Abstract
Expression of the cell surface sialomucin CD34 is common to many adult stem cell types, including muscle satellite cells. However, no clear stem cell or regeneration-related phenotype has ever been reported in mice lacking CD34, and its function on these cells remains poorly understood. Here, we assess the functional role of CD34 on satellite cell-mediated muscle regeneration. We show that Cd34−/− mice, which have no obvious developmental phenotype, display a defect in muscle regeneration when challenged with either acute or chronic muscle injury. This regenerative defect is caused by impaired entry into proliferation and delayed myogenic progression. Consistent with the reported anti-adhesive function of CD34, knock out satellite cells also show decreased motility along their host myofiber. Altogether, our results identify a role for CD34 in the poorly understood early steps of satellite cell activation, and provide the first evidence that beyond being a stem cell marker, CD34 may play an important function in modulating stem cell activity.
Keywords: CD34, satellite cell activation, muscle regeneration
Introduction
Skeletal muscle exhibits a remarkable capacity to regenerate and completely restore its mass and function rapidly after injury. Upon muscle damage, muscle stem cells, known as satellite cells, exit a normally quiescent state to self-renew and produce myoblasts, which then commit to terminal differentiation and fuse with each other or to existing myofibers to repair damage [1]. Although composing only a small fraction of the nuclei found in adult muscle, satellite cells are the primary source of new myogenic nuclei that contribute to efficient hypertrophy and regeneration, overall having a tremendous capacity to repair damage [2–7].
During regeneration, satellite cells migrate from a necrotic area towards the periphery as well as in the opposite direction, from the viable area to the site of damage [8, 9]. Recently, Siegel et al. used time-lapse imaging of satellite cells on single fibers to show that satellite cells become extremely motile, crossing the basement membrane to leave their niche as early as 12 hours after culture initiation [10]. Since the initial observation of satellite cell migration [11], there has been a concerted effort to identify factors that regulate this process. While numerous proteins have been proposed to modulate satellite cell migration [12–16], their specific roles have been difficult to define.
In this study, we focus on the sialomucin CD34. Although it is a marker commonly used to identify and purify satellite cells [17–21], its role in skeletal muscle regeneration remains to be explored. A report by Jankowski et al. showed that CD34 could be used to separate defined subpopulations of preplated myogenic progenitors, with CD34+ cells having the greatest regenerative capacity [22]. Furthermore, Beauchamp et al. reported a rapid decrease in CD34 mRNA expression in satellite cells from cultured single fibers early in myogenic progression [18]. Overall, these led us to hypothesize that, as proposed in other cell types [23–28], CD34 could function during activation, initial proliferation, or migration of adult skeletal muscle progenitors.
Here, we describe the regulation of CD34 expression on myogenic cells in vitro and in vivo. Furthermore, we use Cd34-deficient (Cd34−/−) mice to show that CD34 is essential for efficient satellite cell-mediated muscle regeneration in vivo. Our analysis of satellite cells on single fibers and sorted myogenic progenitor cells (MPCs) from Cd34−/− animals reveals a role for CD34 in promoting efficient myogenic progression, specifically in satellite cell migration and entry into cell cycle. Together, our results provide novel insights into the significance and function of CD34 in muscle regeneration, as well as in the early steps underlying this complex process.
Materials and Methods
Mice
Animals were housed in the animal facility of the Biomedical Research Centre in the University of British Columbia (UBC). Mice were kept under sterile conditions, bred in-house, and handled following guidelines approved by the UBC Animal Care Committee. Cd34−/− mice were provided by Dr. Kelly McNagny. Cd34−/− mice were crossed onto the GFP+CD45.2 background to obtain CD34−/−GFP+CD45.2 mice. LacZ in the Z/AP mice and EGFP expression in the GFP+CD45.2 C56BL/6 mice are both under the control of cytomegalovirus enhancer-chicken beta–actin hybrid promoter. These strains were used as WT controls. The Z/AP and GFP+CD45.2 mice were provided by Dr. Corrinne Lobe (MaRS Centre) and Dr. Irving Weissman (Stanford University), respectively. Mdx mice contain a point mutation in the dystrophin gene yielding complete absence of the protein. Myf5/LacZ animals express the beta-galactosidase gene under the control of the Myf5 promoter. Both strains were provided by Dr. Michael Rudnicki (Ottawa Health Research Institute). Mice genotypes for CD34−/−, mdx, GFP+, and LacZ+ were determined by PCR, fluorescence microscopy, or beta-galactosidase activity using X-gal.
Acute muscle damage
To induce acute damage, 10 μL of notexin (Latoxan# L8104, 10 μg/mL) was injected in the TA muscle. WT and Cd34−/− mice were age (8–9 weeks of age at the time of injection) and sex-matched accordingly. Muscles were harvested at days 5, 7, 10, 14, 21 post-notexin damage, paraffin embedded, and serially sectioned at 5 μm. Slides were H&E stained following standard procedures. Paraffin embedded tissues were sectioned and stained by Wax-it Histology Services, Inc.
Cross-sectional area measurements
Area measurements were performed using images taken from H&E stained slides of cross-sectioned muscle. Images were taken with a light microscope (Zeiss Axioplan2 Imaging) and measurements done on density slices calculated with OpenLab™ software (version 4.0.4). All measured fibers were verified to ensure measurements were done on individual fibers. Regenerating fibers were defined as CNFs. Non-regenerating fibers were defined as fibers with peripherally located nuclei.
MPC preparation, flow cytometry analysis, and FACS isolation
FACS isolation of myogenic progenitors was performed as previously published [29]. Briefly, primary murine myogenic progenitors were obtained from whole hind-limb or TA muscles, carefully harvested from adult mice (6–12 weeks of age), and minced into small pieces. Muscles then underwent enzymatic digestion with 0.2% collagenase type II (Roche# C6885) for 30 minutes followed by collagenase D (Roche# 1088882, 1.5 U/mL) and dispase type II (Roche# 295825, 2.4 U/mL) at 37°C for 1 hour. The homogenized muscle samples were then filtered through a 40 μm cell strainer and the cell suspension stained with antibodies against CD45, CD31, Sca1, and alpha7 integrin, along with Hoechst and PI. All cell surface staining was done on ice. Isotype controls were used to determine gating. Antibodies to CD34 were used when needed. Cells were sorted with BD FACSVantageSE™ (BD FACSDiva™ version 4.0.1.2 software). Purity checks were done to ensure sorting efficiency and accuracy. Analyses of samples was performed using FlowJo (version 8.7)
Cytospin and LacZ stain of sorted MPCs
Following FACS isolation of MPCs, cells were cytospun at 750rpm for 5 minutes. Samples were then fixed with 2% PFA and underwent standard staining for LacZ with X-gal and X-gal staining buffer for detection of beta-galactosidase activity.
