Abstract
Purpose.
To determine whether high light levels, which have a protective effect against form-deprivation myopia, also retard the development of lens-induced myopia in primates.
Methods.
Hyperopic defocus was imposed on 27 monkeys by securing −3 diopter (D) lenses in front of one eye. The lens-rearing procedures were initiated at 24 days of age and continued for periods ranging from 50 to 123 days. Fifteen of the treated monkeys were exposed to normal laboratory light levels (∼350 lux). For the other 12 lens-reared monkeys, auxiliary lighting increased the illuminance to 25,000 lux for 6 hours during the middle of the daily 12 hour light cycle. Refractive development, corneal power, and axial dimensions were assessed by retinoscopy, keratometry, and ultrasonography, respectively. Data were also obtained from 37 control monkeys, four of which were exposed to high ambient lighting.
Results.
In normal- and high-light-reared monkeys, hyperopic defocus accelerated vitreous chamber elongation and produced myopic shifts in refractive error. The high light regimen did not alter the degree of myopia (high light: −1.69 ± 0.84 D versus normal light: −2.08 ± 1.12 D; P = 0.40) or the rate at which the treated eyes compensated for the imposed defocus. Following lens removal, the high light monkeys recovered from the induced myopia. The recovery process was not affected by the high lighting regimen.
Conclusions.
In contrast to the protective effects that high ambient lighting has against form-deprivation myopia, high artificial lighting did not alter the course of compensation to imposed defocus. These results indicate that the mechanisms responsible for form-deprivation myopia and lens-induced myopia are not identical.
Keywords: emmetropization, myopia, hyperopia, refractive error
In contrast to the protective effects that high ambient lighting has against form-deprivation myopia, elevated lighting did not alter the course of compensation to imposed defocus. These results indicate that the mechanisms responsible for deprivation- and lens-induced myopia are not identical.
Introduction
Although ocular growth and emmetropization are actively regulated by visual feedback associated with the eye's effective refractive state,1,2 other visual factors can influence refractive development. In particular, several lines of evidence suggest that ambient light levels can influence the operational properties of the defocus-driven cascade that regulates refractive development and the course of emmetropization.
First, studies of humans indicate that time spent outdoors is associated with the incidence of myopia. Although this relationship has not been observed in very young populations3 or some populations that have a high prevalence of myopia,4 it appears that children who spend more time outdoors have a lower incidence of myopia.5–10 However, once myopia is present, time outdoors does not appear to affect the rate of myopia progression.11 Observations from prospective studies, which have shown that the differences in time spent outdoors between myopes and nonmyopes precede the onset of myopia, suggest that there is a causal relationship between time outdoors and myopia, specifically that greater time spent outdoors is protective against myopia.7,10 The preliminary findings of a randomized control trial to evaluate the protective effects of greater amounts of outdoor time also support a causal relationship between outdoor time and myopia (Morgan IG, et al. IOVS 2012;53:ARVO E-Abstract 2735). However, human studies have not been able to identify the mechanism underlying these protective effects. The protective association between time outdoors and myopia is not dependent on physical or sporting activities, nor is it associated with lower amounts of near work activities.5,8,10 Given that outdoor lighting levels are often 100 times higher than indoor levels and that the progression rates for myopia are lower during the summer than in the winter,12–14 it has been speculated that the protective effects of time outdoors is related to the high ambient lighting levels typically encountered outdoors.9,15,16
The strongest evidence for an influence of ambient lighting levels on refractive development comes from studies of laboratory animals, which have demonstrated that high lighting levels can have a protective effect against myopiagenic viewing conditions (Siegwart JT Jr, et al. IOVS 2012;53:ARVO E-Abstract 3457).16,17 For example, exposing infant monkeys for 6 hours each day to artificial lighting levels that are approximately 2 log units higher than typical indoor lighting levels has a robust protective effect against the phenomenon of form-deprivation myopia. Specifically in monocularly form–deprived monkeys, these high light levels, which were well within the ordinary range of outdoor illuminances, reduced the average degree of myopic anisometropia by 87% in comparison with treated animals exposed to ordinary indoor lighting with the majority of high-light–reared animals actually exhibiting relative hyperopic shifts in their form-deprived eyes. These protective effects against form-deprivation myopia were maintained over a 3.5 month treatment period.17 High ambient lighting conditions have been shown to have qualitatively similar protective effects against form-deprivation myopia in chickens treated for 4 days16 and tree shrews deprived for 11 days (Siegwart JT Jr, et al. IOVS 2012;53:ARVO E-Abstract 3457).
Optically imposed hyperopic defocus, another commonly used strategy to produce experimental myopia, consistently produces compensating axial myopia in animals reared under ordinary lighting levels and light cycles.1,2 In essence, the imposed hyperopic defocus predictably alters the target refractive error for emmetropization. Recent observations in chickens18 and tree shrews (Siegwart JT Jr, et al. IOVS 2012;53:ARVO E-Abstract 3457) show that high ambient lighting can also alter the course of negative lens compensation. However, the protective effects of high ambient lighting may be different for negative lens–induced myopia and form-deprivation myopia. For example, in chickens, high lighting levels slow the rate of myopic compensation for negative lens–induced hyperopic defocus, but, fully compensating changes in the degree of myopia are still achieved by the end of a 6 day treatment period.18 In other words, high light levels slow but do not prevent the development of defocus-induced myopia. On the other hand, the findings in monkeys suggest that the protective effects of high lighting levels against form-deprivation myopia are maintained over extended treatment periods.17
To assess the potential therapeutic benefit of manipulating ambient lighting against myopia in children, it is important to understand the effects of lighting levels on the phenomenon of lens compensation. Because the ocular mechanisms that are responsible for form-deprivation myopia and lens-induced myopia are not identical,19–25 the results obtained for form deprivation may not be applicable to the effects of defocus on refractive development. In this respect, because emmetropization is regulated by defocus, the effects of lighting levels on the phenomenon of lens compensation probably have more implications for normal refractive development and the genesis of common forms of myopia than the effects of lighting levels on the phenomenon of form-deprivation myopia. Therefore, the purpose of this study was to investigate the effects of high ambient lighting on the development and recovery from negative lens–induced myopia in infant monkeys.
