Abstract
Rheumatoid arthritis is a chronic autoimmune immune disease affecting approximately 1% of the population. There has been a renewed interest in the role of B cells in rheumatoid arthritis based on the evidence that B cell depletion therapy is effective in the treatment of disease. This review summarizes the current knowledge of the mechanisms by which B cells contribute to autoimmune arthritis including roles as autoantibody producing cells, antigen-presenting cells, cytokine producing cells, and regulatory cells.
Keywords: B cells, Rheumatoid Arthritis, autoantibodies, co-stimulation, T cells
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease primarily affecting the synovial joints and causing both significant morbidity and increased mortality. The discovery of Rheumatoid Factor (RF) as an autoantibody targeting the immunoglobulin (Ig) Fc region initially defined RA as an autoimmune disease [1]. However, interest in autoantibodies and B cells waned following the discovery that RF did not always correlate with disease. Recently, efficacy of B cell depletion has lead to a resurgent interest in B cells as mediators of RA pathogenesis [2]. There are several different mechanisms by which B cells contribute to autoimmune disease. Defects in B cell tolerance checkpoints lead to autoreactive B cells in RA. Autoreactive B cells act as antigen-presenting cells (APCs) capable of stimulating autoreactive T cell activation. B cells produce both pro-inflammatory and anti-inflammatory cytokines. B cells function as antibody-producing cells. Each of these functions, alone or in combination can contribute to RA pathogenesis.
B cell tolerance checkpoints defective in autoimmune disease
The B cell receptor (BCR) is generated in the bone marrow by the random recombination of Ig variable (V), diversity (D), and joining (J) gene segments from the heavy and light chain alleles. The BCR is further diversified and selected in the periphery by somatic hypermutation. Analysis of individual B cell clones at sequential developmental stages has demonstrated that 75% of early immature B cell precursors display some degree of autoreactivity, and that in healthy individuals these B cells are removed at two different check points [3].
Initially, autoreactive immature B cells are negatively selected within the bone marrow, and are either deleted through apoptosis, undergo BCR editing by secondary recombination events at the IgL chain, or exit the bone marrow in an unresponsive state of anergy or ignorance [4, 5], [6]. Following escape into the periphery, anergized B cells enter into a pool of transitional B cells known as early immigrants where most of them rapidly die [3, 7]. However, elevated percentages of newly emigrated B cells displaying polyreactivity are found in the blood of patients with RA compared to normal donors, suggesting a defect at this initial checkpoint [8]. It is estimated that only 5% of newly formed B cells survive to mature populations in the periphery. Similar to the defects found at the early checkpoint, the naïve population of mature B cells in patients with RA and systemic lupus erythematosus (SLE) patients were found to express a high frequency of autoreactive BCRs suggesting that the second checkpoint in B cell tolerance is also defective [8, 9].
Several different mechanisms control the transition of B cells through this second checkpoint. Differential BCR signaling appears to be involved in the decision of transitional B cell to differentiate further along the pathway towards maturity, fail to differentiate, or be deleted [10, 11]. Assessment of RA and SLE patients B cells, generated from cloned and expressed recombinant antibodies from single B cells, demonstrate a high proportion of self-reactive antibodies indicating a defect in the removal of autoreactive B cells [8, 12]. Furthermore, these autoreactive B cell clones are not a product of ongoing inflammation, as methotrexate and/or anti-TNF-α therapies did not correct the B cell defect [13]. The survival of B cells as they move from the transitional to the mature stage is also determined by interclonal competition [14, 15]. It has been shown that autoreactive B cells are at a competitive disadvantage and only persist in the absence of competition [16]. Therefore, survival signals, either stimulation from extrinsic factors or directly through the BCR, are critical for the entrance of mature naive B cells into the peripheral pool, and represent potential escape mechanisms of autoreactive B cells from peripheral tolerance.
BAFF a pro-survival factor
The peripheral B cell pool is stringently regulated by the pro-survival cytokine BAFF (B-cell activating factor) also called BLyS (B lymphocyte stimulator). BAFF was first discovered as a potential growth factor for B cells, and was subsequently demonstrated to be critical for B-cell development as BAFF−/− mice have a near complete deficiency of peripheral B cells[17, 18, 19]. The findings that mice transgenic for BAFF developed autoimmunity with high levels of rheumatoid factor, circulating ICs, and anti-DNA antibodies indicates that BAFF plays a major role in regulating peripheral B cell homeostasis [20]. BAFF has several receptors; BCMA (B cell maturation antigen), TACI (transmembrane activator and CAML interactor), and BAFF-R (BAFF-receptor). Although BCMA−/− or TACI−/− mice have relatively normal B cell compartments, BAFF-R−/− mice have a B-cell developmental defect similar to BAFF−/− mice indicating that the BAFF survival signal is mediated through BAFF-R [21, 22]. The BAFF-R is initially expressed at the late transitional stage of B cell and increases as B cells mature. Importantly, excessive BAFF rescues self-reactive transgenic B cells from deletion and allows enhanced autoreactive B cell presence in the peripheral pool [23, 24].
In addition to potentiating defects in B cell tolerance checkpoints within mice, BAFF appears to be influential in the pathology of human RA. Serum levels of BAFF are significantly higher in RA patients in comparison to healthy controls [25]. BAFF levels also positively correlate with RF titers suggesting a contribution from BAFF to the survival of autoreactive B cells [25, 26]. BAFF levels are considerably higher in patients with early symptoms of RA in comparison to patients with established RA, and are appreciably higher in synovial fluid in comparison to serum [27], although this was not observed in all studies [28]. The presence of increased survival factors in RA patients may account for the increase in post-switch memory B cells observed in these patients [29, 30]. In patients treated with the B cell depleting anti-CD20 antibody, Rituximab, BAFF levels increased during B cell depletion and declined with the re-emergence of B cells [31, 32]. In addition, BAFF and BAFF-R are widely expressed in RA synovium [33]. BAFF is constitutively expressed by fibroblast-like synoviocytes from RA patients and pro-inflammatory cytokines upregulate BAFF expression. Lymphoid aggregates occasionally form germinal centerlike structures in synovial tissue and these are capable of synthesizing RF and anti-CCP antibodies [34]. BAFF expression likely contributes to survival of B cells in these lymphoid aggregates. Currently, therapies that target BAFF or BAFF receptors are in clinical trails including the anti-BAFF antibody Belimumab and BAFF-R-Ig that selectively block BAFF, along with TACI-Ig (Atacicept), which blocks BAFF binding to TACI [35].
