Abstract
The cyclic nucleotides cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) regulate the activity of protein kinase A (PKA) and protein kinase G (PKG), respectively. This process helps maintain circulating platelets in a resting state. Here we studied the role of cAMP and cGMP in the regulation of megakaryocyte (MK) differentiation and platelet formation. Cultured, platelet-producing MKs were differentiated from fetal livers harvested from 13.5 days postcoital mouse embryos. MK development was accompanied by a dramatic increase in cAMP production and expression of soluble guanylate cyclase, PKG, and PKA as well as their downstream targets vasodilator-stimulated phosphoprotein (VASP) and MENA. Stimulation of prostaglandin E1 receptor/adenylyl cyclase or soluble guanylate cyclase/PKG in cultured MKs increased VASP phosphorylation, indicating that these components share a common signaling pathway. To dissect out the role of cyclic nucleotides in MK differentiation, cAMP/PKA and cGMP/PKG signaling were alternately blocked in cultured MKs. Down-regulation of cAMP pathway effectors decreased MK numbers and ploidy. Notably, cGMP levels increased at the beginning of MK development and returned to basal levels in parallel with MK maturation. However, inhibition of cGMP pathway effectors had no effect on MK development. In addition, platelet release from mature MKs was enhanced by cGMP and inhibited by cAMP. Our data suggest that cAMP plays an important role in MK differentiation, while cAMP and cGMP have opposite effects on platelet production. Identifying the signaling pathways that underpin MK development and proplatelet formation will provide greater insights into thrombopoiesis and may potentially yield useful therapeutic targets.
To maintain normal hemostasis, approximately one trillion (~1012) platelets circulate in an adult human. Platelets derive from the cytoplasm of megakaryocytes (MKs), large cells that develop from pluripotent hematopoietic stem cells in the bone marrow (BM). Platelets assemble and release from long, intermediate cytoplasmic extensions called proplatelets, which are made by MKs, that have multiple platelet-sized swellings along their length [1]. Proplatelet formation is characterized by extensive MK morphological changes and cytoskeletal reorganization. Microtubules, each of which is a linear polymer assembled from thousands of α- and β-tubulin dimers in concert with associated motor proteins, drive proplatelet elongation [2,3]. By contrast, actin-based forces repeatedly bend and bifurcate the proplatelet shafts, amplifying proplatelet ends, sites of platelet maturation and release [2]. Although the cytoskeletal changes that accompany platelet biogenesis have been studied extensively, little is known about the corresponding signals that regulate cytoskeletal reorganization and platelet production. Signaling pathways implicated in MK development include protein kinase C [4], the Rho-Rock/myosin-IIa pathway [5], and PIP2 signaling [6].
The majority of mature MKs localize near the sinusoids in the vascular niche in the BM, and extend their proplatelets through sinusoid endothelial cells [7,8], indicating that extra-cellular signals can contribute to MK and platelet development. Enhancing the movement of MK progenitors to vascular sinusoids is sufficient to induce their differentiation and platelet production [9]. Although the molecular mechanisms that regulate these processes are unknown, it has been shown that the presence of BM endothelial cells is essential for stromal cell-derived factor-1–induced proplatelet formation [9]. Furthermore, the inhibition of vascular recovery in the BM results in impaired MK regeneration after myelosuppression [10], suggesting that endothelium-derived factors such as nitric oxide (NO) and prostaglandin I2 (PGI2) can play an important role in thrombopoiesis.
In the absence of injury, NO and PGI2 produced by vascular endothelial cells help maintain circulating platelets in a resting state by increasing platelet intracellular levels of the cyclic nucleotides, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). While cAMP is synthesized by a membrane adenylyl cyclase, which is activated by Gs-coupled prostaglandin receptors, cGMP is produced by soluble guanylyl cyclase (sGC) in the cytoplasm. Protein kinase A (PKA) and protein kinase G (PKG), which are activated by cAMP and cGMP, respectively, are the major effectors of platelet inhibitory pathways [11]. Vasodilator-stimulated phosphoprotein (VASP) and MENA are well-characterized substrates of PKA and PKG. PKA preferentially phosphorylates VASP at Ser157, while PKG favorably targets Ser239. Hence, phospho-VASP has been used as a marker of platelet inhibition [12].
Here we report that differentiation of MKs derived from mouse fetal liver cells (FLC) is accompanied by dramatic increases in cAMP levels and augmented expression of proteins involved in cAMP/cGMP signaling. Interestingly, we show that cAMP/PKA signaling promotes MK development, but suppresses platelet production. By contrast, cGMP/PKG signaling has no effect on MK development, but strongly stimulates platelet production.
Materials and methods
Detailed information on reagents, antibodies, and suppliers can be found in Supplementary Methods (online only, available at www.exphem.org).
Isolation of mouse FLC and differentiation of MKs
Pregnant C57/BL6 mice at 13.5 days postcoital were anesthetized and sacrificed by CO2 asphyxiation. Mouse fetal livers were explanted and processed as described previously [13]. A portion of the FLC harvested (day 0) were analyzed by Western blot or fluorescence-activated cell sorting (FACS) analysis before culturing. FLC initially consisted of small cells of 2N to 4N ploidy (Supplementary Figure E1B, C; online only, available at www.exphem.org). A two-step bovine serum albumin (BSA) density gradient was used at culture day 2 or 4 to separate MKs (pellet) from non-MKs (top fraction after gradient). The purity of the different fractions was analyzed by phase-contrast microscopy and flow cytometry. Approximately 13% of FLC expressed CD41 at day 0, while CD61 gradually increased in parallel with MK differentiation (Supplementary Figure E1A; online only, available at www.exphem.org). Therefore, CD61 was used as a MK marker. The portion of MKs (CD61-positive cells) in the enriched gradient population was estimated to be 70% to 80% at day 2, and 80% to 90% at day 4, while only 4% to 6% of the non-MK fractions were CD61-positive (Supplementary Figure E1B; online only, available at www.exphem.org). The ploidy of MKs increased from 32N to 64N between days 2 and 4 (Supplementary Figure E1C; online only, available at www.exphem.org).
Preparation of washed mouse platelets
Washed mouse platelets were prepared as described previously [14]. Platelet concentrations were adjusted to 4 × 108 platelets/mL.
