Abstract
Pancreatic ductal adenocarcinoma (PDAC) is challenging to treat, and better means to detect and/or treat pancreatic cancer are urgently needed to save lives. Cathepsin E (Cath E) is a proteolytic enzyme highly expressed in PDAC. In this study, a novel approach using Cath E activation of a Cath E-specific prodrug was demonstrated. Specific activation of the prodrug is expected to kill pancreatic cancer cells without harming normal pancreatic cells. A novel 5-aminolevulinic acid (5-ALA) prodrug was custom-designed to be activated selectively by endogenous Cath E within the PDAC cells. The 5-ALA prodrug was incubated with Cath E-positive and -negative tumor cells and illuminated with various doses of light. In addition, mice genetically engineered to develop PDAC were injected intravenously with the 5-ALA prodrug, and the pancreas was treated with light irradiation. One day after treatment, PDAC tissue was assessed for apoptosis. The 5-ALA prodrug was activated within the Cath E-positive tumor but not in the normal pancreatic tissue. When used in combination with light treatment, it allowed delivery of selective photodynamic therapy (PDT) to the cancerous tissues, with minimal harm to the adjacent normal tissues. With this novel Cath E activation approach, it is possible to detect pancreatic cancer cells accurately and specifically impair their viability, while sparing normal cells. This treatment could result in fewer side effects than the non-specific treatments currently in use. Cath E is a specific and effective drug activator for PDAC treatment.
Keywords: Pancreatic cancer, Drug Activation, Cathepsin E, Photodynamic Therapy, Prodrug, 5-Aminolevulinic acid
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) remains a devastating disease, with the worst outcome of all major cancers [1]. In the US, PDAC is the fourth leading cause of cancer death, with average survival of less than 1 year from diagnosis [2]. To date, surgery has been the only curative treatment; however, only 20% of patients are candidates for surgical resection [2]. Moreover, subsets of patients who initially appear to have resectable tumors are subsequently found to have locally advanced disease, in which a margin-negative resection is not feasible. Unfortunately, no effective treatments for locally invasive and metastatic PDAC are known at this time.
Currently, photodynamic therapy (PDT) is a widely accepted treatment modality for many cancerous and precancerous lesions, including those in bladder, brain, ovary, and skin [3–5]. It is a minimally invasive treatment that damages the target cells by imparting cytotoxicity through generation of reactive oxygen species. The individual PDT components, including photosensitizer (PS), light, and oxygen, are nontoxic. However, upon illumination of the light-sensitive PS in the presence of oxygen, highly reactive singlet oxygen (1O2) species are generated within the tumor tissue, causing severe damage to all cells in the vicinity of the treated area [5–9]. Although the intravenously administrated photosensitizers accumulate in the intended tumor tissue, they also undergo non-specific distribution to non-target tissues, resulting in unavoidable damage to the normal cells [10]. Therefore, the patients must avoid light for a long period until the concentration of the sensitizer decreases to an acceptable level. To restrict the desired PDT effect to pancreatic cancer cells, specific targeted delivery or a cancer-specific intracellular activation approach would be required.
Among the clinically used photosensitizers [11], 5-aminolevulinic acid (5-ALA) is unique in its phototoxicity. 5-ALA, which has no photosensitivity, is a precursor for the in situ biosynthesis of protoporphyrin IX (PpIX), an essential intermediate of hemoglobin synthesis and a natural PS. 5-ALA has been extensively used in the clinic, due to its suitability as a tumor-imaging agent and its efficacy in PDT [12, 13]. Previously, 5-ALA has been tested in PDAC using preclinical models [14]; however, parallel accumulation of 5-ALA in both malignant and normal cells have hindered efforts to implement its use. In addition to 5-ALA, other non-specific photosensitizers, including hypericin [15], photosan [16], meso-tetrahydroxyphenyl chlorine [17], verteporfin [18], and porfimer sodium [19], also have been tested in PDAC. In all cases, the non-specificity of the PS caused severe adverse side effects, such as duodenal perforation [17]. Enhancing the discriminatory ability of the administered PS becomes key to confining the PDT effect to the tumor tissue and minimizing unwanted damage to normal tissues in close proximity [9].
Recently, we illustrated the overexpression of Cathepsin E (Cath E) in human PDAC, both in early lesions of PDAC, called pancreatic intraepithelial neoplasias (PanINs), as well as in metastases [20]. In addition, we demonstrated that the enzymatic activity of Cath E can be used to image early-stage, late-stage, and metastasized PDAC, using a Cath E-activatable imaging probe [20, 21]. In the current study, we aimed to take advantage of the upregulated Cath E to treat pancreatic cancer. For this purpose, we incorporated a 5-ALA residue at the end of a scissile peptide that has selective Cath E cleavage susceptibility [22]. This simple conjugation of 5-ALA and a Cath E substrate peptide overcomes the non-specificity of 5-ALA. Here, we report the development and validation of this potent prodrug for controlled release of free 5-ALA specifically within the Cath E-rich tumor environment, but not in normal tissue, for site-specific PDT of the cancerous tissues, with minimal impairment to the contiguous normal tissues.
MATERIAL AND METHODS
Synthesis and characterization of the 5-ALA prodrug
The Cathepsin E-activatable 5-ALA prodrug, H-Arg-Gln-Ala-Gly-Phe-Ser-Leu-5-ALA-OH, was synthesized by solid-phase peptide synthesis (SPPS) using standard Fmoc chemistry with HBTU/HOBT coupling chemistry on an automatic synthesizer (Tribute, Protein Technologies). The dipeptide Fmoc-Leu-5-ALA was first prepared in solution phase using 0.4N HCTU/NMM, purified by HPLC, and characterized by LC-MS using an LCQ Fleet mass spectrometer (Thermo Finnigan, West Palm Beach, FL). For better coupling efficiency, peptide chain elongation on 2-chlorotrityl chloride resin (0.1 mmole, 1.3 μmol/mg, Novabiochem, La Jolla, CA) was initiated by coupling the Fmoc-Leu-5-ALA-COOH dipeptide, followed by the rest of the amino acid residues. All protecting groups were removed and the peptide was cleaved from the resin using a deprotection-scavenger cocktail (TFA:DCM:TIS = 95:2.5:2.5, 10 mL/gm peptidyl resin) for three hours. After HPLC purification, the exact mass of the 5-ALA prodrug was confirmed by LC-MS. Enzymatic cleavage susceptibility was assessed by incubating the 5-ALA prodrug with Cath E, Cath D, or Cath B (50 pmole each) in 50 mM sodium acetate for 1 hr to achieve complete digestion, and then the fragments were identified by LC-MS.
