Abstract
Objective
To use objective, nonverbal oculomotor tasks to assess executive function and infer the neural basis of impairments in preterm children.
Study design
Cross-sectional study of preterm children age 9 to 16 years (n = 69; mean gestational age 29 weeks) and full term controls (n = 43). Tasks assessed sensorimotor function (reflexive prosaccades); resistance to peripheral distracters (fixation); response inhibition, response preparation, and execution of a voluntary saccade (antisaccades); and spatial working memory (memory-guided saccades). Group differences were analyzed using ANOVA. We used linear regression to analyze the contributions of age, sex, gestational age, and white matter category to task performance.
Results
Preterm children did not differ from controls on basic sensorimotor function, response inhibition, and working memory. Compared with controls, preterm children showed greater susceptibility to peripheral distracters (p = .008) and were slower to initiate an inhibitory response (p = .003). Regression models showed contributions of age and white matter category to task performance.
Conclusions
Preterm children show intact basic sensorimotor function and demonstrate difficulties in processes underlying executive control, including increased distractibility and prolonged response preparation. These limitations may reflect specific neural abnormalities in fronto-subcortical executive control of behavior.
Keywords: preterm, premature birth, oculomotor, saccade, executive function, response inhibition, response preparation
Premature birth has been associated with impairment on tasks requiring complex abilities classified as executive functions.1 These abilities include filtering out distracters, inhibiting automatic responses, maintaining information, and planning to carry out goal-directed behavior.2 Executive function deficits after prematurity have been linked to impaired reading and academic performance and to decreased parent-rated child function and adaptive skills.3, 4
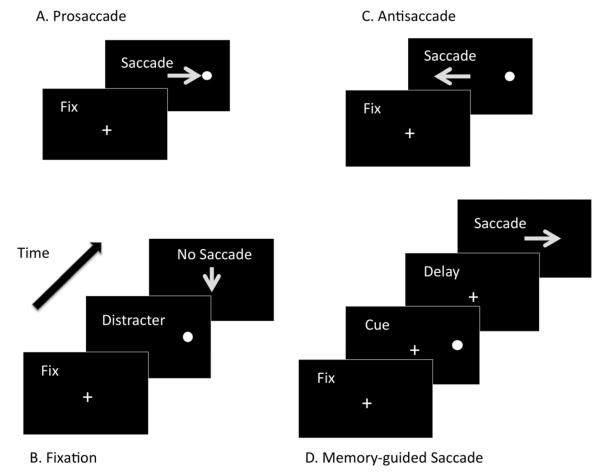
Oculomotor tasks are objective, nonverbal tests that use saccades or eye movements to tap executive function skills. They have been used to infer the status of the underlying brain systems in typically developing populations5 and in populations with neurodevelopmental disorders.6 The tasks are simple, can be performed by infants7 and non-human primates,6 and correlate with other measures of executive function.8 Several tasks comprise the oculomotor battery. (Figure 1) The reflexive prosaccade task requires the subject to fixate a suddenly appearing stimulus9 and serves as a control task to assess underlying basic sensorimotor function. The fixation task requires the subject to look straight ahead despite the appearance of peripheral distracters to assess the ability to resist a response toward the distracters. The antisaccade task requires the subject to inhibit a reflexive response to a suddenly appearing stimulus and instead, to voluntarily look to the mirror location,10 to assess voluntary response inhibition and the ability to prepare and execute the correct voluntary response. The memory-guided saccade task requires the subject to look to the location of a previously presented visual target,11 to assess maintenance of spatial working memory.
Figure 1.
A. Prosaccade task. The subject makes a reflexive saccade to the target. B. Fixation task. The subject looks straight ahead despite the appearance of peripheral distracters. C. Antisaccade task. The subject inhibits a reflexive response to a suddenly appearing stimulus and instead looks to the mirror location. D. Memory-guided Saccade Task. After a delay period, the subject looks to the location of a previously presented visual target.
