Abstract
Background
To study the effects of verapamil on the immediate-early genes (IEGs) expression of bone marrow mesenchymal stem cells (MSCs) stimulated by cyclic mechanical strain, in order to deduce the role of calcium ion channel in the cell signaling responses of MSCs to mechanical strain.
Material/Methods
MSCs were isolated and cultured, and the passage of 3–6 MSCs were stimulated by mechanical strain and pretreated with or without verapamil. After that, flow cytometry was used to measure the fluorescence intensity of intracellular Ca2+ immediately. The expression of early-response genes/proteins (c-fos, c-jun and c-myc) were examined by RT-PCR, immunohistochemistry and Western blot.
Results
Intracellular Ca2+ concentration of MSCs significantly changed when stimulated by cyclic strain, and the expression of c-fos, c-jun and c-myc remarkably increased in both mRNA and protein levels, while verapamil pre-treatment partially inhibited these effects (P<0.01).
Conclusions
The changes of the intracellular calcium concentration of MSCs induced by mechanical strain, dependent on the regulation of calcium channel activation, might play a role in the early response of MSCs to cyclic strain.
Keywords: mesenchymal stem cells, mechanical strain, c-fos, c-jun, c-myc, Verapamil
Background
Mechanical loading is crucial for the regulation of various tissues and cells. Mesenchymal stem cells (MSCs) are the cellular basis of bone remodeling and osteoblast differentiation in vivo[1]. Previous studies in vitro showed that appropriate strain could promote MSCs proliferation and osteoblast differentiation [2,3], while overloaded strain could damage MSCs [4]. However, there is great difference in the cell biological effects when stimulated by different strains [5], and the signal transduction pathway of the responses of MSCs to mechanical strain is still not clear.
Recent studies on cell mechanics in vitro demonstrated that cells produce or activate various second or third messengers after mechanical strain, and various signal molecules interact with each other, constructing the network regulation to activate transcription factors. Then the signals will be transmitted from extra-cellular to intracellular environments, and the expression and distribution of relevant genes or proteins will be initiated or regulated to induce series of biological effects [6]. The early cell response to mechanical strain is complicated [7]. Calcium ion, one of the most important intracellular second messengers, regulates various physiological activities [8]. Intracellular calcium is composed of combined calcium and free calcium. Commonly, most of intracellular calcium is combined calcium (99.9%), distributed in the nuclear, mitochondria, endoplasmic reticulum and membrane, while free calcium is rare. The intracellular calcium significantly increases when the cell is stimulated by physical or chemical factors. As an important second messenger, calcium is combined with its main receptor, calmodulin (CaM), and the Ca2+/CaM compound can regulate the activity of several enzymes. Walker et al. [9] reported that the increase of the intracellular calcium concentration of MSCs is related to the up-regulation of matrix proteins such as osteocalcin, osteopontin. Hutcheson et al. [10] found that strain-dependent accumulation of intracellular Ca2+ was correlated with apoptosis in aortic valve interstitial cells (AVICs), and that those findings indicate early mechano-transductive events that may initiate aortic valve (AV) calcification pathways.
C-fos, c-jun and c-myc are important intermediate products of cell responses to mechanical signals. These genes were activated transiently and rapidly in response to a variety of cellular stimuli. They represent a standing response mechanism that is activated at the transcription level in the first round of response to stimuli, before any new proteins are synthesized. These genes, called immediate-early genes (IEGs) [11], can activate downstream proteases and cytokines and initiate biological effects such as cell proliferation and differentiation.
Verapamil is an L-type calcium channel blocker [12]. In the present study, we observed the expression of Ca2+ and c-fos, c-jun, c-myc after pretreatment with Verapamil on the MSCs stimulated by cyclic strain, in order to explore the dependence of calcium ion channel activation on the early response of MSCs.
Material and Methods
Apparatus and reagents: DMEM-LG culture medium (Gibco), fetal bovine serum (Hyclone), Verapamil (Alexis), Trizol (Sigma), Trypsin and agarose (Amersco), RT and PCR kit (TaKaRa), Calcium ion probe fluo-4/AM (Invitrogen), c-fos, c-jun, c-myc primary antibody (Santa Cruz), Primers (Shanghai Yingjun gene biological technology Co., LTD), cell protein extraction agents, BCA Protein Assay Kit, etc; Main experimental instruments included inverted phase-contrast microscope (OLYMPUS, Japan), flow cytometry (BD,USA), ultraviolet spectrophotometer (SHIMADZU 1240, Japan), PCR instrument (Eppendorf, USA), vertical electrophoresis cells, and electroporator.