Cell Lines and Cell Culture
Two established pancreatic cancer cell lines, MPanc96-FG30 and MPanc96-CTSE, with low and high Cath E expression, respectively, were used in this study [20, 21]. Both cell lines were cultured routinely in DMEM supplemented with 10% FBS and 1× Pen/Strep (100 U/mL penicillin, 100 μg/mL treptomycin). All cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Cellular validation of Cathepsin E-mediated release of 5-ALA
Mpanc96-CTSE and Mpanc96-FG30 cells (1.2 × 104) were treated in 96-well plates with various concentrations (0.1, 0.5, 1, and 5 μM in 100 μL) of 5-ALA prodrug, incubated for 30 minutes at 37°C, and washed several times with PBS. Fluorescence images of the cells were acquired using an inverted epifluorescence microscope and a TRITC (tetramethyl rhodamine isothiocyanate) filter (λex=557 nm and λem=576 nm). Free 5-ALA and untreated cells were included as controls. The possible light damage done in the absence of photosensitizer was evaluated by irradiating Cath E-positive Mpanc96-CTSE cells with various doses (2.5, 5, 10, 15, and 18 J/cm2) of light using a diode laser system (652 nm; B&W TEK, Newark, DE). Phototoxicity of the 5-ALA prodrug was assessed by incubating Mpanc96-CTSE and Mpanc96-FG30 cells in 96-well plates with 0.1 μM 5-ALA prodrug for 30 minutes at 37°C. The cells were washed several times with PBS, the medium was replaced with growth medium, and the cells were treated with a single dose of 10 J/cm2. After PDT, the treated cells and controls were incubated overnight followed by imaging to detect changes in cell morphology.
Cell Viability and Apoptotic Cell Damage Assays
To determine the number of viable cells after light treatment, a homogeneous colorimetric method, the MTS Cell cytotoxicity assay (Cell Titer 96® Aqueous, Promega, Madison, WI) was used. MPanc96-CTSE and MPanc96-FG30 (2 × 104) cells were incubated with various concentrations (0.1, 0.5, 1, and 5 μM) of the 5-ALA prodrug for 30 minutes, followed by treatment with a range of light doses (2.5, 5, 10, and 15 J/cm2) and incubated overnight at 37°C and 5% CO2 in 96-well plates. Cells treated only with light or the 5-ALA prodrug were used as negative controls. To each well containing PDAC cells in 100 μL of phenol-free culture medium, 20 μL MTS solution was added and incubated for 1 hr. The formation of colored formazan was measured directly in 96-well assay plates at 490 nm absorbance without additional processing. All assays were performed in triplicate. Membrane permeability and dead cell apoptosis assay kit (Life Technologies, Carlsbad, CA) were used for staining cells with YO-PRO-1 and propidium iodide (PI) dyes. After PDT, MPanc96-CTSE cells were washed with PBS buffer, treated with 1 μL YO-PRO-1 and PI stock solution per milliliter medium and incubated on ice for 25 minutes, followed by fluorescence imaging using an inverted epifluorescence microscope. Free 5-ALA was included as a positive control and untreated cells as a negative control.
Animal Preparation and In vivo Photodynamic Therapy
All animal studies were performed in compliance with the approved animal protocols and guidelines of Institutional Animal Care and Use Committee of University of Texas MD Anderson Cancer Center. Transgenic mice were genetically engineered to develop pancreatic cancer in 6–8 weeks after birth by crossing LSL-KRasG12D mice with floxed p53 mice and pancreatic specific cre (Pdx-1-Cre) mice to yield mice that possessed a conditional p53 deletion and endogenous mutant KRasG12D. Littermates without PDAC served as controls. LSL-KRasG12D, p53-floxed, and Pdx-1-Cre genetic mice were obtained from the Mouse Models for Human Cancer Consortium Repository (Rockville, MD). Mice were divided into three groups of five transgenic mice with PDAC tumors and three normal littermate controls. Mice were injected with a single dose of saline, free 5-ALA, or the 5-ALA prodrug (1 mg 5-ALA equivalent/kg, 50 μL) intravenously through the tail vein using a 30 gauge needle. Mice were incubated for 60 minutes in a full metabolically active environment and anesthetized by intraperitoneal injection of sodium pentobarbital (Nembutal), at a dose of 50–90 mg/kg diluted to 6 mg/ml. The pancreas was exposed through a left abdominal incision (1 cm, laparotomy) and treated with a 10-J/cm2 dose of light using a diode laser (652 nm) and incidence fluence rate of 50 mW/cm2 for 3.5 minutes. After PDT, the pancreas was carefully returned to the peritoneal cavity and the abdomen was closed. One day after light treatment, animals were sacrificed and the pancreases were harvested for subsequent evaluation.