B cells as APCs
Upon activation by cognate antigen, B cells rapidly endocytose, process and present antigen to CD4+ helper T-cells in an effort to stimulate an immune response. In fact, B cells function as APCs 103-104 more efficiently than other antigen-presenting cells [36-38]. In the context of RA, self-reactive B cells may exacerbate or perpetuate disease through the activation of autoreactive T cells. As the number of antigen-specific B cells increases over the course of an immune response these B cells likely play an increasing important role in autoreactive T cell activation. Accordingly, B cell depletion in mouse models of arthritis and in RA is associated with reduction in T cell activation [39-41]. In the proteoglycan (PG)-induced arthritis (PGIA) model, specific recognition of PG by the BCR is necessary for the activation of autoreactive T cells. In the absence of PG-specific B cells activation of PG-specific T cells and development of arthritis is absent [42]. Interestingly, this activation of PG-specific T cells occurred in the absence of secreted antibody demonstrating that presentation of antigen is through direct binding of PG to the BCR, not through immune complexes (ICs) [42].
The interaction between B and T cells may occur systemically in peripheral lymphoid tissue or locally within the synovium where lymphoid aggregates optimize antigen recognition [43]. It is now clear that multiple autoantigen-specific antibodies are found associated with joint tissue in RA [44]. How these accumulate in the joint is unclear, but joint inflammation or damage could cause the release of macromolecules inducing the activation of B cells present in the synovial tissue. Alternatively, released macromolecules may travel to the draining lymph nodes leading to activation of cognate B and T cells. The presence of polyreactive B cells that have escaped B cell tolerance checkpoints in draining lymph nodes or locally within inflamed synovium are a potential source of antigen-specific APCs for T cell activation. Although autoantigens in RA patients are defined by the presence of autoantibody, a B cell product, it is anticipated that autoreactive T cells specific for these same autoantigens are present in RA patients, however, this is yet to be examined.
B cell expression of CD80/86 is necessary for induction of arthritis
CD4+ T cell activation is a two step process. Initially, the T cell receptor recognizes peptides in association with MHC II on the surface of antigen-presenting cells (APCs), followed by a secondary co-stimulatory signal from CD28 expressed on T cells through ligation with B7-1/B7-2 (CD80/CD86) molecules on APCs [45-47]. Studies using blocking antibodies or mice deficient in either CD28 or CD80/CD86 mice support the importance of this pathway in CD4+ T cell activation [48-50]. CTLA-4, a second ligand for CD80/CD86 that binds with higher affinity, functions as an inhibitory receptor [51]. CTLA-4-Ig (Abatacept) has been successfully developed to interfere with the interaction of CD28 with its ligands CD80/CD86 in autoimmune diseases [52]. Abatacept was approved by the FDA in 2007 for the treatment of RA.
Expression of CD80 and CD86 is minimal on resting B cells, but is increased on activation. Increased expression of CD80/CD86 by the B cells permits interactions with CD28-expressing T cells to induce T cells activation and/or receive T-helper signals [49, 53-55]. In CIA interference with CD80, CD86 or CD28 ameliorates disease [56-58]. These findings were confirmed in PGIA, demonstrating that the complete absence of CD80/86 prevents the development of arthritis [59]. Is the expression of CD80/86 on activated B cells necessary for the development of arthritis? This question was addressed in the PGIA with the creation of mixed bone marrow chimeras that have a B-cell-specific deletion of the CD80/86 molecule while APCs expression remained normal. In this context, expression of CD80/86 on macrophages and dendritic cells is not sufficient to induce PGIA [59]. Reductions in T cell recall responses along with an inability of T cells to adoptively transfer arthritis indicated PG-specific T cell activation is severely defective in the absence of B cells expressing CD80/86 [59]. Interestingly, B cell expression of CD80/86 is not required for the development of autoantibody responses as PG-specific autoantibodies are normal in the B cell-specific CD80/86 deficient chimeras [59]. Cross-linking of the BCR accompanied by signals provided by CD154 (CD40L) on activated T cells and the CD40 receptor on B cells leads to up-regulation of CD80/86 and MHC II on B cells [60, 61]. Previous studies have shown that B cell expression of CD40 is required for antibody production, which may account for the unperturbed autoantibody response in these mice [62].
