Abstract
Background
Salvia divinorum (Salvia) is an increasingly popular recreational drug amongst adolescents and young adults. Its primary active ingredient, Salvinorin A (SA), a highly selective agonist at the kappa opiate receptor (KOR), is believed to be one of the most potent naturally occurring hallucinogens. However, there is little experimental data on the effects of SA in humans.
Methods
In a 3-day, double-blind, randomized, crossover, counterbalanced study, the behavioral, subjective, cognitive, psychophysiological and endocrine effects of 0 mg, 8 mg and 12 mg of inhaled SA were characterized in 10 healthy individuals who had previously used Salvia.
Results
SA produced psychotomimetic effects and perceptual alterations including dissociative and somaesthetic effects, increased plasma cortisol and prolactin and reduced resting EEG spectral power. SA administration was associated with a rapid increase of its levels in the blood. SA did not produce euphoria, cognitive deficits or changes in vital signs. The effects were transient and not dose-related. SA administration was very well tolerated without acute or delayed adverse effects.
Conclusions
SA produced a wide range of transient effects in healthy subjects. The perceptual altering effects and lack of euphoric effects would explain its intermittent use pattern. Such a profile would also suggest a low addictive potential similar to other hallucinogens and consistent with KOR agonism. Further work is warranted to carefully characterize a full spectrum of its effects in humans, to elucidate the underlying mechanisms involved and to explore the basis for individual variability in its effects.
Keywords: Salvinorin A, Salvia, kappa-opioid, hallucinogen, perception, psychosis
Introduction
Salvia divinorum (Salvia) is an increasingly popular recreational drug amongst adolescents and young adults. Salvia, a member of the mint family, has been used for centuries in traditional Mexican religious and medicinal rituals (1, 2). Chewing or smoking Salvia leaves produces depersonalization and auditory and visual hallucinations. Salvinorin A (SA), the primary psychoactive component of Salvia is a potent and highly selective agonist at kappa opiate receptors (KOR) (3). SA has no activity at other receptors systems including dopaminergic, serotonergic or NMDA receptors that are involved in the mechanism of other drugs that produce perceptual abnormalities (3).
Several lines of evidence point to the rising popularity of recreational Salvia and SA use in the US (4–8). National Survey on Drug Use and Health (NSDUH) (2006) data suggest that the rates of SA use among adolescents (0.6%) and young adults (1.7%) are greater than that of other common hallucinogenic drugs such as LSD, ketamine, PCP and DMT (9). These rates of SA exposure have increased from 1.5% in 2006 to 3.7% by 2010. Salvia products are readily available both locally and via the Internet. Salvia and SA are not federally regulated in the US, although the DEA has listed them as “drugs of concern” and 13 states have begun to regulate their use.
Human Data on the effects of SA
The human literature on SA effects is limited by a preponderance of anecdotal reports (1, 5–7, 9–11). Salvia produces a rapid onset of transient mood alterations, dissociative symptoms and psychotomimetic effects. The anecdotal literature is difficult to interpret because of the use of variable doses and routes of administration, the use of other drugs before, with or after SA use, variable set and setting and a lack of characterization of the subject samples.
Experimental data with Salvia/SA in humans include one study that developed a method of to detect SA in biological fluids after smoking Salvia (12) and four on the effects of SA (13–16). Seibert (1994) described subjective effects of oral, sublingual, and inhaled Salvia and SA administration in an open-label, uncontrolled study in twenty subjects (15). Mendelson et al reported no effects and undetectable SA blood levels with SA administered sublingually at doses up to 4 mg in eight subjects (14). The lack of effects in this study was likely due to low bioavailability of sublingual SA. Johnson et al administered 16 doses of inhaled SA in a fixed-order, ascending-dose, placebo-controlled, single-blind study of four subjects (13). Subjects experienced a rapid onset of transient hallucinogenic effects without any physiological changes. Finally, Addy (2011), studied 30 healthy subjects who self-administered 1017 µg of inhaled SA on dried Salvia leaves or placebo (unenhanced dried Salvia leaves) in a partially blinded manner (blinded only to the first dose) (16). The latter two studies, while demonstrating the hallucinatory effects of SA, were also limited in the lack of randomization or objective outcomes, the use of fixed ascending order of doses (13) and the use of Salvia leaves as the vehicle and control (16).
SA has been reported to produce behavioral effects, cognitive impairments and prolactin elevations in animals. Other KOR agonists have been reported to increase prolactin and cortisol levels in rodents (17, 18) and humans (19), and to reduce resting EEG power in rats (20). Resting EEG is a potentially informative as it is sensitive to drug-induced changes in consciousness (21–23) and is altered in psychotic (24, 25). Finally, the pharmacokinetics of SA have not been studied in humans. SA is rapidly metabolized to Salvinorin B (SB), which is a much less potent KOR agonist (26). However, these outcomes have not been studied thus far in humans.
The behavioral, subjective, cognitive, endocrine and psychophysiological effects of SA and it’s pharmacokinetic profile in humans were characterized in a controlled study to address the limitations and gaps in the existing literature.
Methods
This study was approved by the IRBs at Yale University and the VA Connecticut Healthcare System and the FDA, and was carried out in accordance with the Helsinki Declaration of 1975.
Study Design
This double blind, randomized, placebo controlled, counterbalanced, cross over, 3-day study was conducted at the Neurobiological Studies Unit (VA Connecticut Healthcare System, West Haven, CT).
Subjects
As detailed in the Supplement, a rigorous screening was conducted to include medically and psychiatrically healthy subjects, aged between 18 and 55 years with previous exposure to Salvia. Since Salvia users characteristically use other drugs (27), subjects with exposure to other drugs were included in order that the sample be representative. History provided by subjects was corroborated with an outside informant nominated by the subject. Subjects were instructed to refrain from alcohol, illicit drugs or prescription drugs from a week before the first test day until study completion. Subjects were paid $225 per test day for their participation.
General Procedure and Test Days
Subjects presented to the research unit, about 1 hour prior to the scheduled time of administration of drug during which they underwent a urine toxicology exam and pregnancy test (in women), had an intravenous (IV) line placed and underwent baseline ratings. In-study safety procedures were in place as described previously (28). Prospective safety assessments were performed the day after the first and last test days and 1 and 3 months after study completion.
Drugs
Subjects on each test day inhaled one of 2 doses of active SA or placebo (in an aluminum container) administered through a commercially available vaporizer (see Supplement for details). SA was obtained from the laboratory of Dr. Bruce M. Cohen, McLean Hospital, Belmont, MA and stored in the research pharmacy at VA Connecticut Healthcare System, West Haven, CT. On the morning of each test day, the SA dose was prepared in the designated container by the research pharmacists. Placebo consisted of the container devoid of any SA. Subjects and raters were blinded to the dose administered.