Histochemical Staining of Mouse Tissues
Formalin-fixed and paraffin-embedded tissue sections of normal and cancerous pancreas were then examined for apoptosis by TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate- (dUTP-) biotin nick end labeling) staining using ApopTag Peroxidase in situ apoptosis detection kit (EMD Millipore, Billerica, MA). Tissues included 5 samples of PDAC treated with 5-ALA precursor probe, 5 samples of PDAC treated with free 5-ALA as a positive control, and 5 samples of PDAC treated with normal saline as a negative control. We also included 8 normal litter-control pancreas samples including 3 samples of normal pancreas treated with the 5-ALA prodrug, 3 samples of normal pancreas treated with free 5-ALA, and 2 samples of normal pancreas treated with normal saline. Two tissue samples were treated with DNase for use as positive standards in the TUNEL assay.
Statistical Analysis
Statistical Package (version 13, SPSS, Chicago, IL) was used to assess the statistical mean error using the paired-samples t-test with two-tailed p-values.
RESULTS
5-ALA prodrug is efficiently cleaved by Cath E in vitro
The 5-ALA prodrug was designed to release free 5-ALA upon selective Cath E cleavage of the scissile bond between Leu- and 5-ALA residues (Supplementary Figure 1A). The biochemical selectivity of the 5-ALA prodrug was systematically tested using a panel of purified proteolytic enzymes, i.e., Cath E, Cath D, and Cath B (50 pmol each). The LC-MS results indicated that Cath E was the only tested protease capable of cleaving the 5-ALA prodrug to two major metabolic fragments, corresponding to H-Arg1-Gln2-Ala3-Gly4-Phe5-Ser6-Leu7-OH and released free 5-ALA [8] (Supplementary Figure 1B). A higher concentration of Cath B and D (100 pmol) showed no sign of cleavage of the 5-ALA prodrug at the link between 5-ALA and the rest of the peptide substrate, according to LC-MS analysis (data not shown). High energy MS/MS confirmed the production of the metabolic fragments characteristic of selective Cath E-mediated degradation of the 5-ALA prodrug (Supplementary Figure 2A, B).
Cath E is a potent activator of drug release in Cath E-expressing cells
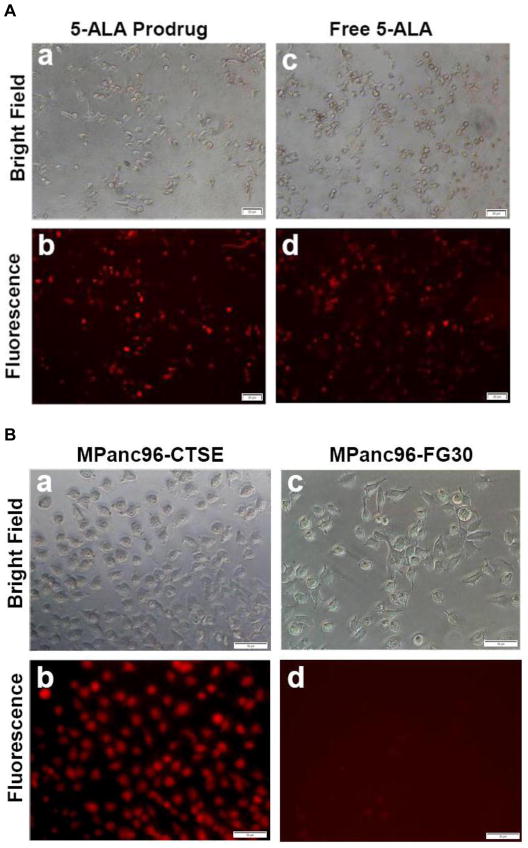
It was shown previously that Cath E expression is upregulated in all stages of PDAC [20], including PanIN lesions of human tumor samples and engineered animal models. To explore the potential use of Cath E as a drug activator, the release of model drug 5-ALA was imaged in cells. Although free 5-ALA is not fluorescent, it is the key component for spontaneous synthesis of the fluorescent PpIX molecule. Therefore, Cath E-positive cells, MPanc96-CTSE, were incubated with the 5-ALA prodrug, or free-5-ALA as a positive control, and imaged. Microscopic images of the unfixed MPanc96-CTSE cells in the TRITC channel showed significant PpIX fluorescence signal originating from cells treated with the 5-ALA prodrug (Figure 1A, panel b). These signals were comparable to those obtained from cells treated with the positive control, free 5-ALA (Figure 1A, panel d). To further confirm the Cath E-mediated pathway, the 5-ALA prodrug was tested with PDAC cells with a different level of Cath E expression. Pronounced fluorescent signals were observed within PDAC cells with high levels of Cath E enzyme, indicating efficient Cath E-mediated release of 5-ALA (Figure 1B, panel b). In contrast, PDAC cells of the parent cell line, MPan96-FG30, with limited Cath E expression, failed to show comparable fluorescent signals within the cells, which strongly suggested the lack of free 5-ALA within the cells (Figure 1B, panel d). This result indicates that Cath E is required to release free 5-ALA and initiate the subsequent synthesis of PpIX.
Figure 1. 5-ALA prodrug is efficiently activated by Cath E-expressing PDAC cells.
(A) Representative microscopic images of unfixed human pancreatic cancer cells (MPanc96-CTSE) after treatment with 0.5 μM 5-ALA prodrug (a, and b) free 5-ALA as a positive control (c, d). Cells imaged on an inverted epifluorescence microscope in bright field (top) and TRITC (Tetramethyl Rhodamine isothiocyanate) channel (λex=557 nm, λem=576 nm) (bottom). Images show significant fluorescence signals originating from cells treated with the 5-ALA prodrug, comparable to that obtained with free 5-ALA, indicating the efficient release of 5-ALA from the prodrug. (B) 5-ALA prodrug activation is Cath E-dependent. Representative microscopic images of unfixed PDAC cells expressing Cath E (MPanc96-CTSE) (a, b) and parent cell line (MPanc96-FG30) (c, d) after treatment with 0.5 μM 5-ALA prodrug for 1hr at 37°C. The pronounced fluorescence signal observed within the MPanc96-CTSE cells indicates strong Cath E-mediated release of 5-ALA, which enables cells visualization in the TRITC channel (b). In contrast, MPanc96-FG30 cells, with limited Cath E expression, failed to show an appreciable fluorescence signal, strongly suggesting the lack of free 5-ALA within the cells (d).
