Abstract
Romidepsin is the second histone deacytelase inhibitor (HDACi) approved for the treatment of advanced stages of cutaneous T cell lymphoma (CTCL). Recent in vitro data suggests that HDACi suppress immune function although these findings have not been confirmed in patients. Thus, we serially examined the cellular immune function of eight CTCL patients undergoing treatment with three cycles of romidepsin. We measured the patient’s natural killer (NK) and dendritic cell (DC) function, and performed an in vitro TUNEL assay to measure cellular apoptosis. Patients’ NK cell cytolytic activity decreased from baseline to the third cycle of treatment (P=.018) but stimulation with a TLR agonist increased this activity (P=.018). At baseline a TLR agonist could both activate patients’ DC (P=.043) and stimulate IL-12 protein production (P=.043) but both were suppressed after the first cycle of romidepsin. Finally, we observed increased specificity for romidepsin induced CD4+ tumor cell apoptosis and dose dependent increases in cellular apoptosis of healthy cells in multiple lineages (P<.05). These findings raise concern that HDACi suppress immune function in CTCL patients and support the concurrent use of multiple immune stimulatory agents to preserve the host immune response.
Keywords: Histone deacetylase inhibitor, cutaneous T-cell lymphoma, romidepsin
Introduction
Cutaneous T cell lymphomas (CTCL) are a heterogenous group of clonal proliferative disorders of skin-trafficking T cells. Sézary syndrome (SS), the leukemic variant of CTCL, is characterized by diffuse skin involvement (erythroderma), lymphadenopathy, and circulating malignant T cells.[1, 2] SS patients have immunologic defects that increase with the severity of their disease and are typified by profoundly impaired Th1 responses and diminished cell mediated immunity. Patients have decreased production of Th1-type cytokines such as interferon-γ (IFN-γ) and interleukin-2 (IL-2) along with decreased natural killer (NK) cell and CD8+ T cell activity.[3] These impairments are partially caused by increased production of IL-4, IL-5, and IL-10 by the malignant T cells, which inhibit Th1 cytokine production, and skew the patients’ immune system towards a Th2 response.[4–7] Moreover, it has been observed that patients have decreased numbers of dendritic cells (DCs) as well as defects in the production of DC derived Th1 stimulatory cytokines, such as IL-12 and IFN- α.[8]
There is substantial evidence that preserving the patient’s immune response is critical to effectively controlling the progression of CTCL at all stages of disease.[9, 10] Multi-modality immune therapy designed to boost the patient’s cell-mediated immunity and anti-tumor response has been associated with a high clinical response rate and, in some cases, a durable remission for patients with advanced leukemia.[11, 12] Treatment of CTCL patients with immune stimulatory cytokines that patients have difficulty producing, such as IFN-α, IFN-γ and recombinant IL-12, has been shown to be clinically efficacious.[13] The efficacy of these cytokines alone or in combination supports using therapies that activate multiple populations of the innate immune system.[14, 15]
Advanced stages of CTCL may progress rapidly and durable remissions are difficult to achieve. Cytotoxic chemotherapy may be employed to rapidly reduce the tumor burden in these patients. Chemotherapy response rates range from 60–80% but the median duration of the response is typically measured in a few months.[16] Given this short duration of response, it is important to pursue agents with novel mechanisms of action to improve care. In 2009, romidepsin was the second histone deacetylase inhibitor (HDACi) approved for the treatment of advanced CTCL after an international multi-center pivotal trial showed that 38% of patients responded to treatment with a median duration of response of 15 months.[17, 18]
HDACis exhibit potent anti-proliferative effects on tumor cells by increasing the expression of pro-apoptotic genes and down regulating the expression of anti-apoptotic genes to induce cell cycle arrest and apoptosis.[19–21] There is also increasing evidence that HDACis impact immune responses by suppressing innate immunity. [22] This suppression affects the activation and function of dendritic and NK cells resulting in defects in adaptive immunity. [22–24]
Although the pivotal multi-center clinical trial of romidepsin did not report any increase of opportunistic infections[18], there have been multiple case reports of DNA virus infections in association with HDACi treatment.[25, 26] In vitro experiments have also shown that HDACis enhance human regulatory T cell (Treg) suppressive function and increase the proportion of CTLA4hi Tregs in suppression assays.[27] Consistent with these findings, HDACis have shown therapeutic benefit in animal models of autoimmune diseases and we previously reported the rapid resolution of an autoimmune blistering disorder in a CTCL patient treated with the HDACi vorinostat.[28–32]
As an intact immune response is important to effectively control CTCL, we sought to measure romidepsin’s effects on the immune function of CTCL patients. We collected serial blood samples prior to and during three months of treatment with IV romidepsin and measured a variety of cellular immune functions over the course of treatment. We also tested ex vivo if a toll-like receptor (TLR) 7/8 agonist, which broadly stimulates the immune response, could activate these patients’ remaining immune cells. Our results provide new evidence that romidepsin suppresses cell-mediated immunity in CTCL patients and that multi-modality treatment, with immune-stimulating agents, may improve clinical outcomes by sustaining cellular immunity.
Materials and methods
Patients
Sézary syndrome (SS) patients were diagnosed on the basis of clinical, histopathologic and immunohistologic criteria.[33] Flow cytometric analysis of peripheral blood samples with assessment of numbers of CD4+/CD26−/CD7− cells was used to quantify the numbers of circulating malignant T cells.[34] Staging of SS patients was based on revised criteria proposed by the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) using the Tumor-Node-Metastasis-Blood (TNMB) classification.[35] Seven patients were stage IV (A or B) and one patient was stage IIB with large cell transformation in the skin. All patients were refractory to multiple systemic therapies (Table 1). Donation of peripheral blood samples by patients was undertaken according to protocols approved by the Stanford University Institutional Review Board (IRB). For the in vitro NK cell assay (Figure 2), and TUNEL assay (Figure 4), PBMC from healthy donors were collected as part of the Wistar Institute blood donation program and approved by the Wistar IRB and chosen randomly from the available donors in the Philadelphia community. All samples were collected according to the Declaration of Helsinki and written informed consent was obtained from all donors prior to sample collection.
Table 1. CTCL patients’ diagnosis, stage, and systemic therpies prior to romidepsin.