Cell culture
MSCs were acquired from patients with congenital hip dysplasia undergoing pelvic osteotomy (n=10, 3–9 years old, mean age 5.2, with the signed informed consent of their parents). The institutional ethics committee approved the procedure and written informed consent was obtained from each patient. During surgery, samples were collected by iliac crest puncture. MSCs were isolated using Percoll solution gradient centrifugation (Sigma, St. Louis, MO) and were seeded into DMEM-LG medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 100 U/mL penicillin and 100 U/mL streptomycin (North China Pharmaceutical Factory, Beijing, China) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Culture medium was replaced and cells were passaged as necessary. The second generation of MSCs was harvested to identify CD34, CD44 and CD105 antigens via flow cytometry (BD, Franklin Lakes, NJ). Cells (3rd to 6th generations) were seeded into the 6-well culture plates with elastic silicone membrane (Flexcell, Hillsborough, NC). Once reaching about 80% confluence, cells were grouped, and then placed in a home-made mechanical strain device, to be stimulated as described in our previous publication [13]. The principle is that the objects are moved up and down by a micromotor, and make the BioFlex cell culture dish elastic membrane deform; accordingly, the cells cultured on the plate are indirectly stretched. In the control groups, cells were cultured on similar plates and kept in the same incubator conditions without mechanical strain loading. The treatments were repeated 3 times.
Effect of stretch and Verapamil on intracellular Ca2+
The passage 3–6 MSCs were inoculated in 6-well cell culture silicone plates (Flexcell, USA) at a density of 1.5×105/2 ml per well, and the cells adhered the next day. After completely covering the culture medium membrane, the cells were treated with Verapamil 30 min before strain in the concentration of 20 μmol/l[14] as shown in Table 1. The mechanics parameter is sinusoidal wave at 1 Hz for 12%. The cells in different groups came from the same source. A no-stimulation group was conducted under the same conditions except for the mechanical strain stimulation. All the experiments were conducted in the incubator. The intracellular calcium was detected immediately after stimulation. After washing twice by extracellular fluid, the fluo-4/AM was added and incubated for 30 min at 37°C, protected from light. The supernatant was then removed and cells were washed 3 times to eliminate the rudimental probe. Cells were collected after trypsinization and centrifugation, and then cell suspensions were prepared. The fluoresce density was detected by flow cytometry in 488 nm.
Table 1.
Groups and treatments.
| Groups | Stimulation times | Verapamil pretreatment (−/+) | Groups | Stimulation times | Verapamil pretreatment (−/+) |
|---|---|---|---|---|---|
| a0 | 0 min | + | b0 | 0 min | − |
| a1 | 1 min | + | b1 | 1 min | − |
| a3 | 3 min | + | b3 | 3 min | − |
| a5 | 5 min | + | b5 | 5 min | − |
| a10 | 10 min | + | b10 | 10 min | − |
Effect of stretch and Verapamil on the intracellular c-fos, c-jun and c-myc expression
MSCs were cultivated in conditioned silicone dishes. After the cells covered approximately 80%, they were respectively loaded with strain at 12% intensity for 0.5 h, 1 h, 2 h, and 4 h. The samples were then collected to detect the gene expression of c-fos, c-jun and c-myc. In the above experiments, the frequency of the strain is sinusoidal wave at 1 Hz. A no-stimulation group was conducted under the same conditions, except for the mechanical strain stimulation, and all the experiments were conducted in the incubator. Other cells were divided into 4 groups: A (strain−, V−), B (strain−, V+), C (strain+, V+) and D (strain+, V−). The strain group was loaded cyclic strain for 1 h and the other parameters were as above. Samples were then collected to detect the expression of c-fos, c-jun and c-myc by RT-PCR, immunochemistry, and Western blot. The sequences of PCR primers were shown in Table 2. The imformation of primers came from the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/LocusLink). Primers were synthesized by Yingjun Company (China). RNAs were extracted by Trizol, and RT-PCR was conducted. The PCR products were electrophoresed in agarose gel, scanned with ultraviolet transilluminator (GelDocXR) and the bands were analyzed. After the cell experiment, the membranes were washed 3 times, fixed by 4% paraformaldehyde for 30 min, and then washed twice. The silicone membrane was cut into small pieces. The cells in the fragments were incubated for 20 min at room temperature in 0. 1% Triton-X100”. According to the instruction manual of the kit, the blocking solution, first antibody (1:80 dilution), and second antibody were added in order. Immunoreactive products were visualized by 3, 3-diaminobenzidine (DAB) and the sections were observed and analyzed by cell staining intensity. After the cell experiment, the cells were washed 3 times by cold PBS and the proteins were extracted by protein extraction kit. BCA protein assay kit was used to determine the concentration, and the concentration in each group was regulated to be consistent. Equal amounts of protein (20 μg) of each sample were loaded per well. Electrophoresis was performed. Proteins were transferred overnight, blocked for 2 h, and then incubated for 24 h at 4°C with the first antibody (1:1000). After rinsing 3 times in TBS-T(10 mmol/L Tris-Cl+100 mmol/L NaCI+0.1% Tween20), the sections were incubated with secondary antibody (1:500) for 1 h at room temperature in a blocking solution of 3% normal goat serum in 0.01 M PBS with 0.3% Triton-X 100 (NGST). After 3 rinses in TBS-T, the band was visualized by chemiluminescence reagents with Hyperfilm ECL films.
