Abstract
Ovarian cancer is the most lethal gynecological malignancy affecting American women. Current hypotheses concerning the etiology of ovarian cancer propose that a reduction in the lifetime number of ovulations decreases ovarian cancer risk. Advanced serous carcinoma shares several biomarkers with fallopian tube epithelial cells, suggesting that some forms of ovarian carcinoma may originate in the fallopian tube. Currently, the impact of ovulation on the tubal epithelium is unknown. In CD1 mice, ovulation did not increase tubal epithelial cell proliferation as measured by bromodeoxyuridine incorporation and proliferating cell nuclear antigen staining as compared to unstimulated animals. In superovulated mice, an increase in the number of pro-inflammatory macrophages was detected in the oviduct. Ovulation also increased levels of phospho-γH2A.X in tubal epithelial cells, indicating that these cells were susceptible to double-strand DNA breakage following ovulation. To determine which components of ovulation contributed to DNA damage in the fallopian tube, an immortalized baboon tubal epithelial cell line and a three-dimensional organ culture system for mouse oviduct and baboon fallopian tubes were developed. Tubal epithelial cells did not proliferate or display increased DNA damage in response to the gonadotropins or estradiol alone in vitro. Oxidative stress generated by treatment with hydrogen peroxide or macrophage-conditioned medium increased DNA damage in tubal epithelial cells in culture. Ovulation may impact the fallopian tube epithelium by generating DNA damage and stimulating macrophage infiltration, but does not increase proliferation through gonadotropin signaling.
Keywords: ovulation, fallopian tube, serous cancer, inflammation
Introduction
Ovarian cancer is the most lethal gynecological malignancy and the fifth leading cause of cancer death among women, due in part to a lack of early detection methods (Jemal, et al. 2009). As a result, approximately 62% of ovarian cancers are not detected until after the tumors have metastasized, resulting in high mortality rates and few treatment options (Altekruse, et al. 2010). The most common histotype of epithelial ovarian cancer is serous carcinoma, which has previously been reported to arise from precursor lesions involving the ovarian surface epithelium (OSE) or ovarian epithelial inclusion cysts (Auersperg, et al. 2002; Scully 1995). Emerging evidence indicates that the fallopian tube epithelium may be an alternative site of origin for serous ovarian cancers (Altekruse et al. 2010; Bell 2005). A striking resemblance between the fallopian tube epithelium and serous ovarian cancer has been observed; this includes similar gene expression profiles, cell morphologies, and expression of E-cadherin, all of which are distinct from the OSE (Auersperg, et al. 2008).
Several lines of evidence support the fallopian tube as a source of neoplastic cells for the initiation of serous cancer. The majority of women with advanced stage serous cancer exhibit preneoplastic lesions in the fallopian tube in addition to a dominant ovarian mass (Jarboe, et al. 2008b). A ‘p53 signature’ has been identified, which marks precursor lesions of the distal fallopian tube that are composed of morphologically benign epithelium exhibiting elevated levels of p53 protein expression (Lee, et al. 2007). Identical p53 mutations have been observed in tumors of the distal fallopian tube and in ovarian or peritoneal serous carcinomas, suggesting a common source (Jarboe, et al. 2008a). Women with BRCA1 or BRCA2 mutations are more likely to exhibit expression of the p53 signature as well as primary fallopian tube carcinoma, indicating that an association may exist between the BRCA mutation phenotype and serous cancer originating from the fallopian tube (Cass, et al. 2005). However, much is still unknown about the association between neoplastic lesions of the fallopian tube and development of serous pelvic or ovarian cancer, largely due to a lack of tools to model neoplasia in the fallopian tube. The laying hen has been used as an animal model for spontaneous ovarian and tubal cancer, and although these animals exhibit primarily the endometrioid histotype rather than the serous histotype of adenocarcinoma, a recent analysis depicted oviductal genes expressed at high levels in cancers as the grade increased (Hakim, et al. 2009; Trevino, et al. 2010). A human fallopian tube ex vivo culture system was developed, which allows for co-culture of both secretory and ciliated cells of the fallopian tube (Levanon, et al. 2010). This represents significant improvements over previous tubal epithelial cell (TEC) culture systems, but does not allow for analysis of TEC in the context of their physiological microenvironment in contact with stroma and extracellular matrix, or for any in vivo analysis of how ovulation might damage tubal cells.
Serous ovarian cancer is largely a spontaneous disease, although approximately 10% of cases are linked to mutations in BRCA1 and BRCA2 (Crum, et al. 2007a). Risk factors for spontaneous serous cancer include nulliparity, infertility, and an early onset of puberty or late menarche, while pregnancy, breastfeeding, and the use of oral contraceptives are protective against ovarian cancer (Auersperg et al. 2008). An increased incidence of non-familial, spontaneous ovarian cancer is linked to an increased number of lifetime ovulations, and several hypotheses have been proposed linking events associated with ovulation to ovarian cancer initiation and progression. The first hypothesis is Fathalla’s tear-and-repair hypothesis, which states that as a result of repetitive proliferation of the OSE to repair the ovulation-induced wound in the ovarian surface, spontaneous DNA replication errors accumulate, leading to neoplasia and ovarian cancer (Fathalla 1971). OSE have been shown to proliferate in response to ovulation and to exhibit signs of DNA damage (Burdette, et al. 2006; Murdoch, et al. 2001), but the effects of ovulation on TEC proliferation and DNA damage are unknown. A second hypothesis linking ovulation to ovarian cancer concerns the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are thought to contribute to the initiation and progression of ovarian cancer by stimulating the growth of the OSE and/or inhibiting apoptosis of damaged cells (Konishi, et al. 1999). Both the OSE and TEC have been shown to express receptors for FSH, LH, and estradiol, though the exact role of these hormones in stimulating TEC in vivo is not known (Zhang, et al. 2001; Zheng, et al. 1996). Third, inflammation induced by ovulation may generate oxidative stress that is further exacerbated by cellular proliferation and the effects of FSH and LH, leading to DNA damage and replication errors. Ovulation generates inflammatory molecules such as bradykinin, prostaglandins, and leukotrienes through ischemia-reperfusion associated with wound repair, as well as through recruitment of leukocytes to sites of ovulation (Murdoch 2008). However, an association between ovulation and inflammation in TEC has not been examined.
The purpose of this study was to evaluate common hypotheses regarding ovulation and the initiation of serous epithelial cancer to determine how proliferation (tear and repair), the gonadotropins, and inflammatory molecules influence normal TEC. Following ovulation, TEC might possibly be sloughed onto the ovarian surface due to the close proximity of the distal fimbriae of the fallopian tube to the ovarian rupture site by a process similar to retrograde menstruation. Sloughed cells might form inclusion cysts in which the epithelial cells are exposed to increased levels of hormones and growth factors (Kurman and Shih Ie 2010). The process of ovulation releases growth factors, steroid hormones, and inflammatory molecules, and the fallopian tube is in close proximity to the source of these factors. The current study reports that ovulation does not increase TEC proliferation in vivo. Moreover, using a novel organ culture system to study mouse TEC and baboon fimbriae TEC, as well as a newly generated immortalized baboon tubal epithelial cell line (TEC40), FSH and LH do not induce proliferation of TEC. TEC from ovulated animals exhibit increased levels of DNA damage and macrophage infiltration into the oviduct. Oxidative stress generated by hydrogen peroxide or macrophage-conditioned media increased DNA damage in TEC40.
Materials and Methods
Animals
Day 25 female CD1 mice were used for in vivo ovulation studies. For organ culture experiments, mouse oviducts were isolated from CD1 day 16 female pups. All mice were acquired through in-house breeding, and breeders were purchased from Harlan (Indianapolis, IN). Fimbriae tissue was obtained from adult female baboons (Papio anubis) housed at the University of Illinois at Chicago under the supervision of Dr. Fazleabas. All animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the established institutional animal use and care protocol at the University of Illinois at Chicago. Animals were housed in a temperature and light controlled environment (12L:12D) and were provided food and water ad libitum.
