Abstract
Because diabetic women appear not to be protected by estrogen in terms of propensity to cardiovascular disease, we tested the possibility that chronic hyperglycemia modulates the effects of E2 on vascular cell growth in vitro. Human endothelial cells (E304) and vascular smooth muscle cells (VSMC) were grown in normal glucose (5.5 mmol/l), high glucose (22 mmol/l) or high manitol (22 nmol/l; an osmotic control) for 7 days. In endothelial cells glucose per se stimulated DNA synthesis. However E2- (but not RAL-) stimulated [3H] thymidine incorporation was attenuated in the presence of high glucose. In parallel, E2-dependent MAP-kinase-kinase activity was blocked in the presence of high glucose. High glucose increased basal creatine kinase (CK) specific activity, but E2-stimulated CK was not significantly impaired in the presence of high glucose. In VSMC, high glucose prevented the inhibitory effect of high E2 (but not of high RAL) concentrations on DNA synthesis. High glucose also prevented E2-induced MAP-kinase-kinase activity. In contrast, while high glucose augmented basal CK, the relative E2-induced changes were roughly equal in normal and high high glucose media. Hence, high glucose blocks several effects of E2 on vascular cell growth, which are mediated, in part, via the MAP-kinase system and are likely contributors to E2’s anti-atherosclerotic properties. Since RAL’s estrogen-mimetic effects on human vascular cell growth were independent of MAP-kinase activation and were not affected by hyperglycemia, the potential use of RAL to circumvent the loss of estrogen function induced by hyperglycemia and diabetes in the human vasculature should be further explored.
Keywords: Glucose, Vascular smooth muscle cells, Endothelial cells, MAP-kinase, MAP-kinase-kinase, Creatine kinase
1. Introduction
Gender-related protection from atherosclerosis in pre-menopausal women is apparently attributable to estradiol’s multiple favorable interactions with the arterial wall, lipid metabolism and fibrinolytic system [1–4]. Several studies have indicated that a premenopausal status does not confer cardiovascular protection in diabetic women [5–8]. Whether this apparent loss of estradiol-dependent functions results from the effects of glucose per se or some other diabetes-associated factors such as dyslipidemia, hyperfib-rinogenemia or hypertension is presently unknown. There is, however, a growing body of evidence that glucose can directly affect vascular smooth muscle cells (VSMC) and endothelial cells both in vitro and in vivo. For example, high extracellular glucose increases VSMC proliferation [9,10], activates the β2 and δ isoforms of VSMC protein kinase C [11,12], p38 mitogen-activated protein (MAP)-kinase and extracellular signal regulated kinases (ERK) 1/2 [11,13], increases endothelin secretion [14] and enhances the expression of intracellular adhesion molecule-1 in endothelial cells [15]. The possibility that high glucose levels interfere with estradiol’s effects in human vascular cells has not been directly tested. Nevertheless, recent evidence suggests that hormone replacement therapy induces less endothelial-dependent vasodilatation in the microcirculation in diabetic women compared with normal postmenopausal female patients [16].
In a series of previous reports we have shown that human umbilical artery smooth muscle cells, the cell type, which is the subject of the present report as well, express, both α and β estradiol receptors as detected by immunohistology and by western blot analysis [17,18]. We observed that estradiol exerts a biphasic effect on human VSMC growth, such that low concentrations stimulate whereas higher concentrations (in the range observed in premenopausal women) inhibit VSMC proliferation [17,18]. Additionally estradiol stimulated DNA synthesis in an endothelial cell line (E304) derived from the human umbilical vein in a dose-related fashion [17,18]. This dual effect of estradiol may favorably affect vascular response to injury in that it is consistent with better capacity for reendothelialization along with attenuation of post-injury myointimal proliferation. In the present study we examined the possibility that these presumably favorable effects of estradiol on cell growth are not operative in the presence of a high glucose concentration. To gain further insight into this question, we compared the effects of E2 in hyperglycemic conditions on growth in vascular cells to a better understood and well established classical genomic effect of E2, i.e., stimulation of creatine kinase (CK) specific activity.
2. Materials and methods
2.1. Cell cultures
2.1.1. Umbilical artery smooth muscle cells (VSMC)
Human umbilical artery VSMC was prepared as previously described with minor modifications [17,18]. Cells were used only at passages 1–3 when expression of smooth muscle actin was clearly demonstrable. To obtain “high glucose” conditions, the medium including the FCS, was supplemented with glucose up to a final concentration of 22 mmol/l (4.5 g/l). Glucose concentration in the regular medium was 5.5 mmol/l (1 g/l). Cells were grown to sub confluence (4–7 days) at the different glucose concentrations in the same medium containing FCS.
2.1.2. Endothelial cells (E304)
E304 cells, an endothelial cell line derived from a human umbilical vein, were purchased from American Type Culture Collection, Manassas, VA, USA (ATCC) and grown in medium 199 containing 10% FCS, glutamine and antibiotics. To obtain “high glucose” conditions, the medium including the FCS, was supplemented with glucose up to a final concentration of 22 mmol/l (4.5 g/l). Glucose concentration in the regular medium was 5.6 mmol/l (1 g/l). Cells were grown to sub confluence (4–7 days) at the different glucose concentrations in the same medium containing FCS.
2.2. Assessment of DNA synthesis
Cells were grown until subconfluence and then treated with various hormones or agents as indicated. Twenty-two hours later, [3H] thymidine was added for 2 h. Cells were then treated with 10% ice-cold trichloroacetic acid (TCA) for 5 min and washed twice with 5% TCA and then with cold ethanol. The cellular layer was dissolved in 0.3 ml of 0.3N NaOH, samples were aspirated and [3H] thymidine incorporation into DNA was determined [17,18].
2.3. Creatine kinase extraction and assay
To compare the effect of E2 on growth to the more classical effects of E2, we measured creatine kinase brain type specific activity, an established genomic response marker of E2.
Cells were treated for 4 h with the various hormones and agents as specified, and were then scraped off the culture dishes and homogenized by freezing and thawing three times in an extraction buffer as previously described [17,18]. Supernatant extracts were obtained by centrifugation of homogenates at 14,000 × g for 5 min at 4 °C in an Eppendorf microcentrifuge. Creatine kinase activity (CK) was assayed by a coupled spectrophotometric assay described previously [17,18]. Protein was determined by Coomasie blue dye binding using bovine serum albumin (BSA) as the standard.
2.4. Reagents and solutions
All reagents used were of analytical grade: chemicals, steroids, activated monophosphorylated threonine ERK-1/2, anti-MAP-kinase antibody (clone # ERK-PT115) and goat anti-rabbit peroxidase were purchased from Sigma (St. Louis, MI). General anti-MAP-kinase rabbit antibody [ERK-2 (c-14), sc-154] was purchased from Santa-Cruz Biotechnology (Santa-Cruz, CA). Enhancement solution was purchased from Wallac (Turku, Finland). The europium-chelating agent was a generous gift from I. Hem-milla (Wallac, Tkurku, Finland). TMB substrate kit for peroxidase and horse anti-mouse peroxidase were from Vector Lab. (Burlingame, CA). The MAP-kinase inhibitor UO126 was purchased from Alexis Corporation (Lausen, Switzerland).