Patients’ stages are classified as tumor-node-metastasis-blood (TNMB) according to the ISCL-EORTC revisions and prior treatments are listed chronologically--beginning with the most recent.[35]
| Patient, TNMB stage, disease |
Previous systemic therapies |
|---|---|
| 1) IVA T3NXM0B2 Sezary Syndrome |
Anti-CCR4 (KW-0761) antibody |
| Denileukin diftitox | |
| Gemcitabine | |
| Forodesine | |
| Photopheresis + bexarotene + interferon-alpha | |
| 2) IVB T4N0B2M1 gastric + Sezary Syndrome |
Denileukin diftitox |
| Photopheresis + interferon-alpha | |
| Narrow band UVB phototherapy + vorinostat | |
| Romidepsin | |
| Bexarotene | |
| Cyclophosphamide, doxorubicin, vincristine, prednisone | |
| 3) IVA T3/4N3M3M0B1 Large cell transformation in lymph nodes and skin |
Anti-CCR4 (KW0761) antibody |
| Forodesine | |
| Vorinostat | |
| Bexarotene | |
| methotrexate | |
| Cyclophosphamide, doxorubicin, vincristine, prednisone | |
| 4) IVA T4N0M0B2 |
Methotrexate |
| Photopheresis + bexarotene | |
| 5) IVA T4N0M0B2 Sezary Syndrome |
Anti-CCR4 (KW-0761) antibody |
| Vorinostat | |
| Photopheresis + interferon-alpha | |
| Bexarotene | |
| 6) IIB T3N0M0B0 Large cell transformation in skin |
Anti-CCR4 (KW-0761) antibody |
| Sapacitabine | |
| Chlorambucil | |
| Denileukin diftitox | |
| Pralatrexate | |
| Forodesine | |
| Vorinostat | |
| Methotrexate | |
| Alemtuzumab | |
| photopheresis + bexarotene | |
| Alemtuzumab | |
| Bexarotene | |
| 7) IVB T4N0B2M1 bone marrow+ Sezary Syndrome |
Vorinostat |
| Anti-CCR4 (KW-0761) antibody | |
| Pralatrexate | |
| Methotrexate + interferon-alpha | |
| Forodesine | |
| CpG/XRT immunotherapy | |
| Bexarotene | |
| Psoralen/UVA phototherapy+ bexarotene | |
| Anti-CD4 (zanolimumab) antibody | |
| 8) IVA T4N0M0B2 Sezary Syndrome |
Photopheresis + methotrexate |
| Photopheresis + interferon-alpha | |
| Photopheresis | |
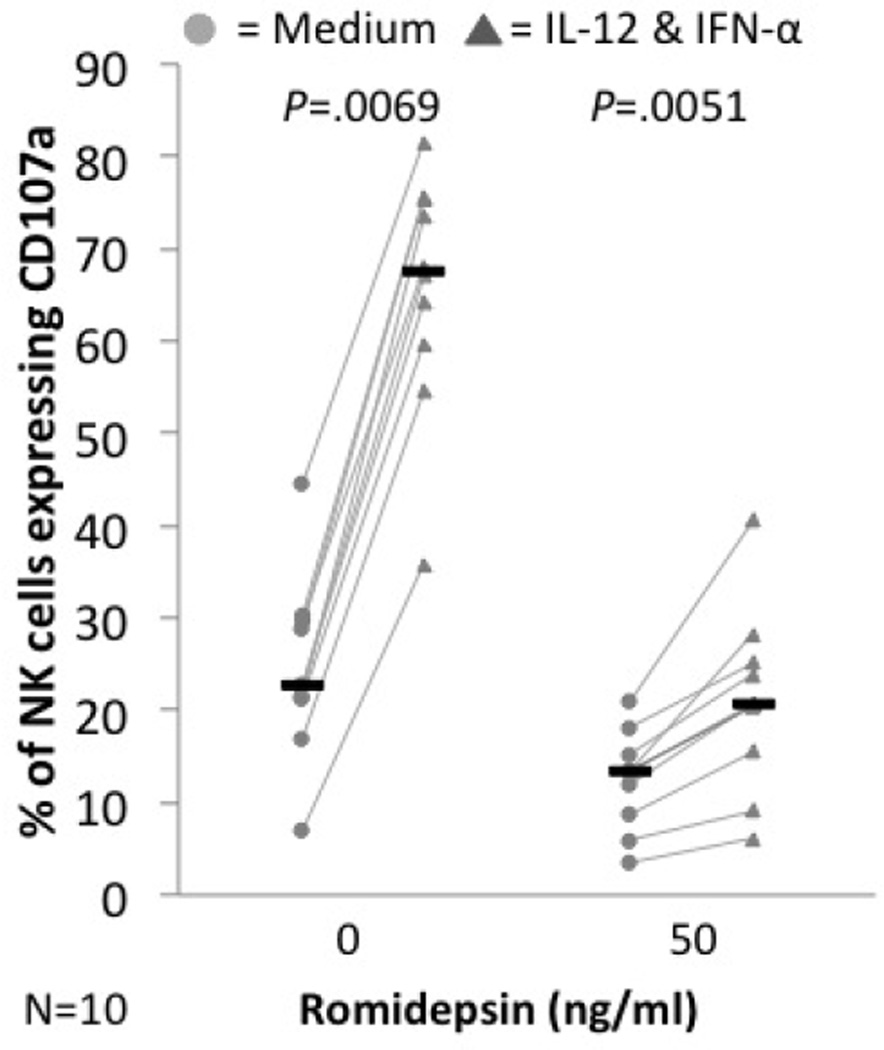
Figure 2. In vitro romidepsin treatment decreases the cytolytic activity of NK cells from healthy donors but treated cells remain responsive to IL-12 and IFN-γ.
PBMC from healthy donors were cultured for 24h in different concentrations of romidepin in the absence (light circles) or presence of IL-12 & IFN-α (dark triangles). CD107a expression was measured after 4h of incubation with K562. Black bars represent the median for each group.
Figure 4. Romidepsin induces apoptosis of different lymphoid populations.
PBMC from healthy donors were cultured in medium with increasing doses of romidepsin for 24h. A TUNEL assay was performed and the cells were labeled to identify CD4+, CD8+, and CD56+ cell populations by flow cytometry. The median and interquartile ranges (error bars) for each group are shown.