Experimental design of ovulation study
Induction of ovulation was performed essentially as described previously and in Figure 1 (Burdette et al. 2006). Control mice were injected i.p. with phosphate buffered saline (PBS) at 0900 h on d25 and 0900 h on d27. Ovulated mice were injected i.p. with 5 IU of pregnant mare serum gonadotropin (PMSG) (Sigma Aldrich, St. Louis, MO) at 0900 h on d25 and 5 IU of human chorionic gonadotropin (hCG) (Sigma Aldrich) at 0900 h on d27. Injections containing 100 mg/kg bromodeoxyuridine (BrdU) (Sigma Aldrich) were given i.p. either at the time of the first and second hormone or PBS injections or solely with the second injection. Upon first injection of BrdU, animals were given water containing 0.8 mg/ml BrdU to allow for continuous labeling. At 2100 h on d 27.5, animals were euthanized using CO2 asphyxiation and cervical dislocation. Total basal proliferation occurring over 60 h was assessed by injecting mice (n=6) with PBS and BrdU at 0900 h on d25, followed by continuous labeling with BrdU until 2100 h on d27.5. Abridged basal proliferation was measured by injecting mice (n=3) with PBS and BrdU at 0900 h on d27, with labeling until 2100 h on d27.5 for a labeling time of 12 hours. Total ovulatory proliferation was measured by ovulating animals (n=6) with PMSG and hCG, with BrdU labeling from 0900 h on d25 to 2100 h on d27.5. At 2100 h on d27.5, ovaries and oviducts, including fat pad, bursa, and part of the uterine tube, were collected. For analysis of tissues at 16 hours post-hCG, animals were injected with PBS (n=4) or PMSG (n=5) at 1700 h on d25, followed by injection of PBS or hCG at 1700 h on d27, and collection of tissues at 0900 h on d28. Tissues were fixed in 4% paraformaldehyde (PFA) for 24 h, dehydrated with ethanol, paraffin embedded, and serial sectioned at 5 µm.
Figure 1. TEC do not proliferate in response to ovulation in vivo.
(A) CD1 mice were injected with buffer (PBS) as a control or hormones (PMSG and hCG) to induce ovulation. BrdU was added to control injections on either day 25 or day 27 to measure cellular proliferation during a 12 hour (abridged basal) or 60 hour (total basal) labeling period. To measure total cellular proliferation (total ovulatory) or proliferation in response to hCG stimulation and the process of ovarian surface rupture (post-ovulatory) was measured by adding BrdU to the d25 and d27 injections, respectively. (B) Oviductal sections (5 µm) were processed for immunohistochemistry and stained with antibodies against CK8 to mark OSE or TEC and antibodies against BrdU or PCNA to mark proliferating cells. Asterisks mark proliferating TEC. (C) The percentage of CK8-positive TEC with BrdU incorporation was scored as a percentage of the total number of CK8-positive TEC. Significant differences (P<0.05) are between groups labeled “a” and “b.” (D) The percentage of CK8-positive, PCNA-positive TEC was scored as a percentage of the total number of CK8-positive TEC. No significant difference was detected between groups.
Organ culture
Oviducts from d16 prepubertal mice were dissected free of the ovary and uterus and gently uncoiled with forceps in dissecting medium composed of Leibovitz (L15) medium with 2mM L-glutamine, 100 U penicillin, and 100 µg streptomycin (Invitrogen, Carlsbad, CA). Each oviduct was cut in four pieces, termed organoids. Fallopian tube fimbriae from adult baboons were collected into dissecting medium and the distal ends of the baboon fimbriae were isolated using a scalpel (baboon organoids). Each organoid was placed into a 0.5% alginate (w/v in PBS) droplet formed on a mesh fiber as described previously (Jackson, et al. 2009). The alginate-encapsulated organoid was placed into 50 mM calcium chloride for 2 min to crosslink the alginate into a gel. The encapsulated organoid was then placed into growth media for 7 days. Growth media was composed of alpha-MEM (Invitrogen), 100 U penicillin and 100 µg streptomycin, and either 3 mg/ml BSA (MP Biomedicals, Solon, OH), 10 % v/v FBS (Invitrogen), 10 mIU/ml or 100 mIU/ml FSH (Sigma), 10 mIU/ml or 100 mIU/ml LH (Sigma), or 10 nM estradiol (Sigma). BrdU was added to the media at a final concentration of 10 µM 24 h prior to fixation.
Cell culture
Fallopian tube fimbriae from adult baboons were rinsed briefly in 70% ethanol and placed into Leibovitz (L15) medium with 1000 U collagenase (Sigma) and 2X trypsin-EDTA (Invitrogen) for 1hr at 37 °C. Tissue was vortexed briefly and the media was collected into a new microcentrifuge tube and centrifuged at 3000 rpm for 3 min to pellet cells. Cells were maintained in MEM with 10% FBS, 2mM L-glutamine, 100 µM non-essential amino acids, 1mM sodium pyruvate, 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen).
To immortalize the baboon TEC, cells were transfected with pW2T+t+ to express the SV40 large and small T antigens (Dr. Kathy Rundell, Northwestern University, Chicago, IL) and Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). Following transfection and subsequent passaging, untransfected cells died, leaving immortalized cells expressing SV40. Cells were passaged for several months in culture, and were verified for expression of SV40, secretory, and ciliated markers at passage number 16. The immortalized baboon TEC cell line was named TEC40, for tubal epithelial cells immortalized with SV40.
For proliferation assays, TEC40 cells were seeded into 96 well plates at 5 × 104 cells/ml. The next day, complete media containing 10% FBS and 10 mIU/ml or 100 mIU/ml FSH or LH was added to plates and the cells were allowed to grow for 4 d. Proliferation was measured with CellTiter 96 Aqueous One Solution (Promega, Madison, WI) according to the manufacturer’s directions. Spectrophotometric analysis at 490 nm was completed using a Biotek EL312e microplate reader (Fisher Biotek, Pittsburgh, PA).
To prepare macrophage-conditioned medium (MCM), RAW264.7 cells were maintained in DMEM with 10% FBS, 100 U penicillin, and 100 µg streptomycin. Conditioned medium was prepared as previously described (An, et al. 2009). Cells were plated at 5 ×105 cells/ml in a 6-well plate 24 h prior to treatment with E. coli lipopolysaccharide serotype O55:B5 (Fisher Scientific) in serum-free media. Cells were treated with serum-free media or serum-free media containing 5 µg/ml lipopolysaccharide for 24 hours before conditioned media was collected.
For analysis of phospho-γH2A.X foci, TEC40 cells were serum starved for 18 hours prior to treatment with hormones, hydrogen peroxide, or MCM. FSH (10 mIU/ml or 100 mIU/ml), LH (10 mIU/ml or 100 mIU/ml), estradiol (10 nM), hydrogen peroxide (Sigma-Aldrich; 1 mM) or MCM was added to fresh serum-free medium. TEC40 were treated for 20 min, followed by fixation and immunofluorescent staining as described below.
Luciferase assays
TEC40 cells were plated in triplicate in phenol red-free DMEM with 10% charcoal-stripped FBS (Gibco), 100 U/ml penicillin, and 100 µg/ml streptomycin into 12-well plates 24 hr prior to transfection. Cells were co-transfected with 0.25 µg pCMV Sport-βgal plasmid and 2.5 µg of equal amounts of the ERE-luc reporter plasmid and ERα or pGL3-Basic plasmids using the calcium phosphate precipitation method (Sambrook, et al. 1989). The ERE-luc reporter plasmid and ERα expression plasmids were gifts from Dr. Benita Katzenellenbogen, University of Illinois, Champaign, IL. After cells were incubated with plasmids for 4 h, cells were washed, glycerol shocked, and incubated in media with or without 50 nM estradiol for 20 h. Cells were harvested in reporter lysis buffer and luciferase activity measured relative to β-galactosidase activity as previously described (Jaffe, et al. 2003).
RNA Isolation and RT-PCR
Total RNA was extracted from 1 mg of adult mouse ovary, d16 mouse oviduct, baboon fimbriae, or 1×106 TEC40 cells using a QIAGEN Qiashredder column and QIAGEN RNeasy kit according to manufacturer’s directions (QIAGEN Inc., Valencia, CA). cDNA was prepared by reverse transcribing 1 µg total RNA using MMLV-RT (Fermentas Inc., Glen Burnie, MD). cDNA was used for PCR amplification using the following primers: mouse FSHR forward (CCTTGCTCCTGGTCTCCTTG), mouse FSHR reverse (CTCGGTCACCTTGCTATCTTG); mouse LHR forward (CGCCCGACTATCTCTCACCTA), mouse LHR reverse (GACAGATTGAGGAGGTT GT CAAA); mouse ERα forward (CAGATAGGGAGCTGGTTCATATG), mouse ERα reverse (GCCAGACGAGACCAATCAT); mouse GAPDH forward (AGGTCGGTGTGAACGG ATTTG); mouse GAPDH reverse (TGTA GACCATGTAGTTGAGGTCA). For PCR amplification of baboon cDNA, the following human primers were used: human FSHR forward (GTCATCATCGGATCTGT CACTG); human FSHR reverse (CATTCCTCGGGAGGTCAGAAG); human LHR forward (GGAGGCCACGTTGACTTACC); human LHR reverse (CAGTTCACTCTCAGCAAGCAT); human ERα forward (TCCTACCAGACCCTTCAGTG); human ERα reverse (GGTCAAATCCACAAAGCCTG); human GAPDH forward (ATGGGGAAGGTGAAGGTCG); human GAPDH reverse (GGGGTCATTGATGGCAACAATA). Cycling conditions for reactions were 94°C 2 min; 34 cycles of 94°C for 30 sec, 55°C for 30 sec, 68°C for 30 sec; 68°C for 5 min. Reactions were run on a 1% (w/v) agarose gel and visualized using an Alpha Innotech gel documentation system (Santa Clara, CA).