Table 2.
Primers design of c-fos,c-jun,c-myc and GAPDH.
| Gene | Primer sequences | PCR product | Annealing temperature |
|---|---|---|---|
| c-fos | Sense: 5’ TCCGAAGGGAAAGGAATA 3’ Antisense: 5’ TGCATAGAAGGACCCAGAT 3’ |
476bp | 52°C |
| c-jun | Sense: 5’ AGCAACGGGCACATCACC 3’ Antisense: 5’ TTTTCGGCACTTGGAGGC 3’ |
564bp | 56°C |
| c-myc | Sense: 5’ ATCATCCAGGACTGTATGTGG 3’ Antisense: 5’ GGCTGTGAGGAGGTTTGC 3’ |
496bp | 54°C |
| GAPDH | Sense: 5-GAAGGTGAAGGTCGGAGTC-3 Antisense: 5-GAAGATGGTGATGGGATTTC-3 |
226bp | 52°C |
Statistical analysis
Using SPSS10.0 statistical software, single-factor analysis of variance was performed. A value of P<0.05 was considered statistically significant.
Results
Morphology of the MSCs under microscope
The primary MSCs adhered to the flask wall 2–4 days after seeding, and were fibroblast-like and spindle-shaped. Clustered, rapid cell proliferation was observed after the first medium replacement at days 3 and 4. After multiple medium replacements and PBS washing, hematopoietic cells were gradually eliminated. At days 12–14, cell confluence reached 80%. After passage, the growth rate of the MSCs increased and confluence was reached after 7 to 10 days. The cells were slender, spindle-shaped, and were spirally distributed until cell fusion. The immunopositive rates of CD34, CD44, and CD105 of the MSCs were 2.3, 97.5, and 96.1%, respectively, which is consistent with the known phenotypic characteristics of MSCs [15]. Cells were inoculated in 6-well cell culture plates covered with silicone. After 48 h, cell confluence reached 80%. The cell morphology showed no significant difference among groups, and no cast-off cells or ruptured cells were detected.
Effect of various stimuli on the intracellular Ca2+
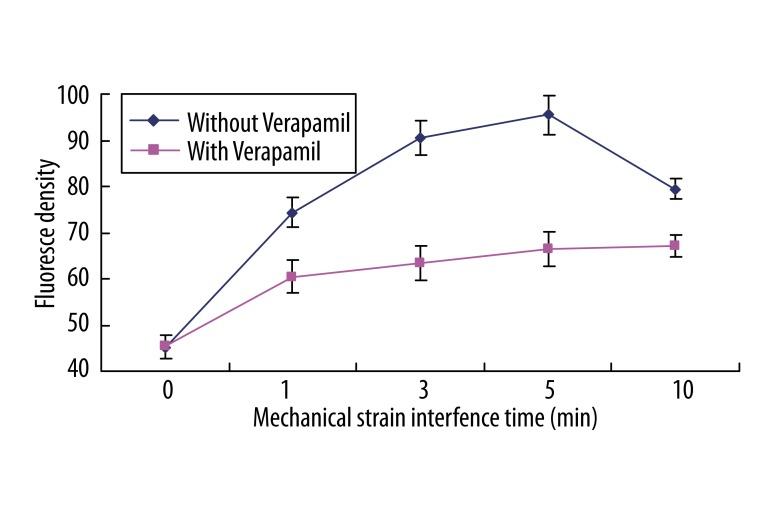
As shown in Figure 1, after mechanical strain application in different times, the fluorescence intensity of the intracellular Ca2+ increased in different degrees. The intracellular Ca2+ concentration increased at 1 min after stretch, and the fluorescence intensity was 164.63% of the control group. This increase was more apparent at 3 and 5 min, with the fluorescence intensity of 200.06% and 211.21%, respectively, compared with the control group. The magnitude of the up-regulation of the Ca2+ concentration showed a decreasing tendency after 10 min, with the intensity of 175.91% of the control. Pretreatment with calcium channel blocker Verapamil 30 min before strain partially blocked the increase of intracellular Ca2+. But along with the time extension, the blocking effect became weaker, with the intensity of 133.62%, 140.27%, 146.77%, and 148.31%, respectively, of the control.
Figure 1.