Reagents
The synthetic imidazoquinoline, 007, a TLR 7/8 agonist, was a gift of Graceway Pharmaceuticals (Exton, PA and Bristol, TN). Romidepsin (Celgene Corporation, Summit, NJ) was received as a gift from the multicenter international pivotal clinical trial of romidepsin (NCT00106431), conducted in part at the University of Pennsylvania.
Preparation and culture of mononuclear cells
Peripheral blood samples were collected from eight CTCL patients prior to romidepsin treatment (baseline) and through three cycles of treatment: on day 7 of the first cycle and then on day 1 of cycles two and three. Each cycle of treatment consisted of a four hour 14 mg/m2 IV infusion of romidepsin on days 1, 8, and 15 in a 28-day period. Peripheral blood mononuclear cells (PBMC) from these samples were isolated and cryopreserved prior to use for in vitro experiments. PBMC were cultured in Gibco RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with Hyclone 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA), Penicillin/Streptomycin and L-glutamine (Invitrogen). Recombinant TLR 7/8 agonist, 007, was used at 10 µg/ml to stimulate cells.
Assessment of natural killer cell, dendritic cell and T cell functions
For NK cell assay, PBMC samples from CTCL patients and healthy controls were thawed, maintained in growth medium overnight and then plated at 5×105 cells/well in a 96 well U-bottom plate. Cells were cultured in growth medium, ±50 ng/ml of romidepsin, ± 007 at 10 µg/ml or ± recombinant IL-12 at 1 ng/ml and IFN-α at 10 ng/ml (R&D Systems, Minneapolis, MN) for 48 hours. The supernatants were collected for cytokine assay. The cells were washed once and fresh growth medium with 10 µl of anti-CD107a-PE antibody, 0.6 µl/ml of BD GolgiStop (BD Biosciences, Franklin Lakes, NJ) and 5×104 K562 cells was added. Cells were cultured for 4 hours before being stained with anti-CD56/16-FITC, anti-CD3-PerCp, and anti-CD69-APC antibody. The concentration of 50 ng/ml was selected because it is within the serum range achieved with in vivo dosing. [36].
For the dendritic and T cell assays, treated patients’ PBMC and PBMC from healthy controls were maintained in growth medium overnight, plated at 3×106 cells/well in a 24 well plate, and then cultured for 48 hours in growth medium ± 007 (healthy controls were also treated with 50 ng/ml of romidepsin ± 007). The supernatants of the wells were then collected for the cytokine assay. The cells were stained as follows: dendritic cells (CD11c-APC) were stained with anti-Lin-FITC (lineage cocktail containing FITC labeled antibodies against CD3, CD14, CD16, CD19, CD20, and CD56), anti-CD80-PE, and anti-HLA-DR-PerCP. T cells were stained with anti-CD25-FITC, anti-CD4-PE, anti CD8-PerCP, and anti-CD69-APC. Murine immunoglobulins of appropriate isotypes were used as a control. All antibodies were purchased from BD Biosciences.
Detection of cytokines
Culture supernatants were harvested and an ELISA was performed according to manufacturer’s recommendation to test for the presence of IL-12p70 (sensitivity: 10 pg/ml; R&D Systems). Levels of IL-12 p40 were also assessed.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
PBMC samples from CTCL patients with a medium tumor burden (circulating Sézary cells between 21%–49% of total lymphocytes) and healthy controls were plated at 3×106 cells/well on a 24 well tissue culture plate overnight. The cells were cultured either in medium alone, 10 ng/ml of romidepsin, or 50 ng/ml of romidepsin for 48 hours. Then a terminal transferase (Roche, Mannheim, Germany) with Cy5 dUTP-APC (GE Healthcare, Little Chalfont, UK) was added per manufacturer’s directions. Cells were stained with anti-CD-56-FITC, anti-CD8-PE, anti-CD4-PerCP, and anti-Cy5 dUTP-APC. Cells were then analyzed as described above.
Flow cytometric analysis
Flow cytometric analysis of NK cells, dendritic cells and TUNEL assay was performed at the University of Pennsylvania. Cells were analyzed with a BD FACSCalibur (BD Biosciences) flow cytometer using BD CELLQuest software (BD Biosciences) at the Flow Cytometry and Cell Sorting Core, Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA. 120,000 events were collected to analyze NK cells and T cells. 150,000 events were collected to analyze DCs. FlowJo software (Treestar, Ashland, OR) was used further for more detailed analysis.
CD4+ FoxP3+ Treg cell enumeration was carried out by immunofluorescent staining of intracellular FoxP3 at Stanford University. PBMC were analyzed fresh or frozen and the following antibodies were added to each sample: anti-CD4-FITC, anti-FoxP3-PE, anti-CD45-PerCP, anti-CD3-APC and anti-CD8 APC-Cy7. The number of FoxP3 expressing CD4+ cells were assessed serially and compared with the absolute number of CD4+ and CD8+ conventional Tcon cells by flow cytometry. All antibodies used in FoxP3 enumeration were obtained from Biolegend, San Diego, CA. Flow cytometric analysis was performed at Stanford University using an Influx flow cytometer (BD Biosciences) and data analysis was performed with FCS Express (DeNovo Software, Los Angeles, CA).
Statistical analysis
Statistical significance was determined using the Wilcoxon signed-rank and the Wilcoxon rank-sum tests performed on Stata/IC 11.1 (StataCorp LP, College Station, TX) with p<.05 considered statistically significant and two-sided tests of hypotheses used in all analyses. Data are expressed as medians of tested individuals. Error bars indicate the interquartile range of each group calculated with Microsoft Excel 2011 (Microsoft, Redwood, WA).
