Abstract
Background
Integrin β1 subunit and its downstream molecule, focal adhesion kinase (FAK), have been demonstrated to be indispensible to the promotion of cell proliferation and survival and anti-apoptosis in cardiomyocytes via activation of their downstream pro-survival signaling molecule, AKT. As a component of the integrin pathway, Dock180 (dedicator of cytokinesis 1) protein is also thought to be involved in the promotion of cell proliferation and survival and anti-apoptosis in the H9C2 cardiomyocytes.
Material/Methods
Rat-derived H9C2 cardiomyocytes were transfected with pCXN2-flag-hDock180, a human Dock180 overexpression eukaryotic recombinant plasmid. The rat and human Dock180 mRNA and protein expression, apoptosis and cell proliferation and survival were analyzed in the H9C2 cardiomyocytes treated with either hypoxia/reoxygenation (H/R) or not, respectively.
Results
Human Dock180 mRNA overexpression could significantly increase the Dock180 protein expression in the H9C2 cardiomyocytes, no matter whether treated with H/R or not. Dock180 overexpression could promote the cell proliferation and survival and anti-apoptosis, and relieve the cell proliferative and survival inhibition and apoptosis induced by H/R in the H9C2 cardiomyocytes via activation of its downstream pro-survival signaling molecule AKT.
Conclusions
Dock180 could act as a pro-survival molecule in H9C2 cardiomyocytes via activation of its downstream pro-survival signaling molecule, AKT.
Keywords: Dock180, apoptosis, cell proliferation, cardiomyocyte, H9C2
Background
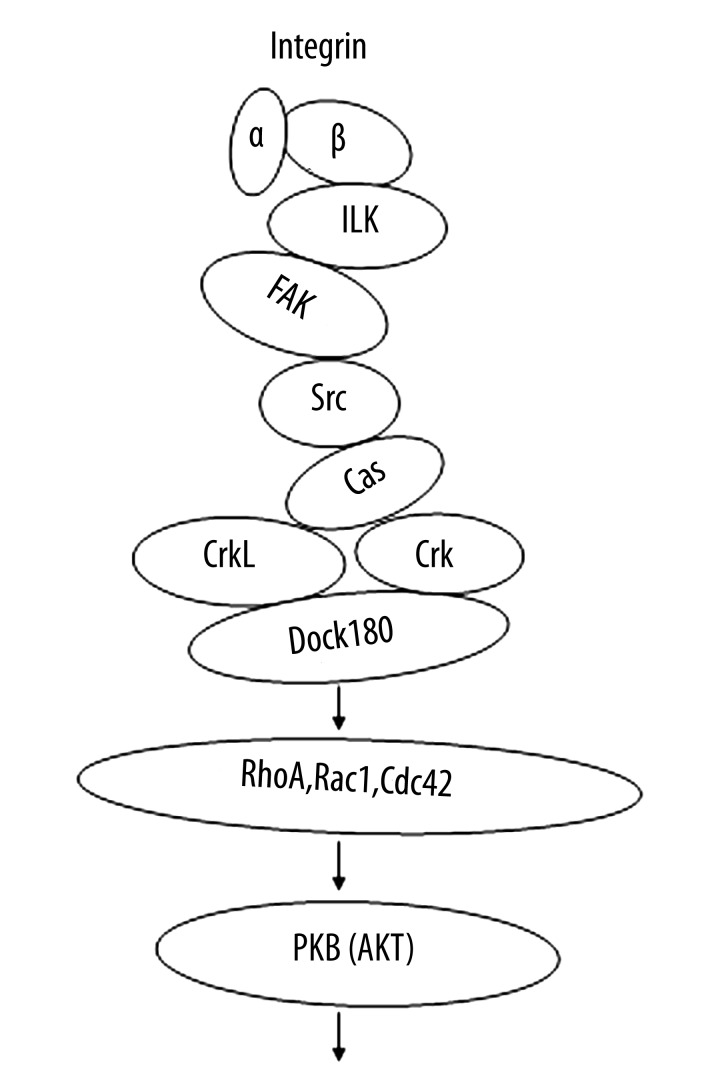
Integrin β1 subunit [1] and its downstream molecule, focal adhesion kinase (FAK) [2], have been demonstrated to be essential in inhibiting post-infarction cardiac remodeling, ischemic cardiomyopathy, and heart failure due to their pro-survival and anti-apoptotic effects on the cardiomyocytes, which are probably mediated by activation of their downstream pro-survival signaling molecules such as protein kinase B (AKT) [3]. Integrin signaling was also found to be involved in the cytoskeletal rearrangement and cardiomyocyte proliferation (Figure 1) [4–6]. Guanine nucleotide exchange factor (GEF) Dock180 protein also acts as an integrin pathway component [7]. However, the roles of Dock180 in the apoptosis, actin cytoskeleton polymerization, proliferation, and survival in cardiomyocytes are poorly understood.