Outcome measures (see Supplement for greater detail):
Subjective and behavioral effects
Subjective feeling states such as “high”, “anxious”, “drowsy”, “irritable”, and “nervous” were measured using a self-reported visual analog scale (VAS). Psychotomimetic symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) (29) and the Psychotomimetic States Inventory (PSI) (30). Perceptual alterations were measured using the Clinician Administered Dissociative Symptoms Scale (CADSS) (31) and the Hallucinogen Rating Scale (HRS)(32, 33).
Cognitive effects
Phonological processing, working memory and attention were assessed using a simple cognitive battery comprising of the Digits Forward and Backward and Letter Number Sequence tasks of the WAIS-R (34).
Neuroendocrine effects
Plasma cortisol and prolactin were assayed at various time points before and after SA inhalation. Levels were analyzed in duplicate by the Yale Center for Clinical Investigation, Yale University, New Haven, CT.
SA and SB levels
Both SA and SB levels were analyzed by Dr. E. Thomas Everhart at the Drug Dependence Research Center (Langley Porter Psychiatric Institute, University of California) using a slightly modified liquid-chromatographic-atmospheric pressure chemical ionization-tandem mass spectrometric method (14) (see Supplement for details). The limits of quantitation were 0.5 ng/ml for both SA and SB in plasma and urine.
Psychophysiological effects
Three minutes of resting state EEG was obtained as subjects sat still with their eyes closed immediately following SA inhalation.
Data Analysis
Initially, data were examined descriptively using means, standard deviations and graphs. Each outcome was tested for normality using Kolmogorov-Smirnov test statistics and normal probability plots. All PANSS, PSI, HRS and cognitive battery outcomes were approximately normally distributed. These outcomes were analyzed using linear mixed models, which included SA dose (placebo, low (8mg), and high (12mg)) and time (pre- vs. post-inhalation) as within-subjects explanatory factors and random subject effects. The best-fitting variance-covariance structure was chosen based on information criteria. Significant interactions between dose and time were interpreted by appropriate post-hoc tests. Similar models were used to compare physiological measures and serum SA and hormone (log) levels across time. All CADSS and VAS outcomes were highly skewed. Thus, these non-normal outcomes were analyzed using the nonparametric approach for repeated measures data, in which data are ranked and then fitted using a mixed-effects model with an unstructured variance-covariance matrix and p-values adjusted for ANOVA-type statistics (ATS)(35). In these models, SA (placebo, low dose, high dose and time (pre- vs. post-treatment)) were included as within-subjects explanatory factors. EEG power frequencies were compared using linear mixed models with dose and electrode (Cz, Pz, Oz) as within-subjects factors. All data were analyzed using SAS, version 9.2 (Cary, NC).
Results
Subjects were young (23.8 ± 3.2 years), predominantly male (90%), with 15.3(± 1.2) years of education, intelligence quotient scores of 117.2 (± 7.1) and low (2.8 ± 2.8) psychosis proneness scores on the Schizotypal Personality Questionnaire (Table S2 in the Supplement). Nine subjects completed all three test days and one dropped out after his second test day. All 10 subjects were included in the analyses. None of the subjects met criteria for alcohol or substance dependence. All subjects had previous exposure to SA) and other illicit substances (Table S3 in the Supplement). For parsimony, only positive results are reported in detail here.
Subjective reports: Listed below are quotations from subjects describing SA-induced changes.
Somaesthetic changes: “I felt a cold prickling feeling on my legs” “…tingling in my fingers” “…felt a pattern sweep over me like a wave… I felt as well as saw the waves…”
Feelings of dissociation: “I felt like I was on a different planet…”
Feelings of detachment: “I could see you and hear you, but I felt separated and distant from you…”
Heightened awareness of visual and/or auditory stimuli: “the patterns on the curtain appeared more prominent…the contrast was more vivid”, “the air-conditioner seemed louder…”
Withdrawal into Self: “…I wished I didn’t have to answer questions…”, “‥wished I was left alone…”
Changes in concentration/increased distractibility: “‥felt distracted by background sounds” “I felt mesmerized by the pattern on the door”
Increased intrusive thoughts (interfering with ability to concentrate): “‥lot of thoughts about my day…”
Changes in mood: “calmer”, “more comfortable”
Subjective effects (VAS)
There was a main effect of SA administration on feeling “drowsy” [ATS=4.55, num df=1.91, p=0.01] such that both low [ATS=4.9, num df=1, p=0.03] and high [ATS=3.99, num df=1, p<0.05] doses of SA produced less drowsiness compared to placebo. SA administration did not produce any changes on the VAS for feeling “high”, “calm”, “sad”, “irritable” or “anxious”.
Psychotomimetic Effects
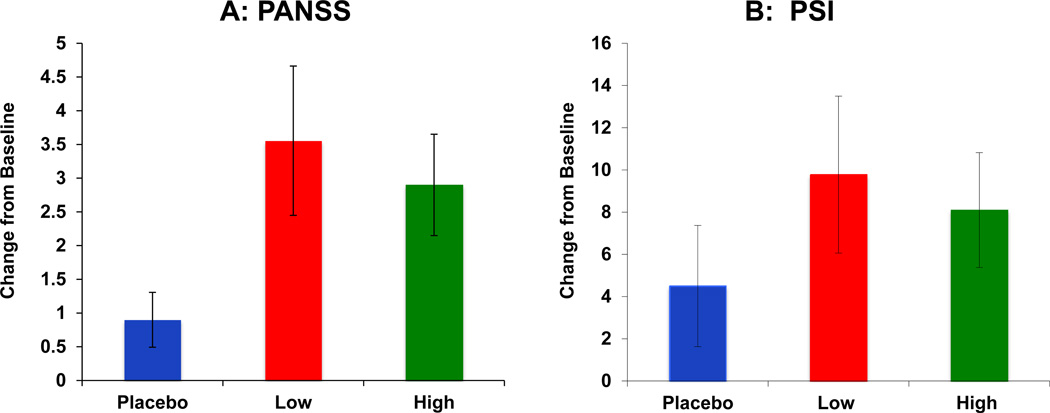
PANSS (Figure 1A)
Figure 1.
Salvinorin A (SA) administration produced transient psychotomimetic effects measured as increases on PANSS positive subscale (Figure 1A) and the PSI (Figure 1B).
 Placebo
Placebo  Salvinorin A Low Dose (8 mg)
Salvinorin A Low Dose (8 mg)  Salvinorin A High Dose (12 mg) SA Doses are depicted as bars along the X axis.
