Abstract
Brain function depends upon complex metabolic interactions amongst only a few different cell types, with as-trocytes providing critical support for neurons. Astrocyte functions include buffering the extracellular space, providing substrates to neurons, interchanging glutamate and glutamine for synaptic transmission with neurons, and facilitating access to blood vessels. Whereas neurons possess highly oxidative metabolism and easily succumb to ischemia, astrocytes rely more on glycolysis and metabolism associated with synthesis of critical intermediates, hence are less susceptible to lack of oxygen. Astrocytoma and higher grade glioma cells demonstrate both basic metabolic mechanisms of astrocytes as well as tumors in general, e.g. they show a high glycolytic rate, lactate extrusion, ability to proliferate even under hypoxia, and opportunistic use of mechanisms to enhance metabolism and blood vessel generation, and suppression of cell death pathways. There may be differences in metabolism between neurons, normal astrocytes and astrocytoma cells, providing therapeutic opportunities against astrocytomas, including a wide range of enzyme and transporter differences, regulation of hypoxia-inducible factor (HIF), glutamate uptake transporters and glutamine utilization, differential sensitivities of monocarboxylate transporters, presence of glycogen, high interlinking with gap junctions, use of NADPH for lipid synthesis, utilizing differential regulation of synthetic enzymes (e.g. isocitrate dehydrogenase, pyruvate carboxylase, pyruvate dehydrogenase, lactate dehydrogenase, malate-aspartate NADH shuttle) and different glucose uptake mechanisms. These unique metabolic susceptibilities may augment conventional therapeutic attacks based on cell division differences and surface receptors alone, and are starting to be implemented in clinical trials.
Keywords: Astrocyte, glycolysis, hypoxia-inducible factor, lactate, metabolism, mitochondria
1. Introduction
In healthy brain, astrocytes demonstrate a critical integrative role in regulating brain function. Neuronal-glia interactions are crucial for regulating brain energy metabolism, neuronal excitability and synaptic activity [1-5]. Due to their highly dynamic nature astrocytes can both adjust their metabolism and refine their shape in response to extracellular signals to better support neuronal function. Astrocytes also critically regulate substrate delivery from the blood to neurons, to maintain an adequate rate of oxidative metabolism in response to metabolic demand. In addition, astrocytes can favor either glycolysis or oxidative metabolism by sensing the availability of oxygen and energy substrates in the extracellular space [6-10]. Here we explore how metabolic pathways that facilitate the adaptation of astrocytes in response to various environmental stimuli may also be critical to astrocytoma development, malignant transformation, and tumor growth. Thus, the considerable adaptability demonstrated by normal astrocytes can be subverted to enhance astrocytoma survival and proliferation.
In this review we first briefly discuss the characteristics of astrocytes that lend inherent plasticity to CNS function, and which also facilitate the nervous system to respond to metabolic challenges. Since we have previously briefly reviewed a wide range of inherent astrocyte characteristics which are critical to overall CNS metabolic functioning [11], we now focus in on several metabolic features which already have been demonstrated to show a transition towards treatment possibility in astrocytoma models.
The origin of astrocytomas continues to be uncertain, but one approach considers that changes in the control of gene expression by DNA binding proteins along with specific DNA mutations can induce malignant transformation in astrocytes [12] as well as in neural stem cells (nestin positive stem cells which typically develop into astrocytes) [13, 14]. Thus, astrocytomas may occur via missteps in the differentiation cascade from primary stem cells in the CNS to astrocytes, or alternatively, from astrocytes that have acquired characteristics of undifferentiated glia and eventually progress into malignant cells [12-14]. Moreover, advanced astrocytomas or glioblastomas consistently demonstrate the presence of tumor stem cells, particularly in highly hypoxic regions, which maintain some characteristics of normal neural stem cells (such as the ability to form neurospheres and to develop into nervous system cells in vitro). However, these tumor stem cells also demonstrate abnormal proliferative ability, aberrant expression of differentiation markers and chromosomal abnormalities; and strongly support tumor maintenance and recurrence [15-17]. Thus, astrocytomas may initially function similar to a population of astrocytes, but with growth may increasingly demonstrate a number of abnormal metabolic pathways, which could further enhance their growth in an opportunistic manner. We next summarize possible targets of vulnerability for metabolic assaults on astrocytomas.
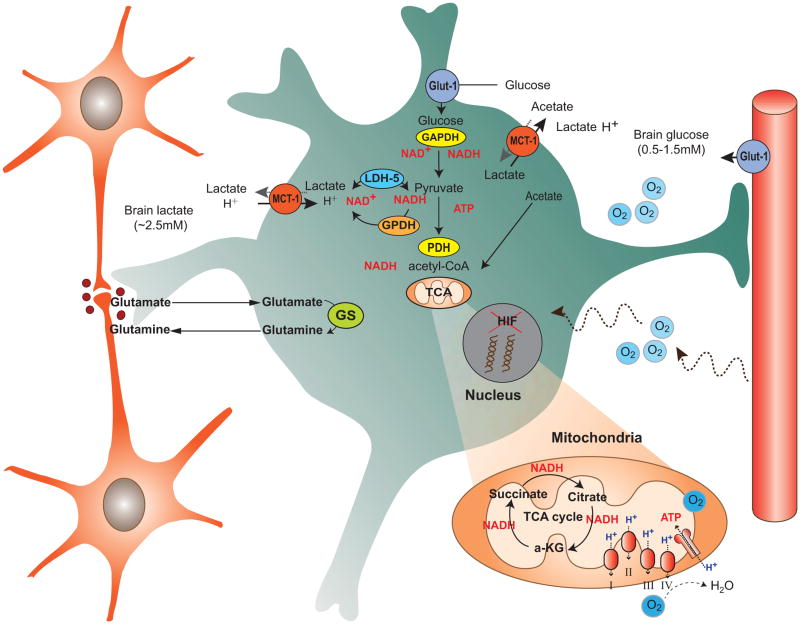
2. Astrocyte - Metabolism and Vascular Interactions (Fig. 1)
Fig. 1.
This diagram shows the important metabolic interactions between the cellular elements of the brain: two neurons are shown on the left (orange), whereas the primary roles of astrocytes (green) are illustrated in terms of metabolic processing (center), as an interface to blood vessels and blood brain barrier (right). Neurons are shown to release glutamate, which is then transported into astrocytes, converted to glu-tamine and exported back for neuronal packaging back into glutamate (using glutamine synthetase, GS). Metabolites present in the extracellular space are shown (glucose: 0.5-1.5 mM concentration; brain lactate ∼ 2.5 mM; systemic lactate ∼ 0.75 mM) as well as transporters (glucose GLUT1 on endothelium and astrocytes, monocarboxylate transporter (MCT1) on endothelium and astrocytes). Astrocytes are net exporters of lactate, derived from glycolysis, maintaining a high extracellular level of lactate for neuronal uptake and metabolism. Oxygen is readily available in the extracellular space for both neuronal and astrocytic oxidative metabolism, even though considerable astrocytic ATP is derived from glycolysis.
2.1. Metabolic Properties of Astrocytes
Astrocytes are at the boundary between the vascular network and neurons, and hence regulate substrate transport between blood vessels, the extracellular space, and neurons, by reinforcing the blood brain barrier. The end-feet processes of astrocytes are adjacent to blood vessels and modulate many blood brain barrier attributes, including strengthening the tight junctions between endothelial cells and facilitating substrate transport. Astrocytes are thought to be critically involved with glucose and monocarboxylate transport into the extracellular space, expressing similar glucose (Glut1) and monocarboxylate transporters (MCT1) to those present on blood vessels, to maintain sufficient levels of glucose (i.e. 0.5-1.5 mM), lactate and acetate in the extracellular space [9, 18, 19]. Numerous transporters expressed in astrocytes contribute to maintenance of extracellular homeostasis, for example NKCC1 (Na+, K+, 2Cl- co-transporter), NA+-K+-ATP-ase, EAAT1 and 2 (l-glutamate and l-aspartate transporter), which function to remove excess, Na+, K+, and glutamate from the extracellular space, respectively. Astrocytes communicate via gap junctions that contribute to the formation of an extended astrocytic network, facilitating redistribution of lactate, glucose, glutamate, Ca2+, and K+ to different cellular or extracellular locations along their concentration gradient or in response to specific stimuli. Calcium waves that propagate within the glial syncytium, demonstrated both in vitro [9, 20] and in vivo, may be mediated by ATP release, and often are associated with changes in neuronal activity and modulation of cerebral blood vessels as well [21].
By buffering the extracellular space, astrocytes can detect and modulate the level of synaptic activity and send appropriate signals to nearby blood vessels to increase blood flow and substrate delivery, utilizing a wide range of chemical (i.e, K+) and diffusible substances (ie, NO, O2, H2O2, etc.). Astrocytes, unlike neurons, are considered able to maintain ATP levels through mainly glycolytic metabolism; therefore under glucose deprivation, ATP production by astrocytes is significantly decreased. On the other hand, astrocytes can survive during hypoxia if there is an adequate supply of glucose. This low sensitivity to hypoxia is evident by the well-known capability to culture astrocytes (or astrocytoma cells) for up to 24 hours from adult brain tissue (even cadaveric), whereas neurons survive less than 3-5 min in an anoxic environment [22].