Figure 1.
The diagram of integrin pathway. Note: α – integrin α subunit; β – integrin β subunit; ILK – integrin-linked kinase; FAK – focal adhesion kinase; Cas – Crk-associated substrate; Crk – chick embryo sarcoma virus CT-10 regulator of kinase; CrkL – Crk-like; Dock180 – dedicator of cytokinesis 1; PKB – protein kinase B (AKT).
In the present study, for the potential clinical application, human Dock180 gene was transfected into rat-derived H9C2 cardiomyocytes to investigate its effects on the apoptosis, actin cytoskeleton, cell proliferation, and survival in the cardiomyocytes treated with either hypoxia/reoxygenation (H/R) or not. Exogenous human Dock180 overexpression was observed to promote the anti-apoptosis, actin cytoskeleton polymerization, cell proliferation, and survival, and alleviate the apoptosis, actin cytoskeleton depolymerization and cell proliferative and survival inhibition induced by H/R in the H9C2 cardiomyocytes via activation of its downstream pro-survival signaling molecule, AKT.
Material and Methods
H9C2 cardiomyocyte culture
The rat-derived cardiomyocyte line H9C2 (purchased from the American Type Culture Collection, Manassas, VA, USA) was maintained in Dulbecco’s modified Eagle’s growth medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 5.5 mM glucose, L-glutamine (2 mM), streptomycin (100 μg/ml), and penicillin (100 IU/ml) (all from Gibco-Invitrogen Corp., Carlsbad, CA, USA). The cells were incubated in a humidified incubator at 37°C, 95% oxygen, and 5% CO2[8].
H9C2 cardiomyocytes treated with pCXN2-flag-hDock180 transfection and hypoxia/reoxygenation, and experiment protocol
The medium was replaced with serum-free medium, and the H9C2 cardiomyocytes at ~60% confluence were transfected with blank or pCXN2-flag (empty plasmid) or pCXN2-flag-hDock180 (human Dock180 overexpression eukaryotic recombinant plasmid, which was generously provided by Prof. Shinya Tanaka at Hokkaido University in Japan to Dr. Hua Linghu at Chongqing Medical University in P. R. China), respectively. The transfection was mediated by lipofectamine™ LTX (Invitrogen Co., Carlsbad, CA, USA). Six hours later, the medium was replaced with growth medium, and the cells were cultured for another 18 hours. Then each group (blank group, empty plasmid group, and Dock180 overexpression group) were divided into 2 parts and treated with either H/R or not, respectively. For those treated without H/R, the medium was replaced with new growth medium, and the culture continued for another 48 hours under the normal culture condition (37°C, 95% air and 5% CO2). Meanwhile, for those treated with H/R, the medium was replaced with serum-free, glucose-free phosphate-buffered saline (PBS), and the cells were transferred into hypoxic chamber (Forma Scientific, Freehold, NJ, USA) and maintained at 37° with humidified 1% air, 94% N2 and 5% CO2. Three hours later, the medium was replaced with growth medium, and the normal culture condition was restored, and the culture continued for another 45 hours [2]. Therefore, the cells were finally randomly divided into blank group, empty plasmid group, Dock180 overexpression group, blank + H/R group, empty plasmid + H/R group, and Dock180 overexpression + H/R group. At the above indicated time point (72 hours after transfection), the medium was removed, and the cells were washed with PBS, and harvested for analysis by RT-PCR, Western blot, flow cytometry (FCM), fibrillar actin (F-actin) cytoskeleton staining and MTT assay, respectively.
RT-PCR analysis for Dock180 mRNA level
Seventy-two hours after transfection, total RNA was isolated from the ×106 cells using a commercial RNA extraction kit (RNAiso Plus, TaKaRa Biotechnology Co., Ltd., Dalian, China), and quantified by absorbance at 260 nm. RNA (2.5 μg) was reversely transcribed into cDNA with the PrimerScript RT reagent Kit (TaKaRa Biotechnology Co., Ltd., Dalian, China; reaction condition: 30°C 10 minutes, 42°C 20 minutes, 95°C 5 minutes) according to the manufacturer’s instructions. The 2 microliters of cDNA generated was amplified using specific primers designed for human Dock180, rat Dock180, and β-actin genes, respectively. The nucleotide sequences and Tm values for all primers are given in Table 1. PCR products were visualized under UV light with ethidium-bromide staining after 2% agarose gel electrophoresis. Images were captured with ChemiDoc XRS (Bio-Rad, Hercules, CA, USA), and then the band intensity was determined with Quantity One software (Version 4.6.2.) [2].
Table 1.