Results
Romidepsin suppresses the cytolytic activity of NK cells from CTCL patients
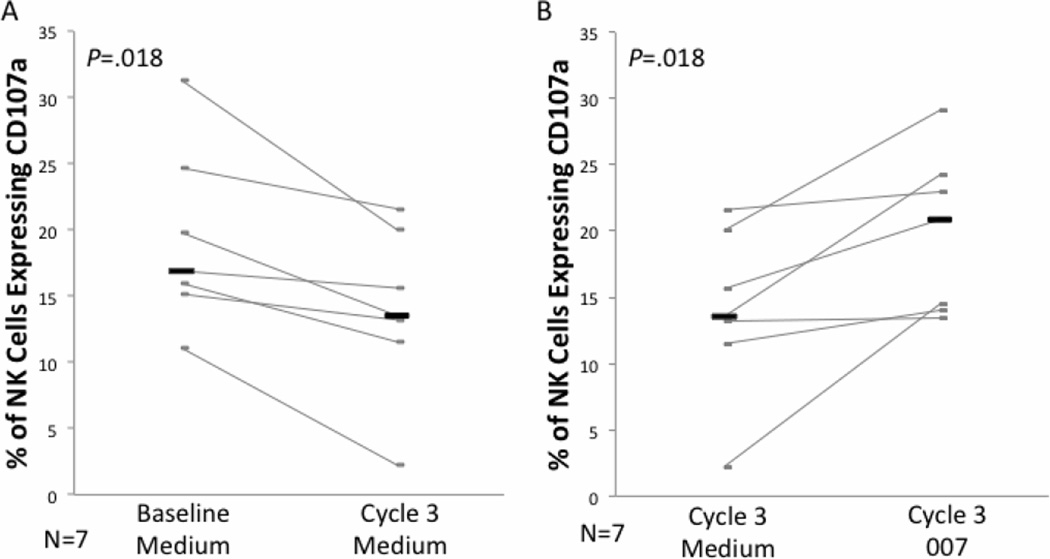
We have previously shown that the HDACi vorinostat suppresses the cytolytic activity of NK cells in vitro in both CTCL patients and healthy donors [25]. Here, we measured the ex vivo NK cell cytotoxicity of CTCL patients using a degranulation marker, CD107a, that we confirmed in numerous assays closely correlates with the level of NK cell activity and target cell death as measured by CR51 release assay (data not shown)(CD107a reference).[37, 38] We found that degranulation of NK cells significantly decreased during romidepsin therapy (Figure 1A). The median percentage of CD107a+ NK cells after culture with K562 was 16.5% at baseline and 13.9% at the beginning of third cycle of treatment (P=.018).
Figure 1. Cytolytic activity of NK cells after romidespin treatment is depressed but augmentable by the TLR agonist 007.
PBMC from CTCL patients at baseline and the beginning of the third cycle of romidepsin were cultured without (A) or with 007 (B), followed by 4h culture with K562 target cells. The cells were stained with CD107a for measurement of NK cell degranulation by flow cytometry. Data for individual patients are shown in gray and the black bars represent the median for each group.
We have previously shown that stimulation of CTCL patients’ PBMC with the synthetic TLR 7/8 agonist, 007, significantly enhances NK cell cytolytic activity [15]. Therefore, we tested whether 007 can increase the cytolysis of NK cells isolated during romidespin treatment. As shown in Figure 1B, the degranulation of NK cells was significantly increased by ex vivo stimulation with 007 (unstimulated median= 13.5%, 007 stimulated median= 20.9%; P=.018).
In vitro romidepsin treatment decreases the cytolytic activity of NK cells from healthy donors
To further characterize romidepsin’s effect on NK cells, we performed an in vitro experiment on PBMC from healthy controls. PBMC from healthy controls were plated in growth medium with or without IL-12 and IFN-γ Degranulation of NK cells was measured by staining for CD107a after K562 incubation. As shown in Figure 2, treatment with 50 ng/ml of romidepsin, significantly suppressed the cytotoxicity of NK cells from healthy donors (untreated median=22.65%, romidepsin median= 13.4%; P=.0069). However, cytokine stimulation with IL-12 and IFN-γ significantly increased the cytolytic activity of these healthy NK cells even in romidepsin treated cells (unstimulated median= 13.4%, IL-12 & IFN-α stimulated median= 20.7%; P=.0051). It is notable that the magnitude of increase in the cytolytic activity stimulated by IL-12 and IFN-γ was much smaller in the romidepsin-treated cells.
Romidepsin treatment decreases dendritic cell activation and cytokine production in response to the TLR agonist 007
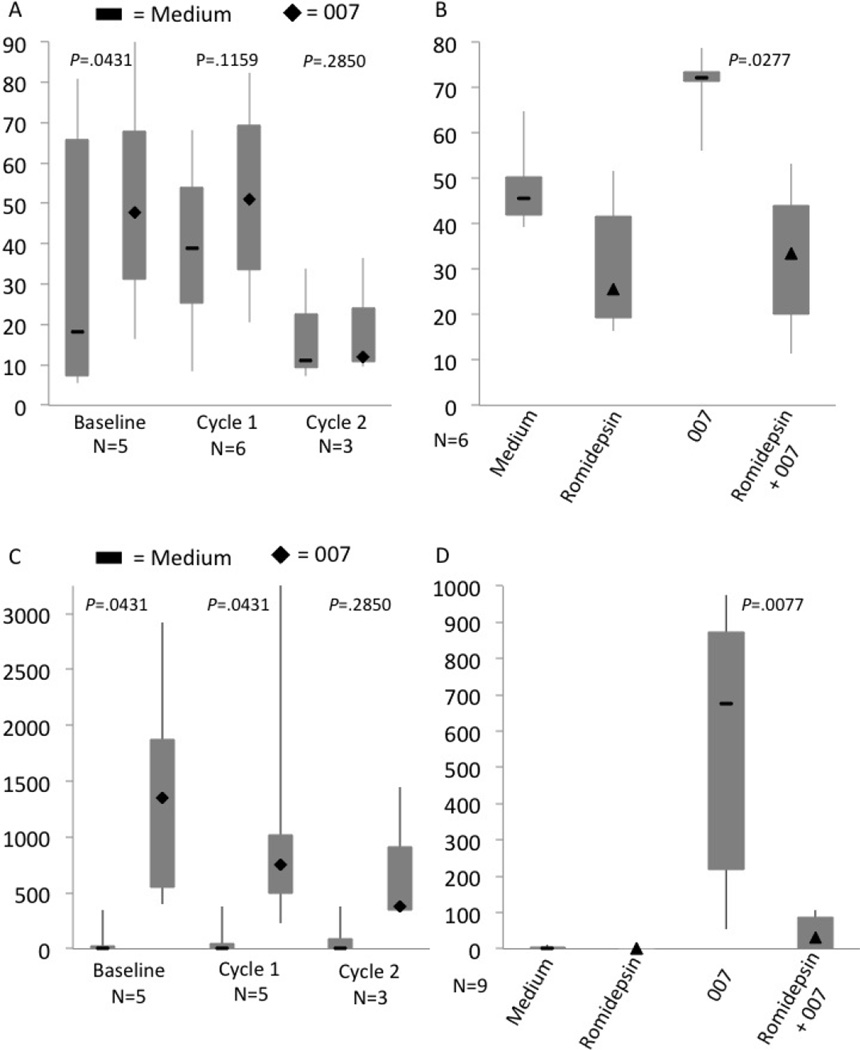
As dendritic cell activation is critical for the patient’s immune system to effectively generate an anti-tumor immune response, we examined the effects of romidepsin on the patients’ CD11c+ myeloid dendritic cells. We measured the activation of dendritic cells by staining for CD80 after TLR 7/8 agonist, 007, stimulation. In samples collected at baseline, prior to romidepsin treatment, the percentage of dendritic cells expressing CD80 significantly increased when the cells were stimulated with 007 (Figure 3A: unstimulated median= 18.2%, 007 stimulated median= 47.7%; P=.0431). In the samples from day 7 of cycle 1, stimulation with 007 up-regulated CD80 expression in five out of the six patients but this trend was not statistically significant (Figure 3A: unstimulated median= 39%, 007 stimulated median= 51.05%; P=.1159). On the other hand, 007 stimulation completely failed to up-regulate CD80 on myeloid dendritic cells