Immunohistochemistry
Organoids were removed from culture media, re-crosslinked in 50 mM calcium chloride for 2 min, and fixed in 2% PFA with 50 mM sodium cacodylate and 10 mM calcium chloride for 16–24 h. All reagents were obtained from Vector Laboratories, Inc. (Burlingame, CA) unless otherwise indicated. Xylene and ethanol were obtained from Surgipath Medical Ind. Inc. (Richmond, IL). Antigen retrieval was performed using 10 mM sodium citrate, pH 6.0. Slides were washed in Tris-buffered saline (TBS) with Tween 20 [20 mM Tris, 500 mM NaCl, 0.1% Tween 20, pH 7.4]. When staining for BrdU, tissues were treated with 4M hydrochloric acid for 10 min, followed by 0.1 M sodium tetraborate for 10 min. Tissues were blocked for 15 min in 3% hydrogen peroxide (Fisher Scientific, Pittsburgh, PA) followed by avidin and biotin blocking according to manufacturer’s instructions. Slides were incubated in TBS-3%BSA-10% serum of the secondary antibody host for 1 h at room temperature. After blocking, slides were incubated overnight at 4°C in primary antibody in 3% BSA-TBS-10% serum. Control slides received serum block instead of the primary antibody. The primary antibodies against BrdU (rat, 1:200 dilution; Abcam, Cambridge, MA), CK8 (CK8 TROMA-1 antibody, rat, 1:200; Developmental Studies Hybridoma Bank, Iowa City, IA), E-cadherin (rabbit, 1:50; Cell Signaling Technology, Denver, MA), F4/80 (rat, 1:100; Abcam), acetylated tubulin (mouse, 1:500; Sigma Aldrich), oviductal glycoprotein 1 (OVGP1, rabbit, 1:250; Abcam), Pax8 (rabbit, 1:250 Proteintech Group, Chicago, IL), phospho-γH2A.X (rabbit, 1:100; Cell Signaling Technology), or SV40 T Ag (rabbit 1:50; Santa Cruz Biotechnology, Santa Cruz, CA) were incubated on tissue sections overnight at 4°C. Slides were rinsed three times for 5 min in TBS-Tween and then incubated at room temperature for 30 min in biotinylated secondary antibody in 3% BSA-TBS. After washing slides in TBS-Tween, avidin/biotin complex (ABC) reagent was added and incubated for 30 min at room temperature. Slides were washed in TBS and antigen-antibody-horseradish peroxidase complex was visualized using diaminobenzidine (DAB) reagent for 5 min. Slides were counterstained with hematoxylin.
Immunofluorescence
TEC40 cells were plated in an 8-well chamber slide at a density of 5 × 104 cells per well. Cells were fixed in 4% PFA for 5 min, washed with PBS, and permeabilized with 0.2% Triton-X100 in PBS for 10 min. Cells were then washed twice with PBS, and blocked with 10% goat serum in PBS for 10 min. Cells were incubated in primary antibody diluted in 10% serum in PBS for 1 h at room temperature. Cells were washed and incubated with anti-rat AlexaFluor-594 conjugated secondary antibodies (Invitrogen) for 30 min to detect cytokeratin 8 (CK8) staining. For double immunofluorescent staining of CK8 and phospho-γH2A.X, cells were incubated with anti-rat AlexaFluor 594 and anti-rabbit AlexaFluor 488 for 30 min. For all other double immunofluorescent staining, cells were incubated with both AlexaFluor594 and a biotinylated anti-rabbit secondary antibody for 30 min. Cells were washed with PBS and incubated with ABC reagent for 30 min. After washing with PBS, cells were fluorescently labeled with AlexaFluor488 using a streptavidin –horseradish peroxidase tyramide signal amplification kit (TSA kit #22, Invitrogen) for 10 min at room temperature. Cells were washed in distilled water and slides were mounted with Vectashield Mounting Medium with DAPI (Vector Laboratories). The proliferating cell nuclear antigen antibody (PCNA; rat) was used according to the manufacturer’s protocol (Zymed, San Francisco, CA).
Western blotting
TEC40 cells were serum-starved overnight prior to treatment with FSH (10 mIU/ml or 100 mIU/ml) or LH (10 mIU/ml or 100 mIU/ml) in serum-free media for 24 hr. Cells were lysed in modified RIPA buffer [25mM Tris-HCl pH 7.6, 150 mM NaCl, 1% (v/v) Triton-X, 1% (v/v) sodium deoxycholate, 0.1% SDS, 1X Complete Mini Protease Inhibitor Cocktail tablets (Roche, Indianapolis, IN), and 1X Phosphatase Inhibitor Cocktail III (Sigma)]. Protein concentration was determined using the bicinchonic acid assay (Pierce, Rockford, IL). 30 µg of cell lysate was run on a 10% SDS-PAGE gel under reducing conditions and transferred to polyvinylidene fluoride membrane. Membranes were blocked for 1 hr at RT in 5% bovine serum albumin (for pERK1/2) or 5% nonfat dry milk (for actin) in TBS-T. Primary antibodies against phospho-p44/42 MAPK (Erk1/2) (Thr 202/Tyr204) (rabbit, 1:1000 dilution; Cell Signaling Technology) or actin (rabbit, 1:1000 dilution; Sigma) were incubated on blots overnight at 4°C. After washing, membranes were incubated with goat anti-rabbit horseradish peroxidase secondary antibody (Cell Signaling) at 1:1000 in blocking buffer for 1 hour. Membranes were washed and incubated with SuperSignal West Femto substrate (Thermo Scientific, Rockford, IL) before imaging on an Alpha Innotech gel documentation system (Santa Clara, CA).
Imaging and counts
Immunohistochemistry or immunofluorescent images were acquired on a Nikon E600 microscope using a DXM1200 digital camera and NIS Elements software (Nikon Instruments, Melville, NY). Immunofluorescent images were also acquired on a Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss MicroImaging, LLC, Thornwood, NY). Using ImageJ software (National Institutes of Health, Bethesda, MD), the number of CK8-positive tubal cells that were also positive for either BrdU, PCNA, or phospho-γH2A.X were counted and expressed as a percentage of total number of CK8-positive tubal cells. At least three random fields from at least two independent experiments were counted.
Statistics
All values are expressed as the mean ± standard error. Tukey’s multiple comparison tests were used to assess differences between experimental groups and control groups. For comparison between two groups, a Students t-test was used. P < 0.05 was considered statistically significant.
Results
Ovulation does not drive proliferation of TEC in vivo
In order to investigate whether ovulation increases proliferation of TEC, incorporation of BrdU was quantified in control CD1 mice or mice that were hormonally induced to ovulate (Figure 1a). Day 25, immature mice were utilized to ensure that no previous ovulations had occurred, leaving the ovaries and oviducts undamaged by previous ovulation-related inflammation, wound repair, or the influence of hormones. Mice were injected with either PBS as a control or a combination of 5 IU of PMSG and hCG to induce ovulation (n= 6) (Burdette et al. 2006). Ovulation was verified to have occurred by the presence of corpora lutea within the ovary. Proliferation in response to both PMSG and hCG (BrdU treatment on d25, 60 hr labeling period) and post-ovulatory proliferation in response to hCG injection (BrdU treatment on d27, 12 h labeling period) were determined and compared to proliferation in PBS-treated animals. To quantify the percentage of proliferating TEC, serial sections of oviduct were stained with antibodies against CK8 to mark epithelial cells, or BrdU to mark proliferating cells (Figure 1b). Tubal cells positive for both CK8 and BrdU were scored as a percentage of total CK8-positive tubal cells (Figure 1b and 1c). For TEC, animals in the abridged basal group showed 3.5% proliferating cells compared to 6.3% proliferating cells in the post-ovulatory group, over the course of a 12 h labeling period. The total basal animals exhibited 18.5% proliferating epithelial cells compared to 17.7% in the total ovulatory animals, both of which underwent labeling for 60 h. Significant differences were not detected when comparing ovulated animals to unstimulated animals and controlling for labeling time in vivo.