Magnetic resonance spectroscopy studies using [2-13C]-acetate demonstrate that astroglia may contribute up to15% of the total oxidative metabolism in the brain [23]. However, the majority of glucose is oxidized in the neuronal compartment [24] and less in the glia [25]: specifically, 65% of glucose oxidative metabolism occurs in neurons vs 35% in as-trocytes [24]. Astrocytic oxidative metabolism is restricted to the cell body and larger astrocytic processes because certain astrocytic compartments, such as the filopodia, are too thin to accommodate mitochondria. These thin filopodia and astrocytic processes therefore depend exclusively on anaerobic glycolysis for ATP production, by utilizing glucose either taken up directly from the extracellular space or derived from glycogen breakdown, with lactate release [26-30] as the endpoint [3]. Accumulation of lactate can inhibit glycolysis at the level of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) step, due to competition with cytosolic NAD+. Therefore, to maintain a high rate of glycolysis during increased metabolic demand and especially during hypoxia, astrocytes export lactate to the extracellular space via low affinity monocarboxylate transporters MCT1 and MCT4 that are preferentially expressed in astroglia cells (see below).
The relative contribution of specific metabolic pathways in astrocytes to energy production is dependent on relative substrate availability and the metabolic state of the cell. For example, in astrocytic cultures exposed to high glucose concentration the rate of oxidative metabolism is low; but when glucose concentration in the extracellular media was reduced from 22 to 2 mM the rate of oxidation of glucose and lactate increased significantly [31]. A similar increase in the rate of glucose and lactate oxidation in astrocytes occurred with aspartate incubation due to the intense metabolic demand [31].
In contrast to neurons, astrocytes can also store reserve energy as glycogen; glycogen deposits have been observed particularly in small astrocytic processes [3, 6, 7]. Utilization of glycogen-derived glucose-6-phospate occurs in situations where there is a rapid increase of energy demand, such as intense neuronal stimulation or spreading depression, as well as during hypoglycemia [6]. Pyruvate produced as a result of glycolytic metabolism can enter a variety of metabolic pathways. In the cytosol pyruvate can be converted to lactate in a reaction catalyzed by lactate dehydrogenase (LDH) or to alanine by transamination. In the mitochondria pyruvate, after being oxidized to acetyl coenzyme A (acetyl-CoA) by pyruvate dehydrogenase (PDH), can enter the TCA cycle and be degraded to CO2 and water. In neurons, metabolic reactions within the TCA cycle are primarily directed toward the ATP formation via oxidative degradation of pyruvate to CO2 and water, but also the generation of synthetic intermediates.
In astrocytes, however, TCA cycle metabolic intermediates more often leave the cycle to serve as precursors for the synthesis of amino acids (particularly glutamate), phospholipids (for synthesis of membranes) or for gluconeogenesis. For this reason new metabolic intermediates are continuously synthesized to sustain the carbon flux along the TCA cycle as well as oxidative phosphorylation. This process of replenishment of metabolic substrates for internal use within the TCA cycle is termed anaplerosis; in contrast, cataplerosis indicates when intermediates are removed from the TCA cycle for synthesis of structural and metabolic compounds. Astrocytes, unlike neurons, can carry out a net synthesis of TCA cycle intermediates via a reaction mediated by pyruvate carboxylase (PC) that adds CO2 to pyruvate (pyruvate carboxylation) to form oxaloacetate. Condensation of oxaloacetate with acetyl-CoA will result in the formation of citrate and eventually α-ketoglutarate (α-KG), succinate, and malate. Because pyruvate carboxylase is expressed in astrocytes but only minimally in neurons [32], this reaction is essential for replenishing TCA cycle intermediates particularly in astrocytes (astrocyte intermediates are eventually transferred also to neurons) [3].
A large aspect of astrocyte functioning is focused on glutamate replenishment after its release from neuronal terminals upon synaptic activation. Glutamate is rapidly removed by astrocytes from the extracellular space near synapses via glutamate transporters, together with Na+. Rapid glutamate uptake via transporters into astrocytes regulates the activity of glutamate at the synapses and prevents its potential toxic effects [3, 10, 33]. To complete this cycle, astrocytes are able to replenish neuronal glutamate stores via the glutamate-glutamine cycle, which requires the conversion of glutamate to glutamine (via glutamine synthetase) and glutamine extrusion for subsequent neuronal uptake. After entering neurons, glutamine is converted back to glutamate via the enzyme glutaminase. However, glutamate can be recycled only a few times in the glutamine-glutamate cycle [3] and is degraded first by oxidative deamination and formation of a-ketoglutarate in the TCA cycle. Therefore astrocytes need to synthesize new glutamate via TCA cycle intermediates, which requires two pyruvate molecules, one for oxaloacetate formation and the other for synthesis of acetyl CoA; together they generate a ‘new’ 6-carbon compound that is decarboxylated to form a-ketoglutarate, and transaminated to form glutamate [3].
2.2. Glia-Neuronal Vascular Interactions
Vascular coupling between neurons, astrocytes and blood vessels is regulated by diffusible factors, particularly by the generation of NO by nNOS (neuronal nitric oxide synthetase) in response to neuronal activity, but also by changes in the NADH/NAD+ ratio and a number of enzymatic cascades [34, 36, 37]. NO can mediate vasodilation directly via cyclic guanine monophosphate (cGMP) increase in arterioles, and indirectly by modulating astrocytes signaling pathways. Direct astrocyte signaling to blood vessels includes the release of K+ from the end-feet directly to the blood vessel and the production and release of arachidonic acid (AA) and its metabolites epoxyeicosatrienoic acids (EETs) and prostaglandin (PGs). The initiation of these signaling pathways depends on glutamate release from neurons and the activation of metabotropic glutamate receptors (mGLUR) expressed on astrocytes, which stimulates Ca2+ influx into the astrocytes [35]. While the release of EET, PG, and K+ mediate vasodilation, AA release leads to vasoconstriction after being converted to 20-hydroxyeicosatetraenoic acid by ω-hydroxylases in the vascular smooth muscle. Oxygen itself, among other diffusible factors, can also regulate the vascular tone in the brain. As a signaling molecule, O2 modulates the activity of nNOS and the synthesis of PG and EETs from AA. In addition, changes in pO2 induce metabolic alterations that modulate the neurovascular response. For example, lower oxygen concentration results in a drop in ATP levels as a consequence of decreased synthesis of ATP, and with enhanced degradation of ATP to ADP there is a secondary increase of adenosine levels in the extracellular space; this increased extracellular adenosine can bind to A2 adenosine receptors in the smooth muscle causing vasodilation [34]. Moreover, because lactate injections in vivo directly increase the NADH/NAD+ ratio and tissue blood flow, investigators have proposed that increased NADH/NAD+ ratio in the brain, which occurs as a consequence of the elevated lactate/pyruvate ratio present after neuronal activation, promotes vasodilation to restore the cellular redox state and maintain ATP production to meet metabolic demand [36, 37].
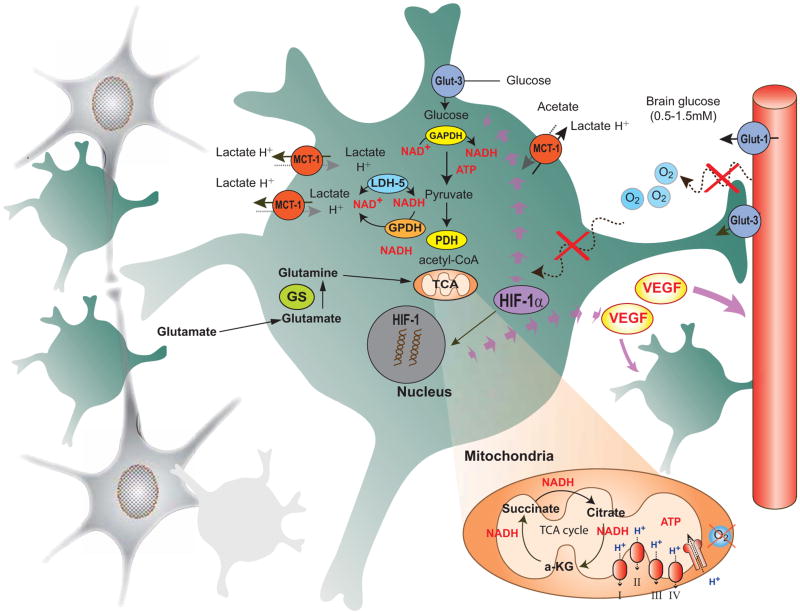
3. Neuronal-Astrocyte-Vascular Metabolic Unit Vulnerabilities - Targets for Treatment (Fig. 2)
Fig. 2.
This diagram illustrates the subversion of normal cellular metabolic interactions for the maintenance and growth of glioma cells. Rapid astrocytoma tumor growth and expansion lead to additional glial cells (as shown by numerous green astrocytes), and neurons are dele-teriously affected by tumor growth, lack of oxygen, and extracellular toxicity. The rapid tumor growth leads to lack of blood vessels, hypoxia and depletion of extracellular glucose, causing a switch to the more sensitive GLUT3 glucose transporter, increases in extracellular lactate and promotion of migration. Upregulation of hypoxia inducible factor-1 HIF1signaling pathways results in the upregulation of various hypoxia regulated genes including vascular endothelial growth factor (VEGF) to enhance glycolysis, angiogenesis, glia proliferation, and blood-brain barrier breakdown. Due to rapid tumor growth and dependence on glycolysis extracellular lactate is enhanced and there is decreased oxidative phosphorylation present, even though the mitochondrial TCA cycle continues the synthesis of amino acids for cell growth and maintenance. Many ordinary characteristic astrocyte enzymatic and cellular processes are enhanced with this transformation into a tumor phenotype, yet still may provide susceptible points of metabolic treatments.