from three samples collected at the beginning of the second cycle of treatment (Figure 3A: unstimulated median= 11%, 007 stimulated median= 11.9%; P=.2850). In a control assay performed on PBMC from healthy donors, stimulation with 007 significantly increased the percentage of CD11c dendritic cells expressing CD80 (Figure 3B: unstimulated median=45.55%, 007 stimulated median= 72.2%; P= .0277). Romidepsin treatment in vitro significantly decreased CD80 expression in these stimulated cells (3B: non-romidepsin treated median= 72.2%, romidepsin treated median= 33.45%; P= .0277).
Figure 3. Romidepsin decreases activation and cytokine production by dendritic cells in response to the TLR agonist 007.
(A) Patients’ PBMC from baseline, day 7 of cycle 1, and day 1 of cycle 2 were cultured for 48 hours in growth medium (flat bars) or 007 (diamonds). CD11c+ dendritic cells were stained for CD80 as an activation marker. (B) PBMC from healthy controls were cultured for 48 hours in medium ± 007 (flat bars) or treated with 50 ng/ml of romidepsin ± 007 (triangles). CD11c dendritic cells were stained for CD80. (C) IL-12 production in medium alone (flat bars) or induced by 007 stimulation (diamonds) at different time points during romidepsin treatment. (D) PBMC from healthy controls were cultured for 48 hours in medium ± 007 (flat bars) or treated with 50 ng/ml of romidepsin ± 007 (triangles). IL-12 production was measured by ELISA. The medians (black markers), minimum value, 1st quartile, 3 quartile and maximum value are shown for each group.
We have previously shown that 007 stimulation of DCs from CTCL patients significantly increases the amount of IL-12 cytokine produced in cell culture.[15] Given this observation, we measured the amount of IL-12p70 produced from patient samples collected at different time points during romidepsin therapy. As shown in Figure 3C, 007 stimulation significantly increased the concentration of IL-12p70 protein at baseline (unstimulated median= 0 pg/ml, 007 stimulated median= 1350pg/ml; P=.0431). However, treatment with romidepsin seemed to suppress the production of IL-12 as 007 stimulation no longer significantly increased IL-12p70 production at the beginning of the second cycle of romidepsin (2nd cycle unstimulated median= 0 pg/ml, 2nd cycle 007 median= 375 pg/ml; P=.2850). Similarly, using cells from healthy volunteer stimulated with 007, romidepsin treatment significantly decreased the in vitro production of IL-12 (3D: untreated median= 676, romidepsin treated median= 31; P= .0077)
Romidepsin treatment significantly increases apoptosis of lymphocytes from healthy donors
We hypothesized that the immune suppression in romidepsin-treated patients may be due, in part, to the death of immune cells caused by the drug. To further examine this, we performed a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay to measure cellular apoptosis in different cell populations from healthy controls. As shown in Figure 4, apoptosis increased in a dose dependent manner with in vitro romidepsin treatment of CD4+ T cell, CD8+ T cell, and NK cell populations from healthy volunteers (Figure 4; P<.05). CTCL patients had a similar increase with significantly increased percentage of CD4+ and CD8+ T cells undergoing apoptosis when the cells were treated with 50 ng/ml of romidepsin (data not shown).
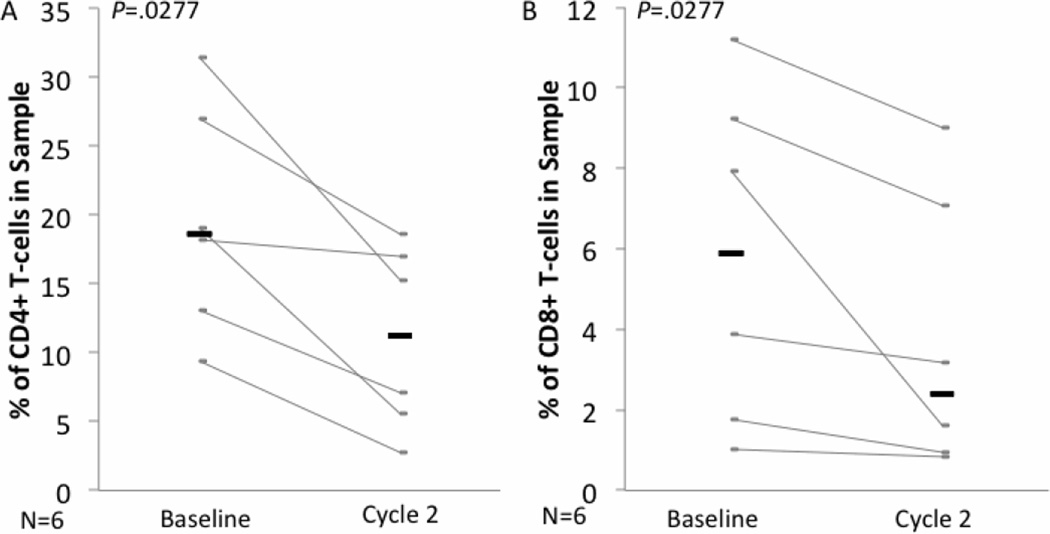
We also observed a decrease in the percentage of CD4+ and CD8+ T cells in the peripheral blood of CTCL patients treated with romidepsin. At the beginning of the second cycle of treatment the percentage of CD4+ and CD8+ T cells among total mononuclear cells was significantly lower then at baseline (Figure 5A–B: CD4+: baseline median= 18.65%, 2nd cycle median=11.195%, P=.0277; CD8+: baseline median= 5.9%, 2nd cycle median= 2.39%, P=.0277).