Assay buffer and wash solution for the DELFIA assay were prepared as previously described [19]. The coating solution for the DELFIA assay was phosphate buffered saline (PBS), pH 7.4. Two buffers were used for Western immunoblotting: TBS (20 mM Tris, pH 7.6, and 137 mM NaCl) and T-TBS: (TBS containing 0.05% Tween-20).
2.5. Europium labeled reagents
Activated anti-MAP-kinase antibody (0.2 mg IgG in 0.9 ml PBS) was dialyzed against 50 mmol/l carbonate/bicarbonate buffer for 2 h. Europium labeling reagent (300 nmole in 100 µl of water) was then added. The reaction mixture was incubated overnight at 4°C and the labeled protein was purified by gel filtration on Sephadex G-25M. The europium labeled antibody was eluted with 50 mmol/l Tris–HCl buffer (pH 7.5) and stored at 4 °C until use.
2.6. Preparation of cell extracts for MAP-kinase
Each treatment was performed in quadruplet. Cells were grown until sub confluence and then tested with the different hormones as indicated. Effects on MAP-kinase were tested in cells, 5 days after the medium has been changed, at a time in which replacement with fresh medium would have taken place. Following 15 min exposure to the various hormones, cells were washed twice with calcium-and magnesium-free cold phosphate buffered saline (PBS). Subsequently, 0.3 ml of lysis buffer was added to each plate. Lysis buffer consisted of 20 mM Hepes, pH 7.5, containing 150 mM NaCl, 1% Triton-X 100, 10% glycerol, 1 mM EDTA, 1 mM EGTA, 10 mM NaF, 10 mM β-glycerol phosphate, 2 mM phenylmethylsulfonyl fluoride and protease inhibitors (1 mM benzamidine, 2 nM sodium vanadate, leupeptin 10 µg/ml, Aprotinin 10 µg/ml and pepstatin (10 µg/ml). The plates were gently agitated at 4°C for 10 min The cells were then scraped from each plate, and transferred to Eppendorf tubes. After centrifugation of the tubes at 4°C for 10 min at 14,000 × g, the supernatants (lysates) containing total cell extracts were removed. The cell lysate corresponding to each treatment was combined and divided into three aliquots. One aliquot was used for protein determination with Coomassie blue using BSA as the standard. Another aliquot of the cell lysate was used for Western immunoblotting while the third aliquot was used for a two-site MAP-kinase assay.
2.7. Two-site MAP-kinase assay
Microtiter strips (Labsysterms, Oy, Helsinki, Finland) were coated over 70 h at 4 °C with the general anti-MAP-kinase antibody (2.5 µg/ml PBS, pH 7.4, 200 µl per well). The antibody solution from each well was then decanted, and the microtiter strips were blocked with 200 µl per well blocking buffer (PBS containing 2% BSA) for 2 h at room temperature. The micro titer strips were then washed twice with buffer after which the cell lysates were then transferred (100 µl per well) in triplicate to the micro titer wells. Assay buffer (100 µl) was then added to each well and the strips were incubated overnight at 4°C and washed three times. Subsequently, Europium labeled, activated anti-MAP-kinase antibody (192 ng per well in 200 µl of assay buffer) was added, and the strips were incubated under shaking conditions for two hours at room temperature. The strips were then washed four times and processed for time-resolved fluorescence as described previously [19]. This assay actually determines the net change due to the combined effects on both kinase and phosphatase activities.
2.8. Western blot analysis
For Western blot immunoblotting equal amounts (30 µg) of cell extracts and molecular weight markers were subjected to SDS-PAGE as previously described [19]. Proteins were transferred to nitrocellulose membranes and stained with Ponceau Red to verify equal protein loading and transfer. After blocking with TBS containing 2% BSA for 2 h, membranes were incubated overnight with the general anti-MAP-kinase (1 g/ml T-TBS containing 1% goat serum) or the activated anti-MAP-kinase antibody (1 g/ml TBS containing 1% horse serum and 1% BSA) or TBS alone. Membranes were subsequently washed three times in T-TBS and incubated with secondary antibodies for 2 h (horse anti-mouse peroxidase at 1:10,000 dilution) in T-TBS containing 1% horse serum and 1% BSA for membranes probed with the activated anti-MAP-kinase antibody or the goat anti-rabbit peroxidase at 1: 10,000 dilution in T-TBS containing 1% goat serum or 1% BSA for membranes probed with the general anti-MAP-kinase antibody. Membranes were washed 3 times with T-TBS and processed for staining with the 3,3′,5,5′,-tetramethyl-benzoidine (TMB) substrate for peroxidase. Signals were then quantified by densitometry using the Quantify 1 program of Bio-Rad.