B cell expression of CD80/86 is necessary, but not sufficient for induction of arthritis
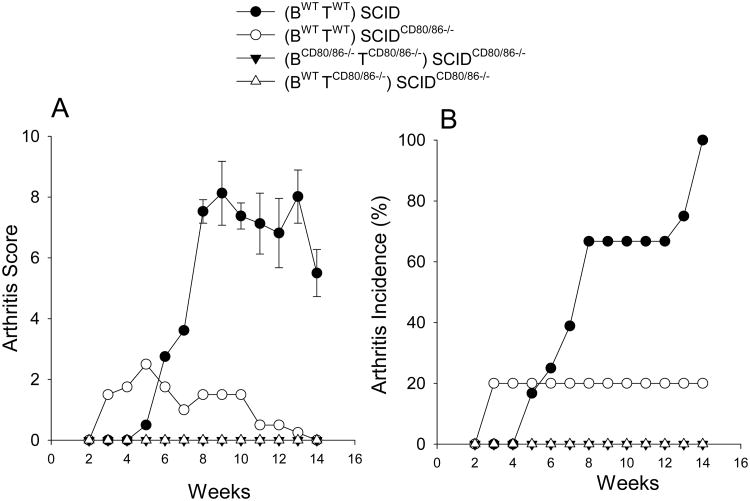
Several APCs are capable of expressing CD80/CD86 [63]. However, lack of CD80/CD86 specifically by B cells impairs PG-specific activation and PGIA pathogenesis [59]. This finding is suggestive that B cells exclusively are required for autoreactive T cell activation and arthritis pathogenesis. Therefore, the question arises if B cells alone are sufficient to activate autoreactive T cells and the development of arthritis or do other APC's also play a role? We addressed this question by asking whether CD80/CD86 expression only on B cells is sufficient for activation of autoreactive T cells and the induction of arthritis. Mice were generated in which CD80/86 was expressed only on B cells and not other APCs. To do this, SCID mice were bred to CD80/86−/− mice and selected for homozygous SCID mutation and CD80/86−/−, designated as SCIDCD80/86−/−. Either SCID or SCIDCD80/86−/− mice were reconstituted with B cells from either wild type (WT) or CD80/CD86−/− mice (BWT and BCD80/86−/−, respectively) along with B cell-depleted splenocytes from either WT or CD80/CD86−/− mice (TWT and TCD80/86−/−, respectively). Four groups of mice were generated; positive control for CD80/CD86 expression on all cells, (BWTTWT)→ SCID, control for loss of CD80/CD86 expression on SCID recipients, (BWTTWT)→ SCIDCD80/86−/−, negative control for absence of CD80/CD86 expression on donor and recipient, BCD80/86−/− TCD80/86−/−→ SCIDCD80/86−/−, and the experimental group where only B cells express CD80/CD86, BWTTCD80/86−/−→ SCIDCD80/86−/−. Reconstituted mice were immunized with PG and the development of arthritis monitored overtime (Figure 1). SCID mice reconstituted with WT cells, (BWTTWT)→ SCID, developed severe inflammatory arthritis with 100% incidence of disease. Transfer of WT cells into CD80/86 deficient SCID recipients, (BWTTWT)→ SCIDCD80/86−/−, had a dramatic effect on the induction of arthritis as only 20% of the recipient mice developed arthritis and the severity was very low. Similarly, transfer of WT B cells and B cell-depleted splenocytes from CD80/86-deficient mice, BWT TCD80/86−/−→ SCIDCD80/86−/− did not develop arthritis These data demonstrate that CD80/86 expression is required on APCs other than B cells, and that in the SCID transfer protocol, expression of CD80/86 on the transferred cells is insufficient to induce disease in the absence of CD80/86 expression in the recipient.
Figure 1.
B cell-exclusive expression of CD80/86 was not sufficient to induce arthritis. SCID and SCIDCD80/86−/− were repopulated with purified B cell (1 × 107) and T-cells (B cell-depleted splenocytes) (1 × 107) from WT and CD80/86−/− mice (BWTTWT)→ SCID, (BWTTWT)→SCIDCD80/86−/−, BCD80/86−/−TCD80/86−/−→ SCIDCD80/86−/− and BWT TCD80/86−/−→ SCIDCD80/86−/− One wk after repopulation, mice were immunized with PG in adjuvant i.p. 3 times at 3 wk intervals. (A) Arthritis score was based on the degree of erythema and swelling. Paws were scored on a scale of 1 to 4. (B) Arthritis Incidence denotes percentage of mice that develop arthritis. Data represent the mean and SEM (n=7). All groups are statistically significantly different p<0.05 than the control group (BWTTWT)→ SCID.
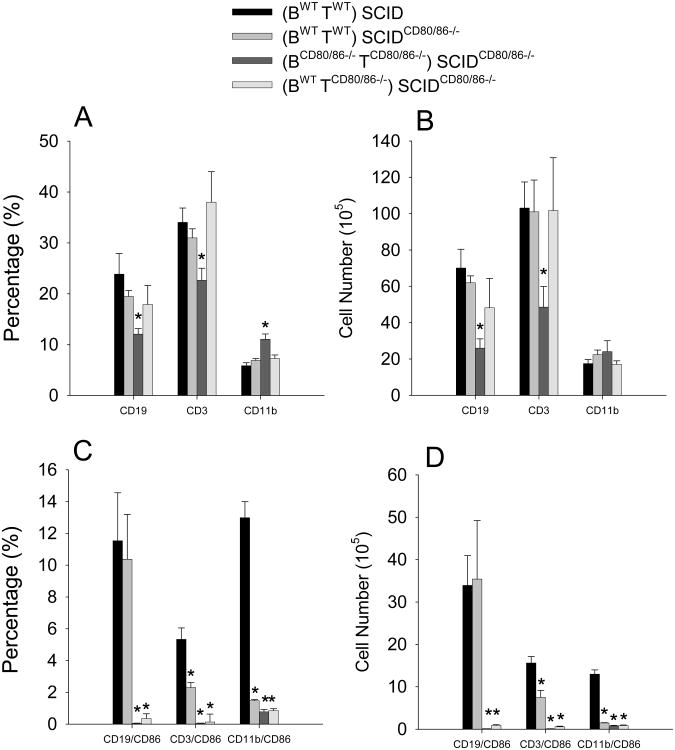
We reported that CD80/86 expression confers a survival advantage in bone marrow chimeric mice [59]. Therefore, it is possible that different survival or repopulation rates of WT or CD80/86 deficient cells in SCIDCD80/86−/− recipient mice accounted for the difference in arthritis severity. At the termination of the experiment, spleen cells were assessed for the percentage and number of B cells (CD19), T cells (CD3), and macrophages/dendritic cells (CD11b) and for the expression of CD86 on these various cell population. There was a significant reduction in the percentage and number of B cells and T cells only when donor cells were deficient in CD80/86 expression (Figure 2 B & D). The increase in the percentage of CD11b positive cells was due to the reduction in percentage of B cells and T cells (Figure 2 A & C). These data show that insufficient repopulation of donor cell in the SCIDCD80/86−/− recipients does not account for the reduction in arthritis.