To verify TEC proliferation using an endogenous marker of proliferation, serial sections were stained for CK8 or PCNA and counted as described above (Figure 1b). Basal animals included both total basal and abridged basal animals, as the only difference between these groups was the time of BrdU incorporation, which was not necessary for measuring PCNA (n=9). Ovulated animals included both total ovulatory and post-ovulatory animals (n=12). In accordance with the measurement of BrdU labeling as an indicator of cell proliferation, basal animals showed 7.0% PCNA-positive cells, compared to 9.0% for ovulated, which was not significantly different (Figure 1d). Therefore, ovulation does not increase TEC proliferation in vivo.
Three-dimensional oviduct culture system retains markers of secretory and ciliated cells
To determine the effects of individual components of ovulation, such as the gonadotropins and inflammation, on TEC proliferation and DNA damage, a three-dimensional organ culture system was developed to study normal primary TEC in their microenvironment. Alginate, a widely used biomaterial in tissue engineering, was used as described previously (Jackson et al. 2009) to maintain the physiological architecture of the oviduct, with the TEC lining the lumen of the tube. Sexually immature d16 CD1 mice were utilized in order to study oviductal epithelium that had not been affected by ovulations. Each oviduct was separated into 4 pieces with a scalpel and placed into an alginate droplet (Figure 2), and cultured for 7 d in basal media (BSA or FBS) or serum-free media supplemented with FSH, LH, or estradiol at cycling or menopausal concentrations (King, et al. 2011). Oviducts were analyzed by immunohistochemistry for normal endogenous markers of TEC and compared to uncultured mouse oviducts (Figure 3a and Supplemental Figure 1). The oviduct culture system retained cytoplasmic markers of normal oviductal epithelium, CK8 and E-cadherin. As assayed by PCNA staining, cultured and uncultured oviducts exhibited similar TEC proliferation rates (Supplemental Figure 1). Cultured oviducts continued to express oviductin (OVGP1), a secreted glycoprotein characteristic of the oviduct (Umezu, et al. 2003). A subpopulation of TEC from cultured and uncultured oviducts stained positively for nuclear expression of Pax8, a marker of secretory cells in the oviduct, while a separate subpopulation of TEC stained positively for acetylated tubulin, a marker of ciliated cells.
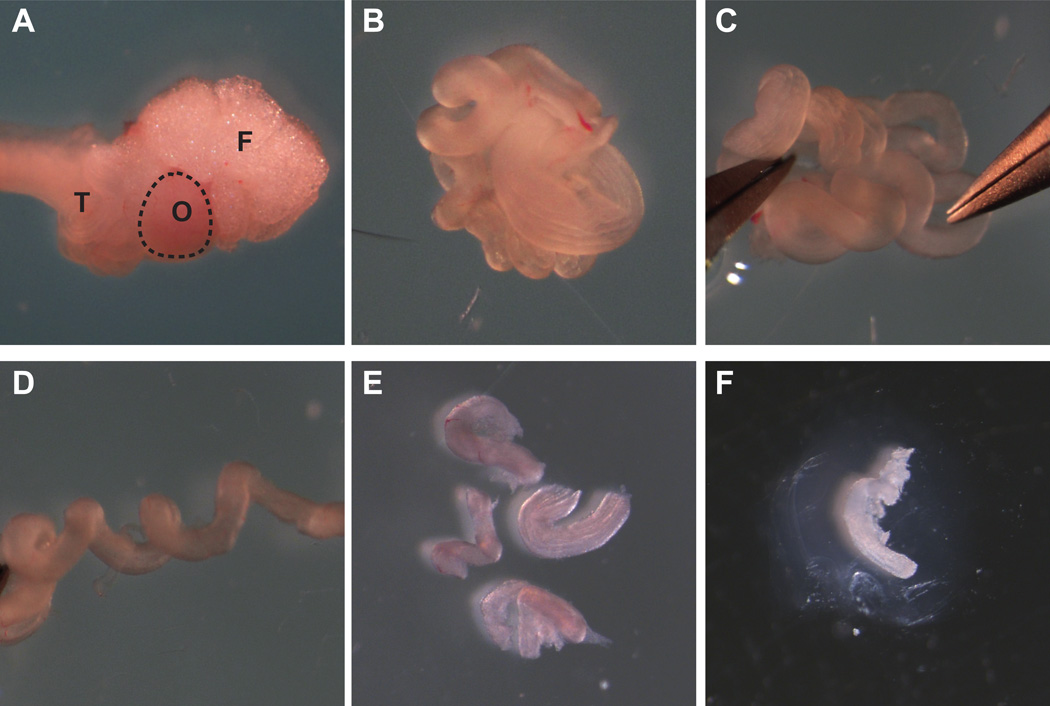
Figure 2. Establishment of a three-dimensional mouse oviduct culture system.
(A) Ovary with fat pad and bursal membrane, oviduct, and part of the uterine horn was dissected from sexually immature CD1 mice. F, fat pad; O, ovary; T, oviduct. (B) Ovary and uterine horn were dissected away, leaving oviduct. (C and D) Using forceps, oviduct was gently uncoiled. (E) Each oviduct was cut into 4 pieces with a scalpel. (F) Each segment of oviduct was embedded in alginate hydrogel and cultured for 7 days.
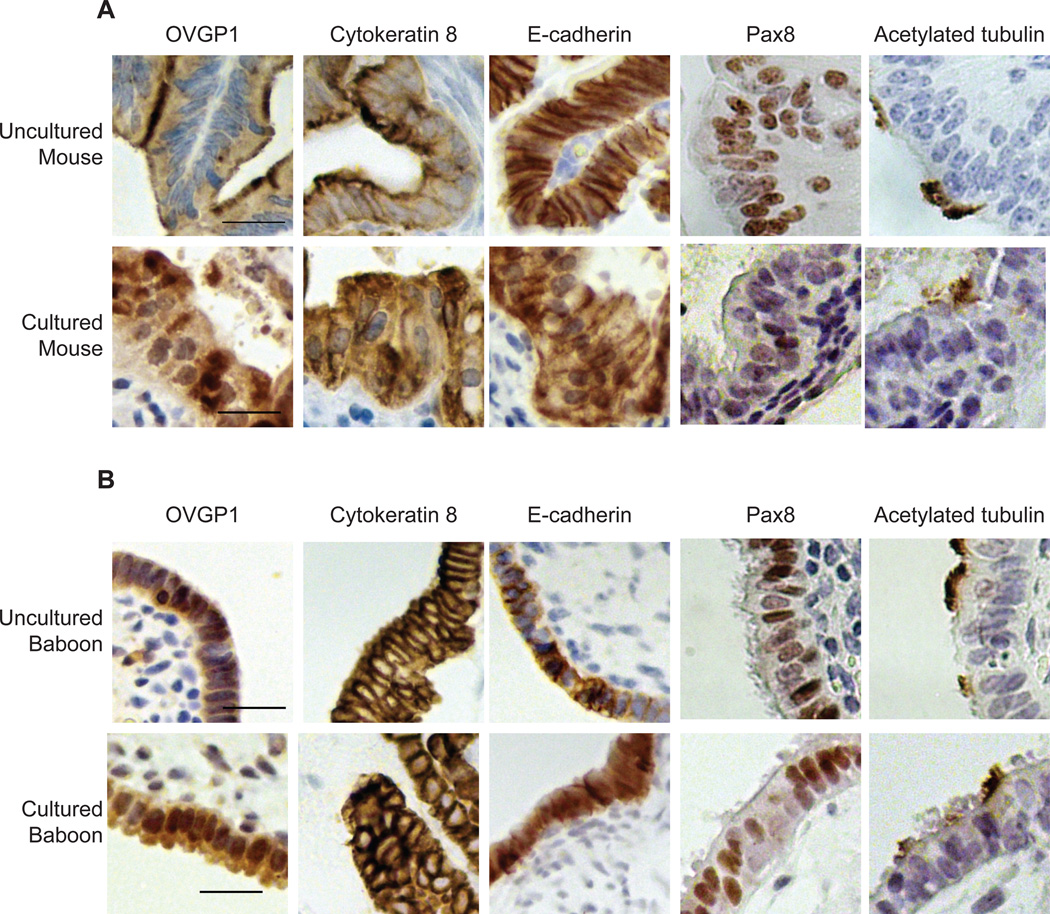
Figure 3. Verification of a three-dimensional mouse oviduct and baboon fimbria organ culture system.
(A) Tissues from uncultured mouse oviducts (top panel) or oviducts cultured for 7 days (bottom panel) were processed for immunohistochemistry and stained with antibodies against OVGP1 (oviductin, tubal epithelium), CK8 (simple epithelium), and E-cadherin (tubal epithelium). Serial sections were stained for Pax8 (secretory tubal epithelium) and acetylated tubulin (ciliated tubal epithelium). (B) Tissues from uncultured baboon fimbriae (top panel) or fimbriae cultured for 7 days (bottom panel) were processed for immunohistochemistry and stained with antibodies against OVGP1, CK8, and E-cadherin. Serial sections were stained for Pax8 and acetylated tubulin. Calibration bars, 20 µm.