Salvinorin A High Dose (12 mg) SA Doses are depicted as bars along the X axis.
Change in PANSS (1A) and PSI scores (1B) are on the Y-axis. Error bars represent S.E.M.
PANSS positive subscale scores range from 1–7 per item × 7 items. PSI scores range from 0–3 × 48 items.
SA produced increases in psychotomimetic effects as measured by the PANSS Positive scores. The dose X time interaction was significant [F(2,43)= 3.12, p= 0.05]. Post hoc analyses revealed that low dose SA increased positive symptoms significantly relative to placebo [F(1,43)= 4.62, p= 0.04], while these increases trended toward significance for the high dose [F(1,43)= 3.37, p= 0.07]. SA produced an increase in PANSS General Psychopathology scores: the dose X time interaction was significant [F (2, 43)= 3.52, p= 0.04], driven by an increase in general symptoms for low dose SA [F (1,43)= 4.65, p= 0.04]. Finally, SA also produced an increase in PANSS total scores: the dose X time interaction trended toward significance [F(2,43)= 2.83, p= 0.07]. Post hoc analyses revealed that this effect was driven by increases due to low dose SA [F(1,43)= 4.1, p< 0.05].
PSI (Figure 1B)
The PSI which also measured SA-induced psychotomimetic effects, showed a dose X time interaction [F(2,43)= 3.11, p= 0.05] driven by increases on PSI scores due to both low [F(1,43)= 8.01, p< 0.01] and high dose SA [F(1,43)= 10.29, p< 0.01].
Perceptual Alterations
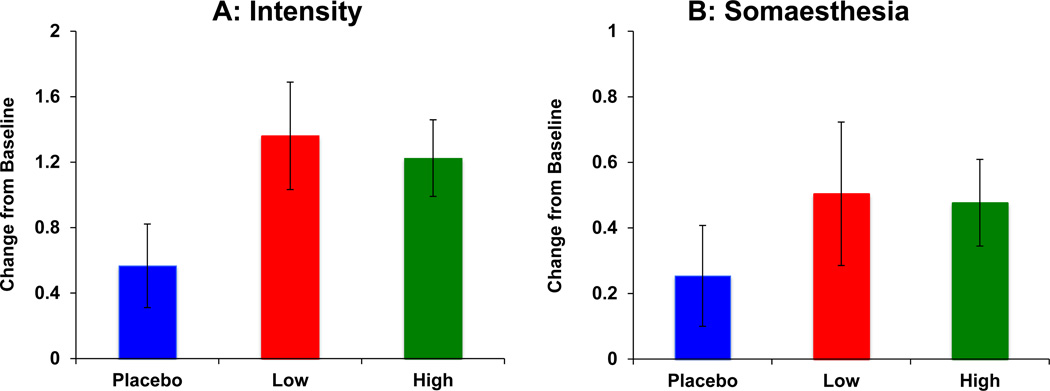
HRS (Figure 2)
Figure 2.
Salvinorin A (SA) administration produced transient perceptual alterations measured as increases on the Hallucinogen Rating Scale (HRS) “Intensity” (Figure 2A) and “Somaesthesia” (Figure 2B) subscales.
 Placebo
Placebo  Salvinorin A Low Dose (8 mg)
Salvinorin A Low Dose (8 mg)  Salvinorin A High Dose (12 mg) SA Doses: Placebo, Low (8mg) and High (12mg) are depicted as bars along the X axis.
Salvinorin A High Dose (12 mg) SA Doses: Placebo, Low (8mg) and High (12mg) are depicted as bars along the X axis.
Change in HRS scores is on the Y-axis. Error bars represent S.E.M.
HRS scores range from 1–4.
SA administration induced perceptual alterations measured by the HRS subscales for Intensity, Somaesthesia and Perception. On the “Intensity” subscale (Figure 2A), there was a main effect of dose [ATS= 3.71, df= 1.45, p = 0.04], driven by increases in scores due to the low dose [ATS= 4.24, df= 1, p = 0.04]. On the “Somaesthesia” subscale (Figure 2B) there was a main effect of dose [ATS= 4.11, df= 1.9, p = 0.02], primarily driven by increases due to the low dose [ATS= 11.4, df= 1, p < 0.001]. There was also a main effect of dose on “Perception” [ATS= 3.35, df= 1.65, p = 0.04], again driven by increases due to low dose [ATS= 4.13, df= 1, p = 0.04].
CADSS
SA administration did not produce any significant changes on the CADSS patient rated [ATS= 0.96. df= 1.73, p= 0.37] or clinician rated sub-scales [ATS= 0.73, df=1.36, p=0.43].
Cognitive Battery
SA administration did not produce any effects on performance on the Digit Forward [F(2,9)= 0.4, p= 0.68], Digit Backward [F(2,17)= 0.33, p= 0.73], or Letter Number Sequencing tasks [F(2,17)= 0.54, p= 0.59].
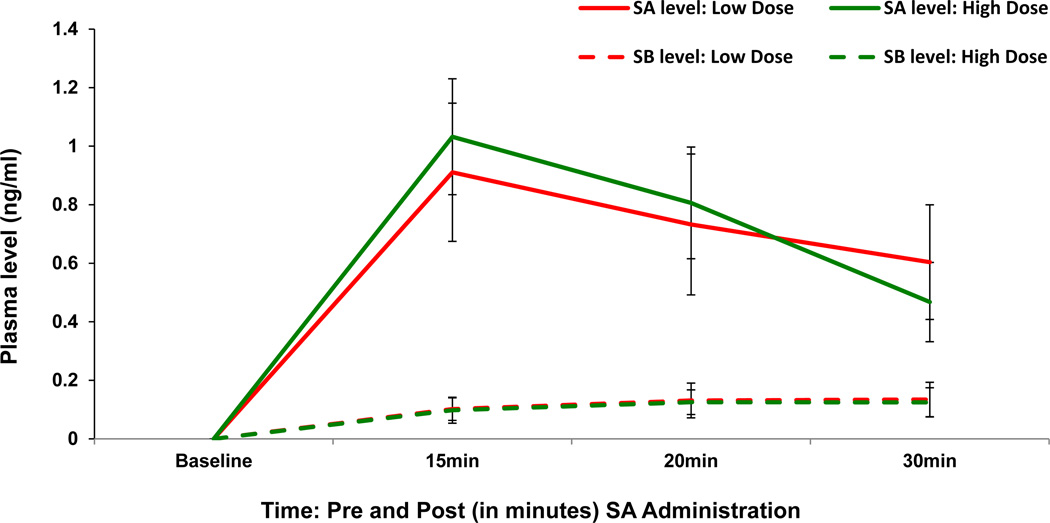
Plasma SA and SB levels (Figure 3)
Figure 3.