Methods
Subjects
The primary subject group consisted of 12 infant rhesus monkeys (Macaca mulatta) that were reared with an optically imposed anisometropia and exposed on a daily basis to high artificial lighting levels. Nine animals were reared with the same optically imposed anisometropia, but were housed under ordinary laboratory lighting. Comparison data were available from previous studies for (1) six additional negative lens–reared animals that were housed under ordinary laboratory lighting, (2) 18 monocularly form–deprived monkeys reared under ordinary lighting,26–28 and (3) eight monocularly form–deprived monkeys reared under the same high light regimen that was employed in this study.17 Data were also available from previous studies for 33 control animals reared under ordinary lighting conditions. Twenty-nine of these control animals were reared with unrestricted vision and four control monkeys were reared wearing helmets that held zero powered spectacles in front of both eyes. In addition, in this study we present data for four control monkeys reared under high ambient lighting. All of the animals were obtained at 1 to 3 weeks of age and, following the initial biometry measurements at approximately 3 weeks of age, were assigned to their respective subject groups on a random basis. Although the different subject groups were studied at different times over a period of several years, the experimental methods were identical. The details of the nursery care for our infant monkeys have been described previously.29
Hyperopic anisometropia was produced by fitting the experimental monkeys with goggles that secured a −3.0 diopter (D) spectacle lens over one eye and a zero powered lens in front of the fellow eye. The spectacle lenses were held at a vertex distance of approximately 11 mm providing monocular fields of view of 80° and 87° in the horizontal and vertical meridians, respectively, and resulting in approximately 2.9 D of relative hyperopic defocus in the treated eyes, which was well within the range of optically imposed refractive errors that normally produce compensating axial growth in infant monkeys.29,30 For the animals exposed to the high light regimen, the lens-rearing procedures were initiated at 24 ± 3 days of age and the animals wore the goggles continuously until they were 75 ± 5 (n = 4) or 145 ± 8 days of age (n = 8). For the experimental monkeys exposed to ordinary laboratory lighting levels, the lens-rearing period was very similar. Specifically, for the normal-light–reared monkeys, the anisometropic rearing procedures were initiated at 23 ± 2 days and continued until the ages of 72 ± 11 (n = 4) or 146 ± 5 days of age (n = 11). The shorter treatment durations for eight of the lens-reared monkeys were employed to support biochemical experiments associated with a separate investigation.
The housing areas for all of the infant monkeys were illuminated with fluorescent tubes (F32T8/TL735, correlated color temperature = 3500 K; Philips Lighting US, Somerset, NJ) maintained on a 12 hour light/12 hour dark cycle. The fluorescent lighting provided illuminances that ranged from 15 (back walls of lower cages) to 630 lux (ceilings of upper cages), with an average of approximately 350 lux for the front walls of all cages. The auxiliary lighting system employed to produce high ambient lighting was similar to that employed in our previous study of the effects of high lighting levels on the phenomenon of form-deprivation myopia.17 Specifically, the housing area for the lens-reared monkeys in the high ambient lighting group was illuminated by four, 1000 W metal halide lamps (MH1000; Plusrite, Ontario, CA) positioned above the animals' cages and two 400 W metal halide lamps (ProBuilt Professional Lighting, LLC, Mundelein, IL) positioned on the floor. The metal halide lamps had major output peaks throughout the visible spectrum resulting in a correlated color temperature of 4200 K. The light from the lamps was filtered to eliminate wavelengths below 360 nm and was delivered indirectly to the animals' cages resulting in illuminances that varied from 18,000 to 28,000 lux, which were well within the range of illuminances commonly encountered in outdoor settings. The auxiliary lights were turned on for 6 hours each day during the middle of the 12 hour lights on cycle. The high light regimen was implemented at the start of the lens-rearing period. In order to evaluate the effects of high ambient lighting on the ability of animals to recovery from any optically induced refractive errors, the high light regimen was continued for at least 5 months following the removal of the goggles for eight of the high-light–reared monkeys. Additional air ducts were installed in the high light caging area to ensure that the temperature (74° ± 10°F) and humidity (50% ± 5%) were maintained within normal limits.
Ocular Biometry
The refractive status, corneal power, and axial dimensions were measured for each eye of each subject using methods described previously.29 The first measurements were obtained at ages corresponding to the start of lens wear, subsequently measurements were obtained at approximately weekly intervals for the first 2 months of the lens-rearing period for all of the high-light–reared animals and for nine of the lens-reared monkeys exposed to normal laboratory lighting levels. Thereafter, ocular dimensions and refractive status were determined every 2 to 4 weeks throughout the remainder of the observation period. To make these measurements, the monkeys were anesthetized (intramuscular injection: ketamine hydrochloride, 15–20 mg/kg, and acepromazine maleate, 0.15–0.2 mg/kg; topical: 1–2 drops of 0.5% tetracaine hydrochloride) and cyclopleged (1% tropicamide). The refractive status of each eye was measured independently by two experienced investigators using a streak retinoscope (Welch Allyn Elite Streak Retinoscope; Welch Allyn Inc., Skaneateles Falls, NY) and averaged.31 An eye's refractive error was defined as the spherical-equivalent spectacle-plane refractive correction32; no corrections were made for the small-eye artifact associated with retinoscopy.33 The anterior radius of curvature of the cornea was measured by keratometry (Hand-held Auto-keratometer; Alcon, Inc., Fort Worth, TX) and central corneal power was calculated from the average of three readings using an assumed refractive index of 1.3375.34 The eyes' axial dimensions were measured by A-scan ultrasonography (Image 2000; Mentor, Norwell, MA); 10 separate measurements were averaged.29
All of the rearing and experimental procedures were reviewed and approved by the University of Houston's Institutional Animal Care and Use Committee, and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Statistical Methods
Mann-Whitney U tests were used to compare the median refractive errors between subject groups. Paired Student's t-tests were employed to examine interocular differences within a given group. Two-sample t-tests were used to compare interocular differences in refractive error and vitreous chamber depth between groups. All of the above analyses were executed using Minitab software (Release 12.21; Minitab Inc., State College, PA). Mixed-design, repeated measures ANOVAs (SuperANOVA; Abacus Concepts, Inc., Berkeley, CA) were used to detect if there were differences in the rates of lens compensation and the rates of recovery from the induced refractive errors between the high and normal light groups. Probability values were adjusted with the Geisser-Greenhouse correction.
Results
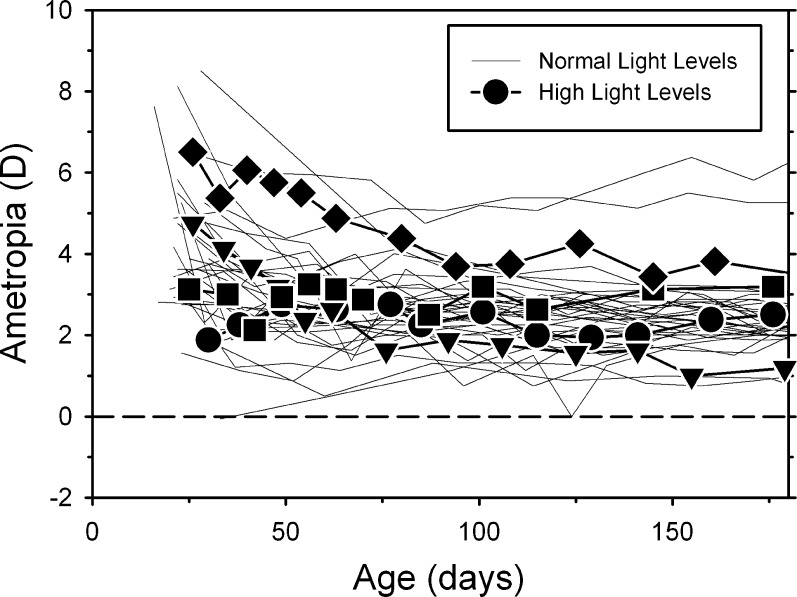
Although the high light regimen reduced the average pupil diameter by 42% (3.3 ± 0.3 mm vs. 1.9 ± 0.2 mm), it did not obviously alter refractive development in control monkeys reared with unrestricted vision. Figure 1 shows refractive error plotted as a function of age for the control monkeys reared under the normal (thin lines) and high ambient lighting conditions (filled symbols). Although the number of high light controls is too small to support a rigorous analysis, the data for the high light control animals were always within the range of data for the animals reared under ordinary laboratory lighting. There were no systematic differences in the course of emmetropization in the two groups of control monkeys.