2.9. Statistical analysis
The significance of differences between the mean values obtained from experimental and controls were evaluated by two ways analysis of variance (ANOVA).
3. Results
3.1. Effect of high glucose on the interaction between estradiol and E304 cells
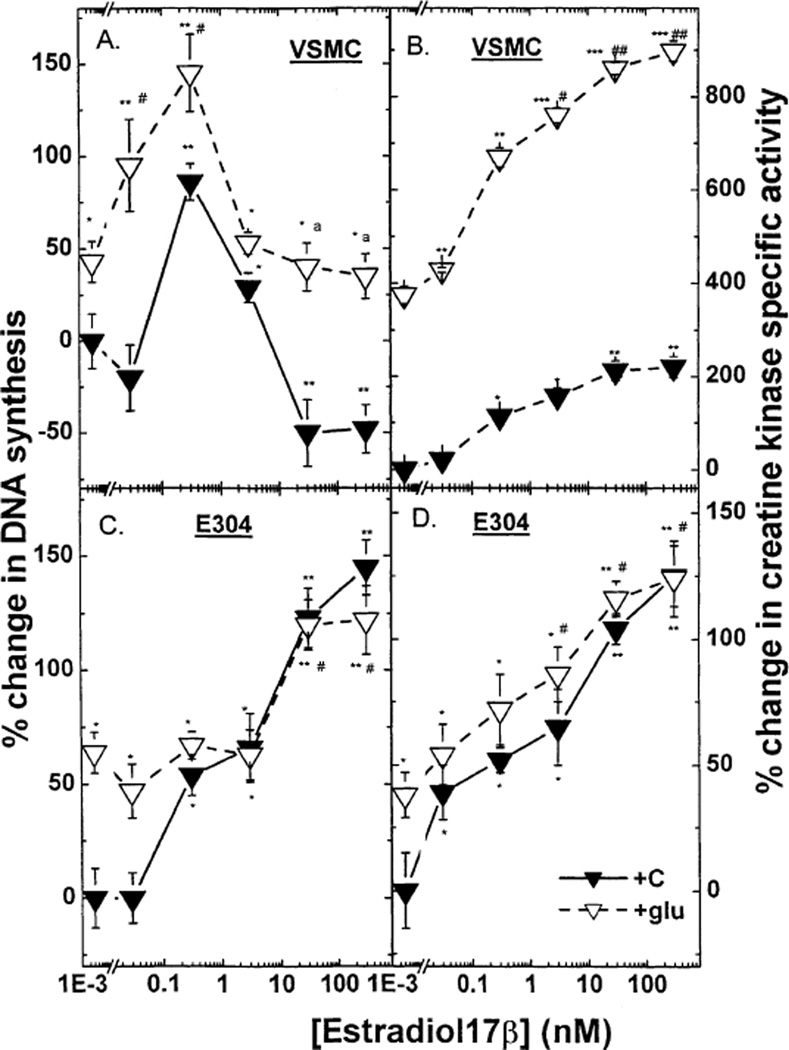
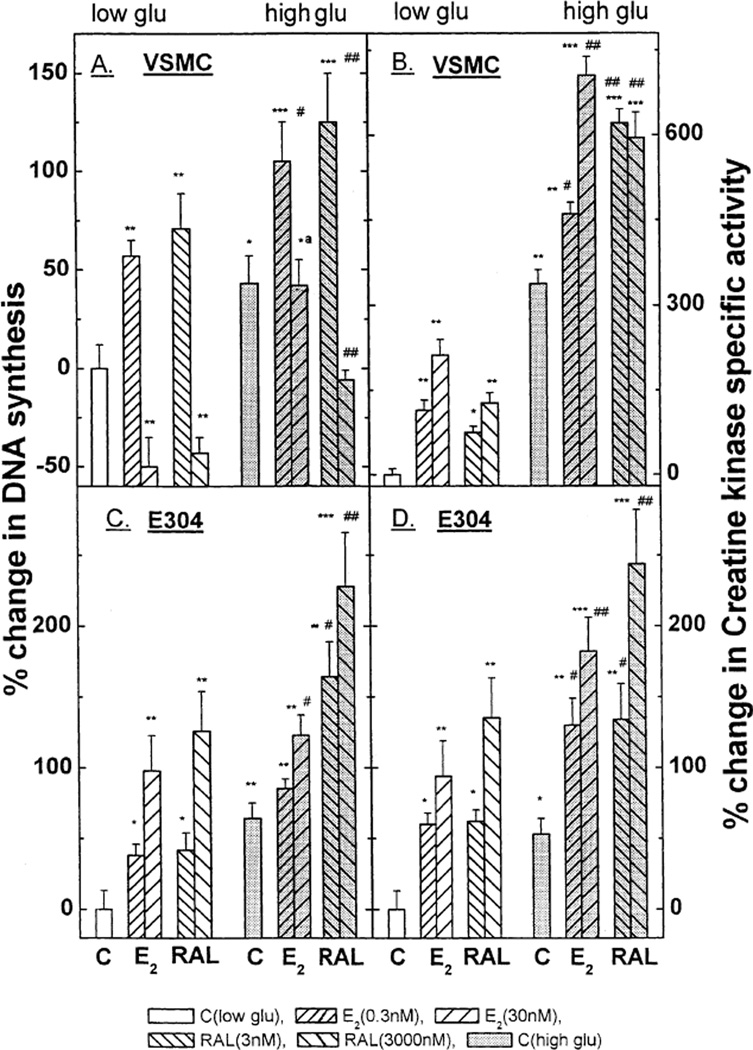
As shown in Fig. 1 (lower panels), high glucose modified the effect of E2 on both DNA synthesis and CK activity in E304 cells. High glucose per se increased [3H] thymidine incorporation into endothelial cells by ~ 65% (* P < 0.05). However, the growth response to estradiol was markedly attenuated such that even at the highest concentrations the relative increment induced by E2 was less than half of that seen under normal glucose conditions [40% versus 98% (* P < 0.01), respectively]. High glucose also increased basal CK activity by ~50% (* P < 0.01) (Fig. 1). However, E2-stimulated CK activity was not significantly impaired in the presence of high glucose, and indeed CK activity attained under these conditions was similar to the response to 3–300 mM of E2 under normal glucose concentrations.
Fig. 1.
The effect of estradiol 17β (0.03–300 nM) in the presence or absence of high glucose on 3[H] thymidine incorporation in VSMC (A), E304 cells (C), and on creatine kinase specific activity in VSMC (B) and E304 cells (D). Cells were prepared, grown and hormonally treated as described in the experimental section. Results are means ±S.E.M. of 4–12 incubates from 2 to 4 experiments and are expressed as the ratio between 3[H] thymidine incorporation or as the ratio between enzyme activity in hormone-treated and control (without hormone) cells. * P < 0.05; ** P < 0.01; *** P < 0.001 for the comparison with control values at low glucose; and # P < 0.05 and ## P < 0.01 for the comparison with control values at high glucose; a P < 0.05 for the comparison between the effect of E2 with and without glucose. The statistical analysis was done by ANOVA. The basal levels of creatine kinase specific activity in VSMC and in E304 cells were 0.050 ± 0.001 and 0.116 ± 0.017 µmol/min/mg protein respectively. The basal levels of 3[H] thymidine incorporation into DNA in VSMC and in E304 cells were 7200 ± 1080 and 94100 ± 12233 dpm per well, respectively.
To determine whether these effects of high glucose are restricted to estradiol, the proliferation and CK responses to IGF-1 and PDGF were also assessed as both growth factors were previously shown by us to stimulate cell growth in E304 and human umbilical VSMC [17]. High glucose had no effect on IGF-1-or PDGF-induced CK activity but slightly increased PDGF induced DNA synthesis, without affecting IGF-1 stimulated cell proliferation in E304 cells (Table 1).
Table 1.
Effect of high glucose on the interaction of growth factors with cell proliferation and CK specific activity in human vascular cells
|
3[H] thymidine incorporation into DNA (% change) | ||||
|---|---|---|---|---|
| E304 |
VSMC |
|||
| Low glucose | High glucose | Low glucose | High glucose | |
| C | 0 ± 13 | 0 ± 19 | 0 ± 17 | 0 ± 14 |
| IGF1 | 100 ± 20 | 110 ± 15* | 340 ± 25*** | 248 ± 30***,# |
| PDGF | 148 ± 10** | 186 ± 15**,# | 275 ± 30** | 227 ± 20*** |
| Creatine kinase specific activity (% change) | ||||
| C | 0 ±15 | 0 ± 9 | 0 ± 3 | 0 ± 19 |
| IGF1 | 68 ± 12* | 88 ± 15* | 68 ± 8* | 61 ± 23* |
| PDGF | 155 ± 12** | 133 ± 35** | 58 ± 8* | 110 ± 20**,## |
Effects of IGF1 (5 ng/ml) and PDGF (50 ng/ml) in the presence or absence of high glucose on 3[H] thymidine incorporation and on creatine kinase specific activity in VSMC and in E304 cells. Results are means±S.E.M. of eight incubates from two experiments and are expressed as percent change of 3[H] thymidine incorporation or enzyme activity in hormone-treated and control cells.
P < 0.05.
P < 0.01.
P < 0.001 for the comparison with control values at either low or high glucose.
P < 0.05.
P < 0.01 for the comparison of values of stimulation at low and high glucose. High glucose stimulated DNA in VSMC by 43 ± 14% and in E304 by 64 ± 9% and CK in VSMC by 375 ± 19% and by 38 ± 9% in E304. The statistical analysis was done by ANOVA.