Salvinorin A (SA) produced increases in plasma levels of SA and Salvinorin B (SB) measured in a subsample of subjects (n=7).
 Placebo
Placebo  Salvinorin A Low Dose (8 mg)
Salvinorin A Low Dose (8 mg)  Salvinorin A High Dose (12 mg) Neither SA nor SB levels were detectable prior to drug administration (baseline).
Salvinorin A High Dose (12 mg) Neither SA nor SB levels were detectable prior to drug administration (baseline).
Time is on the X axis as Baseline (Pre) and 15, 20 and 30 minutes after SA administration
Plasma levels of SA and SB are on the Y-axis.
Separate lines depict the low and high dose of SA. Error bars represent S.E.M.
Only samples from active dose conditions were analyzed for SA and SB levels; the main comparison was between blood levels before and after drug administration. Both doses of SA produced a rapid increase in SA levels compared to pre-administration levels [F (3,38)= 29.4, p< 0.0001] but without significant differences between the two active doses. The levels of SA peaked at + 15 minutes post administration. Both doses of SA also produced an increase in SB levels compared to pre-administration levels [F(3,38)= 8.66, p= 0.0002].
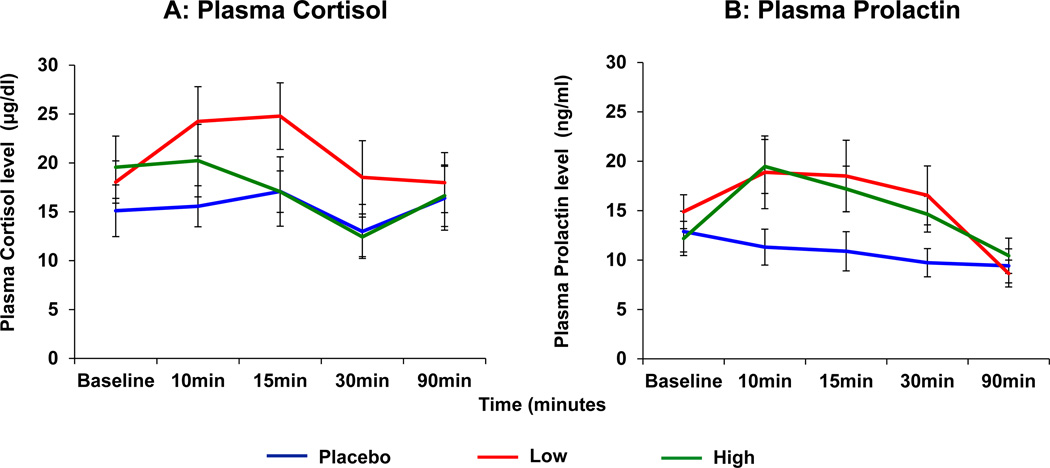
Neuroendocrine Effects (Figure 4)
Figure 4.
Salvinorin A (SA) administration produced elevations in plasma Cortisol (Figure 4A) and Prolactin (Figure 4B).
Time is on the X axis as Baseline (Pre) and 10, 15, 30 and 90 minutes after SA administration
Plasma Cortisol (µg/dl) and Prolactin (ng/ml) levels are on the Y-axis.
Separate lines depict the doses of SA. Error bars represent S.E.M.
Cortisol Levels (Figure 4A): Low dose SA significantly elevated plasma cortisol levels [F(2,120)= 3.11, p< 0.05], which returned to baseline 60 minutes after SA inhalation [F(4,120)=18.69, p<0.0001].
Prolactin Levels (Figure 4B): Both doses of SA significantly elevated plasma prolactin levels [F(8,120)=4.07, p= 0.0003], which also returned to baseline by 60 minutes after administration.
Physiological Effects
Neither dose of SA produced any significant changes in heart rate, systolic or diastolic blood pressure in any subject.
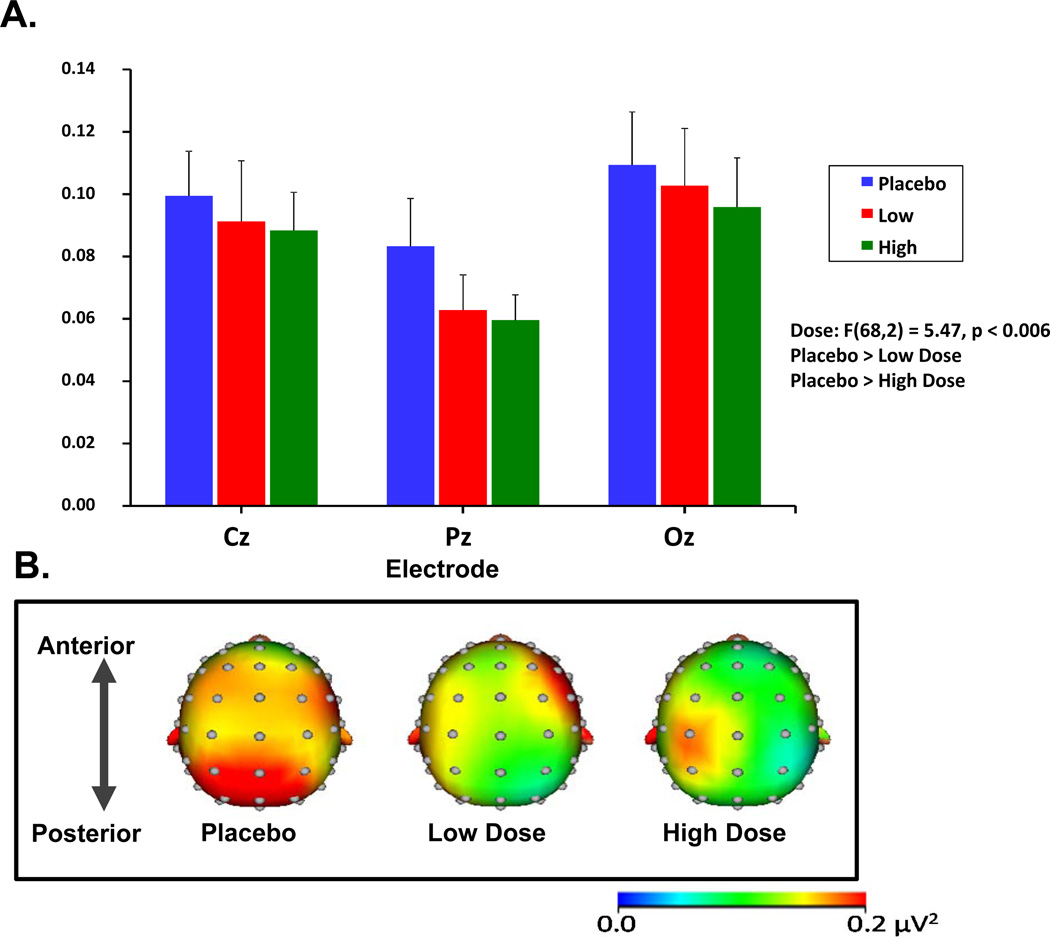
Resting State Electroencephalography (Figure 5)
Figure 5.
