SUMMARY
Older adults have high prevalence rates of insomnia symptoms, yet it is unclear if these insomnia symptoms are associated with objective impairments in sleep. We hypothesized that insomnia complaints in older adults would be associated with objective differences in sleep compared to those without insomnia complaints. To test this hypothesis, we conducted a cross-sectional study in which older adults with insomnia complaints (cases, n=100) were compared to older adults without insomnia complaints (controls, n=100) using dual-night in-lab nocturnal polysomnography, study questionnaires and seven days of at-home actigraphy and sleep diaries. Cases were noted to have reduced objective total sleep time compared to controls (25.8 minutes +/− 8.56, p=0.003). This was largely due to increased wakefulness after sleep onset (WASO), and not increased sleep latency. When participants with sleep-related breathing disorder or periodic limb movement disorder were excluded, the polysomnography total sleep time difference became even larger. Cases also had reduced slow-wave sleep (5.10 +/− 1.38 vs 10.57 +/− 2.29 minutes, effect size −0.29, p=0.04). When comparing self-reported sleep latency and sleep efficiency to objective polysomnographic findings, cases demonstrated low, but statistically significant correlations, while no such correlations were observed in controls. Cases tended to under-estimate their sleep efficiency by 1.6% (+/− 18.4%), while controls over-estimated their sleep efficiency by 12.4% (+/− 14.5%). In conclusion, we noted that older adults with insomnia complaints have significant differences in several objective sleep findings relative to controls, suggesting that insomnia complaints in older adults are associated with objective impairments in sleep.
Keywords: Insomnia, aged, polysomnography, actigraphy, sleep apnea, sleep-state misperception, sleep diary
INTRODUCTION
Many older adults report insomnia symptoms, with prevalence rates ranging from 23.2% to 33.7% in one study of 9,282 elders across three communities (Foley et al., 1995). Several other studies have shown similar high prevalence rates (Ohayon, 2002, Bloom et al., 2009). In these studies, insomnia has been defined using different diagnostic criteria (Ohayon, 2002); however, one common element is the emphasis on self-reported insomnia symptoms.
This raises an important question: Is the self-report complaint of insomnia symptoms associated with objective differences in sleep in older adults? Often, poor correlations between insomnia symptoms and objective sleep parameters have been noted amongst insomniacs of various ages (Orff et al., 2007, Unruh et al., 2008, Rosa and Bonnet, 2000, Vgontzas et al., 1994, Edinger et al., 2000, McCrae et al., 2005). For older adults, the high prevalence of age-related sleep changes suggests that there may be a greater likelihood of underlying objective impairment in sleep initiation or maintenance. Older adults may have unique characteristics that increase the likelihood of this objective sleep impairment relative to younger patients.
For example, older adults may have comorbid medical conditions that are associated with higher rates of insomnia (Katz and McHorney, 1998, Vitiello et al., 2002). Even in relatively healthy elders without insomnia or other sleep disorders, advancing age can result in a reduced objective sleep efficiency (Walsleben et al., 2004, Ohayon et al., 2004) or sleep duration (Klerman and Dijk, 2008). Furthermore, the circadian output that influences sleep-wake cycles may be weaker in older adults, thus leading to more frequent objective sleep-to-wake transitions (Klerman et al., 2004). While several studies have compared the subjective and objective polysomnographic sleep characteristics of older adults with insomnia complaints, most have relied on a single-night polysomnography assessment,(Unruh et al., 2008, Bastien et al., 2003) included middle-aged research study participants,(Means et al., 2003) excluded study participants with other medical conditions,(Wohlgemuth et al., 1999, Edinger et al., 1997) or did not include a control arm (Riedel and Lichstein, 1998, Riedel et al., 2001, Libman et al., 1997). While significant self-report differences in sleep time between older adults with and without insomnia complaints have been observed in most of these studies, the objective sleep findings have been more equivocal. Indeed, some studies have found no statistically significant differences in polysomnographic sleep parameters between older adults with and without insomnia (Bastien et al., 2003)(Wohlgemuth et al., 1999, Edinger et al., 1997). These studies had approximately 30 or fewer older adults in each group, thus while there were potential clinically significant differences in some objective domains, they were not statistically significant.
Our study aim was to understand the subjective and objective sleep findings associated with insomnia symptoms. Our specific hypothesis was that insomnia symptoms in the elderly are associated with significant deficits in sleep quality that manifest not only as impairments in self-report sleep measures, but also as objective polysomnographic changes. Using a case-control methodology, we identified a large cohort of older adults with (n=100) and without insomnia complaints (n=100) and examined their objective sleep characteristics using dual-night polysomnography. Identifying the objective changes in sleep parameters associated with insomnia symptoms in older adults is crucial to understanding insomnia in older adults.
METHODS
Study Population
Participants in the study were community-dwelling adults 65 years or older who resided in the greater Philadelphia metropolitan area. They were recruited from a database of 120,000 older adults who had received out-patient or in-patient care at any site within the University of Pennsylvania Health System (60.1% of screened study participants) as well as via radio/print media advertisements (39.9% of screened study participants). Potential research study participants were recruited solely for this research study and were not drawn from a specific clinic or convenience sample. Five hundred and forty-nine potential research participants were contacted, and of these, 145 (26.4%) were not interested. Forty-one (7.5%) were interested in participating but declined due to active medical issues. Sixty-eight (12.4%) were ineligible due to study exclusion criteria (medical exclusions, active use of sedative-hypnotics, non-English speaking, presence of depression or dementia (the specific criteria are defined in more detail in the following paragraph)). Twenty-six (4.7%) did not meet insomnia case or control status (described below). After completing the screening and consent process, another sixty-nine (12.6%) dropped out prior to beginning the study protocol, with the majority of these citing scheduling conflicts. Screening was performed until 100 cases and 100 controls had been enrolled. All study participants completed written informed consent and the University of Pennsylvania IRB approved the study protocol.
Initial research participant screening consisted of the Geriatric Depression Scale (score>11)(Yesavage et al., 1982) to exclude study participants with depression and the Short Blessed Examination Score (score>6) for cognitive impairment (Davis et al., 1990). Individuals with depression were excluded because depression is associated with prominent alterations of sleep parameters in the majority of patients; sleep disruption, for example, is a diagnostic symptom of depression. Furthermore, there is a bi-directional causal relationship between insomnia and depression such that having one increases the risk of developing the other. Potential study participants with cognitive impairment were excluded due to the potential inaccuracy of their self-report of insomnia. Additional screening used the CAGE questionnaire to identify individuals with heavy alcohol use (defined as being CAGE positive and consuming more than 21 drinks per week) in order to exclude these potential study participants (Buchsbaum et al., 1992).
