Abstract
Macrophages play a central role in the pathogenesis of rheumatoid arthritis (RA). There is an imbalance of inflammatory and antiinflammatory macrophages in RA synovium. Although the polarization and heterogeneity of macrophages in RA have not been fully uncovered, the identity of macrophages in RA can potentially be defined by their products, including the co-stimulatory molecules, scavenger receptors, different cytokines/chemokines and receptors, and transcription factors. In the last decade, efforts to understand the polarization, apoptosis regulation, and novel signaling pathways in macrophages, as well as how distinct activated macrophages influence disease progression, have led to strategies that target macrophages with varied specificity and selectivity. Major targets that are related to macrophage development and apoptosis include TNF-α, IL-1, IL-6, GM-CSF, M-CSF, death receptor 5 (DR5), Fas, and others, as listed in Table 1. Combined data from clinical, preclinical, and animal studies of inhibitors of these targets have provided valuable insights into their roles in the disease progression and, subsequently, have led to the evolving therapeutic paradigms in RA. In this review, we propose that reestablishment of macrophage equilibrium by inhibiting the development of, and/or eliminating, the proinflammatory macrophages will be an effective therapeutic approach for RA and other autoimmune diseases.
Keywords: Macrophage, Polarization, Rheumatoid arthritis, Therapy, Management, Reform, Removal, Joint recruitment, Inflammation, Depletion, TRAIL
Introduction
Rheumatoid arthritis (RA) is a common autoimmune disease in which macrophages potentially play a central pathogenic role, whereas fibroblasts, T and B lymphocytes, and neutrophils might exhibit secondary dysfunctions. An emerging concept that defines the role of macrophages in RA is their high plasticity during development. The nomenclature of general macrophage polarization has been proposed in the last decade, in which M1 (classical, inflammatory) and M2 (alternative, antiinflammatory, tissue repair) are referred to the two extremes of a spectrum of possible macrophage activation status [1]. The relatively rigid M1/M2 nomenclature is reminiscent of the Th1/Th2 T cell dichotomy and has been extended to encompass the broader spectrum of macrophage activation. Recently, a new functional grouping of macrophage populations on the basis of three different functional activities—host defense, wound healing, and immune regulation—has emerged, and the “blending” of distinct macrophage populations and the impacts in maintaining homeostasis have been emphasized [2]. Although the investigation in this field has rapidly expanded from tumors to infection, atherosclerosis, obesity, and other disorders, exploration of macrophage polarization in RA has just commenced, with numerous unsolved mysteries. Furthermore, discrepancies and nuances for macrophage homeostasis regulation between RA and other pathogenic conditions have barely been investigated.
In this review, we describe the perspective of the phenotypic and functional heterogeneity of macrophages in RA synovium, research tools and therapeutic strategies for direct or indirect macrophage depletion and reestablishment of macrophage functional equilibrium in RA.
Monocyte/Macrophage Lineage Development and Joint Recruitment
The mononuclear phagocyte system comprises bone marrow progenitors, circulating monocytes, and tissue macrophages that populate peripheral lymphoid and nonlymphoid tissues, including the spleen, lymph node, liver, lung, gut, skin, kidney, and joints. The differentiation of hematopoietic stem cells gives rise to the common myeloid lineage, which generates erythrocyte progenitors and the granulocyte-macrophage progenitor (GMP), with monocytes deriving from the latter. In this differentiation process, colony-stimulating factors (CSFs), including the macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage stimulating factor (GM-CSF), are the key players [3, 4••].
The current dogma indicates that circulating monocytes that arise from the bone marrow give rise to the tissue macrophages; however, there is no direct and consistent evidence that circulating monocytes contribute to the macrophage in the synovium in RA patients. Bone marrow abnormalities that have been reported in RA include hyperactivity of myeloid cells, increased promonocyte DNA synthesis, increased number of HLA-DR+CD14+ cells, and high CSF activity. However, there are conflicting reports about the peripheral CD14low CD16+ monocyte subpopulation in RA patients [5••]. Further clarification of monocyte/macrophage trafficking among bone marrow, circulation, and synovium is critical for developing strategies that can control the RA synovial macrophage abundance.
Macrophage Polarization and Heterogeneity in RA Synovium
Myelomonocytic lineage cells develop into several cell types that critically contribute to the pathogenesis of RA, which include monocyte/macrophages, dendritic cells, and osteoclasts. High plasticity is one of the characteristics of these cell populations, and it is regulated by the balance of a number of cytokines and growth factors. Imbalances of the mononuclear phagocyte system can occur not only in the affected joints, but also in the peripheral blood, bone marrow, and cardiovascular system of RA patients.
As was mentioned above, heterogeneity and plasticity are hallmarks of monocyte/macrophage lineage cells. To mirror the Th1/Th2 nomenclature, polarized macrophages are referred to as M1/M2 cells. Classically activated M1 macrophages have been known to be induced by IFN-γ, LPS, GM-CSF, and TNF-α, whereas alternatively activated M2 macrophages are induced by IL-4, IL-13, M-CSF, immune complexes, IL-10, and glucocorticoid [1]. In general, M1 macrophages express a high level of TNF-α, IL-1, IL-6, IL-23, IL-12, type I IFN, reactive nitrogen intermediate (RNI), reactive oxygen intermediate (ROI), and CXCL9, 10, and 11, and M2 macrophages express a high level of IL-4, IL-10, CD163, CD206, and CCL16, 17, 18, 22, and 24 [1, 2]. The M1/M2 model is a useful scheme, but it lacks the flexibility and does not reflect the diversity of the monocyte/macrophage activation. To overcome this, a new grouping system based on the function of activated macrophages has been proposed, which includes classically activated macrophages, wound-healing macrophages, and regulatory macrophages [2]. Potentially, the principles of these two nomenclatures can be applied to macrophages in RA. In addition, development of osteoclasts from monocyte/macrophage precursor cells is one of the unique characteristics in RA.