Quantitative real-time and RT-PCR primers
Probes for qPCR analysis of CD34 were purchased from Applied Biosystems (Mm00519283_m1*). RT-PCR primers used to distinguish transcripts for CD34FL and CD34CT isoforms were 5′-AGCACAGAACTTCCCAGCAA-3′ in exons5/6 and 5′-CCTCCACCATTCTCCGTGTA-3′ in exon8.
Transplantation and engraftment
Freshly sorted MPCs were obtained from Z/AP+, Cd34−/−GFP+CD45.2, and GFP+CD45.2 adult mice (8–12 weeks of age). 20,000 GFP+ cells from either WT or Cd34−/− animals were mixed with 40,000 LacZ+ cells in PBS. Mixed cells were then injected into the TA muscles of adult WT mice in a 20 μL volume of PBS. Recipient mice were sacrificed 3 weeks post-transplant and perfused with PBS+10mM EDTA followed by 4% PFA. Hind limb muscles were harvested and left overnight in 20% sucrose at 4°C. Serial 20 μm sections of OCT embedded muscles were analyzed for engraftment of GFP+ and LacZ+ cells. A ratio was then obtained by taking the maximum number of GFP+ fibers and dividing it by the maximum number of LacZ+ fibers on an adjacent section.
BrdU analysis
Following NTX damage, mice were i.p. injected twice daily with 200 μL BrdU (10 mg/mL) for the first 10 days following muscle damage and once daily after day 10. BrdU (0.8 mgl/mL) was also added to the drinking water. All mice were analyzed on the same day to ensure consistency among samples. Individual TAs were maintained as separate samples. Muscle tissues were processed as per our normal MPC protocol. Permeabilization and denaturation with 0.1% saponin and DNAse (300 μg/mL) treatment were performed followed by addition of anti-BrdU antibody. All data samples were collected by flow cytometry using a BD FACSLSRII™ machine (BD FACSDiva™ version 4.0.1.2 software). Analysis of samples was performed using FlowJo (version 8.7). NTX damaged TAs from mice that did not receive BrdU were used as controls.
Single fiber isolations and confocal analysis
Single fiber isolations were performed as per standard protocol. Briefly, the EDL muscle was gently harvested following sacrifice of the mouse. Collagenase I (Worthington# LS004197, 400 U/mL) digestion for approximately 1 hour in 37 °C was performed to obtain single fibers. Fibers were then cultured, harvested, and fixed with 4% PFA at specific time-points. Immunofluoresecent staining was done using antibodies to Pax7 (DSHB), MyoD (clone C20, Santa Cruz# sc-304), and CD34 (clone RAM34, eBioscience# 13-0341) diluted in 0.3% TritonX. Analysis was done by confocal microscopy (Nikon C1 laser scanning confocal microscope).
Doubling time
Calculation of doubling time was performed using the algorithm provided by Roth V. 2006 http://www.doubling-time.com/compute.php.
3D video timelapse microscopy
Real-time video imaging and analysis was performed on WT and Cd34−/− single fiber cultures as initially described in Siegel, et al., 2009 [10].
Statistical analysis
Student’s two-tailed t-test was used on all statistical analyzes performed between groups. Statistical significance was set at p ≤ 0.05.
Results
CD34 is necessary for efficient muscle regeneration in adult mice
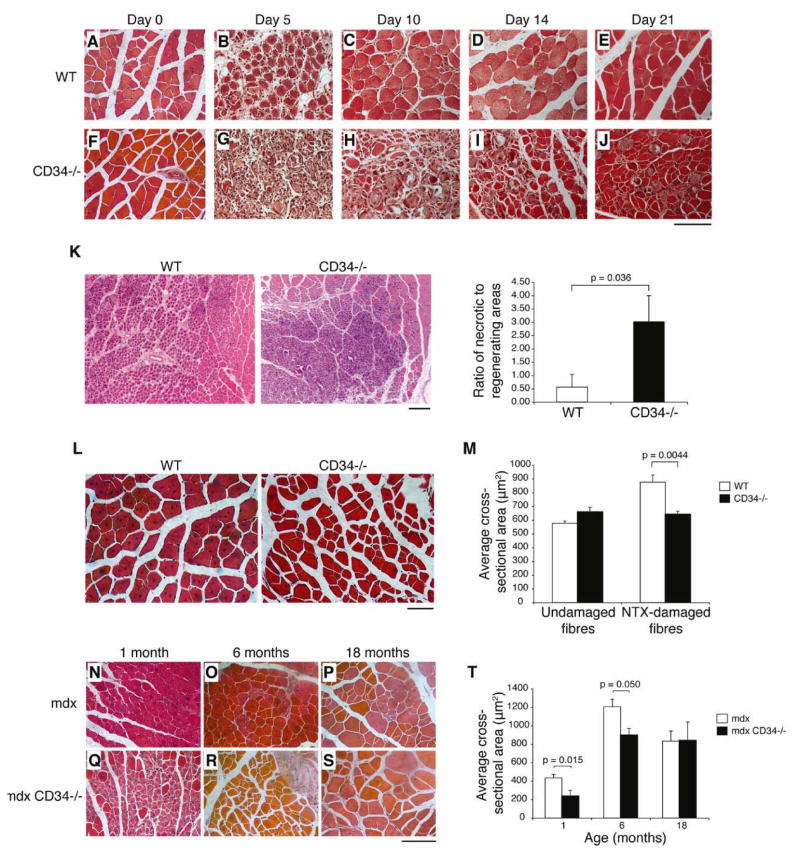
Cd34−/− mice show no obvious phenotypes under homeostatic conditions [30, 31]. To assess whether CD34 is required for skeletal muscle regeneration, we tested the response of these mice to acute damage induced by notexin (NTX) injection [32, 33]. Tibialis anterior (TA) muscles from adult wild-type (WT) and Cd34−/− mice were harvested at days 0, 5, 10, 14, and 21 post-damage (Fig. 1A–J). No differences between WT and Cd34−/− muscle were noticeable prior to damage (Fig. 1A, F). At day 5 post-damage, although the size of the damaged areas in the two groups was comparable, a significant increase in the amount of necrotic myofibers was visible in Cd34−/− animals (Fig. 1K). Elevated levels of necrosis can be observed in Cd34−/− animals at all post-damage time points analyzed, indicating a consistent difference in regeneration efficiency between WT and Cd34−/− animals. Furthermore, the appearance of centrally nucleated fibers (CNFs), characteristic of regenerating myofibers, was delayed in Cd34−/− groups (Fig. 1B vs. 1H) and consistently appeared to be smaller in size (Fig. 1C–E vs. 1H–J). Cross-sectional area (CSA) measurements on non-damaged fibers and regenerating, centrally nucleated fibers (CNFs) from both WT and Cd34−/− animals confirmed that WT CNFs, but not undamaged fibers, are significantly larger than those from Cd34−/− animals (Fig. 1L–M). This suggests that although Cd34−/− animals are capable of initiating repair, their regenerating fibers fail to undergo hypertrophy, a necessary component of muscle regeneration [3, 34].