Participants who met these initial screening criteria were then identified as cases or controls based on the presence or absence of insomnia symptoms, respectively, as determined by the following questions: “In the past 12 months, have you had trouble falling asleep, staying asleep or waking up too early (insomnia)?” If they responded “never”, they were considered a control (no insomnia). If they responded “rarely”, and reported that this problem had not occurred in the past three weeks, they were still considered controls. If they responded “frequently” and reported that it occurred more than three times a week for the past three weeks, they were considered “cases” (National Institute of Mental Health, 1984). Potential participants who did not have a clear history of insomnia (for example, they had frequent insomnia complaints but had not had them for the past few weeks) were considered to be ineligible; as noted earlier, this applied for 26 (4.7%) of screened study participants.
DATA COLLECTION
In addition to demographic questionnaires and a detailed clinical history, study participants also completed the Functional Outcomes of Sleepiness Questionnaire (FOSQ). This measure was designed to examine the general impact of sleepiness on various domains of daytime functioning. It yields a global score ranging from 5 to 20 with lower scores indicating worse functioning (Weaver et al., 1997). It has been predominantly used in obstructive sleep apnea research, but is also applicable for other sleep disorders (Weaver et al., 1997, Gooneratne et al., 2003). Sleep quality was also assessed using the Pittsburgh Sleep Quality Index (PSQI), a widely used measure of sleep that captures both sleep quality and sleep efficiency, as well as other sleep metrics (Buysse et al., 1989).
Objective measures include overnight polysomnography and wrist actigraphy. Study participants spent two nights in the Center for Sleep and Respiratory Neurobiology sleep laboratory in the Hospital of the University of Pennsylvania. They had their sleep measured by a 16-channel polysomnography which included electroencephalogram, electrooculogram, electrocardiogram, snoring, chin and limb electromyelogram, chest/abd respiratory belts, finger oximetry, and airflow monitoring with nasal and oral thermistors. Sleep records were manually scored in 30-second epochs according to standard criteria (Rechtschaffen and Kales, 1968). The first night was used to adapt study participants to the sleep laboratory due to the potential for a first-night effect that could lead to reduced total sleep time and this data was not used for analysis (Riedel et al., 2001, Riedel and Lichstein, 1998). Data from the first and second nights was not combined, and the second night data was used for this analysis.
Individuals with an AHI ≥ 15 events per hour were considered to have sleep-related breathing disorder; this threshold is commonly used in epidemiological studies of sleep (Young et al., 2002). A criteria of greater than 15 periodic limb movements per hour was used to identify possible Periodic Limb Movement Disorder (American Academy of Sleep Medicine, 2005). In addition, wrist-actigraphy data and a concurrent sleep diary were recorded by the participant for the seven day period prior to their objective in-lab polysomnography testing. The wrist-actigraphy data was collected using the Actillume (Ambulatory Monitoring, Inc., Ardsley, New York).
Statistical Analysis
Cases and controls were compared descriptively using chi-squared tests for categorical measures and t-tests for continuous measures. Additionally, in analytical settings where multiple testing (e.g., assessing subscales of Pittsburgh Sleep Quality Index (PSQI)) may have been a concern, we constructed bootstrap adjusted p-values to ensure the correct Type I Error rate (alpha=0.05) for comparisons done with that instrument or test. We also constructed effect sizes: Mean scores for each measure were converted to effect sizes (‘case score’ – ‘control score’) as a standardized measure comparing cases to controls (Cohen, 1988). Additional nested analyses were done consistent with the a priori hypotheses in our research study protocol; these consisted of nested analyses that excluded participants with other sleep disorders and also took into account the presence of daytime functional impairments from sleepiness. All statistical analyses were two-sided and conducted at the alpha=0.05 level of significance using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
The study group consisted of 200 older adults, with 100 cases and 100 controls. Their demographic characteristics are provided in Table 1: The only demographic characteristic that differed between cases and controls was age, with cases an average of 1.4 years younger than the controls (p=0.04). This difference is small.
TABLE 1.
Demographic characteristics of the study population
| Cases (n=100) |
Controls (n=100) |
P | |
|---|---|---|---|
|
| |||
| Female, N (%) | 68 (68) | 63 (64) | 0.52 |
|
| |||
| Race, N (%) | |||
| White | 48 (53) | 51 (54) | 0.73 |
| African American | 41 (45) | 41 (44) | |
| Asian | 0 (0) | 1 (1) | |
| Hispanic | 1 (1) | 1 (1) | |
| Other | 1 (1) | 0 (0) | |
|
| |||
| Marital Status, N (%) | |||
| Married | 37 (40) | 38 (40) | 0.85 |
| Single | 8 (9) | 11 (12) | |
| Separated/Divorced | 18 (19) | 19 (20) | |
| Widowed | 30 (16) | 26 (14) | |
|
| |||
| Education, N (%) | |||
| Junior high or less | 9 (10) | 5 (5) | 0.18 |
| High school | 41 (45) | 37 (39) | |
| College | 23 (25) | 36 (38) | |
| Graduate School | 18 (20) | 17 (18) | |
|
| |||
| Age, years, mean +/− se | 71.15 +/− 0.46 | 72.59 +/− 0.53 | 0.04 |
|
| |||
| BMI, kg/m2, mean +/− se | 28.71 +/− 0.59 | 28.31 +/− 0.46 | 0.59 |
Subjective differences in sleep
We first examined subjective differences in sleep using the PSQI and Sleep Diary (Table 2). The Pittsburgh Sleep Quality Index global score was less than five for controls (4.31 +/− 0.30), and higher than five for cases (9.53 +/− 0.39). This finding supports the use of the insomnia case-control questionnaire in our study since values less than five are considered to be suggestive of good sleepers.(Buysse et al., 1989) Effect sizes (ES) for the PSQI subscales ranged from small-to-medium (“Use of medications”, ES=0.40) to large (“Subjective sleep quality”, ES=1.72).
TABLE 2.
