Abstract
Apo E mutants are associated with type III hyperlipoproteinemia characterized by high cholesterol and triglycerides levels. Autosomal Dominant Hypercholesterolemia (ADH), due to mutations in the LDLR, APOB or PCSK9 genes, is characterized by an isolated elevation of cholesterol due to high levels of low-density lipoproteins (LDL).
We now report an exceptionally large family including 14 members with ADH. Through genome wide mapping, analysis of regional/functional candidate genes and whole exome sequencing, we identified a mutation in the APOE gene, p.Leu167del previously reported associated with sea-blue histiocytosis and familial combined hyperlipidemia. We confirmed the involvement of the APOE p.Leu167del in ADH, with (1) a predicted destabilization of an alpha-helix in the binding domain; (2) a decreased apo E level in LDL; and (3) a decreased catabolism of LDL.
Our results show that mutations in the APOE gene can be associated with bona fide ADH.
Keywords: Apolipoproteine E, Autosomal Dominant Hypercholesterolemia (ADH), mutation, low-density lipoproteins (LDL), linkage analysis
Apolipoprotein E (apo E) is a 34 kDa glycosylated and excreted protein which is a component of chylomicrons, VLDL (very low density lipoproteins), IDL (intermediate density lipoproteins), LDL (low density lipoproteins), and HDL (high density lipoproteins), (OMIM +107741). Apo E is a ligand for the scavenger receptor B type 1 (SR-B1), and for all members of the LDL receptor family. The APOE gene accounts for a significant fraction of the variation in plasma cholesterol levels (Eichner JE et al. 2002). Apo E presents three major isoforms: E3 (the most frequent one), E2 and E4. Whereas apo E3 and apo E4 bind with similarly high affinity to the LDL receptor, the binding of apo E2 is 50- to 100- times weaker (Weisgraber KH et al. 1982). Apo E2 isoform is associated with lower LDL cholesterol, except for 5% of E2/E2 carriers who develop type III hyperlipoproteinemia (OMIM +107741), a relatively rare disease characterized by severely elevated cholesterol and triglyceride levels. Apo E4 isoform is associated with elevated total and LDL-cholesterol levels, due to the higher affinity of apo E4 for VLDL and LDL, whereas apo E2 and apo E3 lipoprotein-binding preference is for HDL (Hatters DM et al. 2006). This relative enrichment of apo E4 on VLDL particles is thought to lead to their accelerated uptake and the consequent down-regulation of LDL receptor expression. This, in turn, would account for the increased levels of LDL in the plasma of apo E4 subjects. In addition to isoforms E2, E3 and E4, rare apo E mutants have been described associated with type III hyperlipoproteinemia or lipoprotein glomerulopathy (OMIM +611771). Finally, a few rare apo E variants (p.Glu21Lys, p.Pro102Arg and, p.Leu270Glu) have been observed in probands with hyperLDLemia (type IIa hyperlipoproteinemia), but none co-segregated with hyperLDLemia in the families (Wardell MR et al. 1991, van den Maagdenberg AM et al. 1993).
The APOE p.Leu167del mutation was reported to be associated with sea-blue histiocytosis (OMIM #26960), characterized by splenomegaly, mild thrombocytopenia, and, in the bone marrow, numerous histiocytes containing cytoplasmic granules which stain bright blue with the usual hematologic stains. The APOE p.Leu167del mutation was also reported in subjects with familial combined hyperlipidemia (FCHL, type III dyslipoproteinemia) (Solanas-Barca M et al. 2012). FCHL (OMIM #144250) is characterized by elevated levels of either plasma cholesterol or triglyceride or both in members of the same family. And from time to time the profile can change in a given person. FCHL was shown to be distinct from autosomal dominant hypercholesterolemia (ADH, OMIM #143890) that is characterized by an isolated elevation of cholesterol bound to LDL giving rise to tendon xanthomas and premature morbidity and mortality from cardiovascular complications. ADH is genetically heterogeneous and associated with mutations in the well-known LDLR (low density lipoprotein receptor) (FH, OMIM #606945), APOB (apolipoprotein B-100) (FDB, OMIM # 144010) and PCSK9 (Proprotein Convertase Subtilisin/Kexin type 9, Abifadel M et al. 2003) genes. Subsequent work from our team has shown further heterogeneity with at least 19% of ADH families in which the disease is not associated with a mutation within one of these three genes (Marduel M et al. 2010). Indeed, new ADH loci were recently mapped at 16q22.1, HCHOLA4 (Marques-Pinheiro A et al. 2010), and 8q24.22 (Cenarro A et al. 2011). While trying to identify the HCHOLA4 gene, we investigated an exceptionally large ADH French kindred that proved to be unlinked to any of the known genes or loci. To identify this new ADH gene, we combined two strategies: the classic genome wide mapping/candidate gene analysis and the novel whole exome sequencing design (methods in supplemental data).