Expression of GM-CSF and M-CSF is increased in synovial fluid from RA patients, and they form a cytokine network between myeloid cells and neighboring cells, contributing to the inflammatory lesions in the joints. The basic biology of GM-CSF and M-CSF has been extensively reviewed [3, 6]. GM-CSF has a broad spectrum of action on neutrophils, eosinophils, macrophages, and dendritic cells, whereas M-CSF acts more specifically on the macrophage lineage. GM-CSF-deficient mice do not have a marked deficiency in myeloid cell development. On the other hand, M-CSF-deficient mice exhibit deficiencies of several macrophage populations, including osteoclasts [3]. This is best demonstrated in the Csf1op/op mice, which develop osteoporosis due to a deficiency of osteoclast development. Due to the critical role of M-CSF in macrophage development, it has been proposed that macrophage populations are normally exposed to constitutive production of M-CSF in the absence of inflammation and M-CSF maintains study-state levels of M2-like polarized macrophages. In the presence of inflammatory stimuli, GM-CSF, plus other signaling factors (TLR ligands, IFN-γ, TNF-α), promotes development of proinflammatory M1 macrophages that further produce inflammatory cytokines, including TNF-α, IL-12, and IL-23.
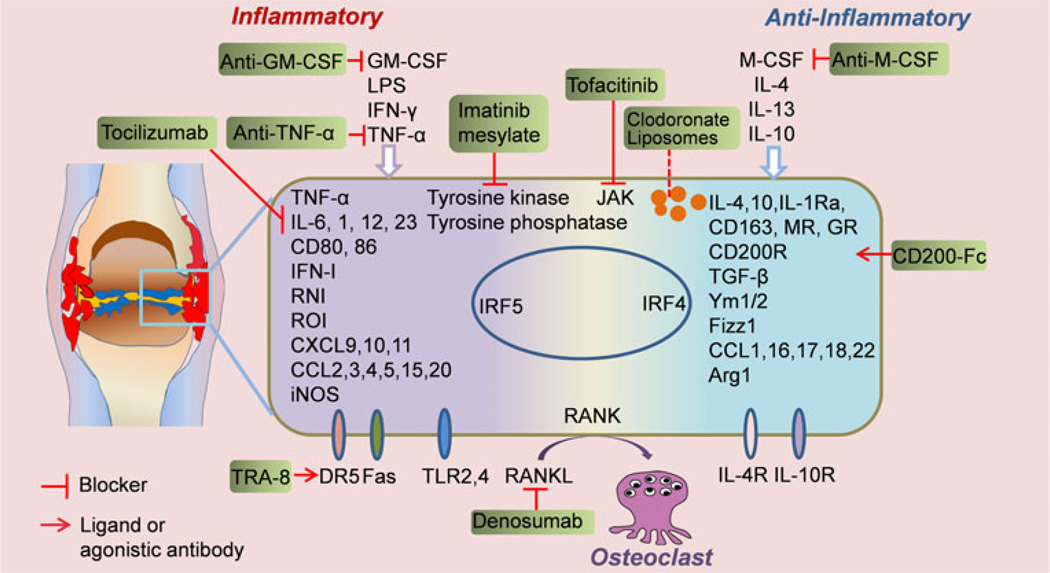
During development of arthritis, TNF-α and IL-1 promote production of GM-CSF and M-CSF by synovial fibroblasts and chondrocytes, indicating that both GM-CSF and M-CSF play a pathogenic role in arthritis. Both GM-CSF-deficient and Csf1op/op mice were resistant to development of CII-induced arthritis [7]. Consistently, blockade of GM-CSF or M-CSF inhibits the development of arthritis [7, 8]. The oral inhibitor of M-CSF receptor 27 reduced the arthritis progression by inactivating the tyrosine kinase [9], which can also be achieved by imatinib mesylate (Glivec/Novartis) [10]. Since depletion of both M-CSF and GM-CSF is effective, several therapies are in phase 1 or phase 2 clinical trials or preclinical studies using GM-CSF-specific antibodies, GM-CSF receptor-specific antibodies, or antibodies directed at M-CSF or its receptor [3, 11••]. JAK inhibitors, including tofacitinib, may also act to effect macrophage development [12]. Macrophage polarization and related therapeutic targets and novel agents are summarized in Fig. 1 and Table 1. As is described below, it is likely that the most efficacious macrophage inhibition or depletion therapy will be the one that specifically targets proinflammatory M1rather than antiinflammatory M2 macrophages.
Fig. 1.
Macrophage polarization in RA synovium and related therapeutic targets. This figure summarizes the environmental cues that control macrophage polarization, the biomarkers for macrophage identity determination, and the therapeutic targets that modify the macrophage polarization and heterogeneity in RA. GM-CSF, granulocyte macrophage-colony stimulating factor; M-CSF, macrophage-colony stimulating factor; LPS, lipopolysaccharide; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; RNI, reactive nitrogen intermediate.; ROI, reactive oxygen intermediate; iNOS, inducible nitric-oxide synthase; CXCL, CXC-chemokine ligand; CCL, CC-chemokine ligand; TGF-β, transforming growth factor β; Ym1/2, chitinase like protein 1/2; Fizz, resistin like molecule; MR, mannose receptor; GR, galactose receptor; Arg1, arginase 1; JAK, Janus kinase
Table 1.