Figure 5. Romidepsin treatment in vivo decreases the percentage of CD4+ and CD8+ T cells among circulating mononuclear cells in CTCL patients.
PBMC from CTCL patients were collected at baseline and day 1 of the second cycle of romidepsin treatment. They were cultured for 48h in medium before being stained for analysis of the percentage of CD4+ (A) and CD8+ (B) cells by flow cytometry. Gray bars represent individual patients and black bars represent the median for each group.
No change in the numbers of circulating Foxp3/CD25Hi T-regulatory cells over the course of romidepsin treatment
Murine studies suggest that HDACi may cause immune suppression by increasing T-regulatory cell numbers or function.[27] To examine this, we measured the number of circulating CD4+, CD25Hi T-regulatory cells (Treg) in CTCL patients over the course of romidepsin treatment. The percentage of Treg cells did not statistically differ over the course of treatment with romidepsin (results not shown). An additional test with cytoplasmic FoxP3 staining, to more clearly define Treg cells, also did not show significant changes over the course of treatment (results not shown).
Discussion
Our results demonstrate that romidepsin broadly inhibits cellular immune function both in vivo and in vitro. In patients treated with three cycles of romidepsin, we observed a decline in their NK cell cytolytic function and the ability of their DCs to be activated or produce IL-12. These findings are consistent with our previous report that in vitro vorinostat, the first HDACi approved for the treatment of CTCL, inhibits NK cell cytotoxicity, DC activation, and DC cytokine production in CTCL patients.[25] Together these findings are powerful evidence of the immunosuppressive effects of HDACis in humans.
How HDACis suppress immune function is not yet fully understood as the mechanism of immune regulation by HDACs is still being actively investigated. In humans, HDACs in class I and II have been shown in vitro to play a role in the regulation of many cytokines including IL-6, IL-12, IFN-α/β, TNF-α and development of monocytes and dendritic cells.[22, 39–41] Class I HDACs also regulate IFN-γ signaling by promoting the expression of STAT1, which suggests they play an important role in the activation of NK cells and T cells.[42, 43] Also, HDAC6, which is in class IIb, has been shown to promote the production of IFN-α/β in response to viral infection by controlling the transcription of interferon regulatory factors (IRF).[44] Dendritic cell function in humans also seems to be controlled by HDACs as the expression of HDAC 11, which is in class IV, enhances DC function by increasing expression of CD86, CD40, increasing IL-12 and decreasing IL-10 production.[45] It is also hypothesized that HDACs play a role in NK cell activation and degranulation as HDACi have been shown to suppress the expression and function of the NK cell activating receptors NKp46 and NKp30 while also inhibiting the release of NK cell cytolytic granules.[23] Romidepsin is thought to primarily inhibit class I HDACS and this is consistent with our findings of suppression in multiple arms of the patients’ immune system.[32, 46, 47]
Another mechanism by which HDACis could cause immune suppression is by increasing Treg cell suppression. In vitro experiments in mice and humans have shown that HDACis increase Treg cell suppression of T cell division, activation, and promote T cell anergy.[27, 48] Furthermore, HDACis have been shown to down-regulate pro-inflammatory cytokines in mouse models of autoimmune diseases such as, arthritis, asthma, colitis, graft versus host disease and systemic lupus erythematosus.[29, 30, 49–53] Similar effects have been observed in humans and we previously reported the resolution of the autoimmune blistering disease bullous pemphigoid in a CTCL patient treated with an HDACi.[28] There are also case reports associating the reactivation of latent herpes viruses with HDACi treatment suggesting that HDACis suppress anti-viral immunity.[25, 26] While the promotion of Treg function and the inhibition of Th1 and Th17 responses does not benefit cancer patients, it may benefit patients with autoimmune diseases. Given the fact that we did not find a significant increase in the number of CD4+CD25+ cells in patients treated with romidepsin, we assume that an increase in conventional Tregs may not be responsible for decreased immune responses in our CTCL patients. Nevertheless, we did not test in this study whether romidepsin increases the individual cell potency of the prevailing Tregs.
Previous studies of apoptosis with vorinostat (Duvic) have suggested that the malignant CD4+ T-cells derived from CTCL patients may have heightened sensitivity to this HDACi with increased cell death.[54] The results from our TUNEL assays with romidepsin demonstrate increased death of NK cells, as well as both CD4 and CD8 T-cells. These findings suggest that the immune suppression may be due, at least in part, to the apoptosis of healthy immune cells, which could blunt the overall potency of the immune response. [55]
We observed that after three cycles of romidepsin, patients’ NK cells could still increase their cytolytic activity with in vitro stimulation with the TLR 7/8 agonist, 007. These findings are consistent with our previous case series reporting the benefit of treating CTCL patients concurrently with both vorinostat and an immune potentiating agent, IFN-γ.[56] As we have previously reported, IFN-γ demonstrates a strong priming effect when combined with a TLR agonist on many different cell populations including dendritic cells and NK cells resulting in enhanced anti-tumor responses. [57, 58] There is substantial evidence that using a multimodality approach to optimize the patients’ anti-tumor response and cell mediated immunity improves clinical outcomes.[9–11] We are actively investigating novel multimodality treatment strategies to optimize clinical outcomes. Our findings suggest that similar approaches with romidepsin deserve further exploration.
Table 2. Organ specific response of patients to treatment with romidepsin.