ADH families were recruited by the French National Research Network on Hypercholesterolemia. For probands, the following inclusion criteria were used: total and LDL-cholesterol values above the 95th percentile when compared to a sex- and age-matched French population (STANISLAS cohort (Siest G et al. 1998), B. Herbeth, G. Siest & S Visvikis-Siest, personal communication 2009), triglycerides below 1.5 mmol/L, personal and/or documented familial xanthomas and/or early coronary artery disease. Non LDLR/-APOB/-PCSK9 probands were selected after direct sequencing of the LDLR and PCSK9 gene coding sequences, MLPA analysis of the LDLR gene, and direct sequencing of parts of the APOB gene encoding the binding domain (exons 26 and 29). Norwegian dyslipidemic probands were recruited with the following inclusion criteria: non-LDLR/-APOB/-PCSK9 carriers, total cholesterol above 8.5 mmol/L, LDL-cholesterol above 5.5 mmol/L and triglycerides below 3.0 mmol/L. The investigation performed here conforms with the principles outlined in the Declaration of Helsinki.
Among 9 non-LDLR/-APOB/-PCSK9/-HCHOLA4 families we have identified, one was large enough to enable genome wide mapping of a new ADH gene (Marques-Pinheiro A et al. 2010). The recruitment of the HC126 family was initiated through the female proband II-2, and expanded to 27 individuals over 3 generations, thus exploring 23 meioses (Figure 1A). Several cardiovascular accidents were reported in the previous generations and the affected proband’s brother II-7 underwent a double bypass at age 43. Family members II-5 and II-15 were scored as “unknown” for linkage analyses because their lipid values without medication were not available. One hundred thousand simulations were performed to evaluate the power and the relevance of a genome wide linkage scan in HC126. The av.ELOD score was 2.1, with a max.ELOD score of 4.8, therefore indicating that the statistically significant threshold of 3 could be reached. The genome-wide scan provided a maximum LOD score of 2.55 (θ=0) for rs718087 on chromosome 19 (Supp. Table S1). Fine regional microsatellite markers provided positive two-point LOD scores : D19S900, D19S903, D19S574, and D19S217 (Supp. Table S1). The 19q13.31–13.32 multipoint analysis gave a maximum LOD score of 3.15 between rs1988065 and rs3513657 (19:43,374,601–45,439,163) (Supp. Table S1, Supp. Figure S1). Regional haplotype construction allowed the identification of a common region for affected members from D19S417 to D19S908 (Figure 1A). Phenotypically unclassified patient II-5 did not carry the familial-disease haplotype, as well as the two affected members I-3 and II-14 who highly probably display a phenocopy form of ADH and suggesting the coexistence of another lipid abnormality in the family, which remains to be identified.
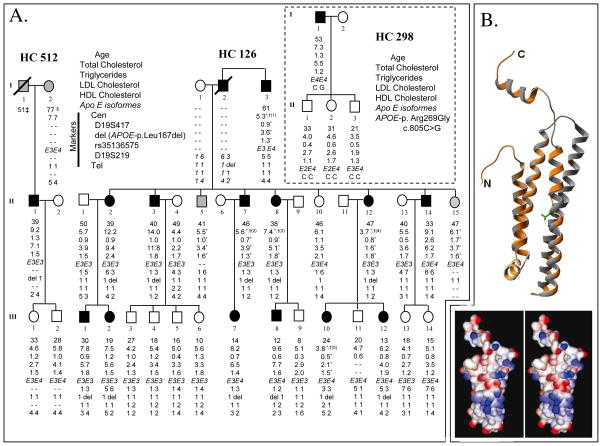
Figure 1. A. Lipid values and regional haplotype of the 19q13.31–13.32 locus in the HC126, HC298 and HC512 families.