Figure 2.
Donor cell sufficiently repopulated the recipients; however, CD86 was reduced on donor cells. At the termination of the experiment (A & C) percentage and (B & D) number of presence of B cell, T cells, and macrophages (A & B) along with their expression CD86 (C & D) were determined by flow cytometry of spleen cells. Data represent the mean and SEM (n=7). * represents statistically significantly different p<0.05 than the control group (BWTTWT)→ SCID.
CD80/86 co-stimulatory molecules are increased after cell activation. We assessed whether CD86 was up-regulated on cells repopulating SCID recipient mice as an indicator of cell activation. We found that the percentage and number of T cells and macrophage/dendritic cells expressing CD86 was significantly reduced in all groups when donor cells transferred into SCIDCD80/86−/− recipient mice were either WT or CD80/86 deficient (Figure C & D). Interestingly, the percentage and number of B cells expressing CD86 were similar to controls when both B cells and B cell depleted splenocytes were WT, but not when either the B cells or the B cell depleted splenocytes were CD80/86-deficient (Figure 2B & D). These data demonstrate that despite the maintenance of high expression of CD86 on B cells in (BWTTWT)→ SCIDCD80/86−/− mice, donor cells were unable to induce severe arthritis when recipient mice were CD80/86-deficient. The reduction in CD86 expression in the WT B cells transferred with CD80/86 B cell depleted splenocytes in BWT TCD80/86−/−→ SCIDCD80/86−/− mice suggests that interaction between B cells and other cell populations of the B cell depleted splenocytes was necessary for CD86 expression.
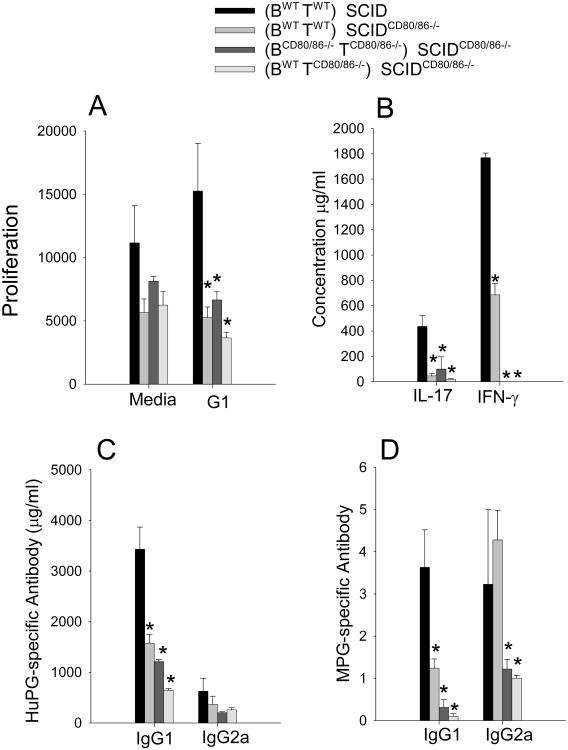
To determine whether reduction in T cell responses account for the failure to induce severe arthritis, we examined T cell proliferation and cytokine production in vitro. In comparison to the positive control, (BWTTWT)→ SCID, PG-specific proliferation and production of the proinflammatory cytokines IFN-γ and IL-17 were significantly suppressed in all the other groups (BWTTWT)→ SCIDCD80/86−/−, BCD80/86−/−TCD80/86−/−→ SCIDCD80/86−/−, and BWTTCD80/86−/−→ SCIDCD80/86−/− (Figure 3 A & B) These data demonstrate that CD80/CD86 expression on SCID APCs is necessary for the priming of T cells. The donor cells expressing CD80/CD86 either by B cells or B cell depleted splenocytes were insufficient to activate autoreactive T cells in vivo.
Figure 3.
Decrease in T cell and antibody in SCIDCD80/86−/− recipient mice. (A) T cell proliferation was measured by 3H-thymine incorporation in PG activated spleen cell cultures, (B) cytokine levels in supernatants from PG activated spleen cells, (C) serum human PG-specific antibody response, (D) serum mouse PG-specific. Data represents the mean and SEM (n=7). Data represent the mean and SEM (n=7). * represents statistically significantly differents p<0.05 from the control group (BWTTWT)→ SCID.
We have reported that a B cell-specific deficiency of CD80/CD86 does not affect the initiation of autoantibodies despite a reduction in T cell activation [59]. To determine whether a reduction in autoantibodies contributes to a decrease in arthritis severity, serum antibodies specific for human and naïve mouse PG were assessed. The IgG1 anti-human and anti-mouse PG antibodies were significantly reduced in immunized SCIDCD80/CD86−/− mice reconstituted with either WT or CD80/CD86−/− donor cells whereas only the IgG2a anti-mouse was significantly decreased in CD80/CD86−/− recipient mice (Figure 3 C & D). These data demonstrate that the decrease in T cell and antibody responses coincided with the reduction in arthritis in SCIDCD80/CD86−/− mice reconstituted with either WT or CD80/CD86−/− donor cells. Collectively, these data demonstrate that the CD80/86 expression by the recipient APCs is necessary for T and B cells activation and indicate that B cell expression of CD80/86 is not sufficient to overcome the lack of expression of CD80/86 by other APCs. These data suggest that B cells may be more effective at activating memory T cells than priming of naïve T cells.