Astrocytoma cells maintain and extend the usual characteristics of normal astrocytes, with a high level of glycolytic functioning and lactate extrusion, but also demonstrate growth and invasion into brain, even in a relatively anoxic environment [38]. Astrocytoma cells likely share more characteristics with immature astrocytes than mature ones, but basic metabolic functions remain common to both. As tumor cells deviate metabolically from astrocytes a number of changes occur due to the relative anoxia, particularly development of a high rate of glycolysis and enhancement of TCA cycle synthetic function sufficient to boost cell replication; collectively, these changes are termed the Warburg effect [39, 40]. The cellular metabolism of astrocytomas depends on glucose utilization via glycolysis and scavenging of other substrates such as glutamate and glutamine for energy; the rate of glucose uptake shows an increase even in the presence of oxygen and is higher than in normal astrocytes, suggesting that targeting glycolytic metabolism (and its byproducts, such as a high lactate level) may be effective in limiting tumor growth.
In astrocytomas and in many other cancers the majority of pyruvate produced from glucose oxidation during glycolysis is reduced to lactate, which is then transported into the extracellular space. This reaction, typical of anaerobic glycolysis in cancer cells, can occur also in the presence of oxygen (aerobic glycolysis) and is enabled by two key enzymes, LDH and pyruvate dehydrogenase kinase. Lactate generation via LDH and subsequent extrusion is crucial for glioma cells to maintain high levels of NAD+ in the cytoplasm and high rate of glycolysis. Otherwise, the accumulation of intracellular lactate will drive the activity of LDH toward pyruvate production, shifting the NADH/NAD+ ratio toward reduction, thus inhibiting glycolysis at the level of GAPDH [41].
In addition to enhancing their ability to survive in hypoxic conditions, maintaining high glycolytic rates gives cancer cells other metabolic advantages that ultimately support cellular proliferation. Many glycolytic intermediates can also be diverted from glycolysis to parallel anabolic pathways. For example, glucose-6P can enter the pentose phosphate pathway which contributes to the formation of NADPH, necessary for the biosynthesis of lipids, nucleotides (such as ribose-5-phospate, a nucleotide precursor), and glutathione. As described for normal astrocytes, in astrocytomas mitochondrial metabolism in the TCA cycle is not strictly tied to oxidative phosphorylation, but critically provides intermediates for lipid, amino acid and nucleotide biosynthesis. However, these features are amplified and distorted within astrocytoma cells. In this way cancer cells can rely on endogenous synthesis of nucleotides and other crucial structural components to meet their proliferating needs even under condition of poor vascularization and limited access to the exogenous pool of nutrients.
The hypothesis of this review is that astrocytoma cells retain the basic metabolic characteristics of immature astrocytes, but many of these characteristics are distorted and exaggerated, with less metabolic control possible, but which may provide unique pathways of susceptibility for possible understanding of invasiveness and treatment. We highlight some of these pathways, many of which are now being considered for clinical investigation in the treatment of astrocytomas.
3.1. HIF Signaling Pathway
Oxygen and glucose levels in the brain are tightly regulated to ensure an adequate oxygen supply to support neuronal function, and cellular metabolism rapidly responds to variation of oxygen and glucose levels to maintain optimal ATP production. A decrease in oxygen tension in the brain stimulates an immediate increase in oxygen supply by regulating blood flow as well as a series of signaling pathways that promote anaerobic metabolism (glycolysis), angiogenesis, and cell survival. In particular, a change in oxygen tension, via the key oxygen sensor prolyl-4 hydroxylase (PHD), regulates the activity of the hypoxia-inducible transcription factor HIF-1, which initiates the adaptive cellular response to hypoxia [42]. HIF-1 is a heterodimeric nuclear transcription factor formed by a HIF-1 β-subunit that is constitutively expressed in the nucleus, and a HIF-1-α-subunit that is regulated by oxygen levels [43].
During normoxia the HIF-1α subunit is expressed and rapidly hydroxylated by PHD, ubiquitinated by E3 ubiquitin protein ligase, which is part of the von Hippel-Lindau (vHL) tumor suppressor protein complex, and then targeted for degradation in the proteasome. Oxygen, together with ascorbate and Fe2+, is one of the cofactors that regulate PHD activity, with a Km for O2 of 230-250 μM for human HIF-PHD1 and HIF-PHD2 [44, 45]. Therefore, exposure to relatively high pO2 levels, close to atmospheric oxygen pressure, will result in a large degree of hydroxylation and HIF-1α degradation. In contrast, during hypoxia when oxygen is limited, the ubiquitination is prevented, HIF-1α is stabilized and translocates to the nucleus to form a dimer with HIF-1β [43]. The heterodimer binds to the hypoxia responsive elements (HRE) in the promoter regions of a number of hypoxia-inducible genes; for example genes regulating the expression of vascular endothelial growth factor (VEGF), erythropoietin (EPO), glucose transporters (GLUT-1 and GLUT-3), and glycolytic enzymes [46]. Two additional isoform of HIF-1α have been identified, HIF-2α is and HIF-3α; the first is structurally and functionally similar to HIF-1α, while HIF-3α may be involved in a negative regulation of the response [43].
Upon exposure to a sublethal hypoxic or ischemic episode, neurons and astrocytes both respond to the insult with the activation of the HIF-1-signaling pathway, which improves their ability to survive at low oxygen levels and increases their tolerance to the second ischemic episode [42, 47]. Although astrocytes are normally more resistant to hypoxic-cell death, exposure to hypoxia has been shown to affect astroglia growth and metabolism. For example, after exposure to intermittent hypoxia astrocytes in the hippocampus and the cerebral cortex demonstrate elevated glial fibrillary acidic protein (GFAP) immunostaining, which is a marker of astroglia hypertrophy and, hyperplasia, as well as increased expression of glial derived trophic factor S100B [48]. These changes are likely mediated by HIF-1, which expression is increased in astrocytes following exposure to an hypoxic insult [46]. Increased immunostaining for HIF-1 was accompanied by the activation of HIF-1 downstream target genes, such as VEGF and GLUT-1, following exposure to low oxygen concentrations. In this scenario, the response of astrocytes to hypoxia appears to be important in promoting neuronal recovery by releasing trophic factors such as S100B and energy substrates. Moreover, HIF-1 dependent expression of the neurotrophic factor EPO in astrocytes can mediate hypoxic preconditioning by inhibiting the activation of apoptotic pathways [49]. However, cellular pathways activated by HIF-1 that promote cell survival in hypoxic conditions can also contribute to cancer proliferation and invasion. Potential mediators of tumor progression and invasion that are dependent on HIF-1 include matrix metalloproteinase 2 (MMP2), VEGF, GLUT-3 upregulation, urokinase type plasminogen activator (uPA), urokinase type plasminogen activator receptor (uPAR) and cyclooxygenase-2 (COX-2), pyruvate dehydrogenase kinase (PDK) [47, 54, 55].
Although HIF-1 is upregulated in various solid tumors, the regulation of HIF-1 signaling pathways in astrocytomas has attracted particular interest. Analysis of HIF-1α mRNA and protein expression in brain samples obtained from patients with various grades of glioma revealed a significant increase of HIF-1α expression in glioblastoma multiforme (GBM) when compared with low grade glioma as well as anaplastic astrocytoma [50, 51]. Aggressive angiogenesis is a prominent feature in GBM and is associated with tumor aggression and poor prognosis. Analysis of tissue resected from GBM patients demonstrates that the level of expression of GLUT-1 and VEGF is directly correlated with overall patient survival [52]. In addition, several studies have shown that survival becomes shorter with higher staining for HIF-1α in patients that underwent surgery for GBM [53, 54]. Increased HIF-1 expression near the areas of necrosis in the glioblastoma confirms that hypoxia plays a key role in the development and aggressiveness of gliomas [50, 54]. In addition, HIF-1-dependent upregulation of a number of proteins that support glycolytic metabolism, including LDH-A, has been directly demonstrated in human derived GBM cells [55].
In primary GBM-derived cells HIF-1α expression is dependent on low oxygen tension: sudden exposure to higher oxygen levels (such as 20% O2) resulted in a rapid degradation of HIF-1α as well as activation of the bone morphogenetic protein-2 (BMP2) signaling pathway, which normally promotes differentiation in astrocytes [56]. Hypoxic conditions together with HIF-1 activation appear to cooperate for creating a micro environment that is ideal for inducing a stem cell phenotype in gliomas [15] as well as for maintaining glioma stem cells, possibly by de-sensitizing cells to pro-differentiating stimuli which can be mediated by BMP2 [54, 56]. This hypotheses is further supported by the finding that knock down of HIF-1α gene expression with small interfering RNAs (siRNAs) restored the ability to undergo differentiation, in response to the application of either the pro-differentiating agent forskolin [51] or BMP2 [54], in both C6 murine glioma cells as well in primary human malignant glioma cells, despite the presence of hypoxia-like conditions.
Similar studies have shown that astrocytoma stem cells contribute to GBM resistance to treatment and recurrence; in addition to HIF-1α, many stem cells also express HIF-2α. In these cells HIF-2α expression is stimulated within a wide range of oxygen levels [57] contributing to the maintenance of the highly aggressive glioma tumor phenotype even at higher pO2 levels.