In primates, the TEC of the distal fimbriae of the fallopian tube are comparable to the TEC of the mouse oviduct (Critoph and Dennis 1977; Komatsu and Fujita 1978). However, TEC are found on the outer surface of the fimbriae and are in contact with the ovary and face the peritoneal cavity, rather than facing the lumen of the oviduct as in mice. To account for these anatomical differences between mice and primates, a three-dimensional culture system for distal baboon fimbriae was developed using the alginate hydrogel method described above (Jackson et al. 2009). Adult female baboons (n=3) were sacrificed and individual fimbria were placed into alginate hydrogel droplets and cultured for 7 d. Cultured and uncultured fimbriae were evaluated by immunohistochemistry for markers of secretory and ciliated cells (Figure 3b and Supplemental Figure 1). Fimbria cultures retained markers of TEC (CK8, E-cadherin), oviductin expression, and similar levels of cell proliferation (PCNA). A subpopulation of baboon fimbriae TEC were positive for expression of acetylated tubulin, while other TEC were positive for expression of Pax8, a marker of secretory cells. Therefore, the three-dimensional organ culture system for mouse oviduct and baboon fallopian tube fimbriae retains markers of the cell types normally present in these tissues.
Immortalization of baboon fimbriae TEC allows co-culture of secretory and ciliated cells
An immortalized baboon fimbriae TEC cell line (TEC40; tubal epithelial cells immortalized with SV40) was generated to observe the effects of individual aspects of ovulation on cultured TEC. Development of a two-dimensional immortalized TEC cell line allows for comparison of immortalized TEC to immortalized OSE cells (IOSE cells) and to previous studies using traditional two-dimensional culture on plastic of normal and cancer cell lines. TEC40 cells retained an epithelial morphology and were analyzed for retention of ciliated and secretory cell markers by immunofluorescence at passage number 16, after several weeks in culture (Figure 4a–f). As demonstrated in the baboon fimbriae organ culture system, TEC40 retained the epithelial cell markers CK8, E-cadherin, and expression of the secreted glycoprotein OVGP1. A subset of cells was positive for Pax8, while another subset was positive for acetylated tubulin. TEC40 exhibited immunofluorescence staining for SV40 T/t, indicating that the cultured cells were immortalized by expression of the viral oncogenes that disrupt p53 and retinoblastoma.
Figure 4. TEC40 tubal epithelium cell line retains markers of secretory and ciliated cells.
Immortalized TEC40 were plated into 8-well chamber slides, fixed, and analyzed by immunofluorescence for epithelial cell markers OVGP1, CK8, and E-cadherin (A–C), secretory cell marker Pax8 (D), and ciliated cell marker acetylated tubulin (E) as well as for expression of SV40 T/t antigen (F). Calibration bar, 10 µm. (G) TEC40 cells treated with FSH or LH for 24 h were analyzed for expression of pERK1/2 or actin as a loading control by western blotting. (H) TEC40 cells were analyzed for activation of ERE-luciferase construct relative to activation of β-galactosidase reporter construct in response to estradiol or cotransfection of ERα. Data represents average ±SEM. * indicates P<0.05.
To determine if TEC40 were responsive to FSH and LH signaling, cells were treated with cycling or menopausal levels of hormones and analyzed for expression of phospho-MAPK (pERK1/2), which is downstream of both FSH and LH signaling in OSE and granulosa cells (Choi, et al. 2009; Choi, et al. 2002). Cells treated with FSH or LH showed a dose-dependent accumulation of phospho-MAPK (pERK1/2), indicating that FSH and LH signaling is present in TEC40 cells (Figure 4g). Additionally, transcripts for FSHR and LHR were detected in TEC40 cells (Supplemental Figure 2).
To determine if TEC40 cells were responsive to estradiol, cells were co-transfected with pCMV Sport-βgal and ERE-luciferase reporter plasmids, with or without co-expression of a plasmid expressing ERα. TEC40 exhibited no significant change in activation of the ERE-luciferase reporter relative to the β-galactosidase reporter when cells were treated with 50 nM estradiol. This corresponds to a lack of expression of ERα in TEC40 cells as assayed by RT-PCR (Supplemental Figure 2). When ERα was overexpressed, TEC40 cells exhibited approximately 4-fold induction of the ERE-luciferase reporter (Figure 4h).
TEC do not proliferate in response to FSH, LH, or estradiol
The mouse oviduct and baboon fimbriae organ culture systems were used to evaluate the effects of FSH, LH, and estradiol on TEC proliferation in vitro. For mouse oviduct cultures, no significant increase in proliferation was seen in response to FSH, LH, or estradiol compared to BSA or FBS controls. Cycling levels of FSH or estradiol suppressed TEC cell proliferation (Figure 5a). For baboon fimbriae cultures, no significant difference in proliferation was seen for tissues cultured with FSH, LH, or estradiol (Figure 5b). To verify the presence of FSHR, LHR, and ERα in these tissues, RNA was extracted from adult mouse ovary as a positive control, d16 mouse oviduct, and adult baboon fimbriae and subjected to RT-PCR. Transcripts for FSHR, LHR, and ERα were detected in the mouse ovary and oviduct and the baboon fimbriae, though FSHR shows weak expression in mouse oviduct (Supplemental Figure 2).
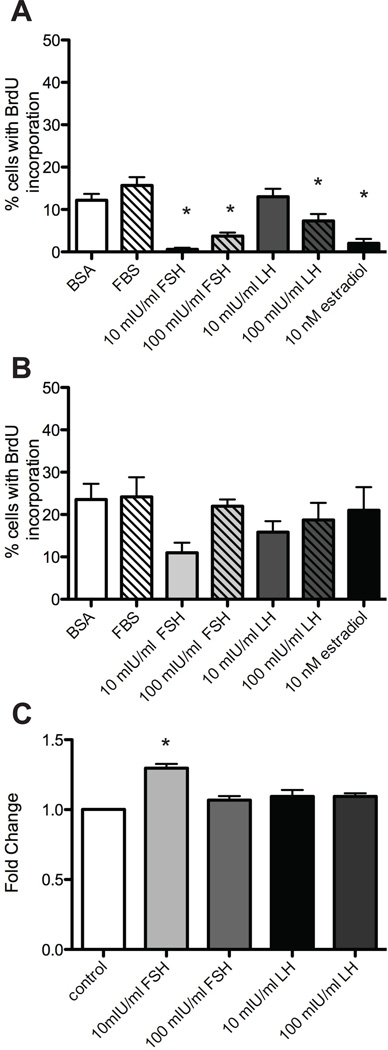
Figure 5. Gonadotropins do not increase proliferation in TEC.
(A and B) After 7 days in culture medium containing 10% FBS, 3 mg/ml BSA, cycling levels (10 mIU/ml) or menopausal levels (100 mIU/ml) of FSH or LH, or 10 nM estradiol, tissues were processed for immunohistochemistry. Serial sections were stained with antibodies against CK8 and BrdU and the percentage of CK8-positive TEC with BrdU incorporation was scored relative to the total number of CK8-positive TEC. (A) Cell proliferation in mouse oviduct culture. (B) Cell proliferation in baboon fimbriae culture. (C) TEC40 cells were cultured with cycling levels (10 mIU/ml) or menopausal levels (100 mIU/ml) of the gonadotropins FSH and LH for 3 days. Proliferation in response to hormones was measured by MTS assay for viability. Data represents average fold change ± SEM compared to control. * indicates P<0.05.
Since TEC40 cells were responsive to FSH and LH, but not estradiol (Figure 4g and h), TEC40 cells were analyzed for proliferation in response to cycling and menopausal levels of FSH and LH. After 4 d in culture, cells cultured in cycling (10mIU/ml) levels of FSH showed a slight, but statistically significant increase in proliferation (Figure 5c). No difference in proliferation was observed at higher concentrations of FSH or LH.
Ovulation induces inflammation and oxidative stress in vivo
As ovulation did not stimulate TEC proliferation in vivo through a tear-and-repair process (Figure 1), and menopausal levels of gonadotropins did not induce proliferation in either mouse oviduct organ culture, baboon fimbriae organ culture, or immortalized TEC40 cells (Figure 5), ovulation-induced inflammation in TEC was evaluated. Tissues from control mice or ovulated mice were analyzed for nuclear phospho-γH2A.X foci 12 h or 16 h after hCG injection (Figure 6a). Phospho-γH2A.X is a histone protein incorporated at sites of double-strand DNA breaks, which are frequently formed from exposure to oxidative damage (Bartkova, et al. 2005). In control animals, 6.5% of TEC exhibited phospho-γH2A.X foci compared to 20.0% of TEC from ovulated animals at 12 h post-hCG and 16.3% of TEC from ovulated animals at 16 h post-hCG (Figure 6b). No difference in proliferation rates were observed in TEC from ovulated animals at 12 h compared to 16 h post-hCG (data not shown).
