Abstract
Background
This study was designed to investigate whether pretreatment of bone marrow mesenchymal stem cells (BMSCs) with 5-azacytidine (5-aza) or double intravenous infusion could enhance their therapeutic potential for dilated cardiomyopathy (DCM).
Material/Methods
BMSCs were cultured for 2 weeks in the presence or absence of 5-aza and DCM serum. The cultured BMSCs (Groups 1 and 2), 5-aza-induced BMSCs (Groups 3 and 4), and medium alone (model control) were transplanted into 80 female Wistar rats by intravenous tail vein injection. Double infusion of BMSCs with 1-day time-interval was carried out in Groups 2 and 4. Postmortem histological analysis and evaluation of heart function were performed at 4 weeks post-transplantation.
Results
Some transplanted BMSCs engrafted into myocardial tissue and were positive for cardiac marker troponin T. The hearts containing transplanted BMSCs secreted a larger amount of vascular endothelial growth factor. Cardiac function parameters and serum level of brain natriuretic peptide (BNP) did not differ among Groups 1, 3, and the model control. As compared with model control, BMSC transplantation in Groups 2 and 4 significantly decreased the serum level of BNP and improved cardiac contractile function, as evidenced by reduced left ventricular end-diastolic and end-systolic diameter, elevated ejection fraction, and fractional shortening.
Conclusions
BMSC transplantation is a promising strategy for the treatment of DCM. Pretreatment of BMSCs with 5-aza and DCM serum does not enhance their therapeutic efficacy, and the double intravenous BMSC infusion method is superior to single infusion for preserving cardiac contractile function in a rat model of DCM.
Keywords: mesenchymal stem cells, dilated cardiomyopathy, 5-azacytidine, intravenous cell transplantation
Background
Dilated cardiomyopathy (DCM) is a common cardiac disease of genetic and acquired etiology, and is characterized by cardiomyocyte hypertrophy, necrotic death of cardiomyocytes, and interstitial fibrosis [1,2]. Left or right ventricular systolic pump function of the heart is impaired, leading to progressive cardiac enlargement and hypertrophy, a process known as cardiac remodeling that leads to congestive heart failure (CHF). Several medications, such as angiotensin-converting enzyme inhibitors, beta-blockers, diuretics and calcium antagonists, have emerged as some of the most effective therapeutic regimes for improving left ventricle (LV) function, CHF, and long-term outcome for patients with DCM; however, the mortality rate for these patients remains high (60–70% within 5–10 years of the initial diagnosis) [3]. Heart transplantation is the only effective therapy for end-stage DCM. Unfortunately, a severe shortage of cadaveric organ donors remains a major obstacle to treating end-stage DCM patients.
Bone marrow mesenchymal stem cells (BMSCs) are pluripotent stem cells residing within the bone marrow microenvironment. BMSCs represent an attractive stem cell source for cell-based therapy, based on the following advantageous features: convenient collection, rapid proliferation and expansion in vitro, persistent self-renewal capacities in vivo, and lack of questionable ethical issues. Since BMSCs are derived from the embryonic mesoderm, they have the potential to differentiate into lineages of mesenchymal tissues, including bone, cartilage, fat, tendon, muscle, and marrow stroma [4]. BMSCs have been reported to effectively differentiate into vascular endothelial cells both in vitro and in vivo[5,6]. Additionally, BMSCs can be induced by 5-azacytidine treatment to express cardiac-specific markers and exhibit spontaneous beating and measurable action potential [7]. Accumulating data in the literature has demonstrated that transplanted BMSCs may improve cardiac function in the rat model of acute or chronic myocardial infarction (MI) [8–10]. Therefore, cell therapy with BMSCs has been attempted to attenuate LV remodeling and preserve cardiac function in the rat model of DCM [11,12]. More significantly, a randomized double-blind and placebo-controlled trial also demonstrated the safety and efficacy of autologous BMSCs transplantation in human patients with DCM [13]. However, before envisaging cell therapy for improving myocardial dysfunction of DCM, several unresolved issues still need to be clarified: 1) the efficient approach to delivery; 2) the appropriate amount of administered cells; 3) the multiple administration of stem cells to obtain an optimal cellular homing and retention by the recipient organ; and 4) the best pretreatment approach to achieve structural and functional integration of the transplanted cells with the host tissue.
Intravenous delivery of BMSCs is an attractive minimal-invasive strategy that allows for repeated infusion of large amounts of cells. Moreover, it may be applicable to patients with diffuse myocardial disease. We hypothesized that double infusion of BMSCs is superior to the single infusion approach for preserving heart function by enhancing the cellular uptake by myocardial tissue. The purpose of the present study, therefore, was to test this hypothesis. 5-azacytidine, a DNA demethylation reagent, has been reported to induce BMSCs to differentiate toward the cardiomyogenic phenotype [7,14,15]. Thus, we postulated that in vitro induction of BMSCs with 5-aza might direct the differentiation into cardiomyogenic cells and further strengthen the structural and functional integration of implanted cells with the host cardiomyocytes. In this study, freshly-isolated BMSCs were initially induced toward the cardiomyogenic phenotype by culturing with a combination of 5-aza and serum from DCM rats. This study also investigated whether the pretreatment of BMSCs with 5-aza and DCM serum was able to strengthen their therapeutic efficacy in DCM rats.
Material and Methods
The animals used in this study received humane care in compliance with the Guide to the Care and Use of Experimental Animals that was formulated by the Medical Ethical Committee on Animal Experiments of Jilin University (Jilin, China).
Preparation of BMSCs
Bone marrow was harvested from anesthetized female Wistar rats (n=10; 8–10 g in weight; 5 d) by flushing the femoral and tibial cavities with phosphate-buffed saline (PBS). Marrow cells were transferred to a sterile tube and mixed with essential medium supplemented with 10% fetal bovine serum and antibiotics. The tube was centrifuged at 2000 rpm for 5 min and the cell pellet was resuspended in 5 mL culture medium. To separate BMSCs and red blood cells, gradient density centrifugation was performed as previously described [16]. The isolated cells were incubated in cell culture medium for 24 hours. Upon medium replacement, non-adherent hematopoietic cells were washed away.
For convenient identification of the transplanted BMSCs, cells were labelled with bromodeoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO, USA). Briefly, 10 μL of a 1 mM BrdU working solution (1:30 dilution of BrdU stock solution in tissue culture media) was directly added to each mL of tissue culture media on the sixth day of culture. The treated cells are then incubated for 24 hours.
Immunohistochemical staining of cultured BMSCs
The BMSCs were cultured for a total 14 days. 5-azacytidine (5 μmol/L) was added into CCM on the third day and incubated with BMSCs for 24 hours. The BMSCs were then incubated in CCM containing 20% of serum from DCM rats for an additional 11 days.