Subjective differences in sleep for cases and controls
| Mean +/− SE |
Cases (N=100) |
Controls (N=100) |
Effect Size |
P |
P (bootstrap) |
|---|---|---|---|---|---|
| Pittsburgh Sleep Quality Index* | |||||
| Subjective Sleep Quality | 1.74 +/− 0.08 | 0.59 +/− 0.06 | 1.72 | <0.0001 | <0.0001 |
| Sleep Latency | 1.65 +/− 0.12 | 0.68 +/− 0.09 | 0.96 | <0.0001 | <0.0001 |
| Sleep Duration | 1.64 +/− 0.15 | 0.70 +/− 0.10 | 1.15 | <0.0001 | <0.0001 |
| Habitual Sleep Efficiency | 1.66 +/− 0.13 | 0.41 +/− 0.08 | 1.22 | <0.0001 | <0.0001 |
| Sleep Disturbances | 1.41 +/− 0.06 | 1.11 +/− 0.06 | 0.53 | 0.0005 | 0.0041 |
| Use of Sleep Medications | 0.47 +/− 0.10 | 0.15 +/− 0.06 | 0.40 | 0.0071 | 0.0496 |
| Daytime Dysfunction | 0.94 +/− 0.08 | 0.60 +/− 0.06 | 0.49 | 0.0010 | 0.0071 |
| PSQI (global) | 9.53 +/− 0.39 | 4.31 +/− 0.30 | 1.56 | <0.0001 | <0.0001 |
|
| |||||
| Sleep Diary | |||||
| Sleep Quality (mean)** | 49.16 +/− 1.58 | 71.54 +/− 1.67 | −1.40 | <0.0001 | <0.0001 |
| Sleep Quality (standard deviation across nights)** |
18.69 +/− 0.78 | 14.33 +/− 0.77 | 0.57 | 0.0001 | <0.0001 |
| Sleep Latency (min) | 37.56 +/− 4.94 | 19.77 +/− 2.16 | 0.67 | 0.0009 | 0.0029 |
| Wakefulness After Sleep Onset (min) |
98.75 +/− 10.77 |
59.71 +/− 7.20 | 0.61 | 0.0027 | 0.0075 |
| Sleep Efficiency (percent) | 74.62 +/− 2.16 | 86.19 +/− 1.36 | −0.84 | <0.0001 | 0.0001 |
| Total Sleep Time (min) | 349.7 +/− 9.55 | 422.8 +/− 6.61 | −1.16 | <0.0001 | <0.0001 |
Higher scores indicate worse sleep quality
Lower scores indicate worse sleep quality
Participants were also asked to complete sleep diaries to provide at-home estimates of sleep quality and sleep parameters. Sleep quality: This consisted of one question which asked study participants to rate their sleep quality on a 0-100 analogue scale each night for seven nights using a sleep diary (Table 2). Cases reported significantly worse sleep quality and also had greater variability in their night-to-night sleep as indicated by the increased standard deviation noted in the mean value across the seven nights of the sleep diary. Sleep parameters: This section consisted of several questions (time in bed, time asleep, time awake, time out of bed, number and duration of nocturnal awakenings) that were then used to calculate sleep efficiency, total sleep time, wakefulness after sleep onset and sleep latency: 55% of cases and 64% of controls completed all the questions on at least five of seven nights. There was no significant difference in demographic characteristics, PSQI, polysomnographic sleep efficiency, sleep apnea or periodic limb movement index in study participants who did or did not fill out their sleep diaries completely. Overall, cases reported significant impairments in perceived sleep efficiency, sleep latency, wakefulness after sleep onset and total sleep time. Thus, there were robust differences in self-report of sleep quality between those with insomnia complaints (cases) and those without (controls).
Subjective differences in daytime functioning
The proposed Research Diagnostic Criteria for insomnia include both the complaint of difficulty sleeping and daytime consequences from insomnia.(Buysse et al., 2006) To explore this further, we used the Functional Outcomes of Sleepiness Questionnaire which provides an assessment of daytime consequences of sleepiness. Using ROC analysis, we determined that a cutoff of 18.6 for the FOSQ global score provided the largest discriminatory ability. However, the overall predictive utility of this criteria is poor: With this cutoff, we noted that 59.8% of cases had FOSQ global scores <18.6 (i.e., daytime dysfunction from sleepiness) and 60.6% of controls had FOSQ scores above or equal to this threshold (Parameters and 95% C.I.: Area-under-the-curve 0.65 (0.58, 0.71); Positive Predictive Value 0.59 (0.50, 0.69), Negative Predictive Value 0.61 (0.51, 0.71)).
Objective differences in sleep
Our study also included several objective measures of sleep. Polysomnography demonstrated several differences in sleep patterns between cases and controls (Table 3). Cases spent 25.8 +/− 8.56 minutes (p=0.003) less time asleep per night, translating into a 4.7% +/− 1.20 (p=0.005) difference in sleep efficiency as compared to controls. This was largely due to differences in wakefulness after sleep onset, and not due to differences in sleep latency (Table 3). Cases also spent less time in Stage 3/4 sleep, suggesting reduced slow wave sleep in cases. There were no differences noted in arousals during sleep except in Stage 3/4 sleep. While the Periodic Limb Movement Index was higher in controls, there was no significant difference in the index for Periodic Limb Movements with Arousals. Attempts to define a polysomnographic sleep efficiency threshold to robustly distinguish cases with insomnia complaints from controls were unsuccessful.
TABLE 3.