Flow cytometry and Western blotting
Cell cultures were stained with an anti-CD41 (Becton Dickinson, Heidelberg, Germany), an anti-CD61 (Emfret, Wüurzburg, Germany) antibody, or an isotype control and analyzed on a Becton Dickinson FACSCalibur using CellQuest software, version 3.1f (Heidelberg, Germany). For protein analysis, MK and platelet lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting was performed as described previously [15], using antibodies against VASP (generated in our laboratory), phospho-VASP Ser157, phospho-VASP Ser239 (Nanotools, Teningen, Germany), β-sGC (Sigma-Aldrich, Steinheim, Germany), PKG-I (generated in our laboratory), PKA-C (catalytic subunit), phospho-JAK2 Tyr1007/1008, JAK2 (Cell Signaling, Frankfurt, Germany), MENA (generously provided by T. Renne, Stockholm, Sweden), glyceraldehyde phosphate dehydrogenase (GAPDH; Chemicon, Munich, Germany), phosphodiesterase 2A (PDE2A), PDE3A (GeneTex, Hiddenhausen, Germany), or PDE5 (generously provided by S. Rybalkin, Seattle, WA, USA). Digitized Western blots from three independent experiments were quantified by densitometry (ImageJ software, National Institutes of Health, Bethesda, MD, USA), and the data were expressed as relative density compared with freshly isolated FLC.
MK ploidy measurements
MKs were resuspended in citrate buffer (40 mM sodium citrate [pH 7.4], 0.25 M sucrose), stained with 15 μg/mL propidium iodide (0.4% NP-40, 0.4 mM EDTA, 0.4 mg/mL RNase) and analyzed by FACS.
cAMP and cGMP measurements
Levels of cAMP and cGMP were measured using an enzyme immunoassay according to manufacturer’s recommendations (Sigma-Aldrich, Steinheim, Germany). Results were normalized to the protein concentration of samples.
Platelet formation counts
At day 3 post-explant, MKs were separated using a BSA gradient and seeded in a 24-well plate (105/mL) in the presence or absence of compounds, as indicated. Released platelets were collected by centrifugation after 24 hours. The morphology of platelets stained for β-tubulin was analyzed by fluorescent microscopy and platelet-sized particles positive for the CD61 marker were counted with beads in the gate adjusted for mouse platelets by FACS, as described previously [16,17].
Data analysis
All experiments were performed at least in triplicate and data are expressed as means ± standard deviation. Data were analyzed using either a t test or an analysis of variance followed by Bonferroni post hoc tests. Differences were considered significant when p < 0.05.
Results
Increased expression of sGC, PKG, PKA, VASP, and MENA during MK differentiation
Mouse MKs developed from fetal livers enlarge and become increasingly polyploid as they grow in culture, until the onset of proplatelet formation on day 4 (Supplementary Figure E1; online only, available at www.exphem.org). To investigate the roles of the NO and PGI2 pathways in MK maturation and platelet generation, we analyzed the expression levels of endothelial NO synthase (eNOS), sGC, PKG, PKA, VASP, and MENA at the different stages of MK development and compared these expression levels to those in mouse platelets. In all experiments, a BSA gradient was used to enrich for the MK fraction, which was then compared to the non-MK fraction.
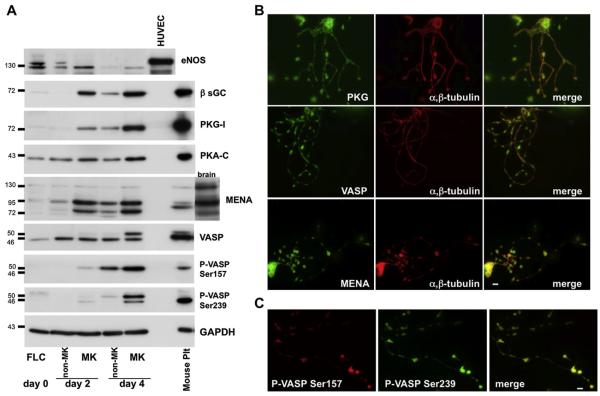
We found that the levels of sGC, PKG-I, PKA-C, VASP, and MENA expressed at low levels in the progenitor cells (FLC) increased dramatically as MKs differentiated (Fig. 1A, Table 1). The expression levels of sGC, PKG-I, PKA-C and VASP in mature MKs at day 4 were comparable to those found in platelets. Only MENA expression was down-regulated in platelets. Increased expression of PKA and PKG correlates with increased kinase activity in the MK fraction, as determined by VASP phosphorylation. Levels of VASP phosphorylated at Ser157 and Ser239 were below the detection threshold in the initial cell cultures. However, VASP phosphorylation at Ser157 was enhanced in MKs on day 2, while the phosphorylation of both Ser157 and Ser239 increased on day 4. These results suggest a major role for the PKA and PKG signaling pathways in MK differentiation. Endothelial NOS became undetectable as the MKs matured (Fig. 1A, Table 1), in agreement with published observations that mouse and human platelets do not express functional NOS proteins[14,18]. Other NOS isoforms (inducible NOS and neuronal NOS) were not expressed in the initial cultures or in mature MKs or platelets (data not shown).
Figure 1.
Increased expression of β-sGC, PKG-I, PKA-C, VASP, and MENA during differentiation of mouse FLC into MKs. (A) FLC and MKs separated from non-MK cells after 2 and 4 days in culture and mouse platelets (Plt) were analyzed for expression levels of endothelial NOS (eNOS), β-sGC, PKG-I, PKA-C (catalytic subunit), MENA, VASP, and phospho-VASP (Ser157, Ser239) by Western blotting. Human umbilical vein endothelial cells were used as a control for eNOS and mouse brain lysate as a control for MENA expression. GAPDH was used as a loading control. Blots representative of four independent experiments are shown. (B) Proplatelets contain PKG-I, VASP, and MENA. Immunofluorescence images of MKs with proplatelets stained for PKG-I, VASP, MENA, or β-tubulin. The images are representative of four independent experiments. (C) Colocalization of VASP phosphoforms in proplatelets. Immunofluorescence images of proplatelets stained with mouse anti–phospho-VASP Ser157 and rabbit anti–phospho-VASP Ser239 representative of three independent experiments are shown. Scale bar: 5 μm.
Table 1.