Figure 1. .
Spherical-equivalent spectacle-plane refractive corrections plotted as a function of age for control monkeys reared under ordinary laboratory lighting (thin lines; n = 33) and under the high ambient lighting conditions (filled symbols; n = 4).
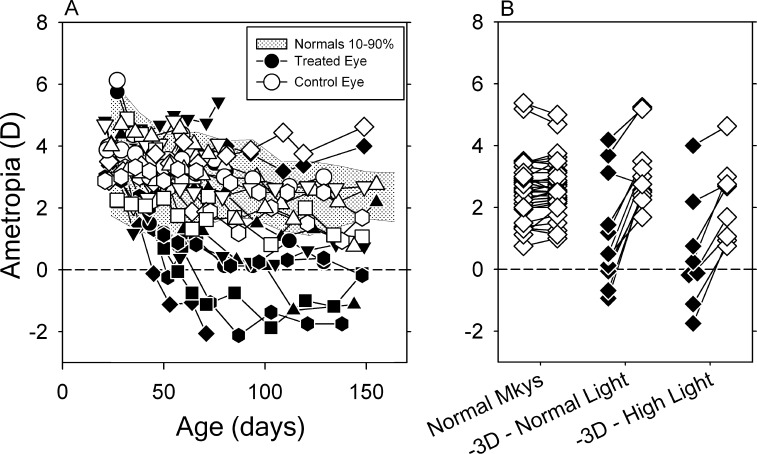
At the start of the observation period, the left and right eyes of the lens-reared monkeys in the high light group were moderately hyperopic (OS: +3.88 ± 1.01 D; OD: +3.80 ± 0.93 D) and there were no interocular differences between the treated and control eyes in refractive error, corneal power, anterior chamber depth, lens thickness, or vitreous chamber depth (P = 0.19–0.96). However, as illustrated in Figure 2A, shortly after the onset of lens wear, all of the high-light–reared animals developed significant degrees of anisometropia. For approximately the first 100 days of lens wear, the refractive errors for the control eyes (open symbols) were within the 10th to 90th percentiles of ametropias exhibited by control monkeys (shaded area). For 11 of the 12 high light animals, the treated eyes became more myopic than their fellow control eyes and these animals manifest anisometropias that compensated, at least in part, for the imposed defocus. It is interesting that the treated eye of one lens-reared monkey exhibited relative hyperopic shifts in refractive error resulting in an anisometropia that exaggerated the defocus imposed by the treatment lenses (down pointing triangle, MKY461). Other than refractive error, there was nothing in the history of this animal that distinguished it from the other high light animals. However, atypical responses have been previously reported in both lens-reared35,36 and form-deprived monkeys27,37 exposed to ordinary laboratory lighting.
Figure 2. .
(A) Spherical-equivalent spectacle-plane refractive corrections plotted as a function of age for the treated (filled symbols) and control eyes (open symbols) of the individual lens-reared monkeys reared under high ambient lighting. The shaded area shows the 10th to 90th percentile range of ametropias for control monkeys. (B) Refractive errors obtained at ages corresponding to the end of the lens-rearing period for the left and right eyes of individual, control monkeys (open symbols) and the treated (filled symbols) and fellow control eyes (open symbols) of individual lens-reared monkeys exposed to normal and high ambient lighting. The lines connect the treated (or right eyes) and fellow eyes (or left eyes) of individual animals.
The refractive errors obtained at ages corresponding to the end of the lens-rearing period are shown in Figure 2B for both eyes of individual control monkeys and the treated (filled symbols) and control eyes (open symbols) of individual lens-reared monkeys exposed to the normal and high light conditions. At approximately 150 days of age, 80% percent of the control monkeys exhibited refractive errors between +1.65 and +3.17 D and the refractive errors in the two eyes of the control monkeys were well matched (average ± SD: OD = +2.57 ± 1.01 D; OS = +2.63 ± 0.90 D; t = −1.36, P = 0.19). Although the refractive errors for the control eyes of the high light–reared animals were not as tightly grouped as the ametropias in normal monkeys, the median control eye refractive error for the high light monkeys (+2.19 D) was not significantly different from the median ametropia for the control monkeys (+2.50 D; P = 0.29). On the other hand, the treated eyes of the high-light–reared monkeys were significantly less hyperopic/more myopic than the eyes of control monkeys (+0.06 D vs. +2.50 D; P = 0.003). There were, however, no differences in either the control eye (medians: +2.19 D vs. +2.90 D; P = 0.12) or treated eye refractive errors (medians: +0.06 D vs. +0.47 D; P = 0.37) between the lens-reared monkeys housed under the high and normal light conditions.
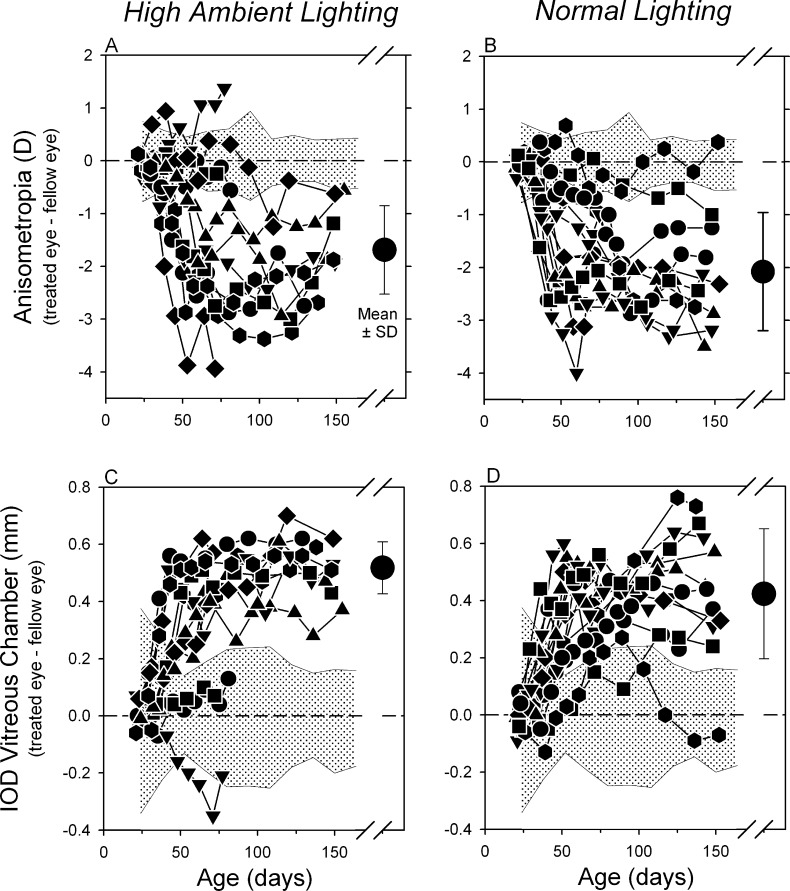
Figures 3A and 3B compare the longitudinal interocular differences in refractive error between the lens-reared monkeys housed under high ambient lighting and ordinary laboratory lighting. The pattern of results was very similar in both groups of experimental monkeys. Almost every animal in both lighting groups developed a myopic anisometropia. By the end of the lens-rearing period (approximately 145 days of age), all eight of the high light monkeys and 11 of the 12 normal light subjects exhibited myopic anisometropias that were more than 2 SDs away from the mean interocular differences in control monkeys and the mean anisometropias for both experimental groups were significantly different from that in control monkeys (−0.06 ± 0.24 D vs. −1.69 ± 0.84 D for high light animals, t = −5.41; P = 0.001; −0.06 ± 0.24 vs. −2.08 ± 1.12 D for normal light animals; t = −5.92; P = 0.0001). However, the anisometropias in the two groups of lens-reared monkeys were not significantly different (t = 0.87; P = 0.4).