Polysomnographic differences in sleep for cases and controls
| Mean +/− SE | Cases (N=100) |
Controls (N=100) |
Effect Size |
P-value |
|---|---|---|---|---|
| Sleep Efficiency (%) | 71.76 +/− 1.20 | 76.50 +/− 1.14 | −0.43 | 0.005* |
| Wake fulness after sleep onset (WASO, minutes) |
114.96 +/− 5.37 | 94.82 +/− 4.94 | 0.39 | 0.0063 |
| Stage 1 (minutes) | 38.89 +/− 20.3 | 47.22 +/− 3.26 | −0.31 | 0.03* |
| Stage 2 (minutes) | 223.26 +/− 5.12 | 231.67 +/− 5.41 | −0.16 | 0.26 |
| Stage 3/4 (minutes) | 5.10 +/− 1.38 | 10.57 +/− 2.29 | −0.29 | 0.04* |
| Stage REM (minutes) | 78.06 +/− 2.78 | 81.67 +/− 2.97 | −0.13 | 0.38 |
| Total Sleep Time (minutes) | 345.31 +/− 6.07 | 371.13 +/− 6.07 | −0.43 | 0.003* |
| Stage 3/4 arousals index (events/hr) | 0.15 +/− 0.04 | 0.08 +/− 0.01 | 0.47 | <.0001* |
| Stage REM arousals index (events/hr) | 0.40 +/− 0.03 | 0.44 +/− 0.03 | −0.14 | 0.76 |
| Stage NREM arousals index (events/hr) | 0.40 +/− 0.02 | 0.42 +/− 0.03 | −0.10 | 0.80 |
| Latency to Stage 1 (minutes) | 20.35 +/− 2.42 | 18.57 +/− 1.95 | 0.08 | 0.57 |
| Latency to Stage 2 (minutes) from Stage 1 | 4.47 +/− 0.72 | 5.35 +/− 0.66 | −0.09 | 0.53 |
| Latency to Stage 3/4 (minutes) from Stage 1 | 33.61 +/− 7.30 | 20.44 +/− 5.03 | 0.24 | 0.15 |
| PLM Index (events/hr) | 8.06 +/− 1.59 | 13.12 +/− 2.87 | −0.22 | <0.001* |
| PLM Arousal Index (events/hr) | 1.28 +/− 0.38 | 1.19 +/− 0.25 | 0.03 | 0.86 |
| Respiratory Disturbance Index (events/hr) | 12.44 +/− 1.37 | 14.56 +/− 1.38 | −0.15 | 0.28 |
p<0.05
Wrist-activity data collected at home demonstrated no significant differences between the sleep efficiency of cases (67.1% +/− 8.8%) and controls (67.8% +/− 10.9%, t=0.5, p=0.6). No differences were noted as well in night-to-night variability of the sleep efficiency: cases 8.1% +/− 2.8% vs. controls 8.5% +/− 3.4%, t=0.94, p=0.3. Other actigraphy measures, such as total time spent awake during the nocturnal period, number of actigraph observed awakenings, total nocturnal time asleep, activity level during nocturnal sleep time, and daytime sleep, showed no significant differences.
Correlation between objective and subjective perception of sleep
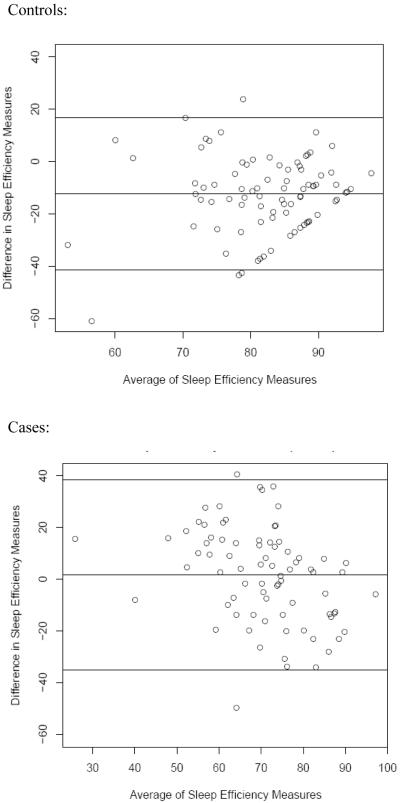
The relationship between polysomnography observed measures of sleep and subjective self-report was also considered (Table 4). While the correlation between subjective and objective sleep efficiency were statistically significant for cases, it was not statistically significant for controls. A similar pattern was noted for sleep latency. However, for all groups, the correlation was low (<0.3). Bland-Altman comparisons were conducted to determine if there were trends in the differences between cases and controls when comparing objective sleep efficiency and subjective (perceived) sleep efficiency (Figure 1): we observed that cases tended to under-report their sleep efficiency by 1.6% while controls tended to over-report their sleep efficiency by 12.4%. Both groups had large standard deviations, with cases having a larger standard deviation than controls. Cases also tended to over-report their actual sleep latency and wakefulness after sleep onset.
TABLE 4.
Correlation and differences in sleep parameters between polysomnography and self-report of general sleep patterns during the month prior to the polysomnography.*
| Parameter | Cases | Controls | Comparison |
|---|---|---|---|
| Sleep Efficiency | |||
| Correlation | 0.29 (p=0.009) | 0.15 (p=0.18) | |
| Difference | 1.6% (SD 18.4%) | −12.4% (SD 14.5%) | t-value = 5.32 (p<0.001) |
| Wakefulness After Sleep Onset (WASO) |
|||
| Correlation | 0.11 (0.38) | 0.19 (0.14) | |
| Difference | −4.1 min (SEM 11.8 min) |
33.7 min (SEM 8.3 min) |
t-value = −2.61 (p=0.01) |
| Sleep Latency | |||
| Correlation | 0.27 (0.016) | 0.09 (0.42) | |
| Difference | −17.4 min (SEM 4.9 min) |
5.8 min (SEM 2.9 min) |
t-value = −4.06 (p<0.001) |
Differences were calculated by subtracting the self-report parameter of interest from the polysomnography parameter of interest (eg: Polysomnography sleep efficiency - self-report sleep efficiency). Self-report sleep parameters for the prior month were derived from the Pittsburg Sleep Quality Index.
Figure 1.
Bland-Altman plot comparing differences in objective (polysomnography) and subjective (self-report) derived sleep parameters in cases and controls. The middle line on each graph represents the mean difference (polysomnography minus self-report), and the upper and lower lines are the limits of agreement (mean difference +/− 2 standard deviations of the difference).
Nested Analyses: Insomnia + daytime impairment
We conducted a nested analysis in which the definition of case and control also included daytime dysfunction from sleepiness (Table 5). A FOSQ cutoff of 18.6 was used as discussed earlier. In the nested analysis, cases thus had both insomnia complaints and daytime dysfunction from sleepiness, while controls did not have insomnia complaints and did not have significant daytime dysfunction from sleepiness. In general, we observed even larger effect size differences between cases and controls when daytime symptoms were included for self-reported PSQI items (Table 2 in comparison to Table 5). Sleep diary findings remained fairly similar in this nested analysis (Table 2 in comparison to Table 5). The polysomnography-determined sleep efficiency and wakefulness after sleep onset also showed larger differences between cases and controls; differences in sleep latency between cases and controls remained non-significant (Table 3 in comparison to Table 5).