In GBM stem cells, unlike normal subventricular zone cells (SVZ), down-modulation of HIF-1 by high oxygen and BMP2 was only transient. HIF-1α recovered over time, suggesting the presence of other factors contributing to non-hypoxic HIF-1α stabilization [54]. In vitro culture of glioblastoma cells showed increased lactic acid production and decreased cytochrome-c oxidase activity compared to normal SVZ cells. Investigators have hypothesized that impaired mitochondrial function contributes to malignancy and pseudo-hypoxic activation of HIF-1 mediated signals via non-hypoxic pathways [54]. For example, mutation of the nuclear gene encoding complex II of the mitochondrial transport chain, called succinate dehydrogenase (SDH), may lead to mitochondrial dysfunction by slowing oxidative phosphorylation and contributing to succinate accumulation first in mitochondria and then in the cytosol; this mutation is noted in pheochromocytoma. Because the activity of the oxygen sensor PHD is negatively modulated by succinate, this accumulation will inhibit HIF-1α degradation [58].
Beside hypoxia, other factors can also activate the HIF system, including insulin, insulin -like growth factor-1 (IGF-1), epidermal growth factor (EGF) and mutant RAS and Src kinase pathways [43]. Because these stimuli promote cellular growth and are involved with tumorigenesis they may also play a role in the aberrant and dysregulated amplification of the HIF-1α signal.
Recently, HIF-1α expression/stabilization, and the activity of HIF-1α dependent signaling molecules have been investigated as potential targets for GBM therapy. For example, the application of SNS-032, a selective inhibitor for cyclin-dependent kinases (CDKs), in human glioblastoma cell lines inhibits hypoxia mediated HIF-1a expression and prevents the HIF-1 induced transcription of important proteins, such as MMP2, VEGF, uPAR, and COX-2, that are required for glioma cell invasion [59]. Similarly, RAS inhibitor trans-fernesylthiosalycilic acid (FTS) caused the disappearance of HIF-1α protein, together with a significant down-regulation of key enzymes involved in glycolysis, leading to ATP decrease and energy failure [60].
Silencing HIF-1α expression with siRNA also prevented the activation of signaling pathways promoting cell proliferation and invasion in vitro and in vivo and blocked the hypoxia-induced migration of neural stem cells. In addition to energy failure, silencing HIF-1α may promote cell death by activating apoptotic cell death signals such as cytochrome-c release, caspase-3 activation and PARP cleavage, further supporting the idea that HIF-1α siRNA applications may provide potential therapeutic strategies against malignant glioma [59].
3.2. Monocarboxylate Transporters
Transport of monocarboxylates, such lactate, pyruvate, and ketone bodies, across cellular and mitochondrial membranes is facilitated by proton-linked monocarboxylate transporters (MCT) [25]. Because the transporter first binds a proton and then lactate or other monocarboxylates in the anionic form, both the pH and monocarboxylate concentration can activate the transporter activity. Astroglial cells express predominately low affinity MCTs MCT1 (lactate Km 3.5-8 mM) [28, 61], and MCT4 (lactate Km25-35 mM) [27], compared with neurons which express predominantly high affinity MCT2 (lactate Km 0.7 mM) [61]. In addition to astrocytes, MCT1 expression has also been reported in endothelial cells forming blood vessels, ependymal cells, and some neurons. Significant expression of MCT1 in cerebral blood vessels indicates that monocarboxylates can cross the blood brain barrier and serve as energy substrates especially during condition of hypoglycemia, starvation, ketogenic diet or when lactate levels are elevated, as well as during infancy [62].
Due to the presence of low affinity transporters MCT1 and MCT4, astrocytes can rapidly extrude lactate into the extracellular space to maintain high glycolytic rate during increased metabolic demand or during hypoxia. When extracellular lactate is present at the normal level of ∼ 2.5 mM, astrocytic lactate uptake is minimal. However, if lactate levels rise to ∼ 5-8 mM the concentration gradient can reverse and astrocytes can uptake lactate via MCT1 and either utilize lactate in the mitochondria or redistribute it to a different cellular location via the astrocytic syncytium [63].
Expression of MCTs is thought to be regulated by several stimuli. For example, during embryonic development and in infants, MCT1 and MCT2 expression in astrocytes and blood vessels is much higher than in adults to facilitate the uptake of ketone bodies and lactate as energy substrate during suckling period (instead of glucose) [62]. Later in life upregulation of MCTs in adults can occur in response to changes in metabolic conditions (i.e. during the ketogenic diet over 2-3 weeks) or with certain diseases. Increased expression of MCT1 has been reported in rat brain after chronic hyperglycemia or exposure to hypoxic/ischemic insult. For example, after transient global ischemia (21 days), MCT1 immunore-activity was increased in neurons, in astroglia, in endothelial cells and in the adjacent ependymal lining [64]. Similarly, after exposure to middle cerebral artery occlusion (6hr), an increase in MCT1 mRNA expression, but not MCT4 and MCT2, was observed in astrocytes and microvessels within the peri-infarct region [65]. Parallel upregulation of GLUT1 and LDH in the same regions indicate that the regulation of MCT1 mRNA expression is probably mediated by the hypoxia-inducible transcription factor HIF-1 [65, 66]. In contrast, Ullah and collaborators [67] have demonstrated in HeLa cells that exposure to hypoxia induced overexpression of MCT4, but not MCT1; this likely occurs via an HIF-1-dependent increase of MCT4 gene transcription, suggesting that hypoxia-induced upregulation of specific subtypes of MCT may vary among different tissues.
Based on this relationship between hypoxia, HIF-1α stabilization and overexpression of HIF-1-target genes (see above), investigators have postulated that in solid tumors, where oxygen availability is reduced, the induced overexpression of MCTs together with glycolytic enzymes and glucose transporters may play a key role in tumor cell survival and proliferation.
Overexpression of MCT, especially MCT1, has been reported in neoplastic human tissue including the most aggressive forms of glial cell tumors. In high grade glial neoplasms such as anaplastic astrocytoma and glioblastoma multiforme, MCT1 immunoreactivity was significantly higher than in low grade astrocytoma [68]. Consistently, western blot analysis has demonstrated overexpression of MCT1 and MCT2 in GBM tissue collected from patients during surgery and in cell lines derived from human malignant astrocytoma compared with tissue from normal brain. Interestingly, in one study, MCT4 protein was not detected in any of the tumor tissue or cell line [69].
Overexpression of MCTs is likely an adaptive response of astrocytoma cells that helps tumor expansion at different levels. First, it helps glioma cells maintain a high rate of glycolysis, by exporting lactate rapidly to extracellular space. Moreover, lactate extrusion, which is coupled with H+ extrusion, contributes to the acidic extracellular microenvironment within astrocytomas, further enabling tumor growth and invasion of nearby tissues through TGF-mediated regulation of extracellular matrix and MMP2 [70].
Recently, studies using C6 rat models of glioma have reported that the immunoreactivity for MCT1 is more intense at the periphery of the glioma tumor near blood vessels while MCT4 is more homogenously expressed within the tumor. Based on this spatial organization of astrocytoma investigators have proposed that lactate exported at the hypoxic core may be taken up by glioma cells in more oxygenated regions via MCT1 and oxidized in the mitochondria [71], similar to lactate spillover in muscle and eventual utilization by red fibers (see below on large scale tumor structure also).
Interestingly, in glioma-derived cells an increase in intra-cellular concentration of lactate was accompanied by a progressive inhibition of both glycolysis and the TCA cycle, due to a switch of the redox state towards reduction (ie, more NADH) that cannot be compensated for by oxidative phos-phorylation, which is limited due to low oxygen levels in poorly vascularized areas of the tumor [72]. These observations suggest that increased lactate levels may render cancer cells more vulnerable to cell death, hence investigators have tested the effect of pharmacological as well as molecular interventions to inhibit lactate extrusion in experimental models of glioma. The application of siRNA, to target the expression of both MCT1 and MCT2 in human malignant astrocytoma cell line U-87 MG, resulted in an 85% decrease of lactate efflux as well as a significant drop in the intracellular pH (from 7.4- to 6.8) [69]. This event was also accompanied by significant decrease in cell viability and increased occurrence of cell death in the targeted cell population. Although promising, treatment using siRNA is still challenging. Therapeutic application of siRNA requires a more effective delivery system (i.e. direct administration into the brain or brain tumor), siRNA stabilization, specific cell recognition, and ability to reach the subcellular molecular target. Possible adverse effects of this therapy include a certain degree of toxicity and the activation of innate immunity (such as an interferon response). CNS treatment can be facilitated with direct delivery into the nervous system as opposed to systemic delivery, where areas around a tumor could be flooded directly with the siRNA molecules in the extracellular space, enhancing uptake and lar space, enhancing uptake and avoiding systemic delivery issues [69].
The effect of the MCT blocker alpha-cyano-4-hydroxycinnamate (4-CIN) on lactate transport and cell viability in human malignant astrocytoma cells has also been tested (U-87 MG) [73]. The main effect of 4-CIN in this preparation at high doses was the inhibition of lactate transport at the plasma membrane, in contrast to pyruvate entry into the mitochondria, which was not affected, as 4-CIN was not internalized into the cells after up to 18 hr incubation. Progressive accumulation of markers for apoptotic and ne-crotic cell death such as membrane blebbing and shrunken cell bodies was observed after exposure to increasing concentration of 4-CIN ranging from 5 to11 mM (for 24 hr). As a result of lactate accumulation and increased NADH levels the synthesis of metabolites such as taurine and glutathione was also inhibited. In addition, pretreatment of glioma cells with 4-CIN 6 hr before low-dose therapeutic irradiation significantly enhanced the efficacy of radiation therapy, leading to a greater inhibition of clonogenic survival compared to radiation therapy alone [73]. Thus, inhibition of lactate transport may provide substantial adjuvant treatment when combined with other modalities, such as radiation therapy.