3.2. Effect of high glucose on the interaction between estradiol and VSMC
Under normal glucose conditions E2 alone had a biphasic affect on [3H] thymidine incorporation by VSMC such that low concentrations stimulated, whereas high concentrations of E2 inhibited DNA synthesis (Fig. 1, upper panels). Further, in a high glucose medium a low concentration of E2 elicited a similar increment in DNA synthesis as in normal glucose medium. In contrast, the suppression of [3H] thymidine uptake induced by high concentrations of E2 in the presence of normal glucose was not observed under high glucose conditions (Fig. 1).
E2 induced a dose-dependent increase in VSMC CK activity. This response was clearly augmented in the presence of high glucose [nearly eightfolds versus fourfold increase over basal activity in a normal glucose medium (* P < 0.001)]. However the relative E2-induced changes were roughly equal in normal and high glucose media (4 versus 3.5 fold increase, respectively) (Fig. 1).
IGF-1—but not PDGF—induced [3H] thymidine incorporation was reduced under high glucose conditions (Table 1). However high glucose markedly enhanced the CK response to PDGF.
3.3. Effect of E2 on MAP-kinase expression and MAP-kinase-kinase activity in VSMC and E304 cells in normal and high glucose media
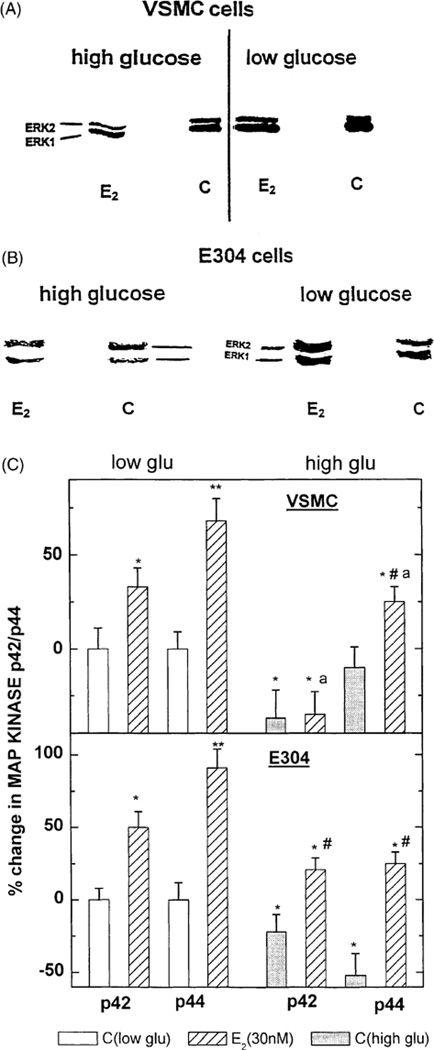
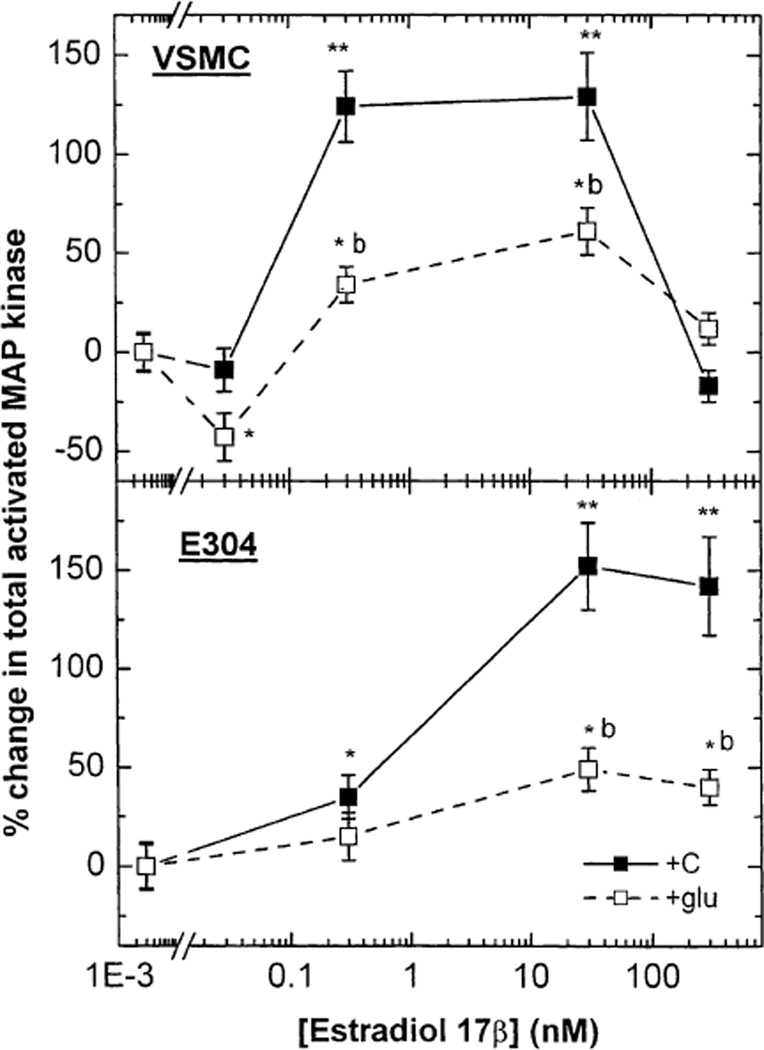
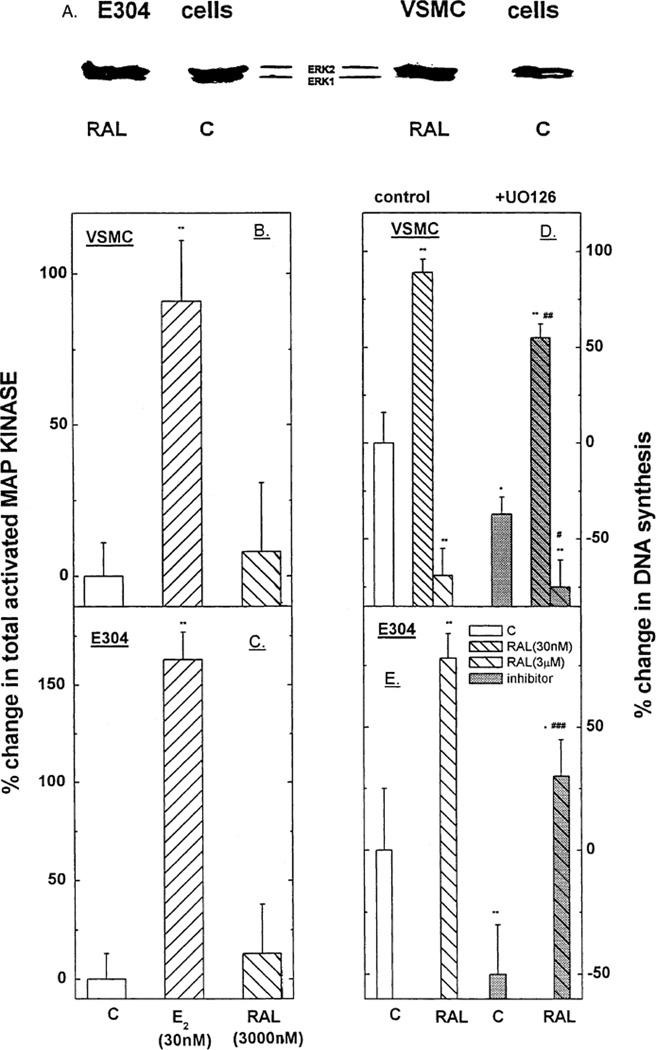
In both VSMC and E304 cell, E2 (0.3 and 30 or 30 nmol/l, respectively) increased the expression of P 42/44 MAP-kinase (ERK1/2) as detected by Western immunoblotting (Fig. 2A–C). The two-site MAP-kinase assay, which quantifies total phosphorylated MAP-kinase, revealed an E2-induced dose-dependent increase in MAP-kinase-kinase activity in both cell types (Fig. 3). In VSMC, high glucose concentration reduced baseline ERK1/2 and attenuated the stimulatory effect of E2 on ERK1/2 expression (Fig. 2A and C). Furthermore, under high glucose, the E2-dependent increase in total activated MAP-kinase in VSMC was reduced by more than 50% (* P < 0.05) (Fig. 3). In E304 cells, high glucose also suppressed basal ERK1/ERK/2 (P42/P44) expression (Fig. 2B and C) and reduced the levels attained following exposure to E2 below those seen under normal glucose conditions. Additionally, the E2-dependent increase in total activated MAP-kinase (MAP-kinase-kinase) in E304 cells was also reduced by high glucose (Fig. 3).