B cells in RA synovium
The synovial tissue of RA patients can be divided into those with diffuse lymphocyte aggregates, those with T and B cells aggregates, and those with highly developed germinal centers designated as tertiary lymphoid tissue (TLT) that contain follicular dendritic cells, and segregated T and B cell areas [64, 65]. The development of TLTs is not unique to RA, but has been described to occur in other inflammatory conditions and is associated with chronic activation of the immune system [66]. Chemokines and cytokines, many of which are necessary for secondary lymphoid organ development, regulate TLT neogenesis, complexity and size. In synovium there is an increase in the local expression of lympotoxin (LT) α, LTβ, CXCL13, CCL21, CCL20, and CXCL12 where TLTs are present in comparison to tissue that forms diffuse lymphoid aggregates [65, 67-70]. Accordingly, the expression level of CXCL13 and LTβ is highly predictive of the presence of TLT [65]. However, the role of CXCL13 may be secondary to LTβ for TLT neogenesis, as ectopic lymphoid cluster development in CXCL13 transgenic mice is dependent on B cells expression of LTβ [71]. In a model of chronic arthritis, the CXCL13 ligand, CXCR5, and the CCL20 and CCL19 ligand, CXCR7, are necessary for the development TLT [72]. B cell expression of LTα and LTβ is also important for the maintenance of B cell follicles [73]. Interestingly, patients treated with anti-TNF-α (entanercept) that binds to both TNF and LTα have disrupted peripheral lymphoid germinal centers and reduced synovial TLT neogenesis [74, 75]. Fewer patients were responsive to anti-TNF therapy if they were positive for TLT, however, abrogation of TLT features following anti-TNF therapy was associated with a clinical response [75].
B cells in germinal centers of secondary lymphoid tissues are capable of undergoing affinity maturation through somatic hypermutation and class-switch recombination of the Ig genes, and potentially differentiating into memory B cells and antibody-secreting plasma cells [76, 77]. B cells accumulating in the synovium display highly mutated V regions suggestive of somatic hypermutation events [78]. In addition, activation-induced cytodine deaminase (AID), which is important for somatic hypermutation and class-switch recombination is present in the TLT indicating that TLTs are able to sustain the diversification of the B cell repertoire [79]. Evidence suggests that TLTs contribute to the production of pathogenic autoantibodies in the synovium as synovial tissue from patients with lymphoid aggregates are enriched for IgM RF and anti-CCP IgG antibodies in comparison to patients with diffuse lymphoid infiltration [80]. There is also significant accumulation of plasma cells in TLTs [81, 79]. Co-stimulatory molecules CD80/86 and CD40 are expressed in varying levels by macrophages, DC, and B cells in the joint, which presumably permits these APCs to activate T cells [82].
B cells in the TLTs are also necessary for the activation and effector function of synovial T cells suggesting that B cells function as APCs in TLTs [83]. The importance of B cells in lymphoid aggregates is best seen in B cell depletion studies (Rituximab) where positive clinical responses were observed in patients with decreased synovial B cells, plasma cell and Ig in the synovium [84-86]. Interesting, synovial B cells are specifically reduced in patients responding to treatment with CTLA-Ig (Abatacept) [86] and anti-TNF therapy (Adalimumab) with coincidental reduction of CD86 expression on peripheral B cells [87]
B cells as cytokine producing cells
Human and mouse B cells produce a variety of different pro- and anti-inflammatory cytokines. Similar to the delineation of CD4+ T-helper (Th) cell subsets upon individual effector functions, B cell in mice can be functionally divided into separate populations by the type of immune response they incite, and are designated as Be1, Be2, and regulatory B cells [88]. Be1 cells, primed by Th1 cytokines, produce IFN-γ, IL-12 (p40), IL-6, TNF, and IL-10, but not IL-4, IL-13, or IL-2, whereas Be2 cells primed by Th2 cells secrete IL-4, IL-3, IL-2, IL-10, TNF, IL-6, and small amounts of IFN-γ and IL-12 [89]. Human B cells produce a similar variety of cytokines [90], however, whether human can be divided into Be1 and Be2 is not as clear. In assessing cytokine mRNA transcripts from different cell populations isolated from RA patients synovial fluid it was found that B cells from all patient express transcripts for IL-12p35, IL-12p40, IL-23p19, IL-7, IL-15, TNF-α, LT-β, BAFF, APRIL and RANKL whereas only some patients B cells expressed IFN-γ, IL-2, IL-1P, IL-21, IL-18, IL-6, and IL-10 [91]. Interestingly, RANKL expressing B cells were found in synovial fluid of RA patients suggesting that depletion of B cells may contribute to the inhibition of bone erosion by Rituximab [92]
Regulatory B cells, also referred to as B10 cells, are activated by anti-CD40L and TLR ligands to produce IL-10, the hallmark cytokine of regulatory B cell populations [93-95]. There are two main populations of regulatory B cells in mice; a marginal zone transitional B cells (CD19+CD21hiCD23hiCD1dhi) demonstrated to prevent induction of arthritis [96], and a B10 population (CD19+CD5+CD1d+) described to inhibit T cell-mediated inflammation (contact hypersensitivity) [97, 98]. In humans an IL-10-producing B cell is contained within the immature B cell subset (CD19+CD24+CD38+) [99]. This population is present in healthy individual, but defective in SLE patients with defective CD40-induced IL-10 production [99]. A similar population of regulatory B cells was observed in RA patients [100].