Figure 1.
Impaired skeletal muscle regeneration in Cd34−/− mice. (A–J) H&E staining of WT and Cd34−/− muscles following acute NTX damage. TA muscles were analyzed at days 0 (A, F), 5 (B, G), 10 (C, H), 14 (D, I), and 21 (E, J) post-NTX damage (n=5–6 mice). Scale bar=100 μm. (K) Quantification of necrotic and regenerating areas of damage 5 days after NTX. Error bars represent ± SEM for n=4–6 mice. Scale bar=200 μm. (L) H&E staining of muscle sections showing centrally nucleated myofibers 21 days after NTX damage. Scale bar=50 μm. (M) Myofiber CSA measurements were performed on undamaged (day 0) and damaged (day 21) myofibers. Error bars represent ± SEM for n=5–6 animals with >200 fibers per animal. (N–S) H&E staining of mdx and mdx/Cd34−/− muscle sections at 1 (N, Q), 6 (O, R), and 18 (P, S) months of age. Scale bar=100 μm. (T) CSA measurements performed on regenerating myofibers of mdx and mdx/Cd34−/− muscles at 1, 6, and 18 months of age. Error bars represent ± SEM for n=3–5 mice with > 200 fibers per animal.
Since the kinetics of satellite cell activation and proliferation may differ between acute and chronic muscle damage, we asked whether the regeneration defect observed in acutely damaged Cd34−/− mice is also present during chronic damage, as for example seen in mdx mice, the murine model for Duchenne muscular dystrophy. Thus, we subjected mdx and mdx/Cd34−/− animals of different ages to the same histological and morphometric analyses. H&E staining shows histological differences reminiscent of those observed after NTX damage (Fig. 1N–S). In addition, relative to mdx controls, CSA measurements showed a significant decrease in regenerating myofiber sizes of mdx/Cd34−/− animals at 4 weeks of age, a time when the first wave of myodegeneration takes place, and at 6 months of age. Interestingly, when 18-month old mice were analyzed, no significant differences were observed between the two groups (Fig. 1T). Together, our data from acute and chronic damage models support a key role for CD34 in ensuring efficient muscle repair.
Prospective isolation of adult skeletal MPCs and characterization of CD34 expression
Fluorescence-activated cell sorting (FACS) can be used to prospectively isolate myogenic progenitors from adult mice [19, 20, 29, 35, 36]. Thus, we used FACS to explore the expression of CD34 on purified myogenic progenitors, isolated as Hoechst+, propidium iodide− (PI), CD45−, CD31−, Sca1−, alpha7 integrin+ cells, hereafter referred to as myogenic progenitor cells (MPCs) (Fig. S1A). This population contains all myogenic activity found in adult skeletal muscle [21, 29].
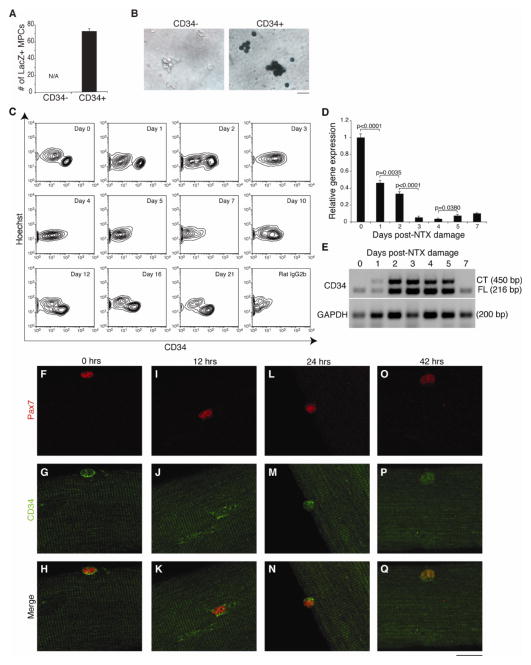
As CD34 expression has been reported on most, but not all, myogenic cells, we assessed the myogenic potential of the CD34+ and CD34−MPC fractions. MPCs isolated from Myf5LacZ mice, which express beta-galactosidase in satellite cells under the control of the Myf5 promoter [37], showed beta-galactosidase activity only in the CD34+ fraction (Fig. 2B–C). Furthermore, limiting dilution assays showed that the frequency of cells capable of initiating colonies containing multinucleated, myosin heavy chain (MyHC) positive, myotubes is negligible within the CD34− fraction (1 in 31 cells in CD34+ fraction vs. 1 in 2921 cells in CD34− fraction). In summary, our results show that essentially all myogenic activity in sorted MPCs is contained within the CD34+ subset.
Figure 2.
CD34 is expressed on myogenic cells and this expression is dynamically regulated during in vivo and in vitro myogenesis. (A) CD34− and CD34+ fractions were isolated from Myf5LacZ animals and stained for LacZ to assess β–galactosidase activity. Quantification of LacZ+ cells was performed. Error bars represent ± SEM for n=3 mice. (B) Representative image of LacZ-stained CD34− and CD34+ MPCs is shown (LacZ+, blue). Scale bar=16 μm. (C) Representative FACS plots showing regulated CD34 expression on MPCs following NTX damage (n=3–5 mice per time-point). An isotype antibody control was used to verify specificity. (D) qRT-PCR analysis of total CD34 expression in purified MPCs after NTX damage. Error bars represent ± SEM for n=3 independent experiments with 10–20 mice per timepoint. (E) End-point RT-PCR analysis of CD34FL and CD34CT isoform expression from purified MPCs. (F–Q) Analysis of CD34 localization (G, J, M, P) in Pax7+ cells (F, I, L, O) on WT fibers at 0, 12, 24, and 42 hours post-culture (Pax7, red; CD34, green; Hoechst, blue). Scale bar=20 μm.
CD34 expression on myofiber-associated satellite cells is extinguished shortly after the isolated fibers are placed in culture [18]. As the conditions used in these cultures promote satellite cells expansion, likely by mimicking the environment of damaged muscle, we hypothesized that a similar down-regulation may be observed following damage in vivo. Indeed, FACS analysis revealed that CD34 is down-regulated from the surface of MPCs starting at day 3 post-damage, and is essentially absent by day 5. At day 10, a time that follows the cessation of their proliferation [29], MPCs begin to re-express CD34 on their surface and by day 21, CD34 surface expression is fully restored (Fig. 2C). Quantitative real-time PCR (qRT-PCR) analysis of sorted MPCs confirmed the down-regulation of total CD34 mRNA soon after damage (Fig. 2D). In contrast, the expression of CD34 on Sca1+ alpha7 integrin− fibro-adipogenic progenitors (FAPs) remains constant throughout the timecourse (data not shown), despite the fact that FAPs proliferate to the same extent as myogenic cells [29], indicating CD34 regulation is specific to MPCs.