The 5-ALA prodrug, in combination with light, is an effective phototoxic agent that selectively induces PDAC cell death in vitro
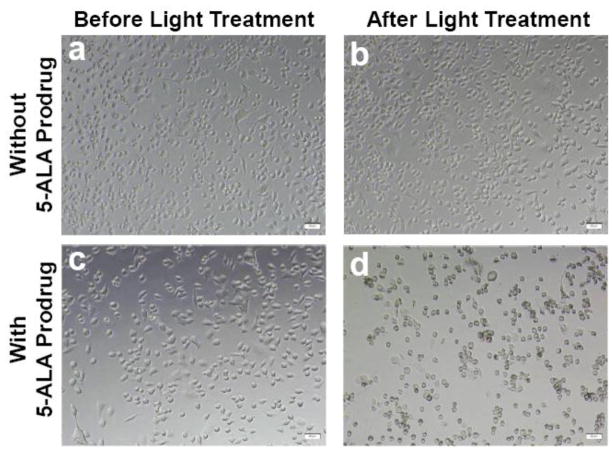
The potential usefulness of the 5-ALA prodrug was then examined, in combination with light treatment, as a therapeutic agent for PDAC. Cath E-rich cells, MPanc96-CTSE, were treated with the 5-ALA prodrug for 30 minutes, washed, imaged and illuminated. After light treatment, cells were imaged again to assess the effect of the photodynamic treatment. The cells images show no signs of morphological change upon incubation with 5-ALA prodrug without light treatment (Figure 2, panel c). Similarly, in the absence of the 5-ALA prodrug, the images of cells with or without light illumination showed no change in morphology, even with a high irradiation dose of 90 mW/cm2 (Figure 2, panels a, b). On the other hand, 5-ALA prodrug-treated cells underwent significant cell death upon treatment with light (Figure 2, panel d). The images indicate that 5-ALA prodrug alone does not have any apparent cellular toxicity, whereas, upon light treatment, massive damage to the cells was observed. Most cells were greatly altered or destroyed. From these results, we can infer that the light treatment imposes its phototoxic effect only in the event of intracellular release of 5-ALA from the 5-ALA prodrug by Cath E enzyme cleavage.
Figure 2. 5-ALA prodrug, in combination with light treatment, is an effective phototoxic agent that selectively and sensitively induces cell death in vitro.
Microscopic image of unfixed human pancreatic cancer cells (MPanc96-CTSE) untreated (top) and treated with 0.1 μM 5-ALA prodrug (bottom), before (a, c) and after (b, d) illumination with doses of 18 J/cm2 (top) and 10 J/cm2 (bottom). The cells show no sign of morphological changes upon exposure to high doses of light alone. However, massive cell destruction is observed upon treatment with the 5-ALA prodrug and exposure to a low dose of light.
To further verify the results, light was applied to the 5-ALA prodrug-treated cells with a different level of Cath E. The images display greater damage to the PDAC cells over-expressing Cath E, MPanc96-CTSE cells (Figure 3A, right), compared to those having limited Cath E expression, MPanc96-FG30, (Figure 3A, left). As supported by the fluorescence images (Figure 1B), the extent of cell damage corresponded to the level of Cath E expression in both cell lines. These results reflect the role of Cath E expression in cleaving the 5-ALA prodrug sequence, resulting in controlled release of 5-ALA and subsequent PpIX synthesis within the tumor cells, which causes cell damage upon light treatment.
Figure 3. 5-ALA prodrug is an effective phototoxic agent in cells in the presence of light.
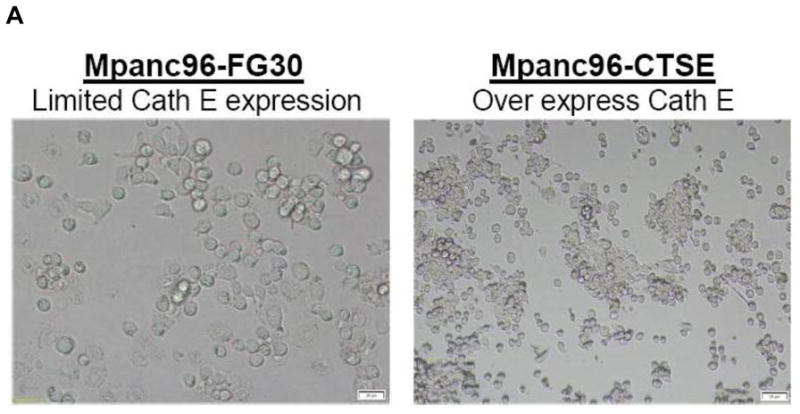
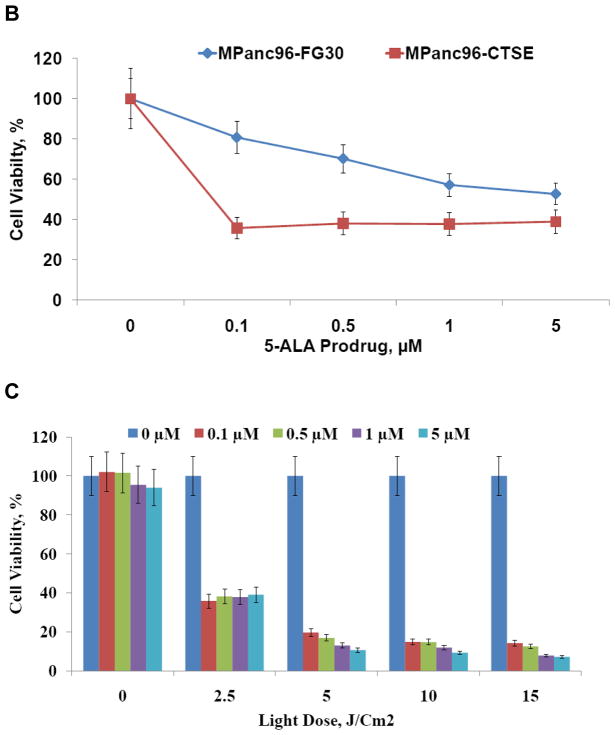
(A) Human pancreatic cancer cells, MPanc96-FG30 (left) and MPanc96-CTSE (right), treated with 0.1 μM 5-ALA prodrug after light exposure (10 J/cm2). The images show morphological changes in MPanc96-CTSE cells and, to a lesser extent, in the parental MPanc96-FG30 cells, which have limited expression of Cath E. This strongly suggests that the enzymatic activity of Cath E plays a major role in the efficiency of treatment. (B) Viability of MPanc-FG30 and MPanc96-CTSE cells treated with light dose of 2.5 J/cm2 with various concentrations (0.1, 0.5, 1, and 5 μM) of 5-ALA prodrug. Quantitation of cell viability demonstrated that the PDT was more highly phototoxic in MPanc96-CTSE, compared to Mpanc-FG30, cells under all conditions. (C) Viability of MPanc96-CTSE cells treated with a range of light doses (2.5, 5, 10, and 15 J/cm2) at various concentrations (0.1, 0.5, 1, and 5 μM) of 5-ALA prodrug. Quantitation of cell viability illustrated that the phototoxic effect of PDT on the cells increased with increasing concentration of 5-ALA prodrug and light dose.