Increased expression of β-sGC, PKG-I, PKA-C, VASP, and MENA during MK differentiation
| Day 0 |
Day 2 |
Day 4 |
||||
|---|---|---|---|---|---|---|
| FLC | non-MK | MK | non-MK | MK | Plt | |
| eNOS | 1 | 0.31 (0.02)a | 0.41 (0.12)a | 0.03 (0.01)a | 0.07 (0.02)a | |
| β sGC | 1 | 0.23 (0.11) | 26.88 (5.33)a | 8.28 (2.29) | 52.15 (2.94)a | 63.56 (2.35) |
| PKG I | 1 | 1.65 (0.87) | 26.42 (10.03) | 13.89 (3.92) | 66.62 (16.62)a | 127.8 (27.8) |
| PKA C | 1 | 1.69 (0.16) | 2.65 (0.21)a | 1.83 (0.32) | 3.56 (0.64)a | 4.87 (0.34) |
| MENA | 1 | 3.17 (0.21) | 21.98 (4.03)a | 11.59 (1.91)a | 26.03 (2.05)a | 10.91 (2.52) |
| VASP | 1 | 3.07 (0.25) | 3.52 (0.60) | 2.51 (0.49) | 7.45 (1.50)a | 12.22 (2.69) |
| P-VASP Ser157 | 1 | 0.08 (0.01) | 9.62 (2.75) | 37.37 (8.99)a | 73.12 (2.7)a | 24.23 (2.95) |
| P-VASP Ser239 | 1 | 1.14 (0.11) | 3.31 (2.30) | 2.53 (0.19) | 43.41 (1.76)a | 37.15 (1.97) |
Digitized bands as from Figure 1A were quantified using ImageJ (National Institutes of Health) software. Each value represents the relative expression level in four independent experiments and are mean ± standard deviation. The value from FLC at day 0 is 1. Plt = platelets.
Statistical significance of p<0.05 compared to FLC. Values for positive control cells were: human umbilical vein endothelial cells, 1.91 (0.22); brain, 30.76 (0.54).
Localization of PKG, VASP, and MENA in proplatelet-producing MKs was evaluated by immunofluorescence staining. Both the MK cell bodies and elaborated proplatelets contained PKG, VASP, and MENA (Fig. 1B). Staining of all proteins was cytoplasmic and present along the shafts and platelet-like swellings (proplatelets were stained for β-tubulin to highlight their morphology). Because phosphorylation of VASP at Ser239 occurs at the height of proplatelet formation on day 4, we examined the localization of the two phospho-VASP isoforms in proplatelet-producing MKs. Proplatelets were stained similarly for both phospho-VASP Ser157 and phospho-VASP Ser239 (Fig. 1C), indicating no spatial separation of the different phospho-VASP isoforms.
Differential regulation of cAMP and cGMP levels during MK development
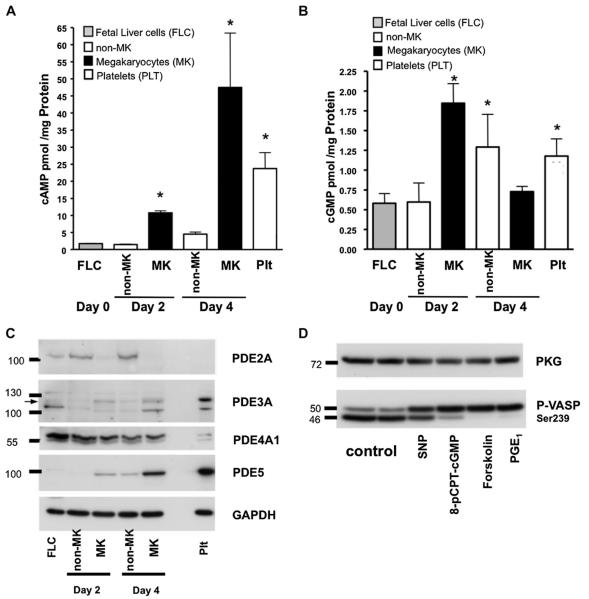
To address the roles of PKA and PKG in MK maturation and platelet formation, we measured cAMP and cGMP levels in FLC, non-MK cells, and MKs on 2 and 4 days post-explant. Compared to the initial FLC, the levels of cAMP in MKs increased by fivefold on day 2 (from 1.8 pmol/mg protein in FLC to 10 pmol/mg protein in MKs, n = 3; p < 0.05, t test) and 25-fold on day 4 (to 48 ± 17 pmol/mg protein, n = 3; p < 0.05, t test). The levels of cAMP in MKs were double those found in mature mouse platelets (Fig. 2A). This augmentation of cellular cAMP levels explains the sequential phosphorylation of VASP at the Ser157, followed by Ser239 site. We did not observe an increase in cAMP levels in the non-MK cell fraction, which had cAMP levels that were similar to those in the initial FLC culture (4.5 ± 1.5 pmol/mg protein in non-MK vs 1.8 ± 0.2 in FLC pmol/mg protein, n ± 3; p > 0.05), indicating that the rise of cAMP levels was MK-specific. Similarly, levels of cGMP (Fig. 2B) increased threefold in MKs (1.8 ± 0.4 pmol/mg protein, n = 3; p < 0.05, t test) on day 2 compared to the initial FLC (0.58 ± 0.29 pmol of cGMP/mg protein), but subsequently decreased to near basal levels (0.75 ± 0.07 pmol/mg protein, n = 3; p > 0.05). In addition, cGMP levels in MKs at day 4 were lower than those found in platelets (1.2 ± 0.2 pmol/mg protein, n = 3; p > 0.05). The rise in cytoplasmic cGMP levels at day 2 paralleled the increased expression of sGC (Fig. 1A).
Figure 2.
cAMP and cGMP levels and PDEs expression changes during MK development. (A) cAMP and (B) cGMP levels were measured in FLC, day 2 and 4 MK, non-MK cells, and mouse platelets (Plt) by electroimmunoassay. The results, normalized to cell proteins, are representative of three independent experiments and are means ± standard deviation. *A t test significance of p < 0.05 compared to FLC at day 0, n = 3. (C) Expression levels of PDE2A, PDE3A, PDE4A1, and PDE5 were analyzed by Western blotting in FLC, MK, non-MK cells after 2 and 4 days in culture, and mouse platelets. Representative blots from three independent experiments are shown. (D) PKG and PKA stimulation in MK cultures. Gradient purified MKs were preincubated for 1 hour in serum-free Dulbecco’s modified Eagle medium without TPO and then treated for 5 min with sodium nitroprusid (SNP; 10 μM), 8-pCPT-cGMP (25 μM), forskolin (5 μM), or PGE1 (1 μM). Anti–phospho VASP Ser239 antibody was used to analyze PKG and PKA activation. Ser157 is a PKA preferential phosphorylation site that shifts VASP mobility from 46 to 50 kDa. Therefore two bands, or a shifted band, indicate phosphorylation of both sites. PKG-I was used as a loading control. Blots representative of three independent experiments are shown.