The nucleotide sequences and Tm value of the primer of Dock180 and β-actin.
| Gene | Sequences of primers | Size of products (bp) | Tm (°C) |
|---|---|---|---|
| Human Dock180 | Forward:5’-AATCATGCCCTCAAGTCTGG-3’ Reverse: 5’-CCAATGACGTTCATCACCTG-3’ |
405 | 57 |
| Rat Dock180 | Forward: 5’-AGTTGATGAAGGTGGATGGTG-3’ Reverse: 5’-GTTTCGTGTAGGCTAATGTCG-3’ |
231 | 58 |
| Rat β-actin | Forward:5’-AGATGACCCAGATCATGTTTGA-3’ Reverse: 5’-TTGGCATAGAGGTCTTTA-3’ |
535 | 54 |
Western blot analysis for Dock180 and p-AKT protein levels
Seventy-two hours after transfection, the cells were harvested and lysed with cell lysis buffer (Beyotime Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions, and protein concentration was measured using BCA Protein Assay Kit (Beyotime Biotechnology Co., Ltd., Shanghai, China), and standardized (aliquots of supernatants containing equal amounts of proteins). An equal amount (50 μg) of protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred electrophoretically to a polyvinylidene difluoride (PVDF) membrane (Immobilon Transfer Membranes, Millipore Co., Bedford, MA, USA). Non-specific sites were blocked by incubation of the membrane in blocking buffer (3% bovine serum albumin in T-TBS) for 2 hours at room temperature. The membranes was then incubated with the indicated primary antibodies [Anti-human and rat Dock180 (H-4) mouse monoclonal antibody: (1:500), No. sc-13163, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; anti-rat-p-AKT (Ser473) (D9E) XP® rabbit monoclonal antibody: (1:500), No. 4060, Cell Signaling Technology Inc., Danvers, MA, USA; Anti-rat β-actin rabbit polyclonal antibody: (1:500), No. bs-0061R, Boster Biosynthesis Biotechnology Co., Ltd., Wuhan, China; Anti-flag mouse polyclonal antibody: (1:200), No. AF519, Beyotime Biotechnology Co., Ltd., Shanghai, China, respectively] overnight at 4°C, and then with horseradish peroxidase-conjugated anti-mouse or anti-rabbit second antibody (IgG, 1:1500, Boster Biosynthesis Biotechnology Co., Ltd., Wuhan, China) for 1 hour at room temperature. The immunoreactive bands were visualized by incubation with DAB in BeyoECL Plus (Beyotime Biotechnology Co., Ltd., Shanghai, China) and photographed by ChemiDoc XRS detection system (Bio-Rad, Hercules, CA, USA). Absorbance of the bands was analyzed using image analysis software (Quantity One 4.6.2). The densities of Dock180 and p-AKT proteins in relation to β-actin were expressed as Dock180/β-actin and p-AKT/β-actin, respectively, which represent the relative expression levels of Dock180 and p-AKT proteins, respectively [8].
Flow cytometry analysis for H9C2 cardiomyocyte apoptosis
Seventy-two hours after transfection, the apoptotic and necrotic cells were labeled with annexin V-FITC in Annexin V-FITC Apoptosis Detection Kit (Beyotime Biotechnology Co., Ltd., Shanghai, China) and propidium iodide (PI, No. P4864, Sigma-Aldrich Co., St. Louis, MO, USA), respectively, according to the manufacturer’s instruction. Then the sorting of fluorescence-activated apoptotic cells was performed by flow cytometer (BD FACSVantage™ SE, BD Biosciences Co., Franklin Lakes, NJ, USA). The cells with annexin V-positive and PI-negative were defined as the apoptotic cells. The apoptotic rate was calculated as the percentage of the number of the annexin V-positive and PI-negative cells over the number of the total cells [8].
F-actin cytoskeleton staining and observation
Seventy-two hours after transfection, the cells were washed with PBS, fixed in 4% paraformaldehyde solution in PBS for 5 minutes, washed extensively in PBS, permeabilized with 0.1% Triton-100 in PBS, and washed again in PBS. Then the F-actin in the cells was stained with a 50 μg/ml phalloidin-FITC solution (No. P5282, Sigma-Aldrich Co., St. Louis, MO, USA) in PBS (containing 1% DMSO from the original stock solution) for 40 minutes at room temperature, and washed several times with PBS to remove unbound phalloidin-FITC. Then the F-actin in the cells was restained with a 50 μg/ml PI solution for 5 minutes, and washed several times with PBS to remove unbound PI. The F-actin in the cells was observed under a laser scanning confocal microscope (Leica TCS SP2, Leica Microsystems Co., Wetzlar, Germany) [4].