To quantitate cell viability upon photodynamic treatment with the 5-ALA prodrug, PDAC cells were subjected to homogeneous, colorimetric MTS cytotoxicity assay. In this assay, the conversion of MTS into the aqueous soluble formazan product is accomplished only in metabolically active cells. After incubating PDAC cells with the tetrazolium compound, living PDAC cells in culture were quantified directly by measuring the absorbance of the produced formazan product at 490 nm. The cytotoxicity assay results show a rapid decrease in MPanc96-CTSE cell viability, to approximately 40% of the original value, upon PDT using 0.1 μM 5-ALA prodrug and a dose of 2.5 J/cm2 light (Figure 3B). Meanwhile, a slow and steady decrease in cell viability was observed in MPanc96-FG30, which have limited Cath E expression, upon PDT under the same conditions (Figure 3B). Increasing the light dose from 2.5 to 5 J/cm2 resulted in a decrease in MPanc96-CTSE cell viability from approximately 40% to approximately 20% (Figure 3C). Higher light doses (10 and 15 J/cm2) showed no appreciable enhancement of cellular toxicity.
The possible pathways of PDAC cell damage were studied by staining the cells using membrane permeability and dead cell apoptosis kit. The PDAC cells were stained with YO-PRO-1 and PI after PDT with the 5-ALA prodrug. Untreated cells were stained as well, for comparison. Images of the PDAC cells after PDT with 5-ALA prodrug showed that the apoptotic cells stained with green fluorescence while dead cells stained with red and green fluorescence (Figure 4, panels d, f). The apoptotic populations were easily distinguished from the necrotic ones. In contrast, images of cells incubated with the 5-ALA produg without light treatment failed to show appreciable YO-PRO-1 and PI fluorescence signals (Figure 4, panels c, e). The results suggest that apoptosis is the main pathway of the Cath E-mediated cellular damage imparted by PDT with 5-ALA prodrug.
Figure 4. Mechanism of 5-ALA prodrug-induced cell death.
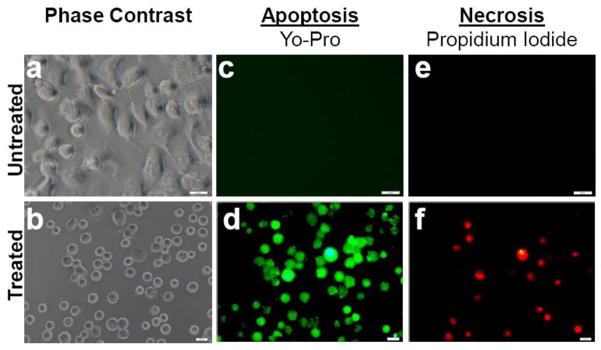
Representative microscopic images of untreated (top) and treated (bottom) MPanc96-CTSE cells, illustrating apoptotic and necrotic cell damage that occurred during PDT. The images indicate that apoptosis is the primary mechanism of cell death. Cells were stained with both Yo-Pro (apoptosis) and PI (necrosis) immediately after PDT.
Photodynamic therapy with 5-ALA prodrug causes selective PDAC cell death in vivo
To examine the selectivity and efficiency of Cath E activation in vivo, the 5-ALA prodrug was tested in genetically engineered mouse models (GEMM). One group of mice with normal pancreases and a second group of mice with PDAC were injected intravenously with saline, free 5-ALA, or 5-ALA prodrug. One hour after IV injection, the abdomen was surgically opened and the exposed pancreases were illuminated locally by a laser. The opening was closed with stitches and the animals allowed to recover for 24 hrs. Tissue sections of normal pancreas and PDAC were stained with TUNEL to identify apoptotic cell damage. We found that tissue sections from mice injected with saline showed no sign of brown staining, indicating that the procedure caused insignificant cell damage (Figure 5, panels a, d). Similarly, tissue sections from normal mice treated with the 5-ALA prodrug showed no signs of brown staining, reflecting the lack of apoptosis in these tissues (Figure 5, panel c and Supplementary Figure 3, right). In contrast, multiple brownstained spots (highlighted with black arrows) were observed in the tissue sections of normal pancreas from mice treated with free 5-ALA (Figure 5, panel b and Supplementary Figure 3, left). Furthermore, multiple apoptotic cells (brown-stained spots) were found in tissue sections from all mice with PDAC treated with free 5-ALA or the 5-ALA prodrug (Figure 5, panels e, f). This result revealed that free 5-ALA was nonspecifically picked up by normal pancreatic cells and pancreatic cancer cells, and utilized for PpIX synthesis. Therefore, light-induced apoptosis was seen in all 5-ALA-treated tissues. Detailed pathological examination of 5-ALA-treated tissues showed non-specific cell damage in areas adjacent to the islets of Langerhans as well as in normal acinar cells (Supplementary Figure 3). However, the results with the 5-ALA prodrug were completely different. Because 5-ALA can only be released from the 5-ALA prodrug in Cath E-expressing PDAC and not in normal pancreas, non-specific damage to normal pancreatic tissues were not seen in tissues treated with the 5-ALA prodrug. Therefore, Cath E does play a pivotal role in specific activation of prodrugs design to be activated by this enzyme.