Figure 3. .
Interocular differences (treated eye − fellow eye) in refractive error (top) and vitreous chamber depth (bottom) plotted as a function of age for individual lens-reared monkeys exposed to the high- (left) and normal-light conditions (right). The shaded areas in each plot represent ±2 SDs from the mean values for control monkeys. The large symbols to the right in each plot represent the mean ± SD for the treated monkeys at the end of the lens-rearing period and the start of the recovery period.
The myopic anisometropias in the lens-reared animals were caused by a relative acceleration in the axial elongation rates of their treated eyes. At the end of the lens-rearing period, the treated and control eyes of the high light animals exhibited similar corneal powers (treated eye = +54.41 ± 1.05 D; control eye = +54.37 ± 1.15 D; t = 0.28; P = 0.79) and anterior chamber depths (treated eye = 3.15 ± 0.18 mm; control eye = 3.15 ± 0.18; t = −0.65; P = 0.53). The mean interocular difference in lens thickness was marginally larger in the treated eyes (3.71 ± 0.09 mm vs. 3.68 ± 0.11; t = 2.38; P = 0.05), but this difference contributed little to the overall increase in axial length. On the other hand, as illustrated in Figures 3C and 3D, the interocular differences in vitreous chamber depth were substantial and mirrored the interocular differences in refractive error in both the normal and high light animals. At the end of the treatment period, the mean interocular difference in vitreous chamber depth for the high-light–reared animals (0.52 ± 0.09 mm) was significantly larger than that observed in control monkeys (−0.01 ± 0.10 mm; t = 14.23; P < 0.0001), but similar in magnitude to that observed in the lens-reared monkeys exposed to the normal lighting regimen (0.42 ± 0.23 mm; t = 1.24; P = 0.24).
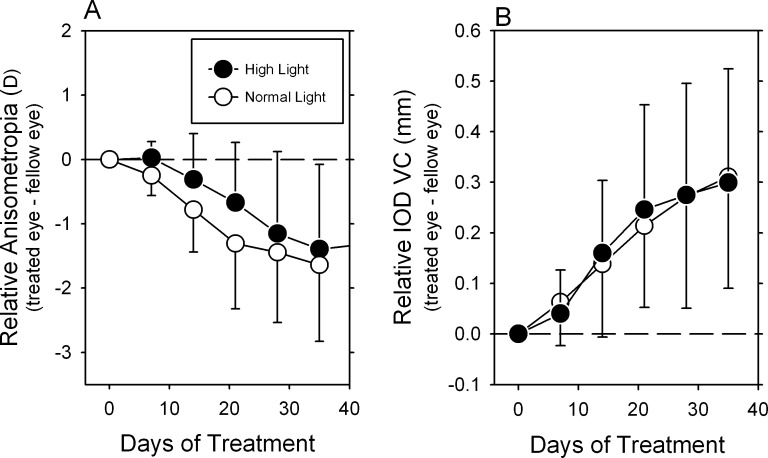
In the majority of animals in both the normal and high light groups, the compensating changes occurred rapidly following the onset of lens wear and then after approximately 50 to 75 days of age the interocular differences in refractive error and vitreous chamber depth remained relatively stable. In order to compare the rates at which the lens-reared monkeys in the two lighting groups compensated for the optically imposed anisometropia, the interocular differences in refractive error, and vitreous chamber depth were normalized to the values obtained at the start of lens wear and time was expressed in terms of days of treatment. The mean (±SD) relative interocular differences in refractive error and vitreous chamber depth for the critical early stages of the treatment period are shown, respectively, in Figures 4A and 4B. There was a trend for the high light animals to exhibit slightly slower compensating myopic changes during the early treatment period, however, these differences were not statistically significant (F = 0.90; P = 0.39). The mean measures of relative vitreous chamber elongation (Fig. 4B) were virtually identical in the two groups of lens-reared monkeys during the early treatment period (F = 0.28; P = 0.71). Over the first 5 weeks of lens wear, the vitreous chamber data for the high and normal lighting groups overlapped at each of the early time points.
Figure 4. .
Average (±SD) relative interocular differences (treated eye − fellow eye) in refractive error (A) and vitreous chamber depth (B) for the lens-reared monkeys exposed to the high- (filled symbols) and normal-light regimens (open symbols) plotted as a function of the days of treatment. The data were normalized to the age at the onset of lens wear and the initial interocular differences in refractive error.
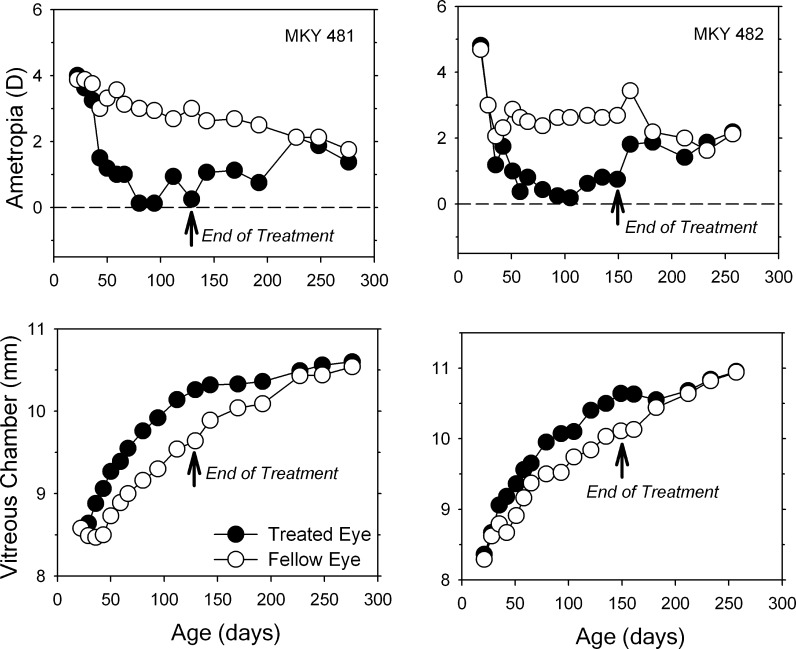
Under normal laboratory lighting, infant monkeys can recover from vision-induced refractive errors,38,39 a process that is regulated by visual feedback associated with the eye's effective refractive status.40,41 Figure 5 shows longitudinal data from two lens-reared monkeys that demonstrate that the recovery process takes place in a qualitatively similar manner in animals exposed to high ambient lighting. As observed in treated animals reared under ordinary lighting,38,39 the myopic compensation that occurred in response to monocularly imposed hyperopic defocus came about primarily as a result of acceleration in the vitreous chamber elongation rates of the treated eyes. Following removal of the treatment lenses and the onset of unrestricted vision, there was a distinct decrease in the vitreous chamber elongation rate in the treated eyes, which subsequently became less myopic as the corneas and lenses in the treated eyes underwent their normal age-related reductions in power. Once, isometropia was reestablished, the treated eyes began elongating again, but this time at the same rate as the fellow eyes.