Changes of free calcium of MSCs after stimulus (According to the SPSS 10.0 Test, a1 ~a10 vs. a0 (P<0.01), b1 ~b10 vs. b0 (P<0.01); a0 vs. b0, P>0.05, no significance; b1 vs. a1, b3 vs. a3, b5 vs. a5, b10 vs. a10, P<0.01).
Expression of c-fos, c-jun and c-myc of MSCs by RT-PCR
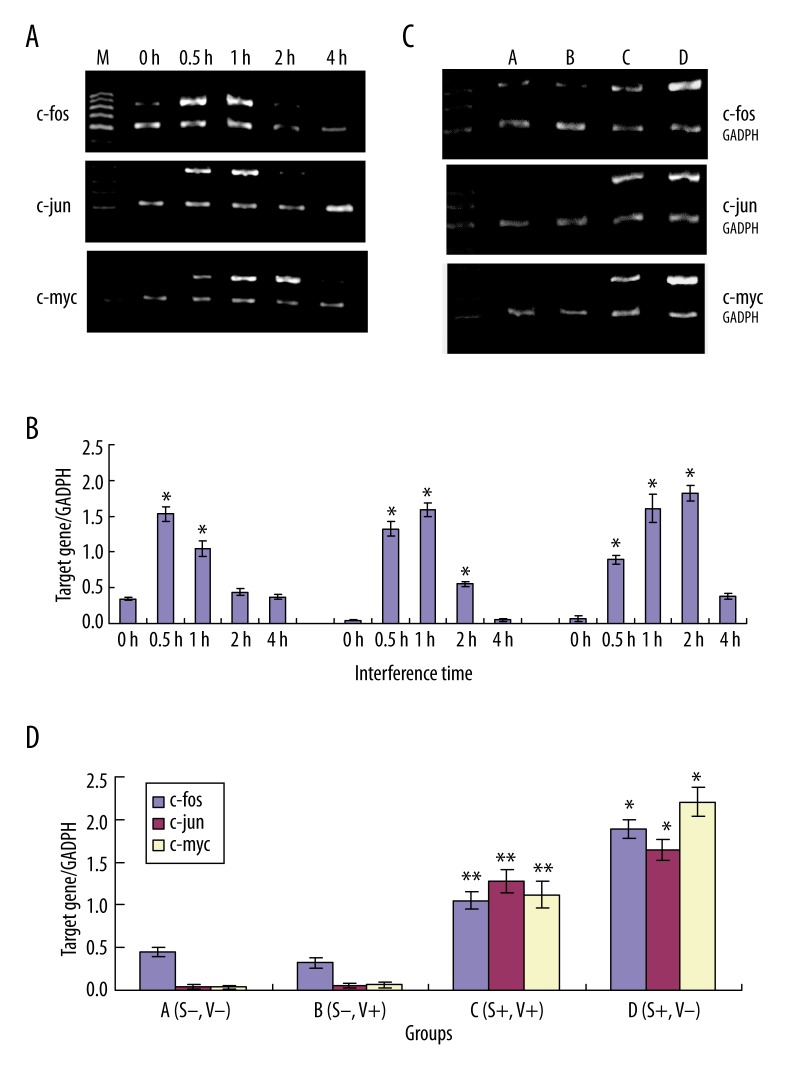
As indicated by Figure 2A, 2B, there was little or no expression of c-fos, c-jun and c-myc in the no-strain group. After short-term (0.5 h, 1 h, 2 h, 4 h) loading of the strain, gene expression of the c-fos, c-jun and c-myc significantly increased. With the time extension the changes showed the tendency was increase- keeping on- reversion. But there was some difference in the time course and density, of which the increase of c-myc lasted for a long time. After 4 h, the expression of c-fos, c-jun and c-myc recovered to the former level.
Figure 2.
Effects of strain on the expression of c-fos, c-jun and c-myc of MSCs by RT-PCR. (A) After different times (0.5 h, 1 h, 2 h, 4 h)loading of the strain, gene expression of the c-fos, c-jun and c-myc significantly increased in MSCs. (The above bands is the objective product and the low band is the inner control product.) (B) The relative gray value of the c-fos (left), c-jun (mediate), c-myc (right)at different times. (vs. control group, *P<0.01, with significant statistically significance). (C,D) Effects of various treatment on the expression of c-fos, c-jun and c-myc of MSCs by RT-PCR. A (strain−, V−), B (strain−, V+), C (strain+, V+), D (strain+, V−), D group vs. A group, * P<0.01; C group vs. D group, ** P<0.01.
As demonstrated in Figure 2C, 2D, after loading with the strain for 1 h, the gene expression of the c-fos, c-jun and c-myc significantly increased. Pretreatment with Verapamil partially blocked the increased expression, with the inhibition of 44.29%, 22.18% and 49.26%, respectively. The inhibition of c-fos and c-myc was comparatively apparent.