Although still controversial, limitation of lactate extrusion (i.e., leading to accumulation of intracellular lactate) may contribute to cell death by inhibiting aerobic glycolysis and by increasing intracellular acidity, which is toxic for cancer cells. In addition, due to inhibition of glycolysis, lactate buildup inside the cells may force astrocytoma cells to use oxidative metabolism for energy production, which ultimately increases ROS generation and enhanced their cellular vulnerability to apoptosis.
In addition to interfering with lactate transport, therapy may also target intracellular lactate metabolism in astrocytoma cells. Lactate extrusion into the extracellular space is important for acidification and enhancement of cell migration, but as its level rises (above 5 mM) lactate transport can reverse, leading to lactate uptake by neighboring astrocytoma cells. Astrocytoma cells may then secondarily scavenge lactate as a metabolite through a number of pathways in addition to LDH mediated conversion to pyruvate. The cytoplasmic isoforms of LDH differ in function between astrocytes and neurons: LDH4-5 in astrocytes favors pyruvate to lactate conversion followed by lactate extrusion [74] whereas neuronal LDH is biased towards lactate to pyruvate conversion after lactate uptake into neurons. Utilization of lactate in mitochondria is dependent on sufficient NAD+ being present to allow LDH to convert lactate to pyruvate in neurons [74-77]. However, LDH inhibition in astrocytoma cells will block the conversion of pyruvate to lactate forcing the cells into oxidative metabolism (see PDH section below) and eventually inhibit glycolysis by preventing NADH to NAD+ recycling [78].
3.3. Acetate
In addition to glucose, astrocytes can use acetate as an alternative energy substrate, especially under certain metabolic conditions such as the ketogenic diet, which consists of acetate precursors, such as acetoacetate. Acetate is transported from the blood to the brain and enters into the cells via H+-coupled MCTs which are expressed in endothelial cells as well as in neurons and astrocytes. Because accumulation of acetate occurs preferentially in astrocytes rather than in neurons, astrocytes may express a different isoform of monocarboxylate transporter that has a specific affinity for acetate [79]. Both astrocytes and neurons express the enzyme acetyl-CoA syntethase-3, which is necessary to convert acetate into acetyl-CoA before entering the TCA cycle; however, due to these unique transport characteristics acetate itself (as opposed to acetoacetate) is exclusively metabolized in astrocytes. As an example of this selective glial/astrocytoma uptake, investigators have utilized [2-13C]-labeled acetate in NMR spectroscopy study to estimate the contribution of astrocytes' oxidative metabolism to total substrate oxidation [24] as well as the trafficking of metabolites from glia to neurons.
In addition, the metabolic poison 2-fluoroacetate and its highly toxic metabolite 2-fluorocitrate have been used to study glial metabolism and glial cell involvement in nervous system transmission or transmitter release [3, 80]. Two-fluoroacetate is a specific inhibitor of astrocyte metabolism, and after being converted to 2-fluorocitrate in the TCA cycle, it specifically and irreversibly inhibits the enzyme aconitase resulting in a significantly decreased carbon flux in glial TCA cycle. This toxin leads to loss of glutamate synthesis and hence astrocytes are unable to replenish neuronal glutamate, resulting in a loss of neuronal synaptic transmission, as much as inhibition of astrocytic metabolism.
More recently investigators have tested the use of 1-11C-acetate and 2-14C-acetate as alternative radiopharmaceuticals to 18FDG for PET scan for the detection of brain tumors, especially astrocytoma. Preliminary studies of 2- 14C-acetate uptake performed in rats transplanted with C6-glioma tumors in vivo and in human astrocytoma tumor slices in vitro reported that 2- 14C-acetate was a sensitive glioma marker with high tumor/tissue uptake ratio, and in some cases, more sensitive than 18FDG which gives high background activity [81]. In patients, 1-11C-acetate uptake has been demonstrated in various brain tumors, including astrocytoma, meningioma, and metastatic tumors. Compared to PET scan using 18FDG, 1-11C-acetate was better at visually detecting astrocytoma due to the low uptake in the normal brain [82]. With 1-11C-acetate PET, 85% of the tumors were clearly demonstrated as hot lesions compared to 16% with 18FDG, suggesting that acetate can be a sensitive tracer for detecting and localizing glioma tumors. In one study using 1-11C-acetate PET, investigators were even able to differentiate between low grade versus high grade astrocytoma [83]. However, in this study the number of patients was limited and further research is necessary to confirm this result. Thus, the use of acetate may be excellent for imaging astrocytomas and other glial tumors but specific toxins for treatment linked to acetate are not yet clinically relevant.
3.4. Enzyme Differences in Astrocytomas Reflect Subverted Metabolism
Several astrocytic metabolic preferences may be subverted to support astrocytoma formation. In neurons, for example, the TCA cycle critical intermediates are constantly recycled, with only a small need for replenishment of these intermediates (such as oxaloacetate, succinate, α-KG) due to net loss or degradation. In contrast, anaplerosis mediated by PC is normally very active in glial cells to replace intermediates exiting the TCA cycle, for structural and synthetic use throughout the cell, including aminoacid, lipids, proteins and nucleic acids [32]. We outline differences in several enzymes with astrocytomas, which may exert an effect towards promoting tumor growth and metabolism.
3.5. Pyruvate Dehydrogenase (PDH)
In astrocytes a critical entry enzyme into the TCA cycle (pyruvate dehydrogenase complex: PDH) is selectively down-regulated so glycolysis is encouraged as a primary source of energy, leading to net lactate extrusion [41]; the PDH complex converts pyruvate into acetyl-CoA. This normal glial metabolic behavior becomes accentuated in astrocytomas [84, 85], leading to the predominant glycolytic phe-notype [45, 86]. This glycolytic phenotype may also protect against apoptosis due to an overall decrease of mitochondrial stress; mitochondria are hyperpolarized and there is less oxidative leakage of oxygen into reactive oxygen species [85]. Thus, in astrocytomas the decreased emphasis on oxidative metabolism is a function of the inherent astrocyte-regulated phenotype leading to PDH suppression, the hypoxic tumor environment, altered and scavenged substrate supply, and evasion of apoptosis, together with fast growth and high need for synthetic intermediates for rapid cellular mitotic activity.
Dichloroacetate (DCA), a generic drug currently used for treatment of lactic acidosis, regulates PDH directly. DCA enhances PDH and entry of pyruvate into the TCA cycle, thus directly reducing lactate efflux into the extracellular space [41, 84, 85].
Because, PDH is normally downregulated in astrocytes and astrocytoma cells (as part of the glycolytic phenotype), dichloroacetate application results in a decrease in the rate of lactate formation by enhancing acetyl-CoA synthesis and glucose oxidation. However, in a hypoxic environment such enhancement may not necessarily result in increased oxidative metabolism but appears likely to enhance apoptosis, generate additional reactive oxygen species, and possibly hasten tumor cell death. Because dichloroacetate is a generic compound (but unapproved for tumor treatment), this possible antitumor effect is currently being tested on astrocytomas but there are significant peripheral nerve side-effects from its use, particularly peripheral neuropathy. However, a recent study, conducted in a small number of patients (n=5) reported that DCA treatment was associated in some GBM patients with prolonged radiologic stabilization or tumor regression and limited side effects (at the therapeutic dose used for this study) [84, 85]. There is also a political undertone (but completely unproven) due to the generic nature of dichloroacetate, since in some lay publications the lack of drug company support has been viewed as a potential suppression of a possibly valuable therapy for astrocytoma. DCA remains promising awaiting further clinical trials of efficacy and side effects.
Interestingly, neural crest tumors show markedly different metabolic abnormalities than astrocytomas, for example inherited pheochromocytomas demonstrate critical mutations to the mitochondrial protein succinate dehydrogenase (SDH; complex II on the electron transport chain) which directly interferes with oxidative metabolism (ie, SDH or von Hippel-Lindau protein [VHL] mutations with loss of function) [87]. These mutations reduce respiratory function leading to a forced dependence on glycolysis for energy generation and cell survival. However, the large protein screens which led to identification of IDH1 mutations [88, 89] failed to demonstrate the presence of such specific mutations of mitochondrial proteins in astrocytomas that could lead to mitochondrial failure. Therefore, in astrocytomas the glycolytic phenotype occurs as a consequence of the hypoxic environment which limits respiration and the requirement for rapid tumor growth. Both these mechanisms of impaired mitochondrial function lead to the Warburg effect [39].