Figure 5. .
Spherical-equivalent spectacle-plane refractive corrections (top) and vitreous chamber depth plotted as a function of age for the treated (filled symbols) and control eyes (open symbols) of two high light, lens-reared monkeys that exhibited relatively high degrees of myopic anisometropia. The first data point in each plot represents the onset of lens wear. The arrows mark the end of the treatment period and the onset of unrestricted vision.
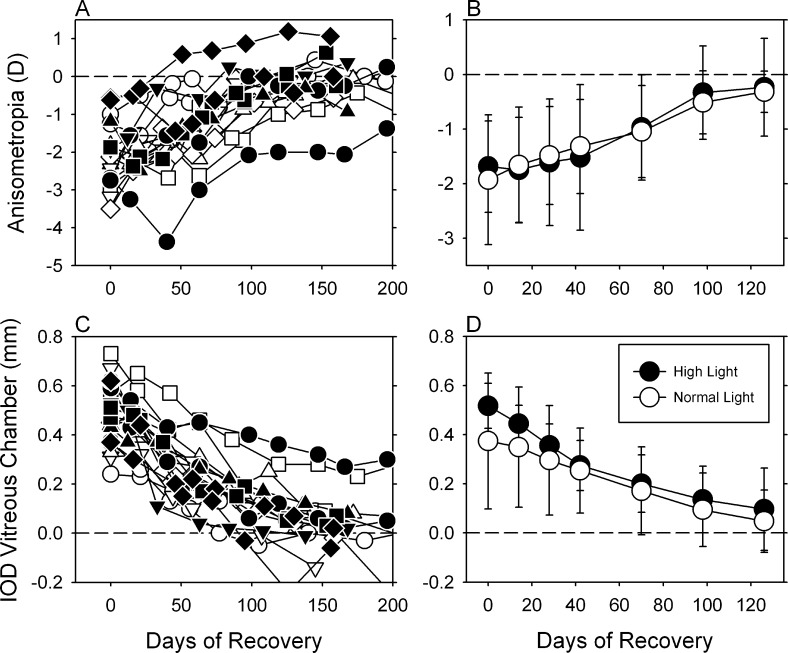
Figure 6 compares the recovery process in the lens-reared monkeys housed under the normal (n = 10; open symbols) and high lighting regimens (n = 8; filled symbols). Following the onset of unrestricted vision, the experimentally induced anisometropias (top panels) in both groups of animals decreased systematically as the interocular differences in vitreous chamber depth decreased (bottom panels). The data for individual animals in the normal and high light groups overlapped throughout the recovery period (left panels). As illustrated in the right two panels, which shows the mean (±SD) interocular differences in refractive error and vitreous chamber depth, high ambient lighting did not significantly alter the ability of animals to recover from optically induced myopia or the rate at which they recovered (anisometropia: F = 0.56, P = 0.64; vitreous chamber: F = 1.16, P = 0.32).
Figure 6. .
Left. Interocular differences (treated eye − fellow eye) in refractive error (top) and vitreous chamber depth (bottom) plotted as a function of age for individual lens-reared monkeys exposed to the high- (filled symbols) and normal-light conditions (open symbols) during the recovery period. The leftmost symbols in each plot represent the end of the lens-rearing period. Right. Means ± SDs plotted as a function of time during the recovery period.
Discussion
The main finding of this study was that daily exposure to high levels of artificial lighting did not alter the course of negative lens compensation in infant monkeys. Specifically, as in animals maintained under ordinary laboratory lighting, optically imposed hyperopic defocus accelerated vitreous chamber growth resulting in a relative myopic shift in the refractive errors of the treated eyes. The highlight regimen did not affect the final degree of compensating myopia in the treated eyes or the rate at which the treated eyes responded to the imposed hyperopic defocus. In addition, the data obtained during the recovery period showed that high lighting levels did not alter the eye's ability to respond to relative myopic defocus. As in animals exposed to ordinary laboratory lighting levels, myopic defocus decreased axial growth rates resulting in relative hyperopic shifts in refractive error. Moreover, the degree of recovery and the time course for these refractive changes were not affected by exposure to elevated ambient lighting.
Interspecies Comparisons
The effects of elevated ambient lighting on compensation for experimentally induced defocus in infant monkeys are comparable in several respects to earlier observations in chickens18 and some recent observations in tree shrews (Siegwart JT Jr, et al. IOVS 2012;53:ARVO E-Abstract 3457). For example, in both chickens and monkeys, high illuminances did not alter the end point for emmetropization (i.e., the degree of compensation produced by negative lens–induced hyperopic defocus was the same in animals exposed to high lighting levels versus ordinary lighting levels). In addition, like chickens reared with positive lens–induced defocus, infant monkeys with experimentally induced myopia and exposed to high lighting levels exhibited the same degree of compensation during the recovery process as infant monkeys maintained under ordinary lighting conditions. Similarly, preliminary observations from two animals suggest that tree shrews reared in elevated lighting also fully compensate for optically imposed hyperopic defocus (Siegwart JT Jr, et al. IOVS 2012;53:ARVO E-Abstract 3457).
In contrast to observations in chickens18 and tree shrews (Siegwart JT Jr, et al. IOVS 2012;53:ARVO E-Abstract 3457), the high light regimen did not appear to alter the rate of defocus-induced refractive compensation in infant monkeys. For instance, high light regimens, which were similar in intensity and daily exposure duration to those employed in this study, slowed compensation for negative lenses in both chickens18 and tree shrews (Siegwart JT Jr, et al. IOVS 2012;53:ARVO E-Abstract 3457), and accelerated compensation for myopic defocus in chickens.18 However, the time scale for the alterations in the rate of lens compensation in chickens and tree shrews was very short. In chickens, high light levels reduced the degree of compensation observed after 2 days of treatment, but as in the normal-light–reared animals, complete compensation was achieved after only 5 days of treatment. In tree shrews, reduced rates of compensation were documented over an 11 day treatment period, but full compensation was achieved in two animals after 16 and 23 days of treatment. In comparison, the time required to achieve full compensation in infant monkeys is approximately 6 weeks, which is substantially longer than that required for comparable degrees of imposed defocus in chickens and tree shrews. In comparison to our typical experimental protocols, we increased the frequency of our measurements during the early part of the treatment period in order to more accurately assess the time course of lens compensation in our infant monkeys. Accordingly, at least five measurements were obtained during the very rapid phase of compensation in the lens-reared monkeys. When considered in relation to the time when compensation is normally complete, the time points when data were collected from our monkeys were comparable to the sample times in chickens and tree shrews. Nevertheless, on an absolute scale, the measurement schedule for the infant monkeys was much less frequent than those employed in the chicken and tree shrew studies. As a consequence, if the effects of the high light regimen on the rate of compensation were restricted to the first few days of treatment, it is unlikely that these effects would have been detected by our measurement protocol.