Additionally, we assessed if CD34 regulation also occurs at the level of isoform expression. Two different isoforms of CD34 have been described, a full-length (CD34FL) and a truncated version (CD34CT), which lacks most of the cytoplasmic domain [38]. A switch in expression from CD34CT to CD34FL has been reported on cultured satellite cells [18]. Using end-point RT-PCR, our investigation of CD34 isoform expression in vivo shows that in non-damaged muscle, MPCs exclusively express CD34FL. Upon satellite cell activation and proliferation (days 1–5 post-NTX damage), both CD34FL and CD34CT are co-expressed. By day 7, when regeneration is well underway, the CD34FL again becomes the predominant isoform (Fig. 2E). Since CD34FL and CD34CT are distinct in their ability to interact with cytoplasmic proteins [39, 40], a change in isoform expression may lead to changes in CD34 localization. However, confocal analysis of CD34 localization on Pax7+ satellite cells associated to cultured single fibers from the extensor digitorum longus (EDL) muscle failed to reveal any obvious changes beyond surface down-regulation (Fig. 2F–Q).
Our analysis confirms that CD34 expression is rapidly down-regulated on satellite cells following damage. This tight regulation, together with the observed muscle regeneration defect in Cd34−/− mice, provides support to the notion that CD34 plays a fundamental role in the myoregenerative process. As CD34 is no longer expressed in MPCs entering differentiation, such role is likely to be in satellite cell activation, migration, or proliferation.
Defective engraftment of Cd34−/− MPCs
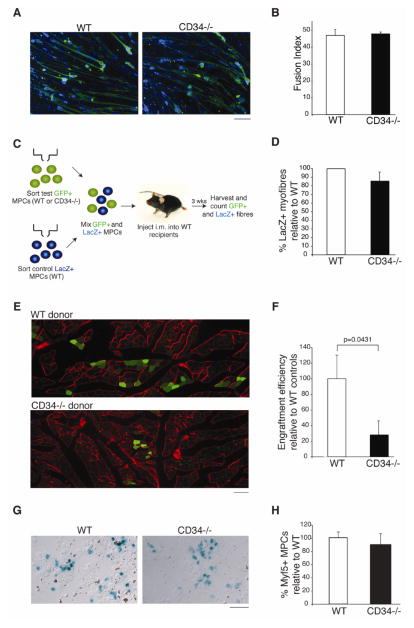
Many CD34+ cell types [29, 38, 41, 42] have been proposed to participate in muscle regeneration. To test whether the observed muscle regeneration defect in Cd34−/− animals reflects a direct effect of CD34 loss in MPCs, we functionally compared WT and Cd34−/− MPCs. We began by testing their ability to differentiate in vitro. Sorted cells were expanded under growth conditions for 5 days and then exposed to differentiation conditions for 5 days. Multinucleated, MyHC+ myotubes were readily observed in both WT and Cd34−/− samples (Fig. 3A). Fusion index calculations, a standard measure of differentiation efficiency, confirmed that Cd34−/− MPCs differentiate in vitro as efficiently as WT (Fig. 3B). These results are consistent with the observation that CD34 disappears from the cell surface prior to the appearance of mature myofibers, suggesting it would have little impact on the ability of MPCs to differentiate.
Figure 3.
In vitro and in vivo functional assessment of WT and Cd34−/− MPCs. (A) Representative images showing WT and Cd34−/− differentiated MPCs forming multinucleated myotubes (MyHC, green; Hoechst, blue). Scale bar=65 μm. (B) Fusion index (percent of total nuclei found in myotubes) for WT and Cd34−/− myotubes. Error bars represent ± SEM for n=3 mice with 15 random fields of view per animal. (C) Schematic of the transplant experiment. (D) Direct enumeration and comparison of WT LacZ+ donor-derived myofibers used as internal standards in transplantation experiments. Bar graphs displaying the relative amount of LacZ+ fibers injected with WT/GFP+ or Cd34−/−/GFP+ MPCs. Error bars represent ± SEM for n=3 mice. (E) Representative image showing engraftment of WT/GFP+ and Cd34−/−/GFP+ MPCs 3 weeks following injection into non-damaged WT recipients (GFP, green; Laminin, red). Scale bar=95 μm. (F) Quantification of engraftment. GFP+ donor-derived myofibers were counted and normalized to the number of LacZ+ donor fibers. Ratios were then normalized to WT controls. Error bars represent ± SEM for n=3–5. (G) MPCs from WT and Cd34−/− mice on a Myf5LacZ background were sorted, cytospun, and stained for LacZ to assess β-galactosidase activity. Representative images displaying LacZ+ cells in both groups are shown. Scale bar=50 μm. (H) Frequency of LacZ+ cells in WT and Cd34−/− sorted MPCs. Error bars represent ± SEM for n=3 mice.
Nevertheless, because in vitro differentiation conditions may not faithfully recapitulate the in vivo environment [43], we further tested the myogenic potential Cd34−/− MPCs in vivo using cell transplantation. MPCs were freshly sorted from three groups of mice: (1) Z/AP mice ubiquitously expressing LacZ, (2) WT mice ubiquitously expressing GFP (WT/GFP+), (3) GFP+ mice lacking CD34 (Cd34−/−/GFP+). LacZ+ MPCs, initially used as internal standards, were mixed with either WT/GFP+ or Cd34−/−/GFP+ MPCs prior to transplantation into non-damaged TA muscles of WT recipients. Engraftment was assessed 3 weeks later by quantifying donor-derived, GFP+ myofibers (Fig. 3C). Comparable numbers of LacZ+ fibers were observed in all samples (Fig. 3D, Fig. S2A), confirming that no bias was introduced prior to injection. In support of a cell autonomous role of CD34 in MPCs, our results reveal that Cd34−/−/GFP+ MPCs engraft with significantly decreased efficiency compared to WT/GFP+ MPCs (Fig. 3E–F, Fig. S2B).
To exclude a difference in the frequency of myogenic cells contained within WT and Cd34−/−MPCs as the cause for our observation, we sorted MPCs from WT and Cd34−/− mice carrying a Myf5LacZ transgene. No significant difference in the number of beta-galactosidase+ cells, indicative of MPC frequency, was noted between the two groups (Fig. 3G–H, Fig. S2C). Overall, these data demonstrate that CD34 expression on MPCs is required for the efficient generation of myofibers following transplant and suggest that the muscle regeneration defect seen in Cd34−/− mice can be attributed to a cell autonomous defect of MPCs.