The oculomotor system involved in these tasks has been well-characterized and consists of a widely distributed network, including frontal and brainstem regions,6 which overlaps with the circuitry underlying attention and executive function.12 Given their vulnerability to brain injury,13 oculomotor tasks may contribute to our understanding of the neural basis of executive function deficits in preterm children. Response inhibition deficits on antisaccade tasks14, 15 and difficulty maintaining fixation when required for other eye movement tasks have been reported in preterm children.14 These studies are limited by small sample size, focus on children with cerebral palsy, a limited battery of oculomotor tasks, and failure to study task components.
The goal of this study was to use oculomotor tasks in preterm children without major motor deficits to evaluate aspects of executive function and infer the neural basis of impairments. We hypothesized that compared with full term controls, preterm children would show intact sensorimotor processing, susceptibility to peripheral distracters, and impairments in response inhibition, response preparation, and working memory.
METHODS
Participants were part of a two-site neuroimaging study conducted in Pittsburgh, PA and Palo Alto, CA. Subjects, age 9 to 16 years, had gestational age (GA) < 36 weeks and birth weight (BW) < 2500 g (n = 69). Controls had GA ≥ 37 weeks (n = 43). Exclusion criteria for all participants included active seizures, complications of ventriculoperitoneal shunt for hydrocephalus, congenital malformation, meningitis or encephalitis, sensory impairments, inability to perform an MRI study, and non-English speaker. The presence of these conditions would complicate interpretations of imaging findings in relation to outcomes in the preterm group. Neonatal medical complications in the preterm group were as follows: 10 had abnormal findings on head ultrasound or MRI (at least grade 2 intraventricular hemorrhage, echodensities, or cystic lesions), 9 had mildly abnormal findings (either grade 1 hemorrhage or choroid plexus cyst); 19 had respiratory distress syndrome and 8 developed chronic lung disease; and 4 were small for gestational age (defined as ≤ 3rd percentile in BW for GA). Conventional MRI scans (T1- and T2- weighted images) were obtained on 54 preterm children as part of the larger study and scored by a board-certified neuroradiologist, blinded to group status, using a 5 to 15 point scale in which higher scores indicated more severe injury.16 MRI scan variables are in the Appendix (available at www.jpeds.com).
Children were recruited by letters to former patients, participants in early intervention programs, and by fliers in the community.4, 17, 18 Full term children were group-matched to preterm children for age, sex, and race. Maternal education, dichotomized as < 4-year college degree versus college degree or higher in this relatively high-SES sample, was used as the measure of SES. Due to small numbers of individual ethnic/racial groups, race was dichotomized as white versus non-white. There were no differences between sites of testing for age, sex or race. SES was higher at the Palo Alto site than the Pittsburgh site, X2(1) = 12.0, p = .001.
The study was approved by institutional review boards at the University of Pittsburgh and Stanford University. A parent or legal guardian provided informed consent, and children provided assent. Participants were compensated for participation. GA, BW, and medical complications were gathered from parent report and medical records. IQ was estimated using the Wechsler Abbreviated Scale of Intelligence, a nationally standardized test of general intellectual ability that measures verbal and nonverbal cognitive ability.19
Eye Movement Test Procedures
Participants were tested in a dark room, seated 56 cm from a 17″ PC monitor where stimuli were displayed using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA). Movement was minimized with a table-mounted chin rest and head restraint. Eye movement measurements were acquired with an Applied Science Laboratories model 504 (ASL, Bedford, MA) table mounted near-infrared eye tracker with a sampling rate of 60 Hz. A technician monitored eye movements during task performance in real-time using E5WIN software (ASL, Bedford, MA).
Eye Movement Tasks
Participants were given standardized instructions and completed five practice trials correctly before testing began to ensure comprehension. Correct performance was emphasized rather than speed of responding. Tasks were presented in fixed order.