Lens-Induced Myopia Versus Form-Deprivation Myopia
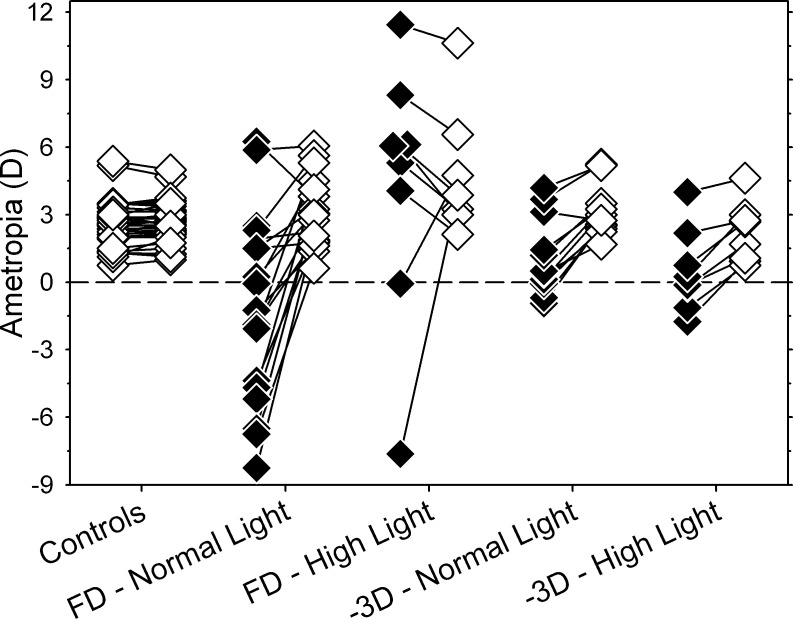
In infant monkeys, high ambient lighting has dramatically different effects on the phenomena of lens-induced myopia and form-deprivation myopia. Figure 7 illustrates the refractive errors obtained at ages corresponding to the end of the treatment period for form-deprived17 and negative lens–reared monkeys. Data are shown for experimental groups reared under ordinary laboratory lighting and under high ambient lighting. The measurement methods, the rearing periods, and the normal and high light regimens for the previous study of ambient light effects on form-deprivation myopia17 were virtually identical to those employed in this study.
Figure 7. .
Refractive errors for the two eyes of individual monkeys are shown for different treatment groups. All of the data were obtained at ages corresponding to the end of the lens-rearing period for the high light animals. The open and filled symbols represent untreated control eyes and treated eyes, respectively. The data for the monocularly form-deprived monkeys exposed to the normal- and high-light regimen were replotted from Smith et al.17
Whereas the high lighting levels did not significantly alter the responses of infant monkeys to negative lens–induced defocus, high light levels largely prevented the development of form-deprivation myopia. At the end of the treatment period, the deprived eyes of 17 of the 18 monocularly form–deprived monkeys reared under ordinary laboratory lighting were less hyperopic/more myopic than their fellow control eyes (i.e., they consistently exhibited form-deprivation myopia). In the high light group, only two of eight form-deprived animals developed relative myopic errors in their treated eyes; in the other six high-light–reared monkeys, the deprived eyes were more hyperopic than their fellow control eyes. It is important to note that the protective effects of high light against form-deprivation myopia were maintained over a prolonged treatment period (3–4 months). In contrast, negative lens imposed defocus produced relative myopia in 11 of the 12 normal-light–reared monkeys and in all eight of the high-light–reared monkeys. These results are important because they demonstrate that high ambient lighting does not generally retard all forms of vision-induced myopia, at least not in a quantitatively similar manner. Instead the protective effects of high ambient lighting appear to be specific for or more robust for form-deprivation myopia.
The data available from chickens and tree shrews, although not conclusive, are in agreement the idea that high lighting levels affect form-deprivation myopia and lens-induced myopia in different ways. For example, over a 4 day treatment period, exposure to high lighting levels reduces the degree of form-deprivation myopia in chicks by approximately 70%. However, it is not known whether extending the treatment period by several days would eliminate the differences in deprivation myopia between normal- and high-light–reared chickens as it did in negative lens–reared animals.16,18 Similarly, over an 11 day treatment period, elevated lighting levels significantly reduced the degree of form-deprivation myopia in tree shrews. Again, it is not known if longer treatment periods would eliminate the differences between form-deprived tree shrews reared under ordinary and high lighting levels because, as pointed out above, with longer treatment periods, two high-light–reared tree shrews fully compensated for the imposed hyperopic defocus (Siegwart JT Jr, et al. IOVS 2012;53:ARVO E-Abstract 3457).
Although many of the anatomic changes produced by form deprivation and hyperopic defocus are similar in nature (e.g., choroidal thinning and axial elongation),2 and many components of the visually driven signal cascade are common to both form-deprivation and lens-induced myopia (e.g., as revealed by the similar effects of muscarinic antagonists),42 a series of experiments, primarily conducted using chickens, have demonstrated that the mechanisms responsible for form-deprivation myopia and lens-induced myopia are not identical.2 For example, interrupting the parasympathetic inputs to the eye reduces the axial myopia produced by form deprivation,19,43,44 but does not alter the compensating axial myopia produced by negative lens–induced hyperopic defocus.19,45 Form deprivation produces some rapid, transient changes in retinal RNA transcript expression that are not observed with negative lens–induced defocus.46 Serotonin antagonists reduce lens-induced myopia, but not deprivation-induced myopia.25 And exposure to brief periods of stroboscopic lighting attenuates form-deprivation myopia much more than negative lens–induced myopia, whereas light exposure during the night preferentially attenuates lens-induced myopia, possibly reflecting differences in the roles of circadian factors in deprivation- and defocus-induced myopia.21 In this respect, the comparisons in Figure 7 provide clear evidence that there are also important differences in the vision-dependent mechanisms that are responsible for form-deprivation myopia and negative lens–induced myopia in primates.
Several observations indicate that the protective effects of high light against form-deprivation myopia involve the retinal dopamine system. First, form deprivation decreases the synthesis and release of retinal dopamine,47–49 which is a strong inhibitor of axial growth. Dopamine agonists inhibit the axial elongation normally produced by form deprivation.24,50 Dopamine antagonists block the protective effects that brief daily periods of unrestricted vision have on form-deprivation myopia.20,51 Retinal dopamine release is enhanced by light stimulation in an intensity dependent manner.52 and flickering lights, which also stimulate retinal dopamine release,53 retard the axial myopia produced by form deprivation.21 Most importantly, the protective effects of high ambient lighting on form-deprivation myopia are blocked by selective dopamine D2 antagonists.18 These results suggest that high ambient light produces a signal that effectively stops the unregulated growth associated with the open-loop viewing conditions produced by form deprivation. In essence, in the absence of visual signals that normally regulate ocular growth (e.g., defocus) the light-stimulated release of retinal dopamine retards the development of form-deprivation myopia, possibly by reducing the intrinsic, default growth rate of the eye.