TABLE 5.
Nested analysis comparing cases with daytime functional impairment from sleepiness to controls without functional impairment (functional impairment from sleepiness is based on the Functional Outcomes of Sleepiness questionnaire (FOSQ) cutoff of 18.6)
| Mean +/− SE | Cases with FOSQ < 18.6 (N=63) |
Controls with FOSQ >= 18.6 (N=57) |
Effect Size |
P | P (bootstrap) |
|---|---|---|---|---|---|
|
| |||||
| Pittsburgh Sleep Quality Index | |||||
| Subjective Sleep Quality | 1.69 +/− 0.10 | 0.46 +/− 0.07 | 1.99 | <.0001 | <.0001 |
| Sleep Latency | 1.49 +/− 0.15 | 0.58 +/− 0.11 | 0.91 | <.0001 | 0.0001 |
| Sleep Duration | 1.61 +/− 0.13 | 0.67 +/− 0.09 | 1.13 | <.0001 | <.0001 |
| Habitual Sleep Efficiency | 1.65 +/− 0.18 | 0.34 +/− 0.10 | 1.31 | <.0001 | <.0001 |
| Sleep Disturbances | 1.40 +/− 0.07 | 0.98 +/− 0.06 | 0.85 | <.0001 | 0.0003 |
| Use of Sleep Medications | 0.29 +/− 0.11 | 0.09 +/− 0.05 | 0.33 | 0.0844 | 0.4413 |
| Daytime Dysfunction | 1.13 +/− 0.10 | 0.46 +/− 0.08 | 0.94 | <.0001 | 0.0001 |
| PSQI (global) | 9.27 +/− 0.47 | 3.58 +/− 0.34 | 1.85 | <.0001 | <.0001 |
|
| |||||
| Sleep Diary | |||||
| Sleep Latency (min) | 37.66 +/− 5.48 | 19.69 +/− 2.48 | 0.74 | 0.0028 | 0.0111 |
| Wakefulness After Sleep Onset (min) |
103.54 +/− 12.91 | 63.84 +/− 9.90 | 0.60 | 0.0159 | 0.0518 |
| Sleep Efficiency (percent) | 74.63 +/− 2.67 | 85.84 +/− 1.88 | −0.77 | 0.0009 | 0.0030 |
| Total Sleep Time (min) | 350.79 +/− 12.52 | 423.76 +/− 8.33 | −1.09 | <.0001 | <.0001 |
|
| |||||
| PSG sleep parameters | |||||
| Sleep efficiency (%) | 72.99 +/− 1.40 | 78.39 +/− 1.17 | −0.54 | 0.0041 | 0.0143 |
| Total Sleep Time (minutes) | 352.51 +/− 6.98 | 379.42 +/− 6.10 | 0.53 | 0.0048 | 0.0161 |
| Wakefulness after sleep onset (minutes) |
110.66 +/− 5.74 | 86.23 +/− 5.07 | 0.58 | 0.0020 | 0.0063 |
| Latency to Stage I (minutes) | 19.16 +/− 2.73 | 18.34 +/− 2.45 | 0.04 | 0.8262 | 0.9937 |
| Latency to Stage II (minutes) | 24.40 +/− 3.08 | 23.46 +/− 2.54 | 0.04 | 0.8143 | 0.9947 |
Nested Analyses: Insomnia + daytime impairment when excluding sleep-related breathing disorder and periodic limb movement disorder
Since sleep-related breathing disorder and periodic limb movements can also lead to daytime sleepiness complaints, we next considered these parameters as part of our a priori analyses. A criteria of a respiratory disturbance index greater than or equal to 15 events/hour was used for sleep-related breathing disorder and a periodic limb movement index greater than 15 events/hour was used for periodic limb movement disorder (Table 6). For cases, 24 study participants had these conditions (14 had sleep-related breathing disorder and 14 had a periodic limb movement disorder, with 4 having both), and for controls, 20 had these conditions (20 had sleep-related breathing disorder and 15 had periodic limb movement disorder, with 15 having both). When study participants with these other sleep disorders were excluded, there were again more prominent differences in most subjective parameters on the PSQI as suggested by a larger effect size metric, however the PSQI sleep latency, sleep efficiency and sleep duration subscale effect sizes were slightly reduced (Table 2 in comparison to Table 6). For the sleep diary parameters (sleep efficiency, sleep latency, total sleep time and wakefulness after sleep onset), the effect sizes were also reduced, and the differences became non-significant for the sleep latency, sleep efficiency and wakefulness after sleep onset (Table 2 in comparison to Table 6). For objective polysomnography parameters, on the other hand, the difference between cases and controls was even further increased for sleep efficiency, total sleep time and WASO, but sleep latency remained non-significant (Table 3 in comparison to Table 6).
TABLE 6.