3.6. NAD(P)H Isocitrate Dehydrogenases (IDH)
The recent, unexpected discovery with large-scale protein analysis of a mutated, cytoplasmic isocitrate dehydrogenase mutation (IDH1) has led to considerable interest in metabolic understanding of astrocytomas [88]. Newer articles have started to understand the role of the mutated cytoplasmic enzyme, which appears to lead to less NADPH generation due to the loss of function [90], and the association of the mutation with improved survival, since cells with the mutation generate less glutathione and hence are less well protected from internally generated reactive oxygen species. One possible explanation for this correlation with survival is that the mutated enzyme leads to loss of function, particularly less NADPH generation, and thus may lead to enhanced sensitivity to treatments which generate reactive oxygen species and hence enhancement of apoptosis and cell death, such as radiation therapy. In addition, there is a gain of 2-hydroglutarate levels due to this mutation, which might serve as additional enhancement to maintain the tumor phenotype. The mitochondrial form of NADPH IDH2, for example, specifically leads to enhancement of mitochondrial glutathione, so a loss of function mutation of IDH2 would also enhance reactive oxygen species and secondary apoptosis. The other mitochondrial isoform of this enzyme (NADH-dependent IDH 3) specifically catalyzes the oxidative decarboxylation of isocitrate to α-KG in the TCA cycle, and is critical for TCA cycle function [91]. IDH1 localizes to the cytoplasm, whereas IDH2 and IDH3 are found in mitochondria; only IDH3 is used for actual ATP generation and energy utilization; IDH1 and 2 share 70% sequence homology.
The NADPH produced by IDH1 arises from cytosolic α-KG formation, and appears to be more localized to astrocytes than neurons [92]. IDH1 deficiency leads to increased lipid peroxidation, oxidative DNA damage, peroxide generation, and decreased cell survival [93]. Mutations in IDH1, particularly at arginine codon 132, are found in 50-94% of WHO grade 2 and 3 astrocytomas and secondary glioblastomas that arise from lower grade astrocytomas [91]. IDH1 mutations are thought to occur before TP53 mutations, one of the more common genetic alterations in these tumors, leading to the theory that IDH1 mutations arise in the transition from a normal astrocyte (or stem cell) to a tumor cell.
The functional consequences of IDH1 mutations may create vulnerabilities that can be taken advantage of for astrocytoma therapies. Loss of normal enzymatic activity is clearly established via dominant inhibition of any wild-type enzymes during dimerization. More interesting is the possibility of a neomorphic or abnormal gain of function effect. Unbiased metabolite profiling has revealed that expression of IDH1 arginine132 mutations leads to production of 2-hydroxyglutarate (2-HG) and increased HIF production (as described in detail above). Additionally, IDH1 mutations would increase mutagenesis that favors tumorigenesis. Unchecked oxidative stress would lead to mutations from ROS exposed to the genome. Thus, IDH1 mutations continue to be explored in terms of the actual metabolic effects and the likelihood of using this marker for tumor outcome.
3.7. Pyruvate Carboxylase
As previously described this enzyme is selectively expressed in astrocytes, and is mostly responsible for direct synthesis of oxaloacetate from glucose-derived pyruvate and CO2 an anaplerotic process that replenishes the TCA cycle intermediates that often leave the cycle to serve as the precursors for the synthesis of glutamate or other neurotransmitter utilized by neurons, fatty acids and nucleotides [3]. The selective presence of this enzyme within astrocytes facilitates a much greater flexibility for glucose utilization than is present within neurons. Interestingly, in astrocytomas pyruvate carboxylase may be down-regulated [94] compared to astrocytes, but other metabolic intermediates can be scavenged and utilized for anaplerosis, particularly glutamine. Glutamine is nearly as abundant as glucose and provides an additional example of astrocytoma cells scavenging for any suitable substrate that can be utilized for ATP supply as well as building stocks for tumor growth, particularly fatty acid (membranes) and amino acids (protein synthesis). To enter the TCA cycle glutamine must be converted first to glutamate and then to α-ketoglutarate [94]. Due to lack of neurons within tumors and the complete disruption of the brain architecture and metabolism due to tumor growth in glioblastoma the glutamate - glutamine cycle between neurons and astrocytes is interrupted, making glutamine a crucial substrate available to supplement a deficient PC pathway and support tumor growth.
3.8. NADH and NADPH Regulation and Cytoso-lic/mitochondrial “Shuttles”
Both NAD+/NADH and NADP+/NADPH are inherent cofactors for many synthesizing and energy-generating enzymes, and NADH carries the energy from the reactions within mitochondria to the electron transport chain for ATP generation, whereas NADPH is critical for lipid and protein synthesis in the cytosol and glutathione regeneration [95]. In both cases these metabolic cofactors control the rate of multiple enzymes as well as ultimately most energy (ie, ATP) generation within cells. These cofactors also indirectly influence multiple additional reactions throughout cells, particularly cell proliferation, and several tumor therapeutic drugs may involve reduction of NAD+ and NADH levels with secondary inhibition of ATP from glycolysis [95]. Further, NAD+ treatment, which can be administered directly or as the precursor: niacin or nicotinamide, can also enhance oxidative stress within astrocytoma cells in culture, decreasing their survival [96]. Additional reactions which are NAD+ dependent include regulation of many of the sirtuin gene regulators, which can sense cytosolic NAD+ levels and then regulate cell cycle function [97]. Specifically, SIRT2 regulates cell cycle in gliomas and has been identified as a possible gene target for tumor treatment to slow down mitotic activity. However, how NAD+ and energy can regulate these sirtuin molecules remains unclear.
Although considerable NADH is generated by glycolysis, with complete glycolysis this NADH is utilized by LDH for lactate production, leading to net balance of NAD/NADH ratio. To maintain this net balance lactate needs to be extruded. However, extra NADH accumulates in the cytoplasm during either aerobic glycolysis, when pyruvate enters the mitochondria, or when exogenous lactate is transported into the cell and converted into pyruvate. In these conditions, to prevent NADH –induced inhibition of glycolysis, reducing equivalents need to be transferred into mitochondria to regenerate NAD+. For this purpose there are two types of “shuttle” mechanisms that transfer the NADH “reducing equivalents” to the mitochondria, where the resulting NADH or FADH2 can be oxidized in the electron transport chain for generation of ATP [98]. The neuronal shuttle for this purpose is the reversible malate-aspartate exchange shuttle, which effectively transfers NADH from the cytoplasm to the mitochondria, creating a molecule of oxaloacetate in the process. However, in astrocytes an alternative shuttle exists, the glycerol phosphate shuttle, which transfers the reducing equivalent to FADH2; this shuttle is irreversible as compared to the neuronal malate-aspartate shuttle. It is unclear how the phenotype of this important enzyme changes with the development of astrocytomas but does provide a clear therapeutic target which differentiates astrocytes and astrocytomas from neurons.
4. Tumor Structure: Structural and Metabolic Gradients in Astrocytomas
As astrocytomas enlarge they develop a three-dimensional structure with vascular and metabolic gradients; this is the visible tumor apparent on MRI scans and at the time of surgery. This structure depends upon development of new blood vessels resulting in a marked oxygen gradient, which vary from the outer rim (which is heavily vascularized) to the inner hypoxic core [71]. This large-scale tumor structure is quite evident on MRI scans of higher grade astrocytomas, for example, which show a necrotic (non-enhancing) center, a diffuse, irregular area of contrast enhancement likely significant for the fast-growing tumor region, and a diffuse, mottled “edematous” area representing a surround area of spread and invasion but with less metabolic activity. For example, the inner necrotic core of the tumor can begin to develop due to hypoxia and acidity, as tumor growth increases in relationship to blood vessels growing in from the periphery. The hypoxia then progressively stimulates HIF induction and thus the inner core is highly glycolytic.
These large-scale gradients have been studied in humans as well as rat models of gliomas, with the findings of higher lactate and lower glucose within the clear tumor region, compared to the brain around the tumor [99]. High lactate levels have been noted in both MRI spectroscopy studies of astrocytomas as well as direct extracellular dialysis studies of these tumors [74-77]. Such high lactate levels are clearly predicted by the highly active glycolysis scheme predicted in the Warburg effect [39]. Oxygen gradients may result in HIF and metabolic gradients as well. For example, lactate levels may be high extracellularly within the core hypoxic region but the lactate may then diffuse towards more oxygenated regions away from the core, creating an extracellular lactate shuttle, similar to that observed in muscle, for example [71, 99]. Thus, the excess lactate generated in the hypoxic core due to glycolysis can be successfully scavenged at the rim of the tumor for metabolism, creating an economy of metabolism across the large macro scale of a tumor. Lactate can be moved both extracellularly via diffusion as well as via gap junctions in the syncytium. As extracellular lactate levels rise, eventually the gradient for lactate extrusion can reverse (at 6-8 mM) and lactate can accumulate intracellularly and be secondarily used for metabolism. Astrocytoma are generally devoid of neurons, which is one reason why the extracellular lactate levels rise and extracellular acidity increases within the tumor beds; normally neurons would use the extruded lactate as their primary energy substrate.
As astrocytomas undergo malignant transformation they progress from lower grade to more infiltrative and invasive higher grade astrocytomas culminating in glioblastoma, which is characterized by large areas of necrosis in the core of the tumor, occurring as a result of hypoxia and abundant lactate release. Higher grade astrocytomas also show a significant vascular proliferation, with secondary blood vessel generation and loss of the blood brain barrier due to the amplification of the HIF-1α -dependent signaling pathways.