Blackened symbols represent affected subjects, white for unaffected subjects while individuals with an undetermined status are in grey. Genotypes are provided in straight, while deduced genotypes are provided in italics. Lipid values are given in mmol/L. * Values under hypocholesterolemic drug therapy. † Total cholesterol without drug : (1) 8.8 mmol/L at 55 years old, (2) 7.98 mmol/L at 43, (3) 14.8 mmol/L at 30, (4) 11.2 mmol/L at 45, (5) 7.98.8 mmol/L at 16. ‡ Died from myocardial infarction at 51 years old. § Presented myocardial infarction at 77 years old. B. In silico analysis of the putative impact of the p.Leu167del mutation. Top: Model obtained. In orange: structural prediction of pLeu167del mutation. In grey: human wild type apo E structural prediction. Leu166 and 167 are shown in green. Bottom: Electrostatic potential of wild-type apo E and p.Leu167del mutant. Charge distribution is represented as a gradient in which positive potentials are drawn in blue and negative in red.
To identify the disease gene, we combined two sequencing strategies. For the first strategy, an inventory of all the genes at 19:43,374,601–45,439,163 was drawn up from online data (Supp. Table S2). We investigated the APOE/APOC1/APOC4/APOC2 gene cluster, as well as the two hepatic control regions (HCR-1,-2), in HC126-II-7, HC126-III-1 and the c.500_502delTCC (p.Leu167del, formerly called Δ149Leu) variation in the APOE gene (RefSeq NM_000041.2) was found (Supp. Figure S2). For the second strategy, whole exome sequencing was performed for three affected members of HC126 (III-2, III-7 and III-8), to possibly identify a mutation within another regional gene that had not been investigated in the first strategy. Four variants were found, the c.437G>A (p.Arg146Gln) in the CCDC123 gene, the c.1151A>G (p.Gln384Arg) in the RHPN2 gene, the c.657G>C (p.Asn219Lys) in the CEACAM8 gene and the c.500_502delTCC (p.Leu167del) in the APOE gene. The two first variants were shown to be frequent genomic variants (//snp.gs.washington.edu/EVS/, //www.1000genomes.org), excluding them from being a possible disease-causing mutation. The CEACAM8 variant is rare, 3/10755 in the Exome Variant Server and not reported by the “1000 genome” project. However, tissue expression profile and reports of alterations of this gene’s expression in acute lymphoblastic leukemias (Lasa A et al. 2008) excluded this variant as a possible ADH disease-causing mutation. Finally, the p.Leu167del in the APOE gene was the only possible disease-causing mutation within the candidate locus.