Expression of c-fos, c-jun and c-myc of MSCs by immunochemistry
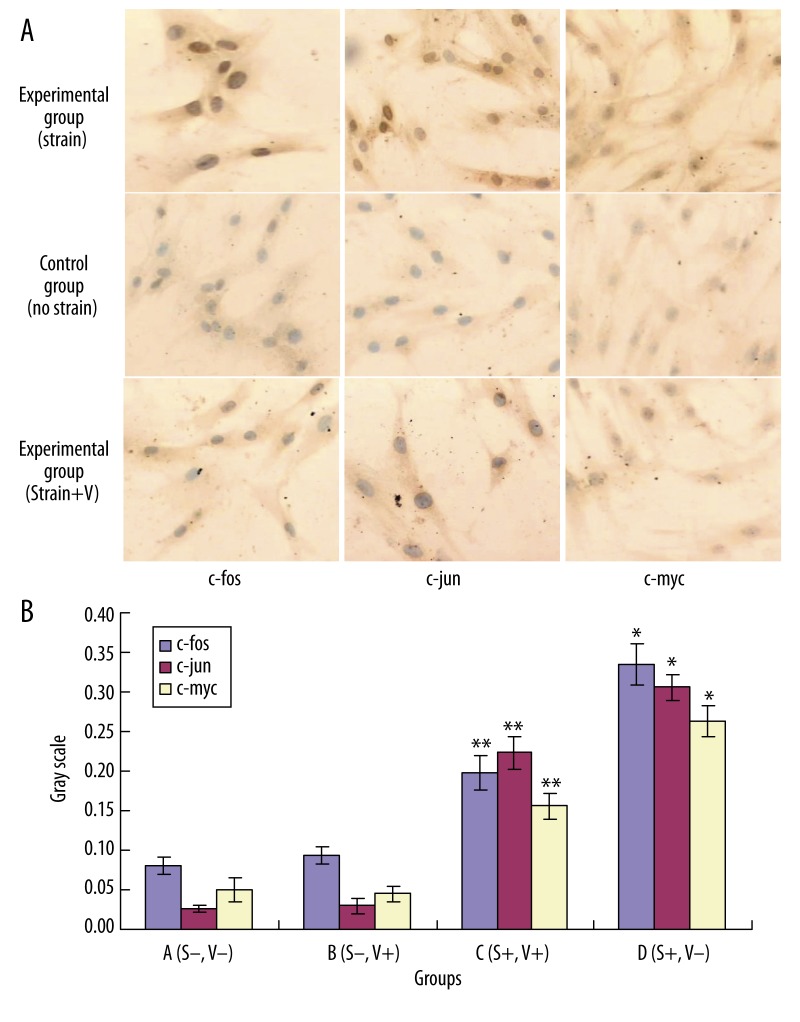
The immunochemical staining of MSCs cytoplasm and nucleus without strain (with or without Verapamil pretreatment) was mild, and the staining of c-fos, c-jun and c-myc was similar. The nuclear staining became brown after strain for 1 h. The staining was attenuated by Verapamil pretreatment, with deep staining at the edge of the nuclear membrane (Figure 3).
Figure 3.
Effects of various treatment on the protein expression of c-fos, c-jun and c-myc of MSCs by immunochemisty. A (strain−, V−), B (strain−, V+), C (strain+, V+), D (strain+, V−), D group vs. A group, *P<0.01; C group vs. D group ** P<0.01.
Protein expression of c-fos, c-jun and c-myc of MSCs by Western blot
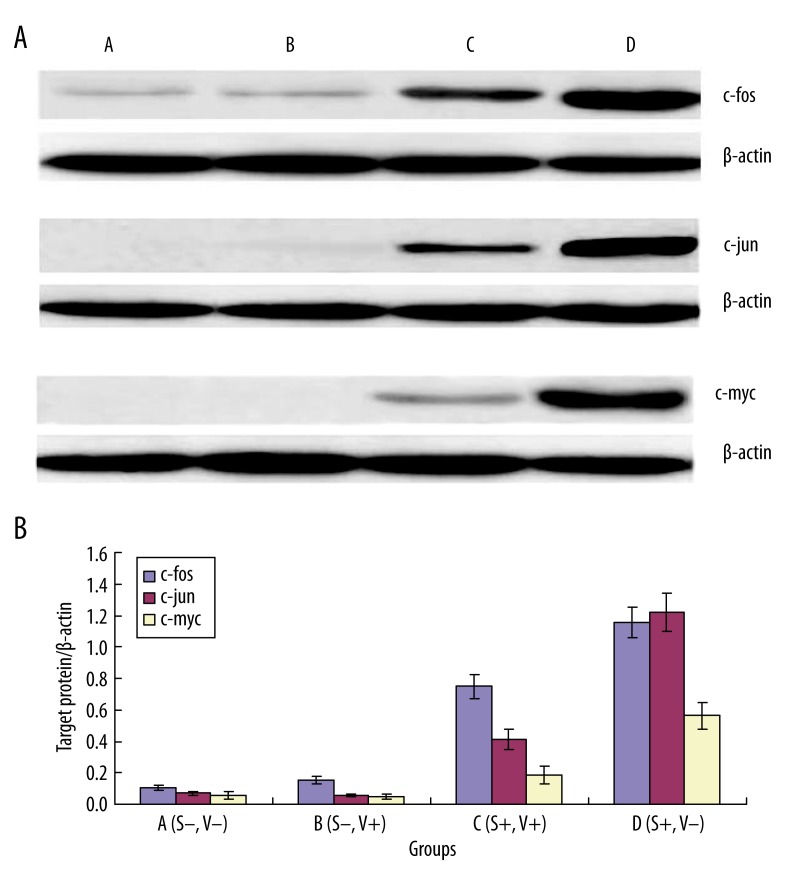
As demonstrated in Figure 4, there was little or no expression of c-fos, c-jun and c-myc in the no-strain group, with or without Verapamil. After loading with the strain for 1 h, the expression of the c-fos, c-jun and c-myc significantly increased, and pretreatment with Verapamil partially blocked the up-regulation of the expression.