Thus, the classic high grade glial neoplasm has a diffuse advancing front, consisting of infiltrative cells demonstrating an “edema-like” signal on MRI scans. Though this signal may superficially resemble the extracellular edema noted in meningiomas and extra-axial tumors from VEGF changes in blood vessel permeability, the cell density on biopsy is higher than lower as would be expected for edema, confirming the presence of the abnormal astrocytoma advancing front, which is highly invasive throughout the brain. The tumor core shows a contrast-enhancing rim, which is the forefront of VEGF induced neovascularity and active tumor boundary; resection of the contrast enhancing area can prolong survival since this is the fastest growing region with the most mitotic activity. Various metabolic treatments may have more impact on the higher grade tumor, particularly the contrast-enhancing rim region, as compared to either the core hypoxic region (with necrosis) or the low density tumor invading diffusely into the surrounding brain Thus multiple different treatments may be needed, both to synergistically take advantage of several susceptibilities as well as to treat these different macro regions within human tumors.
5. Summary
We have focused on a few unique metabolic differences between neurons, astrocytes and astrocytoma cells, which may form the basis of treatments aimed at metabolic susceptibility. Several of these treatment approaches are in preclinical studies or some even in early clinical trials to assess efficacy and toxicity. For example, direct infusion of siRNAs has been suggested to alter protein or enzyme function, in addition to the use of some approaches as adjuvant treatments for more ordinary clinical approaches, such as radiation therapy. The area of metabolic tumor treatment is now a rapidly growing field and may contribute significantly to astrocytoma therapy.
Acknowledgments
This work was supported by NIH Grants R01 NS051856 and R01 AG037599, and a VA Merit Review Award.
Abbreviations
- α-KG
α-keto-glutarate
- 2-HG
2-hydroxyglutarate
- AA
Arachidonic acid
- acetyl-CoA
Acetyl coenzyme A
- BMP2
Morphogenetic protein-2
- COX-2
Cyclooxygenase-2
- EETs
Epoxyeicosatrienoic acids
- EPO
Erythropoietin
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GBM
Glioblastoma multiforme
- HIF
Hypoxia-inducible factor
- IDH
Isocitrate dehydrogenase
- LDH
Lactate dehydrogenase
- MCT
Monocarboxylate transporters
- MMP2
Matrix metalloproteinase 2
- PC
Pyruvate carboxylase
- PDH
Pyruvate dehydrogenase
- PDK
Pyruvate dehydrogenase kinase
- PG
Prostaglandin
- PHD
Prolyl-4-hydroxylase
- SDH
Succinate dehydrogenase
- siRNA
Small interfering RNA
- uPA
Urokinase type plasminogen activator
- uPAR
Urokinase type plasminogen activator receptor
- VEGF
Vascular endothelial growth factor
Footnotes
Conflict Of Interest: The authors confirm that this article content has no conflicts of interest.
References
- 1.Aubert A, Pellerin L, Magistretti PJ, et al. A coherentneurobiological framework for functional neuroimaging providedby a model integrating compartmentalized energy metabolism. ProcNatl Acad Sci USA. 2007;104(10):4188–93. doi: 10.1073/pnas.0605864104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escartin C, Valette J, Lebon V, et al. Neuron-astrocyte interactions in the regulation of brain energy metabolism: a focus on NMR spectroscopy. J Neurochem. 2006;99(2):393–401. doi: 10.1111/j.1471-4159.2006.04083.x. [DOI] [PubMed] [Google Scholar]
- 3.Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27(2):219–49. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- 4.Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24(2):321–9. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- 5.Verkhratsky A, Toescu EC. Neuronal-glial networks as substrate for CNS integration. J Cell Mol Med. 2006;10(4):826–36. doi: 10.1111/j.1582-4934.2006.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 6.Brown AM, Sickmann HM, Fosgerau K, et al. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res. 2005;79(1-2):74–80. doi: 10.1002/jnr.20335. [DOI] [PubMed] [Google Scholar]
- 7.Gruetter R. Glycogen: the forgotten cerebral energy store. J Neurosci Res. 2003;74(2):179–83. doi: 10.1002/jnr.10785. [DOI] [PubMed] [Google Scholar]
- 8.Mangia S, Giove F, Tkac I, et al. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab. 2009;29(3):441–63. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouach N, Koulakoff A, Abudara V, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 10.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16(3):877–85. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner DA, Adamson DC. Neuronal-astrocyte metabolicinteractions: understanding the transition into abnormalastrocytoma metabolism. J Neuropathol Exp Neurol. 2011;70(3):167–76. doi: 10.1097/NEN.0b013e31820e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moon JH, Kwon S, Jun EK, et al. Nanog-induced dedifferentiation of p53-deficient mouse astrocytes into brain cancer stem-like cells. Biochem Biophys Res Commun. 2011;412(1):175–81. doi: 10.1016/j.bbrc.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 13.Dai C, Celestino JC, Okada Y, et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligoden-drogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15(15):1913–25. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhrbom L, Dai C, Celestino JC, et al. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62(19):5551–8. [PubMed] [Google Scholar]
- 15.Heddleston JM, Li Z, McLendon RE, et al. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–84. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina JR, Hayashi Y, Stephens C, et al. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12(6):453–63. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar EE, Lin A, Mahairaki V, et al. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177(3):1491–502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnoni S, Ghisoni L, Locatelli M, et al. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J Neurosurg. 2003;98(5):952–8. doi: 10.3171/jns.2003.98.5.0952. [DOI] [PubMed] [Google Scholar]
- 19.Abi-Saab WM, Maggs DG, Jones T, et al. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab. 2002;22(3):271–9. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Tabernero A, Medina JM, Giaume C. Glucose metabolism and proliferation in glia: role of astrocytic gap junctions. J Neurochem. 2006;99(4):1049–61. doi: 10.1111/j.1471-4159.2006.04088.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuga N, Sasaki T, Takahara Y, et al. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31(7):2607–14. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbons HM, Dragunow M. Adult human brain cell culture for neuroscience research. Int J Biochem Cell Biol. 2010;42(6):844–56. doi: 10.1016/j.biocel.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Lebon V, Petersen KF, Cline GW, et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22(5):1523–31. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zielke HR, Zielke CL, Baab PJ, et al. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem. 2007;101(1):9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
- 25.Hertz L, Yu AC, Kala G, et al. Neuronal-astrocytic and cytosolic-mitochondrial metabolite trafficking during brain activation, hyperammonemia and energy deprivation. Neurochem Int. 2000;37(2-3):83–102. doi: 10.1016/s0197-0186(00)00012-7. [DOI] [PubMed] [Google Scholar]
- 26.Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res. 2001;66(5):824–38. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- 27.Dimmer KS, Friedrich B, Lang F, et al. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–27. [PMC free article] [PubMed] [Google Scholar]
- 28.Broer S, Rahman B, Pellegri G, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. Journal of Biol Chem. 1997;272(48):30096–102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- 29.Korf J. Is brain lactate metabolized immediately after neuronal activity through the oxidative pathway? J Cereb Blood Flow Metab. 2006;26(12):1584–6. doi: 10.1038/sj.jcbfm.9600321. [DOI] [PubMed] [Google Scholar]
- 30.Shurr A. Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow & Metab. 2006;26:142–52. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- 31.Abe T, Takahashi S, Suzuki N. Oxidative metabolism in cultured rat astroglia: effects of reducing the glucose concentration in the culture medium and of D-aspartate or potassium stimulation. J Cereb Blood Flow Metab. 2006;26(2):153–60. doi: 10.1038/sj.jcbfm.9600175. [DOI] [PubMed] [Google Scholar]
- 32.Hassel B. Carboxylation and anaplerosis in neurons and glia. Mol Neurobiol. 2000;22(1-3):21–40. doi: 10.1385/MN:22:1-3:021. [DOI] [PubMed] [Google Scholar]
- 33.Oz G, Berkich DA, Henry PG, et al. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24(50):11273–9. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon GR, Choi HB, Rungta RL, et al. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456(7223):745–9. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mintun MA, Vlassenko AG, Rundle MM, et al. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc Natl Acad Sci USA. 2004;101(2):659–64. doi: 10.1073/pnas.0307457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ido Y, Chang K, Williamson JR. NADH augments blood flow in physiologically activated retina and visual cortex. Proc Natl Acad Sci USA. 2004;101(2):653–8. doi: 10.1073/pnas.0307458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudard S, Boitier E, Miccoli L, et al. Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res. 1997;17(3C):1903–11. [PubMed] [Google Scholar]
- 39.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 40.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itoh Y, Esaki T, Shimoji K, et al. Dichloroacetate Effects on Glucose and Lactate Oxidation by Neurons and Astroglia in vitro and on Glucose Utilization by Brain in vivo. Proc Natl Acad Sci USA. 2003;100(8):4879–84. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acker T, Acker H. Cellular oxygen sensing need in CNS function: physiological and pathological implications. J Exp Biol. 2004;207(Pt 18):3171–88. doi: 10.1242/jeb.01075. [DOI] [PubMed] [Google Scholar]