MTT assay of H9C2 cardiomyocyte proliferation
At the indicated time points (24 hours and 72 hours) after transfection, 20 ul MTT (5 mg/ml) working solution (No. 0793, Amresco Inc., Solon, OH, USA) was added to the cells in each well, and followed by a further incubation at 37°C for 4 hours. The supernatants were carefully removed. Two hundred microliters of dimethyl sulfoxide (DMSO) was added to each well, and the well plate was shaken for 30 minutes at room temperature. The absorbance of the samples was then measured at a wavelength of 570 nm in a multi-well spectrophotometer microplate reader (Multiskan MK3, Thermo Fisher Scientific Inc., Vantaa, Finland). The proliferative rate was calculated as (the absorbance at 72 hours – the absorbance at 24 hours)/the absorbance at 24 hours, and expressed as a percentage [8].
Statistical analysis
All of the experiments were repeated at least 3 times. Data are expressed as mean values ± S.D. All analyses were performed using the SPSS17.0 statistical software. The statistical significance among groups was evaluated by one-way ANOVA, followed, in case of significance, by a two-sided Tukey test for multiple comparisons. A value of P<0.05 was considered statistically significant.
Results
Expression of Dock180 mRNA
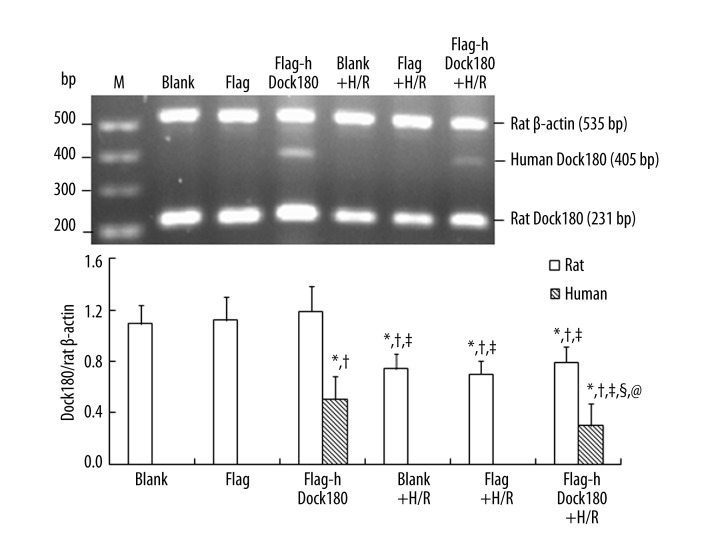
Both rat and human Dock180 mRNA in the H9C2 cardiomyocytes presented with significantly decreased levels after H/R treatment either in the blank, empty plasmid, or Dock180 overexpression groups (All P<0.05, Figure 2); However, as compared with blank and empty plasmid groups, Dock180 overexpression could significantly increase human Dock180 mRNA level in the H9C2 cardiomyocytes, either treated with H/R or not (All P<0.05, Figure 2).
Figure 2.
The expression of Dock180 mRNA in H9C2 cardiomyocytes examined by RT-PCR. Note: M – DNA marker; Blank – blank group; Flag – empty plasmid group; Fag-hDock180 – Human Dock180 overexpression group; Blank + H/R – blank + H/R group; Flag + H/R – empty plasmid + H/R group; Flag-hDock180 + H/R – Human Dock180 overexpression + H/R group; Compared with blank group, * P<0.05; Compared with empty plasmid group, †P<0.05; Compared with human Dock180 overexpression group, ‡P<0.05; Compared with blank + H/R group, §P<0.05; Compared with empty plasmid + H/R group, @P<0.05.
Expression of Dock180 and p-AKT proteins
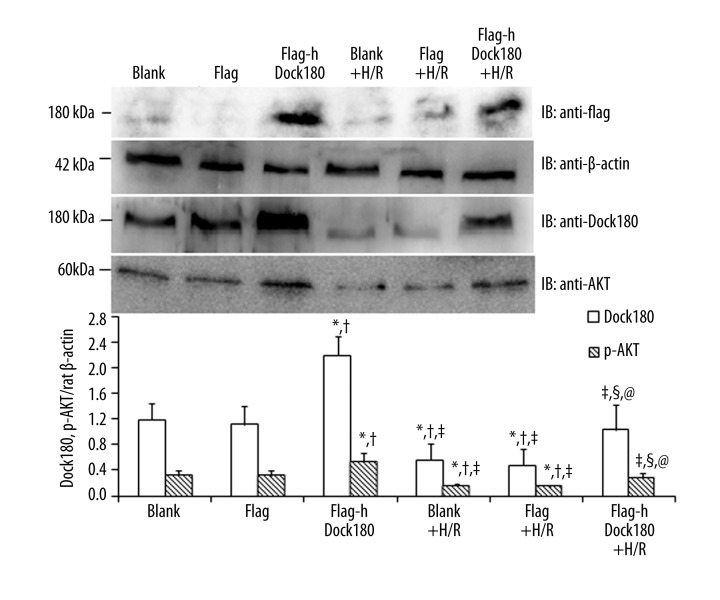
Consistent with the above results, significantly decreased Dock180 and p-AKT protein levels in the H9C2 cardiomyocytes were found after treatment with H/R either in the blank, empty plasmid or Dock180 overexpression groups (All P<0.05, Figure 3); However, as compared with blank and empty plasmid groups, Dock180 and p-AKT protein levels in the H9C2 cardiomyocytes were detected to be significantly increased in the Dock180 overexpression group, either treated with H/R or not (All P<0.05, Figure 3).