Figure 5. 5-ALA prodrug in combination with light treatment caused selective pancreatic cancer cell death in vivo.
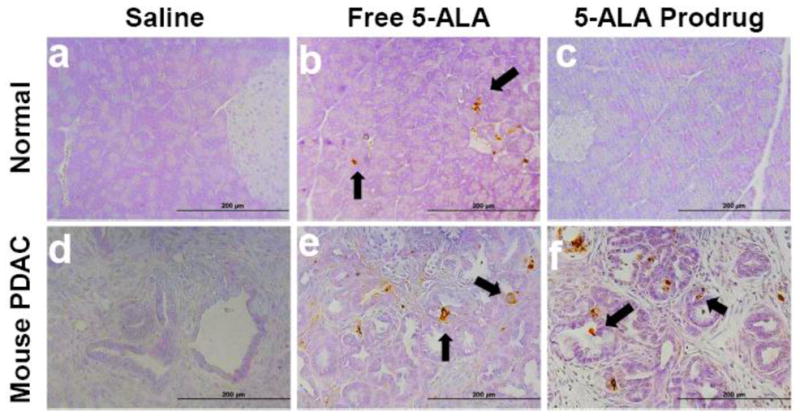
(a–c) Normal pancreas and (d–f) mouse PDAC from genetic mouse model (p53 conditional deletion/LSL-KrasG12D/Pdx1-Cre) treated with 10 J/cm2. (a, d) negative control saline, (b, e) positive control free 5-ALA, and (c, f) 5-ALA prodrug (1 mg 5-ALA equivalent/kg). Formalin-fixed, paraffin-embedded tissue sections were examined for apoptosis by TUNEL using ApopTag Peroxidase Kit. TUNEL staining of PDAC pancreas showed no apoptotic cells in mice treated with saline and PDT (d). However, multiple brown-stained cancer cells were observed in pancreas of animals treated with free 5-ALA and 5-ALA prodrug, indicating apoptosis (e and f, arrows). Tissue sections of normal pancreas of mice treated with PDT using 5-ALA prodrug showed no apoptotic staining, 5-ALA prodrug similar to the negative saline control (a, c). In contrast, scattered brown-stained spots were observed in normal acinar cells in the tissue sections of normal pancreas of mice treated with PDT using free 5-ALA (b, arrows).
DISCUSION
The unique expression of Cath E and its proteolytic activity in neoplastic cells has drawn great attention to its potential use as a biomarker for PDAC. Recently, we demonstrated that elevated levels of Cath E expression in pancreatic cancer could be exploited for PDAC detection using a novel fluorescent imaging probe [20, 21]. To expand the utilization of the distinctive expression of Cath E beyond imaging to specifically kill pancreatic cancer cells, a Cath E-mediated prodrug concept was demonstrated. The novel prodrug model consists of a 5-ALA residue and a Cath E-sensitive peptide sequence. 5-ALA is the key precursor for hemoglobin. In cells, 5-ALA is utilized spontaneously to synthesize a fluorescent intermediate, PpIX, and then the subsequent chelation of iron forms the final non-fluorescent product, hemoglobin [13]. 5-ALA by itself is not toxic or fluorescent, but the formed intermediate PpIX is fluorescent and phototoxic. Therefore, in its intact state, the 5-ALA prodrug is insensitive to light illumination and not imageable. Upon selective proteolytic cleavage by the endogenous Cath E, the 5-ALA residue was released and then used to produce PpIX, resulting in a marked PpIX fluorescent signal originating within the neoplastic cells expressing Cath E. This fluorescent signal enabled visualization of the Cath E-expressing tumor cells (Figure 1A, panel b and Figure 1B, panel b). In addition, when light illumination at the appropriate wavelength is applied, phototoxicity specific to PDAC cells expressing Cath E is observed and its efficiency is dependent on Cath E expression level (Figure 2, panel d and Figure 3A, B). Moreover, promising in vivo PDT effects on Cath E-expressing PDAC cells in GEMM have been shown with a small amount of 5-ALA prodrug (1 mg 5-ALA equivalent/kg) and low light dose (10 J/cm2) (Figure 5, panel f). Most importantly, selective, targeted cell death of PDAC cells expressing Cath E, but not of normal pancreatic cells, was demonstrated (Figure 5, panel c). As expected, unmodified 5-ALA photosensitizer cause noticeable nonspecific damage to normal pancreatic tissue, such as normal acinar cells (Figure 5, panel b) and pancreatic tissue in close proximity to the islets of Langerhans (Supplementary Figure 3, top, left). The current study is the first clear demonstration of targeted therapy of pancreatic cancer in GEMM using a novel prodrug specific for Cath E endogenous activity that detects and treats PDAC tumors with minimal damage to the adjacent normal pancreatic tissue. These results validate the utility of developing new Cath E-mediated diagnostic and therapeutic agents as a new clinically relevant approach for PDAC management.