Additional nested analysis in which cases with daytime functional impairments from sleepiness are compared to controls without daytime functional impairments, excluding those with sleep-related breathing disorders (SRBD, apnea-hypopnea index ≥ 15 events/hr) and those with periodic limb movement disorder (PLMD, periodic limb movement index ≥ 15 events/hr). Functional impairment from sleepiness is defined by a Functional Outcomes of Sleepiness Questionnaire (FOSQ) score cutoff of 18.6
| Mean +/− SE | Cases with FOSQ < 18.6 (N=39) |
Controls with FOSQ >= 18.6 (N=37) |
Effect Size |
P | P (bootstrap) |
|---|---|---|---|---|---|
|
| |||||
| Pittsburgh Sleep Quality Index | |||||
| Subjective Sleep Quality | 1.64 +/− 0.11 | 0.51 +/− 0.08 | 1.83 | <0.0001 | <.0001 |
| Sleep Latency | 1.38 +/− 0.18 | 0.68 +/− 0.15 | 0.70 | 0.0031 | 0.0185 |
| Sleep Du ration | 1.54 +/− 0.17 | 0.66 +/− 0.11 | 1.04 | <0.0001 | 0.0002 |
| Habitual Sleep Efficiency | 1.47 +/− 0.21 | 0.31 +/− 0.12 | 1.12 | <0.0001 | 0.0001 |
| Sleep Disturbances | 1.38 +/− 0.08 | 1.03 +/− 0.07 | 0.75 | 0.0018 | 0.0101 |
| Use of Sleep Medications | 0.23 +/− 0.11 | 0.08 +/− 0.06 | 0.29 | 0.2320 | 0.8190 |
| Daytime Dysfunction | 1.10 +/− 0.12 | 0.46 +/− 0.11 | 0.91 | 0.0002 | 0.0014 |
| PSQI (global) | 8.72 +/− 0.55 | 3.70 +/− 0.43 | 1.64 | <0.0001 | <.0001 |
|
| |||||
| Sleep Diary | |||||
| Sleep Latency (min) | 32.45 +/− 5.18 | 23.33 +/− 3.20 | 0.45 | 0.1340 | 0.3551 |
| Wakefulness After Sleep Onset (min) |
96.78 +/− 15.39 | 67.91 +/− 12.84 | 0.43 | 0.1544 | 0.4009 |
| Sleep Efficiency (percent) | 76.44 +/− 2.92 | 85.55 +/− 2.57 | −0.63 | 0.0236 | 0.0746 |
| Total Sleep Time (min) | 356.62 +/− 14.38 |
425.72 +/− 10.72 | −1.04 | 0.0003 | 0.0011 |
|
| |||||
| PSG constructs | |||||
| Sleep efficiency | 72.67 +/− 1.82 | 79.44 +/− 1.48 | −0.64 | 0.0059 | 0.0158 |
| Total Sleep Time | 351.64 +/− 8.87 | 385.60 +/− 7.88 | 0.64 | 0.0060 | 0.0159 |
| Wakefulness after sleep onset | 115.4 0 +/− 7.52 | 82.44 +/− 6.60 | 0.74 | 0.0017 | 0.0045 |
| Latency to Stage I | 17.31 +/− 3.31 | 17.34 +/− 2.94 | −0.00 | 0.9950 | 1.0000 |
| Latency to Stage II | 21.28 +/− 3.65 | 21.46 +/− 3.07 | −0.09 | 0.9715 | 1.0000 |
DISCUSSION
This is one of the largest studies to date comparing subjective and objective sleep patterns in older adults with and without insomnia complaints based on a dual night polysomnography paradigm. We observed statistically significant reductions in polysomnography-derived objective sleep efficiency resulting in approximately 25.8 minutes less sleep per night (on average) in cases with insomnia symptoms relative to controls. When excluding study participants with co-existing sleep disorders, such as sleep-related breathing disorder, this difference became even larger. However, no specific cutoff could be determined that objectively defined insomnia by sleep efficiency. There were reductions in stage 3/4 sleep time and increased arousals from stage 3/4 sleep in older adults with insomnia symptoms compared to controls. We also noted that on average, older adults with insomnia complaints had self-reported sleep parameters that more closely matched objective findings than those who did not have insomnia complaints, but there was greater inter-individual variability amongst those with insomniac complaints.
While we observed polysomnography-confirmed differences in sleep efficiency between cases and controls, this reduced sleep efficiency was predominantly due to increases in wakefulness after sleep onset (WASO) and not to increases in sleep latency. This finding is interesting in that a meta-analysis of sleep during the lifespan showed that sleep latency tended to remain fairly stable, while wakefulness after sleep onset tended to increase, thus resulting in a lower sleep efficiency (Ohayon et al., 2004). Possible etiologies of this increased wakefulness after sleep onset include a higher rate of comorbid medical conditions that lead to fragmentation of sleep (such as increased urinary frequency due to benign prostatic hypertrophy, congestive heart failure or other disorders), or reduced arousal thresholds in older adults. While this appears to suggest that asking a patient how long it takes them to fall asleep each night may be less revealing a question than asking about their wakefulness after sleep onset, we noted that both cases and controls tended to inaccurately report the amount of time spent awake after sleep onset.
Another observation of note was that by excluding participants with other sleep disorders, such as sleep-related breathing disorders or periodic limb movement disorder, we found a more prominent difference in polysomnography sleep efficiency between those with insomnia complaints and those without. For example, when excluding study participants with these two conditions in a nested analysis, those with insomnia complaints and daytime impairments from sleepiness (FOSQ<18.6) had a polysomnography total sleep time that was 33.96 minutes less than controls (Table 6). One possible mechanism to explain the fact that sleep efficiency differences are larger between insomnia participants and non-insomnia participants when we exclude those with sleep-related breathing disorders is that sleep-related breathing disorders may lead to increased levels of sleepiness, thus improving sleep efficiency (Gooneratne et al., 2006). Overall, sleep-related breathing disorders may affect up to 20% or more of older adults with insomnia complaints (Beneto et al., 2009, Morin et al., 1999, Lichstein et al., 1999, Gooneratne et al., 2006). Thus it is possible that the current body of insomnia literature that relies on self-reported symptoms alone, and thereby includes undiagnosed sleep-related breathing disorder or periodic limb movement disorder participants, may underestimate the true severity of sleep efficiency impairment in older adults with insomnia. While some studies have suggested that polysomnography provides little additional information in the evaluation of insomnia,(Vgontzas et al., 1995) polysomnography may be particularly useful for older adults with insomnia (Edinger et al., 1989, Gooneratne et al., 2006) as older adults in general have a higher prevalence of sleep-related breathing disorders than younger patients (Young et al., 2002).
While we noted a statistically significant decrease in polysomnography-determined total sleep time between cases and controls of 25.8 minutes (Table 3), the clinical significance of this difference is worth considering. One way to judge this clinical aspect is to consider the treatment differences noted in insomnia randomized controlled trials as a potential measure of a clinically meaningful change. Non-benzodiazepine drugs that are currently FDA approved for insomnia treatment improve polysomnography total sleep time by 11.4 minutes based on a meta-analysis of randomized controlled trials (Buscemi et al., 2007). It is also worth noting that the objective differences we observed, while statistically significant, had an effect size of 0.43, which may be considered a small to medium effect size, although it increased to 0.64 in the nested analysis, a medium effect size (Cohen, 1988). Furthermore, these objective differences in sleep are modest while subjective differences are very large and robust.