Figure 3.
The expression of Dock180 and p-AKT proteins in H9C2 cardiomyocytes examined by Western blot. Note: Blank – blank group; Flag – empty plasmid group; Fag-hDock180 – Human Dock180 overexpression group; Blank + H/R – blank + H/R group; Flag + H/R – empty plasmid + H/R group; Flag-hDock180 + H/R – Human Dock180 overexpression + H/R group; Compared with blank group, * P<0.05; Compared with empty plasmid group, †P<0.05; Compared with human Dock180 overexpression group, ‡P<0.05; Compared with blank + H/R group, §P<0.05; Compared with empty plasmid + H/R group, @P<0.05.
Apoptotic rate of H9C2 cardiomyocytes
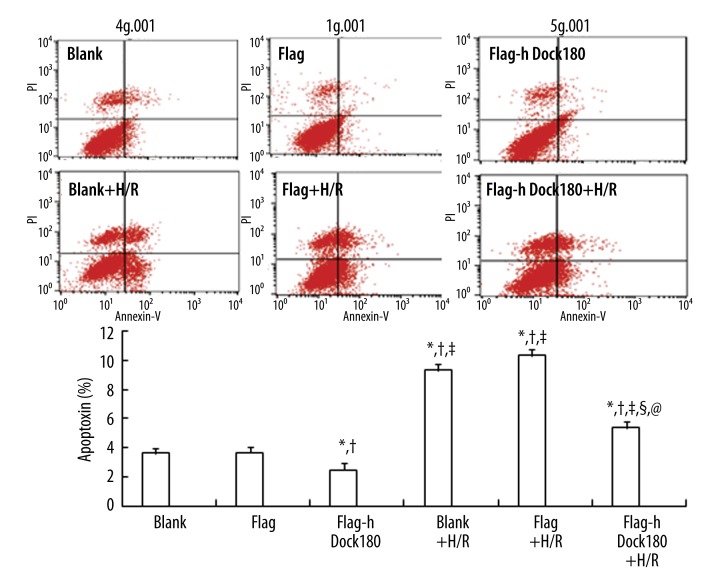
As indicated in Figure 4, H/R treatment could significantly increase the ratio of apoptotic H9C2 cardiomyocytes in the blank, empty plasmid, and Dock180 overexpression groups, respectively (All P<0.05, Figure 4); However, the apoptotic rate in the Dock180 overexpression group was significantly less than that in the blank and empty plasmid groups, either treated with H/R or not (All P<0.05, Figure 4).
Figure 4.
The apoptotic rate of H9C2 cardiomyocytes examined by flow cytometry. Note: Blank – blank group; Flag – empty plasmid group; Fag-hDock180 – Human Dock180 overexpression group; Blank + H/R – blank + H/R group; Flag + H/R – empty plasmid + H/R group; Flag-hDock180 + H/R – Human Dock180 overexpression + H/R group; Compared with blank group, * P<0.05; Compared with empty plasmid group, †P<0.05; Compared with human Dock180 overexpression group, ‡P<0.05; Compared with blank + H/R group, §P<0.05; Compared with empty plasmid + H/R group, @P<0.05.
F-actin staining
As indicated in Figure 5, after H/R treatment, the chaotically rearranged F-actin fiber cytoskeleton could be observed; significantly decreased fluorescence intensity of the F-actin fiber cytoskeleton was found while the atrophied and deformed cardiomyocytes appeared; However, as compared with the blank and empty plasmid groups, the F-actin fiber in the Dock180 overexpression group presented with thicker, longer and more longitudinally arranged cytoskeleton, and the fluorescence intensity of the F-actin fiber cytoskeleton was increased greatly in the Dock180 overexpression group, either treated with H/R or not.
Figure 5.
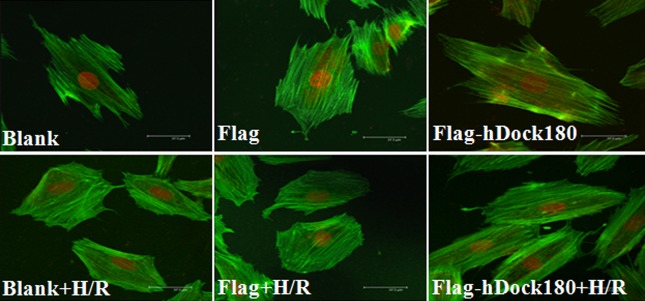
The F-actin cytoskeleton of H9C2 cardiomyocytes examined by laser scanning confocal microscope. Note: Blank – blank group; Flag – empty plasmid group; Fag-hDock180 – Human Dock180 overexpression group; Blank + H/R – blank + H/R group; Flag + H/R – empty plasmid + H/R group; Flag-hDock180 + H/R – Human Dock180 overexpression + H/R group.