In all four tasks, peripheral targets were presented in the horizontal plane at randomized locations 4 or 8 degrees of visual angle right or left from central fixation.
Prosaccade (48 trials)
Participants fixated a green central fixation cross and then looked toward the peripheral light (target), which appeared for 1s. Dependent measures included latency (reaction time, measured as the interval from fixation offset to the initiation of the saccade to the target) and accuracy of saccades to peripheral locations (measured in degrees of visual angle).
Fixation (48 trials)
Participants fixated a blue central fixation cross for a variable delay and held gaze at the central fixation area after the cross was extinguished for a 200msec gap period. The gap was followed by the appearance of a small circular target that appeared at a randomized location for 1s. Any trial containing a movement from central fixation greater than 50 pixels toward a peripheral target was considered an error. The dependent measure was proportion of errors, or the proportion of trials with breaks from fixation.
Antisaccade (48 trials)
Participants fixated a red central fixation cross for a variable delay. The fixation cross was extinguished for a 200msec gap after which a peripheral target appeared for 1s. Subjects directed their gaze to the mirror location of the target. Dependent measures were proportion of inhibitory errors and latency of correct primary saccades away from the peripheral targets. Response preparation period, defined as the delay period between the instructional cue and the appearance of the stimulus (0.5s, 2s, 4s, or 6s), was varied to assess its impact on latency and performance of the task (proportion of errors).
Memory-guided saccade (32 trials)
Participants fixated a yellow central fixation cross. After 1.925s a small target appeared at a randomized location in the horizontal meridian for 75msec. Participants should not look at the peripheral target but instead remember its location during the ensuing working memory delay period of 2.5 or 7.5s. After the delay, the central fixation cross was extinguished, and participants had 2s to saccade to the remembered location. Dependent measures were latency to initiate a correct response and accuracy of initial and final resting saccade to the remembered location on correct trials. Trials in which the subject made a saccade toward the peripheral target prior to the end of the delay period were considered failed trials (failures of response inhibition), which were reported as proportion of errors.
Eye Movement Analysis
Eye movement recordings were analyzed offline using a combination of ILAB20 and in-house programs written in MATLAB (MathWorks, Inc.). Results of algorithm-based measurements were presented graphically and numerically online to a technician, blind to group membership, for inspection of measurements from each saccade of each trial of every task. Saccades were identified using a velocity algorithm employing a 30 deg/sec criterion, which reliably detects 0.25 degree saccades. Trials with saccade latencies of < 80msec were omitted to exclude any express saccade responses that were not guided by task stimuli. Rare blink artifacts, which occurred on < 10% of trials and occasionally resulted in failure of the software to identify primary saccades, were also excluded.
Data Analysis
Trials within each experimental condition were averaged for each subject. Data were missing for 2 full term subjects on the fixation task and 3 preterm subjects on fixation and memory-guided saccade tasks due to technical difficulty. Appropriate adjustments in the degrees of freedom are reported for these tasks. Repeated measures ANOVA with target location (near, 4 degrees, vs. far, 8 degrees) and response preparation or delay period as repeated factors were applied to the data. Differences due to target location occurred for latency on the memory-guided saccade task and are discussed only for that task. Group (preterm vs. full term) was considered a between-subjects factor and planned comparisons were used. Response preparation period was analyzed for group-by-factor interactions on latency and proportion of errors on the antisaccade task and for latency on the memory-guided saccade task. Linear interpolation was used for the few missing data points as has been used before.5, 21 All tests were 2-tailed; significance was set at p < .05.
Within the preterm group, we used forced entry linear regression analyses to identify factors associated with the proportion of errors for fixation, antisaccade, and memory-guided saccade tasks. Predictor variables included age, sex, GA, and white matter injury category (dichotomized as normal vs. abnormal) from conventional MRIs.