The BMSCs were fixed with cold methanol for 30 min, washed 3 times with PBS, and blocked by incubating with 2% goat serum for 30 min at room temperature. The BMSCs were then incubated with rabbit anti-rat troponin T (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37°C for 1 hour. For the negative control, PBS was substituted for the primary antibody. After 3 washes with PBS, slides were incubated with biotinylated goat anti-rabbit IgG for 30 min at 37°C. Finally, avidin D-horseradish peroxidase (HRP) was added and incubated for 30 minutes at 37°C. The peroxidase activity was detected by incubating with the 3,3’ diaminobenzidine (DAB) substrate system for 5 minutes.
Western blotting
Total protein concentration of cells was determined using the Bradford assay with Coomassie Protein Assay Reagent Kit (Pierce Biotechnology, Rockford, IL); 50 μg of each were boiled for 5 minutes and loaded onto a 12% SDS polyacrylamide gel for resolution by electrophoresis and subsequent electroblotting to nitrocellulose membranes. After blocking, membranes were incubated with primary rabbit anti-rat antibody against troponin T (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at room temperature. After 3 washes with TTBS buffer, a second incubation was performed with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:5000) for 2 hours at room temperature. Immunoreactive bands were detected by an enhanced chemiluminescence kit (Amersham Life Science, Arlington Heights, IL, USA).
Animal model of DCM and cell transplantation
Eighty female Wistar rats (210–240 g in weight) were treated with Adriamycin by intraperitoneal injection of 2.5 mg/kg dose twice per week for 6 weeks, in order to induce DCM. Fifteen additional rats received intraperitoneal injections of an equivalent volume of 0.9% saline alone and served as normal controls.
At 2 weeks after successful establishment of DCM, 65 surviving rats were randomized into 5 groups (listed below) and the tail veins were cannulated for infusion of BMSCs or CCM. Rats in the Model Group (n=17) received intravenous infusion of 100 μL CCM alone. Rats in Group 1 (n=12) underwent intravenous infusion of 5×106 BMSCs/100 μL in CCM. Rats in Group 2 (n=12) underwent double intravenous infusion of BMSCs using the same dosage twice and a 1-day time-interval. Rats in Group 3 (n=12) underwent intravenous infusion of 5×106 5-aza-induced BMSCs/100 μL in CCM. Rats in Group 4 (n=12) received double infusions of 5-aza-induced BMSCs using the same dosage twice and a 1-day time-interval.
Echocardiographic examination
Transthoracic echocardiography was performed at 4 weeks post-transplantation using a 13-MHz phased-array transducer (Aloka 5500; Aloka Ltd., Tokyo, Japan) by an investigator who was blinded to the treatment-groups. M-mode tracing of LV was obtained by imaging of the heart beating in the 2-dimensional mode with short-axis at the level of the papillary muscle. LV end-systolic diameter (LVESD) and end-diastolic diameter (LVEDD) were measured according to the American Society of Echocardiography leading-edge method using at least 3 consecutives cardiac cycles. Fractional shortening (FS) was calculated as [((LVEDD-LVESD)/LVEDD) ×100]. LV ejection fraction (LVEF) was calculated on the basis of the Teichholtz formula.
Histological examination
Frozen rat hearts were embedded in the OCT compound and transversely cut into 8-μm thick slices in the direction from apex to base. Tissue sections were fixed with 4% paraformaldehyde and stained with Masson’s trichrome and hematoxylin-eosin (HE). Twenty randomly selected fields were microscopically analyzed in each Masson’s trichrome-stained section. After each field was scanned and digitized with Image ProPlus software (version 6.0; Media Cybernetics, Silver Springs, MA, USA), the collagen volume fraction (CVF) was calculated as the sum of all areas containing fibrotic tissue divided by the total area of the image. HE-stained sections were analyzed under a light microscope by a pathologist who was blinded to the design of the experiment.
Immunohistochemical analysis of tissue sections
Tissue sections were fixed with 4% paraformaldehyde for 10 min at room temperature, washed 3 times with PBS containing 0.3% Triton X-100, and blocked with 3% bovine serum albumin (BSA) for 30 min at room temperature. Slides were then incubated with primary antibodies: rabbit anti-rat BrdU (1:200; Santa Cruz Biotechnology) and mouse anti-rat cTnT (1:200; Santa Cruz Biotechnology) at 4°C overnight. After 3 washes with PBS, slides were incubated with Cy3-conjuagted goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG for 30 min at 37°C. After 3 more washes with PBS, the slides were stained with Hochest 33342 to detect nuclei. Laser scanning confocal microscopy (FV-1000; Olympus, Japan) was used to identify the florescence distribution. The infused BMSCs or troponin T positive BMSCs were counted in 6 high power fields of vision under ×200 magnification for each section.
Tissue sections were incubated with the preliminary rabbit anti-rat antibody against vascular endothelial growth factor (VEGF) (1:200; Dako, Cambridge, UK) at room temperature overnight. After washing 3 times in Tris-buffered saline (TBS), the sections were incubated with biotinylated mouse anti-rabbit IgG (1:5000; Abcam, Cambridge, MA) for 30 minutes and followed by 3 5-minute washes in TBS. The final incubation was for 30 minutes with avidin D-HRP at 37°C. The peroxidase was detected by incubating with DAB substrate for 5 minutes. The sections were counterstained with hematoxylin and mounted using neutral gum medium.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from rat heart tissue using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. One microgram of RNA was reverse transcribed using SuperScript III reverse transcriptase (Invitrogen). The resultant cDNA was applied as the template for PCR amplification. The β-actin gene was amplified as an internal reference. The primers used for VEGF were upstream primer 5’-TCTTCAAGCCGTCCTGTG-3’ and downstream primer 5’-AAATGCTTTCTCCGCTCT-3’; for β-actin were upstream primer 5’-GTCAGGTCATCACTATCGGCAAT-3’ and downstream primer 5’-AGAGGTCTTTACGGATGTCAACGT-3’. Thermal cycle conditions were as follows: 1 cycle of initialization at 94°C for 5 minutes, 28 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and elongation at 72°C\for 45 seconds. The resultant PCR products were visualized on 1% agarose gels stained with ethidium bromide. The band intensity was evaluated with Band Scan 5.0 software.
Measurement of brain natriuretic peptide (BNP)
Blood was collected from every rat at 4 weeks after BMSC transplantation. Since the active BNP are rapidly cleaved and have a half-life of 3–4 min, we determined the serum level of N-terminal BNP using an electrochemiluminescence immunoassay (ProBNP Elecsys; Roche Diagnostics GmbH, Germany). The N-terminal BNP levels were measured with an ELISA kit (Assaypro, St. Charles, MO, USA); reproducibility and precision of this assay is well below 5%, even when using high concentrations of N-terminal BNP.