The cytoplasmic levels of cyclic nucleotides are also regulated by PDE-mediated degradation. Therefore, we evaluated the expression of the PDEs known to exist in platelets, namely PDE2A, PDE3A, and PDE5. Only PDE3A and PDE5 increased with MK maturation (Fig. 2C, Table 2). PDE5 is activated by cGMP and is highly specific for cGMP hydrolysis [19]. Therefore, the fall of cGMP at day 4 in MKs may be due to increased PDE5 expression. PDE2A, an enzyme that hydrolyzes both cAMP and cGMP, was detectable in the initial FLC (Fig. 2C, Table 2), but decreased below the detection threshold as MKs matured. In addition, PDE2A was not found in mouse platelets, although it has been identified in human platelets (data not shown). Cyclic AMP undergoes hydrolysis by PDE1A and PDE4A, as well as by PDE2A and PDE3A. Although we failed to detect PDE1A (data not shown), a down-regulated, 66-kDa PDE4A1 splice variant was detected in maturing MKs. Therefore, a slight increase in PDE3A expression, coupled with a down-regulation of PDE2A and PDE4A1 expression, results in increased cAMP levels in developing MKs. Taken together, these results indicate that cAMP and cGMP are differentially regulated during MK development.
Table 2.
Expression of PDE2A, PDE3A, PDE4A1, and PDE5 during MK differentiation
| Day 0 |
Day 2 |
Day 4 |
||||
|---|---|---|---|---|---|---|
| FLC | Non-MK | MK | Non-MK | MK | Plt | |
| PDE2A | 1 | 2.31 (0.28)a | 0.54 (0.14) | 3.12 (0.41)a | 0.12 (0.03)a | 0.023 (0.01) |
| PDE3A | 1 | 0.18 (0.03) | 1.66 (0.16) | 0.70 (0.08) | 3.26 (0.44)a | 6.34 (0.73) |
| PDE4A1 | 1 | 0.93 (0.04) | 0.79 (0.07) | 0.66 (0.05)a | 0.41 (0.10)a | 0.19 (0.07) |
| PDE5 | 1 | 0.47 (0.18) | 13.31 (2.55)a | 4.72 (1.85) | 44.3 (4.36)a | 56.5 (3.63) |
Digitized bands as in Figure 2C were quantified using ImageJ (National Institutes of Health) software. Each value represents the relative expression of the protein from four independent experiments, expressed as mean ± standard deviation, setting the value from FLC at day 0 as 1. Plt = platelets.
Statistical significance of p < 0.05 compared to FLC.
To further explore the functions of the cAMP/PKA and cGMP/PKG pathways during MK maturation, the extent of VASP Ser239 phosphorylation was analyzed after stimulating adenylyl cyclase (forskolin), the prostaglandin receptor (PGE1), sGC (sodium nitroprusid), or PKG (8-pCPT-cGMP). Stimulation of cAMP/PKA or cGMP/PKG signaling markedly increased the levels of phospho-VASP in the cells (Fig. 2D). Phosphorylation of Ser157 on VASP shifts its apparent molecular weight from 46 kDa to 50 kDa in sodium dodecyl sulfate polyacrylamide gel electrophoresis [20]. Therefore, the detection of two bands, or a band shifted to 50 kDa, reports phosphorylation of both sites when probing with an anti–phospho-VASP (Ser239) antibody.
Although thrombopoietin (TPO) is required to induce MK maturation and platelet formation, it does not directly signal to increase cAMP or cGMP (Supplementary Figure E2A, B; online only, available at www.exphem.org). In addition, PKA or PKG activation had no effect on TPO/c-Mpl signaling because phosphorylation of JAK2, a kinase that initiates TPO/c-Mpl signaling in MKs, was unaffected by increased levels of cAMP or cGMP (Supplementary Figure E2C; online only, available at www.exphem.org). These data demonstrate that PKA/PKG signaling is not directly controlled by TPO in MKs, although TPO is required to induce protein expression by activating critical upstream transcription factors.
The cAMP/PKA pathway is important for MK differentiation
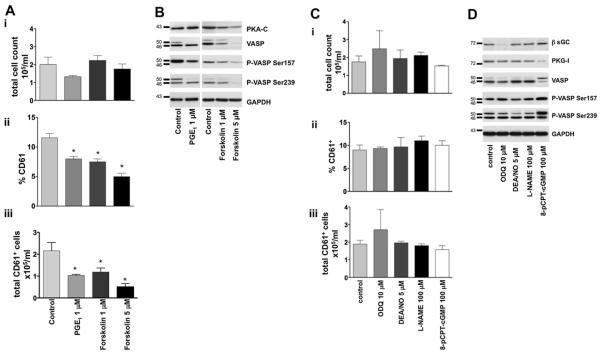
Because cyclic nucleotide levels increase during MK development, we sought to determine whether cAMP/PKA or cGMP/PKG are essential for differentiation. A pharmacological approach that is based on long-term stimulation was used to down-regulate either the cAMP/PKA or cGMP/PKG systems. This approach has been described for PKC inhibition with phorbol 12-myristate 13-acetate [21], and has also been shown for PKG [22]; the continuous (each day during 4 days of culture) application of forskolin or PGE1 down-regulates proteins in the cAMP/PKA pathway, while 8-pCPT-cGMP and oxadiazolo- [4,3-a]quinoxalin-1-one (ODQ) down-regulate protein effectors of the cGMP/PKG pathway. The MK cell cultures were analyzed on day 4 for the following markers of MK differentiation: number of CD61-positive cells (absolute number and percent of total), proliferation (total cell count), ploidy of MKs, and MK expression levels of cAMP and cGMP pathway proteins.
Treatment of cultures with increasing concentrations of forskolin decreased expression of PKA and VASP and attenuated VASP phosphorylation (Fig. 3B, Supplementary Figure E3A; online only, available at www.exphem.org). PGE1 (1 μM) also decreased the amount of total VASP and VASP phosphorylation, indicating that PGE1 inhibited PKA activity. GAPDH, a housekeeping protein unaffected by pharmacological inhibition, was used as a loading control. In addition, the drugs used did not affect cell viability, as assessed by trypan blue staining. Critically, forskolin and PGE1 inhibited MK differentiation in a dose-dependent manner, as determined by a decrease in the percentage and absolute number of CD61-positive cells (Fig. 3Aii and iii). The impaired MK differentiation was not the result of diminished proliferation, as there was no significant change in the total number of cells in the culture (Fig. 3Ai).