Fig. 2.
(A–C) Western blot depicting the effect of estradiol 17β at 30 nM in the presence or absence of high glucose on p42/p44 MAP-kinase in VSMC (A) and in E304 cells (B). Densitometric analysis of these gels corrected by the concentration of cell protein applied to the gels is shown in (C). Results are means of Western blot analysis of gels from two incubates from three experiments and are expressed as the ratio between the various treatments and control cells at a low glucose. * P < 0.05; ** P < 0.01 for the comparison with control values at low glucose; # P < 0.05 for the comparison with control values at high glucose; and a P < 0.05 for the comparison between the effect of E2 with and without glucose. The statistical analysis was done by ANOVA.
Fig. 3.
The effect of estradiol 17β (0.03–300 nM) in the presence or absence of high glucose on total activated MAP-kinase in VSMC and in E304 cells. Results are means of fluorometric assay of extracts from 2 to 3 incubates from 3 experiments and are expressed as the ratio between hormone-treated and control cells at a low glucose concentration. * P < 0.05; ** P < 0.01 for the comparison with control values at low glucose; # P < 0.05 for the comparison with control values at high glucose; and b P < 0.01 for the comparison between the effect of E2 with and without glucose. The statistical analysis was done by ANOVA as explained in the Materials and Methods section.
3.4. Effect of MAP-kinase-kinase inhibition on E2-induced DNA synthesis
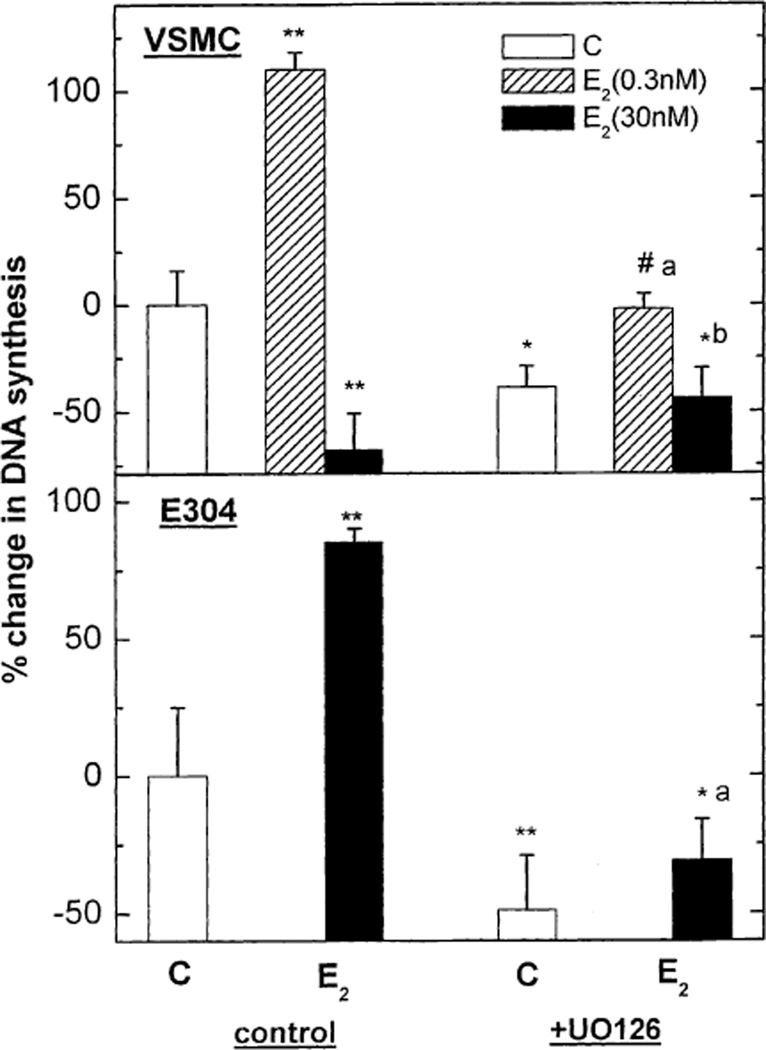
To further clarify the role of MAP-kinase in the estrogenic modulation of cell growth, VSMC and E304 were co-treated with E2 (0.3 and 30 nmol/l) and the MAP-kinase-kinase inhibitor UO126 (10−6 M). E2-stimulated MAP-kinase-kinase was significantly reduced in the presence of the inhibitor in both cell types [from 145 ± 23% to 35 ± 12% over basal activity (p < 0.05) in E304 cells, and from 110 ± 25% to 5 ± 12% over basal activity (P < 0.05) in VSMC cells]. The MAP-kinase-kinase inhibitor UO126 reduced both basal and E2-induced increase in DNA synthesis in E304 cells (Fig. 4). In VSMC, UO126 reduced basal [3H] thymidine incorporation and attenuated the increase in DNA synthesis elicited by low E2 concentrations. However, MAP-kinase-kinase inhibition had no significant effect on the relative stimulation (approximately twofold) of VSMC growth elicited by the low concentrations of E2 (Fig. 4). Finally, MAP-kinase-kinase inhibition entirely blocked the suppression of [3H] thymidine incorporation in VSMC elicited by a high concentration of E2.
Fig. 4.