RESULTS
By design, preterm and full term groups differed in GA and BW and were matched in SES, sex, and race (Table I). Preterm children had mean IQ scores in the average range, but were significantly lower than full term controls. Among the preterm children, 25% were receiving special education. MRI scans were categorized as normal for 25 children; 28 of 29 children were categorized as having mild injury, therefore white matter category was classified as normal vs. abnormal.
Table 1.
Participant Characteristics
| Preterm (n = 69) |
Full Term (n = 43) |
p | |||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Range | Mean (SD) | Range | ||
| Age (years) a | 12.3 (1.8) | 9.2-16.1 | 12.7 (2.1) | 9.2-16.9 | .287 |
| Perinatal Data | |||||
| GA (weeks) | 29 (2.7) | 24-35.5 | 39.7 (1.2) | 37-42 | < .001* |
| Birthweight (grams) | 1230 (473) | 482-2495 | 3466 (489) | 2438-4422 | < .001* |
| Academic Scores | |||||
| IQ | 103 (15.3) | 69-136 | 114 (13) | 86-142 | < .001* |
|
| |||||
| Demographicsb | Preterm | Full Term | p | ||
|
| |||||
| Race, n (%) | |||||
| White | 49 (71) | 31 (72) | .902 | ||
| Sex | |||||
| Male | 31 (45) | 21 (49) | .702 | ||
| Maternal Education, n (%) | |||||
| < college degree | 25 (36) | 21 (49) | .237 | ||
Data analyzed by t-test
Data analyzed by chi-square (asymptotic or exact significance: 2-sided)
Significance p < .05
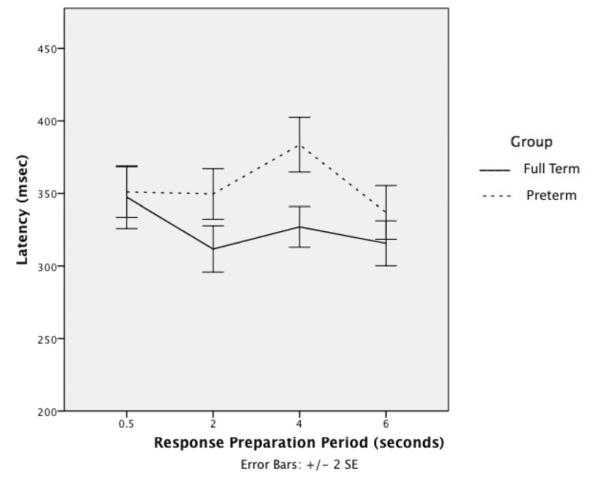
Oculomotor results are detailed in (Table II). There were no group differences in proportion of errors, latency, or accuracy for prosaccades. The preterm group showed more errors than the full term group for fixation. Group differences in proportion of inhibitory errors did not reach statistical significance, and there were no significant interactions for antisaccade. For latency, there were main effects of group, response preparation period, and group by response preparation period interaction. (Figure 2) The preterm group overall had slower latency than the full term group. Post-hoc planned comparisons showed that the preterm group had longer reaction times than the full term group at the 2s and 4s periods. These results indicate that the preterm group had longer latencies than the full term group when given intermediate lengths of time to prepare responses. For inhibitory errors (i.e., made a saccade to the peripheral target before the end of the delay period), the preterm group showed a trend for more errors compared with the full term group for memory-guided saccades. For latency, there was no main effect of group, but there were main effects of location, and group by location interaction. Both groups showed shorter latency for far locations. The preterm group showed longer latency for near locations. There was a main effect of delay with both groups showing longer latency with 2.5s delay, but no group by delay interaction. There were no differences between groups in the accuracy of initial or resting saccades.
Table 2.