To confirm the involvement of the APOE gene in ADH, we screened the APOE p.Leu167del through a direct fluorescent PCR method in a control group and we sequenced the whole APOE gene in other ADH or hyperlipidemic probands. The APOE p.Leu167del was not detected in over 220 controls, in agreement with Faivre et al. who had investigated 50 French controls, and indicating that this apo E variation is not a frequent polymorphism. Furthermore, we sequenced the APOE gene in a sample of 153 non-LDLR/-APOB/-PCSK9 probands (55 French ADH and 98 Norwegian hyperlipidemics). Within the French ADH probands, the p.Leu167del variation, and p.Arg269Gly (c.805C>G) were found for family HC512 and HC298, respectively (Figure 1, Supp. Figure S2). The p.Arg269Gly apo E mutant has been previously reported in a proband with type IIb hyperlipoproteinemia, differing from ADH by elevated plasma triglyceride levels (Wardell MR et al. 1991). Within the Norwegian hyperlipidemic cohort, p.Arg235Trp (c.703C>T) was found in a 56 years old man with 9.4, 2.7, 6.4 and 1.8 mmol/L of total cholesterol, triglycerides, LDL and HDL, respectively, and the E3E4 apo E isoformes (Supp. Figure S2). The two missense variations, p.Arg269Gly and p.Arg235Trp, were absent in over 500 French controls, indicating that they are not frequent. Their predicted effect was “not tolerated” with SIFT or, “possibly damaging” and “probably damaging”, with Polyphen and Polyphen-2, respectively, providing relevant effects on the protein activity. The identification of these other APOE mutations for ADH probands is a major finding that confirms the involvement of apo E alterations in the pathogenesis of ADH. To evaluate the possible effect of the p.Leu167del mutation on apo E function, we performed homology modeling of the human wild type apo E protein and of the deleted mutant. Leu167 of apoE is not a conserved amino acid, but is the fourth residue in a 6 amino acid motif of which positions 1, 2, 3 and 6 are very well conserved from Xenopus tropicalis to Homo sapiens (Supp. Figure S3). It is thus very likely that a deletion of an amino acid in this motif would alter the general structure of this highly conserved part of apo E. Because human apo E is 76.4% identical in amino acid sequence with mouse apo E, we used the crystal structure 1YA9 to realize the predicted model shown in Figure 1B. The p.Leu167del variation interrupts one α helix in a group of four helices stabilized by a leucine zipper. Disruption of one helix in the group very probably alters the interaction with the three others. The electrostatic surface charges are altered in apo E p.Leu167del, that also likely influences interaction with lipids and affinity of apo E to its receptors. Nguyen et al. performed a competitive receptor binding test in vitro with apo E isolated from VLDL of one p.Leu167del carrier and observed no significant binding defect. However, their proband was heterozygous for apo E p.Leu167del, thus, the effect of the deleted mutant could not be observed alone. Thus, a LDL receptor-binding defect could not directly be excluded under the hypothesis of a compensatory increased activity of the normal apoE in the presence of the deleted mutant. The other mutations, p.Arg235Trp and p.Arg269Gly, are at the origin of a loss of charge in the first and second α helices of the C-terminal domain that very probably change each helix’s properties, interaction with lipoproteins, and receptor binding.
Total apo E levels were measured for p.Leu167del carriers, HC126-II-2 (apo E = 4.09 mg/dL, under rosuvastatin), HC126-III-2 (apo E = 4.03 mg/dL), and were both in the normal range (2.30–6.30 mg/dL in French controls). Apo E distribution in each lipoprotein fraction showed a higher proportion in HDL (77% vs 63–69%), and a lower proportion in LDL (12% vs 18–25%) (Supp. Table S3). However, these data need to be confirmed because they are based on the study of only two patients, including one under a lipid lowering therapy.
In order to evaluate if LDL bearing the apo E p.Leu167del display an altered catabolism in vivo, we performed kinetic studies of apo B-100-containing lipoproteins in HC126-II-3 after a 2-month wash out of any hypolipidemic treatment (Table 1). After this period, the lipid values for this patient were: 3.7, 10.5, 8.1 and 0.7 mmol/L for triglycerids, total-, LDL- and HDL-cholesterol respectively. VLDL and IDL kinetic parameters were in accordance with the high triglyceride level only observed for this measurement, and never before in this patient, showing similarities with a type III hyperlipoproteinemia (E2E2 genotype) such as the dramatically increased VLDL and IDL pool due to a decreased conversion rate (Ooi EM et al. 2010). Interestingly, the increased VLDL and IDL pool for the HC126 patient was also due to an increased production rate, that was not observed for E2E2 patients, but already reported for patients carrying a PCSK9 gene mutation (Ouguerram K et al. 2003). LDL kinetic parameters were similar to those from FH patients (mutation in the LDLR gene) with an increased LDL pool, which was the consequence of both an increase in LDL production rate and a decrease in LDL catabolism (Table 1). Despite the decreased conversion of VLDL to IDL, the very important VLDL production implies that a higher than normal proportion of lipoprotein follows the metabolic cascade leading to an increased apo B-100 flux into the next lipoprotein fractions. Accordingly, IDL production is increased and, combined to the low IDL catabolism, leads to an increased LDL production. Finally, there is an increased LDL pool due to the combination of this abnormal metabolic cascade and decreased LDL catabolism. LDL kinetic parameters of apo E mutant carrier were similar to those from FH patients strengthening the very probable effect of LDL bearing an apo E mutant on their catabolism. Two hypotheses may be put forward to explain the increased LDL pool observed. The first one is a decreased affinity of the apo E mutant to the LDL receptor as suggested by our in silico structural prediction and in vitro lipoprotein composition analysis. The second hypothesis is that the increased LDL production is related to the increased apo B-100 flux from VLDL as suggested here by in vivo kinetic studies. Altogether, both hypotheses are in agreement with a decreased catabolism of LDL bearing apo E p.Leu167del and thus explaining the hyperLDLemia observed in the family. However, these data need to be confirmed because they are based on the study of only one patient.