Novel macrophage-related therapeutic agents for rheumatoid diseases
| Agent | Mechanisms | Development phase | Reference |
|---|---|---|---|
| Antihuman DR5 antibody, TRA-8(CS-1008) | Induces apoptosis of targeted cells | Preclinical | [27••] |
| Tumor necrosis factor-converting enzyme (TACE) inhibitors, TMI-005 and BMS-561392 | Inhibits the release of soluble TNF from its membrane-bound precursor | Clinical Phase II | [51] |
| GM-CSF and GM-CSF receptor antibodies | Blocks the binding of GM-CSF to its receptor and downstream signaling | Clinical Phase II | [3, 11••] |
| JAK inhibitor, Tofacitinib and Ruxolitinib | Abrogated TNF-induced STAT1 activation and expression of genes encoding inflammatory chemokines | Clinical Phase III | [12] |
| Tyrosine kinase inhibitor, Imatinib and mastinib | Inhibits tyrosine kinases, including platelet-derived growth factor receptor (PDGFR), Kit, and Fms related kinases | Clinical Phase II–III | [52, 53] |
| Anti-IL-12/IL-23 monoclonal antibody, ustekinumab | Blocking binding of IL-12/23 to their receptors and the downstream signaling | Approved for psoriasis and psoriatic arthritis | [54] |
| Clodronate liposomes | Deplete synovial macrophages by intraarticular administration | Open study in patients who were scheduled for knee joint replacement | [49] |
Recent work has identified several novel signaling pathways that regulate myeloid cell differentiation. It was discovered that IL-10 induced a defect in RANK signaling that preferentially affected calcium-dependent signaling pathways, including a rapidly activated CaMK-MEK-ERK pathway. IL-10 suppressed RANK signaling and human osteoclastogenesis in part by inhibiting expression of the triggering receptor expressed on myeloid cells 2 (TREM-2) by a transcriptional mechanism, thereby suppressing TREM-2-mediated costimulation. These results reveal a new mechanism of inhibition of osteoclastogenesis and identify an early step in osteoclast differentiation that can be effectively targeted for inhibition [13]. In addition to this, the immune receptor expressed on myeloid cells 1 (IREM-1/CD300F) has been shown to inhibit various inflammatory process in myeloid cells, and it is related to the immunoreceptor tyrosine-based inhibition motifs (ITIM) signaling [14•]. An inhibitory molecule composed of five ITIM-like sequences in the cytoplasmic tail of IREM-1 were synthesized in conjugation with human immunodeficiency virus-transactivator of transcription (HIV-TAT), which was added to promote internalization of the peptides. All these TAT–ITIM fusion peptides inhibited TLR-mediated production of proinflammatory molecules, including MMP-9, TNF-α, monocyte chemotactic protein-1 (MCP-1), and IL-8. TAT–ITIM peptides blocked both myeloid differentiation factor 88 (MyD88) and Toll-interleukin 1 receptor (TIR) domain-containing adapter-inducing interferon-β (TRIF)-mediated TLR signalling pathways. The inhibitory effects of these TAT–ITIM peptides require activation of Src homology 2 (SH2) containing tyrosine phosphatase (SHP) and/or phosphoinositide3-kinase (PI3K) [14•]. Co-stimulatory molecules are important for interaction between antigen presenting cells (DC, macrophages) and T cells. Costimulatory molecules have recently been shown to be regulated by Tenascin-C, which is an extracellular matrix glycoprotein not expressed on healthy synovium but which is elevated in the rheumatoid joint where high levels are produced by myeloid cells [15]. Dendritic cells that do not express tenascin-C produce lower levels of inflammatory cytokines than do cells from wild-type mice and exhibit specific defects in Th17 cell polarization. Moreover, tenascin-C null mice display ablated IL-17 levels in the joint during experimental arthritis [15].
In normal synovium, an equilibrium is established that maintains normal joint function. This equilibrium can be shifted toward an inflammatory pathway by two conditions. First, growth factors such as GM-CSF that promote M1 macrophage development can be increased, and second, inflammatory cytokines such as TNF-α and IL-6 that further favor M1 macrophage development can be elevated. Both elevated GM-CSF and increased IL-6 can be observed months before onset of RA [16••]. The presence of M1 macrophages can establish a positive feedback loop by producing cytokines that promote their development. This positive feedback loop is normally limited by endogenous physiological factors that attempt to restore a normal balance. When this fails and arthritis develops, normal equilibrium between the macrophage subpopulations can be reestablished either by pharmacological intervention that directly depletes inflammatory macrophages or by blockade of factors that promote their development, such as GM-CSF or TNF-α inhibitor therapy.
Macrophage Subpopulation Analysis in Arthritis
One of the well-recognized features of RA synovium is an increased abundance of macrophages at the intimal and subintimal compartments [17]. CD68 and CD163 are two commonly used markers for identification of synovial macrophages in RA synovium. CD68 is a scavenger receptor that binds to oxidized low-density lipoprotein and may also be involved in the cell–cell interaction [18]. CD68 can be detected on both cell surface and lysosomal membranes [19]. It has been shown that changes in the number of synovial sublining CD68+ macrophages correlate with clinical improvement independently of the therapeutic strategy, and it can be used as sensitive biomarker to predict the possible efficacy of new antirheumatic treatments [20]. CD163 is a type I transmembrane protein that belongs to the group B scavenger receptor cysteine-rich superfamily [21]. One of the important functions of CD163 is its capacity to bind and internalize haemoglobin–haptoglobin (HbHp) complexes. leading to the release of IL-10 and carbon oxide (CO), which exert strong antiinflammatory effects [22]. Its function repertoire also includes being a receptor of tumor necrosis factor-like weak inducer of apoptosis (TWEAK), as well as a receptor for various pathogens and as an immunomodulator. It has been demonstrated that CD163 has major advantages as a macrophage marker, as compared with CD68, in RA synovium because it discriminates between synovial macrophages and synovial intimal fibroblasts, which also stain positive for CD68 in disease tissue [23]. Additionally, soluble CD163 in sera is a promising diagnostic marker for untreated new-onset systemic juvenile idiopathic arthritis and macrophage activation syndrome, which is characterized by an overwhelming inflammatory reaction driven by excessive expansion of T cells and hemophagocytic macrophages [24].