Figure 4.
Effects of various treatment on the protein expression of c-fos, c-jun and c-myc of MSCs by Western Blot. A (strain−, V−), B (strain−, V+), C (strain+, V+), D (strain+, V−), D group vs. A group, * P<0.01; C group vs. D group ** P<0.01.
Discussion
The basic theory of the response of the organism to the mechanics is that cells receive mechanical signals in the microenvironment, and then transform the signals into series of intracellular biochemical reactions, including the reception of the strain, production of the biological signals, transduction of the intracellular signals, and changes of gene expression [7,16,17]. Different extracellular signal stimuli had different signal transduction pathways, and several mediate messengers were involved. The present study found that transient strain could significantly increase the fluoresce density of the intracellular Ca2+of MSCs. The effect was time-dependent within a certain range, and the magnitude of the up-regulation of the Ca2+ concentration decreased after 10 min. After pre-treatment with Verapamil, the Ca2+ concentration still significantly increased very early (1 min), and then the increase became gradual. Verapamil partly blocked the increase of intracellular Ca2+ of MSCs, but the effect became weaker over time, thus we deduce that the rapid increase of Ca2+ in the very early time could be attributed to the release of Ca2+ from the Ca2+ pool by the strain stimulus. As well, the direct reception of the membrane stretch sensitivity calcium channel to the mechanical signal could promote changes in Ca2+ concentration. The subsequent gradual increase in Ca2+ concentration suggests the exhaustion of the Ca2+ pool. Also, the internal flow of Ca2+ was blocked by the inhibition of the L-type Ca2+ channel by Verapamil. These two factors resulted in the development of the “platform stage” of Ca2+ increase.
There are 3 cis-acting elements in the promoter of c-fos: cAMP-response element (CRE), serum response element (SRE), and sis-inducible element (SIE). The synergistic reaction of the above elements plays an important role in the correct expression of c-fos [18]. Ordinarily, the phosphorylated sites are phosphorylated by GSK-3 and CK-2, and are dephosphorylated immediately when receiving the growth stimulus signal to remove the inhibition to the cytokines maintaining the rest state of cells, and promote the cell growth, which allows fast induction of c-jun. The c-fos and c-jun proteins consisted of 380 and 331 amino acid residues, respectively, and were synthesized when the cells were stimulated. The c-fos and c-jun proteins return to the nuclei after phosphorylation to compose the AP-1 transcription factor, which integrated the upstream signals and combined to the AP-1 attachment region with high affinity to affect the expression of the target genes [19,20]. The AP-1 transcription factor may involve the signal transduction and influence the cell proliferation, and could be considered as the early marker of biosynthesis. Many genes (e.g., osteocalcin, osteopontin, type I collagen protein and Runx-2) contain the AP-1 combining sites (TGACTCA) [21,22]. C-myc [23] is different from the gene family of c-fos, c-jun (AP-1 gene family). The C-myc gene product is a nuclear protein related to cell proliferation, which is synthesized in the cytoplasm and composed of an oligomer with another protein such as Max. The nuclear localization signal of c-myc makes it locate in the nucleus, but c-myc will activate or inhibit several gene transcriptions when combined with the specific DNA site, so as to grow or differentiate. In the normal cells without stimulus, the expression of c-fos, c-jun, and c-myc is very low. Some studies [24] reported that the fos protein transiently increased in the osteoblast of perichondrium and periosteum of the skull and femur in newborn mice, suggesting the important role of c-fos in the normal osteoblasts differentiation. Also, immediate- early protein is the inhibitor of immediate-early gene expression. In the case of c-fos, for instance, the reduction of the c-fos gene transcription after induction is attributed to the inhibition of the protein product of c-fos itself. Therefore, in the present study, the immediate-early genes of MSCs significantly increased after transitory mechanical strain, while the genes rapidly returned to the former level with the time extension. This tendency is in accordance with the other studies on other cells [23,25–27].