Salvinorin A (SA) administration induced reductions in resting beta-band EEG power.
Figure 5A depicts the grand-averaged resting EEG spectral power in the beta range (13–29 Hz) at midline electrode sites. Error bars represent S.E.M.
Figure 5B depicts the topographic maps indicating grand-averaged beta power across the placebo and the two active SA doses.
SA administration decreased resting state EEG spectral power across all frequencies examined (although not all frequency bands reached significance). Compared to placebo, SA was associated with lower beta power at both doses [F(68,2) = 5.47, p < 0.006]. SA also lowered theta power with a trend toward significance [F(68,2) = 2.44, p = 0.09]. The effects of SA on delta, alpha, and gamma frequencies were not statistically significant.
Safety
No serious adverse events (death, hospitalization, or emergency room visit) occurred during or after the study. One subject dropped out for unspecified reasons after reporting no effects on either of the test days in which he participated. No test days were terminated prematurely nor were rescue medications necessary. Exit interviews conducted in a subsample of subjects revealed that subjects felt they had been adequately informed about the risks of the study. Follow-up assessments at 1, 3, and 6 months revealed no new psychiatric symptoms or increased Salvia consumption.
Discussion
This is the first report to our knowledge on a wide range of dose-related subjective, behavioral, cognitive, cardiovascular, psychophysiological and neuroendocrine effects and safety of inhaled SA in a randomized, double-blind, placebo-controlled, crossover, counterbalanced study in healthy humans.
Onset and duration of effects
As expected, SA produced very short lasting psychoactive effects with some psychotomimetic features, most notably somaesthetic changes, dissociative effects and perceptual alterations. Consistent with anecdotal data and experimental reports (13, 16), the onset of SA effects was very rapid (within seconds to minutes) as captured on the HRS “Intensity” subscale, with a peak within 10 minutes and a return to baseline within 30 minutes. No subjects reported any lingering effects at the time of discharge (90 minutes after inhalation) or any persistent or recurrent effects during the safety follow-ups.
Magnitude of effects
The magnitude of psychotomimetic effects induced by SA as measured by the PANSS positive subscale (3.5 point increase) and PSI (10 point increase) was comparable to the effects of delta-9-tetrahydrocannabinol and ketamine on those measures (28, 30, 36).
Comparison of SA administration in this study to recreational use by subjects
Peak effects in this study were rated as only 20% to 30% of the peak effects experienced with recreational SA use. A number of factors might account for the differences, the most obvious being that the drug was delivered in this study more slowly and at lower doses than characteristic of recreational use. While a vaporizer reaches the target temperature within minutes, the typical recreational method of delivery (in which subjects apply direct heat to a glass pipe or aluminum foil containing Salvia) attains this temperature instantaneously. This factor should also be taken into consideration while comparing these data with other studies of inhaled SA (13, 16). Secondly, in this study subjects received pure SA, whereas with recreational use either salvia leaves or extract-enhanced leaves are used. The contribution of other psychoactive compounds present in these preparations may alter subjective effects. Finally, the combination of variable strength of Salvia products and variability in the amount used recreationally makes accurate estimation of recreational dose near impossible. This limits the ability to accurately compare the doses in this study with recreational doses. These considerations notwithstanding, subjects were asked to compare effects in this laboratory study to those associated with recreational use in order to infer how doses used in this study compared to doses used recreationally.
Endocrine effects of SA
Elevations in serum prolactin are a well-recognized biomarker of KOR agonism in rodent and non-human primates (37–39). This study is the first to demonstrate endocrine effects of SA in humans and thus, provides clear objective evidence of the centrally-mediated effects of SA. KORs are abundantly distributed in the hypothalamus (40, 41) and KOR agonists are known to increase prolactin levels, but the exact mechanism remains unclear. One possibility is that SA via KOR agonism may lower dopamine levels in the tuberoinfundibular pathway similar to the effects of KOR agonism on dopamine in other brain regions (42–44).
This is the first report to our knowledge on the cortisol elevating effects of SA in humans; this effect is consistent with the cortisol elevating effects of other KOR agonists observed in animals and humans (17, 19). The cortisol stimulatory effect in nonhuman primates was shown to be specific to KOR agonism, not produced by Mu or Delta opioid agonists and was blocked by a selective KOR antagonist (17). Collectively, the results of the current study and previous studies demonstrate that similar to other KOR agonists, SA stimulates the hypothalamic-pituitary axis (HPA) activity in humans.
Psychophysiological (EEG) Effects
No previous study has examined the psychophysiological effects of SA in humans. While all doses of SA decreased broadband resting-state EEG spectral power, the reductions were significant in the beta-band (13 to 29 Hz), and trended toward significance in the theta band (4 to 7 Hz). These effects are consistent with a previous human study showing that the KOR agonist pentazocine decreased resting EEG power in the theta, alpha, and beta frequency bands (45). However, the pattern of SA effects on resting EEG are different from that of other hallucinogens such as mescaline, ketamine and ayahuasca (46–48) which are associated with increases or no change in beta power. These differences serve to highlight the fact that SA produces its effects via a unique mechanism and thus may have a distinct psychophysiological profile. While the neurochemical mechanisms of these changes, as well as their functional implications remain unclear, the current findings suggest that resting EEG may provide an objective, behaviorally-independent index of KOR agonist effects on brain function.
Thus the inclusion of outcomes such as resting EEG, hormonal levels and SA and SB levels in this study provide objective biological correlates of SA effects in humans. The method of delivery, doses of SA and overall study design are validated by effects detected on subjective as well as objective outcomes. This is particularly crucial, given the wide variability in subjective effects that may be reported in such a study.
Relevance to Abuse
SA is now recognized as a potential drug of abuse with increasing use especially amongst youth. However, several lines of evidence suggest that in contrast to other drugs of abuse with addictive liability, SA is less likely to be used compulsively, repetitively or persistently. In this study SA did not produce euphoria, an effect that is common to most addictive drugs. Furthermore, the findings from surveys of SA users (5) and reports from our subjects suggest that recreational Salvia use is sporadic, in contrast to the compulsive, repetitive use and persistent use pattern of addictive drugs.