Although macrophages from RA synovial tissue or synovial fluid can exhibit both proinflammatory and antiinflammatory phenotypes, they have not been classified as M1 and M2 macrophages. In RA, proinflammatory effector molecules include MIF, TNF-α, IL-1, IL-15, IL-18, IL-23, IL-27, nitric oxide/reactive oxygen species, and different chemokines. On the other hand, they also generate IL-1Ra, IL-10, TGF-β, and soluble TNF-R, which are inhibitory mediators. RA synovial macrophages also produce proteinases (MMP-1, 2, 9) and proteinase inhibitors (TIMP-1, 2) that antagonize the effects of MMPs. These molecules can potentially be used to determine the macrophage heterogeneity in RA synovium or synovial fluid. Very recently, an in vitro polarization study using human monocytes validated that CD80 is the most robust phenotypic marker for human M1 macrophages polarized by IFN-γ, whereas CD200R was upregulated and CD14 was specifically downregulated on M2 macrophages polarized by IL-4. CD163 and CD16 were found to be specific markers for macrophages polarized by IL-10 [25]. However, macrophages retain their high plasticity during the differentiation and respond to the environmental cues (Fig. 1) rapidly, and thus, reversely, reliance on a single marker to determine the identity of a specific macrophage population is not feasible and might be misleading, resulting in the mislabel of “hybrid” macrophage subsets.
Recent studies indicated that IRF5 expression in macrophages was reversibly induced by inflammatory stimuli and contributed to the plasticity of macrophage polarization. High expression of IRF5 was characteristic of M1 macrophages, which suggested a critical role for IRF5 in M1 macrophage polarization and defined a previously unknown function for IRF5 as a transcriptional repressor [26••]. Our group has observed that the expression of death receptor 5 (DR5) is upregulated in the CD11b+Ly6C+ inflammatory macrophages in the mouse collage induced arthritis (CIA) model [27••], and its expression is correlated with that of IRF5. Consistent with this, DR5 expression is significantly higher in mouse bone marrow derived M1 macrophages polarized by GM-CSF, as compared with M2 macrophages polarized by M-CSF. A similar pattern was also confirmed in human polarized macrophages (Jun Li et al., unpublished observation).
Resolution of Inflammation by Macrophage Depletion
As a key mechanism for reestablishing equilibrium, macrophages undergo apoptosis by signaling through TNF-R1, CD95 (Fas), or DR5 (TRAIL-R) [28•]. These apoptosis pathways all signal through the death domain, leading to activation of Caspace-8 and the mitochondrial-related apoptosis pathway, including BCL-2 family members. The balance between activation through GM-CSF or M-CSF and the signaling of apoptosis pathways determines production of proinflammatory and antiinflammatory cytokines. Thus, regulation of activation, as compared with apoptosis, can be a major determinant in the onset, progression, and resolution of RA.
TNF-R1 and TNF-RII Signaling of Apoptosis and Inflammation Macrophages
TNF-RI possesses a death domain capable of signaling apoptosis, whereas TNF-RII does not have a death domain. One of the first investigations of the role of TNF-R1 in mediating apoptosis of mononuclear cells was carried out by crossing C57BL/6-lpr with TNFRI−/− mice [29]. This leads to a greatly increased lympho-proliferative autoimmune disease with massive expansion of mononuclear cells in LN, spleen, and synovium. The authors concluded that TNF-R1 plays an important compensatory role for apoptosis in the absence of Fas. This is consistent with previous observations that TNF-α administration could inhibit nephritis in NZB/W and NOD mice, but early administration is essential for the beneficial effect [30].
In established RA, TNF-α acts as a positive feedback signal to further promote development and survival of macrophages. Under these circumstances, which are usually encountered in clinical practice, blockade of TNF-α signaling in macrophages can promote depletion of macrophages [31]. Etanercept and infliximab have been shown to induce apoptosis in monocytes and macrophages in both synovial fluid and peripheral blood in vivo [32]. Furthermore, treatment with TNFα blockers reduces the number of infiltrating synovial granulocytes and macrophages, as well as reducing the expression of chemokines, IL-8, and monocyte chemotactic protein-1 [33]. Anti-TNF-α therapy has also been shown to potentially ameliorate arthritis by upregulating TGF-β and reversing the functional defect of regulatory T cells in RA, which can then inhibit the activity of inflammatory macrophages [34]. The development of other biological therapies, such as the anti-IL-6 receptor antibody, tocilizumab, exhibits the therapeutic effect, in part, by inhibition of macrophage development and function [35].
The relative importance of TNF-R1 and TNF-RII signaling has been investigated in induction of inflammation. Blüml and co-workers investigated the mechanisms leading to the influx of inflammatory hematopoietic cells into the synovial membrane and the role of TNF-RI and TNF-RII in this process in an animal model of rheumatoid arthritis (RA) [36••]. The absence of TNF-RI on hematopoietic cells did not affect joint inflammation but markedly attenuated erosive bone destruction via reduced synovial accumulation of osteoclast precursors. In contrast, the absence of TNFRII on hematopoietic cells increased joint inflammation as well as erosive bone destruction via the regulation of osteoclast precursor apoptosis. The findings indicate that selective blockade of TNF-RI, leaving the antiinflammatory effects of TNF-RII unaltered instead of unselectively blocking TNF-α, might be advantageous in patients with RA.