Figure 6. Ovulation induces double strand DNA breaks in vivo and increases oviduct-associated macrophages.
(A) Oviducts from basal or ovulated mice (12 h or 16 h post-hCG) were sectioned and analyzed by immunohistochemistry for CK8 (red), phospho-γH2A.X (green), and DAPI (blue). Calibration bars, 10 µm. Lower panels show CK8 staining (brown), which indicates the location of TEC cells. Upper panels are higher magnification of boxed area in lower panel. L, lumen of oviduct; P, periphery of oviduct. Yellow arrows point to damaged nuclei. (B) Positive cells were scored as CK8-positive TEC cells with nuclear phospho-γH2A.X nuclear foci, relative to the total number of CK8-positive TEC cells. Data represents average fold change ± SEM compared to control. Significant differences (P<0.05) are between groups labeled a and b. (C) Oviducts from basal or ovulated mice (12 h or 16 h post-hCG) were sectioned and analyzed by immunohistochemistry for F4/80 macrophage glycoprotein (brown staining indicates antigen). COC, cumulus oocyte complex. Top panel, total magnification 400×. Bottom panel, total magnification 200×. Calibration bars, 20 µm.
It is possible that the DNA damage observed in vivo in response to ovulation may be due to the inflammatory reaction associated with ovulation. Macrophage infiltration into human fallopian tube mucosa has previously been demonstrated in response to ovulation, and macrophages are known to secrete pro-inflammatory mediators (Gaytan, et al. 2007). Therefore, oviducts from control or ovulated mice were analyzed for expression of F4/80 antigen, a surface glycoprotein that is expressed by macrophages during maturation and activation in response to inflammation (Austyn and Gordon 1981; Robinson-Smith, et al. 2007). Control animals showed no macrophage infiltration, while macrophages were found at the periphery of oviducts of ovulated animals at 12 h after hCG injection (Figure 6c). At 16 h after hCG injection, macrophages were found at the periphery of oviducts, adjacent to cumulus-oocyte complexes, and associated with the tubal epithelium. Therefore, macrophage infiltration and phospho-γH2A.X foci are detected in the oviduct after ovulation in vivo.
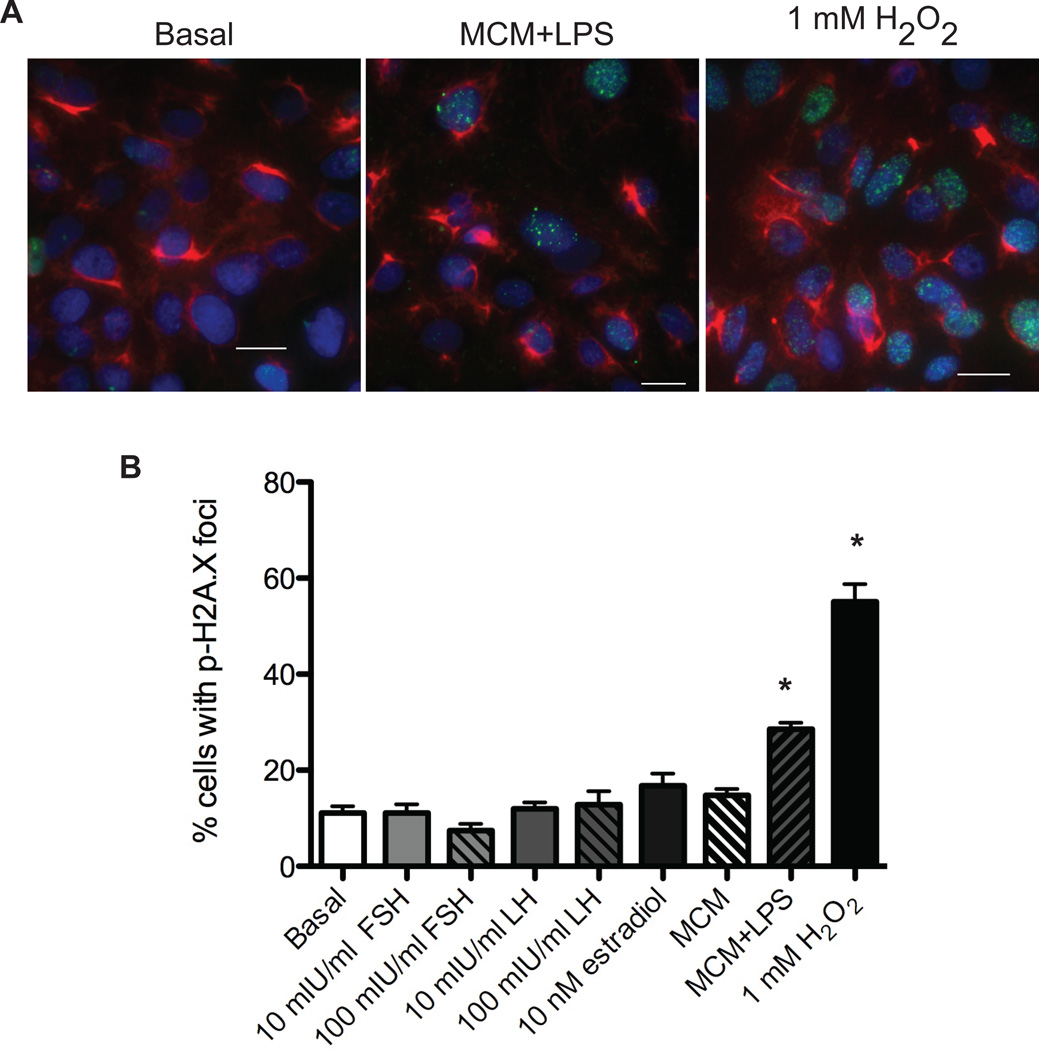
To determine which aspect of ovulation correlates with increased DNA damage, TEC40 cells were treated with cycling or menopausal levels of FSH or LH, or 10 nM estradiol to examine the impact of hormonal components of ovulation on DNA damage. To analyze the effects of oxidative stress associated with inflammation, 1mM hydrogen peroxide, macrophage-conditioned media from RAW264.7 cells (MCM), or MCM from RAW264.7 cells stimulated with E. coli lipopolysaccharide (MCM+LPS) was added to TEC40 cells. Cells were analyzed for the presence of phospho-γH2A.X foci after treatment with basal media, hormone, hydrogen peroxide, MCM, or MCM+LPS (Figure 7). In response to hydrogen peroxide or MCM+LPS, a significant increase in DNA damage was observed.
Figure 7. Oxidative stress induces double strand DNA breaks in TEC40 cells.
TEC40 cells were plated into 8-well chambers slides and incubated with serum-free medium, serum-free medium containing cycling concentrations (10 mIU/ml) or menopausal concentrations (100 mIU/ml) of FSH or LH, 10 nM estradiol, 1 mM hydrogen peroxide, MCM, or MCM+LPS for 20 minutes. (A) Cells were analyzed by immunofluorescence for CK8 (red), phospho-γH2A.X (green), and DAPI (blue). Calibration bars, 20 µm. (b) Positive cells were scored as those that exhibited phospho-γH2A.X nuclear foci, relative to the total number of TEC40 cells as analyzed by DAPI staining. Data represents average fold change ± SEM compared to basal. * indicates P<0.05.
Discussion
In addition to a family history of breast and ovarian cancer, a primary risk factor for serous ovarian cancer is an increased number of lifetime ovulations (Auersperg, et al. 1997). While the OSE is a commonly accepted site of origin for many histotypes of ovarian cancer, emerging evidence suggests that the TEC may be an alternative source for high-grade serous cancer (Crum, et al. 2007b). The goal of this study was to examine the impact of ovulation on TEC by evaluating three commonly accepted hypotheses regarding the origin of ovarian cancer – the tear and repair hypothesis, the gonadotropin hypothesis, and the inflammation hypothesis. Despite well-documented increases in OSE proliferation in the CD1 mouse strain 12 hr post-hCG injection (Burdette et al. 2006), TEC in the present study did not proliferate in response to ovulation in vivo, at either 12 hr or 16 hr post-hCG injection. However, it is possible that TEC proliferation may occur at a more distant time point following ovulation. Although no increase in TEC proliferation was observed at these early time points following ovulation, heightened DNA damage and macrophage infiltration was detected.