No splenomegaly or thrombocytopenia were present in the 12 APOE p.Leu167del carriers of the HC126 family nor in the proband of HC512 indicating that these subjects did not present the sea-blue histiocytosis disease. It seems thus reasonable to hypothetisize that sea-blue histiocytosis develops only for carriers of two mutations, the APOE-p.Leu167del and another mutation in another gene. The hyperLDLemia observed in the HC126 family would thus reveal the pure effect of the p.Leu167del mutation. The digenic mechanism hypothesis could explain the only two reports in the literature of the association between the p.Leu167del in the APOE gene and sea-blue histiocytosis (Nguyen TT et al. 2000, Faivre L et al. 2005).
The APOE p.Leu167del mutation was reported in subjects with familial combined hyperlipidemia (FCHL, type III dyslipoproteinemia) (Solanas-Barca M et al. 2012) and in the HC126 family reported here with a bona fide ADH. This overlap between the FCHL and ADH phenotype has also been reported for mutations in the LDLR gene (Civiera et al. 2008). Hypertriglyceridemia can sometimes be observed in ADH subjects, mainly because of the many common genetic and environmental factors contributing to triglyceride elevation (Talmud PJ 2001). Mutations in the LDLR or APOE gene may amplify the effect of these factors, and thus, according to the number or the nature of these factors, could be associated with an overlapping phenotype between FCHL and ADH.
In conclusion, our results show that screening of the APOE gene is warranted in the setting of molecular diagnosis of ADH along with the LDLR, APOB and PCSK9 genes.
Supplementary Material
Acknowledgments
We thank family members for their cooperation. The authors wish to thank Pr. Jean Davignon (IRCM, Montréal), Pr. Michel Krempf (Institut du Thorax, Université de Nantes), Dr. Laurence Faivre (Centre de Génétique, CHU Dijon) and Dr. Maryse Guerin (INSERM, UMRS 939, Paris) for their helpful comments; the Center for Clinical Investigation (University Hospital of Lille), MC Federspiel (Service de Biochimie Métabolique, Groupe Hopsitalier Pitié-Salpêtrière, AP-HP) for their technical assistance, and Drs. Stacey Gabriel and James Pirruccello for performing exome sequencing and analyzing the data. Exome sequencing and analysis was funded through NIH R01HL107816 to Dr. Sekar Kathiresan. This work was supported by grants from GIS-Maladies Rares, PHRC (AOM06024) and ANR (ANR-05-PCOD-017, ANR-06-MRAR-038, ANR-08-GENO-002-01). M.M. and A.M.-P. were supported by a grant from Ministère de l’Education Nationale et de la Technologie (France). M.A is supported by grants from Région Ile de France and Conseil de la Recherche de l’Université Saint-Joseph (Beirut, Lebanon).