Fas Apoptosis as a Mechanism for Selective Depletion of Macrophages
Fas and Fas ligand (FasL) were the first autoimmune genes that acted in an autosomal recessive fashion to promote autoimmune disease in lymphoproliferative (lpr) and generalized lymphoproliferative disease (gld) mice [37, 38]. Since these mice develop massive lymphoproliferation and a greatly expanded population of unusual B220+ CD3+ CD4−CD8− T cells, FasL-Fas interactions were first thought to inhibit autoimmune diseases by limiting the development of self-reactive T cells. However, targeting deletion of the Fas defect to T cells using a CD4-Cre and Floxed Fas resulted in mice that developed inflammatory diseases and early death. However, autoimmune disease and autoantibody production was greatly increased by targeting the Fas defect to subpopulations of macrophages. This was first explored by Stranges et al. [39], who utilized a Floxed exon 9 of Fas (Fas knock-in, FasKI) crossed to Cd11c-Cre, which was compared with the FasKI crossed with the CD19-Cre, Lck-Cre, and granzyme B (Gzmb) Cre transgenic mice. The results demonstrated that the FasKI Cd11c-Cre mice in which the loss of Fas expression was restricted to CD11c+ dendritic cells developed systemic autoimmunity, including hyperimmunoglobulinemia, splenomegaly, and histologic changes in the spleen and liver. FasKI Cd11c-Cre mice did not exhibit an expansion of the CD4−CD8−B220+ T cell population [39]. More recently, Perlman and colleagues [40] targeted deletion of Fas specifically in CD11b+ myeloid cells in the CreLysM Fasflox/flox mice, which greatly enhanced the number of activated proinflammatory CD11b macrophages. In the CreLysM Fasflox/flox mice, there were markedly increased numbers of F4/80+Gr-1+ macrophages and also increased numbers of CD11b+F4/80+Gr-1intermediate and CD11b+F4/80+Gr-1low macrophages. These macrophages exhibited an enhanced response to TLR4 ligation-induced endotoxin shock, which was associated with increased signaling of the nuclear factor (NF)-κB phospho-p65 and the mitogen-activated protein (MAP) kinase pathway p38, as well as the AKT pathway. It was proposed that in the absence of Fas, an increased innate immune response leads to activation of macrophages and adaptive immune responses.
Selective deletion of proinflammatory, but not antiinflammatory, macrophages is necessary to prevent or treat inflammatory diseases. This was previously demonstrated by Burnett et al [41], who showed that targeted depletion of macrophages using drug-inducible suicide of Fas-mediated apoptosis in CSF-1 receptor (c-fms)+ macrophages led to splenomegaly and lymphadenopathy. The molecular mechanism associated with these abnormal phenotypes in transgenic macrophage Fas-induced apoptosis (Mafia) mice was not identified. It is noteworthy that CSF-1 is one of the factors that promote the development of M2 antiinflammatory macrophages [41]. Thus, selective depletion of inflammatory macrophages while sparing suppressor or tolerogenic macrophages would be desirable. Previous work has shown that specific cytokines such as GM-CSF, M-CSF, and G-CSF promote the development of inflammatory M1 macrophages, antiinflammatory M2 macrophages, and granulocytes, respectively [42•].
Tumor Necrosis Factor Related Apoptosis Inducing Ligand (TRAIL)–Death Receptor 5 Pathway and Apoptosis of Synovial Macrophages and Fibroblasts
Expression of DR5 or TRAIL on RA synovial fibroblast and macrophages has been controversial. Perlman and coworkers have reported that TRAIL R1 and R2, which are required for signal transmission by TRAIL, were not detected on RA SF lymphocytes, macrophages, or synovial fibroblasts [43]. Normal peripheral blood-derived monocyte-differentiated macrophages expressed TRAIL-R2, but not TRAIL or the other TRAIL receptors. Adenoviral-mediated delivery of TRAIL had no effect on the survival of normal macrophages or RA synovial fibroblasts but readily induced apoptosis in the prostate cancer cell line (PC-3) that expressed TRAIL-R1 and -R2.
However, Zhou [44] and colleagues found that an anti-DR5 antibody TRA-8 could induce apoptosis of RA synovial fibroblasts in vitro and in vivo, whereas OA fibroblasts were resistant to apoptosis. Using two agonistic monoclonal antibodies specific for TRAIL-R1 (DR4) and TRAIL-R2 (DR5), the expression and function of these death receptors in RA synovial fibroblast cells were examined. The synovial tissues and primary synovial fibroblast cells isolated from patients with RA, but not those isolated from patients with osteoarthritis, selectively expressed high levels of cell surface DR5, and were highly susceptible to anti-DR5 antibody (TRA-8, Daiichi Sankyo, Inc.) mediated apoptosis. In contrast, RA synoviocytes did not show increased expression of TRAIL-R1 (DR4), nor was there any difference in expression of Fas between RA and osteoarthritis synovial cells. In vitro TRA-8 induced apoptosis of RA synovial cells and inhibited production of matrix metalloproteinases induced by proinflammatory cytokines. In vivo TRA-8 effectively inhibited hypercellularity of a SV40-transformed RA synovial cell line and completely prevented bone erosion and cartilage destruction induced by these cells. These results indicate that increased DR5 expression and susceptibility to DR5-mediated apoptosis are characteristic of the proliferating synovial cells in RA.
Strategies to selectively and directly promote apoptosis of inflammatory macrophages have only recently been reported by Li et al. [27]. Using a type-II collagen-induced arthritis (CIA) model, we have demonstrated that depletion of M1 inflammatory macrophages using an antihuman DR5 (TRA-8) therapy in CreLysM human/mouse DR5 transgenic (Tg) mice prevented development of arthritis, as well as ameliorating established arthritis. The results also showed that depletion of inflammatory macrophages reduced the numbers of Th17 cells and increased the numbers of Treg cells, suggesting that elimination of inflammatory macrophages may have beneficial global immune modulatory effects that lead to suppression of autoimmunity. Interestingly, achievement of selective depletion of inflammatory macrophages in this study was most likely due to the use of a 3-kb promoter of the mouse MK1 (Dr5) gene to induce authentic expression of the chimeric hu/mo DR5 Tg. The authors have identified that, under these conditions, CD11b+Ly6C+ inflammatory macrophages exhibited the highest expression of the chimeric DR5 Tg and, thus, became the target of TRA-8 depletion during the course of CIA.