Addictive drugs share in common the capacity to increase dopamine in the nucleus accumbens (NAcc). SA and synthetic kappa opioid agonists (U-69593, U-50488 and R-84760) decrease dopamine levels in the nucleus accumbens of rodents (43, 49–52). Synthetic KOR agonists and SA induce conditioned place aversion (53–57). KOR agonists reduce cocaine self-administration (58–61), cocaine-induced hyperlocomotion (57, 62–64), cocaine-induced reinstatement of drug self-administration (61, 65–67), and cocaine-induced behavioral sensitization (62, 68–71). However, one study did show intracerebroventricular SA self-administration and conditioned place preference in mice at relatively low doses (72). KOR agonists also reduce intracranial self-stimulation (ICSS) (73) consistent with a profile of aversive effects.
Collectively the evidence suggests that SA and other KOR agonists are likely to have low addiction liability. In fact, KOR agonists have been studied as potential treatment for addictions (74–78), but further development has been hampered by adverse effects (73, 77, 79–84). Most likely, SA is used for its perceptual altering effects. Since the concept of drug “abuse” includes “use for nontherapeutic effects,” SA may be considered an agent with recreational abuse liability similar to Lysergic Acid Diethylamide (LSD). The intensity of SA effects reported by recreational users is highly variable ranging from mild perceptual alterations to frank psychosis prompting contact with poison control or necessitating emergency care and hospitalization (85, 86). In the current study too, the intensity of SA effects showed significant inter-individual variability. Finally, in individuals who may be vulnerable to psychotic illnesses or with an established psychotic disorder, SA exposure may have particularly devastating consequences.
Relevance to Psychosis
The results of this study are also relevant to understanding the pathophysiology of psychosis and to drug development. According to the dominant DA hypothesis, increased mesolimbic dopamine is implicated in the pathophysiology of the positive symptoms of psychosis (87). SA induces psychosis-like effects but decreases dopamine in several brain regions (43, 44); which arguably was indirectly reflected in the increased prolactin levels observed in this study. Furthermore, the DA D2 receptor antagonist haloperidol does not attenuate the deficits in prepulse inhibition produced by KOR activation (88). SA’s only known mechanism of action is KOR agonism. It does not have affinity for serotonin (5-HT2), dopamine (DA), cannabinoid (CB1R) or N-Methyl-D-Aspartate (NMDA) receptor systems that have been implicated in the mechanism of other drugs that produce psychotomimetic effects (3). Therefore, KOR agonism may be relevant to the pathophysiology of psychosis and the study of the KOR system using a probe such as SA may shed more light on the involvement of this system in the pathophysiology of psychosis. Further studies are necessary to investigate the precise mechanism/s underlying the psychotomimetic effects of SA. Finally, while admittedly simplistic and speculative, the association between KOR agonism and psychosis raises the possibility that KOR antagonists might have antipsychotic potential.
Strengths and limitations
Important strengths of this study include the double-blind, randomized, placebo-controlled, crossover design, the use of multiple doses, the estimation of blood levels, and the use of a range of objective and subjective measures. While the standardized set, setting and validation of method of delivery using objective measures and blood levels are strengths of this experimental approach, they limit generalizability of these findings to recreational use. Finally, the lack of differences in both plasma levels and responses between the two doses did not permit characterization of the dose-response profile of SA.
Future directions
Future studies should focus on characterizing the safety, tolerability and effects of a wider dose range of SA in humans. Further, although preclinical data suggest that SA acts solely via the KOR, whether this is indeed the case in humans is unclear. Studies examining the effects of KOR blockade on the effects of SA, and receptor-imaging studies will help answer these questions.
Supplementary Material
ACKNOWLEDGMENTS
This research project was funded in part by a NARSAD Young Investigator Award to MR and a grant from NIDA (R21 DA029826- 01A1 to MR. The authors wish to acknowledge support from the (1) Department of Veterans Affairs, (2) National Institute of Mental Health, (3) National Institute of Drug Abuse, (4) National Institute of Alcoholism and Alcohol Abuse (NIAAA) and (5) the Yale Center for Clinical Investigation (YCCI). The authors also thank Angelina Genovese, R.N.C., M.B.A.; Michelle San Pedro, R.N.; Elizabeth O’Donnell, R.N.; Brenda Breault, R.N., B.S.N.; Sonah Yoo, R.Ph.; Rachel Galvan, R.Ph.; and Willie Ford at the VA Connecticut Healthcare System, West Haven Campus for their contributions to the success of this project. The authors thank Drs Bruce Cohen and Thomas Munro of McLean Hospital for the supply of Salvinorin A and the analyses to support the IND application for this study. The authors also thank Dr Thomas Everhart for conducting the analysis of SA and SB levels and YCCI for conducting the hormonal analyses.