Loss of CD34 on MPCs leads to impaired proliferation in vivo following acute damage
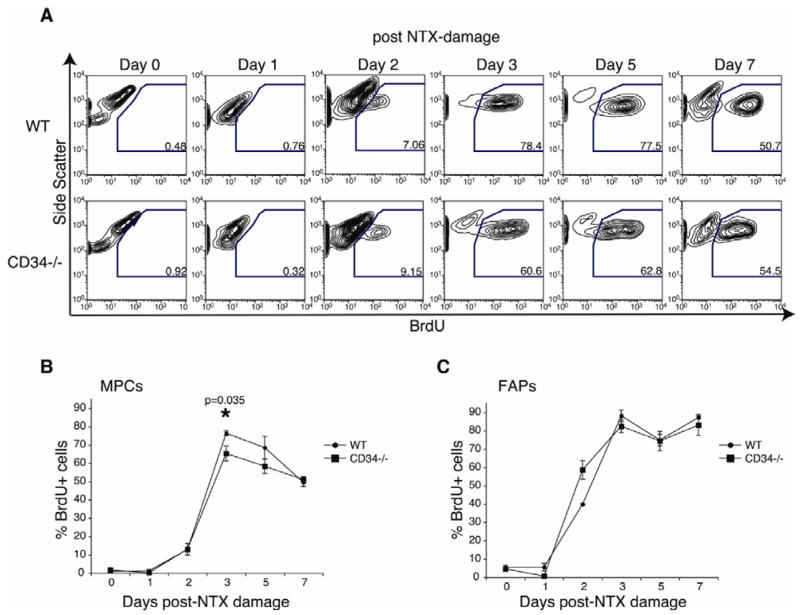
Recent publications suggest that a burst of proliferation occurs following transplant of MPCs to recipient muscle [19, 44]. Since WT and Cd34−/− MPCs are equally able to generate myotubes in vitro (Fig. 3A–B), we hypothesized that inefficient proliferation of Cd34−/− MPCs may underlie the phenotype observed in Cd34−/− mice. To assess MPC proliferation in vivo, mice were treated with BrdU and injected with NTX. FACS analysis show significantly decreased BrdU incorporation in Cd34−/− MPCs 3 days post-damage, when WT MPCs proliferation is maximal (Fig. 4A–B). A similar trend of inefficient Cd34−/− MPC proliferation is observed at day 5. This proliferation defect is not observed in FAPs, providing further evidence that CD34 plays a selective role in MPC function (Fig. 4C).
Figure 4.
Inefficient in vivo proliferation of MPCs lacking CD34. (A) Representative FACS plots showing detection of a distinct BrdU+ MPC population at 0, 1, 2, 3, 5, and 7 days following NTX damage in WT and Cd34−/− animals. (B) Graph showing the frequency of BrdU+ WT and Cd34−/− MPCs at 0, 1, 2, 3, 5, and 7 days following NTX damage. Error bars represent ± SEM for n=3–5 mice per time-point. (C) Frequency of BrdU+ WT and Cd34−/− FAPs at 0, 1, 2, 3, 5, 7 days post-NTX damage. Error bars represent ± SEM for n=3–5 mice per time-point.
The lower frequency of BrdU+ MPCs could reflect either a reduction in their proliferative response or the selective loss of Cd34−/− cells. To distinguish between these possibilities, we used TUNEL staining of WT and Cd34−/− muscle sections to detect apoptotic cells 3 days post-damage. We observed little to no apoptosis in either groups (data not shown), indicating that the decreased amount of Cd34−/− MPCs is not due to increased apoptosis. Thus, our results suggest that CD34 is required for the efficient expansion of MPCs after muscle injury in vivo.
CD34 is necessary for the efficient entry of satellite cell into proliferation
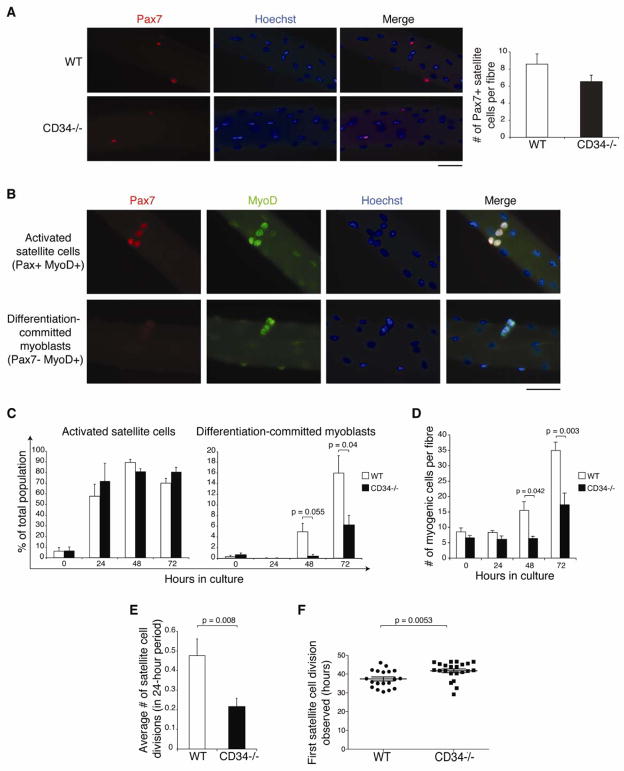
One potential explanation for the observed reduction in proliferating MPCs could be delayed satellite cell entry into proliferation. To test this hypothesis, we cultured fibers from adult EDL muscles of WT and Cd34−/− animals. No difference in numbers of Pax7+ satellite cells was apparent immediately after fiber isolation (Fig. 5A), indicating that CD34 is dispensable for the maintenance of satellite cell numbers during homeostasis.
Figure 5.
Defective activation of Cd34−/− satellite cells. (A) Immunofluorescent detection and enumeration of satellite cells on WT and Cd34−/− single fibers (Pax7, red; Hoechst, blue). Scale bar=50 μm. Error bars represent ± standard deviation for n=5–7 animals. (B) Combined Pax7 and MyoD staining on single fibers identify two subpopulations of myogenic progenitors: Pax7+ MyoD+ as activated satellite cells and Pax7− MyoD+ as differentiation-committed myoblasts (Pax7, red; MyoD, green; Hoechst, blue). Scale bar=50 μm. (C) Quantification of activated satellite cells and differentiation committed myoblasts on WT and Cd34−/− cultured fibers. Error bars represent ± standard deviation for n=3–7 animals per time-point. (D) Total myogenic cells counted per fiber. Error bars represent ± SEM for n=3–7 animals per time-point. (E) Number of satellite cell divisions detected using time-lapse microscopy between 24 and 48 hours after fiber culture initiation. Error bars represent ± SEM for n=42–107 satellite cells. (F) Timing of first division of individual satellite cell. Line represents the mean values ± SEM for n=19–22 satellite cells in 5–7 mice.