In order to examine the components of ovulation that may contribute to fallopian tube neoplasia, such as the influence of gonadotropins and inflammatory factors on TEC proliferation and DNA damage, novel tools were developed. Using an alginate hydrogel matrix, sections of mouse oviduct or baboon distal fimbriae were cultured in a three-dimensional organ culture system, allowing for precise control of hormones or growth factors in contact with TEC. This system offers certain advantages over traditional cell culture, including retention of the underlying stromal tissue, and culture of non-transformed, non-immortalized cells. However, luminal structures are particularly sensitive to constriction within an alginate matrix, as has been observed for follicular antrum formation (West-Farrell, et al. 2009; Xu, et al. 2006). Compared to uncultured mouse oviducts, those cultured in alginate for 7 d showed a reduction in the thickness of the epithelial layer lining the interior of the oviduct, which likely reflects compression of the oviductal lumen due to growth within the alginate gel. Despite this compression, the cultured mouse oviducts showed similar rates of proliferation of TEC as observed in uncultured oviducts and retained both secretory and ciliated epithelium, with the highest proportion of ciliated cells found in sections of the oviduct closest to the ovary (infundibulum and ampulla) and decreasing towards the uterus (isthmus). The present study also utilized baboon fallopian tube epithelium, which is structurally similar to human tissue with the TEC on the outer surface of the tissue. As a corollary to the organ culture system, an immortalized baboon fallopian tube cell line was generated that retained both secretory and ciliated TEC, allowing for comparative analysis of the effects of the gonadotropins on cell growth on two-dimensional plastic versus three-dimensional organ culture, as well as a renewable source of cells for studies on downstream signaling pathways and neoplastic transformation in TEC. The TEC40 cells were immortalized with SV40, which allows direct comparison to the IOSE cell lines which were generated in a similar manner (Auersperg, et al. 1994; Karst, et al. 2011). Similarly to the IOSE cells and immortalized human TEC, the TEC40 cells do not form colonies in soft agar (data not shown), indicating that they could be used to model early transformative events (Karst et al. 2011).
Using three-dimensional organ culture to evaluate the gonadotropin hypothesis of ovarian cancer demonstrated that although FSH and LH at the tonically high levels observed during menopause induce proliferation of OSE, no increase in proliferation or DNA damage was detected in TEC, despite a common embryonic origin for these cells in the coelomic epithelium (Auersperg 2011; Auersperg et al. 2008). Transcripts for FSHR, LHR, and ERα were detected in the mouse oviduct and baboon fimbriae, indicating that signaling components responsive to FSH, LH, and estradiol are present in the tissues. Interestingly, in the mouse oviduct cultures the gonadotropins suppressed proliferation of TEC, which may be due to a lack of growth factors in the media permitting analysis of the isolated effects of each hormone, whereas in vivo, the cells are exposed to a variety of cytokines, growth factors, and hormones, resulting in a slightly higher basal rate of proliferation. At cycling levels, FSH caused a significant increase in TEC40 cell proliferation. Although these cells are morphologically normal, expression of SV40 T/t antigen disrupts p53 and retinoblastoma, leading to genetic changes in the cells that may allow for potential alterations in FSH-mediated signaling. The TEC40 cells exhibited little or no expression of ERα, which may also be reflective of altered signaling related to immortalization of the cells with SV40 or loss of receptor in long-term culture.
Oviduct TEC expressed increased levels of phospho-γH2A.X, a marker of double-strand DNA breaks, after ovulation at 12 hr and 16 hr after administration of hCG. Using the oviduct and fimbriae organ culture systems to determine if FSH or LH caused DNA damage revealed no increase in phospho-γH2A.X foci relative to controls as expected, as FSH and LH have not been shown to generate reactive oxygen radicals. Metabolites of estradiol have been shown to generate reactive oxygen species (ROS), increase inflammation, and play a role in progression of hormone-responsive cancers (Straub 2007). Postmenopausal estrogen therapy has been correlated with an increased risk of ovarian cancer, but the effect of estrogens on TEC is unknown (Ness, et al. 2002). However, treatment of TEC with estradiol did not increase proliferation or DNA damage in culture after 7 d. Hydrogen peroxide has been frequently used to model oxidative stress generated by ovulation (Murdoch et al. 2001; Symonds, et al. 2008). TEC40 cells treated with hydrogen peroxide or MCM+LPS exhibited increased levels of phospho-γH2A.X foci, providing a common lesion in cell lines exposed to oxidative stress and in TEC in vivo after ovulation. Induction of H2A.X foci in response to oxidative stress was not likely a result of the altered p53 and Rb status of the TEC40 cells, as OVCA420 cells, which express wild type p53 and Rb, exhibited similar levels of DNA damage in response to hydrogen peroxide or MCM+LPS (data not shown).
Ovulation caused an increase of activated macrophages associated with the oviduct, and suggests one potential mechanism for how cells proximal to the site of ovulation may be susceptible to DNA damage. At 12 h post-hCG administration, activated macrophages were found primarily at the periphery of oviducts, likely associated with blood vessels, where they may be available for extravasation into the tubal epithelium. At 16 h post-hCG, examination of oviducts showed the presence of cumulus-oocyte complexes within the oviductal lumen, and activated macrophages were observed adjacent to and in contact with tubal epithelium near sites of ovulated oocytes. Macrophages associate with cumulus-oocyte complexes (Akkoyunlu, et al. 2003), which together with the expelled follicular fluid from the site of ovulation may contain reactive oxidants that act on the tubal epithelium. The cumulus-oocyte complexes in the oviduct may release cytokines that promote further invasion of the macrophages into the tubal epithelium from local blood vessels (Zolti, et al. 1991). Through release of cytokines and increase in cyclooxygenase (COX) enzymes associated with leukocyte infiltration into the ovary, ovulation increases levels of reactive oxidants, leading to formation of DNA adducts, base modifications, and strand breaks (Croteau and Bohr 1997; Jabbour, et al. 2009; Murdoch 2008). This is the first study to show that ovulation can influence DNA damage in the TEC, perhaps due to the close proximity to the fallopian tube epithelium of the ovary and inflammatory factors released during ovulation. Further studies are needed to conclusively determine if inflammation following ovulation causes DNA damage in the fallopian tube.
TEC are susceptible to DNA damage following ovulation, but the fate of the damaged cells is unknown. Although little or no apoptosis was observed in TEC following ovulation (data not shown), it is likely that some or all of the DNA damage is repaired. This may be reflected in a lack of increase in the percentage of TEC exhibiting H2A.X foci at 12 hr post-hCG compared to 16 hr post-hCG, despite an increase in macrophage association with the TEC. It is possible that by 16 hr after hCG treatment, TEC have begun to repair the double strand DNA breaks observed following ovulation. Cells with unrepaired double strand DNA breaks or DNA replication errors as a result of oxidative damage at critical sites, such as in tumor suppressor genes, may serve as progenitor cells for neoplastic lesions (Schildkraut, et al. 2010). It is possible that the OSE, which undergoes cyclic remodeling during monthly ovulations, is better equipped to process cells damaged in response to ovulation as compared to TEC. Investigation into the pathways necessary to repair DNA strand breaks may provide valuable information regarding the mechanisms of p53 protein regulation as part of the tubal “precursor signature” and how mutations in BRCA increase the risk of high grade serous cancer.
In summary, ovulation impacts the fallopian tube epithelium as well as the OSE, lending support to the correlation between a reduction in the number of lifetime ovulations and a reduced risk of ovarian cancer. Ovulation and its associated hormones did not increase proliferation or directly induce DNA lesions using novel organ culture systems and a newly generated tubal cell line. However, in vivo ovulation induced macrophage infiltration into the fallopian tube epithelium and enhanced DNA damage in the TEC similar to that observed with reactive oxidants in vitro. This study examines the three major hypotheses connecting a long-standing correlation between ovulation and the risk of ovarian cancer in light of emerging evidence suggesting that the fallopian tube epithelium may be a site of origin for serous carcinomas.
Supplementary Material
Acknowledgements
This work was supported by BIRCWH grant K12HD055892, NIH grant R03CA139492, Ovarian Cancer Research Fund Liz Tilberis LT/UIC/01.2011, UIC Cancer Center Grant, and UIC Center for Clinical and Translational Science grant to JEB. This research was supported by Eunice Kennedy Shriver NICHD/NIH grant 5U54HD040093-10 to ATF as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
SMK carried out immunohistochemistry and immunofluorescence analyses, participated in chemically-induced ovulation studies, carried out organ culture studies, performed western blot analyses and RT-PCR, and drafted the manuscript. TSH carried out MTS analyses and immunofluorescence analyses. LYW participated in immunohistochemistry analyses. RCJ performed luciferase analysis of TEC40 cells and critical reading of the manuscript. ATF provided baboon tissue and critical reading and revision of the manuscript. JEB conceived of the study design, participated in its design and coordination, and was involved in manuscript preparation and revision. All authors read and approved the final manuscript.