Figure 3.
The cAMP, but not cGMP, enhances MK development. (A) To modulate the cAMP pathway, FLC were cultured with TPO and treated daily with forskolin (1 and 5 μM) and PGE1 (1 μM). Cultures were analyzed on day 4 for proliferation (total cell count [i]), percent CD61 expressed (ii), and for counts of CD61+ cells (iii). (B) Expression of PKA-C, VASP, phospho-VASP 157Ser, phospho-VASP 239Ser, and GAPDH (loading control) in MKs. (C) To modulate the cGMP pathway, FLC were cultured with TPO, stimulated daily with ODQ (10 μM), DEA/NO (5 μM), L-NAME (100 μM), and 8-pCPT-cGMP (100 μM), and analyzed on day 4 for total cell counts (i), percent CD61 expressed (ii), and counts of CD61+ cells (iii). (D) Expression of β-sGC, PKG-I, VASP, phospho-VASP 157Ser, phospho-VASP 239Ser, and GAPDH, as loading controls, were evaluated in MKs. *Statistically significant differences compared to control; p < 0.05, n = 6.
The manipulation of cell cultures to induce deregulation of cGMP production failed to impact either MK differentiation or proliferation (Fig. 3C). Cells treated with 8-pCPT-cGMP showed reduced PKG expression, while ODQ treatment decreased β-sGC expression (Fig. 3D, Supplementary Figure E3B; online only, available at www.exphem.org). VASP expression and its phosphorylation at both sites (Ser157 and Ser239) remained intact after ODQ treatment and were only slightly increased after 8-pCPT-cGMP treatment, due to the cAMP/PKA system. Similarly, DEA/NO and NG nitro-l-arginine methyl ester (L-NAME; NOS inhibitor) did not affect protein expression or MK differentiation (Fig. 3C, D).
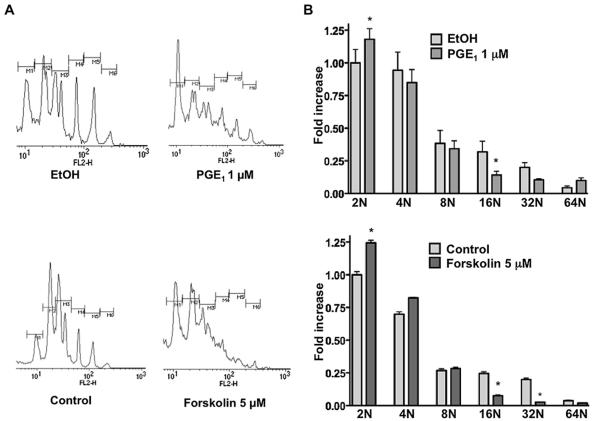
The same pharmacological treatments were employed to study MK ploidy. Manipulation of the cAMP pathway to induce PKA down-regulation/inhibition (Fig. 3B) resulted in decreased MK ploidy. Most of the cells were locked at the 2N to 4N stages in the presence of forskolin and PGE1 and the number of 16N to 32N MKs was markedly decreased (Fig. 4A, B; *p < 0.05 as compared to control, or the solvent delivery control, ethanol for PGE1, n = 3). Conversely, when cultures were treated with DEA/NO or ODQ, the MKs matured in a normal manner, as judged by ploidy (data not shown). Our results suggest an important role for PKA/cAMP, but not for PKG/cGMP, in the process of MK maturation.
Figure 4.
PGE1 and forskolin inhibit MK polyploidization. FLC were cultured with TPO, stimulated daily with forskolin (5 μM), PGE1 (1 μM), or 0.01% ethanol, as a solvent delivery control. The ploidy of MKs on day 4 was assessed by propidium iodide staining. (A) Histograms representative of three experiments are presented. (B) Ploidy is expressed as fold increase relative to control 2N, mean ± standard deviation of three experiments. *Significance of p < 0.05 compared to the ploidy of the control, n = 4.
cGMP increases platelet production
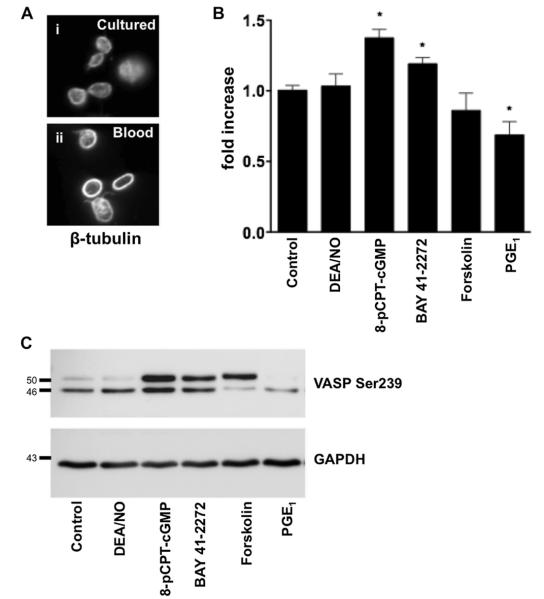
MK maturation culminates in proplatelet elaboration and platelet release. We therefore investigated the roles of the cAMP and cGMP pathways in determining these final events. To examine platelet release, day 3 MKs were harvested using a BSA gradient and cultured for an additional 24 hours in the presence/absence of indicated substances (Fig. 5). Platelets released into culture were identified by their size and microtubular coil staining for β-tubulin (Fig. 5A). Released platelets (CD61+) were counted by FACS and P-VASP was analyzed to monitor the activity of the cAMP and cGMP pathways. Stimulation of PKG with 8-pCPT-cGMP and sGC with BAY41-2272 increased platelet production by 37% (p < 0.05, n = 6) and 20% (p < 0.05, n = 4), respectively (Fig. 5B). Both treatments induced VASP phosphorylation as expected (Fig. 5C). Hence, platelet release was enhanced by the continuous presence of the cGMP analogue (8-pCPT-cGMP) or the increased production of cytosolic cGMP (BAY41-2272). By contrast, the NO donor DEA/NO has a short half-life in solution (2 min) and had no effect on VASP phosphorylation or platelet production (Fig. 5B, C). The opposite effect was observed after induction of cAMP/PKA signaling. Despite its short half-life, PGE1 reduced platelet production by ~30% (p < 0.05, n = 6). Forskolin treatment reduced platelet production by ~15% (p > 0.05, n = 6), although this was not statistically significant. These results suggest that cAMP and cGMP have distinct and opposing roles in the regulation of platelet release from MKs.