B cells as autoantibody-producing cells in RA
In RA, autoantibodies directed against self-antigens including RF and cyclic citrullinated peptides (CCP) are found in the serum of patients many years before the onset of clinical symptoms of disease [101]. Recent assessment of sera from patients with RA using synovial proteome microarrays have confirmed these autoantbodies, and identified new candidate autoantigens [102]. Autoantigens have also been identified to deposite on the surface of cartilage in the form of immune complexes (IC) [44]. Although it is unclear if these antibodies are pathogenic in RA, murine models of RA have demonstrated the pathogenic potential of autoantibodies. Anti-collagen type II and anti-glucose-6-phosphate isomerase (GPI) are sufficient to induce joint destruction [103, 104]. ICs and activated complement components deposited on cartilage surfaces promote activation of Fc receptors and subsequent local inflammation in the joint. The requirement for activation of the alternative complement pathway in the development of arthritis is observed in collagen-induced arthritis (CIA), K/BxN, and in our model, proteoglycan-induced arthritis (PGIA) [105, 106]. Additionally, Fc gamma receptors (FcyR) play a critical role in arthritis development in animal models of RA [105, 107-111]. A deficiency in FcγRIII, an activating receptor, inhibits arthritis while a deficiency in the inhibitory FcγRII exacerbates disease. Thus, the depletion of autoantibodies after B cell depletion therapy in RA patients is at least one possible mechanism contributing to the observed reduction in disease symptoms.
Conclusion
Since the initial discovery of RF, research has greatly advanced our understanding of how B cells contribute to RA. The finding that B cell depletion is an effective therapy in autoimmune diseases has been the impetus to explore the potential for targeting more specific functions of these cells for the treatment of autoimmunity and other immune mediated conditions.
Acknowledgments
This work is supported by grants from NIH NIAMS A.F. R01AR04657 and R01AR056999. K.M.H is supported by NIHT32HL007605.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Franklin EC, Holman HR, Muller-Eberhard HJ, et al. An unusual protein component of high molecular weight in the serum of certain patients with rheumatoid arthritis. J Exp Med. 1957;105(5):425–38. doi: 10.1084/jem.105.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JC, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 2001;40(2):205–11. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- 3.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 4.Goodnow CC, Crosbie J, Adelstein S, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334(6184):676–82. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 5.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337(6207):562–6. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 6.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177(4):1009–20. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double-transgenic model. J Exp Med. 1994;179(1):125–34. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuels J, Ng YS, Coupillaud C, et al. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201(10):1659–67. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurasov S, Wardemann H, Hammersen J, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–11. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Clarke SH. Evidence for a ligand-mediated positive selection signal in differentiation to a mature B cell. J Immunol. 2003;171(12):6381–8. doi: 10.4049/jimmunol.171.12.6381. [DOI] [PubMed] [Google Scholar]
- 11.Wang LD, Lopes J, Cooper AB, et al. Selection of B lymphocytes in the periphery isdetermined by the functional capacity of the B cell antigen receptor. Proc Natl Acad Sci U S A. 2004;101(4):1027–32. doi: 10.1073/pnas.0307040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yurasov S, Tiller T, Tsuiji M, et al. Persistent expression of autoantibodies in SLE patients in remission. J Exp Med. 2006;203(10):2255–61. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menard L, Samuels J, Ng YS, et al. Inflammation-independent defective early B cell tolerance checkpoints in rheumatoid arthritis. Arthritis Rheum. 2011;63(5):1237–1245. doi: 10.1002/art.30164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean AR, Rosado MM, Agenes F, et al. Resource competition as a mechanism for B cell homeostasis. Proc Natl Acad Sci U S A. 1997;94(11):5792–7. doi: 10.1073/pnas.94.11.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Boer RJ, Freitas AA, Perelson AS. Resource competition determines selection of B cell repertoires. J Theor Biol. 2001;212(3):333–43. doi: 10.1006/jtbi.2001.2379. [DOI] [PubMed] [Google Scholar]
- 16.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371(6496):389–95. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 17.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 18.Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 19.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293(5537):2108–11. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 22.Rahman ZS, Rao SP, Kalled SL, et al. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. 2003;198(8):1157–69. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20(4):441–53. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 24.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20(6):785–98. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Seyler TM, Park YW, Takemura S, et al. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115(11):3083–92. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pers JO, Daridon C, Devauchelle V, et al. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci. 2005;1050:34–9. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 27.Moura RA, Cascao R, Perpetuo I, et al. Cytokine pattern in very early rheumatoid arthritis favours B-cell activation and survival. Rheumatology (Oxford) 2011;50(2):278–82. doi: 10.1093/rheumatology/keq338. [DOI] [PubMed] [Google Scholar]
- 28.Gottenberg JE, Miceli-Richard C, Ducot B, et al. Markers of B-lymphocyte activation are elevated in patients with early rheumatoid arthritis and correlated with disease activity in the ESPOIR cohort. Arthritis Res Ther. 2009;11(4):R114. doi: 10.1186/ar2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moura RA, Weinmann P, Pereira PA, et al. Alterations on peripheral blood B-cell subpopulations in very early arthritis patients. Rheumatology (Oxford) 2010;49(6):1082–92. doi: 10.1093/rheumatology/keq029. [DOI] [PubMed] [Google Scholar]