Oculomotor Task Results*
| Task | Preterm | Full Term | F or t | p |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Prosaccade | (n=69) | (n=43) | ||
| Proportion of Errors | .017 ± .022 | .018 ± .025 | .040 | .841 |
| Latency (msec) | 216 ± 36.1 | 204 ± 36.9 | 2.83 | .095 |
| Accuracy (degrees of visual angle) |
3.25 ± 1.99 | 3.53 ± 1.91 | .522 | .472 |
| Fixation | (n=66) | (n=41) | ||
| Proportion of Errors | .24 ± .23 | .13 ± .15 | 7.23 | .008** |
| Antisaccade | (n=69) | (n=43) | ||
| Proportion of Errors | .55 ± .22 | .48 ± .22 | 2.23 | .138 |
| Latency (msec)*** | 4.90 | .003** (group by response preparation period interaction) |
||
| 0.5s | 351 ± 72 | 347 ± 71 | ||
| 2s | 350 ± 73 | 312 ± 53 | ||
| 4s | 384 ± 77 | 327 ± 46 | ||
| 6s | 337 ± 76 | 316 ± 51 | ||
|
Memory-Guided
Saccade |
(n=66) | (n=43) | ||
| Proportion of Errors | .47 ± .27 | .39 ± .19 | 3.1 | .079+ |
| Latency (msec)++ | 13.6 | <.001 (main effect of location) |
||
| Far Location | 513 ± 147 | 496 ± 145 | 4.6 | .034** (group by location interaction) |
| Near Location | 563 ± 141 | 513 ± 147 | ||
| Latency++ | 12.5 | .001** (main effect of delay) |
||
| Short Delay | 558 ± 143 | 526 ± 153 | ||
| Long Delay | 500 ± 138 | 483 ± 137 | ||
| Accuracy (degrees of visual angle) |
||||
| Initial Saccade | 4.65 ± 2.0 | 4.68 ± 2.0 | .003 | .959 |
| Resting Saccade | 4.50 ± 2.1 | 4.54 ± 2.0 | .013 | .909 |
Data analyzed using t-test or ANOVA
p significant at < .05
trend for significance
Antisaccade latency: Repeated-measures ANOVA with response preparation period as repeated factor and group as between-subjects variable showed main effect of group, F(1, 109) = 7.6, p = .007, response preparation period, F(3, 107) = 10.4, p < .001, and group by response preparation period interaction. Post-hoc comparisons showed that preterm differed from full term at 2s, t(110) = −3.1, p = .003) and 4s, t(110) = −4.3, p < .001, (bold).
Memory-guided saccade latency: Repeated-measures ANOVA with location and delay as repeated factors and group as between-subjects variable showed a significant interaction between group and location. Post-hoc comparisons showed that the interaction was driven by preterm children, t(130) = 1.99, p < .05, who had slower latency for near locations (bold). There was a main effect of delay on latency for both groups, F(1, 110) = 12.5, p = .001 (bold).
Figure 2.
Antisaccade latency: group by response preparation period interaction.
Contributors to Task Performance
The models predicting to proportion of errors on each task were significant. For fixation, R2 = .194, p = .032; age was the only significant predictor (β = −.356, p =.011), indicating fewer errors as age increased. For antisaccade, R2 = .411, p < .001; white matter category was significant (β = .304, p = .011), indicating that abnormal white matter category was associated with more errors on the task. Age was also significant (β = −.47, p < .001). For memory-guided saccade, R2 = .265, p = .005; white matter category was the only significant predictor in the model (β = .361, p =.008). There was a trend for age as a predictor (β = −.245, p = .076). Sex and GA were not significant predictors in any of the models.
DISCUSSION
Lack of differences between groups on the prosaccade task indicates that impaired performance on the other oculomotor tasks cannot be attributed to sensorimotor impairment and are therefore likely indications of cognitive limitations.