Regulators of Apoptosis in RA Synovial Fibroblasts and Macrophages
While most macrophages express Fas, TNF-R1, or DR5 and their natural ligands, Fas-L, TNF, or TRAIL, are present, apoptosis does not always occur, due to the presence of inhibitors. A major antiapoptosis pathway is NFκB, which signals inhibitors of apoptosis, including XIAP, as well as the PI3 kinase-AKT pathway, which blocks several apoptosis pathways, including Apaf-1 and BCL2 pathways. A relative resistance to apoptosis in RA synovium is associated with increased expression of prosurvival Bcl-2 family members [45]. Perlman and co-workers determined the importance of endogenous Bcl-2 by using an adenoviral vector expressing a hammerhead ribozyme to Bcl-2 (Ad-Rbz-Bcl-2) mRNA to reduce Bcl-2 expression [46]. This resulted in decreased cell viability in RA and OA synovial fibroblasts and increased mitochondrial permeability transition, cytochrome c release, activation of caspases 9 and 3, and DNA fragmentation. Mcl-1 was strongly expressed in the synovial lining and was increased in the sublining fibroblasts of patients with RA, as compared with control synovial tissue [47]. Expression of Mcl-1 was increased in CD14+ macrophages from the SF of patients with RA, as compared with normal in vitro differentiated macrophages [48]. Inhibition of the PI3-kinase/Akt-1 or STAT-3 pathways significantly reduced the percentage of CD14+ cells within the SF and resulted in the reduction of Mcl-1 and the induction of apoptosis of synovial macrophages. Transfection of RA synovial macrophages with Mcl-1 siRNA resulted in apoptotic cell death. Mcl-1 was also critical for the survival of RA synovial fibroblasts, because the forced reduction of Mcl-1 using a Mcl-1 antisense-expressing adenoviral vector induced apoptotic cell death, which was mediated through Bax, Bak, and Bim [47].
Macrophage Depletion by Clodronate Liposome
Clodronate-encapsulated liposome is one of the most effective agents at depleting mononuclear phagocytic populations in rodents. The liposomes are taken out by macrophages, and once inside, the clodronate diphosphonate induces apoptosis. It has been shown that a single intraarticular administration of clodronate liposomes leads to macrophage depletion and decreased expression of adhesion molecules in the synovial lining in patients with RA [49]. However, such therapy targets all mononuclear phagocytes, including monocytes, macrophages, and dendritic cells, and possibly antiinflammatory macrophages are depleted as well when it is administrated systematically.
Genetic Mouse Models for Studying Macrophage Depletion
Genetic mouse models have been essential for understanding the differentiation and homeostatic regulation of the macrophages. Macrophages can be depleted by several different approaches by using genetically engineered mice. The most successful one is the use of diphtheria toxin receptor (DTR) transgenic mice. The human diphtheria receptor is 104 more sensitive to diphtheria toxin than is the mouse DTR. When the human DTR is expressed on mouse cells, these cells can be depleted in the presence of diphtheria toxin. Different promoters have been used to drive the expression of DTR in mice, which include Cd11b, Cd169, and Cd11c promoters. The Cd11b-DTR results in a broad depletion of the myeloid cells; Cd169-DTR leads to the more specific depletion of marginal metallophilic and marginal zone macrophages of the spleen and macrophages in lymph nodes and bone marrow. In addition to depletion of DC, Cd11c-DTR also depletes alveolar macrophages, splenic marginal zone and metallophilic macrophages, and some CD8+ T cells [50]. A novel bacterial artificial chromosome transgenic mouse model was generated in which the diphtheria toxin receptor is expressed under the CD11c promoter. In these mice, efficient DC depletion can be achieved over prolonged periods of time by multiple injections of diphtheria toxin. Another genetic conditional depletion model is the “MAFIA” (macrophage FAS-induced apoptosis) mouse line, which expresses a suicide construct based on the FK506-binding protein (FKBP) and FAS (CD95) downstream of the Csf1r promoter [41]. When mice are treated with the FKBP-dimerizing drug AP20187, cells that express CSF1R are ablated.
Most of the currently available genetic mouse models for studying macrophage depletion are not highly cell-type specific, and the killing mechanisms are not related to the pathogenesis of diseases. To achieve the better targeting, we have generated a mouse DR5 3 kb promoter/Floxed STOP/human mouse chimeric DR5 transgenic mouse. The expression of the transgenic DR5 in this mouse is dependent not only on the Cre expression, but also on the mouse DR5 3 kb promoter, which improves the cell specificity. More important, the killing effect is controlled by the anti-DR5 antibody, as well as the DR5 signaling in the specific cells. This strategy leads to the targeted depletion of the inflammatory macrophage, whereas other macrophage subsets are spared and macrophage equilibrium is reestablished [27].
Conclusions
The ultimate goal of effective and safe therapy in RA is to selectively target the central causes of the disease and reestablish the cellular and functional homeostasis of joints, essentially curing the disease or maintaining a sustained, drug-free remission. In this review, we propose that the central cause of RA is the factors that initially lead to a subclinical increase in pathogenic macrophages. Depending on the magnitude or chronicity of the extrinsic factor, the balance between proinflammatory and antiinflammatory macrophages is tipped to the point where a pathogenic positive feedback loop is maintained. Therefore, early suppression of the amplifying loops is the most important therapeutic strategy in RA. It can be accomplished by blocking the factors that promote development of inflammatory macrophages, such as GM-CSF and TNF-α inhibitors, or by directly targeting pathogenic macrophages with apoptosis-inducing agents such as anti-DR5, as well as other approaches listed in Table 1. Some of these macrophage-related agents will lead to the reestablishment of macrophage functional equilibrium and, thus, will likely be developed into more effective or better tolerated therapies and provide important additions to the current RA treatment paradigms.
Acknowledgments
Disclosure Funding for this research was made possible by the American College of Rheumatology (ACR) Research and Education Foundation and the Lupus Research Institute (LRI). Dr. Li has received grant support (Fellowship Award) from the Arthritis Foundation. Dr. Hsu has received grant support from the LRI and NIH 1 R01 AI083705. Dr. Mountz has received grant support from the ACR, NIH RO1 AI071110-01A1, VA 1I01BX000600, and Daiichi-Sankyo Co Ltd.
Contributor Information
Jun Li, Division of Clinical Immunology and Rheumatology, Department of Medicine, University of Alabama at Birmingham, SHEL Bldg., Suite 337, 1825 University Blvd., Birmingham, AL 35294-2182, USA, junliuab@uab.edu.