Statistical analysis
Statistical analysis was performed with SPSS software, version 13.0 (SPSS, Chicago, IL, USA). All values are expressed as the mean ± standard deviation (SD). Chi-square test (X2) was used to compare the parameters among groups.
Results
Ex vivo cardiomyogenic transdifferentiation of pretreated BMSCs
Representative negative control and pretreated BMSCs stained for cardiac marker troponin T are shown in Figure 1A and 1B, respectively. A representative Western blot of cardiac troponin T is shown in Figure 1C. Positive expression of troponin T was visualized as a cytoplasmically localized granular brown-yellow precipitate. As expected, positive staining for troponin T was not present in the negative control. The BMSCs pretreated with 5-aza and DCM serum stained positively on the 14th day for cardiac-specific troponin T. Western blotting identified troponin T as a 32-kDa band that was slightly larger in the pretreated BMSCs than in the negative control. This finding indicated that the freshly-isolated BMSCs were successfully induced toward the cardiomyogenic phenotype by a combination of 5-aza and serum from DCM rats.
Figure 1.
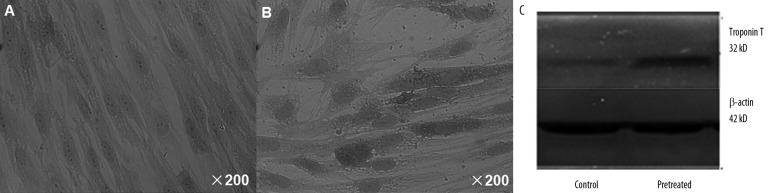
The negative control BMSCs showed negative staining for troponin T (A), but BMSCs pretreated with 5-aza and DCM serum showed positive staining for troponin T (B). Troponin T, a large protein band, was present in the pretreated BMSCs (C).
Body weight and mortality rate of treated rats
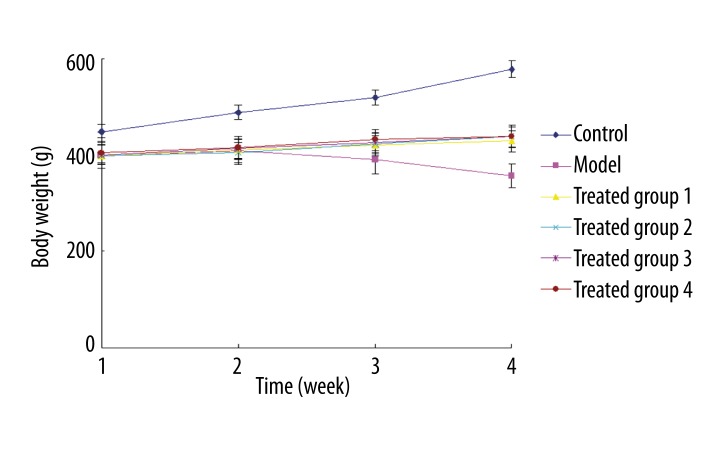
Figure 2 shows of the variations in body weight (BW) during the 4 post-transplantation weeks for each of the 6 treatment groups. The mortality rates of treated rats are summarized in Table 1. The normal group showed progressively increased BW and no occurrence of death. The model group exhibited idleness, anorexia, and diarrhea. The BW of the model group reduced progressively with the duration of Adriamycin treatment, and the mortality rate of this group was 58.8% (10/17). In Group 1, BW slightly increased with the duration of feeding, and the mortality rate was 25% (3/12). In Group 2, BW moderately increased with the duration of feeding, and the mortality rate was 8.3% (1/12). In Group 3, BW slightly increased with the duration of feeding, and mortality rate was 33.3% (4/12). In Group 4, BW moderately increased with the duration of feeding, and mortality rate was 16.7% (2/12). These findings not only indicated that DCM was associated with reduced BW and death of animals, but also suggest that BMSC transplantation contributed to recovery of BW and reduction in mortality rates, and that double infusion of BMSCs was more efficacious than single infusion.
Figure 2.
The normal group (dark blue) showed progressively increased BW. BW was gradually decreased with the duration of adriamycin treatment in the model group (pink). BW slightly increased with the duration of feeding in Groups 1 (yellow) and 3 (purple), and. BW moderately increased with the duration of feeding in Groups 2 (light blue) and 4 (brown).
Table 1.
Mortality rates of the six groups of rats.
| Normal | Model | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|---|
| Mortality | 0 | 58.8* | 25# | 8.3#,& | 33.3# | 16.7#,& |
| Rate | (0/15) | (10/17) | (3/12) | (1/12) | (4/12) | (2/12) |
Data is presented as% (n/total);
P<0.05 compared with Normal control;
P<0.05 compared with Model group;
P<0.05 compared with Group 1 or Group 3.
In vivo potential cardiomyogenic transdifferentiation of BMSCs
The nuclei of the cells were labelled with BrdU prior to transplantation. Representative staining sections for troponin T are shown in Figure 3. No BrdU-stained cells were observed in the model group. At 4 weeks post-transplantation, BrdU-stained cells were found to have engrafted into the heart, where they were discretely distributed throughout the myocardial regions. Some of the transplanted BMSCs stained positively for troponin T and appeared to have fused with native cardiomyocytes. These findings suggested that transplanted BMSCs may be capable of differentiating into the cardiomyogenic phenotype.
Figure 3.
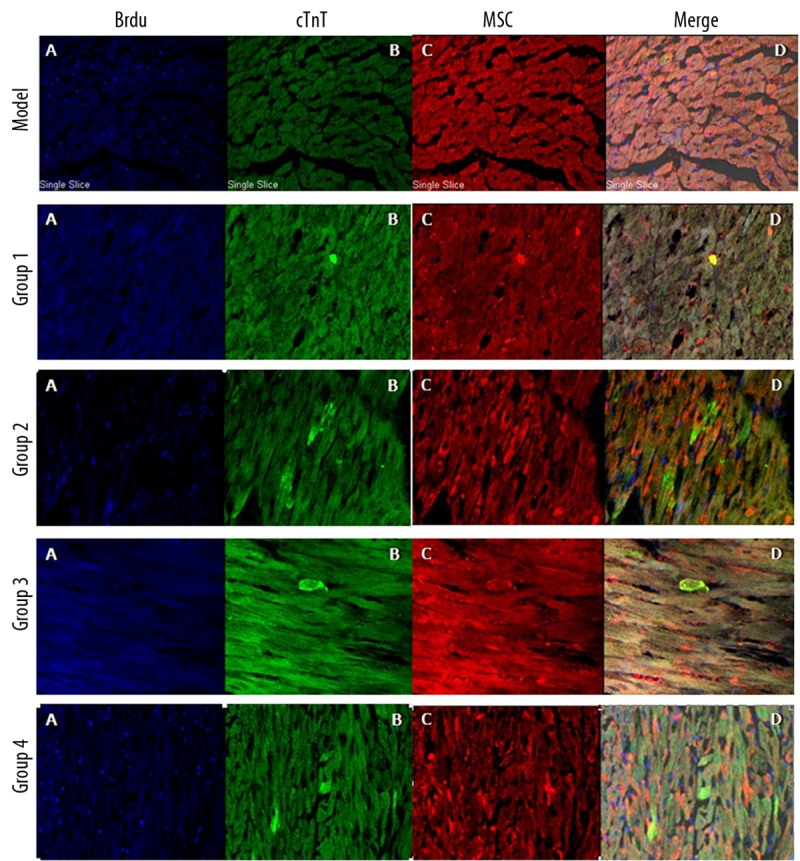
No BrdU-stained cells were present in the model group. BrdU-labelled cells (A) were discretely distributed throughout the myocardial regions of Groups 1, 2, 3 and 4. Some of transplanted BMSCs were also positive for troponin T (B–D).