- 30.Souto-Carneiro MM, Mahadevan V, Takada K, et al. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis Res Ther. 2009;11(3):R84. doi: 10.1186/ar2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cambridge G, Stohl W, Leandro MJ, et al. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54(3):723–32. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- 32.Vallerskog T, Heimburger M, Gunnarsson I, et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8(6):R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima K, Itoh K, Nagatani K, et al. Expression of BAFF and BAFF-R in the synovial tissue of patients with rheumatoid arthritis. Scand J Rheumatol. 2007;36(5):365–72. doi: 10.1080/03009740701286615. [DOI] [PubMed] [Google Scholar]
- 34.Masson-Bessiere C, Sebbag M, Durieux JJ, et al. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin Exp Immunol. 2000;119(3):544–52. doi: 10.1046/j.1365-2249.2000.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daridon C, Burmester GR, Dorner T. Anticytokine therapy impacting on B cells in autoimmune diseases. Curr Opin Rheumatol. 2009;21(3):205–10. doi: 10.1097/BOR.0b013e32832a0760. [DOI] [PubMed] [Google Scholar]
- 36.Vidard L, Kovacsovics-Bankowski M, Kraeft SK, et al. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J Immunol. 1996;156(8):2809–18. [PubMed] [Google Scholar]
- 37.Lanzavecchia A, Bove S. Specific B lymphocytes efficiently pick up, process and present antigen to T cells. Behring Inst Mitt. 1985;77:82–7. [PubMed] [Google Scholar]
- 38.Abbas AK, Haber S, Rock KL. Antigen presentation by hapten-specific B lymphocytes. II. Specificity and properties of antigen-presenting B lymphocytes, and function of immunoglobulin receptors. J Immunol. 1985;135(3):1661–7. [PubMed] [Google Scholar]
- 39.Hamel K, Doodes P, Cao Y, et al. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180(7):4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- 40.Bouaziz JD, Yanaba K, Venturi GM, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104(52):20878–83. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, et al. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum. 2011;63(6):1507–16. doi: 10.1002/art.30314. [DOI] [PubMed] [Google Scholar]
- 42.O'Neill SK, Shlomchik MJ, Glant TT, et al. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174(6):3781–8. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 43.Weyand CM, Goronzy JJ, Takemura S, et al. Cell-cell interactions in synovitis. Interactions between T cells and B cells in rheumatoid arthritis Arthritis Res. 2000;2(6):457–63. doi: 10.1186/ar128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monach PA, Hueber W, Kessler B, et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106(37):15867–72. doi: 10.1073/pnas.0908032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkins MK, Taylor PS, Norton SD, et al. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147(8):2461–6. [PubMed] [Google Scholar]
- 46.Linsley PS, Brady W, Grosmaire L, et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173(3):721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azuma M, Ito D, Yagita H, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366(6450):76–9. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 48.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265(5176):1225–7. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 49.Lenschow DJ, Sperling AI, Cooke MP, et al. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153(5):1990–7. [PubMed] [Google Scholar]
- 50.Khoury SJ, Akalin E, Chandraker A, et al. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155(10):4521–4. [PubMed] [Google Scholar]
- 51.Linsley PS, Brady W, Urnes M, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229(1):307–21. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 53.Valle A, Aubry JP, Durand I, et al. IL-4 and IL-2 upregulate the expression of antigen B7, the B cell counterstructure to T cell CD28: an amplification mechanism for T-B cell interactions. Int Immunol. 1991;3(3):229–35. doi: 10.1093/intimm/3.3.229. [DOI] [PubMed] [Google Scholar]
- 54.Constant S, Schweitzer N, West J, et al. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155(8):3734–41. [PubMed] [Google Scholar]
- 55.Stack RM, Lenschow DJ, Gray GS, et al. IL-4 treatment of small splenic B cells induces costimulatory molecules B7-1 and B7-2. J Immunol. 1994;152(12):5723–33. [PubMed] [Google Scholar]
- 56.Tellander AC, Pettersson U, Runstrom A, et al. Interference with CD28, CD80, CD86 or CD152 in collagen-induced arthritis. Limited role of IFN-gamma in anti-B7-mediated suppression of disease. J Autoimmun. 2001;17(1):39–50. doi: 10.1006/jaut.2001.0527. [DOI] [PubMed] [Google Scholar]
- 57.Iwai H, Kozono Y, Hirose S, et al. Amelioration of collagen-induced arthritis by blockade of inducible costimulator-B7 homologous protein costimulation. J Immunol. 2002;169(8):4332–9. doi: 10.4049/jimmunol.169.8.4332. [DOI] [PubMed] [Google Scholar]
- 58.Webb LM, Walmsley MJ, Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7-1 and B7-2. Eur J Immunol. 1996;26(10):2320–8. doi: 10.1002/eji.1830261008. [DOI] [PubMed] [Google Scholar]
- 59.O'Neill SK, Cao Y, Hamel KM, et al. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179(8):5109–16. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 60.Barrett TB, Shu G, Clark EA. CD40 signaling activates CD11a/CD18 (LFA-1)-mediated adhesion in B cells. J Immunol. 1991;146(6):1722–9. [PubMed] [Google Scholar]
- 61.Yellin MJ, Sinning J, Covey LR, et al. T lymphocyte T cell-B cell-activating molecule/CD40-L molecules induce normal B cells or chronic lymphocytic leukemia B cells to express CD80 (B7/BB-1) and enhance their costimulatory activity. J Immunol. 1994;153(2):666–74. [PubMed] [Google Scholar]
- 62.Lumsden JM, Williams JA, Hodes RJ. Differential requirements for expression of CD80/86 and CD40 on B cells for T-dependent antibody responses in vivo. J Immunol. 2003;170(2):781–7. doi: 10.4049/jimmunol.170.2.781. [DOI] [PubMed] [Google Scholar]
- 63.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 64.Schroder AE, Greiner A, Seyfert C, et al. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996;93(1):221–5. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167(2):1072–80. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 66.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6(3):205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 67.Manzo A, Paoletti S, Carulli M, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35(5):1347–59. doi: 10.1002/eji.200425830. [DOI] [PubMed] [Google Scholar]
- 68.Page G, Miossec P. Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression. J Pathol. 2004;204(1):28–38. doi: 10.1002/path.1607. [DOI] [PubMed] [Google Scholar]
- 69.Gregorio A, Gambini C, Gerloni V, et al. Lymphoid neogenesis in juvenile idiopathic arthritis correlates with ANA positivity and plasma cells infiltration. Rheumatology (Oxford) 2007;46(2):308–13. doi: 10.1093/rheumatology/kel225. [DOI] [PubMed] [Google Scholar]
- 70.Timmer TC, Baltus B, Vondenhoff M, et al. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 2007;56(8):2492–502. doi: 10.1002/art.22748. [DOI] [PubMed] [Google Scholar]