Hui-Chen Hsu, Division of Clinical Immunology and Rheumatology, Department of Medicine, University of Alabama at Birmingham, SHEL Bldg., Suite 311, 1825 University Blvd., Birmingham, AL 35294-2182, USA, rheu078@uab.edu; Department of Medicine, Birmingham VA Medical Center, Birmingham, AL 35233, USA.
John D. Mountz, Division of Clinical Immunology and Rheumatology, Department of Medicine, University of Alabama at Birmingham, SHEL Bldg., Suite 307, 1825 University Blvd., Birmingham, AL 35294-2182, USA, jdmountz@uab.edu Department of Medicine, Birmingham VA Medical Center, Birmingham, AL 35233, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 4. Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. The authors discuss how recent discoveries in the origin of the DC and macrophage lineage help establish key functional differences between tissue DC and macrophage subsets. The authors also emphasize the need to further understand the functional heterogeneity of the tissue DC and macrophage lineages to better comprehend the complex role of these cells in tissue homeostasis and immunity.
- 5. Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum. 2009;60:1210–1221. doi: 10.1002/art.24505. This is one of the most comprehensive review discussing the macrophage heterogeneity in autoimmunity.
- 6.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 7.Campbell IK, Rich MJ, Bischof RJ, et al. The colony-stimulating factors and collagen-induced arthritis: Exacerbation of disease by m-csf and g-csf and requirement for endogenous m-csf. J Leukoc Biol. 2000;68:144–150. [PubMed] [Google Scholar]
- 8.Cook AD, Braine EL, Campbell IK, et al. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (gm-csf): Requirement for gm-csf in the effector phase of disease. Arthritis Res. 2001;3:293–298. doi: 10.1186/ar318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohno H, Uemura Y, Murooka H, et al. The orally-active and selective c-fms tyrosine kinase inhibitor ki20227 inhibits disease progression in a collagen-induced arthritis mouse model. Eur J Immunol. 2008;38:283–291. doi: 10.1002/eji.200737199. [DOI] [PubMed] [Google Scholar]
- 10.Ando W, Hashimoto J, Nampei A, et al. Imatinib mesylate inhibits osteoclastogenesis and joint destruction in rats with collagen-induced arthritis (cia) J Bone Miner Metab. 2006;24:274–282. doi: 10.1007/s00774-006-0684-1. [DOI] [PubMed] [Google Scholar]
- 11. Cornish AL, Campbell IK, McKenzie BS, et al. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:554–559. doi: 10.1038/nrrheum.2009.178. The authors postulate that antagonism of G-CSF or GM-CSF could represent a novel therapeutic approach for a variety of autoimmune-mediated inflammatory diseases, including rheumatoid arthritis.
- 12.Anna Yarilina KX, Chan C, et al. Regulation of macrophage-mediated chronic inflammation by jak inhibitors. Arthritis Res Ther. 2012;14:53–54. [Google Scholar]
- 13.Park-Min KH, Ji JD, Antoniv T, et al. IL-10 suppresses calcium-mediated costimulation of receptor activator nf-kappa b signaling during human osteoclast differentiation by inhibiting trem-2 expression. J Immunol. 2009;183:2444–2455. doi: 10.4049/jimmunol.0804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SM, Suk K, Lee WH. Synthetic peptides containing itim-like sequences of irem-1 (cd300f) differentially regulate myd88 and trif-mediated tlr signalling through activation of shp and/or pi3k. Clin Exp Immunol. 2012;167:438–446. doi: 10.1111/j.1365-2249.2011.04528.x. The authors describe the generation of immunomodulatory molecules that can regulate the inflammatory activation of macrophages.
- 15.Ruhmann M, Piccinini AM, Kong PL, et al. Endogenous activation of adaptive immunity: Tenascin-c drives il-17 synthesis in arthritic joint disease. Arthritis Rheum. 2012 doi: 10.1002/art.34401. [DOI] [PubMed] [Google Scholar]
- 16. Kokkonen H, Soderstrom I, Rocklov J, et al. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383–391. doi: 10.1002/art.27186. The data show that prior to development of RA, "prepatient" individuals already had significantly increased levels of GM-CSF, M-CSF, and other proinflammatory cytokines and chemokines, indicating activation of the immune. system, as compared with controls, who did not develop RA. After disease onset, the involvement and activation of the immune system was more general and widespread.
- 17.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 18.Ramprasad MP, Terpstra V, Kondratenko N, et al. Cell surface expression of mouse macrosialin and human cd68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1996;93:14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strobl H, Scheinecker C, Csmarits B, et al. Flow cytometric analysis of intracellular cd68 molecule expression in normal and malignant haemopoiesis. Br J Haematol. 1995;90:774–782. doi: 10.1111/j.1365-2141.1995.tb05195.x. [DOI] [PubMed] [Google Scholar]
- 20.Haringman JJ, Gerlag DM, Zwinderman AH, et al. Synovial tissue macrophages: A sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:834–838. doi: 10.1136/ard.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47:1650–1660. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor cd163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca JE, Edwards JC, Blades S, et al. Macrophage subpopulations in rheumatoid synovium: Reduced cd163 expression in cd4+ t lymphocyte-rich microenvironments. Arthritis Rheum. 2002;46:1210–1216. doi: 10.1002/art.10207. [DOI] [PubMed] [Google Scholar]
- 24.Bleesing J, Prada A, Siegel DM, et al. The diagnostic significance of soluble cd163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 25.Ambarus CA, Krausz S, van Eijk M, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2011;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 26. Krausgruber T, Blazek K, Smallie T, et al. Irf5 promotes inflammatory macrophage polarization and th1-th17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. The data suggest a critical role for IRF5 in M1 macrophage polarization and define a previously unknown function for IRF5 as a transcriptional repressor.