The response of patients’ skin, blood, lymph nodes and global assessment were recorded. Abbreviations: CR= complete remission, PR= partial remission, SD= stable disease, n/a= not-applicable (patient did not have disease present at this organ at initiation of romidepsin).
| Response | ||||
|---|---|---|---|---|
| Patient | Skin | Blood | Lymph Nodes | Global |
| 1 | SD | CR | n/a | SD |
| 2 | SD | SD | n/a | SD |
| 3 | SD | n/a | SD | SD |
| 4 | PR | CR | n/a | PR |
| 5 | PR | SD | n/a | SD |
| 6 | SD | n/a | n/a | SD |
| 7 | PR | PR | n/a | PR |
| 8 | CR | CR | n/a | CR |
Acknowledgements
This work was supported by a grant from the National Cancer Institute (R01 CA122569), and a Translational Research grant from the Leukemia and Lymphoma Society to A.H.R.; a grant from the National Cancer Institute (R01 CA132098) to L.C.S.; a grant from the Doris Duke Charitable Foundation to the University of Pennsylvania to fund M.J.K-S. as a Clinical Research Fellow; Y.H.K. has no funding sources to report. The funding sources were not involved in the design or completion of this study. The funding sources were not involved in the preparation or submission of this manuscript.
The authors thank Daniel Sell for his excellent technical advice on visualizing and graphing the data. D.W.S. has no affiliations or funding sources to report.
Footnotes
Presented orally, in part, at the 71st Annual Meeting of the Society for Investigative Dermatology, Phoenix, Arizona, May 4–7, 2011.
Authorship and conflict of interest
Contribution: M.J.K-S., Y.H.K., R.A., W-K.W., M.W., and A.H.R. designed the research; M.J.K-S., S.S, B.B, C.H., K.S., R.A., and, W-K.W. collected the data; M.J.K-S., Y.H.K., M.W and, A.H.R analyzed the data; L.C.S. provided essential materials; M.J.K-S., Y.H.K., R.A., W-K.W., L.C.S., M.W., and A.H.R. wrote the manuscript. Y.H.K., M.W., and A.H.R. supervised the experimental work.
Conflict-of-interest disclosure: The authors state no conflicts of interest.
REFERENCES
- 1.Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sezary syndrome. Blood. 1996;88:2385–2409. [PubMed] [Google Scholar]
- 2.Haynes BF, Bunn P, Mann D, et al. Cell surface differentiation antigens of the malignant T cell in Sezary syndrome and mycosis fungoides. J Clin Invest. 1981;67:523–530. doi: 10.1172/JCI110062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood NL, Kitces EN, Blaylock WK. Depressed lymphokine activated killer cell activity in mycosis fungoides. A possible marker for aggressive disease. Arch Dermatol. 1990;126:907–913. [PubMed] [Google Scholar]
- 4.Suchin KR, Cassin M, Gottleib SL, et al. Increased interleukin 5 production in eosinophilic Sezary syndrome: regulation by interferon alfa and interleukin 12. J Am Acad Dermatol. 2001;44:28–32. doi: 10.1067/mjd.2001.109853. [DOI] [PubMed] [Google Scholar]
- 5.Vowels BR, Cassin M, Vonderheid EC, et al. Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol. 1992;99:90–94. doi: 10.1111/1523-1747.ep12611877. [DOI] [PubMed] [Google Scholar]
- 6.Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103:669–673. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- 7.Asadullah K, Docke WD, Haeussler A, et al. Progression of mycosis fungoides is associated with increasing cutaneous expression of interleukin-10 mRNA. J Invest Dermatol. 1996;107:833–837. doi: 10.1111/1523-1747.ep12330869. [DOI] [PubMed] [Google Scholar]
- 8.Wysocka M, Zaki MH, French LE, et al. Sezary syndrome patients demonstrate a defect in dendritic cell populations: effects of CD40 ligand and treatment with GM-CSF on dendritic cell numbers and the production of cytokines. Blood. 2002;100:3287–3294. doi: 10.1182/blood-2002-01-0231. [DOI] [PubMed] [Google Scholar]
- 9.Rook AH, Wood GS, Yoo EK, et al. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood. 1999;94:902–908. [PubMed] [Google Scholar]
- 10.Wood GS. Lymphocyte activation in cutaneous T-cell lymphoma. J Invest Dermatol. 1995;105:105S–109S. doi: 10.1111/1523-1747.ep12316249. [DOI] [PubMed] [Google Scholar]
- 11.Raphael BA, Shin DB, Suchin KR, et al. High Clinical Response Rate of Sezary Syndrome to Immunomodulatory Therapies: Prognostic Markers of Response. Arch Dermatol. 2011 doi: 10.1001/archdermatol.2011.232. In press. [DOI] [PubMed] [Google Scholar]
- 12.Richardson SK, Lin JH, Vittorio CC, et al. High clinical response rate with multimodality immunomodulatory therapy for Sezary syndrome. Clin Lymphoma Myeloma. 2006;7:226–232. doi: 10.3816/CLM.2006.n.063. [DOI] [PubMed] [Google Scholar]
- 13.Rook AH, Kuzel TM, Olsen EA. Cytokine therapy of cutaneous T-cell lymphoma: interferons, interleukin-12, and interleukin-2. Hematol Oncol Clin North Am. 2003;17:1435–1448. ix. doi: 10.1016/s0889-8588(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 14.Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wysocka M, Dawany N, Benoit BM, et al. Synergistic enhancement of cellular immune responses by the novel toll receptor 7/8 agonist 3M 007 and interferon-γ: implications for therapy of cutaneous T-cell lymphoma. Leukemia and Lymphoma. doi: 10.3109/10428194.2011.582202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunn PA, Jr, Hoffman SJ, Norris D, et al. Systemic therapy of cutaneous T-cell lymphomas (mycosis fungoides and the Sezary syndrome) Ann Intern Med. 1994;121:592–602. doi: 10.7326/0003-4819-121-8-199410150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 19.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 20.Richon VM, Garcia-Vargas J, Hardwick JS. Development of vorinostat: current applications and future perspectives for cancer therapy. Cancer Lett. 2009;280:201–210. doi: 10.1016/j.canlet.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Vrana JA, Decker RH, Johnson CR, et al. Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but independent of p53. Oncogene. 1999;18:7016–7025. doi: 10.1038/sj.onc.1203176. [DOI] [PubMed] [Google Scholar]
- 22.Roger T, Lugrin J, Le Roy D, et al. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood. 2011;117:1205–1217. doi: 10.1182/blood-2010-05-284711. [DOI] [PubMed] [Google Scholar]
- 23.Ogbomo H, Michaelis M, Kreuter J, et al. Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS Lett. 2007;581:1317–1322. doi: 10.1016/j.febslet.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 24.Song W, Tai YT, Tian Z, et al. HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells. Leukemia. 2011;25:161–168. doi: 10.1038/leu.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anshelevich A, Wysocka M, Benoit BM, et al. Minimizing the immunosuppressive effects of histone deacetylase inhibitors (HDACi): Implications for therapy of cutaneous T-cell lymphoma (CTCL). First World Congress of Cutaneous Lymphomas; Chicago, IL. 2010. [Google Scholar]