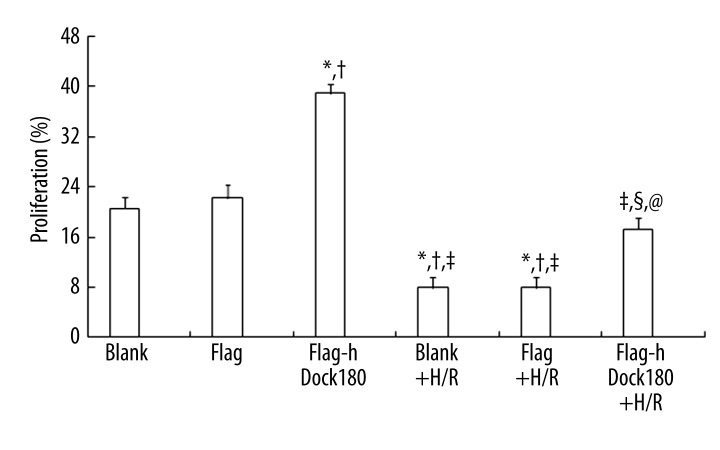
Proliferative rate of H9C2 cardiomyocytes
As demonstrated in Figure 6, H/R treatment could significantly decrease the proliferative rate in the H9C2 cardiomyocytes in all 3 groups (All P<0.05, Figure 6); However, in contrast with the blank and empty plasmid groups, Dock180 overexpression group presented with significantly increased numbers of viable cells, either treated with H/R or not (All P<0.05, Figure 6).
Figure 6.
The proliferative rate of H9C2 cardiomyocytes examined by MTT. Note: Blank – blank group; Flag – empty plasmid group; Fag-hDock180 – Human Dock180 overexpression group; Blank + H/R – blank + H/R group; Flag + H/R – empty plasmid + H/R group; Flag-hDock180 + H/R – Human Dock180 overexpression + H/R group; Compared with blank group, * P<0.05; Compared with empty plasmid group, †P<0.05; Compared with human Dock180 overexpression group, ‡P<0.05; Compared with blank + H/R group, §P<0.05; Compared with empty plasmid + H/R group, @P<0.05.
Discussion
In this study we demonstrated the following findings: (1) Rat-derived H9C2 cardiomyocytes have endogenous Dock180 expression at the mRNA and protein levels, and its expression could be decreased after treatment with H/R. (2) Overexpression of human Dock180 mRNA could significantly increase the Dock180 protein expression in the rat-derived H9C2 cardiomyocytes either treated or untreated with H/R. (3) Human Dock180 overexpression could promote F-actin cytoskeleton polymerization, cell proliferation and survival and anti-apoptosis, and attenuate F-actin cytoskeleton depolymerization, cell proliferative and survival inhibition and apoptosis induced by H/R in the rat-derived H9C2 cardiomyocytes via activation of its downstream pro-survival signaling molecule, AKT.
Survival inhibition and apoptosis in cardiomyocytes are among the major causes of the pathogenesis of many cardiovascular diseases such as diabetic and ischemic ventricular enlarged cardiomyopathy, and systolic heart failure [9–11]. A novel therapy targeting the integrin pathway could be developed to benefit patients with cardiovascular diseases [12] because of its anti-apoptotic and pro-survival effects on the cardiomyocytes [6]. The integrin pathway links extracellular matrix substances to actin cytoskeleton in cardiomyocytes, senses mechanical stretch, and translates it into intracellular biochemical signaling [7]. Total knockout of integrin pathway components such as Cas [13], Crk [14], CrkL [15], and Dock180 [16] could cause most of the mice to die before birth because of actin cytoskeleton depolymerization and severe heart developmental abnormalities. Knockout of other components such as integrin β1 subunit [17], integrin-linked kinase (ILK) [18], FAK [19,20] exclusively in the cardiomyocytes could make most of the embryonic mice survive to birth. However, these knockout mice developed severe dilated cardiomyopathy and spontaneous heart failure after birth, most likely due to actin cytoskeleton disorganization, survival inhibition, and apoptosis in the knockout cardiomyocytes [2,21–23]. When the mice with integrin β1 subunit [17,21], or FAK [19] knockout exclusively in the cardiomyocytes were challenged by isoproterenol, or angiotensin II, or pressure overload, the dilated cardiomyopathy and heart failure in the knockout mice were remarkably accelerated because of increased apoptosis and survival inhibition in the knockout cardiomyocytes. Similarly, the increased apoptosis and survival inhibition in the knockout cardiomyocytes and accelerated post-infarction ischemic cardiomyopathy and heart failure were also described in the mice with integrin β1 subunit- [1] or FAK- [2] knockout exclusively in the cardiomyocytes when they were challenged by myocardial infarction. Isoproterenol or H/R treatment was found to further increase the apoptosis and survival inhibition in the integrin β1 subunit [21] and FAK [2] knockout cardiomyocytes. However, in contrast, ILK overexpression could decrease such apoptosis and proliferative and survival inhibition in the cardiomyocytes, and thus improve post-infarction ischemic cardiomyopathy and heart failure [24].