- 27. Li J, Hsu HC, Yang P, et al. Treatment of arthritis by macrophage depletion and immunomodulation: testing an apoptosis-mediated therapy in a humanized death receptor mouse model. Arthritis Rheum. 2012;64:1098–1109. doi: 10.1002/art.33423. This is the first demonstration and proof of the principle that an anti-human DR5 apoptosis therapy can be used to prevent and treat arthritis in a CIA mouse model. The authors utilized a novel hu/mo chimeric DR5 mouse to demonstrate the efficacy of an antihuman DR5 therapy and showed that induction of apoptosis of macropghages was the major mechanism of action leading to decreased inflammatory macrophages and Th17 cells and increased Treg cells.
- 28. Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun. 2010;2:204–215. doi: 10.1159/000296507. This review summarizes the contribution of the caspases and their regulators in monocyte/macrophage cell fate and discusses how these molecules orchestrate the initiation, maintenance, and resolution of inflammation. More provocatively, we discuss possible strategies for controling inflammation by manipulating leukocyte life span.
- 29.Zhou T, Edwards CK, 3rd, Yang P, et al. Greatly accelerated lymphadenopathy and autoimmune disease in lpr mice lacking tumor necrosis factor receptor i. J Immunol. 1996;156:2661–2665. [PubMed] [Google Scholar]
- 30.Jacob CO, Aiso S, Michie SA, et al. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (tnf): similarities between tnf-alpha and interleukin 1. Proc Natl Acad Sci USA. 1990;87:968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catrina AI, Trollmo C, af Klint E, et al. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis Rheum. 2005;52:61–72. doi: 10.1002/art.20764. [DOI] [PubMed] [Google Scholar]
- 33.Taylor PC, Peters AM, Paleolog E, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory t cells in rheumatoid arthritis and reversal by anti-tnfalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh JA, Beg S. Lopez-Olivo MA Tocilizumab for rheumatoid arthritis: a cochrane systematic review. J Rheumatol. 2011;38:10–20. doi: 10.3899/jrheum.100717. [DOI] [PubMed] [Google Scholar]
- 36. Bluml S, Binder NB, Niederreiter B, et al. Antiinflammatory effects of tumor necrosis factor on hematopoietic cells in a murine model of erosive arthritis. Arthritis Rheum. 2010;62:1608–1619. doi: 10.1002/art.27399. The data indicate that selective blockade of TNFRI, leaving the antiinflammatory effects of TNFRII unaltered instead of unselectively blocking TNF, might be advantageous in patients with RA.
- 37.Takahashi T, Tanaka M, Brannan CI, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe-Fukunaga R, Brannan CI, Copeland NG, et al. Lymphoproliferation disorder in mice explained by defects in fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 39.Stranges PB, Watson J, Cooper CJ, et al. Elimination of antigen-presenting cells and autoreactive t cells by fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuda CM, Agrawal H, Misharin AV, et al. Requirement of myeloid cell-specific fas expression for prevention of systemic autoimmunity in mice. Arthritis Rheum. 2012;64:808–820. doi: 10.1002/art.34317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnett SH, Kershen EJ, Zhang J, et al. Conditional macrophage ablation in transgenic mice expressing a fas-based suicide gene. J Leukoc Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 42. Fleetwood AJ, Dinh H, Cook AD, Hertzog PJ, Hamilton JA. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on type I interferon signaling. J Leukoc Biol. 2009;86:411–421. doi: 10.1189/jlb.1108702. The findings demonstrate that constitutive and LPS-induced type I IFN play significant roles in regulating the differences in phenotype and function between M-CSF-induced macrophages and GM-CSF-induced macrophages.
- 43.Perlman H, Nguyen N, Liu H, et al. Rheumatoid arthritis synovial fluid macrophages express decreased tumor necrosis factor-related apoptosis-inducing ligand r2 and increased decoy receptor tumor necrosis factor-related apoptosis-inducing ligand r3. Arthritis Rheum. 2003;48:3096–3101. doi: 10.1002/art.11302. [DOI] [PubMed] [Google Scholar]
- 44.Ichikawa K, Liu W, Fleck M, et al. TRAIL-R2 (DR5) mediates apoptosis of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2003;171:1061–1069. doi: 10.4049/jimmunol.171.2.1061. [DOI] [PubMed] [Google Scholar]
- 45.Lawlor KE, Smith SD, van Nieuwenhuijze A, et al. Evaluation of the bcl-2 family antagonist abt-737 in collagen-induced arthritis. J Leukoc Biol. 2011;90:819–829. doi: 10.1189/jlb.0311174. [DOI] [PubMed] [Google Scholar]
- 46.Perlman H, Georganas C, Pagliari LJ, et al. Bcl-2 expression in synovial fibroblasts is essential for maintaining mitochondrial homeostasis and cell viability. J Immunol. 2000;164:5227–5235. doi: 10.4049/jimmunol.164.10.5227. [DOI] [PubMed] [Google Scholar]
- 47.Liu H, Eksarko P, Temkin V, et al. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2005;175:8337–8345. doi: 10.4049/jimmunol.175.12.8337. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Huang Q, Shi B, et al. Regulation of mcl-1 expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2006;54:3174–3181. doi: 10.1002/art.22132. [DOI] [PubMed] [Google Scholar]
- 49.Barrera P, Blom A, van Lent PL, et al. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000;43:1951–1959. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 50.Hochweller K, Striegler J, Hammerling GJ, et al. A novel CD11c.DTR transgenic mouse for depletion of dendritic cells reveals their requirement for homeostatic proliferation of natural killer cells. Eur J Immunol. 2008;38:2776–2783. doi: 10.1002/eji.200838659. [DOI] [PubMed] [Google Scholar]
- 51.Moss ML, Sklair-Tavron L, Nudelman R, et al. Tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008;4:300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 52.Walker UA. More about masitinib. Arthritis Res Ther. 2009;11:120. doi: 10.1186/ar2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paniagua RT, Robinson WH. Imatinib for the treatment of rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:190–191. doi: 10.1038/ncprheum0465. [DOI] [PubMed] [Google Scholar]
- 54.Reich K, Yasothan U, Kirkpatrick P. Ustekinumab. Nat Rev Drug Discov. 2009;8:355–356. doi: 10.1038/nrd2878. [DOI] [PubMed] [Google Scholar]