The role of retinal dopaminergic mechanisms may not be exactly the same in negative lens–induced myopia. For example, unlike form deprivation, induced hyperopic defocus does not consistently downregulate retinal dopamine levels.22,54,55 In chickens, the nonselective dopamine agonist, apomorphine, does reduce the axial elongation produced by negative lenses,24,56 however, in contrast to observations in form-deprived animals, D2 receptor antagonists do not prevent the protective effects of brief daily periods of unrestricted vision on lens-induced myopia.20 Possibly the protective effects of apomorphine on lens-induced myopia are mediated via the drug's effects on serotoninergic mechanisms.57 Regardless, the most relevant observations come from a recent parametric comparison of the effects of dopaminergic agents on deprivation-induced and lens-induced myopia in a mammalian species. Specifically, in guinea pigs with similar degrees of experimental myopia, apomorphine reduced form-deprivation myopia in a dose-dependent manner, but did not alter the course of lens-induced myopia even with dosages 100 times higher than those that completely suppressed form-deprivation myopia.22 These observations together with the failure of high ambient lighting to alter the course of lens-induced myopia suggest that light-stimulated release of retinal dopamine may not be sufficient to block axial elongation that is driven by optical defocus (i.e., as recently suggested by Nickla and Totonelly20 and Dong et al.,22 the role of dopaminergic mechanisms may be different in deprivation myopia and defocus-induced myopia).
Limitations of This Study and Implications for Myopia in Humans
The fact that the high light experiments demonstrate that form-deprivation myopia and lens-induced myopia are not mediated by identical mechanisms in primates is important in several respects. First, this observation is in agreement with previous research in other laboratory animals and provides another example of how many of the basic operating characteristics of the vision-dependent mechanisms that influence ocular growth and refractive development have been conserved across species. In one sense, the high degree of agreement between species supports the extrapolation of the results from laboratory animals to human refractive development. However, with respect to the development of myopia in humans, the differences between deprivation-induced and defocus-induced myopia complicates the extrapolation of the animal data to the human condition. For example, the strong protective effects of elevated lighting levels on form-deprivation myopia supports the idea that the protective effects of time outdoors against the onset of myopia in children is due, at least in part, to the higher light levels encountered outdoors. If this is the case, then there could be substantial therapeutic benefit to manipulating ambient lighting levels in efforts to retard myopia development in children. On the other hand, the failure of elevated lighting levels to alter the course of lens-induced myopia, which is likely to be more relevant to common forms of myopia in humans than form-deprivation myopia, suggests that other factors associated with outdoor activities may influence the development of myopia. In particular, it has been argued that the very flat dioptric topographies associated with outdoor scenes may reduce the risk for the development of myopia.58
However, with respect to the potential antimyopic effects of ambient light levels, it is important to recognize the limitations of the current study. We employed a single high light exposure regimen. In order to thoroughly evaluate the potential therapeutic effects of ambient lighting on the development of myopia, it will be necessary to determine how basic exposure parameters (e.g., intensity, duration, frequency, time of day, and spectral composition) influence the effects of ambient lighting levels on both deprivation-induced and defocus-induced myopia and to characterize in detail the time course for any high light effects. It is also important to note that we employed only one lens power in our experiments and that it is likely that our treatment lenses produced consistently higher degrees of defocus, at least initially, than a child would normally encounter. In this respect, it is possible that the antimyopia effects of elevated lighting levels could be more effective for lower initial amounts of hyperopic defocus. Moreover, it is important to keep in mind that our experiments were conducted in very young animals. Specifically, our rearing period corresponded to approximately 2 through 15 months of life for a human infant.59 Although form deprivation and optically induced defocus can produce myopia in both infants and older, adolescent monkeys,60,61 perhaps the potential protective effects of elevated lighting are more significant, at least for defocus-induced myopia, at older ages, possibly ages corresponding to the onset of juvenile myopia. In addition, at present we cannot rule out the possibility that long term exposure to high ambient lighting, possibly over several years, is required to produce protective effects against myopia.
Although form-deprivation myopia may not be analogous to the most common forms of myopia found in children, there is no doubt, as discussed above, that deprivation myopia and defocus myopia involve many of the same ocular elements and processes. Consequently, studies of form-deprivation myopia can certainly provide insights into the operating characteristics of the vision-dependent cascade that mediates the effects of defocus on refractive development. For example, as discussed above, for a given treatment period, the degree of form-deprivation myopia may reflect the eye's intrinsic growth rate, possibly reflecting the gain of the defocus driven feedback loop that regulates eye growth. Regardless, the results of this study emphasize that the differences observed between deprivation myopia and lens-induce myopia should be considered when attempting to develop treatment strategies to retard myopia in children that involve elevated ambient lighting.
Acknowledgments
Supported by National Institutes of Health Grants EY-03611 and EY-07551 and funds from the Vision Cooperative Research Centre and the University of Houston System Foundation.