We then examined the progression of satellite cells through the myogenic program by assessing Pax7 and MyoD expression. Pax7 is highly expressed in quiescent satellite cells; but, upon entry into cycle, these cells initiate expression of MyoD and eventually down-regulate Pax7 as they commit to differentiation [45, 46]. Thus, 2 myogenic progenitor populations can then be identified: activated satellite cells (Pax7+ MyoD+) and differentiation-committed myoblasts (Pax7− MyoD+) (Fig. 5B). Analyses of these populations over time in WT and Cd34−/− single fiber cultures revealed no significant difference in the frequency of Pax7+ cells that activate MyoD expression. However, analysis of the differentiation-committed myoblast population shows that while these cells become evident in WT samples, they are absent in Cd34−/− fibers after 48 hours of culture. At the 72-hour time-point, although differentiation-committed myoblasts are now readily detectable on Cd34−/− fibers, their frequency remains significantly lower than on WT fibers (Fig. 5C). Thus, the lack of CD34 on satellite cells results in a significant delay in their progression along the myogenic program.
We next examined whether their proliferation is also similarly delayed. On WT fibers, an increase in the total number of myogenic cells, defined as cells expressing Pax7 and/or MyoD, is clearly observed at 48 hours following culture initiation. In contrast, no such increase is detected on Cd34−/− fibers until 72 hours following culture. At this point, WT fibers harbor significantly more myogenic cells than Cd34−/− fibers (Fig. 5D). Because these differences were not present initially, they reveal a striking reduction in the ability of Cd34−/− satellite cells to expand. Cd34−/− myogenic progenitors do proliferate, as can be seen by their sharp increase between 48–72 hours in culture. However, this proliferation is delayed when compared to WT fibers, whose associated myogenic progenitors numbers begin to increase between 24–48 hours in culture (Fig. 5D). In addition, the in vitro doubling time of Cd34−/− myogenic progenitors was considerably longer than that of WT cells (45.31 hours vs. 30.81 hours, respectively), providing further evidence that the lack of CD34 on myogenic progenitor indeed results in reduced proliferation.
To further confirm these results, we performed time-lapse imaging of cultured single fibers [10], which allows us to directly monitor satellite cell divisions. We observed fewer divisions on Cd34−/− fibers between 24–48 hours of culture compared to WT controls (Fig. 5E), reflecting a smaller percentage of Cd34−/− cells entering the cell cycle (44.2 vs. 20.6%). Furthermore, the first division of Cd34−/− satellite cells was significant delayed (Fig. 5F). Altogether, these data argue that CD34 is essential for satellite cells to efficiently enter their first cell cycle. Subsequently, the lack of CD34 results in reduced expansion of myogenic cells as well as in a delay in progression through the myogenic program.
CD34 is essential for efficient satellite cell motility
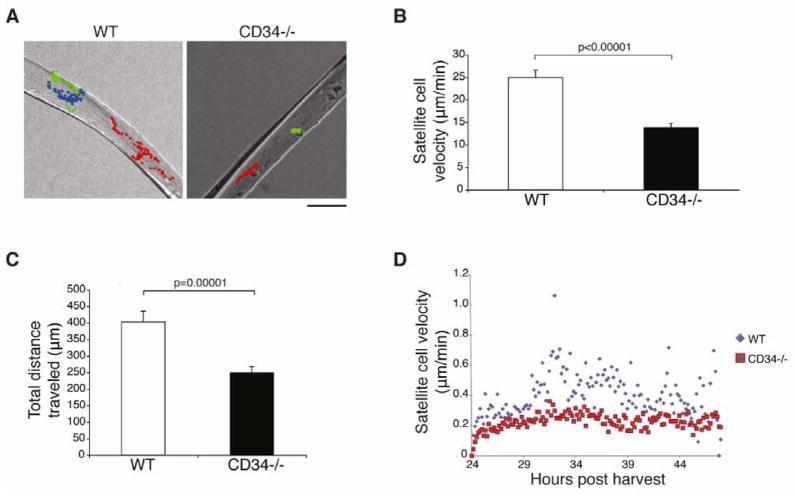
CD34 has been proposed to promote the efficient migration of hematopoetic cells through its anti-adhesive functions [23, 39, 40]. Since Siegel and colleagues [10] have established that satellite cells undergo extensive movement and migratory behavior during culture, we evaluated the motility of Cd34−/− satellite cells on their native substrate, the myofiber.
Live imaging of WT and Cd34−/− single fibers was initiated 24 hours after isolation and continued for an additional 24 hours [10]. During this period, both WT and Cd34−/− cells were found to be actively motile, but WT cells exhibited substantially more movement than Cd34−/− cells (supplemental online videos S1–2). Direct tracking of individual satellite cells over time revealed a dramatic reduction in average speed and overall distance traveled by Cd34−/− cells in comparison to WT controls (Fig. 6A–C). Evaluation of the instantaneous velocities show that WT cells move faster throughout this period, suggesting that the decreased motility of Cd34−/− cells is not due to a delay in initiating movement, but rather to a defect in migration (Fig. 6D). Overall, these data support a key role for CD34 in promoting efficient satellite cell movement.
Figure 6.
CD34 is necessary for efficient satellite cell motility. (A) Representative images of WT and Cd34−/− satellite cell tracking on single fibers based on time-lapse microscopy imaging. Each color represents a different cell tracked. Scale bar=50 μm. (B) WT and Cd34−/− satellite cell velocities as determined using time-lapse microscopy. Error bars represent ± SEM for n=42–107 satellite cells. (C) Total distances traveled for WT and Cd34−/− satellite cell were determined. Error bars represent ± SEM for n=42–107 satellite cells. (D) Measurement of frame-by-frame instantaneous velocities for WT and Cd34−/− cells.
One of the earliest motility-associated phenomena that take place during satellite cell activation is their exit from the niche. To test whether this process is also delayed in Cd34−/− animals, the position of individual Pax7+ satellite cells on cultured fibers relative to the laminin+ basement membrane was assessed by confocal microscopy. We observed no difference in the proportion of WT and Cd34−/− cells located above or below the basement membrane, indicating that the inefficient motility of Cd34−/− satellite cells does not affect their ability to exit from the niche (Fig. S3A).
Discussion
CD34 is a well-known surface marker used to isolate various progenitor cells, yet is also notorious for leaving researchers questioning its exact functional role. Previous reports speculated that CD34 may function as a homing receptor, a blocker of differentiation, a pro-adhesive receptor, or, conversely, an anti-adhesion molecule [40]. Efforts to directly reveal a function for CD34 have been hampered by the fact that CD34 is one member of a functionally redundant family of three sialomucins (CD34, podocalyxin, endoglycan) with an overlapping tissue distribution [40, 47, 48]. This may, in part, explain why Cd34−/− mice show no obvious defects in tissues where its homologues are expressed. Recent studies, however, showed that phenotypes in these mice can be revealed when a specific system is challenged, thus leading to more precise hypotheses of CD34’s function [23, 49–51].
In this study, we evaluated muscle regeneration in adult Cd34−/− animals and observed a clear impairment in both acute and chronic damage models. Notably, in the chronic damage model, this defect was no longer apparent in aged, 18-month old animals. However, since impaired regeneration in mdx mice has been attributed to satellite cell exhaustion [52, 53], the defects in satellite cell function caused by the lack of CD34 could be masked in aged animals.