The effect of MAP-kinase-kinase inhibitor; UO126 on estradiol 17β (0.3 or 300 nM)-dependent 3[H] thymidine incorporation in VSMC and in E304 cells. Results are means ± S.E.M. of 4–12 incubates from 2 to 4 experiments and are expressed as the ratio between 3[H] thymidine incorporation in hormone-treated and control cells. * P < 0.05; ** P < 0.01 for the comparison with control values; # P < 0.05 for the comparison with control values in the presence of UO126; and a P < 0.05; b P < 0.01 for the comparison between the effect of E2 with and without UO126. The statistical analysis was done by ANOVA.
3.5. Effect of high glucose on the interaction between raloxifene and vascular cells
In the presence of a normal glucose concentration the estrogen antagonist raloxifene (RAL) had full estrogen-mimetic action with respect to DNA synthesis and CK activity (Fig. 5) in VSMC and E304 cells. Nevertheless, RAL differed significantly from E2 in VSMC under high glucose conditions: a high concentration of raloxifene was still capable of inhibiting DNA synthesis even under high glucose conditions, an effect not elicited by E2 itself (Fig. 5A). In endothelial cells raloxifene induced the same relative increment in DNA synthesis under low and high glucose conditions (Fig. 5C; in absolute terms RAL-dependent DNA synthesis was enhanced in high glucose medium in parallel to the rise in the base line [3H] thymidine incorporation). The effects of RAL on CK activity in both VSMC and E304 cells closely paralleled those of E2 under both low and high glucose conditions (Fig. 5B and D).
Fig. 5.
Effects of raloxifene (RAL) (3 and 3000 nM) and estradiol 17β (E2) (0.3 and 30 nM) in the presence or absence of high glucose on 3[H] thymidine incorporation (A) and on creatine kinase specific activity (B) in VSMC and in E304 cells) (C and D), respectively). Results are means ± S.E.M. of 4–12 incubates from 2 to 4 experiments and are expressed as the ratio between 3[H] thymidine incorporation or as the ratio between enzyme activity in hormone-treated and control cells. * P < 0.05; ** P < 0.01; *** P < 0.001 for the comparison with control values at low glucose; # P < 0.05 and ## P < 0.01 for the comparison with control values at high glucose; a P < 0.05 for the comparison between the hormonal effect with and without glucose. The statistical analysis was done by ANOVA.
3.6. Effect of raloxifene on MAP-kinase
As shown in Fig. 6A RAL had no effect on ERK1/ERK2 expression in either VSMC or E304 cells. Likewise, total activated MAP-kinase (MAP-kinase-kinase activity) was not affected by RAL in either cell type (Fig. 6B and C). Finally, although the MAP-kinase-kinase inhibitor UO126 (10−6 M) lowered basal DNA synthesis in VSMC and E304 cells, it had no effect on the changes in [3H] thymidine incorporation induced by RAL (Fig. 6D and E). Specifically, the RAL-induced suppression of DNA synthesis in VSMC as well as the RAL-dependent enhancement of [3H] thymidine uptake in E304 cells was preserved in the presence of UO126. Finally, while UO126 reduced basal and RAL-induced DNA synthesis in E304 cells, neither the net change, nor the relative increases in [3H] thymidine incorporation were affected by MAP-kinase activation (Fig. 6E).
Fig. 6.
Western blot depicting the effect of raloxifene at 3000 nM as compared with estradiol 17β at 30 nM in the presence or absence of high glucose on ERK1/2 in VSMC and in E304 cells (A). Total MAP-kinase-kinase specific activity in the cells (B and C) under the stimulation of either E2 or RAL. Results are means ± S.E.M. of 4–12 incubates from 2 to 4 experiments and are expressed as the as the ratio between the various treatments and control cells at a low glucose. The effect of MAP-kinase-kinase inhibitor; UO126 on raloxifene (3 or 3000 nM)-dependent [3H] thymidine incorporation in VSMC and in E304 cells is shown in 6D. (E) Results are means ± S.E.M. of 4–12 incubates from 2 to 4 experiments and are expressed as the ratio between 3[H] thymidine incorporation in hormone-treated and control cells. * P < 0.05; ** P < 0.01; *** P < 0.001 for the comparison with control values; # P < 0.05 ## P < 0.01, ### P < 0.001 for the comparison with control values in the presence of UO126. The statistical analysis was done by ANOVA.
4. Discussion
The key finding in the present study is that chronic exposure to high glucose concentration substantially modifies the effects of estradiol on human vascular cell growth. First, high glucose per se increased DNA synthesis in VSMC. This observation is in accord with earlier reports that short-term incubation in a high glucose medium increases porcine, rabbit and human VSMC replication [8,9,20]. Second, increased glucose concentration did not affect the proliferative response to a low concentration of estradiol (in the range observed in men and postmenopausal women). Third, the inhibitory effect exerted by high E2 concentrations, (such as found in premenopausal women or under hormone replacement therapy) on DNA synthesis in VSMC in a normal glucose medium was not discernible under high glucose conditions. Fourth, high glucose attenuated the stimulatory effect of E2 on DNA synthesis in endothelial cells, whereas it did not moderate IGF1- or PDGF-stimulated endothelial growth. Thus, high glucose apparently blocks potentially important cell growth related effects by which estrogens may confer cardiovascular protection in premenopausal women. In contrast, high glucose did not block E2-induced activation of CK, a classical genomic E2 response marker [17].
Previous studies unveiled several estradiol-inducible pathways that could contribute to E2-mediated suppression of VSMC replication and/or stimulation of endothelial replication. For example, E2 acutely increases NO release in both endothelial cells [21,22] and VSMC [23,24], which, if sustained, could result in attenuation of VSMC proliferation via cyclic GMP-dependent mechanisms. Recent evidence indicates that in endothelial cells these estrogenic effects are rapid [21,25], involve early posttranscriptional activation of MAP-kinase (ERK1/2) [26] and can be induced by cell-membrane impermeant estrogenic ligands which bind to ERα, [21,25,27] thus suggesting a rapid, membrane-dependent, ERα-mediated non-genomic pathway of E2 action.
In contrast to these observations in endothelial cells, two previous studies indicated that E2 as well as several phytoestrogens, presumably acting via estrogen receptor dependent mechanisms, reduce both MAP-kinase activity and VSMC proliferation [28,29]. These results were interpreted as evidence that inhibition of MAP-kinase in VSMC may have a key role in estrogen-dependent suppression of proliferation of VSMC. However, in these studies the effects of E2 or phytoestrogens were tested under serum-stimulated conditions whereas in our experiments the effect of E2 was assessed under serum-deplete conditions (5 days after change of media). Because serum likely contains not only gonadal steroids by also many other growth factors, these apparently discordant results are difficult to compare.
In the present study, E2 elicited dose-dependent and largely similar increases in activated MAP-kinase in both human VSMC and E304 cells, despite discordant effect on DNA synthesis in these two cell types. Inhibition of MAP-kinase activation resulted in nearly complete blockade of E2-induced DNA synthesis in E304 cells. This suggests that in E304 cells, E2-induced DNA synthesis is mediated, at least in part, through MAP-kinase activation. The observation that a high glucose concentration reduces both E2-induced MAP-kinase activation and E2-stimulated [3H] thymidine uptake in endothelial cells is also consistent with this concept.