- 43.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 44.Hirsila M, Koivunen P, Gunzler V, et al. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278(33):30772–80. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 45.Tennant DA, Frezza C, MacKenzie ED, et al. Reactivating HIF prolyl hydroxylases under hypoxia results in metabolic catastrophe and cell death. Oncogene. 2009;28(45):4009–21. doi: 10.1038/onc.2009.250. [DOI] [PubMed] [Google Scholar]
- 46.Chavez JC, Agani F, Pichiule P, et al. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89(5):1937–42. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- 47.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7(8):345–50. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 48.Aviles-Reyes RX, Angelo MF, Villarreal A, et al. Intermittent hypoxia during sleep induces reactive gliosis and limited neuronal death in rats: implications for sleep apnea. J Neurochem. 2010;112(4):854–69. doi: 10.1111/j.1471-4159.2009.06535.x. [DOI] [PubMed] [Google Scholar]
- 49.Ruscher K, Freyer D, Karsch M, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22(23):10291–301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sondergaard KL, Hilton DA, Penney M, et al. Expression of hypoxia-inducible factor 1alpha in tumours of patients with glioblastoma. Neuropathol Appl Neurobiol. 2002;28(3):210–7. doi: 10.1046/j.1365-2990.2002.00391.x. [DOI] [PubMed] [Google Scholar]
- 51.Lu H, Li Y, Shu M, et al. Hypoxia-inducible factor-1alpha blocks differentiation of malignant gliomas. FEBS J. 2009;276(24):7291–304. doi: 10.1111/j.1742-4658.2009.07441.x. [DOI] [PubMed] [Google Scholar]
- 52.Flynn JR, Wang L, Gillespie DL, et al. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113(5):1032–42. doi: 10.1002/cncr.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irie N, Matsuo T, Nagata I. Protocol of radiotherapy for glioblastoma according to the expression of HIF-1. Brain Tumor Pathol. 2004;21(1):1–6. doi: 10.1007/BF02482169. [DOI] [PubMed] [Google Scholar]
- 54.Pistollato F, Rampazzo E, Abbadi S, et al. Molecular mechanisms of HIF-1alpha modulation induced by oxygen tension and BMP2 in glioblastoma derived cells. PLoS One. 2009;4(7):e6206. doi: 10.1371/journal.pone.0006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lal A, Peters H, St Croix B, et al. Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst. 2001;93(17):1337–43. doi: 10.1093/jnci/93.17.1337. [DOI] [PubMed] [Google Scholar]
- 56.Pistollato F, Chen HL, Rood BR, et al. Hypoxia and HIF1alpha repress the differentiative effects of BMPs in high-grade glioma. Stem Cells. 2009;27(1):7–17. doi: 10.1634/stemcells.2008-0402. [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Bao S, Wu Q, et al. Hypoxia-Inducible Factors Regulate Tumorigenic Capacity of Glioma Stem Cells. Cancer Cell. 2009;15(6):501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25(34):4675–82. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 59.Ali MA, Reis A, Ding LH, et al. SNS-032 prevents hypoxia-mediated glioblastoma cell invasion by inhibiting hypoxia inducible factor-1alpha expression. Int J Oncol. 2009;34(4):1051–60. doi: 10.3892/ijo_00000231. [DOI] [PubMed] [Google Scholar]
- 60.Blum R, Jacob-Hirsch J, Amariglio N, et al. Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1alpha, causing glycolysis shutdown and cell death. Cancer Res. 2005;65(3):999–1006. [PubMed] [Google Scholar]
- 61.Broer S, Broer A, Schneider HP, et al. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochemical Journal. 1999;341(Pt 3):529–35. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94(1):1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 63.Gandhi GK, Cruz NF, Ball KK, et al. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem. 2009;111(2):522–36. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng MT, Chan SA, Schurr A. Ischemia-induced changes in monocarboxylate transporter 1 reactive cells in rat hippocampus. Neurol Res. 2003;25(1):83–6. doi: 10.1179/016164103101200978. [DOI] [PubMed] [Google Scholar]
- 65.Zhang F, Vannucci SJ, Philp NJ, et al. Monocarboxylate transporter expression in the spontaneous hypertensive rat: effect of stroke. J Neurosci Res. 2005;79(1-2):139–45. doi: 10.1002/jnr.20312. [DOI] [PubMed] [Google Scholar]
- 66.Enerson BE, Drewes LR. Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J Pharm Sci. 2003;92(8):1531–44. doi: 10.1002/jps.10389. [DOI] [PubMed] [Google Scholar]
- 67.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. Journal of Biol Chem. 2006;281(14):9030–7. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 68.Froberg MK, Gerhart DZ, Enerson BE, et al. Expression of monocarboxylate transporter MCT1 in normal and neoplastic human CNS tissues. Neuroreport. 2001;12(4):761–5. doi: 10.1097/00001756-200103260-00030. [DOI] [PubMed] [Google Scholar]
- 69.Mathupala SP, Parajuli P, Sloan AE. Silencing of monocarboxylate transporters viasmall interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55(6):1410–9. doi: 10.1227/01.neu.0000143034.62913.59. discussion 9. [DOI] [PubMed] [Google Scholar]
- 70.Baumann F, Leukel P, Doerfelt A, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 2009;11(4):368–80. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grillon E, Farion R, Fablet K, et al. The spatial organization of proton and lactate transport in a rat brain tumor. PLoS One. 2011;6(2):e17416. doi: 10.1371/journal.pone.0017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouzier-Sore AK, Canioni P, Merle M. Effect of exogenous lactate on rat glioma metabolism. J Neurosci Res. 2001;65(6):543–8. doi: 10.1002/jnr.1184. [DOI] [PubMed] [Google Scholar]
- 73.Colen CB, Seraji-Bozorgzad N, Marples B, et al. Metabolic remodeling of malignant gliomas for enhanced sensitization during radiotherapy: an in vitro study. Neurosurgery. 2006;59(6):1313–23. doi: 10.1227/01.NEU.0000249218.65332.BF. discussion 23-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Brien J, Kla KM, Hopkins IB, et al. Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem Res. 2007;32(4-5):597–607. doi: 10.1007/s11064-006-9132-9. [DOI] [PubMed] [Google Scholar]
- 75.Marzatico F, Curti D, Dagani F, et al. Enzymes related to energy metabolism in human gliomas. J Neurosurg Sci. 1986;30(3):129–32. [PubMed] [Google Scholar]
- 76.Lemire J, Mailloux RJ, Appanna VD. Mitochondrial lactate dehydrogenase is involved in oxidative-energy metabolism in human astrocytoma cells (CCF-STTG1) PLoS One. 2008;3(2):e1550. doi: 10.1371/journal.pone.0001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lipton P. Effects of membrane depolarization on nicotinamide nucleotide fluorescence in brain slices. Biochem J. 1973;136(4):999–1009. doi: 10.1042/bj1360999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galeffi F, Foster KA, Sadgrove MP, et al. Lactate uptake contributes to the NAD(P)H biphasic response and tissue oxygen response during synaptic stimulation in area CA1 of rat hippocampal slices. J Neurochem. 2007;103(6):2449–61. doi: 10.1111/j.1471-4159.2007.04939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18(14):5225–33. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 1997;21(1):106–13. [PubMed] [Google Scholar]
- 81.Dienel GA, Popp D, Drew PD, et al. Preferential labeling of glial and meningial brain tumors with [2-(14)C]acetate. J Nucl Med. 2001;42(8):1243–50. [PubMed] [Google Scholar]
- 82.Liu RS, Chang CP, Guo WY, et al. 1-11C-acetate versus 18F-FDG PET in detection of meningioma and monitoring the effect of gamma-knife radiosurgery. J Nucl Med. 2010;51(6):883–91. doi: 10.2967/jnumed.109.070565. [DOI] [PubMed] [Google Scholar]
- 83.Tsuchida T, Takeuchi H, Okazawa H, et al. Grading of brain glioma with 1-11C-acetate PET: comparison with 18F-FDG PET. Nucl Med Biol. 2008;35(2):171–6. doi: 10.1016/j.nucmedbio.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99(7):989–94. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2(31):31ra4. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 86.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10(4):267–77. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 87.Favier J, Briere JJ, Burnichon N, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yan H, Bigner DD, Velculescu V, et al. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res. 2009;69(24):9157–9. doi: 10.1158/0008-5472.CAN-09-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324(5924):261–5. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bleeker FE, Atai NA, Lamba S, et al. The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119(4):487–94. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reitman ZJ, Jin G, Karoly ED, et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci USA. 2011;108(8):3270–5. doi: 10.1073/pnas.1019393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minich T, Yokota S, Dringen R. Cytosolic and mitochondrial isoforms of NADP+-dependent isocitrate dehydrogenases are expressed in cultured rat neurons, astrocytes, oligodendrocytes and microglial cells. J Neurochem. 2003;86(3):605–14. doi: 10.1046/j.1471-4159.2003.01871.x. [DOI] [PubMed] [Google Scholar]
- 93.Lee SM, Koh HJ, Park DC, et al. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med. 2002;32(11):1185–96. doi: 10.1016/s0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 94.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104(49):19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mazurek S, Boschek CB, Eigenbrodt E. The role of phosphometabolites in cell proliferation, energy metabolism, and tumor therapy. J Bioenerg Biomembr. 1997;29(4):315–30. doi: 10.1023/a:1022490512705. [DOI] [PubMed] [Google Scholar]
- 96.Zhao C, Hong Y, Han J, et al. NAD+ treatment decreases tumor cell survival by inducing oxidative stress. Front Biosci. 2011;3:434–41. doi: 10.2741/e258. [DOI] [PubMed] [Google Scholar]
- 97.Inoue T, Hiratsuka M, Osaki M, et al. The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle. 2007;6(9):1011–8. doi: 10.4161/cc.6.9.4219. [DOI] [PubMed] [Google Scholar]
- 98.McKenna MC, Waagepetersen HS, Schousboe A, et al. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol. 2006;71(4):399–407. doi: 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 99.Roslin M, Henriksson R, Bergstrom P, et al. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol. 2003;61(2):151–60. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]