To improve PDT efficacy, efforts have been focused on ways to escalate cellular damage by improving the spectroscopic and photochemical properties of the photosensitizers, together with use of a state-of-the-art laser beam [23, 24]. Pairing of macromolecular moieties, such as polymers and antibodies and, more recently, nanoparticles, is the traditional approach to achieve preferential localization in neoplastic tissues [25–29]. However, problems, such as early and general sensitization, low selectivity, undesirable phototoxicity to normal cells, and the inconvenience of sustained photosensitivity remain unaddressed. Investigation of the relative selectivity of the photosensitizer, photofrin, in tumor and normal tissues reveals the lack of an absolute difference in the two types of tissue, which certainly narrows the window of therapeutic application [10]. Improving the targeting efficiency and avoiding the universal sensitization would prevail over limitations of PDT in clinical applications. Previously, we validated the potential use of protease activity in achieving selective imaging [30, 31]. In this study, we modified the photosensitizing moiety 5-ALA through conjugation with a peptide sequence that is specifically sensitive to the endogenous proteolytic activity of Cath E in PDAC cells. Since the 5-ALA prodrug in its intact state is insensitive to light illumination, most side effects typically associated with PDT have been largely avoided, including the universal sensitization that caused unwanted phototoxicity to normal cells. Upon intracellular Cath E proteolytic cleavage, the 5-ALA prodrug showed the distinct capacity to localize the PDAC cancer cells and emit fluorescent signals originating within the neoplastic cells. Such a PDAC-specific fluorescent signal could be used to guide surgical procedures. C ombined with light illumination, 5-ALA prodrug specifically damages the cancer cells, while minimizing harm to the adjacent normal cells. Currently, it is technically challenging for surgeons to detect and treat metastases due to the lack of adequate imaging and treatment agents. Therefore, the cath E-mediated imaging probe or prodrug could help close the gap between the existing clinical needs and the lack of adequate effective detection and therapeutic agents. This new prodrug promises improvement in pancreatic cancer diagnosis and treatment, which are currently great challenges.
CONCLUSIONS
In conclusion, this enzyme-activation approach enables selective detection and treatment of pancreatic cancer while sparing normal pancreatic cells. The results of this study provide essential preclinical evidence, using a GEMM model, that Cath E-mediated therapy using the 5-ALA prodrug in combination with light illumination will be useful for clinical adjuvant and neoadjuvant management of pancreatic cancer. The full utility of this therapeutic approach will not be known until clinical trials are performed. However, demonstration of the power of this approach in effectively targeting and killing cancer cells that express Cath E, but not the contiguous normal pancreatic cells in GEMM that mimic human PDAC, suggest its utility for safely eradicating this lethal cancer. Because this approach will specifically kill only cancer cells but not normal cells, it will result in fewer side effects than currently available treatments. The use of the PDT approach with the newly developed 5-ALA prodrug will help improving survival as it will be able to detect and treat PDAC simultaneously in the same setting or even during surgical procedures. Importantly, the Cath E-activation approach could be applied to other drugs and diseases.
Supplementary Material
(A) General structure of intact 5-ALA prodrug and its metabolic fragments observed upon enzymatic degradation with Cath E. (B) Mass spectrometric characterization of intact 5-ALA prodrug and its metabolites after Cath E digestion.
(A) Fragment Ions (monoisotopic masses) detected in high-energy MS-MS spectra. (B) Fragment Ion table based on the monoisotopic masses.
Multiple brown-stained spots in the vicinity of islets of Langerhans (L) (top, left), and in normal acinar cells (bottom, left), were observed in sections of normal pancreas of mice treated with PDT using free 5-ALA, indicating apoptosis in these tissues. In contrast, sections of normal pancreas of mice treated with PDT using 5-ALA prodrug showed no signs of nonspecific apoptosis.
Acknowledgments
This research was supported, in part, by grants from the National Institutes of Health (NIH) CA135312 (to CT), The Lockton Endowment (to CDL), and M. D. Anderson Cancer Center CCSG CA#16672 (to CDL).
Abbreviations
- Cath E
Cathepsin E
- PDAC
Pancreatic ductal adenocarcinoma
- GEMM
genetically engineered mouse model
- PDT
Photodynamic Therapy
- PS
Photosensitizer
- PI
Propidium iodide
- RP-HPLC
reversed phase high performance liquid chromatography
- MS
mass spectrometry
- UV
ultraviolet
Footnotes
WRA and ZCM: study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content, and technical support. HW: Histological analysis and interpretation of data. CDL and CHT: study concept and design, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript for important intellectual content, obtained funding, technical support, and study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Grochow LB, Abrams RA. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Annals of surgery. 1997;226(3):248–257. doi: 10.1097/00000658-199709000-00004. discussion 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. The lancet oncology. 2004;5(8):497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 4.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chemical reviews. 2010;110(5):2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nature reviews. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 6.Lovell JF, Liu TW, Chen J, Zheng G. Activatable photosensitizers for imaging and therapy. Chemical reviews. 2010;110(5):2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 7.Issa MC, Manela-Azulay M. Photodynamic therapy: a review of the literature and image documentation. Anais brasileiros de dermatologia. 2010;85(4):501–511. doi: 10.1590/s0365-05962010000400011. [DOI] [PubMed] [Google Scholar]