The above findings support the idea that the integrin pathway components play pivotally protective roles in the cardiomyocytes via their effects of anti-apoptosis, pro-proliferation, and survival on the cardiomyocytes, which are probably mediated by activation of their downstream pro-survival signaling molecules such as PI3K/AKT/mTOR/p70S6K [3,18,25,26] and ERK [25–27].
Dock180 is a GEFs family member in the integrin pathway. Rat Dock180 amino acid sequence shares 100% identity with human Dock180 amino acid sequence, although rat Dock180 mRNA has 84% identity with human Dock180 mRNA. Our present study confirmed that the human Dock180 protein has the same molecular mass (180 kDa) as that of rat Dock180 protein. Dock180 binds with N-terminal SH3 domain of its upstream adaptors such as Crk and CrkL following stimulation by signals through integrin, and subsequently activates GTPases such as RhoA, Rac1, and Cdc42. Dock180 regulates apoptosis, cytoskeleton polymerization, and cell proliferation in non-cardiomyocytes [28,29]. However, little is known about its role in apoptosis, F-actin cytoskeleton polymerization, cell proliferation, and survival in the cardiomyocytes.
The results of the present study, to the best of our knowledge, are the first to confirm the endogenous expression of Dock180 in rat-derived H9C2 cardiomyocytes, implying it has potential roles in the pathophysiology and physiology in rat-derived H9C2 cardiomyocytes. Moreover, overexpression of human Dock180 mRNA can significantly increase the Dock180 protein expression in the rat-derived H9C2 cardiomyocytes treated either with or without H/R. We also believe this is the first report to demonstrate that human Dock180 overexpression can promote the anti-apoptosis, F-actin cytoskeleton polymerization, and cell proliferation and survival, and moreover attenuate the apoptosis, F-actin cytoskeleton depolymerization and cell proliferative and survival inhibition induced by H/R in the rat-derived H9C2 cardiomyocytes via activation of its downstream pro-survival signaling molecule, AKT. Ding et al. proved that ILK overexpression could decrease the apoptosis and cell proliferative and survival inhibition in the cardiomyocytes [24]. Hakim et al found that FAK could alleviate the apoptosis and survival inhibition induced by H/R in the cardiomyocytes [2]. Our results are consistent with the findings reported by Ding and Hakim [2,24]. However, the Dock180 downstream of other pro-survival signaling molecules remain to be determined.
Conclusions
Dock180 overexpression could promote the anti-apoptosis and cell proliferation and survival in H9C2 cardiomyocytes via activation of its downstream pro-survival signaling molecule, AKT.
Acknowledgements
We are grateful to Prof. Shinya Tanaka (Hokkaido University in Japan) for providing the recombinant plasmids including pCXN2-flag and pCXN2-flag-hDock180.
Footnotes
Conflict of interest
The authors declare no conflict of interest with respect to this research.