The relationship between MAP-kinase and DNA synthesis is more complex in VSMC. In these cells, MAP-kinase-kinase was also activated by E2. Further, in VSMC, MAP-kinase-kinase inhibition entirely prevented the inhibitory effect of high E2 concentrations on [3H] thymidine incorporation but had no effect on the relative enhancement of DNA synthesis elicited by a low E2 concentration. Finally, high glucose suppressed both E2-stimulated MAP-kinase-kinase activity and E2-induced inhibition of VSMC cell growth. Overall these findings suggest that E2-induced inhibition of cell growth in VSMC requires activation of MAP-kinase, but that the stimulation of VSMC replication induced by low E2 concentrations can be elicited independent of the p42/44 MAP-kinase system. Consistent with the latter is the finding that in VSMC, E2-stimulated DNA synthesis under high glucose conditions (observed with a low E2 concentration) is fully preserved despite the glucose-induced suppression of p42/44 MAP-kinase expression.
The observation that MAP-kinase activation can serve both hormone-induced acceleration of cell growth such as seen in E304 cells and inhibition of DNA synthesis such as appears in our VSMC experiments is not unprecedented. For example, whereas angiotensin II was reported to promote cell replication via ERK1/2 stimulation [30], vanadate was recently shown to inhibit balloon injury-induced proliferation of aortic smooth muscle cells via induction of p42/p44 MAP-kinase activity, which could be prevented by a MAP-kinase-kinase inhibitor [31]. Likewise, inducible nitric oxide synthase was shown to inhibit VSMC proliferation via activation of p42/p44 MAP-kinase, an effect that could be partially blocked inhibition of MAP-kinase activation [32].
Although the effects of raloxifene in VSMC and E304 cells, generally resemble those of E2, our study suggests that there may be a fundamental difference in the mechanisms by which these compounds affect vascular cell replication and that this difference may form the basis for the discordant actions of E2 and raloxifene under high glucose conditions. In contrast to E2, raloxifene did not affect the p42/44 MAP-kinase system, and the MAP-kinase-kinase inhibitor UO126 did not modify its effects on [3H] thymidine incorporation.
To the extent that MAP-kinase activation is a marker of a membrane-dependent pathway of estrogenic action, the MAP-kinase independent effects of raloxifene in human vascular cell growth are more consistent with the classical cytoplasmatic-nuclear model of gonadal steroid effects than with the more recently proposed non-genomic, cell-membrane mediated transduction cascade [21,25–27]. The finding that high glucose, which blocks MAP-kinase activation also inhibits E2’s but not raloxifene’s effects on vascular cell growth is also consistent with a cell-membrane independent mechanism of raloxifene’s action. Finally, the observation that CK activity, a classical marker of E2 genomic effects [33,34] was clearly inducible by both E2 and raloxifene under high glucose conditions suggest that high glucose may not modify estrogenic effects which are mediated by the classical cytoplasmatic-nuclear receptor pathway.
In summary, estrogen-mimetic effects on human vascular cell growth can be elicited by MAP-kinase dependent and independent mechanisms. Activation of the MAP-kinase system facilitates E2-induced endothelial cell growth and is required for E2-dependent inhibition of VSMC proliferation, and since MAP-kinase-kinase is attenuated by high glucose, neither of these two interactions is fully preserved under high glucose conditions. Raloxifene can elicit the same effects apparently via direct nuclear interaction, bypassing the MAP-kinase system, and hence its stimulatory effect on endothelial cell growth and suppressive effect on VSMC proliferation is maintained under high glucose conditions. Thus, estrogen-mimetic effects, which may be important for cardiovascular protection, i.e., stimulation of endothelial cell growth and inhibition of VSMC replication, are not preserved under high glucose conditions with E2 but can still be attained with raloxifene despite hyperglycemia. If these in vitro observations apply in the in vivo setting, the use of raloxifene may comprise a potential strategy to circumvent the presumed loss of E2 function in diabetic women. The possibility that raloxifene may offer better vascular protection than E2 in diabetes and hyperglycemia owing to the preservation of estrogen-mimetic inhibition of VSMC proliferation and acceleration of re-endotheliazation is currently under investigation.
During the submission of our manuscript, a report supporting our finding that the antiproliferative effect of E2 in human internal mammary artery smooth muscle cells was abolished by high glucose appeared in the literature (S. Ling, P.J. Little, M.R.I. Williams, A. Dai, K. Hashimura, J.-P. Liu, PA. Komesaroff, K. Sudhir (2002). High glucose abolishes the antiproliferative effect of 17β-estradiol in human vascular smooth muscle cells. Am. J. Physiol. Endocrinol. Metab. 282: E746-E751).
Acknowledgements
We would like to thank Mrs. Rivka Morris for her expert assistance in preparing this manuscript and Prof. A.M. Kaye for his help in the CK assays and fruitful discussion of the results.
References
- 1.Koh KK, Mincemoyer R, Bui MN, Csako G, Pucino F, Gkuetta V, Waclwin M, Cannon RO. Effects of hormone replacement therapy on fibrinolysis in postmenopausal women. N. Engl. J. Med. 1997;336:683–690. doi: 10.1056/NEJM199703063361002. [DOI] [PubMed] [Google Scholar]
- 2.Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, Szklo M. Association of hormone replacement therapy with various cardiovascular risk factors in postmenopausal women: the Atherosclerosis Risk in Communities Study investigations. N. Engl. J. Med. 1993;28:1069–1075. doi: 10.1056/NEJM199304153281501. [DOI] [PubMed] [Google Scholar]
- 3.Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Hori H. Amelioration of ischemia and reperfusion-induced myocardial injury by 17β-estradiol: role of nitric oxide and calcium-activated potassium channels. Circulation. 1997;96:1953–1963. doi: 10.1161/01.cir.96.6.1953. [DOI] [PubMed] [Google Scholar]
- 4.Williams JK, Honore EK, Adams DUM. Contrasting effects of conjugated estrogens and tamoxifen on dilator responses of atherosclerotic epicardial coronary arteries in nonhuman primates. Circulation. 1997;96:1970–1975. doi: 10.1161/01.cir.96.6.1970. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, McGee DL. Diabetes and cardiovascular disease: The Framingham Study. J. Am. Med. Assoc. 1979;241:2038–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 6.Kaseta JR, Skofar DF, Ram JL, Sowers JR. Cardiovascular disease in the diabetic women. J. Clin. Endocrinol. Metab. 1999;84:1835–1838. doi: 10.1210/jcem.84.6.5735. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Colditz GA, Stampfer MJ, Willet WC, Krolewski AS, Rosner B, Arky RA, Speizer FE, Hennekens CH. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 1991;151:1141–1147. [PubMed] [Google Scholar]