Table 2 summarizes the myocardial distribution density of the infused BMSCs and the troponin T positive BMSCs. The transplanted BMSCs in Group 1 and Group 3 showed similar distribution patterns, and the BMSC distribution density of Group 2 and Group 4 were comparable. As compared with Groups 1 and 3, the transplanted BMSCs were more densely distributed in Groups 2 and 4. These findings indicate that double intravenous infusion significantly enhanced the homing of transplanted BMSCs to the myocardial region, as compared to single infusion.
Table 2.
Myocardial distribution density of infused BMSCs and cTnT positive BMSCs in the four groups of rats.
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| BMSCs | 6.5±0.66 | 15.1±2.33& | 5.0±0.79* | 14.5±2.52#,& |
| cTnT | 1.2±1.1 | 3.2±1.7& | 1.3±1.1* | 3.0±1.6#,& |
Data is presented as mean numbers of cells per ×200 objective fields ±SD.
P>0.05 compared with Group 1;
P>0.05 compared with Group 2;
P<0.05 compared with Group 1 or Group 3.
Serum level of brain natriuretic peptides
The serum levels of BNP detected in the 6 groups of rats are summarized in Table 3. The serum level of BNP was significantly elevated in the model group as compared to that in the normal group. This finding indicates that DCM induced substantial elevation of serum BNP. The serum level of BNP did not differ between Groups 1 and 3, and no difference in BNP level was observed between Groups 2 and 4. These findings indicate that the pretreatment of BMSCs by 5-aza and DCM serum did not influence the serum level of BNP. More importantly, there were no significant differences in the serum level of BNP among Groups 1 and 3 and the model group. In contrast, the serum levels of BNP in Groups 2 and 4 were substantially higher than those in the model group. These indicate that double infusion of BMSCs was superior to single infusion in reducing the serum level of BNP.
Table 3.
Serum BNP level of the six groups of rats.
| BNP (pg/mL) | |
|---|---|
| Normal control (n=15) | 346.1±82.3 |
| Model group (n=10) | 889.1±210.5* |
| Group 1 (n=8) | 751.4±188.2# |
| Group 2 (n=11) | 538.3±132.4@,§ |
| Group 3 (n=8) | 731.6±146.5# |
| Group 4 (n=10) | 562.6±139.3@,§ |
BNP – brain natriuretic peptide.
P<0.05 compared with Normal control;
P>0.05 compared with Model group;
P 0.05 compared with Model groupl;
P<0.05 compared with Group 1 or Group 2.
VEGF factors released from transplanted BMSCs
VEGF expression was visualized as nuclear and cytoplasmic localized granular brown-yellow precipitate. Representative staining sections for VEGF are shown in Figure 4. The brown-yellow staining for VEGF was absent in the normal group. However, staining for VEGF was present in Groups 1–4, and the Model Group; the levels were least obvious in the Model group. These findings indicate that BMSC transplantation could promote the myocardial expression of VEGF.
Figure 4.
VEGF expression was indicated by brown-yellow staining of tissue sections. VEGF staining was not obvious in the normal group. As compared with the model group, Groups 1–4 exhibited a more robust distribution of VEGF expression.
VEGF and β-actin mRNA expression was detected as band intensities of PCR products resolved on gels, and are shown in the upper and lower panels of Figure 5. The VEGF mRNA expression was defined as the gray value of VEGF relative to the β-actin band intensity. The relative VEGF mRNA expressions detected in heart tissues from all 6 groups of rats are summarized in Table 4. The relative VEGF mRNA expression was moderately higher in the Model Group than in the Normal Group, indicating that DCM could induce VEGF mRNA expression. There was no significant difference in the VEGF mRNA expression detected between Groups 1 and 3 or between Groups 2 and 4, indicating that the pretreatment with 5-aza and DCM serum do not enhance myocardial VEGF mRNA expression induced by BMSC transplantation. More importantly, VEGF mRNA expression did not differ among Group 1, 3 and the Model Group. VEGF mRNA expression in Groups 2 and 4 were substantially higher than those detected in the Model Group, indicating that double infusion of BMSCs was better than single infusion in promoting the secretion of VEGF.
Figure 5.
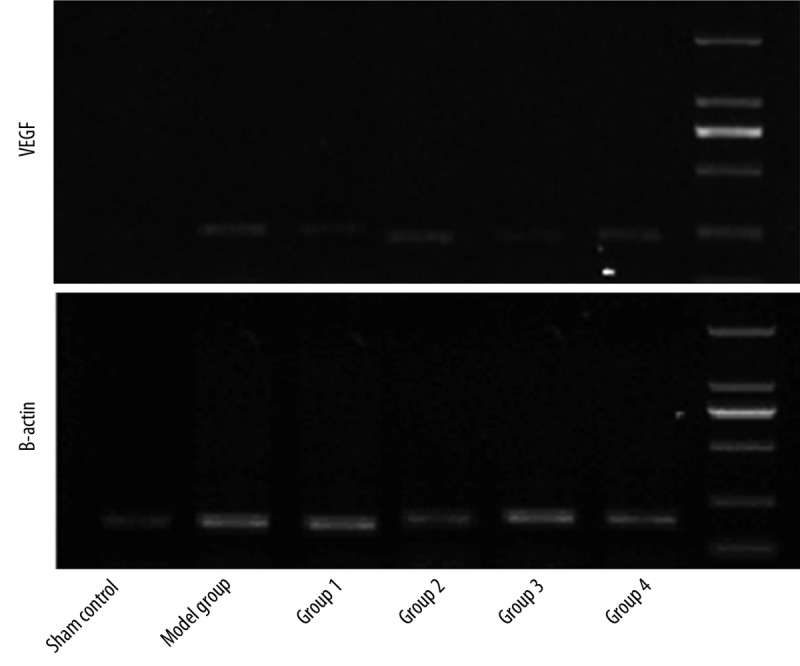
VEGF mRNA expression was slightly higher in the model group than in the normal group. VGEF mRNA expression of Groups 1 and 3 were comparable, as were those of Groups 2 and 4. More importantly, the expression of VEGF mRNA in Groups 2 and 4 was markedly higher than that in Groups 1 and 3.