A detailed analysis of CD34 expression on normal MPCs at steady state and during regeneration following acute damage showed that, consistent with previous results in vitro, CD34 expression is regulated during in vivo regeneration and is down-regulated prior to differentiation. However, upon analysis of CD34 isoform expression, we find our results from sorted MPCs in apparent contradiction with those published using individual satellite cells from single fibres [18], despite our use of the same primers on multiple experiments. One possibility for this could be that some of the cells assayed in Beauchamp et al. may be FAPs, which can contaminate fiber preparations [54]. Nevertheless, in support for our hypothesis that CD34’s role is likely in the early stages of myogenesis, our results show that it is during the stages prior to differentiation, when CD34 is regulated, that defects are observed in Cd34−/− myogenic cells. The defective engraftment of Cd34−/− MPCs in WT recipients conclusively supports the notion that CD34 plays a cell autonomous role in MPCs.
More specifically, our data demonstrates that CD34 is required for MPCs and satellite cells to proceed through the early stages of myogenic progression, defined here as the period spanning from the onset of damage to the first satellite cell division. Three main events take place during this time. (1) Satellite cells exit the niche by crossing the basement membrane. Presumably, this requires the cells to be motile, however no defect in this first step was observed in Cd34−/− fiber cultures, suggesting the effect of CD34 on motility is not generalized. (2) Satellite cells move rapidly at relatively large distances. In vitro, this movement takes place on the outside surface of the basement membrane ensheathing the myofibers, which likely represents the interstitial space in regenerating tissue in vivo. (3) Myogenic cells begin to proliferate. We have found that these latter two processes, movement and proliferation, are clearly defective in Cd34−/− myogenic progenitors.
The relationship between satellite cell movement and proliferation remains debatable. Some published work suggests movement follows division [35], while our data demonstrates that satellite cells can become motile before dividing (Supplementary online videos S1–2). However, it has been shown that proliferation can proceed despite blocked movement [10]. Evidence that both proliferation and motility are affected in Cd34−/− mice suggests that a functional link between the two may exist. But, it is also possible that CD34 plays independent roles in each of these processes. Interestingly, links between CD34 and both proliferation and motility have been independently established in other systems. Cd34−/− mast cells and eosinophils display defective inflammatory migration, an effect that has been ascribed to the loss of CD34 anti-adhesive properties [23, 39]. In support of a role for CD34 in facilitating the entry of quiescent cells into proliferation, Trempus et al. reported that Cd34−/− animals failed to develop papillomas upon DMBA/TPA induction, a phenomenon attributed to inefficient hair follicle stem cell activation [28]. Finally, studies comparing CD34+ and CD34− HSC subsets show a higher percentage of proliferating cells in the CD34+ population [27] providing a link between CD34 and proliferation. Our data lend further support to this concept as we find a clear role for CD34 in mediating activation, proliferation, and motility of myogenic cells.
While the reported anti-adhesive properties of CD34 are likely to be at the basis for its function in promoting cell motility, the molecular link between this molecule and proliferation is still unresolved. Interestingly, another sialomucin, CD164, has been described to regulate myoblast motility and fusion through modulation of satellite cell responses to the chemokine SDF-1 [55]. Moreover, it has been shown that CD34+ myogenic cells express higher levels of the SDF-1 receptor, CXCR4, compared to the CD34− population [56], suggesting a role for CD34 in CXCR4 signaling. However, we failed to detect any change in the ability of Cd34−/− cells to migrate in response to SDF-1 (data not shown), suggesting that CD34 acts through other mechanisms, the elucidation of which requires further investigation.
Conclusion
In summary, we have shown that CD34 is necessary for efficient muscle regeneration in response to both acute and chronic damage. The down-regulation of both mRNA and surface protein levels of CD34 expression on myogenic precursors shortly following damage in combination with the defective engraftment of Cd34−/− MPCs indicates that CD34 plays a cell autonomous role on myogenic progenitors during the early phase of adult myogenesis. Our data demonstrating impaired motility and delayed entry into proliferation of Cd34−/− satellite cells further support this notion. Overall, we show that CD34 is not just a useful marker of MPCs, but it is required for efficient myogenic progression.
Supplementary Material
Acknowledgments
We thank L. Yi, J. Duenas, J. Kang, S. Boumahdi, A. Johnson, J. Wong, the BRC animal facility and BRC Core Staff for their expert technical assistance. We are also grateful to B. Ajami, G. Alfaro, B. Paylor, and Dr. M. Long for help in editing the manuscript. This research was supported by grants from CIHR (MOP-82864) to F.M.V.R, D.D.W.C is supported by the National Institute of Health (NIH, 1R21AR056814-01). L.A.S.A was supported by fellowships from the Natural Sciences and Engineering Research Council of Canada (NSERC, PGSD2-362406-2008) and the Michael Smith Foundation for Health Research (MSFHR, ST-JGS-062(06-1)BM).