Mohini Ranganathan has received research grant support administered through Yale University School of Medicine from Eli Lilly Inc. Deepak Cyril D’Souza received research grant support administered through Yale University School of Medicine from Astra Zeneca, Abbott Laboratories, Eli Lilly Inc., Organon, Pfizer Inc., and Sanofi; he is a consultant for Bristol Meyers Squibb.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE/CONFLICT OF INTEREST
Patrick Skosnik, Ashley Schnakenberg, Brian Pittman, Andrew Sewell, Bruce Cohen report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, et al. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations. The Journal of pharmacology and experimental therapeutics. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez D, Riba J, Bouso JC, Gomez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug and Alcohol Dependence. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford JA, Watkins WC, Blumenstein L. Correlates of Salvia divinorum use in a national sample: findings from the 2009 National Survey on Drug Use and Health. Addictive Behaviors. 2011;36:1032–1037. doi: 10.1016/j.addbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Baggott MJ, Erowid E, Erowid F, Galloway GP, Mendelson J. Use patterns and self-reported effects of Salvia divinorum: an internet-based survey. Drug and Alcohol Dependence. 2010;111:250–256. doi: 10.1016/j.drugalcdep.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Lange JE, Daniel J, Homer K, Reed MB, Clapp JD. Salvia divinorum: effects and use among YouTube users. Drug and Alcohol Dependence. 2010;108:138–140. doi: 10.1016/j.drugalcdep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Nyi PP, Lai EP, Lee DY, Biglete SA, Torrecer GI, Anderson IB. Influence of age on Salvia divinorum use: results of an Internet survey. Journal of Psychoactive Drugs. 2010;42:385–392. doi: 10.1080/02791072.2010.10400701. [DOI] [PubMed] [Google Scholar]
- 8.Sumnall HR, Measham F, Brandt SD, Cole JC. Salvia divinorum use and phenomenology: results from an online survey. Journal of Psychopharmacology. 2010 doi: 10.1177/0269881110385596. [DOI] [PubMed] [Google Scholar]
- 9.Paulzen M, Grunder G. Toxic psychosis after intake of the hallucinogen salvinorin A. J Clin Psychiatry. 2008;69:1501. doi: 10.4088/jcp.v69n0919c. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez D, Riba J, Bouso JC, Gomez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006 doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Vortherms TA, Roth BL. Salvinorin A: from natural product to human therapeutics. Mol Interv. 2006;6:257–265. doi: 10.1124/mi.6.5.7. [DOI] [PubMed] [Google Scholar]
- 12.Pichini S, Abanades S, Farre M, Pellegrini M, Marchei E, Pacifici R, et al. Quantification of the plant-derived hallucinogen Salvinorin A in conventional and non-conventional biological fluids by gas chromatography/mass spectrometry after Salvia divinorum smoking. Rapid Commun Mass Spectrom. 2005;19:1649–1656. doi: 10.1002/rcm.1970. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug and Alcohol Dependence. 2011;115:150–155. doi: 10.1016/j.drugalcdep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendelson JE, Coyle JR, Lopez JC, Baggott MJ, Flower K, Everhart ET, et al. Lack of effect of sublingual salvinorin A, a naturally occurring kappa opioid, in humans: a placebo-controlled trial. Psychopharmacology (Berl) 2011;214:933–939. doi: 10.1007/s00213-010-2103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 16.Addy PH. Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2470-6. [DOI] [PubMed] [Google Scholar]
- 17.Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko MC. Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology. 2008;33:478–486. doi: 10.1016/j.psyneuen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackford SP, Little PJ, Kuhn CM. Mu- and kappa-opiate receptor control of prolactin secretion in rats: ontogeny and interaction with serotonin. Endocrinology. 1992;131:2891–2897. doi: 10.1210/endo.131.6.1332851. [DOI] [PubMed] [Google Scholar]
- 19.Ur E, Wright DM, Bouloux PM, Grossman A. The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. British Journal of Pharmacology. 1997;120:781–784. doi: 10.1038/sj.bjp.0700971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coltro Campi C, Clarke GD. Effects of highly selective kappa-opioid agonists on EEG power spectra and behavioural correlates in conscious rats. Pharmacol Biochem Behav. 1995;51:611–616. doi: 10.1016/0091-3057(94)00384-u. [DOI] [PubMed] [Google Scholar]
- 21.Valenza G, Carboncini MC, Virgillito A, Creatini I, Bonfiglio L, Rossi B, et al. EEG complexity drug-induced changes in disorders of consciousness: A preliminary report. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3724–3727. doi: 10.1109/IEMBS.2011.6090633. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald MK, Roehrs TA. Mu-opioid self-administration vs passive administration in heroin abusers produces differential EEG activation. Neuropsychopharmacology. 2005;30:212–221. doi: 10.1038/sj.npp.1300596. [DOI] [PubMed] [Google Scholar]
- 23.Bocker KB, Hunault CC, Gerritsen J, Kruidenier M, Mensinga TT, Kenemans JL. Cannabinoid modulations of resting state EEG theta power and working memory are correlated in humans. J Cogn Neurosci. 2010;22:1906–1916. doi: 10.1162/jocn.2009.21355. [DOI] [PubMed] [Google Scholar]
- 24.Venables NC, Bernat EM, Sponheim SR. Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophr Bull. 2009;35:826–839. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sponheim SR, Clementz BA, Iacono WG, Beiser M. Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biol Psychiatry. 2000;48:1088–1097. doi: 10.1016/s0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- 26.Hooker JM, Munro TA, Beguin C, Alexoff D, Shea C, Xu Y, et al. Salvinorin A and derivatives: protection from metabolism does not prolong short-term, whole-brain residence. Neuropharmacology. 2009;57:386–391. doi: 10.1016/j.neuropharm.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perron BE, Ahmedani BK, Vaughn MG, Glass JE, Abdon A, Wu LT. Use of Salvia divinorum in a Nationally Representative Sample. The American journal of drug and alcohol abuse. 2011 doi: 10.3109/00952990.2011.600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 29.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 30.Mason OJ, Morgan CJ, Stefanovic A, Curran HV. The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res. 2008;103:138–142. doi: 10.1016/j.schres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 32.Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- 33.Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behavioural Brain Research. 1996;73:121–124. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- 34.Wechsler D. The Psychological Corporation. 3rd ed. San Antonio, TX: 1997. Administration and scoring manual for the Wechsler Adult Intelligence Scale. [Google Scholar]
- 35.Brunner E, Domhof S, Langer F. Nonparametric analysis of longitudinal data in factorial experiments. New York, NY: John Wiley and Sons; 2002. [Google Scholar]
- 36.Krystal JH, Perry E, Gueorguieva R, Belger A, Madonick S, A A-D, et al. Comparative and Interactive Human Psychopharmacologic Effects of Ketamine and Amphetamine: Implications for Glutamatergic and Dopaminergic Model Psychoses and Cognitive Function. Archives of General Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- 37.Butelman ER, Mandau M, Tidgewell K, Prisinzano TE, Yuferov V, Kreek MJ. Effects of salvinorin A, a kappa-opioid hallucinogen, on a neuroendocrine biomarker assay in nonhuman primates with high kappa-receptor homology to humans. The Journal of pharmacology and experimental therapeutics. 2007;320:300–306. doi: 10.1124/jpet.106.112417. [DOI] [PubMed] [Google Scholar]
- 38.Butelman ER, Harris TJ, Kreek M. Apparent efficacy of kappa-opioid receptor ligands on serum prolactin levels in rhesus monkeys. European Journal of Pharmacology. 1999;383:305–309. doi: 10.1016/s0014-2999(99)00640-8. [DOI] [PubMed] [Google Scholar]