Table 4.
VEGF mRNA expression in heart tissues from the six groups of rats.
| VEGF mRNA expression‡ | |
|---|---|
| Normal control (n=15) | 0.12±0.02 |
| Model group (n=10) | 0.34±0.02* |
| Group 1 (n=8) | 0.42±0.02# |
| Group 2 (n=11) | 0.60±0.03@,§ |
| Group 3 (n=8) | 0.38±0.02# |
| Group 4 (n=10) | 0.56±0.02@,§ |
Relative to β-actin;
P<0.05 compared with Sham control;
P>0.05 compared with Model group;
P<0.05 compared with Model group;
P<0.05 compared with Group 1 or Group 3.
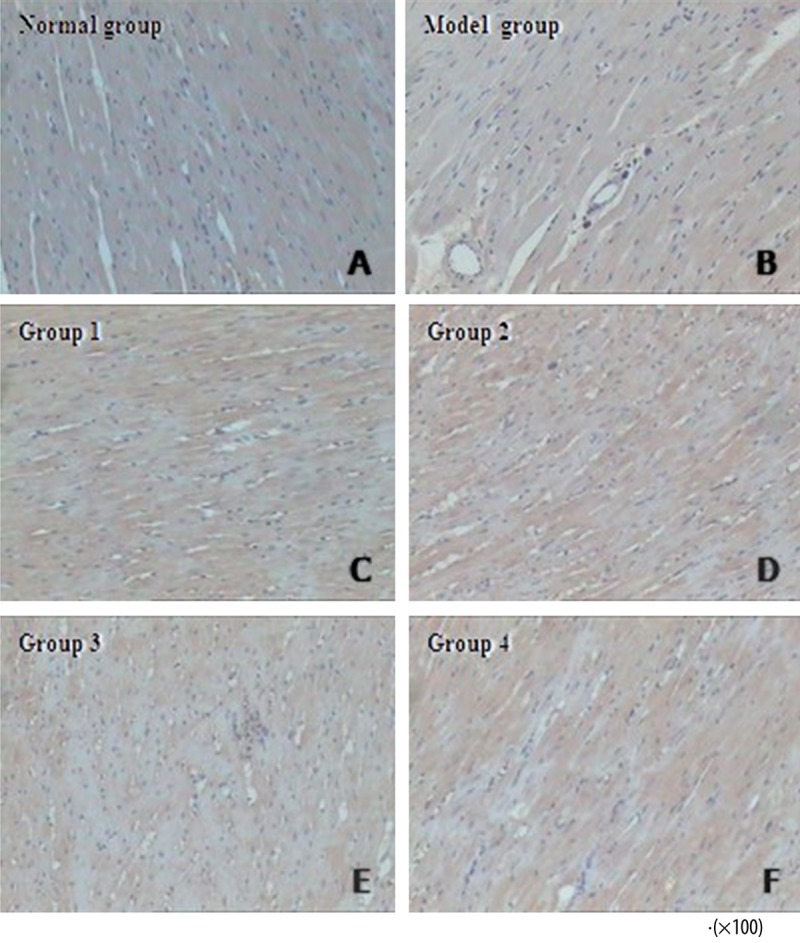
Cardiac fibrosis and collagen volume fraction
Representative Masson’s trichrome stained sections from the 6 groups of rats are shown in Figure 6. The Normal Group was characterized by a discrete distribution of collagen fibers and an intact network of delicate fibers. In the Model Group, however, the deposition of collagen fibers was obvious, exhibiting an interrupted collagen network that was irregularly arranged. In Groups 1 and 3, the collagen fiber formation was still obvious, but the broken fibers were arranged randomly in all directions. In Groups 2 and 4, the collagen fiber components were reduced, and the integrity of the collagen network appeared to be restored with an organized fiber structure.
Figure 6.
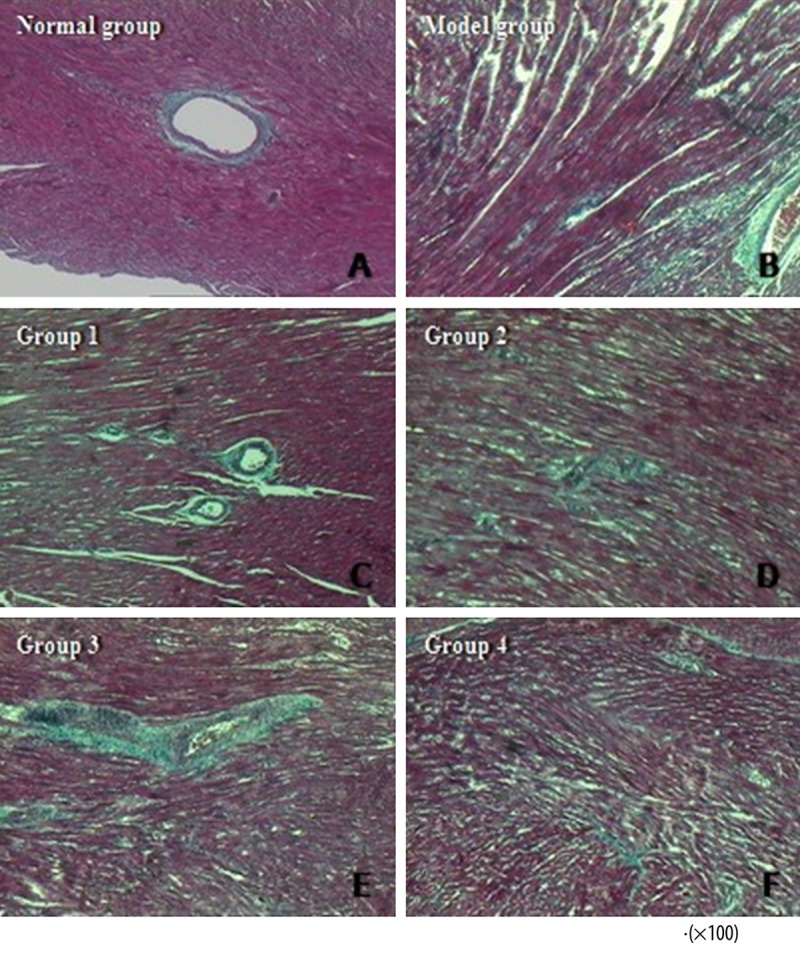
The normal group showed an intact network of delicate fibers (A). Severe cardiac fibrosis was observed in the model group (B). Groups 1 (C) and 3 (E) did not exhibit any reduction in collagen deposition or recovery in the fiber meshwork integrity. However, in Groups 2 (D) and 4 (F), the collagen components were decreased with more well-organized fiber structure.