Fixation requires sustained active engagement. The preterm group showed impaired ability to retain fixation, suggesting that limitations in suppressing distractibility in fixation could be related to limitations in the maintenance of sustained attentional engagement. The fixation task may also be tapping features of response inhibition—failure to inhibit gaze toward peripheral distracters. The ability to maintain fixation continues to improve through childhood22 as does the ability to maintain an inhibitory set;23 these abilities may be delayed or limited with prematurity. This novel finding is consistent with high rates of inattention and impulsivity in preterm children.18
We did not find significant response inhibition failures in the preterm compared with the full term group on the antisaccade task, though there was a trend on the memory-guided saccade. Preterm children had more errors on the antisaccade, but these differences did not reach statistical significance. The antisaccade task generated more errors across groups than did fixation, indicating it was comparatively more difficult. The high difficulty may have limited the ability to find group differences. Crucial to the ability to inhibit prepotent responses is recruitment of fronto-subcortical circuitry,24 and the ability for prefrontal regions to influence other cortical and subcortical regions. The error rate suggests limitations in fronto-subcortical integration in preterm children.
A second novel finding was that variations in response preparation periods on antisaccade resulted in longer latencies in the preterm than full term groups. The response preparation period allows the participant to access preparatory processes. Preterm children had the longest latencies when given intermediate time to prepare a response. Full term children did not change latencies regardless of response preparation time. The longer reaction times of the preterm group suggest that they may have slower processing speed or require a greater preparatory effort, perhaps to control distractibility, undermining inhibitory control. Given that the preparatory period engages frontal and subcortical regions in order to generate a correct inhibitory reponse,24 this result also suggests limitations in this brain circuitry.
A group by location interaction in the memory-guided saccade task indicated that the preterm group was slower to respond with near targets compared with full term children. Near locations are more difficult to inhibit than far locations. A longer latency may reflect added effort to successfully suppress a response and perform accurately. Memory-guided saccade latency results are compatible with antisaccade results, suggesting fronto-subcortical limitations.
We found no spatial working memory deficits in the preterm group. Our results differ from those of other studies using other tests of verbal, visual and visuospatial working memory.1, 25 Our task required maintenance of spatial locations, but no manipulation of working memory. The use of more varied locations outside of the horizontal plane might identify deficits in future work. This result suggests preserved cortical processing of spatial memory known to be supported by prefrontal and parietal regions.26
As expected based on studies showing developmental improvements in executive function,8 age contributed to oculomotor performance in the preterm group. Despite a relatively healthy preterm group, white matter category also contributed to performance on antisaccade and memory-guided saccade tasks, even though the injuries were almost all mild. Other studies of preterm children showed that periventricular leukomalacia and abnormalities on head ultrasound predicted executive functions in 6 to 8 year old children < 1500g27 and in children < 1000g.3 The preterm children in these other studies had lower GA, BW, and SES, and greater white matter injury compared with our study.
Executive functions have been linked to prefrontal cortex as well as parietal and temporal cortices, basal ganglia, and cerebellum.2, 26 Retaining fixation requires active engagement of pause cells in the superior colliculus.9 Increased susceptibility to peripheral distracters in the preterm group suggests that long range white matter tracts from frontal lobes to brain stem regions that carry the information to resist peripheral distraction may be at risk or injured in the preterm group.
Reflexive prosaccades depend primarily on direct projections from visual and parietal cortices to the superior colliculus, whereas volitional antisaccades engage frontal cortex, which projects both directly and indirectly (via basal ganglia) to the superior colliculus and brainstem.28 A functional MRI (fMRI) study of response inhibition and attention in adults with history of preterm birth showed reduced activation in the fronto-parieto-cerebellar network involved in attention allocation and increased activation of posterior regions on a response inhibition task.29 The results here are consistent with less mature functional brain circuitry in the preterm group.
The neural basis of response preparation has been studied with fMRI.30, 31 A study that assessed the preparation period during antisaccade trials found activation of the left dorsolateral prefrontal cortex and anterior cingulate cortex.32 In our study, the preterm group showed differences from the full term group in response preparation that may reflect abnormalities in these regions. Whether the preterm children were less efficient in preparing responses or used response preparation time to compensate for response inhibition difficulties warrants further study.