Source of support: The study was financed by the medical science and technology research project of Health Bureau of Chongqing City, People’s Republic of China (No. 04-2-154 and No. 2009-2-290) and the nature science research fund of Chongqing Science and Technology Commission in Chongqing City, People’s Republic of China (CSTC, No. 2007BB5276)
References
- 1.Krishnamurthy P, Subramanian V, Singh M, Singh K. Deficiency of beta1 integrins results in increased myocardial dysfunction after myocardial infarction. Heart. 2006;92:1309–15. doi: 10.1136/hrt.2005.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim ZS, DiMichele LA, Rojas M, et al. FAK regulates cardiomyocyte survival following ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:241–48. doi: 10.1016/j.yjmcc.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J Biol Chem. 2008;283:35622–29. doi: 10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei L, Wang L, Carson JA, et al. beta1 integrin and organized actin filaments facilitate cardiomyocyte-specific RhoA-dependent activation of the skeletal alpha-actin promoter. FASEB J. 2001;15:785–96. doi: 10.1096/fj.00-026com. [DOI] [PubMed] [Google Scholar]
- 5.Yutao X, Geru W, Xiaojun B, et al. Mechanical stretch-induced hypertrophy of neonatal rat ventricular myocytes is mediated by beta(1)-integrin-microtubule signaling pathways. Eur J Heart Fail. 2006;8:16–22. doi: 10.1016/j.ejheart.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Kuppuswamy D. Importance of integrin signaling in myocyte growth and survival. Circ Res. 2002;90:1240–42. doi: 10.1161/01.res.0000025080.78636.23. [DOI] [PubMed] [Google Scholar]
- 7.Lal H, Verma SK, Foster DM, et al. Integrins and proximal signaling mechanisms in cardiovascular disease. Front Biosci. 2009;14:2307–34. doi: 10.2741/3381. [DOI] [PubMed] [Google Scholar]
- 8.Chou HC, Chen YW, Lee TR, et al. Proteomics study of oxidative stress and Src kinase inhibition in H9C2 cardiomyocytes: a cell model of heart ischemia-reperfusion injury and treatment. Free Radic Biol Med. 2010;49:96–108. doi: 10.1016/j.freeradbiomed.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y. Intracardiac renin-angiotensin system and myocardial repair/remodeling following infarction. J Mol Cell Cardiol. 2010;48:483–89. doi: 10.1016/j.yjmcc.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu Y, Li G, Wang ZH, Zhang CJ. Up-regulation of phosphorylated ATM/ATR substrate/Akt expression by phenylephrine in peri-infarct myocardium in rats. Acta Cardiol Sin. 2011;27:182–88. [Google Scholar]
- 11.Wang LP, Li G, Wang ZH, et al. Elevated expression of C3G protein in the peri-infarct myocardium in rats. Med Sci Monit Basic Res. 2013;19:1–5. doi: 10.12659/MSMBR.883709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lal H, Guleria RS, Foster DM, et al. Integrins: novel therapeutic targets for cardiovascular diseases. Cardiovasc Hematol Agents Med Chem. 2007;5:109–32. doi: 10.2174/187152507780363223. [DOI] [PubMed] [Google Scholar]
- 13.Honda H, Oda H, Nakamoto T, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–65. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 14.Park TJ, Boyd K, Curran T. Cardiovascular and craniofacial defects in Crk-null mice. Mol Cell Biol. 2006;26:6272–82. doi: 10.1128/MCB.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guris DL, Fantes J, Tara D, et al. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nat Genet. 2001;27:293–98. doi: 10.1038/85855. [DOI] [PubMed] [Google Scholar]
- 16.Sanematsu F, Hirashima M, Laurin M, et al. DOCK180 is a Rac activator that regulates cardiovascular development by acting downstream of CXCR4. Circ Res. 2010;107:1102–5. doi: 10.1161/CIRCRESAHA.110.223388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shai SY, Harpf AE, Babbitt CJ, et al. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–64. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 18.White DE, Coutu P, Shi YF, et al. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–60. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng X, Kraus MS, Wei H, et al. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–27. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng X, Wu X, Druso JE, et al. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc Natl Acad Sci USA. 2008;105:6638–43. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamurthy P, Subramanian V, Singh M, Singh K. Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension. 2007;49:865–72. doi: 10.1161/01.HYP.0000258703.36986.13. [DOI] [PubMed] [Google Scholar]
- 22.Hannigan GE, Coles JG, Dedhar S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circ Res. 2007;100:1408–14. doi: 10.1161/01.RES.0000265233.40455.62. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Huang XN, Yan W, et al. Role of the integrin-linked kinase/PINCH1/alpha-parvin complex in cardiac myocyte hypertrophy. Lab Invest. 2005;85:1342–56. doi: 10.1038/labinvest.3700345. [DOI] [PubMed] [Google Scholar]
- 24.Ding L, Dong L, Chen X, et al. Increased expression of integrin-linked kinase attenuates left ventricular remodeling and improves cardiac function after myocardial infarction. Circulation. 2009;120:764–73. doi: 10.1161/CIRCULATIONAHA.109.870725. [DOI] [PubMed] [Google Scholar]
- 25.Balasubramanian S, Kuppuswamy D. RGD-containing peptides activate S6K1 through beta3 integrin in adult cardiac muscle cells. J Biol Chem. 2003;278:42214–24. doi: 10.1074/jbc.M303428200. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Fedak PW, Dai X, et al. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation. 2006;114:2271–79. doi: 10.1161/CIRCULATIONAHA.106.642330. [DOI] [PubMed] [Google Scholar]
- 27.Communal C, Singh M, Menon B, et al. beta1 integrins expression in adult rat ventricular myocytes and its role in the regulation of beta-adrenergic receptor-stimulated apoptosis. J Cell Biochem. 2003;89:381–88. doi: 10.1002/jcb.10520. [DOI] [PubMed] [Google Scholar]
- 28.Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–93. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Linghu H, Wang J, et al. The role of Crk/Dock180/Rac1 pathway in the malignant behavior of human ovarian cancer cell SKOV3. Tumour Biol. 2010;31:59–67. doi: 10.1007/s13277-009-0009-9. [DOI] [PubMed] [Google Scholar]