References
- Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- Besley GT, Broadhead DM, Lawlor E, McCann SR, Dempsey JD, Drury MI, Crowe J. Cholesterol ester storage disease in an adult presenting with sea-blue histiocytosis. Clin Genet. 1984;26(3):195–203. doi: 10.1111/j.1399-0004.1984.tb04367.x. [DOI] [PubMed] [Google Scholar]
- Castoldi G, Grusovin GD, Scapoli G. Sea-blue histiocytosis in hyperlipoproteinemia (cytomorphological, clinical and pathogenetic aspects) Haematologica. 1974;59(1):68–80. [PubMed] [Google Scholar]
- Cenarro A, Garcia-Otin AL, Tejedor MT, Solanas M, Jarauta E, Junquera C, Ros E, Mozas P, Puzo J, Pocovi M, Civeira F. A presumptive new locus for autosomal dominant hypercholesterolemia mapping to 8q24.22. Clin Genet. 2010 doi: 10.1111/j.1399-0004.2010.01485.x. [DOI] [PubMed] [Google Scholar]
- Dergunov AD, Novoselov AV, Visvikis S, Siest G, Yakushkin VV, Tsibulsky V. The composition, structural properties and binding of very-low-density and low-density lipoproteins to the LDL receptor in normo- and hypertriglyceridemia: relation to the apolipoprotein E phenotype. Biol Chem. 2005;386(5):441–52. doi: 10.1515/BC.2005.053. [DOI] [PubMed] [Google Scholar]
- Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155(6):487–95. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- Faivre L, Saugier-Veber P, Pais de Barros JP, Verges B, Couret B, Lorcerie B, Thauvin C, Charbonnier F, Huet F, Gambert P, Frebourg T, Duvillard L. Variable expressivity of the clinical and biochemical phenotype associated with the apolipoprotein E p. Leu149del mutation. Eur J Hum Genet. 2005;13(11):1186–91. doi: 10.1038/sj.ejhg.5201480. [DOI] [PubMed] [Google Scholar]
- Ferrans VJ, Buja LM, Roberts WC, Fredrickson DS. The spleen in type I hyperlipoproteinemia. Histochemical, biochemical, microfluorometric and electron microscopic observations. Am J Pathol. 1971;64(1):67–96. [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Familial Hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabollic Basis of Inherited Diseases. six. New York: McGraw-Hill, Inc; 1989. pp. 1215–1250. [Google Scholar]
- Grau AJ, Weisbrod M, Hund E, Harzer K. [Niemann-Pick disease type C: A neurometabolic disease through disturbed intracellular lipid transport] Nervenarzt. 2003;74(10):900–5. doi: 10.1007/s00115-003-1577-3. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31(8):445–54. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Holland P, Hug G, Schubert WK. Chronic Reticuloendothelial Cell Storage Disease. Am J Dis Child. 1965;110:117–24. doi: 10.1001/archpedi.1965.02090030127002. [DOI] [PubMed] [Google Scholar]
- Lasa A, Serrano E, Carricondo M, Carnicer MJ, Brunet S, Badell I, Sierra J, Aventin A, Nomdedeu JF. High expression of CEACAM6 and CEACAM8 mRNA in acute lymphoblastic leukemias. Ann Hematol. 2008;87(3):205–11. doi: 10.1007/s00277-007-0388-1. [DOI] [PubMed] [Google Scholar]
- Lee YB, Kim H, Park CI, Kim CS, Choi IJ, Kim KY, Moon YH. Sea blue histiocytosis associated with hyperlipoproteinemia type IIb. Yonsei Med J. 1983;24(2):132–40. doi: 10.3349/ymj.1983.24.2.132. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Rall SC., Jr Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res. 1999;40(11):1933–49. [PubMed] [Google Scholar]
- Mahley RW, Rall SC. Type III hyperlipoproteinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabollic Basis of Inherited Diseases. 6. New York: McGraw-Hill, Inc; 1989. pp. 1195–1213. [Google Scholar]