References
- Akkoyunlu G, Korgun ET, Celik-Ozenci C, Seval Y, Demir R, Ustunel I. Distribution patterns of leucocyte subpopulations expressing different cell markers in the cumulus-oocyte complexes of pregnant and pseudopregnant mice. Reprod Fertil Dev. 2003;15:389–395. doi: 10.1071/RD03037. [DOI] [PubMed] [Google Scholar]
- Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, et al. SEER Cancer Statistics Review, 1975–2007. 2010 [Google Scholar]
- An Y, Liu K, Zhou Y, Liu B. Salicylate inhibits macrophage-secreted factors induced adipocyte inflammation and changes of adipokines in 3T3-L1 adipocytes. Inflammation. 2009;32:296–303. doi: 10.1007/s10753-009-9135-1. [DOI] [PubMed] [Google Scholar]
- Auersperg N. The origin of ovarian carcinomas: a unifying hypothesis. Int J Gynecol Pathol. 2011;30:12–21. doi: 10.1097/PGP.0b013e3181f45f3e. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Maines-Bandiera SL, Dyck HG. Ovarian carcinogenesis and the biology of ovarian surface epithelium. J Cell Physiol. 1997;173:261–265. doi: 10.1002/(SICI)1097-4652(199711)173:2<261::AID-JCP32>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Maines-Bandiera SL, Dyck HG, Kruk PA. Characterization of cultured human ovarian surface epithelial cells: phenotypic plasticity and premalignant changes. Lab Invest. 1994;71:510–518. [PubMed] [Google Scholar]
- Auersperg N, Ota T, Mitchell GW. Early events in ovarian epithelial carcinogenesis: progress and problems in experimental approaches. Int J Gynecol Cancer. 2002;12:691–703. doi: 10.1046/j.1525-1438.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Woo MM, Gilks CB. The origin of ovarian carcinomas: a developmental view. Gynecol Oncol. 2008;110:452–454. doi: 10.1016/j.ygyno.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2):S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- Burdette JE, Kurley SJ, Kilen SM, Mayo KE, Woodruff TK. Gonadotropin-induced superovulation drives ovarian surface epithelia proliferation in CD1 mice. Endocrinology. 2006;147:2338–2345. doi: 10.1210/en.2005-1629. [DOI] [PubMed] [Google Scholar]
- Cass I, Holschneider C, Datta N, Barbuto D, Walts AE, Karlan BY. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstet Gynecol. 2005;106:1327–1334. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- Choi JH, Chen CL, Poon SL, Wang HS, Leung PC. Gonadotropin-stimulated epidermal growth factor receptor expression in human ovarian surface epithelial cells: involvement of cyclic AMP-dependent exchange protein activated by cAMP pathway. Endocr Relat Cancer. 2009;16:179–188. doi: 10.1677/ERC-07-0238. [DOI] [PubMed] [Google Scholar]
- Choi KC, Kang SK, Tai CJ, Auersperg N, Leung PC. Follicle-stimulating hormone activates mitogen-activated protein kinase in preneoplastic and neoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2002;87:2245–2253. doi: 10.1210/jcem.87.5.8506. [DOI] [PubMed] [Google Scholar]
- Critoph FN, Dennis KJ. The cellular composition of the human oviduct epithelium. Br J Obstet Gynaecol. 1977;84:219–221. doi: 10.1111/j.1471-0528.1977.tb12559.x. [DOI] [PubMed] [Google Scholar]
- Croteau DL, Bohr VA. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J Biol Chem. 1997;272:25409–25412. doi: 10.1074/jbc.272.41.25409. [DOI] [PubMed] [Google Scholar]
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007a;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007b;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- Gaytan M, Morales C, Bellido C, Sanchez-Criado JE, Gaytan F. Macrophages in human fallopian tube and ovarian epithelial inclusion cysts. J Reprod Immunol. 2007;73:66–73. doi: 10.1016/j.jri.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Hakim AA, Barry CP, Barnes HJ, Anderson KE, Petitte J, Whitaker R, Lancaster JM, Wenham RM, Carver DK, Turbov J, et al. Ovarian adenocarcinomas in the laying hen and women share similar alterations in p53, ras, and HER-2/neu. Cancer Prev Res (Phila Pa) 2009;2:114–121. doi: 10.1158/1940-6207.CAPR-08-0065. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138:903–919. doi: 10.1530/REP-09-0247. [DOI] [PubMed] [Google Scholar]
- Jackson KS, Inoue K, Davis DA, Hilliard TS, Burdette JE. Three-dimensional ovarian organ culture as a tool to study normal ovarian surface epithelial wound repair. Endocrinology. 2009;150:3921–3926. doi: 10.1210/en.2008-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe RC, Donnelly KM, Fazleabas AT. The induction of baboon glycodelin expression by progesterone is not through Sp1. Mol Hum Reprod. 2003;9:35–40. doi: 10.1093/molehr/gag008. [DOI] [PubMed] [Google Scholar]
- Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, Lee Y, Crum CP. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008a;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- Jarboe EA, Folkins AK, Drapkin R, Ince TA, Agoston ES, Crum CP. Tubal and ovarian pathways to pelvic epithelial cancer: a pathological perspective. Histopathology. 2008b;53:127–138. doi: 10.1111/j.1365-2559.2007.02938.x. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A. 2011;108:7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Quartuccio S, Hilliard TS, Inoue K, Burdette JE. Alginate Hydrogels for Three-Dimensional Organ Culture of Ovaries and Oviducts. J Vis Exp. 2011 doi: 10.3791/2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Fujita H. Electron-microscopic studies on the development and aging of the oviduct epithelium of mice. Anat Embryol (Berl) 1978;152:243–259. doi: 10.1007/BF00350523. [DOI] [PubMed] [Google Scholar]
- Konishi I, Kuroda H, Mandai M. Review: gonadotropins and development of ovarian cancer. Oncology. 1999;57(Suppl 2):45–48. doi: 10.1159/000055274. [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch WJ. Ovulatory factor in ovarian carcinogenesis. Adv Exp Med Biol. 2008;622:119–128. doi: 10.1007/978-0-387-68969-2_10. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Townsend RS, McDonnel AC. Ovulation-induced DNA damage in ovarian surface epithelial cells of ewes: prospective regulatory mechanisms of repair/survival and apoptosis. Biol Reprod. 2001;65:1417–1424. doi: 10.1095/biolreprod65.5.1417. [DOI] [PubMed] [Google Scholar]
- Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, Purdie DM, Risch HA, Vergona R, Wu AH. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155:217–224. doi: 10.1093/aje/155.3.217. [DOI] [PubMed] [Google Scholar]
- Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, McFarland-Mancini MM, Drew AF. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 2007;67:5708–5716. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Press; 1989. [Google Scholar]
- Schildkraut JM, Iversen ES, Wilson MA, Clyde MA, Moorman PG, Palmieri RT, Whitaker R, Bentley RC, Marks JR, Berchuck A. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;5:e10061. doi: 10.1371/journal.pone.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully RE. Early de novo ovarian cancer and cancer developing in benign ovarian lesions. Int J Gynaecol Obstet. 1995;49(Suppl):S9–S15. doi: 10.1016/0020-7292(95)02404-z. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Symonds DA, Merchenthaler I, Flaws JA. Methoxychlor and estradiol induce oxidative stress DNA damage in the mouse ovarian surface epithelium. Toxicol Sci. 2008;105:182–187. doi: 10.1093/toxsci/kfn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino LS, Giles JR, Wang W, Urick ME, Johnson PA. Gene Expression Profiling Reveals Differentially Expressed Genes in Ovarian Cancer of the Hen: Support for Oviductal Origin? Hormones and Cancer. 2010;1:177–186. doi: 10.1007/s12672-010-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu T, Hanazono M, Aizawa S, Tomooka Y. Characterization of newly established clonal oviductal cell lines and differential hormonal regulation of gene expression. In Vitro Cell Dev Biol Anim. 2003;39:146–156. doi: 10.1007/s11626-003-0009-9. [DOI] [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80:432–439. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Zhang M, Shi H, Segaloff DL, Van Voorhis BJ. Expression and localization of luteinizing hormone receptor in the female mouse reproductive tract. Biol Reprod. 2001;64:179–187. doi: 10.1093/biolreprod/64.1.179. [DOI] [PubMed] [Google Scholar]
- Zheng W, Magid MS, Kramer EE, Chen YT. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am J Pathol. 1996;148:47–53. [PMC free article] [PubMed] [Google Scholar]
- Zolti M, Ben-Rafael Z, Meirom R, Shemesh M, Bider D, Mashiach S, Apte RN. Cytokine involvement in oocytes and early embryos. Fertil Steril. 1991;56:265–272. doi: 10.1016/s0015-0282(16)54483-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.