The collagen volume fractions in the 6 groups of rats are summarized in Table 5. CVF was significantly elevated in the model group, as compared to the normal group. This finding indicated that DCM was associated with a substantial deposition of collagen. CVF did not differ either between Groups 1 and 3 or between Groups 2 and 4. These findings indicated that pretreatment with 5-aza and DCM serum did not enhance the curative effect of BMSCs on suppressing collagen deposition. More importantly, there was no difference observed in the CVFs among Groups 1 and 3 and the Model Group. CVFs in Groups 2 and 4 were substantially higher than those in the model group, indicating that double infusion of BMSCs was superior to single infusion for depressing collagen deposition.
Table 5.
CVF of hearts in the six groups of rats.
| CVF (%) | |
|---|---|
| Normal control (n=15) | 3.66±0.71 |
| Model group (n=10) | 26.59±3.60* |
| Group 1 (n=8) | 22.89±5.46# |
| Group 2 (n=11) | 16.19±5.71@,§ |
| Group 3 (n=8) | 22.85±6.87# |
| Group 4 (n=10) | 17.22±4.9@,§ |
CVF – collagen volume fraction.
P<0.05 compared with Normal control;
P>0.05 compared with Model group;
P<0.05 compared with Model group;
P<0.05 compared with Group 1 or Group 2.
Analysis of pathological staining
Representative HE staining sections from the 6 groups of rats are shown in Figure 7. In the Normal Group, cardiac muscle fibers were regularly organized in a linear array without interruption, the cardiomyocytes had abundant and clear cytoplasm, and the interstitial compartment appeared normal and had no excessive accumulation of fluid. In the Model Group, cardiac muscle fibers were irregularly arranged and interlaced with one another, while the interstitial compartment was enlarged by excessive fluid accumulation, and the cardiomyocytes exhibited partial vacuolar degeneration. In Groups 1 and 3, the cardiac muscle fibers were arranged irregularly, the interstitial compartment was enlarged by collected edematous fluid, and partial vacuolar degeneration was observed. In Groups 2 and 4, the cardiac muscle fibers appeared to be regularly arranged with no obvious disruption, and observations of interstitial edema and cytoplasmatic partial vacuolar degeneration were less frequent.
Figure 7.
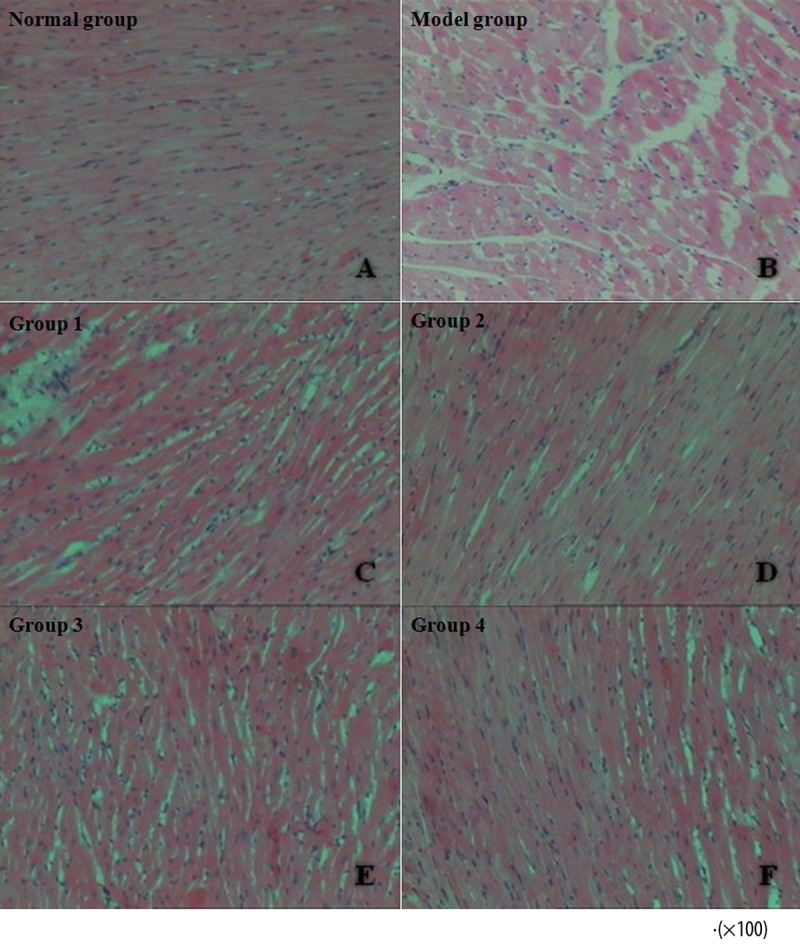
Irregularly organized cardiac muscle fibers, excessive fluid accumulation within the interstitial compartment, and partial vacuolar degeneration of cardiomyocytes were observed in the model group (A). The cardiac histopathological features of Groups 1 (C) and 3 (E) were very similar to those of the model group. However, Groups 2 (D) and 4 (F) exhibited regularly aligned cardiac muscle fibers, discrete interstitial edema, and only occasional partial vacuolar degeneration.
Cardiac function parameters
Table 6 summarizes the cardiac function parameters for each of the 6 groups of rats at 4 weeks after BMSCs transplantation. LVEDD and LVESD were significantly larger in the Model Group, indicating that DCM was accompanied by substantial LV cavity enlargement. However, LVEDD and LVESD did not differ between Groups 1 and 3 or between Groups 2 and 4, indicating that the pretreatment with 5-aza and DCM serum did not enhance the curative effect of BMSCs on attenuating cardiac enlargement. More importantly, there were no differences in LVEDD and LVESD observed among Groups 1 and 3 and the Model Group. LVEDD and LVESD in Groups 2 and 4, however, were substantially less than those in the model group. These findings indicate that double infusion of BMSCs was superior to single infusion in suppressing the LV cavity enlargement.
Table 6.
Cardiac function parameters for the six groups of rats.
| LVEDD (mm) | LVESD (mm) | LVEF (%) | FS (%) | |
|---|---|---|---|---|
| Normal control | 6.3±0.8 | 3.4±0.5 | 81.8±7.6 | 47.7±6.7 |
| Model group | 8.5±0.9* | 5.4±0.6* | 61.0±10.8* | 32.4±4.4* |
| Group 1 | 7.5±0.6# | 4.8±0.6# | 67.5±7.9# | 37.7±3.7# |
| Group 2 | 6.8±0.6@,§ | 3.8±0.6@,§ | 75.4±6.3@,§ | 39.7±6.1@,§ |
| Group 3 | 7.4±0.4# | 4.9±0.4# | 66.6±5.3# | 36.8±5.8# |
| Group 4 | 6.6±0.5@,§ | 3.7±0.5@,§ | 74.8±6.7@,§ | 40.5±3.9@,§ |
LVEDD – left ventricular end-diastolic diameter; LVESD – left ventricular end-systolic diameter; LVEF – left ventricular ejection fraction; FS – fractional shortening.