Figure 5.
Opposite effects of PKG and PKA on platelet formation. (A) Platelets released from the MK cultures (i) were identified by the presence of microtubular coils, the defining characteristic of peripheral blood platelets (ii, β-tubulin staining). (B) Gradient purified day 3 MKs were cultured overnight in the absence or presence of DEA/NO (5 μM), 8-pCPT-cGMP (100 μM), BAY 41-2272 (1 μM), forskolin (5 μM), or PGE1 (1 μM). Platelet production was analyzed by flow cytometry, gating platelet-sized CD61+ particles. Data are presented as the fold increase in platelets released from the untreated MKs (defined as 1). *Significance of p < 0.05 as compared to the control, n = 4. (C) VASP phosphorylation at Ser239 after 24 hours incubation with indicated substances.
Discussion
Platelet adenylyl cyclase and sGC initiate important inhibitory signaling cascades in platelets by producing cAMP and cGMP, which activate PKA and PKG, respectively [23]. VASP is a protein that binds many actin-associated proteins and is a shared downstream substrate for both PKA and PKG. VASP phosphorylation is believed to be critical for the relaxation of the platelet’s actin cytoskeleton [24]. Hence, the loss of VASP expression in knockout mice results in a prothrombotic state mediated by the circulation of hyperactive platelets [25]. A clear role of cyclic nucleotides in thrombopoiesis, however, has not been established.
In the present study, we examined the roles of cyclic nucleotide production systems in the differentiation of mouse MKs and their subsequent ability to produce platelets. We found that MK differentiation is accompanied by a marked increase in expressions of sGC, PKA-C, PKG-I, VASP, and MENA (Fig. 1A, B). This was accompanied by 5- to 25-fold increase of cAMP, and MKs had a much higher amount of VASP in its phosphorylated state (Figs. 1A, 2A). Furthermore, pharmacological manipulation of the cAMP-dependent pathways influenced PKA expression, VASP phosphorylation, and MK differentiation (Fig. 3A, B). cGMP, on the other hand, was increased early in MKs, and then decreased to basal levels (Fig. 2B). Drug-induced down-regulation of sGC and PKG levels had no effect on MK differentiation (Fig. 3C, D). Platelet release was increased by cGMP/PKG stimulation, while cAMP/ PKA stimulation caused the opposite effect (Fig. 5B). From these results, we hypothesize a new role for the cyclic nucleotides in relaxing the MK actin system. This relaxation would prevent unwanted MK spreading and secretion, while permitting the microtubule system to form proplatelets without interference.
Cyclic AMP and its principal target, PKA, modulate numerous cell physiological processes, such as metabolism, growth, differentiation, gene transcription, and synaptic release of neurotransmitters [26]. Increased expression of the PKA regulatory subunit RII has been reported in human CD34+-derived MKs [27]. Increased cAMP has been found to delay apoptosis of CD34+ stem cells and MKs [28,29]. cAMP also increases expression of the chemokine receptor CXCR4, which augments the ability of CD34+ cells to transmigrate to the endothelium and to adhere to stroma [30]. In agreement with our results, over-expression of pituitary adenylate cyclase-activating polypeptide, which increases cAMP formation via Gs-coupled VPAC1 receptor, inhibits MK differentiation in both humans and mice [31]. PKA is required for maximal activation of NF-E2, a critical transcription factor in erythrocytes and MKs [32]. Therefore, PKA may also participate upstream of NF-E2 by up-regulating specific genes that promote MK differentiation.
Cyclic GMP regulates gene expression, and the loss of PKG expression is associated with the dedifferentiation of vascular smooth muscle cells [33]. NO induces apoptosis in Meg-01 cells and platelet counts are decreased by ~50% in inducible NOS knockout mice [34]. Moreover, rats treated with the NOS inhibitors l-nitro-arginine and L-NAME exhibit decreased platelet counts [35]. Our data show that the cGMP/PKG pathway is not required for MK differentiation, but does enhance the final step of platelet production. By contrast, cAMP/PKA stimulation led to a decline in platelet release. Interestingly, anagrelide, a PDE3 inhibitor that increases cAMP in cells, has been found to be helpful in the treatment of essential thrombocythemia by reducing the peripheral platelet count [36].
Movement of MKs to the BM vascular niche is necessary for their final maturation and subsequent release of platelets, a process mediated by the chemokines, fibroblast growth factor–4 and stromal cell-derived factor–1 (CXCR4 ligand), even in the absence of TPO [9]. MKs localize in vivo to sinusoidal BM endothelial cells, elaborating their proplatelets into microvessels, and releasing platelets into the intravascular sinusoidal space [7,8]. MKs in the vascular microenvironment are in intimate contact with the endothelium, whose production of NO and prostaglandins could influence MK differentiation and platelet release. It has been suggested that proplatelets are sheared by blood flow in BM sinusoids [8]. Conceivably, increased levels of cyclic nucleotides in MKs and proplatelets could prevent shear stress-induced activation of the released platelets or on the proplatelet’s endothelial transmigration, where the released platelets are exposed to matrix proteins.
Conclusions
The present data elucidate a role for the cyclic nucleotides cAMP and cGMP in thrombopoiesis. MK development is associated with increased expression of proteins essential for the function of inhibitory platelet pathways. Our results demonstrate the involvement of cAMP/PKA in the maturation of MKs from MK precursors, while the cGMP/PKG pathway stimulates platelet release, the final step in MK maturation. These data should prompt future investigations to define the mechanisms by which cAMP regulates differentiation processes in MKs, as well as the involvement of cGMP in platelet release.
Supplementary Material
Supplementary Figure E1. (A) Expression of CD41 and CD61 in MK cultures. Fetal livers from 13.5 days postcoital embryos were explanted and cultured in Dulbecco’s modified Eagle medium with TPO (10 ng/mL) for 4 days. Cultures were analyzed by FACS for CD41 and CD61 expression (days 0 to 4). FLC are CD41 + on day 0. Expression of CD61 gradually increases as MKs mature in culture. Results are presented as mean 6 standard deviation from three independent experiments. (B) Characterization of FLC, MKs, or non-MKs after 2 and 4 days in culture. FLC, MKs (cells collected in pellet after BSA gradient), and non-MKs (nonsedimented cell fraction of gradient) from day 2 and 4 cultures were analyzed by phase-contrast microscopy and FACS (CD61 +). The starting FLC and non-MKs consisted of small cells that were negative for CD61 or have 4% to 6% CD61 + cells, respectively. Gradient enriched MKs are large cells, 70% to 80% and 80% to 90% CD61 + after days 2 and 4, respectively. (C) FLC were 2N to 4N. Day 2 MKs were ≤32N. Ploidy on day 4 increased to 64N.