- 39.Butelman ER, Kreek MJ. kappa-Opioid receptor agonist-induced prolactin release in primates is blocked by dopamine D(2)-like receptor agonists. European Journal of Pharmacology. 2001;423:243–249. doi: 10.1016/s0014-2999(01)01121-9. [DOI] [PubMed] [Google Scholar]
- 40.Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- 41.Sim-Selley LJ, Daunais JB, Porrino LJ, Childers SR. Mu and kappa1 opioid-stimulated [35S]guanylyl-5'-O-(gamma-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience. 1999;94:651–662. doi: 10.1016/s0306-4522(99)00344-9. [DOI] [PubMed] [Google Scholar]
- 42.Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. The Journal of pharmacology and experimental therapeutics. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 44.Grilli M, Neri E, Zappettini S, Massa F, Bisio A, Romussi G, et al. Salvinorin A exerts opposite presynaptic controls on neurotransmitter exocytosis from mouse brain nerve terminals. Neuropharmacology. 2009;57:523–530. doi: 10.1016/j.neuropharm.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 45.Bromm B, Ganzel R, Herrmann WM, Meier W, Scharein E. Pentazocine and flupirtine effects on spontaneous and evoked EEG activity. Neuropsychobiology. 1986;16:152–156. doi: 10.1159/000118317. [DOI] [PubMed] [Google Scholar]
- 46.Wikler A. Clinical and electroencephalographic studies on the effects of mescaline, N-allylnormorphine and morphine in man; a pharmacologic analysis of the functions of the spontaneous electrical activity of the cerebral cortex. The Journal of nervous and mental disease. 1954;120:157–175. [PubMed] [Google Scholar]
- 47.Riba J, Anderer P, Morte A, Urbano G, Jane F, Saletu B, et al. Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. British Journal of Clinical Pharmacology. 2002;53:613–628. doi: 10.1046/j.1365-2125.2002.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maksimow A, Sarkela M, Langsjo JW, Salmi E, Kaisti KK, Yli-Hankala A, et al. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2006;117:1660–1668. doi: 10.1016/j.clinph.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Donzanti BA, Althaus JS, Payson MM, Von Voigtlander PF. Kappa agonist-induced reduction in dopamine release: site of action and tolerance. Res Commun Chem Pathol Pharmacol. 1992;78:193–210. [PubMed] [Google Scholar]
- 50.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- 52.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;173:146–152. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]
- 53.Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- 54.Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- 56.Mori T, Nomura M, Nagase H, Narita M, Suzuki T. Effects of a newly synthesized kappa-opioid receptor agonist, TRK-820, on the discriminative stimulus and rewarding effects of cocaine in rats. Psychopharmacology (Berl) 2002;161:17–22. doi: 10.1007/s00213-002-1028-z. [DOI] [PubMed] [Google Scholar]
- 57.Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- 58.Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- 59.Glick SD, Visker KE, Maisonneuve IM. Effects of cyclazocine on cocaine self-administration in rats. Eur J Pharmacol. 1998;357:9–14. doi: 10.1016/s0014-2999(98)00548-2. [DOI] [PubMed] [Google Scholar]
- 60.Kuzmin AV, Semenova S, Gerrits MA, Zvartau EE, Van Ree JM. Kappa-opioid receptor agonist U50,488H modulates cocaine and morphine self-administration in drug-naive rats and mice. Eur J Pharmacol. 1997;321:265–271. doi: 10.1016/s0014-2999(96)00961-2. [DOI] [PubMed] [Google Scholar]
- 61.Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- 62.Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–338. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- 63.Vanderschuren LJ, Schoffelmeer AN, Wardeh G, De Vries TJ. Dissociable effects of the kappa-opioid receptor agonists bremazocine, U69593, and U50488H on locomotor activity and long-term behavioral sensitization induced by amphetamine and cocaine. Psychopharmacology (Berl) 2000;150:35–44. doi: 10.1007/s002130000424. [DOI] [PubMed] [Google Scholar]
- 64.Collins SL, Gerdes RM, D'Addario C, Izenwasser S. Kappa opioid agonists alter dopamine markers and cocaine-stimulated locomotor activity. Behav Pharmacol. 2001;12:237–245. doi: 10.1097/00008877-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Schenk S, Partridge B, Shippenberg TS. Reinstatement of extinguished drug-taking behavior in rats: effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology (Berl) 2000;151:85–90. doi: 10.1007/s002130000476. [DOI] [PubMed] [Google Scholar]
- 66.Sun W, Xue Y, Huang Z, Steketee JD. Regulation of cocaine-reinstated drug-seeking behavior by kappa-opioid receptors in the ventral tegmental area of rats. Psychopharmacology (Berl) 2010;210:179–188. doi: 10.1007/s00213-010-1812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morani AS, Kivell B, Prisinzano TE, Schenk S. Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav. 2009;94:244–249. doi: 10.1016/j.pbb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shippenberg TS, LeFevour A, Heidbreder C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- 69.Morani AS, Schenk S, Prisinzano TE, Kivell BM. A single injection of a novel kappa opioid receptor agonist salvinorin A attenuates the expression of cocaine-induced behavioral sensitization in rats. Behav Pharmacol. 2012;23:162–170. doi: 10.1097/FBP.0b013e3283512c1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morani AS, Kivell B, Prisinzano TE, Schenk S. Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav. 2009 doi: 10.1016/j.pbb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 72.Braida D, Limonta V, Capurro V, Fadda P, Rubino T, Mascia P, et al. Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol Psychiatry. 2008;63:286–292. doi: 10.1016/j.biopsych.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 73.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 74.Neumeyer JL, Mello NK, Negus SS, Bidlack JM. Kappa opioid agonists as targets for pharmacotherapies in cocaine abuse. Pharmaceutica acta Helvetiae. 2000;74:337–344. doi: 10.1016/s0031-6865(99)00044-8. [DOI] [PubMed] [Google Scholar]
- 75.Prisinzano TE, Tidgewell K, Harding WW. Kappa opioids as potential treatments for stimulant dependence. The AAPS journal. 2005;7:E592–E599. doi: 10.1208/aapsj070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mello NK, Negus SS. Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann N Y Acad Sci. 2000;909:104–132. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- 78.Rothman RB, Gorelick DA, Heishman SJ, Eichmiller PR, Hill BH, Norbeck J, et al. An open-label study of a functional opioid kappa antagonist in the treatment of opioid dependence. J Subst Abuse Treat. 2000;18:277–281. doi: 10.1016/s0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 79.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 80.Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- 81.Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–566. [PubMed] [Google Scholar]
- 82.Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- 83.Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- 84.Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9:187–198. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vohra R, Seefeld A, Cantrell FL, Clark RF. Salvia divinorum: exposures reported to a statewide poison control system over 10 years. J Emerg Med. 2011;40:643–650. doi: 10.1016/j.jemermed.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 86.Przekop P, Lee T. Persistent psychosis associated with salvia divinorum use. The American journal of psychiatry. 2009;166:832. doi: 10.1176/appi.ajp.2009.08121759. [DOI] [PubMed] [Google Scholar]
- 87.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 88.Bortolato M, Aru GN, Frau R, Orru M, Fa M, Manunta M, et al. Kappa opioid receptor activation disrupts prepulse inhibition of the acoustic startle in rats. Biol Psychiatry. 2005;57:1550–1558. doi: 10.1016/j.biopsych.2005.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.