- 26.Ritchie D, Piekarz RL, Blombery P, et al. Reactivation of DNA viruses in association with histone deacetylase inhibitor therapy: a case series report. Haematologica. 2009;94:1618–1622. doi: 10.3324/haematol.2009.008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akimova T, Ge G, Golovina T, et al. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clinical immunology (Orlando, Fla) 2010;136:348–363. doi: 10.1016/j.clim.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner JM, Evans KG, Goldstein S, et al. Vorinostat for the treatment of bullous pemphigoid in the setting of advanced, refractory cutaneous T-cell lymphoma. Arch Dermatol. 2009;145:985–988. doi: 10.1001/archdermatol.2009.229. [DOI] [PubMed] [Google Scholar]
- 29.Glauben R, Batra A, Fedke I, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. Journal of immunology (Baltimore, Md: 1950) 2006;176:5015–5022. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 30.Mishra N, Reilly CM, Brown DR, et al. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111:539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reilly CM, Mishra N, Miller JM, et al. Modulation of renal disease in MRL/lpr mice by suberoylanilide hydroxamic acid. Journal of immunology (Baltimore, Md: 1950) 2004;173:4171–4178. doi: 10.4049/jimmunol.173.6.4171. [DOI] [PubMed] [Google Scholar]
- 32.Shakespear MR, Halili MA, Irvine KM, et al. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011 doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Murphy GF. Cutaneous T-cell lymphoma. Adv Pathol. 1988;1:131–156. [Google Scholar]
- 34.Introcaso CE, Hess SD, Kamoun M, et al. Association of change in clinical status and change in the percentage of the CD4+CD26- lymphocyte population in patients with Sezary syndrome. J Am Acad Dermatol. 2005;53:428–434. doi: 10.1016/j.jaad.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 36.Celgene Corporation. Istodax (romidepsin) prescribing information. Summit, NJ: 2011. [Google Scholar]
- 37.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. Journal of immunological methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 38.Uhrberg M. The CD107 mobilization assay: viable isolation and immunotherapeutic potential of tumor-cytolytic NK cells. Leukemia. 2005;19:707–709. doi: 10.1038/sj.leu.2403705. [DOI] [PubMed] [Google Scholar]
- 39.Aung HT, Schroder K, Himes SR, et al. LPS regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. Faseb J. 2006;20:1315–1327. doi: 10.1096/fj.05-5360com. [DOI] [PubMed] [Google Scholar]
- 40.Bode KA, Schroder K, Hume DA, et al. Histone deacetylase inhibitors decrease Toll-like receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology. 2007;122:596–606. doi: 10.1111/j.1365-2567.2007.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brogdon JL, Xu Y, Szabo SJ, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 42.Chang HM, Paulson M, Holko M, et al. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:9578–9583. doi: 10.1073/pnas.0400567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer OH, Knauer SK, Greiner G, et al. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev. 2009;23:223–235. doi: 10.1101/gad.479209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nusinzon I, Horvath CM. Positive and negative regulation of the innate antiviral response and beta interferon gene expression by deacetylation. Mol Cell Biol. 2006;26:3106–3113. doi: 10.1128/MCB.26.8.3106-3113.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villagra A, Cheng F, Wang HW, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10:92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradner JE, West N, Grachan ML, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furumai R, Matsuyama A, Kobashi N, et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 48.Wang L, Tao R, Hancock WW. Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance. Immunol Cell Biol. 2009;87:195–202. doi: 10.1038/icb.2008.106. [DOI] [PubMed] [Google Scholar]
- 49.Chung YL, Lee MY, Wang AJ, et al. A therapeutic strategy uses histone deacetylase inhibitors to modulate the expression of genes involved in the pathogenesis of rheumatoid arthritis. Mol Ther. 2003;8:707–717. doi: 10.1016/s1525-0016(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 50.Reddy P, Maeda Y, Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci U S A. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi JH, Oh SW, Kang MS, et al. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin Exp Allergy. 2005;35:89–96. doi: 10.1111/j.1365-2222.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 52.Reddy P, Sun Y, Toubai T, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li N, Zhao D, Kirschbaum M, et al. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc Natl Acad Sci U S A. 2008;105:4796–4801. doi: 10.1073/pnas.0712051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C, Richon V, Ni X, et al. Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. J Invest Dermatol. 2005;125:1045–1052. doi: 10.1111/j.0022-202X.2005.23925.x. [DOI] [PubMed] [Google Scholar]
- 55.Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer. 2003;3:30. doi: 10.1186/1471-2407-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardner JM, Introcaso CE, Nasta SD, et al. A novel regimen of vorinostat with interferon gamma for refractory Sezary syndrome. J Am Acad Dermatol. 2009;61:112–116. doi: 10.1016/j.jaad.2008.11.889. [DOI] [PubMed] [Google Scholar]
- 57.Wysocka M, Newton S, Benoit BM, et al. Synthetic imidazoquinolines potently and broadly activate the cellular immune response of patients with cutaneous T-cell lymphoma: synergy with interferon-gamma enhances production of interleukin-12. Clin Lymphoma Myeloma. 2007;7:524–534. doi: 10.3816/clm.2007.n.037. [DOI] [PubMed] [Google Scholar]
- 58.Wysocka M, Dawany N, Benoit B, et al. Synergistic enhancement of cellular immune responses by the novel Toll receptor 7/8 agonist 3M-007 and interferon-gamma: implications for therapy of cutaneous T-cell lymphoma. Leuk Lymphoma. 2011;52:1970–1979. doi: 10.3109/10428194.2011.582202. [DOI] [PMC free article] [PubMed] [Google Scholar]