclonidine 0.5%. J Clin Pharmacol. 2011; 51: 1690–1695 [DOI] [PubMed] [Google Scholar]
- 37. Brown SM, Khanani AM, McCartney DL. The effect of daily use of brimonidine tartrate on the dark-adapted pupil diameter. Am J Ophthalmol. 2004; 138: 149–151 [DOI] [PubMed] [Google Scholar]
- 38. Fuder H. Functional consequences of prejunctional receptor activation or blockade in the iris. J Ocul Pharmacol. 1994; 10: 109–123 [DOI] [PubMed] [Google Scholar]
- 39. Jumblatt JE. Prejunctional alpha 2-adrenoceptors and adenylyl cyclase regulation in the rabbit iris-ciliary body. J Ocul Pharmacol. 1994; 10: 617–621 [DOI] [PubMed] [Google Scholar]
- 40. Kubo C, Suzuki R. Involvement of prejunctional alpha 2-adrenoceptor in bovine ciliary muscle movement. J Ocul Pharmacol. 1992; 8: 225–231 [DOI] [PubMed] [Google Scholar]
- 41. Tervo T, Tarkkanen A, Tervo K. Innervation of the human pupillary sphincter muscle by nerve fibers immunoreactive to substance P. Ophthalmic Res. 1985; 17: 111–114 [DOI] [PubMed] [Google Scholar]
- 42. Rubin LJ, Nolte JF. Modulation of the response of a photosensitive muscle by beta-adrenergic regulation of cyclic AMP levels. Nature. 1984; 307: 551–553 [DOI] [PubMed] [Google Scholar]
- 43. Neuhuber W, Schrodl F. Autonomic control of the eye and the iris. Auton Neurosci. 2011; 165: 67–79 [DOI] [PubMed] [Google Scholar]
- 44. Adkins JC, Balfour JA. Brimonidine. A review of its pharmacological properties and clinical potential in the management of open-angle glaucoma and ocular hypertension. Drugs Aging. 1998; 12: 225–241 [DOI] [PubMed] [Google Scholar]
- 45. Burke J, Schwartz M. Preclinical evaluation of brimonidine. Surv Ophthalmol. 1996; 41: S9–S18 [DOI] [PubMed] [Google Scholar]
- 46. Cambridge D. UK-14, 304, a potent and selective alpha2-agonist for the characterisation of alpha-adrenoceptor subtypes. Eur J Pharmacol. 1981; 72: 413–415 [DOI] [PubMed] [Google Scholar]
- 47. deSousa JL, Malhotra R. Brimonidine for anisocoria. Ophthalmology. 2007; 114: 1419 [DOI] [PubMed] [Google Scholar]
- 48. Robin AL, Burnstein Y. Selectivity of site of action and systemic effects of topical alpha agonists. Curr Opin Ophthalmol. 1998; 9: 30–33 [DOI] [PubMed] [Google Scholar]
- 49. Besada E, Reed K, Najman P, Shechtman D, Hardigan P. Pupillometry study of brimonidine tartrate 0.2% and apraclonidine 0.5%. J Clin Pharmacol. 2011; 51: 1690–1695 [DOI] [PubMed] [Google Scholar]
- 50. Cambron M, Maertens H, Crevits L. Apraclonidine and my pupil. Clin Auton Res. 2011; 21: 347–351 [DOI] [PubMed] [Google Scholar]
- 51. Dinslage S, Strauss B, Jordan JF, Diestelhorst M, Krieglstein GK. The effect of brimonidine on the pupillary reflex. A pupillographic study in healthy volunteers [in German]. Ophthalmologe. 2005; 102: 879–887 [DOI] [PubMed] [Google Scholar]