In the present study, after loading strain for 1 h on MSCs, the expression of c-fos, c-jun, and c-myc significantly increased, which could be partly blocked by Verapamil pretreatment, detected by PCR, immunochemistry and Western blot. Peake et al. [28] found that the immediate-early genes such as c-fos are downstream of the calcium signal pathway, and their concentrations are affected by calcium. Kletsas et al. [29] demonstrated that the c-fos and c-jun expression increased in the human periodontal ligament (hPDL) osteoblastic cells after straining, and then the AP-1 transcription factor increased to promote the expression of the osteoplastic genes. The mechanical stimulus increased the intracellular calcium concentration, and promoted the combination of Ca2+/CaM and CaMK to activate CaMK, which could enhance the transcriptional activity of c-fos through the phosphorylation of Ser133 and is involved in the nuclear reaction with PKA. There are many kinds of calcium channels [30], such as voltage-sensitive calcium channel (mainly as L-type calcium channel), receptor operated calcium channel, and second operated calcium channel. Verapamil, used in the present study, is an L-type calcium channel, which can specifically block the inflow of the Ca2+ induced by the change of voltage-sensitive calcium channel stimulated by strain, and therefore partially block the immediate-early protein expression. However, the blocking effect is still only partial, because the increase of intracellular calcium is independent of the sole activation of calcium channel, while Verapamil can only block the biological effect induced by an L-type calcium channel. Peake et al. [28] reported that Ca2+ chelator (EDTA) and mechanical strain-sensitive calcium channel blocker could partly inhibit the biological effect of osteoblasts induced by mechanical stimulus. L-type calcium channel nifedipine used by Walker et al. [9] could also partly block the activation of the downstream of calcium pathway. Therefore, we deduce that the increase of intracellular calcium concentration induced by mechanical strain is related to the activation of several calcium channels, the inflow of extracellular Ca2+ and the mobilization of intracellular calcium.
Conclusions
Therefore, we it appears that the mechanical strain on MSCs can initiate several calcium channels, induce extracellular Ca2+ inflow and/or calcium release from the endoplasmic reticulum to increase the intracellular calcium concentration, mediate the expression of immediate- early genes, and may involve biological effects such as signal transduction and cell proliferation, as well as differentiation. However, the specific course should be further investigated.
Acknowledgements
The authors thank FESTO Pneumatic Center, Huazhong University of Science and Technology, Wuhan, China, for development of the mechanical strain device.
Footnotes
Source of support: This work was supported by the National Natural Sciences Foundation of China. (Approval number 81101366)
References
- 1.Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38(Suppl 1):S26–32. doi: 10.1016/j.injury.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Jagodzinski M, Drescher M, Zeichen J, et al. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;7:35–41. doi: 10.22203/ecm.v007a04. [DOI] [PubMed] [Google Scholar]
- 3.Song G, Ju Y, Shen X, et al. Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces. 2007;58:271–77. doi: 10.1016/j.colsurfb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Thorpe SD, Buckley CT, Vinardell T, et al. Dynamic compression can inhibit chondrogenesis of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;377:458–62. doi: 10.1016/j.bbrc.2008.09.154. [DOI] [PubMed] [Google Scholar]
- 5.Koike M, Shimokawa H, Kanno Z, et al. Effects of mechanical strain on proliferation and differentiation of bone marrow stromal cell line ST2. J Bone Miner Metab. 2005;23:219–25. doi: 10.1007/s00774-004-0587-y. [DOI] [PubMed] [Google Scholar]
- 6.Potier E, Noailly J, Ito K. Directing bone marrow-derived stromal cell function with mechanics. J Biomech. 2010;43:807–17. doi: 10.1016/j.jbiomech.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Liu T, Zheng Y, et al. Early responses of osteoblast-like cells to different mechanical signals through various signaling pathways. Biochem Biophys Res Commun. 2006;348:1167–73. doi: 10.1016/j.bbrc.2006.07.175. [DOI] [PubMed] [Google Scholar]