Study limitations include use of a convenience sample of relatively high-functioning preterm children that may not be representative of all preterm children. The study covered a wide range of age and gestational age. In addition, almost all preterm children had either normal or mild white matter injury on conventional MRI that may have limited group differences. Nonetheless, both age and white matter category were predictors of performance in the preterm group.
Our study contributes to our understanding of executive function deficits in preterm children by utilizing a distinctive set of oculomotor paradigms. Prematurity has been associated with compromised white matter development or injury13 including regions associated with executive function and attention,33, 34 which could affect functional connectivity of preserved gray matter regions. Preterm children in our study, free of major sensory and motor deficits, demonstrated intact basic sensorimotor function. Intact spatial memory and planned inhibitory responses in the presence of limitations in suppressing distracters and slowed response initiation suggest intact cortical processing and impaired cortico-subcortical functional integration that may be associated with white matter injury in preterm children. Collectively, our findings indicate that even in high functioning preterm children, oculomotor tasks show subtle indices of compromised executive function. Future work to understand the neural basis of these findings will examine the relationship between oculomotor measures of executive function and properties of the white matter tracts of the brain.
Acknowledgments
We thank the families and children who participated in the study.
Supported by the National Institutes of Health (grant RO1-HD46500 to H.F., and grant 5R01 MH067924 to B.L.), NIH Pediatric Research Loan Repayment Program Award (to I.L.), and the Clinical and Translational Science Award (1UL1 RR025744) for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the National Center for Research Resources, National Institutes of Health.
Abbreviations
- ANOVA
analysis of variance
- BW
birth weight
- FMRI
functional magnetic resonance imaging
- GA
gestational age
- IQ
intelligence quotient
- SES
socioeconomic status
Appendix
Palo Alto, CA Site: MRI data were acquired on a 3T Signa Excite (GE Medical Systems, Milwaukee, WI) at Stanford University. Two high resolution IR-prep 3D FSPGR scans (FOV = 24 × 18 cm, matrix size = 260 × 192, 0.9 mm slices, TI = 300 ms, flip angle = 15 degrees, 1 NEX) and one IR-prep 3D FSPGR scan (FOV = 24 ×15.6 cm, matrix size = 256 × 192, 1.2mm slices, TI = 300 ms, flip angle = 15 degrees, 1 NEX) were collected. The three T1 images were averaged. They were coregistered based on a mutual information algorithm (SPM5, http://www.fil.ion.ucl.ac.uk/spm/). A trained experimenter manually identified the anterior and posterior commissures and mid-sagittal plane, and these points were used to put the image in a canonical orientation.
Pittsburgh, PA Site: Structural magnetic resonance imaging and DTI data were acquired with a Siemens 3T MAGNETOM Allegra (Erlangen, Germany) system with a standard circularity-polarized head coil at University of Pittsburgh. Structural images were acquired first, using a sagittal magnetization-prepared rapid gradient-echo T1-weighted sequence (repetition time 1570ms, echo time 3.04ms, flip angle 8, inversion time 800ms, voxel size 0.78mm × 0.78125mm × 0.78125mm).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of Executive Function and Attention in Preterm Children: A Systematic Review. Developmental Neuropsychology. 2009;34:393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- 2.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 3.Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. Journal of Developmental & Behavioral Pediatrics. 2006;27:459–69. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Loe IM, Lee ES, Luna B, Feldman HM. Executive Function Skills are Associated with Reading and Parent-Rated Child Function in Children Born Prematurely Early Human Development. In Press;doi:10.1016/j.earlhumdev.2011.07.018. [DOI] [PMC free article] [PubMed]
- 5.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA, Luna B, et al. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–72. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 6.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MH. The inhibition of automatic saccades in early infancy. Developmental Psychobiology. 1995;28:281–91. doi: 10.1002/dev.420280504. [DOI] [PubMed] [Google Scholar]