- 8.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1999;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 9.Natarajan R, Gonzales N, Xu L, Nadler J. Vascular smooth muscle cells exhibit increased growth response to elevated glucose. Biochem. Biophys. Res. Commun. 1992;87:552–560. doi: 10.1016/s0006-291x(05)81529-3. [DOI] [PubMed] [Google Scholar]
- 10.Yasunari K, Kohno M, Kano H, Yokokawa K, Miami M, Yoshidawa J. Mechanisms of action of troglitazone in the prevention of high glucose-induced migration and proliferation of cultured coronary smooth muscle cells. Circ. Res. 1997;81:953–962. doi: 10.1161/01.res.81.6.953. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZX, Yamachi T, Kubaki K, Meier M, Rhodes CJ, King GL. Glucose or diabetes activates p38 mitogen activated protein kinase in different pathways. J. Clin. Invest. 1999;103:185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoguchi T, Battan R, Handler E, Spofrtsman JR, Heath W, King G. Preferential Elevation of protein kinase C isoform ββ II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc. Natl. Acad. Sci. USA. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natarajan R, Scott S, Bai W, Yerni KK, Nadler J. Angiotensin II signaling in vascular smooth muscle cells under high glucose conditions. Hypertension. 1999;33:378–384. doi: 10.1161/01.hyp.33.1.378. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T, Ohuaka K, Takayanagi R, Umeda F, Nawata H. Enhanced secretion of endothelin 1 by elevated glucose levels from cultured bovine aortic endothelial cells. FEBS Lett. 1990;267:16–18. doi: 10.1016/0014-5793(90)80276-o. [DOI] [PubMed] [Google Scholar]
- 15.Takami S, Yamashita S, Kihara S, Kamed-Takemura K, Magtszawa Y. High concentration of glucose induces the expression of intercellular adhesion molecule-1 in human umbilical vein endothelial cells. Atherosclerosis. 1998;138:35–41. doi: 10.1016/s0021-9150(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 16.Lim S, Caballero AE, Arora SS, Smakowski P, Bashoff EM, Brawn FM, Logerfro FW, Horton ES, Veves A. The effect of hormone replacement therapy on vascular reactivity and endothelial function of healthy individuals and individuals with type 2 diabetes. J. Clin. Endocrinol. Metab. 1999;84:4159–4164. doi: 10.1210/jcem.84.11.6160. [DOI] [PubMed] [Google Scholar]
- 17.Somjen D, Kohen F, Jaffe A, Amir-Zaltsman Y, Knoll E, Stern N. Effect of gonadal steroids and their antagonist on DNA synthesis in human vascular cells. Hypertension. 1998;32:39–45. doi: 10.1161/01.hyp.32.1.39. [DOI] [PubMed] [Google Scholar]
- 18.Somjen D, Kohen F, Amir-Zaltsman Y, Knoll E, Stern N. Vitamin D analogs modulate the action of gonadal steroids in human vascular cells in vitro. Am. J. Hypertens. 2000;13:396–403. doi: 10.1016/s0895-7061(99)00203-4. [DOI] [PubMed] [Google Scholar]
- 19.Amir-Zaltsman Y, Mazor O, Gayer B, Scherz A, Salomon Y, Kohen F. Inhibitors of protein tyrosine phosphorylation: preliminary assessment of activity by time-resolved fluorescence. Luminescence. 2000;15:377–380. doi: 10.1002/1522-7243(200011/12)15:6<377::AID-BIO619>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Aveno R, Arora S, Carnody BJ, Cosby K, Sidawy AN. Thiamine (Vitamin B1) Protects against glucose-and insulin-mediated proliferation of human infragenicular arterial smooth muscle cell proliferation. Am. Vasc. Surg. 2000;14:37–43. doi: 10.1007/s100169910007. [DOI] [PubMed] [Google Scholar]
- 21.Haynes MP, Shina D, Russel KS, Collinge M, Fulton D, Morales-Ruiz A, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ. Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 22.Russel KS, Haynes MP, Shina D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane-binding sites for estradiol, which mediate rapid intracellular signaling. Proc. Natl. Acad. Sci. USA. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binko J, Majewski H. 17β-Estradiol reduces vasoconstriction in endothelial-denuded rat aortas through inducible Nos. Am. J. Physiol. 1998;274:H853–H859. doi: 10.1152/ajpheart.1998.274.3.H853. [DOI] [PubMed] [Google Scholar]
- 24.Saito S, Aras RS, Lou H, Ramwell PW, Foegh L. Effects of estrogen on nitric oxide synthase expression in rat aorta allograft and smooth muscle cells. Heart Lung Transplant. 1999;18:937–945. doi: 10.1016/s1053-2498(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 25.Stefano GB, Prevot V, Beanvillain JC, Cadet P, Finiani C, Welters I, Frichione GL, Breton C, Lassalle P, Salzet M, Belfinger TV. Cell surface estrogen receptor mediate calcium-dependent nitric oxide release in human endothelia. Circulation. 2000;108:1594–1597. doi: 10.1161/01.cir.101.13.1594. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelson ME, Shaul PW. Estrogen receptor alpha mediates the non-genomic activation of endothelial nitric oxide synthase by estrogen. J. Clin. Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HP, Lee JY, Jeong JK, Bae SW, Lee HK, Jo I. Non-genomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor alpha localized in caveolae. Biochim. Biophys. Res. Commun. 1999;263:257–262. doi: 10.1006/bbrc.1999.1348. [DOI] [PubMed] [Google Scholar]
- 28.Doum GB, Levkau NL, Chamberlain Y, Wang AW, Clower AW. The mitogen-activation protein kinase pathway contributes to vanadate toxicity in vascular smooth muscle cells. Mol. Cell. Biochem. 1998;183:97–103. doi: 10.1023/a:1006820214072. [DOI] [PubMed] [Google Scholar]
- 29.Morey AK, Pedram A, Razandi M, Prims BA, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology. 1997;138:3330–3339. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- 30.Nozawa Y, Matsuura N, Mikyake H, Yamada S, Kimura R. Effects of TH-14277 on angiotensin II-induced proliferation, migration and intracellular signaling in vascular smooth muscle cells and on neointimal thickening after balloon injury. Life Sci. 1999;64:2061–2070. doi: 10.1016/s0024-3205(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 31.Dubey RK, Gillespi DG, Imthurn B, Rosseli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP-kinase activity in human aortic smooth muscle cells. Hypertension. 1999;33:177–182. doi: 10.1161/01.hyp.33.1.177. [DOI] [PubMed] [Google Scholar]
- 32.Kibbe MR, Li J, Nie S, Watkins SC, Lizonova A, Kovesdi I, Simmons RL, Billiar TR, Tzeno E. Inducible nitric oxide synthase (i NOS) expression upregulates p 21 and inhibits vascular smooth muscle cell proliferation through p 42/44 mitogen-activated protein kinase activation and independent of p 53 and cyclic guanosine monophosphate. J. Vasc. Surg. 2000;31:1214–1228. doi: 10.1067/mva.2000.105006. [DOI] [PubMed] [Google Scholar]
- 33.Kaye AM, Weisman Y, Harell A, Somjen D. Hormonal stimulation of bone cell proliferation. J. Steroid. Biochem. Mol. Biol. 1990;37:431–435. doi: 10.1016/0960-0760(90)90494-6. [DOI] [PubMed] [Google Scholar]
- 34.Somjen D, Waisman Y, Harell A, Berger E, Kaye AM. Direct and sex-specific Stimulation by sex steroids of creatine-kinase activity and DNA synthesis in rat bone. Proc. Natl. Acad. Sci. USA. 1989;86:3361–3365. doi: 10.1073/pnas.86.9.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]