Footnotes
Author contributions: L.A.S.A.: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.A.D.: Conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; A.L.S.: Conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; A.S.A.: Collection and/or assembly of data, final approval of manuscript; K.M.M.: Conception and design, provision of study material, data interpretation, manuscript writing, final approval of manuscript; L.A.M.: Conception and design, data interpretation, final approval of manuscript; D.D.W.C.: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support; F.M.V. R: Conception and design, data interpretation, manuscript writing, final approval of manuscript, financial support
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 2.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- 3.Snow MH. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat Rec. 1990;227:437–446. doi: 10.1002/ar.1092270407. [DOI] [PubMed] [Google Scholar]
- 4.Schultz E. Satellite cell proliferative compartments in growing skeletal muscles. Dev Biol. 1996;175:84–94. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- 5.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Snow MH. An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res. 1978;186:535–540. doi: 10.1007/BF00224941. [DOI] [PubMed] [Google Scholar]
- 8.Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985;8:217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- 9.Phillips GD, Hoffman JR, Knighton DR. Migration of myogenic cells in the rat extensor digitorum longus muscle studied with a split autograft model. Cell Tissue Res. 1990;262:81–88. doi: 10.1007/BF00327748. [DOI] [PubMed] [Google Scholar]
- 10.Siegel AL, Atchison K, Fisher KE, et al. 3D timelapse analysis of muscle satellite cell motility. Stem Cells. 2009;27:2527–2538. doi: 10.1002/stem.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bischoff R. Chemotaxis of skeletal muscle satellite cells. Dev Dyn. 1997;208:505–515. doi: 10.1002/(SICI)1097-0177(199704)208:4<505::AID-AJA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Germani A, Di Carlo A, Mangoni A, et al. Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol. 2003;163:1417–1428. doi: 10.1016/S0002-9440(10)63499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin CA, Apponi LH, Long KK, et al. Chemokine expression and control of muscle cell migration during myogenesis. J Cell Sci. 2010;123:3052–3060. doi: 10.1242/jcs.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell. 2009;17:649–661. doi: 10.1016/j.devcel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horsley V, Jansen KM, Mills ST, et al. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 16.Salerno MS, Dyer K, Bracegirdle J, et al. Akirin1 (Mighty), a novel promyogenic factor regulates muscle regeneration and cell chemotaxis. Exp Cell Res. 2009;315:2012–2021. doi: 10.1016/j.yexcr.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Conboy MJ, Cerletti M, Wagers AJ, et al. Immuno-analysis and FACS sorting of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol. 2010;621:165–173. doi: 10.1007/978-1-60761-063-2_11. [DOI] [PubMed] [Google Scholar]
- 18.Beauchamp JR, Heslop L, Yu DS, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacco A, Doyonnas R, Kraft P, et al. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montarras D, Morgan J, Collins C, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 21.Sherwood RI, Christensen JL, Conboy IM, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Jankowski RJ, Deasy BM, Cao B, et al. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci. 2002;115:4361–4374. doi: 10.1242/jcs.00110. [DOI] [PubMed] [Google Scholar]
- 23.Blanchet MR, Maltby S, Haddon DJ, et al. CD34 facilitates the development of allergic asthma. Blood. 2007;110:2005–2012. doi: 10.1182/blood-2006-12-062448. [DOI] [PubMed] [Google Scholar]
- 24.Blanchet MR, McNagny KM. Stem cells, inflammation and allergy. Allergy Asthma Clin Immunol. 2009;5:13. doi: 10.1186/1710-1492-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen JS, McNagny KM. CD34 is a key regulator of hematopoietic stem cell trafficking to bone marrow and mast cell progenitor trafficking in the periphery. Microcirculation. 2009;16:487–496. doi: 10.1080/10739680902941737. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Laver JH, Ogawa M. Reversible expression of CD34 by murine hematopoietic stem cells. Blood. 1999;94:2548–2554. [PubMed] [Google Scholar]
- 27.Shman TV, Savitski VP, Fedasenka UU, et al. Apoptosis and proliferation differences between CD34+ and CD34− leukemic subpopulations in childhood acute leukemia. Hematology. 2007;12:403–407. doi: 10.1080/10245330701393758. [DOI] [PubMed] [Google Scholar]
- 28.Trempus CS, Morris RJ, Ehinger M, et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng J, Baumhueter S, Cacalano G, et al. Hematopoietic defects in mice lacking the sialomucin CD34. Blood. 1996;87:479–490. [PubMed] [Google Scholar]
- 31.Suzuki A, Andrew DP, Gonzalo JA, et al. CD34-deficient mice have reduced eosinophil accumulation after allergen exposure and show a novel crossreactive 90-kD protein. Blood. 1996;87:3550–3562. [PubMed] [Google Scholar]
- 32.Harris JB. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon. 2003;42:933–945. doi: 10.1016/j.toxicon.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Harris JB, Vater R, Wilson M, et al. Muscle fibre breakdown in venom-induced muscle degeneration. J Anat. 2003;202:363–372. doi: 10.1046/j.1469-7580.2003.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams GR. Satellite cell proliferation and skeletal muscle hypertrophy. Appl Physiol Nutr Metab. 2006;31:782–790. doi: 10.1139/h06-053. [DOI] [PubMed] [Google Scholar]
- 35.Kuang S, Kuroda K, Le Grand F, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell KJ, Pannerec A, Cadot B, et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 37.Tajbakhsh S, Bober E, Babinet C, et al. Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibres as well as early embryonic muscle. Dev Dyn. 1996;206:291–300. doi: 10.1002/(SICI)1097-0177(199607)206:3<291::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Krause DS, Ito T, Fackler MJ, et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994;84:691–701. [PubMed] [Google Scholar]
- 39.Drew E, Merzaban JS, Seo W, et al. CD34 and CD43 inhibit mast cell adhesion and are required for optimal mast cell reconstitution. Immunity. 2005;22:43–57. doi: 10.1016/j.immuni.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121:3683–3692. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 41.Baumhueter S, Dybdal N, Kyle C, et al. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood. 1994;84:2554–2565. [PubMed] [Google Scholar]
- 42.Drew E, Merkens H, Chelliah S, et al. CD34 is a specific marker of mature murine mast cells. Exp Hematol. 2002;30:1211. doi: 10.1016/s0301-472x(02)00890-1. [DOI] [PubMed] [Google Scholar]
- 43.Cornelison DD. Context matters: in vivo and in vitro influences on muscle satellite cell activity. J Cell Biochem. 2008;105:663–669. doi: 10.1002/jcb.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X, Yang Z, Liu Q, et al. In vivo fluorescence imaging of muscle cell regeneration by transplanted EGFP-labeled myoblasts. Mol Ther. 2010;18:835–842. doi: 10.1038/mt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 46.Zammit PS, Relaix F, Nagata Y, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 47.Doyonnas R, Kershaw DB, Duhme C, et al. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med. 2001;194:13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sassetti C, Van Zante A, Rosen SD. Identification of endoglycan, a member of the CD34/podocalyxin family of sialomucins. J Biol Chem. 2000;275:9001–9010. doi: 10.1074/jbc.275.12.9001. [DOI] [PubMed] [Google Scholar]
- 49.Maltby S, Wohlfarth C, Gold M, et al. CD34 is required for infiltration of eosinophils into the colon and pathology associated with DSS-induced ulcerative colitis. Am J Pathol. 2010;177:1244–1254. doi: 10.2353/ajpath.2010.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanchet MR, Gold M, Maltby S, et al. Loss of CD34 leads to exacerbated autoimmune arthritis through increased vascular permeability. J Immunol. 2010;184:1292–1299. doi: 10.4049/jimmunol.0900808. [DOI] [PubMed] [Google Scholar]
- 51.Strilic B, Kucera T, Eglinger J, et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17:505–515. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Luz MA, Marques MJ, Santo Neto H. Impaired regeneration of dystrophin-deficient muscle fibers is caused by exhaustion of myogenic cells. Braz J Med Biol Res. 2002;35:691–695. doi: 10.1590/s0100-879x2002000600009. [DOI] [PubMed] [Google Scholar]
- 53.Sacco A, Mourkioti F, Tran R, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starkey JD, Yamamoto M, Yamamoto S, et al. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem. 2011;59:33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bae GU, Gaio U, Yang YJ, et al. Regulation of myoblast motility and fusion by the CXCR4-associated sialomucin, CD164. J Biol Chem. 2008;283:8301–8309. doi: 10.1074/jbc.M706730200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ieronimakis N, Balasundaram G, Rainey S, et al. Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PLoS One. 2010;5:e10920. doi: 10.1371/journal.pone.0010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.