- 8.Nishioka NS. Drug, light, and oxygen: a dynamic combination in the clinic. Gastroenterology. 1998;114(3):604–606. doi: 10.1016/s0016-5085(98)70546-3. [DOI] [PubMed] [Google Scholar]
- 9.Fan BG, Andren-Sandberg A. Photodynamic therapy for pancreatic cancer. Pancreas. 2007;34(4):385–389. doi: 10.1097/mpa.0b013e3180439c50. [DOI] [PubMed] [Google Scholar]
- 10.Hahn SM, Putt ME, Metz J, Shin DB, Rickter E, Menon C, Smith D, Glatstein E, Fraker DL, Busch TM. Photofrin uptake in the tumor and normal tissues of patients receiving intraperitoneal photodynamic therapy. Clin Cancer Res. 2006;12(18):5464–5470. doi: 10.1158/1078-0432.CCR-06-0953. [DOI] [PubMed] [Google Scholar]
- 11.Musiol R, Serda M, Polanski J. Prodrugs in photodynamic anticancer therapy. Current pharmaceutical design. 2011;17(32):3548–3559. doi: 10.2174/138161211798194549. [DOI] [PubMed] [Google Scholar]
- 12.Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE, Nesland JM. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79(12):2282–2308. doi: 10.1002/(sici)1097-0142(19970615)79:12<2282::aid-cncr2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. Journal of photochemistry and photobiology. 1990;6(1–2):143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- 14.Regula J, Ravi B, Bedwell J, MacRobert AJ, Bown SG. Photodynamic therapy using 5-aminolaevulinic acid for experimental pancreatic cancer--prolonged animal survival. British journal of cancer. 1994;70(2):248–254. doi: 10.1038/bjc.1994.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CD, Kwan D, Saxton RE, McFadden DW. Hypericin and photodynamic therapy decreases human pancreatic cancer in vitro and in vivo. The Journal of surgical research. 2000;93(1):137–143. doi: 10.1006/jsre.2000.5949. [DOI] [PubMed] [Google Scholar]
- 16.Xie Q, Jia L, Liu YH, Wei CG. Synergetic anticancer effect of combined gemcitabine and photodynamic therapy on pancreatic cancer in vivo. World J Gastroenterol. 2009;15(6):737–741. doi: 10.3748/wjg.15.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AW. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50(4):549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusuf TE, Matthes K, Brugge WR. EUS-guided photodynamic therapy with verteporfin for ablation of normal pancreatic tissue: a pilot study in a porcine model (with video) Gastrointestinal endoscopy. 2008;67(6):957–961. doi: 10.1016/j.gie.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Topazian M, Zhong N, Baron TH, Vege SS, Wang KK. Photodynamic therapy of intraductal papillary mucinous neoplasm. Endoscopy. 2012;44(2):213–215. doi: 10.1055/s-0031-1291539. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Monserrate Z, Abd-Elgaliel WR, Grote T, Deng D, Ji B, Arumugam T, Wang H, Tung CH, Logsdon CD. Detection of pancreatic cancer tumours and precursor lesions by cathepsin E activity in mouse models. Gut. 2012;61(9):1315–1322. doi: 10.1136/gutjnl-2011-300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abd-Elgaliel WR, Cruz-Monserrate Z, Logsdon CD, Tung CH. Molecular imaging of Cathepsin E-positive tumors in mice using a novel protease-activatable fluorescent probe. Molecular bioSystems. 2011;7(12):3207–3213. doi: 10.1039/c1mb05215b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd-Elgaliel WR, Tung CH. Selective detection of Cathepsin E proteolytic activity. Biochimica et biophysica acta. 2010;1800(9):1002–1008. doi: 10.1016/j.bbagen.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Geel IP, Oppelaar H, Oussoren YG, Schuitmaker JJ, Stewart FA. Mechanisms for optimising photodynamic therapy: second-generation photosensitisers in combination with mitomycin C. British journal of cancer. 1995;72(2):344–350. doi: 10.1038/bjc.1995.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. The lancet oncology. 2000;1:212–219. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 25.Choi Y, McCarthy JR, Weissleder R, Tung CH. Conjugation of a photosensitizer to an oligoarginine-based cell-penetrating peptide increases the efficacy of photodynamic therapy. Chem Med Chem. 2006;1(4):458–463. doi: 10.1002/cmdc.200500036. [DOI] [PubMed] [Google Scholar]
- 26.Peterson CM, Shiah JG, Sun Y, Kopeckova P, Minko T, Straight RC, Kopecek J. HPMA copolymer delivery of chemotherapy and photodynamic therapy in ovarian cancer. Advances in experimental medicine and biology. 2003;519:101–123. doi: 10.1007/0-306-47932-X_7. [DOI] [PubMed] [Google Scholar]
- 27.Brokx RD, Bisland SK, Gariepy J. Designing peptide-based scaffolds as drug delivery vehicles. J Control Release. 2002;78(1–3):115–123. doi: 10.1016/s0168-3659(01)00491-6. [DOI] [PubMed] [Google Scholar]
- 28.Konan YN, Gurny R, Allemann E. State of the art in the delivery of photosensitizers for photodynamic therapy. Journal of photochemistry and photobiology. 2002;66(2):89–106. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 29.Jang B, Park JY, Tung CH, Kim IH, Choi Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS nano. 2011;5(2):1086–1094. doi: 10.1021/nn102722z. [DOI] [PubMed] [Google Scholar]
- 30.Choi Y, Weissleder R, Tung CH. Protease-mediated phototoxicity of a polylysine-chlorin(E6) conjugate. ChemMedChem. 2006;1(7):698–701. doi: 10.1002/cmdc.200600053. [DOI] [PubMed] [Google Scholar]
- 31.Choi Y, Weissleder R, Tung CH. Selective antitumor effect of novel protease-mediated photodynamic agent. Cancer research. 2006;66(14):7225–7229. doi: 10.1158/0008-5472.CAN-06-0448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) General structure of intact 5-ALA prodrug and its metabolic fragments observed upon enzymatic degradation with Cath E. (B) Mass spectrometric characterization of intact 5-ALA prodrug and its metabolites after Cath E digestion.
(A) Fragment Ions (monoisotopic masses) detected in high-energy MS-MS spectra. (B) Fragment Ion table based on the monoisotopic masses.
Multiple brown-stained spots in the vicinity of islets of Langerhans (L) (top, left), and in normal acinar cells (bottom, left), were observed in sections of normal pancreas of mice treated with PDT using free 5-ALA, indicating apoptosis in these tissues. In contrast, sections of normal pancreas of mice treated with PDT using 5-ALA prodrug showed no signs of nonspecific apoptosis.