Supplementary Figure E2. (A, B) MK cAMP and cGMP levels are not affected by TPO. Day 4 enhanced MKs were treated with forskolin (5 μM, 5 min), sodium nitroprusid (SNP; 10 μM, 5 min), or TPO (10 ng/mL, 15 min) and cAMP or cGMP levels measured by electroimmunoassay. Results of three independent experiments were normalized to the protein concentration and are means ± standard deviation. *Significance of p < 0.05, compared to control, n = 3, t test. (C) PKA/PKG activation does not interfere with TPO/c-Mpl signaling. Day 4 enriched MKs were resuspended in serum-free media, starved for 1 hour, and then stimulated for 15 min with TPO (10 ng/mL), 8-pCPT-cGMP (25 μM), or forskolin (5 μM) in the presence or absence of TPO. Phosphorylation of JAK2 (JAK2-P, Tyr 1007/1008) or VASP (VASP-P, Ser 239) was evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting in MKs. Total JAK2 and VASP were used as loading controls. Representative blots from three independent experiments are shown.
Supplementary Figure E3. (A) Signal densities from Figure 3B were quantified and expression levels of PKA-C, VASP, P-VASP Ser157, and P-VASP Ser239 in MKs (day 4) after treatment of cell cultures with PGE1 (1 μM) and forskolin (1 μM, 5 μM) are shown. (B) Signal densities from Figure 3D were quantified and expression levels of β-sGC, PKG-I, VASP, P-VASP Ser157, and P-VASP Ser239 in MKs (day 4) after treatment of cell cultures with ODQ (10 μM), DEA/NO (5 μM), L-NAME (100 μM), and 8-pCPT-cGMP (100 μM) are shown. Each bar represents the relative expression of the proteins indicated after different treatments, and setting the value from control as 1. *Statistically significant compared to control; p < 0.05, analysis of variance.
Acknowledgments
We thank Dr. Hugh Kim and Dr. Markus Bender for critical reading of the manuscript.
Funding disclosure This work was supported in part by the Deutsche For-schungsgemeinschaft (SFB688) and National Institutes of Health grants HL-104145, HL-059561 and HL-68130.
Footnotes
Conflict of interest disclosure No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Italiano JE, Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147:1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecine P, Italiano JE, Jr, Kim SW, Villeval JL, Shivdasani RA. Hematopoietic-specific beta 1 tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2. Blood. 2000;96:1366–1373. [PubMed] [Google Scholar]
- 4.Rojnuckarin P, Kaushansky K. Actin reorganization and proplatelet formation in murine megakaryocytes: the role of protein kinase calpha. Blood. 2001;97:154–161. doi: 10.1182/blood.v97.1.154. [DOI] [PubMed] [Google Scholar]
- 5.Chang Y, Aurade F, Larbret F, et al. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109:4229–4236. doi: 10.1182/blood-2006-04-020024. [DOI] [PubMed] [Google Scholar]
- 6.Schulze H, Korpal M, Hurov J, et al. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107:3868–3875. doi: 10.1182/blood-2005-07-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavassoli M, Aoki M. Localization of megakaryocytes in the bone marrow. Blood Cells. 1989;15:3–14. [PubMed] [Google Scholar]
- 8.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 9.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 10.Kopp HG, Hooper AT, Broekman MJ, et al. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest. 2006;116:3277–3291. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter U, Gambaryan S. cGMP and cGMP-dependent protein kinase in platelets and blood cells. In: Schmidt HHHW, editor. Handbook of Experimental Pharmacology. Springer-Verlag; Berlin/Heidelberg: 2009. pp. 533–548. [DOI] [PubMed] [Google Scholar]
- 12.Reinhard M, Jarchau T, Walter U. Actin-based motility: stop and go with Ena/VASP proteins. Trends Biochem Sci. 2001;26:243–249. doi: 10.1016/s0968-0004(00)01785-0. [DOI] [PubMed] [Google Scholar]
- 13.Shivdasani RA, Schulze H. Culture, expansion, and differentiation of murine megakaryocytes. Curr Protoc Immunol. 2005 doi: 10.1002/0471142735.im22f06s67. Chapter 22:Unit 22F 26. [DOI] [PubMed] [Google Scholar]
- 14.Gambaryan S, Kobsar A, Hartmann S, et al. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost. 2008;6:1376–1384. doi: 10.1111/j.1538-7836.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 15.Begonja AJ, Geiger J, Rukoyatkina N, Rauchfuss S, Gambaryan S, Walter U. Thrombin stimulation of p38 MAP kinase in human platelets is mediated by ADP and thromboxane A2 and inhibited by cGMP/cGMP-dependent protein kinase. Blood. 2007;109:616–618. doi: 10.1182/blood-2006-07-038158. [DOI] [PubMed] [Google Scholar]
- 16.Thon JN, Montalvo A, Patel-Hett S, et al. Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191:861–874. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurak Begonja A, Hoffmeister KM, Hartwig JH, Falet H. FlnA-null megakaryocytes prematurely release large and fragile platelets that circulate poorly. Blood. 2011;118:2285–2295. doi: 10.1182/blood-2011-04-348482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozuyaman B, Godecke A, Kusters S, Kirchhoff E, Scharf RE, Schrader J. Endothelial nitric oxide synthase plays a minor role in inhibition of arterial thrombus formation. Thromb Haemost. 2005;93:1161–1167. doi: 10.1160/TH03-09-0588. [DOI] [PubMed] [Google Scholar]
- 19.Lin CS, Lin G, Xin ZC, Lue TF. Expression, distribution and regulation of phosphodiesterase 5. Curr Pharm Des. 2006;12:3439–3457. doi: 10.2174/138161206778343064. [DOI] [PubMed] [Google Scholar]