- 8.Asato MR, Sweeney JA, Luna B. Cognitive processes in the development of TOL performance. Neuropsychologia. 2006;44:2259–69. doi: 10.1016/j.neuropsychologia.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford University Press; New York: 2006. [Google Scholar]
- 10.Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–96. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- 11.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–84. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- 12.Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences of the United States of America. 1998;95:831–8. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurology. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christ SE, White DA, Brunstrom JE, Abrams RA. Inhibitory control following perinatal brain injury. Neuropsychology. 2003;17:171–8. [PubMed] [Google Scholar]
- 15.Newsham D, Knox PC, Cooke RW. Oculomotor control in children who were born very prematurely. Investigative Ophthalmology & Visual Science. 2007;48:2595–601. doi: 10.1167/iovs.06-1425. [DOI] [PubMed] [Google Scholar]
- 16.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. Journal of Pediatrics. 2003;143:171–9. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee ES, Yeatman J, Luna B, Feldman HM. Specific Language and Reading Skills in School-Aged Children and Adolescents are Associated with Prematurity after Controlling for IQ. Neuropsychologia. 2011;49:906–913. doi: 10.1016/j.neuropsychologia.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loe IM, Lee ES, Luna B, Feldman H. Behavior Problems of 9-16 Year Old Preterm Children: Biological, Sociodemograhic, and Intellectual Contributions. Early Human Development. 2011;87:247–52. doi: 10.1016/j.earlhumdev.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wechsler . Wechsler Abbreviated Scale of Intelligence (WASI) Manual. Harcourt Assessment, Inc.; San Antonio, TX: 1999. [Google Scholar]
- 20.Gitelman DR. ILAB: a program for postexperimental eye movement analysis. Behavioral Research Methods, Instruments and Computers. 2002;34:605–12. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- 21.Loe IM, Feldman HM, Yasui E, Luna B. Oculomotor Performance Identifies Underlying Cognitive Deficits in Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:431–40. doi: 10.1097/CHI.0b013e31819996da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paus T, Babenko V, Radil T. Development of an ability to maintain verbally instructed central gaze fixation studied in 8- to 10-year-old children. International Journal of Psychophysiology. 1990;10:53–61. doi: 10.1016/0167-8760(90)90045-f. [DOI] [PubMed] [Google Scholar]
- 23.Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J Neurosci. 2009;29:12558–67. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J Neurosci. 2010;30:15535–45. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luu TM, Ment LR, Allan W, Schneider K, Vohr BR. Executive and Memory Function in Adolescents Born Very Preterm. Pediatrics. 2011;127:e000. doi: 10.1542/peds.2010-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikkai A, Curtis CE. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 2011;49:1428–34. doi: 10.1016/j.neuropsychologia.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor HG, Klein N, Schatschneider C, Hack M. Predictors of early school age outcomes in very low birth weight children. Journal of Developmental & Behavioral Pediatrics. 1998;19:235–43. doi: 10.1097/00004703-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 28.LeVasseur AL, Flanagan JR, Riopelle RJ, Munoz DP. Control of volitional and reflexive saccades in Tourette’s syndrome. Brain. 2001;124:2045–58. doi: 10.1093/brain/124.10.2045. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence EJ, Rubia K, Murray RM, McGuire PK, Walshe M, Allin M, et al. The neural basis of response inhibition and attention allocation as mediated by gestational age. Hum Brain Mapp. 2009;30:1038–50. doi: 10.1002/hbm.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol. 2008;99:133–45. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeSouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89:1016–23. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- 32.Brown MR, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. J Neurophysiol. 2007;98:1751–62. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- 33.Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–66. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 34.Skranes J, Lohaugen GC, Martinussen M, Indredavik MS, Dale AM, Haraldseth O, et al. White matter abnormalities and executive function in children with very low birth weight. Neuroreport. 2009;20:263–6. doi: 10.1097/wnr.0b013e32832027fe. [DOI] [PubMed] [Google Scholar]