P<0.05 compared with Normal control;
P>0.05 compared with Model group;
P<0.05 compared with Model group;
P<0.05 compared with Group 1 or Group 2.
Both EF and FS were significantly reduced in the Model Group, indicating that DCM was accompanied by substantially impaired cardiac function. EF and FS did not differ between Groups 1 and 3, or between Groups 2 and 4, indicating that pretreatment with 5-aza and DCM serum did not enhance the curative effect of BMSCs on preserving cardiac function. More importantly, there were no differences in EF and FS among Groups 1 and 3 and the Model Group. EF and FS in Groups 2 and 4, however, were substantially higher than those in the model group. These findings indicated that double infusion of BMSCs was superior to single infusion in improving cardiac function.
Discussion
Earlier studies have shown that intramyocardial injection of BMSCs improves cardiac function in an experimental rat model of DCM [12]. However, little information is available about the therapeutic efficacy of intravenous infusion of BMSCs for DCM. Whether pretreatment of BMSCs by 5-aza and DCM serum or double infusion are capable of enhancing their therapeutic potential has not been well-defined. In this study, we demonstrated the following conclusions: 1) 5-aza and DCM serum contribute to in vitro differentiation of BMSC into the cardiomyogenic phenotype; 2) transplanted BMSCs can differentiate into the cardiomyogenic phenotype and secrete a large amount of VEGF; 3) transplanted BMSCs suppress the deposition of collagen and improve cardiac function; 4) pretreatment with 5-aza and DCM serum does not enhance the therapeutic efficacy of BMSCs; and; 5) double infusion of BMSC is superior to single infusion in preservation of cardiac function.
Contrary to our initial hypothesis, we did not observe that pretreatment of BMSCs by 5-aza and DCM serum could attenuate the DCM-associated pathological changes and further improve cardiac contractile function. Our finding appears to disagree with previously published studies. Ling et al. [17] demonstrated that induction of BMSCs by 5-aza improved their in vivo cardiac differentiation and led to a significantly larger increase in LV function in a rat model of MI. Tomita et al. [18] found that BMSCs cultured with 5-aza could differentiate into cardiac-like muscle cells in vivo in an MI model, and that the 5-aza-treated BMSC-transplanted hearts had systolic and developed pressures that were higher than those of BMSC hearts. MI is characterized by continuous loss of contractile cardiomyocytes, which are replaced by non-contractile scar tissue. The 5-aza-treated BMSCs have a more robust ability to differentiate into cardiomyocytes and fuse with native host tissue, further replenishing the lost cardiomyocytes and improving the efficiency of beating cell generation in ischemic MI. However, cardiomyocytes hypertrophy and interstitial fibrosis are the main reasons for impaired cardiac function of DCM. It is reasonable to speculate that differentiation of BMSCs into cardiomyocytes does not significantly influence cardiac hypertrophy and fibrosis. Moreover, Zhang et al. [19] demonstrated that induction of BMSCs by 5-aza results in reduced proliferation speed, potentially impairing the self-renewal capability of transplanted BMSCs in vivo.
Up-regulation of stromal-cell-derived factor 1 and its receptor, CXCR4, is known to play a critical role in recruiting stem cells to damaged myocardium [20,21]. Our study demonstrated that DMSCs homed to and engrafted into the myocardial tissue at 4 weeks after intravenous transplantation. Previous studies also indicated that intravenously transplanted BMSCs were preferentially attracted to and retained in infracted myocardium [22,23]. Thus, intravenous delivery of BMSCs offers a relatively safe, effective and minimally-invasive approach for targeting cell delivery to myocardium. No data are yet available on the optimal cell dosage of intravenous transplantation. Intravenous delivery of 5×106 BMSCs has been showed to improve cardiac function in a rat model of acute MI [22]. Pulmonary passage is a major obstacle to intravenous stem cell delivery [24]. Although the majority of BMSCs are trapped inside the lungs following intravenous infusion, the 5×106 concentration of BMSCs is relatively safe for lungs and does not cause deterioration of lung function in a rat model of chronic MI [25]. Unfortunately, our study indicated that the single intravenous delivery of 5×106 BMSCs did not improve the cardiac function in a rat model of DCM. However, double intravenous delivery of 5×106 BMSCs did significantly improve the cardiac function, as evidenced by decreased LVESD and LVEDD and increased EF and FS. The differential efficacy of single vs. double infusion is presumably attributed to the increased homing and retention of BMSCs in targeted heart. The dosage of intravenous BMSCs has been shown as being positively related to the beneficial effect on cardiac function in a study of infarcted porcine heart [26]. The dose-dependent response relationship has also been observed between the number of transplanted BMSCs and muscular functional regeneration after severe skeletal muscle injury in rats [27].
Serum level of BNP is a well-established independent indicator of LV remodelling and survival in patients with cardiomyopathy [28]. Indeed, in the rat cohort of our study, elevated BNP serum level was a powerful independent marker of death. More importantly, the double infusion of BMSCs resulted in a considerable reduction in BNP serum levels in DCM-induced rats with elevated BNP serum level at baseline. Moreover, the double infusion of BMSCs was associated with a significantly lower mortality during the entire experimental period. Taken together, these data demonstrate that intravenous BMSC transplantation can beneficially interfere with LV remodelling processes and reduce the mortality rates of advanced DCM.
Some transplanted BMSCs have been documented as staining positively for von Willebrand factor and smooth muscle actin, which suggests, respectively, that BMSCs can differentiate into endothelial cells and vascular smooth muscle cells [12]. Previous studies also demonstrated that BMSCs can participate in new blood vessel formation in vivo and promote construction of a complete network of capillary structures in a Matrigel-based in vitro assay system [12,22,29]. Furthermore, engrafted BMSCs induce angiogenesis by supplying a variety of angiogenic factors, including VEGF, hepatocyte growth factor, and insulin growth factor (IGF)-1, which not only induce proliferation of pre-existing vasculature but also suppress ischemic apoptosis of hypertrophied cardiomyocytes [30]. Thus, BMSCs may increase myocardial capillary density not only through their ability to generate capillary-like structures, but also through a paracrine mechanism. DCM alone is associated with a relative decrease in capillary density. Therefore, BMSC-induced neovascularisation would be expected to contribute to improvement of cardiac function. The matrix metalloproteases (MMPs) also play a crucial role in LV remodelling in DCM. In fact, transplanted BMSC-induced inhibition of MMP activities have been shown to attenuate collagenous LV remodelling in a rat model of DCM [12], which is consistent with the findings from our current study. Although the underlying mechanisms of BMSC transplantation remain unclear, it is likely that attenuation of the deposition of extracellular collagen and LV remodelling are involved.