Disclosure: E.L. Smith III, P; L.-F. Hung, None; B. Arumugam, None; J. Huang, None
References
- 1. Smith EL III. Charles F. Prentice Award Lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011; 88: 1029–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43: 447–468 [DOI] [PubMed] [Google Scholar]
- 3. Low W, Dirani M, Gazzard G, et al. Family history, near work, outdoor activity, and myopia in Singapore Chinese preschool children. Br J Ophthalmol. 2010; 94: 1012–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang M, Li L, Chen L, et al. Population density and refractive error among Chinese children. Invest Ophthalmol Vis Sci. 2010; 51: 4969–4976 [DOI] [PubMed] [Google Scholar]
- 5. Dirani M, Tong L, Gazzard G, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009; 93: 997–1000 [DOI] [PubMed] [Google Scholar]
- 6. Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007; 48: 3524–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones-Jordan LA, Mitchell GL, Cotter SA, et al. Visual activity prior to and following the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 2011; 52: 1841–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rose K, Morgan I, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008; 115: 1279–1285 [DOI] [PubMed] [Google Scholar]
- 9. Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw SM. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008; 126: 527–530 [DOI] [PubMed] [Google Scholar]
- 10. Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012; 53: 2856–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones-Jordan LA, Sinnott LT, Cotter SA, et al. Time outdoors, visual acuity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci. 2012; 53: 7169–7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng L, Gwiazda J, Thorn F. Children's refractions and visual acuities in the school year and summer. Optom Vis Sci. 2010; 87: 406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donovan L, Sankaridurg P, Ho A, et al. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci. 2012; 89: 1196–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fulk GW, Cyert LA, Parker DA. Seasonal variation in myopia progression and ocular elongation. Optom Vis Sci. 2002; 79: 46–51 [DOI] [PubMed] [Google Scholar]
- 15. Mutti DO, Marks AR. Blood levels of vitamin D in teens and young adults with myopia. Optom Vis Sci. 2011; 88: 377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 5348–5354 [DOI] [PubMed] [Google Scholar]
- 17. Smith EL III, Hung L-F, Huang J Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012; 53: 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010; 51: 5247–5253 [DOI] [PubMed] [Google Scholar]
- 19. Nickla DL, Schroedl F. Parasympathetic influences on emmetropization in chicks: evidence for different mechanisms in form deprivation vs negative lens-induced myopia. Exp Eye Res. 2012; 102: 93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011; 93: 782–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kee C-s, Marzani D, Wallman J Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001; 42: 757–583 [PubMed] [Google Scholar]
- 22. Dong F, Zhi Z, Pan M, et al. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011; 17: 2824–2834 [PMC free article] [PubMed] [Google Scholar]
- 23. Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995; 35: 1175–1194 [DOI] [PubMed] [Google Scholar]
- 24. Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004; 81: 137–147 [DOI] [PubMed] [Google Scholar]
- 25. George A, Schmid KL, Pow DV. Retinal serotonin, eye growth and myopia development in chick. Exp Eye Res. 2005; 81: 616–625 [DOI] [PubMed] [Google Scholar]
- 26. Smith EL III, Harwerth RS, Wensveen JM, Ramamirtham R, Kee C-s, Hung L-F Effects of brief daily periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002; 43: 291–299 [PubMed] [Google Scholar]
- 27. Smith EL III, Hung L-F Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 2000; 40: 371–381 [DOI] [PubMed] [Google Scholar]
- 28. Huang J, Hung L-F, Ramamirtham R, et al. Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci. 2009; 50: 4033–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith EL III, Hung L-F The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999; 39: 1415–1435 [DOI] [PubMed] [Google Scholar]
- 30. Hung L-F, Crawford MLJ, Smith EL III. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Med. 1995; 1: 761–765 [DOI] [PubMed] [Google Scholar]
- 31. Harris WF. Algebra of sphero-cylinders and refractive errors, and their means, variance, and standard deviation. Am J Optom Physiol Opt. 1988; 65: 794–902 [DOI] [PubMed] [Google Scholar]
- 32. Kee C-s, Hung L-F, Qiao-Grider Y, Roorda A, Smith EL III Effects of optically imposed astigmatism on emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2004; 45: 1647–1659 [DOI] [PubMed] [Google Scholar]
- 33. Hung L-F, Ramamirtham R, Wensveen JM, Harwerth RS, Smith EL III. Objective and subjective refractive error measurements in monkeys. Optom Vis Sci. 2012; 89: 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kee C-s, Hung L-F, Qiao Y, Habib A, Smith EL III Prevalence of astigmatism in infant monkeys. Vision Res. 2002; 42: 1349–1359 [DOI] [PubMed] [Google Scholar]
- 35. Smith EL III, Hung L-F, Huang J Relative peripheral hyperopic defocus alters central refractive development in monkeys. Vision Res. 2009; 49: 2386–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whatham A, Judge S. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001; 41: 267–273 [DOI] [PubMed] [Google Scholar]
- 37. Troilo D, Nickla D. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol Vis Sci. 2005; 46: 1873–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qiao-Gridder Y, Hung L-F. Kee C-s, Ramamirtham R, Smith EL III. Recovery from form deprivation myopia in rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci. 2004; 45: 3361–3372 [DOI] [PubMed] [Google Scholar]
- 39. Huang J, Hung L-F, Smith EL III. Recovery of peripheral refractive errors and ocular shape in rhesus monkeys (Macaca mulatta) with experimentally induced myopia. Vision Res. 2012; 73: 30–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wildsoet CF, Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res. 2000; 40: 3273–3282 [DOI] [PubMed] [Google Scholar]
- 41. Amedo AO, Norton TT. Visual guidance of recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri). Ophthalmic Physiol Opt. 2012; 32: 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diether S, Schaeffel F, Lambrou GN, Fritsch C, Trendelenburg A-U. Effects of intravitreally and intraperitonally injected atropine on two types of experimental myopia in chicken. Exp Eye Res. 2007; 84: 266–274 [DOI] [PubMed] [Google Scholar]
- 43. Shih YF, Fitzgerald MEC, Reiner A. The effects of choroidal or ciliary nerve transection on myopic eye growth induced by goggles. Invest Ophthalmol Vis Sci. 1994; 35: 3691–3701 [PubMed] [Google Scholar]
- 44. Schmid GF, Papastergiou GI, Lin T, Riva CE, Laties AM, Stone RA. Autonomic denervations influence ocular dimensions and intraocular pressure in chicks. Exp Eye Res. 1999; 68: 573–581 [DOI] [PubMed] [Google Scholar]
- 45. Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996; 36: 1023–1036 [DOI] [PubMed] [Google Scholar]
- 46. Ashby RS, Megaw PL, Morgan IG. Changes in retinal alphaB-crystallin (cryab) RNA transcript levels during periods of altered ocular growth in chickens. Exp Eye Res. 2010; 90: 238–243 [DOI] [PubMed] [Google Scholar]
- 47. Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989; 2: 465–471 [DOI] [PubMed] [Google Scholar]
- 48. Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989; 86: 704–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weiss S, Schaeffel F. Diurnal growth rhythms in the chicken eye: relation to myopia development and retinal dopamine levels. J Comp Physiol A. 1993; 172: 263–270 [DOI] [PubMed] [Google Scholar]
- 50. Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991; 32: 1674–1677 [PubMed] [Google Scholar]
- 51. Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigment epithelium. Visual Neurosci. 1993; 10: 447–453 [DOI] [PubMed] [Google Scholar]
- 52. Brainard GC, Morgan WW. Light-induced stimulation of retinal dopamine: a dose-response relationship. Brain Res. 1987; 424: 199–203 [DOI] [PubMed] [Google Scholar]
- 53. Umino O, Lee Y, Dowling JE. Effects of light stimuli on the release of dopamine from interplexiform cells in the white perch retina. Vis Neurosci. 1991; 7: 457–458 [DOI] [PubMed] [Google Scholar]
- 54. Guo SS, Sivak JG, Callender MG, Diehl-Jones B. Retinal dopamine and lens-induced refractive errors in chicks. Curr Eye Res. 1995; 14: 385–389 [DOI] [PubMed] [Google Scholar]
- 55. Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995; 35: 1247–1264 [DOI] [PubMed] [Google Scholar]
- 56. Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res. 2010; 91: 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Newman-Tancredi A, Cussac D, Quentric Y, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(s), receptor subtypes. J Pharmacol Exp Ther. 2002; 303: 815–822 [DOI] [PubMed] [Google Scholar]
- 58. Charman WN. Myopia, posture and the visual environment. Ophthalmic Physiol Opt. 2011; 31: 494–501 [DOI] [PubMed] [Google Scholar]
- 59. Qiao-Grider Y, Hung L-F. Kee C-s, Ramamirtham R, Smith EL III. Normal ocular development in young rhesus monkeys (Macaca mulatta). Vision Res. 2007; 47: 1424–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith EL III, Bradley DV, Fernandes A, Boothe RG Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999; 76: 428–432 [DOI] [PubMed] [Google Scholar]
- 61. Zhong X, Ge J, Nie H, Smith EL III. Compensation for experimentally induced hyperopic anisometropia in adolescent monkeys. Invest Ophthalmol Vis Sci. 2004; 45: 3373–3379 [DOI] [PubMed] [Google Scholar]