- 20.Smolenski A, Bachmann C, Reinhard K, et al. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal anti-body. J Biol Chem. 1998;273:20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 21.Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 22.Perkins WJ, Warner DO, Jones KA. Prolonged treatment of porcine pulmonary artery with nitric oxide decreases cGMP sensitivity and cGMP-dependent protein kinase specific activity. Am J Physiol Lung Cell Mol Physiol. 2009;296:L121–L129. doi: 10.1152/ajplung.90318.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz UR, Walter U, Eigenthaler M. Taming platelets with cyclic nucleotides. Biochem Pharmacol. 2001;62:1153–1161. doi: 10.1016/s0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- 24.Walders-Harbeck B, Khaitlina SY, Hinssen H, Jockusch BM, Illen-berger S. The vasodilator-stimulated phosphoprotein promotes actin polymerisation through direct binding to monomeric actin. FEBS Lett. 2002;529:275–280. doi: 10.1016/s0014-5793(02)03356-2. [DOI] [PubMed] [Google Scholar]
- 25.Hauser W, Knobeloch KP, Eigenthaler M, et al. Megakaryocyte hyperplasia and enhanced agonist-induced platelet activation in vasodilator-stimulated phosphoprotein knockout mice. Proc Natl Acad Sci U S A. 1999;96:8120–8125. doi: 10.1073/pnas.96.14.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–D693. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- 27.den Dekker E, Gorter G, Heemskerk JW, Akkerman JW. Development of platelet inhibition by cAMP during megakaryocytopoiesis. J Biol Chem. 2002;277:29321–29329. doi: 10.1074/jbc.M111390200. [DOI] [PubMed] [Google Scholar]
- 28.Pozner RG, Negrotto S, D’Atri LP, et al. Prostacyclin prevents nitric oxide-induced megakaryocyte apoptosis. Br J Pharmacol. 2005;145:283–292. doi: 10.1038/sj.bjp.0706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negrotto S, Pacienza N, D’Atri LP, et al. Activation of cyclic AMP pathway prevents CD34(+) cell apoptosis. Exp Hematol. 2006;34:1420–1428. doi: 10.1016/j.exphem.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Goichberg P, Kalinkovich A, Borodovsky N, et al. cAMP-induced PKCzeta activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood. 2006;107:870–879. doi: 10.1182/blood-2005-03-0941. [DOI] [PubMed] [Google Scholar]
- 31.Freson K, Peeters K, De Vos R, et al. PACAP and its receptor VPAC1 regulate megakaryocyte maturation: therapeutic implications. Blood. 2008;111:1885–1893. doi: 10.1182/blood-2007-06-098558. [DOI] [PubMed] [Google Scholar]
- 32.Casteel D, Suhasini M, Gudi T, Naima R, Pilz RB. Regulation of the erythroid transcription factor NF-E2 by cyclic adenosine monophosphate-dependent protein kinase. Blood. 1998;91:3193–3201. [PubMed] [Google Scholar]
- 33.Boerth NJ, Dey NB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res. 1997;34:245–259. doi: 10.1159/000159231. [DOI] [PubMed] [Google Scholar]
- 34.Battinelli E, Willoughby SR, Foxall T, Valeri CR, Loscalzo J. Induction of platelet formation from megakaryocytoid cells by nitric oxide. Proc Natl Acad Sci U S A. 2001;98:14458–14463. doi: 10.1073/pnas.241427398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molnar M, Suto T, Toth T, Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol. 1994;170:1458–1466. doi: 10.1016/s0002-9378(94)70179-2. [DOI] [PubMed] [Google Scholar]
- 36.Tomer A. Effects of anagrelide on in vivo megakaryocyte proliferation and maturation in essential thrombocythemia. Blood. 2002;99:1602–1609. doi: 10.1182/blood.v99.5.1602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure E1. (A) Expression of CD41 and CD61 in MK cultures. Fetal livers from 13.5 days postcoital embryos were explanted and cultured in Dulbecco’s modified Eagle medium with TPO (10 ng/mL) for 4 days. Cultures were analyzed by FACS for CD41 and CD61 expression (days 0 to 4). FLC are CD41 + on day 0. Expression of CD61 gradually increases as MKs mature in culture. Results are presented as mean 6 standard deviation from three independent experiments. (B) Characterization of FLC, MKs, or non-MKs after 2 and 4 days in culture. FLC, MKs (cells collected in pellet after BSA gradient), and non-MKs (nonsedimented cell fraction of gradient) from day 2 and 4 cultures were analyzed by phase-contrast microscopy and FACS (CD61 +). The starting FLC and non-MKs consisted of small cells that were negative for CD61 or have 4% to 6% CD61 + cells, respectively. Gradient enriched MKs are large cells, 70% to 80% and 80% to 90% CD61 + after days 2 and 4, respectively. (C) FLC were 2N to 4N. Day 2 MKs were ≤32N. Ploidy on day 4 increased to 64N.
Supplementary Figure E2. (A, B) MK cAMP and cGMP levels are not affected by TPO. Day 4 enhanced MKs were treated with forskolin (5 μM, 5 min), sodium nitroprusid (SNP; 10 μM, 5 min), or TPO (10 ng/mL, 15 min) and cAMP or cGMP levels measured by electroimmunoassay. Results of three independent experiments were normalized to the protein concentration and are means ± standard deviation. *Significance of p < 0.05, compared to control, n = 3, t test. (C) PKA/PKG activation does not interfere with TPO/c-Mpl signaling. Day 4 enriched MKs were resuspended in serum-free media, starved for 1 hour, and then stimulated for 15 min with TPO (10 ng/mL), 8-pCPT-cGMP (25 μM), or forskolin (5 μM) in the presence or absence of TPO. Phosphorylation of JAK2 (JAK2-P, Tyr 1007/1008) or VASP (VASP-P, Ser 239) was evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting in MKs. Total JAK2 and VASP were used as loading controls. Representative blots from three independent experiments are shown.
Supplementary Figure E3. (A) Signal densities from Figure 3B were quantified and expression levels of PKA-C, VASP, P-VASP Ser157, and P-VASP Ser239 in MKs (day 4) after treatment of cell cultures with PGE1 (1 μM) and forskolin (1 μM, 5 μM) are shown. (B) Signal densities from Figure 3D were quantified and expression levels of β-sGC, PKG-I, VASP, P-VASP Ser157, and P-VASP Ser239 in MKs (day 4) after treatment of cell cultures with ODQ (10 μM), DEA/NO (5 μM), L-NAME (100 μM), and 8-pCPT-cGMP (100 μM) are shown. Each bar represents the relative expression of the proteins indicated after different treatments, and setting the value from control as 1. *Statistically significant compared to control; p < 0.05, analysis of variance.