Interestingly, these subjective differences are less robust in the nested analyses that excluded study participants with sleep-related breathing disorder or periodic limb movements. Subjective sleep diary-determined sleep latency, sleep efficiency and wakefulness after sleep onset no longer had significant differences between cases and controls in this nested analysis. This may be due to two factors: When study participants with these two disorders were removed for this nested analyses, there was both an improvement in sleep parameters in cases and a worsening of these same parameters in controls, leading to a reduction in the overall differences between cases and controls. This raises the interesting question of whether there is a differential effect of other sleep disorders, such as sleep-related breathing disorder, on subjective sleep experiences in older adults with insomnia symptoms relative to those without.
While we noticed differences in polysomnography-measured sleep parameters, we did not observe differences in standard wrist-activity (actigraphy) sleep parameters between cases and controls. This finding is not surprising as uncertainty exists regarding the role of actigraphy in assessing sleep parameters in patients with insomnia; a recent American Academy of Sleep Medicine practice parameters review had a recommendation level of “option” signifying “inconclusive or conflicting evidence” in terms of the utility of actigraphy for insomnia assessment (Morgenthaler et al., 2007). Others have noted that objective sleep parameters derived by actigraphy were not associated with next day consequences, such as psychological well-being (McCrae et al., 2008). Research comparing polysomnography to actigraphy has noted good correlations in some sleep metrics, however those with the worst sleep had significant discrepancies between the two methods,(Blackwell et al., 2008) and actigraphy may tend to overestimate sleep efficiency in older adults (Sivertsen et al., 2006).
Insomnia sufferers have frequently been considered to have a significant element of sleep-state misperception underlying their insomnia. There is a tendency to disregard self-reported sleep parameters due to the belief that insomniacs frequently underreport their sleep (Orff et al., 2007). However, some studies have noted that the relationship between subjective and objective sleep may be more complex (Perlis et al., 2001, Tang and Harvey, 2005): while insomnia patients may overestimate their sleep latency, they may underestimate their total sleep efficiency (Libman et al., 1997). In addition, many individuals tend to overestimate sleep time,(Silva et al., 2007) a finding that others and we have noted in our non-insomnia study participants (Vitiello et al., 2004, Rosa and Bonnet, 2000). Thus the problem may not be that older insomniac patients are underestimating their sleep time, but instead that older non-insomniacs are actually overestimating their sleep time. The sleep-state misperception that is felt to characterize older insomnia patients may actually be more common in those older adults who do not complain of insomnia. For example, we observed that the correlation between self-reported sleep efficiency and objective polysomnography sleep efficiency was higher in older adults with insomnia complaints than in older adults without insomnia complaints. This parallels findings from Bastien et al. who noted a statistically significant correlation between several subjective and objective sleep parameters in older insomniac patients, but a lack of correlation in older good sleepers (Bastien et al., 2003). Another study using actigraphy noted the opposite trend, however, with participants who did not complain of insomnia having higher correlations with objective parameters than those who did complain of insomnia (McCrae et al., 2005). It is important to emphasize that while the self-report mean values were closer to objective polysomnography findings for the older adults with insomnia relative to the non-insomniacs, the standard deviation was larger for those with insomnia. Thus, a specific insomnia patient may be less precise than a non-insomnia patient and there may be more variability associated with their estimate. In addition, even though the correlations between subjective and objective measures of sleep efficiency and sleep latency were statistically significant in older adults with insomnia (but as noted earlier were not significant in the non-insomniacs), the overall correlations were still low and less than 0.30, a finding noted by others (Unruh et al., 2008). Future research exploring factors that influence cognitive arousal and perception of sleep, such as attentional bias, may provide useful insights into the pathophysiology of insomnia complaints (Espie, 2007, Spiegelhalder et al., 2010, Unruh et al., 2008, Tang and Harvey, 2005, Means et al., 2003). One potential explanation for the tendency of non-insomniacs to overestimate their sleep time, for example, is that they may have adapted or acclimatized to age-related changes in sleep and do not perceive it as “insomnia” (Zilli et al., 2008, Vitiello et al., 2004, Unruh et al., 2008).
Our study has several limitations. We used a screening paradigm for insomnia that relied upon simple symptom complaints: Our case-control classification did not require that research study participants have a formal diagnosis of insomnia. We chose this approach to maximize the clinical relevance of this study because it reflects the symptom complaint that most patients mention to their primary care providers. We also noted a slight difference in age with our insomnia cases being 1.4 years younger than our controls. While statistically significant, we believe that it is of minimal clinical significance. Our study used in-lab polysomnography as a primary measure of sleep parameters. While at-home polysomnography is another option and more closely reflects at-home conditions, we felt that in-lab polysomnography was a suitable approach for the following reasons: 1) we used an adaptation night to help acclimate research study participants to the lab; and 2) an in-lab study could minimize potential unexpected environmental factors or equipment problems that may occur at-home. The at-home sleep diaries, had a 60% rate of completion for all sleep time questions, thus complete subjective sleep diary metrics (sleep efficiency, etc.) could be calculated only on 60% of all study participants. However, no significant differences were noted on demographic, PSQI and polysomnographic sleep parameters between those study participants who did and did not fully complete the sleep diaries, suggesting that these sleep diary complaint and non-compliant study participants are fairly similar. The study excluded individuals with depression, thus our study findings are not applicable to patients with a diagnosis of depression. In addition, the study conclusions cannot necessarily be extrapolated to other age ranges, and our study design (which enrolled only study participants over the age of 65) means that we cannot determine if the subjective/objective findings are specific to older adults.
The findings from this study demonstrate that sleep patterns in older adults with insomnia complaints have significant objective differences from those of older adults without insomnia complaints. When excluding research study participants with sleep related breathing disorder or periodic limb movement disorder, some of these objective differences, such as sleep efficiency, become more pronounced. The changes do, however, remain modest in comparison to the very robust changes in self-report instruments. Increased wakefulness after sleep onset was one of the key areas that contributed to reduced sleep efficiency in older adults with insomnia symptoms. While sleep state misperception can be one cause of insomnia, we noted that many older adults with insomnia complaints reported sleep times that more closely reflected their objective sleep than did non-insomniacs, who had a tendency to over-report their total sleep time. The findings from this study suggest that the subjective complaint of insomnia in older adults is indeed associated with objective differences in sleep parameters. When an older patient presents with insomnia symptoms, it may be associated with significant and measurable objective impairments in sleep that warrant further evaluation and management.