- 71.Luther SA, Lopez T, Bai W, et al. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12(5):471–81. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 72.Wengner AM, Hopken UE, Petrow PK, et al. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 2007;56(10):3271–83. doi: 10.1002/art.22939. [DOI] [PubMed] [Google Scholar]
- 73.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172(6):3422–7. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 74.Anolik JH, Ravikumar R, Barnard J, et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180(2):688–92. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]
- 75.Canete JD, Celis R, Moll C, et al. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68(5):751–6. doi: 10.1136/ard.2008.089284. [DOI] [PubMed] [Google Scholar]
- 76.Manser T. Textbook germinal centers? J Immunol. 2004;172(6):3369–75. doi: 10.4049/jimmunol.172.6.3369. [DOI] [PubMed] [Google Scholar]
- 77.Honjo T, Kinoshita K, Muramatsu M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu Rev Immunol. 2002;20:165–96. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- 78.Gause A, Gundlach K, Zdichavsky M, et al. The B lymphocyte in rheumatoid arthritis: analysis of rearranged V kappa genes from B cells infiltrating the synovial membrane. Eur J Immunol. 1995;25(10):2775–82. doi: 10.1002/eji.1830251010. [DOI] [PubMed] [Google Scholar]
- 79.Humby F, Bombardieri M, Manzo A, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6(1):e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosengren S, Wei N, Kalunian KC, et al. Elevated autoantibody content in rheumatoid arthritis synovia with lymphoid aggregates and the effect of rituximab. Arthritis Res Ther. 2008;10(5):R105. doi: 10.1186/ar2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim HJ, Krenn V, Steinhauser G, et al. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J Immunol. 1999;162(5):3053–62. [PubMed] [Google Scholar]
- 82.Aarvak T, Natvig JB. Cell-cell interactions in synovitis: antigen presenting cells and T cell interaction in rheumatoid arthritis. Arthritis Res. 2001;3(1):13–7. doi: 10.1186/ar135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takemura S, Klimiuk PA, Braun A, et al. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167(8):4710–8. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 84.Kavanaugh A, Rosengren S, Lee SJ, et al. Assessment of rituximab's immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann Rheum Dis. 2008;67(3):402–8. doi: 10.1136/ard.2007.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teng YK, Levarht EW, Toes RE, et al. Residual inflammation after rituximab treatment is associated with sustained synovial plasma cell infiltration and enhanced B cell repopulation. Ann Rheum Dis. 2009;68(6):1011–6. doi: 10.1136/ard.2008.092791. [DOI] [PubMed] [Google Scholar]
- 86.Buch MH, Boyle DL, Rosengren S, et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann Rheum Dis. 2009;68(7):1220–7. doi: 10.1136/ard.2008.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Catalan D, Aravena O, Sabugo F, et al. B cells from rheumatoid arthritis patients show important alterations in the expression of CD86 and FcgammaRIIb, which are modulated by anti-tumor necrosis factor therapy. Arthritis Res Ther. 2010;12(2):R68. doi: 10.1186/ar2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10(4):236–47. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunol. 2000;1:475–481. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 90.Pistoia V. Production of cytokines by human B cells in health and disease. Immunol Today. 1997;18(7):343–50. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 91.Yeo L, Toellner KM, Salmon M, et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):2022–8. doi: 10.1136/ard.2011.153312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68(2):216–21. doi: 10.1136/ard.2007.085787. [DOI] [PubMed] [Google Scholar]
- 93.Fillatreau S, Sweenie CH, McGeachy MJ, et al. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–50. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 94.Mauri C, Gray D, Mushtaq N, et al. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizoguchi A, Mizoguchi E, Takedatsu H, et al. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 96.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–78. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 97.Yanaba K, Bouaziz JD, Haas KM, et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 98.Yanaba K, Bouaziz JD, Matsushita T, et al. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182(12):7459–72. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 100.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–41. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 102.Hueber W, Kidd BA, Tomooka BH, et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005;52(9):2645–55. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 103.Kouskoff V, Korganow AS, Duchatelle V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87(5):811–22. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 104.Stuart JM, Dixon FJ. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983;158(2):378–92. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ji H, Ohmura K, Mahmood U, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 106.Hietala MA, Jonsson IM, Tarkowski A, et al. Complement deficiency ameliorates collagen-induced arthritis in mice. J Immunol. 2002;169(1):454–9. doi: 10.4049/jimmunol.169.1.454. [DOI] [PubMed] [Google Scholar]
- 107.Kaplan CD, O'Neill SK, Koreny T, et al. Development of Inflammation in Proteoglycan-Induced Arthritis Is Dependent on FcgammaR Regulation of the Cytokine/Chemokine Environment. J Immunol. 2002;169(10):5851–9. doi: 10.4049/jimmunol.169.10.5851. [DOI] [PubMed] [Google Scholar]
- 108.Kaplan CD, Cao Y, Verbeek JS, et al. Development of proteoglycan-induced arthritis is critically dependent on Fcgamma receptor type III expression. Arthritis Rheum. 2005;52(5):1612–9. doi: 10.1002/art.21030. [DOI] [PubMed] [Google Scholar]
- 109.Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, et al. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16(3):391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 110.van Lent PL, Nabbe K, Blom AB, et al. Role of activatory Fc gamma RI and Fc gamma RIII and inhibitory Fc gamma RII in inflammation and cartilage destruction during experimental antigen-induced arthritis. Am J Pathol. 2001;159(6):2309–20. doi: 10.1016/s0002-9440(10)63081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diaz de Stahl T, Andren M, Martinsson P, et al. Expression of FcgammaRIII is required for development of collagen-induced arthritis. Eur J Immunol. 2002;32(10):2915–22. doi: 10.1002/1521-4141(2002010)32:10<2915::AID-IMMU2915>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]