Conclusions
Intravenous infusion of BMSCs is capable of improving cardiac function in a rat model of DCM, possibly through differentiation into the cardiomyogenic phenotype, secretion of angiogenic factors, such as VEGF, and inhibition of cardiac fibrosis. Pretreatment of BMSCs with 5-aza and DCM serum does not enhance their therapeutic efficacy, but double infusion of BMSCs is superior to single transplantation in improving cardiac function in a rat model of DCM.
Acknowledgement
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Footnotes
Source of support: This work was supported by grants from the Science and Technology Department of Jilin Province Youth Fund Project (No. 201101069) and the Jilin Province Development and Reform Commission Project (No. 20100173)
References
- 1.Luk A, Ahn E, Soor GS, Butany J. Dilated cardiomyopathy: a review. J Clin Pathol. 2009;62(3):219–25. doi: 10.1136/jcp.2008.060731. [DOI] [PubMed] [Google Scholar]
- 2.Fidziańska A, Walczak E, Glinka Z, et al. Ultrastructural evidence of myocardial capillary remodeling in peripartum cardiomyopathy. Med Sci Monit. 2010;16(5):CS62–66. [PubMed] [Google Scholar]
- 3.Thiene G, Basso C, Calabrese F, et al. Twenty years of progress and beckoning frontiers in cardiovascular pathology: cardiomyopathies. Cardiovasc Pathol. 2005;14(4):165–69. doi: 10.1016/j.carpath.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Eve DJ. The continued promise of stem cell therapy in regenerative medicine. Med Sci Monit. 2011;17(12):RA292–305. doi: 10.12659/msm.882152. [DOI] [PubMed] [Google Scholar]
- 5.Kashani IR, Golipoor Z, Akbari M, et al. Schwann-like cell differentiation from rat bone marrow stem cells. Arch Med Sci. 2011;7(1):45–52. doi: 10.5114/aoms.2011.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 7.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai T, Li RK, Weisel RD, et al. Autologous heart cell transplantation improves cardiac function after myocardial injury. Ann Thorac Surg. 1999;68(6):2074–80. doi: 10.1016/s0003-4975(99)01148-0. [DOI] [PubMed] [Google Scholar]
- 9.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112(2):214–23. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 10.Assmus B, Honold J, Schächinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355(12):1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 11.Mu Y, Cao G, Zeng Q, Li Y. Transplantation of induced bone marrow mesenchymal stem cells improves the cardiac function of rabbits with dilated cardiomyopathy via upregulation of vascular endothelial growth factor and its receptors. Exp Biol Med (Maywood) 2011;236(9):1100–7. doi: 10.1258/ebm.2011.011066. [DOI] [PubMed] [Google Scholar]
- 12.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–35. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 13.Arnous S, Mozid A, Mathur A. The Bone Marrow Derived Adult Stem Cells for Dilated Cardiomyopathy (REGENERATE-DCM) trial: study design. Regen Med. 2011;6(4):525–33. doi: 10.2217/rme.11.29. [DOI] [PubMed] [Google Scholar]
- 14.Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Papakonstantinou C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact Cardiovasc Thorac Surg. 2007;6(5):593–97. doi: 10.1510/icvts.2007.157875. [DOI] [PubMed] [Google Scholar]
- 15.Balana B, Nicoletti C, Zahanich I, et al. 5-Azacytidine induces changes in electrophysiological properties of human mesenchymal stem cells. Cell Res. 2006;16(12):949–60. doi: 10.1038/sj.cr.7310116. [DOI] [PubMed] [Google Scholar]
- 16.Yablonka-Reuveni Z, Nameroff M. Skeletal muscle cell populations. Separation and partial characterization of fibroblast-like cells from embryonic tissue using density centrifugation. Histochemistry. 1987;87(1):27–38. doi: 10.1007/BF00518721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling SK, Wang R, Dai ZQ, et al. Treatment of rat bone marrow mesenchymal stem cells with a combination of hypergravity and 5-azacytidine enhances therapeutic efficacy for myocardial infarction. Biotechnol Prog. 2011;27(2):473–82. doi: 10.1002/btpr.558. [DOI] [PubMed] [Google Scholar]
- 18.Tomita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(Suppl 19):II247–56. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Chu Y, Shen W, Dou Z. Effect of 5-azacytidine induction duration on differentiation of human first-trimester fetal mesenchymal stem cells towards cardiomyocyte-like cells. Interact Cardiovasc Thorac Surg. 2009;9(6):943–46. doi: 10.1510/icvts.2009.211490. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YL, Zhang HF, Li XL, et al. Increased stromal-cell-derived factor 1 enhances the homing of bone marrow derived mesenchymal stem cells in dilated cardiomyopathy in rats. Chin Med J (Engl) 2010;123(22):3282–87. [PubMed] [Google Scholar]
- 21.Zaruba MM, Franz WM. Role of the SDF-1-CXCR4 axis in stem cell-based therapies for ischemic cardiomyopathy. Expert Opin Biol Ther. 2010;10(3):321–35. doi: 10.1517/14712590903460286. [DOI] [PubMed] [Google Scholar]
- 22.Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287(6):H2670–76. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 23.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–68. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 24.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–92. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Jiang Q, Zhang H, et al. Intravenous administration of bone marrow mesenchymal stromal cells is safe for the lung in a chronic myocardial infarction model. Regen Med. 2011;6(2):179–90. doi: 10.2217/rme.10.104. [DOI] [PubMed] [Google Scholar]
- 26.Wolf D, Reinhard A, Seckinger A, et al. Dose-dependent effects of intravenous allogeneic mesenchymal stem cells in the infarcted porcine heart. Stem Cells Dev. 2009;18(2):321–29. doi: 10.1089/scd.2008.0019. [DOI] [PubMed] [Google Scholar]
- 27.Winkler T, von Roth P, Matziolis G, et al. Dose-response relationship of mesenchymal stem cell transplantation and functional regeneration after severe skeletal muscle injury in rats. Tissue Eng Part A. 2009;15(3):487–92. doi: 10.1089/ten.tea.2007.0426. [DOI] [PubMed] [Google Scholar]
- 28.Kim SW, Park SW, Lim SH, et al. Amount of left ventricular hypertrophy determines the plasma N-terminal pro-brain natriuretic peptide level in patients with hypertrophic cardiomyopathy and normal left ventricular ejection fraction. Clin Cardiol. 2006;29(4):155–60. doi: 10.1002/clc.4960290406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Wei Y, Wagner TE. In vitro endothelial differentiation of long-term cultured murine embryonic yolk sac cells induced by matrigel. Stem Cells. 1999;17(2):72–81. doi: 10.1002/stem.170072. [DOI] [PubMed] [Google Scholar]
- 30.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–36. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]