ACKNOWLEDGMENTS
Supported by NIH grants NIA R01 AG14155, NCCAM R01 AT001521, NIA K23 AG01021, and NCRR CTSA UL1-RR-024134.
The investigators would like to thank Laura Zachweija, Heidi Arner, Phil Gehrman, Jonathan ‘Emeka Nkwuo and Greg Maislin for their assistance with this project.
Footnotes
Financial Disclosure:
Dr. Gooneratne has received grant support from Takeda Pharmaceuticals, Inc. Dr. Dinges has consulted for Pfizer, Inc., Merck & Co., Inc., Takeda Pharmaceuticals, Inc., Sanofi-Aventis, Inc., and Cephalon, Inc.
Conflict of interest: No authors have conflicts of interest related to this publication.
REFERENCES
- American Academy of Sleep Medicine . International classification of sleep disorders, 2nd ed.: Diagnostic and coding manual. American Academy of Sleep Medicine; Westchester, Illinois: 2005. [Google Scholar]
- Bastien CH, Fortier-Brochu E, Rioux I, Leblanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- Beneto A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009 doi: 10.1016/j.smrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57:761–89. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum DG, Buchanan RG, Welsh J, Centor RM, Schnoll SH. Screening for drinking disorders in the elderly using the CAGE questionnaire. J Am Geriatr Soc. 1992;40:662–5. doi: 10.1111/j.1532-5415.1992.tb01956.x. [DOI] [PubMed] [Google Scholar]
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Davis PB, Morris JC, Grant E. Brief screening tests versus clinical staging in senile dementia of the Alzheimer type. J Am Geriatr Soc. 1990;38:129–35. doi: 10.1111/j.1532-5415.1990.tb03473.x. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Glenn DM, et al. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000;68:586–93. [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Sullivan RJ, Jr., et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20:1119–26. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Hoelscher TJ, Webb MD, Marsh GR, Radtke RA, Erwin CW. Polysomnographic assessment of DIMS: empirical evaluation of its diagnostic value. Sleep. 1989;12:315–22. [PubMed] [Google Scholar]
- Espie CA. Understanding insomnia through cognitive modelling. Sleep Med. 2007;8(Suppl 4):S3–8. doi: 10.1016/S1389-9457(08)70002-9. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Gehrman PR, Nkwuo JE, et al. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–8. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Weaver TE, Cater JR, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51:642–9. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- Katz DA, Mchorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158:1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Davis JB, Duffy JF, Dijk DJ, Kronauer RE. Older people awaken more frequently but fall back asleep at the same rate as younger people. Sleep. 2004;27:793–8. doi: 10.1093/sleep/27.4.793. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep--implications for insomnia. Curr Biol. 2008;18:1118–23. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libman E, Creti L, Levy RD, Brender W, Fichten CS. A comparison of reported and recorded sleep in older poor sleepers. J. Clinical Geropsychol. 3(3):199–211. [Google Scholar]; J Clinical Geropsychol. 1997;3:199–211. [Google Scholar]
- Lichstein KL, Riedel BW, Lester KW, Aguillard RN. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67:405–10. doi: 10.1037//0022-006x.67.3.405. [DOI] [PubMed] [Google Scholar]
- Mccrae CS, Mcnamara JP, Rowe MA, et al. Sleep and affect in older adults: using multilevel modeling to examine daily associations. J Sleep Res. 2008;17:42–53. doi: 10.1111/j.1365-2869.2008.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccrae CS, Rowe MA, Tierney CG, Dautovich ND, Definis AL, Mcnamara JP. Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60:P182–9. doi: 10.1093/geronb/60.4.p182. [DOI] [PubMed] [Google Scholar]
- Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003;4:285–96. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. Jama. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health Consensus conference Drugs and insomnia. The use of medications to promote sleep. Jama. 1984;251:2410–4. [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Orff HJ, Drummond SP, Nowakowski S, Perils ML. Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007;30:1205–11. doi: 10.1093/sleep/30.9.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardization Terminology: Techniques and Scoring System for Sleep Stages of Human Subjects. Brain Information Services/Brain Research Institute, University of California at Los Angeles; Los Angeles, California: 1968. [Google Scholar]
- Riedel BW, Lichstein KL. Objective sleep measures and subjective sleep satisfaction: how do older adults with insomnia define a good night’s sleep? Psychol Aging. 1998;13:159–63. doi: 10.1037//0882-7974.13.1.159. [DOI] [PubMed] [Google Scholar]
- Riedel BW, Winfield CF, Lichstein KL. First night effect and reverse first night effect in older adults with primary insomnia: does anxiety play a role? Sleep Med. 2001;2:125–33. doi: 10.1016/s1389-9457(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Rosa RR, Bonnet MH. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosom Med. 2000;62:474–82. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Silva GE, Goodwin JL, Sherrill DL, et al. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B, Omvik S, Havik OE, et al. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29:1353–8. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Kyle SD, Feige B, et al. The impact of sleep-related attentional bias on polysomnographically measured sleep in primary insomnia. Sleep. 2010;33:107–12. doi: 10.1093/sleep/33.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang NK, Harvey AG. Time estimation ability and distorted perception of sleep in insomnia. Behav Sleep Med. 2005;3:134–50. doi: 10.1207/s15402010bsm0303_2. [DOI] [PubMed] [Google Scholar]
- Unruh ML, Redline S, An MW, et al. Subjective and Objective Sleep Quality and Aging in the Sleep Heart Health Study. J Am Geriatr Soc. 2008;56:1218–27. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Kales A, Manfredi RL, Tyson K. Validity and clinical utility of sleep laboratory criteria for insomnia. Int J Neurosci. 1994;77:11–21. doi: 10.3109/00207459408986015. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Kales A, Bixler EO, Manfredi RL, Vela-Bueno A. Usefulness of polysomnographic studies in the differential diagnosis of insomnia. Int J Neurosci. 1995;82:47–60. doi: 10.3109/00207459508994289. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res. 2004;56:503–10. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–9. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27:293–8. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- Wohlgemuth WK, Edinger JD, Fins AI, Sullivan RJ., Jr. How many nights are enough? The short-term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology. 1999;36:233–44. [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- Zilli I, Ficca G, Salzarulo P. Factors involved in sleep satisfaction in the elderly. Sleep Med. 2008 doi: 10.1016/j.sleep.2008.01.004. [DOI] [PubMed] [Google Scholar]