- 8.Sato K, Adachi T, Ueda D, et al. Measurement of local strain on cell membrane at initiation point of calcium signaling response to applied mechanical stimulus in osteoblastic cells. J Biomech. 2007;40:1246–55. doi: 10.1016/j.jbiomech.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Walker LM, Publicover SJ, Preston MR, et al. Calcium-channel activation and matrix protein upregulation in bone cells in response to mechanical strain. J Cell Biochem. 2000;79:648–61. doi: 10.1002/1097-4644(20001215)79:4<648::aid-jcb130>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Hutcheson JD, Venkataraman R, Baudenbacher FJ, David Merryman W. Intracellular Ca(2+) accumulation is strain-dependent and correlates with apoptosis in aortic valve fibroblasts. J Biomech. 2012;45(5):888–94. doi: 10.1016/j.jbiomech.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deleu S, Pirson I, Clermont F, et al. Immediate early gene expression in dog thyrocytes in response to growth, proliferation, and differentiation stimuli. J Cell Physiol. 1999;181:342–54. doi: 10.1002/(SICI)1097-4652(199911)181:2<342::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Catterall WA, Seagar MJ, Takahashi M, Nunoki K. Molecular properties of voltage-sensitive calcium channels. Adv Exp Med Biol. 1989;255:101–9. doi: 10.1007/978-1-4684-5679-0_11. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Chen B, Wang G, et al. Effects of mechanical strain on oxygen free radical system in bone marrow mesenchymal stem cells from children. Injury. 2011;42:753–57. doi: 10.1016/j.injury.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Xue S, Yang P, Du H. Study of transmembrane La3+ movement in rat ventricular myocytes by the patch-clamp technique. Chinese Science Bulletin. 2005;50:968–70. [Google Scholar]
- 15.Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. J Biosci Bioeng. 2005;100:12–27. doi: 10.1263/jbb.100.12. [DOI] [PubMed] [Google Scholar]
- 16.Hughes-Fulford M. Signal transduction and mechanical stress. Sci STKE. 2004;2004:RE12. doi: 10.1126/stke.2492004re12. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg T, Ziegler N, Alonso A, et al. Strain response in fibroblasts indicates a possible role of the Ca(2+)-dependent nuclear transcription factor NM1 in RNA synthesis. Cell Calcium. 2011;49:259–71. doi: 10.1016/j.ceca.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Janknecht R, Cahill MA, Nordheim A. Signal integration at the c-fos promoter. Carcinogenesis. 1995;16:443–50. doi: 10.1093/carcin/16.3.443. [DOI] [PubMed] [Google Scholar]
- 19.Vesely PW, Staber PB, Hoefler G, Kenner L. Translational regulation mechanisms of AP-1 proteins. Mutat Res. 2009;682:7–12. doi: 10.1016/j.mrrev.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–46. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 21.Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 22.Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995;15:1858–69. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shyu KG, Chao YM, Wang BW, Kuan P. Regulation of discoidin domain receptor 2 by cyclic mechanical stretch in cultured rat vascular smooth muscle cells. Hypertension. 2005;46:614–21. doi: 10.1161/01.HYP.0000175811.79863.e2. [DOI] [PubMed] [Google Scholar]
- 24.Machwate M, Jullienne A, Moukhtar M, Marie PJ. Temporal variation of c-Fos proto-oncogene expression during osteoblast differentiation and osteogenesis in developing rat bone. J Cell Biochem. 1995;57:62–70. doi: 10.1002/jcb.240570108. [DOI] [PubMed] [Google Scholar]
- 25.Ying B, Fan H, Wen F, et al. Mechanical strain-induced c-fos expression in pulmonary epithelial cell line A549. Biochem Biophys Res Commun. 2006;347:369–72. doi: 10.1016/j.bbrc.2006.06.105. [DOI] [PubMed] [Google Scholar]
- 26.Haasper C, Jagodzinski M, Drescher M, et al. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Exp Toxicol Pathol. 2008;59:355–63. doi: 10.1016/j.etp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Millward-Sadler SJ, Lin H, et al. Evidence for JNK-dependent up-regulation of proteoglycan synthesis and for activation of JNK1 following cyclical mechanical stimulation in a human chondrocyte culture model. Osteoarthritis Cartilage. 2007;15:884–93. doi: 10.1016/j.joca.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Peake MA, Cooling LM, Magnay JL, et al. Selected contribution: regulatory pathways involved in mechanical induction of c-fos gene expression in bone cells. J Appl Physiol. 2000;89:2498–507. doi: 10.1152/jappl.2000.89.6.2498. [DOI] [PubMed] [Google Scholar]
- 29.Kletsas D, Basdra EK, Papavassiliou AG. Effect of protein kinase inhibitors on the stretch-elicited c-Fos and c-Jun up-regulation in human PDL osteoblast-like cells. J Cell Physiol. 2002;190:313–21. doi: 10.1002/jcp.10052. [DOI] [PubMed] [Google Scholar]
- 30.Dombrowski JE, Bergey DR. Calcium ions enhance systemin activity and play an integral role in the wound response. Plant Science. 2007;172:335–44. [Google Scholar]