- Marduel M, Carrie A, Sassolas A, Devillers M, Carreau V, Di Filippo M, Erlich D, Abifadel M, Marques-Pinheiro A, Munnich A, Junien C, Boileau C, et al. Molecular spectrum of autosomal dominant hypercholesterolemia in France. Hum Mutat. 2010;31(11):E1811–24. doi: 10.1002/humu.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Pinheiro A, Marduel M, Rabes JP, Devillers M, Villeger L, Allard D, Weissenbach J, Guerin M, Zair Y, Erlich D, Junien C, Munnich A, et al. A fourth locus for autosomal dominant hypercholesterolemia maps at 16q22.1. Eur J Hum Genet. 2010;18(11):1236–42. doi: 10.1038/ejhg.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghashpour M, Cualing H. Splenomegaly with sea-blue histiocytosis, dyslipidemia, and nephropathy in a patient with lecithin-cholesterol acyltransferase deficiency: a clinicopathologic correlation. Metabolism. 2009;58(10):1459–64. doi: 10.1016/j.metabol.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Kruckeberg KE, O’Brien JF, Ji ZS, Karnes PS, Crotty TB, Hay ID, Mahley RW, O’Brien T. Familial splenomegaly: macrophage hypercatabolism of lipoproteins associated with apolipoprotein E mutation [apolipoprotein E (delta149 Leu)] J Clin Endocrinol Metab. 2000;85(11):4354–8. doi: 10.1210/jcem.85.11.6981. [DOI] [PubMed] [Google Scholar]
- Ooi EM, Janus ED, Grant SJ, Sinclair LM, PHRB Effect of apolipoprotein E genotype on apolipoprotein B-100 metabolism in normolipidemic and hyperlipidemic subjects. J Lipid Res. 2010;51(8):2413–21. doi: 10.1194/jlr.M004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouguerram K, Magot T, Zair Y, Marchini JS, Charbonnel B, Laouenan H, Krempf M. Effect of atorvastatin on apolipoprotein B100 containing lipoprotein metabolism in type-2 diabetes. J Pharmacol Exp Ther. 2003;306(1):332–7. doi: 10.1124/jpet.103.048991. [DOI] [PubMed] [Google Scholar]
- Parker AC, Bain AD, Brydon WG, Harkness RA, Smith AF, Smith, Boyd DH. Sea-blue histiocytosis associated with hyperlipidaemia. J Clin Pathol. 1976;29(7):634–8. doi: 10.1136/jcp.29.7.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rywlin AM, Lopez-Gomez A, Tachmes P, Pardo V. Ceroid histiocytosis of the spleen in hyperlipemia: relationship to the syndrome of the sea-blue histiocyte. Am J Clin Pathol. 1971;56(5):572–9. doi: 10.1093/ajcp/56.5.572. [DOI] [PubMed] [Google Scholar]
- Sharma P, Kar R, Dutta S, Pati HP, Saxena R. Niemann-Pick disease, type B with TRAP-positive storage cells and secondary sea blue histiocytosis. Eur J Histochem. 2009;53(3):183–6. doi: 10.4081/ejh.2009.e22. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Weng W, de Bruijn IH, de Knijff P, Funke H, Smelt AH, Gevers Leuven JA, van’t Hooft FM, Assmann G, Hofker MH, Havekes LM, Frants RR. Characterization of five new mutants in the carboxyl-terminal domain of human apolipoprotein E: no cosegregation with severe hyperlipidemia. Am J Hum Genet. 1993;52(5):937–46. [PMC free article] [PubMed] [Google Scholar]
- Wardell MR, Rall SC, Jr, Schaefer EJ, Kane JP, Weisgraber KH. Two apolipoprotein E5 variants illustrate the importance of the position of additional positive charge on receptor-binding activity. J Lipid Res. 1991;32(3):521–8. [PubMed] [Google Scholar]
- Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257(5):2518–21. [PubMed] [Google Scholar]
- Solanas-Barca M, de Castro-Orós I, Mateo-Gallego R, Cofán M, Plana N, Puzo J, Burillo E, Martín-Fuentes P, Ros E, Masana L, Pocoví M, et al. Apolipoprotein E gene mutations in subjects with mixed hyperlipidemia and a clinical diagnosis of familial combined hyperlipidemia. Atherosclerosis. 2012;222(2):449–55. doi: 10.1016/j.atherosclerosis.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Civeira F, Jarauta E, Cenarro A, García-Otín AL, Tejedor D, Zambón D, Mallen M, Ros E, Pocoví M. Frequency of low-density lipoprotein receptor gene mutations in patients with a clinical diagnosis of familial combined hyperlipidemia in a clinical setting. J Am Coll Cardiol. 2008;52(19):1546–53. doi: 10.1016/j.jacc.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Talmud PJ. Genetic determinants of plasma triglycerides: impact of rare and common mutations. Curr Atheroscler Rep. 2001;3(3):191–9. doi: 10.1007/s11883-001-0061-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.